Abstract
Objective: Update the available evidence comparing biologic disease-modifying antirheumatic drugs (bDMARDs) in combination with conventional synthetic disease-modifying antirheumatic drugs (CsDMARDs) to bDMARDs in monotherapy in patients with rheumatoid arthritis. Methods: Research was limited to randomized controlled trials. Major outcome: ACR 20 response criteria at 24 weeks. Secondary outcomes: clinical and radiographic criteria at week 24, 52 and 104. Results: 23 trials (6358 patients), including seven bDMARDs and one other molecule: Anbainuo (anti-TNF-R). No study satisfied our search criteria for anakinra, certolizumab and infliximab. Compared to bDMARD monotherapy, combination therapy gives a better ACR 20 at 24 weeks (RR: 0.88 (0.84–0.94)) in fixed and random effect models, and this result is sustained at 52 and 104 weeks. The results were mostly similar for all other outcomes without increasing the risk of adverse effects. Conclusion: This meta-analysis confirms the superiority of combination therapy over monotherapy in rheumatoid arthritis, in accordance to the usual guidelines.
Keywords: rheumatoid arthritis, DMARDs (synthetic), biologic agents, systematic reviews, meta-analysis
1. Introduction
Rheumatoid arthritis (RA) is the most common inflammatory rheumatism in adults [1]. The EULAR and French recommendations stipulate that methotrexate (MTX) should be started as soon as possible after the diagnosis of established RA [1,2]. If remission or low disease activity is not achieved after six months of conventional synthetic DMARDs (CsDMARDs) in patients without factors associated with a poor prognosis, treatment with another CsDMARD may be considered. By contrast, in the presence of a poor prognosis factor, biological treatment should be considered in association with the CsDMARD previously used [3]. In total, ten biological agents (bDMARDs) have been approved for RA treatment. Among them, adalimumab, certolizumab and etanercept have also been approved for use in monotherapy, as have abatacept, anakinra, tocilizumab and sarilumab. New strategies for RA treatment based on the inhibition of Janus kinase (JAK) pathways have been developed, but are not reviewed here. Many RA patients find it difficult to adhere to their CsDMARD prescription because of intolerance or contraindications [4,5,6,7,8,9,10]. It is, therefore, important to evaluate the benefits and harm associated with the use of biological agents in monotherapy. We conducted a systematic review of the literature and a meta-analysis, to update the available evidence already established [11] comparing the use of bDMARD and CsDMARD combination therapy with the use of biotherapy in monotherapy in patients with rheumatoid arthritis.
2. Materials and Methods
A PICOS design (Participants, Interventions, Comparisons and Outcomes) was used for the search strategy. The study selection, assessment of eligibility criteria, data extraction and statistical analyses were performed with a predefined protocol [12]. The reporting of the systematic review and meta- analysis conforms to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) statement [13].
2.1. Literature Search
CENTRAL, EMBASE and MEDLINE were used to identify published reports. Additional randomized controlled trials (RCTs) identified in relevant systematic reviews not retrieved through the electronic databases were then collated.
2.2. Trial Selection
Articles were screened independently by two authors of the review (C.D. and L.F.X.) for inclusion on the basis of their title, abstract and full text if necessary. Disagreements were resolved by consensus or through discussion with a third author (P.H.). Search results were limited to randomized controlled trials (RCTs) with two arms. We included open-label trials in our qualitative and quantitative analysis and performed a meta-regression analysis to determine whether the inclusion of these studies with a lower grade of recommendation modified our findings. We did not include trials for which a full text in English was not available and trials not reporting American College of Rheumatology (ACR) responses. We chose to stop studying inclusion after 104 weeks of follow-up.
2.3. Participants
Adults (>18 years) with RA, according to the 1987 or 2010 classification criteria.
2.4. Types of Interventions
Biologics used alone compared to biologics used in combination with a conventional synthetic DMARD.
2.5. Outcome Measures
We used all the available data published in the selected studies for the meta-analysis. We decided a priori to use the outcome assessment at 24 weeks, 52 and 104 weeks, to determine whether early outcomes were sustained over time. Our major outcome was the ACR 20 response criteria at 24 weeks. The secondary outcomes were: the ACR 20 criteria at 52 and 104 weeks, the ACR 50, 70, 90 response criteria, the DAS 28 remission score (including C-reactive protein (CRP) concentration or erythrocyte sedimentation rate (ESR)), the proportion of Van der Heide-modified Sharp’s scores (mTSS) non-progressor (≤0.5), the proportion of patients withdrawing from the study due to adverse events and for lack of efficacy, improvement in the Health Assessment Questionnaire (HAQ) score > 0.22 and remission according to the Clinical Disease Activity Index (CDAI) and Simple Disease Activity Index (SDAI) scores. Concerning tolerance, we assessed adverse events, serious adverse events, infections, serious infections, cancers and tuberculosis.
2.6. Data Collection and Handling of Missing Data
Data from the trials were independently extracted by two abstractors (C.D. and F.X.L.). We obtained additional information from the online Supplementary Materials of the original RCTs when necessary. For graphic data, we used WebPlotDigitizer-4.2 copyright 2010–2019 Ankit Rohatgi for digital data extraction. Several studies [14,15,16,17] have shown the tool’s reliability and validity to extract data from single-case graphs.
2.7. Risk of Bias
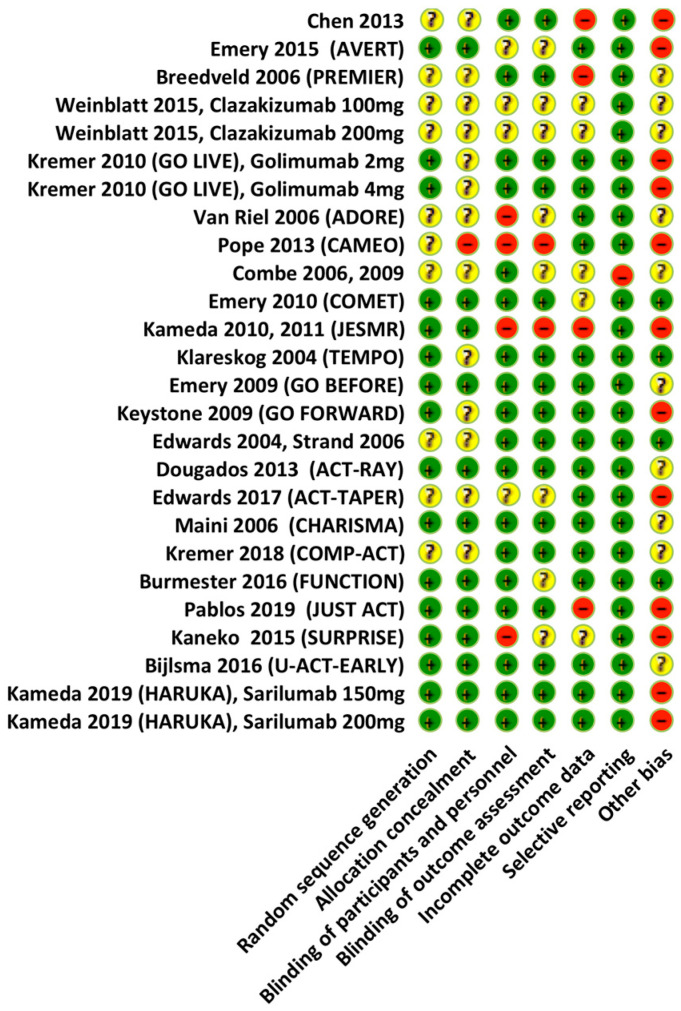
We assessed the risk of bias for each trial included, using the Cochrane ‘Risk of bias’ tool and the following criteria: selection bias, performance bias, detection bias, attrition bias and reporting bias [18]. The risk of bias has been classified as: ‘low’, ‘high’ or ‘unclear’ (due to either a lack of information or uncertainty over the potential for bias). The GRADE score reflects the extent to which we are convinced that the actual effect is close to that estimated in the meta-analysis (Figure 1).
Figure 1.
Assessment of the risk of bias. The Cochrane Collaboration tool used for randomized trials.
2.8. Statistical Analysis
We performed meta-analyses with fixed and random effects models in R version 3.6.1 (5 July 2019) Copyright © 2022 The R Foundation for Statistical Computing. The relative risk (RR) was the metric of choice for binary outcomes, and the mean difference (MD) or the standardized mean difference (SMD) was used for quantitative variables. Inverse variance weighting was used for the pooling of studies [19]. We used the DerSimonian–Laird method to estimate the variance between studies [20]. Between-study heterogeneity was assessed with the Q-test, considering a p-value < 0.05 to be statistically significant. The I² statistic was calculated to quantify the residual heterogeneity, ranging from 0 to 100% [21]. A leave-one-out method was used to identify outlying studies responsible for heterogeneity. Sensitivity analyses were conducted by meta-regression. The criteria included in the meta-regression analysis included: dose titration allowed in the study protocol, mean duration of disease, history of CsDMARD and bDMARD use before inclusion, presence or absence of a disease stabilization phase before randomization, positivity for RF and/or ACPA, severity on the DAS at inclusion, authorization of corticosteroid therapy use during the study and blinding throughout the study. Publication bias was evaluated by a graphical method, with a rank correlation test for funnel plot asymmetry [22].
3. Results
3.1. Study Selection Process
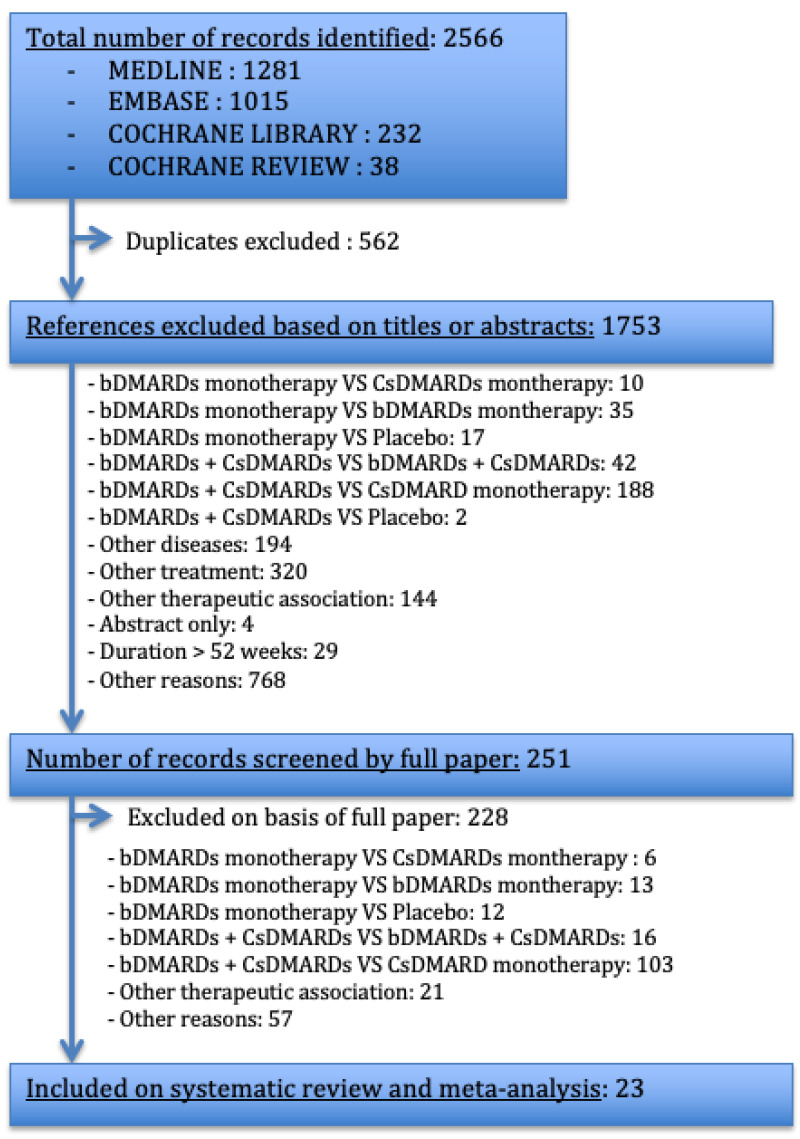
We identified 2566 publications: 1281 from PUBMED, 1015 from EMBASE, 232 from the Cochrane Library and 38 Cochrane reviews (Figure 2). We retained 23 articles, corresponding to 6358 patients. Of these studies, one focused on abatacept [23], one on adalimumab [24], six on etanercept [25,26,27,28,29,30,31,32], one on a similar molecule of etanercept, abainuo [33], three on golimumab [34,35,36,37,38], one on rituximab [39,40], eight on tocilizumab [41,42,43,44,45,46,47,48,49,50], one on sarilumab [51] and one on clazakizumab [52]. Of these studies, four had open-label designs [29,30,32,50]. In the COMET [28], COMP-ACT [48] and JUST-ACT [47] studies, the participants were randomized into the two arms of interest after an initial period of 52 weeks, 24 weeks and 16 weeks, respectively, during which all patients received a combination of bDMARDs + CsDMARDs. In the ACT-TAPER [4] study, patients achieving a good/moderate EULAR response were randomized to a double-blind MTX taper arm, in which the MTX dose was tapered to 5 mg at 16 weeks, with complete withdrawal of MTX at 24 weeks, or to a stable MTX dose arm. We defined the day of inclusion, D0, for this study as the day on which methotrexate was completely withdrawn. We have not identified any studies satisfying our search criteria for anakinra, certolizumab or infliximab.
Figure 2.
Flow diagram for study inclusion/exclusion. CsDMARDs: Conventional synthetic disease-modifying anti-rheumatic drugs; bDMARDs: Biological disease-modifying anti-rheumatic drugs; VS: versus.
3.2. Study Characteristics
The characteristics of the studies included in the meta-analysis are displayed in Table 1. Regarding the history of CsDMARD use, three studies included only patients who had never used CsDMARDs (13%), 16 included patients not naïve for CsDMARD use (69.6%) and four were not selective on the basis of these criteria (17.4%). All but two of the studies used MTX as the CsDMARD. For the history of bDMARD use, nine studies included only patients who had never used bDMARDs (39.1%), eight included patients with a possible history of use before inclusion (34.8%) and five included patients not naïve for bDMARD treatment (21.7%). Of the studies, eight included (26.1%) planned treatment adjustment during the trial a priori, with a rescue treatment administered if the main endpoint was not reached within the time allowed.
Table 1.
Study characteristics.
| Studies | Follow-Up |
CsDMARD
History |
WO
Period |
bDMARD
History |
WO
Period |
RA
Duration |
Treatment | Doses (mg) | Dose Adjustment Defined a Priori | N |
|---|---|---|---|---|---|---|---|---|---|---|
|
Anbainuo,
Chen, 2013 [33] |
24 weeks | Naive | / | Naive | / | ND | Abainuo + MTX Abainuo |
Abainuo: 25 mg SC 2x/Week MTX: 10–15 mg/Week PO |
No | N = 132 N = 132 |
| Abatacept (AVERT), Emery, 2015 [23] | 48 weeks | Naive: MTX-naive or received MTX (≤10 mg/week) for ≤4 weeks |
MTX 1 month |
Naive | / | <2 years | ABA + MTX ABA |
ABA: 125 mg SC/Week MTX: 7.5–20 mg/Week PO |
No | N = 119 N = 116 |
| Adalimumab (PREMIER), Breedveld, 2006 [24] | 48/104 weeks | Naive or not: MTX, cyclophosphamid, CYC, AZA, or 2 other CsDMARDs were excluded |
4 weeks |
Naive | / | <3 years | ADA + MTX ADA |
ADA: 40 mg SC/2 weeks MTX: 7.5–20 mg/Week PO |
Increased dosing with ADA/placebo to weekly if ACR 20 not achieved in 2 consecutive visits after week 16. | N = 268 N = 274 |
| Clazakizumab, Weinblatt, 2015 [53] | 24 weeks | Non naive: MTX failure (>3 months treatment) |
/ | Naive | / | >16 weeks | CLZ + MTX CLZ |
CLZ: 100 mg SC/4 wks MTX: 10–22 mg/Week PO |
If <20% reduction SJC/TJC: receive open-label CLZ 200 mg SC/4 wk + MTX |
N = 60 N = 60 |
| Etanercept, (ADORE), van Riel, 2006 [32] | 16 weeks | Non naive: MTX >12.5 mg/week for >3 months |
12 weeks | Naive | / | ND | ETN + MTX ETN |
ETN: 25 mg SC 2x/Week MTX: >12.5 mg/week PO or SC |
No | N = 155 N = 159 |
| Etanercept, (CAMEO), Pope, 2013 [29] | 24/104 weeks | Non naive: MTX therapy for >12 weeks |
/ | Non naive: ETN + MTX for 6 months |
/ | > 6 months | ETN + MTX ETN |
ETN: 50 mg SC/Week MTX: ≥15 mg/week |
No | N = 107 N = 98 |
| Etanercept, Combe, 2006 [25] | 24/48/104 weeks | Non naive: SSZ for >4 months |
Other than SSZ: 3 month | Naive or not: ineligible if they had received ETN or other TNF antagonists |
bDMARDs or CTX: 6 months |
<20 years | ETN + SSZ ETN |
ETN: 25 mg SC 2x/Week SSZ: 2–2.5–3 g/day PO |
No | N = 101 N = 103 |
| Etanercept (COMET), Emery, 2010 [28] | 52 weeks | Non naive: ETN + MTX for 52 weeks before new randomization. |
No | Non naive: ETN + MTX during 52 weeks before new randomization. |
No | 4 months until 2 years | ETN + MTX ETN |
ETN: 25 mg SC 2x/week MTX 7.5–20 mg/Week PO |
No | N = 111 N = 111 |
| Etanercept, (JESMR), Kameda, 2010 and 2011 [30,31] | 24/52 weeks | Non naive: MTX 6 mg/week for >3 months |
/ | Naive | / | ND | ETN + MTX ETN |
ETN: 25 mg SC 2x/Week MTX: 6–8 mg/week |
No | N = 76 N = 71 |
| Etanercept (TEMPO), Klareskog, 2004 [27] | 24/52/ 104 weeks |
CsDMARD non naive, but MTX naive or not | MTX 6 month |
Naive or not: Ineligible if previously received ETN or other TNF antagonists. |
ISD: 6 months bDMARD: 3 months |
6 months until 20 years. | ETN + MTX ETN |
ETN: 25 mg SC 2x/week MTX: 7.5–20 mg/Week PO |
No | N = 231 N = 223 |
| Golimumab (GO BEFORE), Emery, 2009 [35] | 24/52/104 weeks |
Naive or not: had not received more than 3 weekly doses of oral MTX | / | Naive or not: IFX, ETN, ADA, RTX, NTZ, or cytotoxic agents, and alkylating agents, were excluded |
ANK: 4 weeks alefacept/ efalizumab: 3 months, other: 5 half-lives |
3 months until 3 years | GOL + MTX GOL |
GOL: 100 mg SC/4 weeks MTX: 10–20 mg/Week PO |
If <20% improvement from baseline SJC/TJC entered early escape any time after week 24. | N = 159 N = 159 |
|
Golimumab (GO
FORWARD), Keystone, 2009 [38] |
24/52/104 weeks |
Non naive: had been receiving a stable dose of MTX 15–25 mg/week for at least 4 weeks | Other than MTX 4 weeks | Naive or not: excluded if used anti- TNF agent, RTX, NTZ or cytotoxic agents | ANK: 4 weeks alefacept efalizumab: 3 months |
NR | GOL + MTX GOL |
GOL: 100 mg SC/4 weeks MTX: 15–20 mg/week PO |
If <20% improvement from baseline TJC/SJC escape any time after week 24. | N = 89 N = 133 |
| Golimumab (GO LIVE), Kremer, 2010 [37] | 24/48 weeks | Non naive: MTX for >3 months |
/ | Naive or not: limited to 20% of the study population. (Excluded if RTX, ABA, or NTZ). |
IFX, alefa- Cept/efalizumab: 3 months, ETN/ADA 2 monthsANK/ABA/NTZ. 4 weeks |
<8 years | GOL 2 mg/kg + MTX GOL 4 mg/kg + MTX GOL 2 mg/kg GOL 4 mg/kg |
GOL: 2 mg/kg OR 4 mg/kg IV/12 weeks MTX: 15 mg/Week PO |
At weeks 16 and 24, patients with <20% improvement from baseline in both the SJC and TJC entered early escape and dose regimen |
N = 128 N = 129 N = 129 N = 128 |
| Rituximab, Edwards, 2004, Strand, 2006 [40,41] | 24/48/104 weeks |
Non naive: had failed 1–5 CsDMARDs and MTX with treatment for >16 weeks |
/ | ND | / | ND | RTX + MTX RTX |
RTX: 1000 mg IV on days 1 and 15 all 6 months MTX: 12.5–15 mg/Week PO |
No | N = 40 N = 40 |
| Sarilumab, (HARUKA)Kameda, 2019 [52] | 24/52 weeks | Naive or not: -monotherapy: CsDMARDs naive -combination: CsDMARDs non naive |
/ | Naive or not | CYC, MFMAZA, CTX, bDMARD: 4–12 weeks |
ND | SLM 150 mg + non-MTX CsDMARDs SLM 200 mg + non-MTX CsDMARDs SLM 150 mg SLM 200 mg |
SLM 150 or 200 mg/2 Weeks SC | No | N = 15 N = 15 N = 30 N = 31 |
| Tocilizumab (ACT RAY), Dougados, 2013 [42] | 24/52/104 weeks |
Non naive: MTX for at least 12 weeks |
LEF: 3 moth Other 1 month |
Naive or not | bDMARD 1 month |
ND | TCZ + MTX TCZ |
TCZ: 8 mg/kg IV/4 weeks MTX: 15–20 mg/Week PO |
At week 24, if DAS28 > 3.2; an open-label CsDMARD was added. At week 36, if DAS28 > 3.2, an additional CsDMARD added. |
N = 277 N = 276 |
| Tocilizumab (ACT-TAPER), Edwards, 2017 [50] | 24 weeks | Non naive: had inadequately responded to 2 CsDMARDs, including MTX. |
/ | Non naive | Had have TCZ 8 mg/kg/4 weeks for 24 weeks | ND | TCZ + MTX stable dose TCZ + MTX Tapering dose |
TCZ: 8 mg/kg IV/4 weeks MTX stable dose: 10–15 mg/Week MTX tapering dose S24 to S40: 5 mg /week; S40 to S48: 0 mg. |
No | N = 136 N = 136 |
| Tocilizumab (CHARISMA), Maini, 2006 [54] | 16/20 weeks | Non naive: MTX failure >6 months of treatment |
LEF: 6 months Other 4 weeks |
Naive or not | anti-TNF agents: 12 weeks |
ND | TCZ + MTX TCZ |
TCZ: 8 mg/kg IV/4 weeks MTX: 10–25 mg/Week PO |
No | N = 50 N = 52 |
| Tocilizumab (COMP-ACT), Kremer, 2018 [49] | 24 weeks | Non naive: TCZ + MTX during 24 weeks before new randomization. |
/ | Non naive: TCZ + MTX for 24 weeks before new randomization. |
/ | ND | TCZ + MTX TCZ |
TCZ: 162 mg/week (≥100 kg) or /2 weeks (<100 kg) MTX: >15 mg/week PO |
No | N = 147 N = 147 |
| Tocilizumab (FUNCTION), Burmester, 2016 [45] | 24/52/104 weeks |
CsDMARD-naive or not but MTX-naive | / | Naive | / | <2 years | TCZ + MTX TCZ |
TCZ:8 mg/kg IV/4 wks MTX: 7.5–20 mg/Week PO |
No | N = 291 N = 292 |
| Tocilizumab (JUST ACT), Pablos, 2019 [48] | 12 weeks | Non naive: TCZ + MTX 16 weeks before randomization. |
/ | Non naive: TCZ + MTX 16 weeks before randomization. |
/ | NR | TCZ + MTX TCZ |
TCZ: 8 mg/kg IV/4 wks MTX: >15 mg/Wek PO |
No | N = 83 N = 82 |
| Tocilizumab, (SURPRISE), Kaneko, 2015 [51] | 24/52/104 weeks |
Non naive: MTX ≥6 mg/week for at least 8 weeks |
LEF: 12 weeks, other: 8 weeks |
Naive | Tacrolimus: 4 weeks |
<10 years | TCZ + MTX TCZ |
TCZ: 8 mg/ kg IV/4 wks MTX: >6 mg/Week PO |
No | N = 118 N = 115 |
| Tocilizumab (U-ACT-EARLY), Bijlsma, 2016 [46] | 24/52/104 weeks |
Naive | / | Naive | / | <1 year | TCZ + MTX TCZ |
TCZ:8 mg/ kg IV/4 wks MTX: 10–30 mg/Week PO |
No | N = 106 N = 103 |
ND: not disclosed; N: number; IV: intravenous; IM: intramuscular; PO: per os; SC: subcutaneous; wks: weeks; mths: months; WO: wash out; min: minimum; ACR 20: American college of Rheumatology 20; SJC: swollen joint count; TJC: tender joint count; sDMARDs: conventional disease-modifying antirheumatic drugs; bDMARDs: biologic disease-modifying antirheumatic drugs; ABA: abatacept; ADA: adalimumab; ANK: anakinra; AZA: azathioprine; CLZ: clazakizumab; CTX: cyclophosphamide; CYC: ciclosporine; CZP: certolizumab pegol; ETN: etanercept; GOL: golimumab; HCQ: hydroxychloroquine; IFX: infliximab; ISD: immunosuppressive drug; LEF: leflunomide; MMF: mycophenolate; MTX: methotrexate; NTZ: natalizumab; RTX: rituximab; SLM: sarilumab; SSZ: sulfasalazine; TCZ: tocilizumab.
3.3. Principal Characteristics of the Patients
More than 75% of the patients were women, and the mean age was 55 years (range: 45.4 ± 11.9 to 63.3 ± 10.6 years). Disease duration ranged from 26 days to 12 years, and most of the patients for whom the information was available had tested positive for autoantibodies (RF and/or ACPA). The DAS 28 score at baseline ranged from 2.6 to 6.8, and the mTSS score ranged from 0 ± 0 to 39.6 ± 56.1.
3.4. Primary Efficacy Endpoint: ACR 20 at 24 Weeks
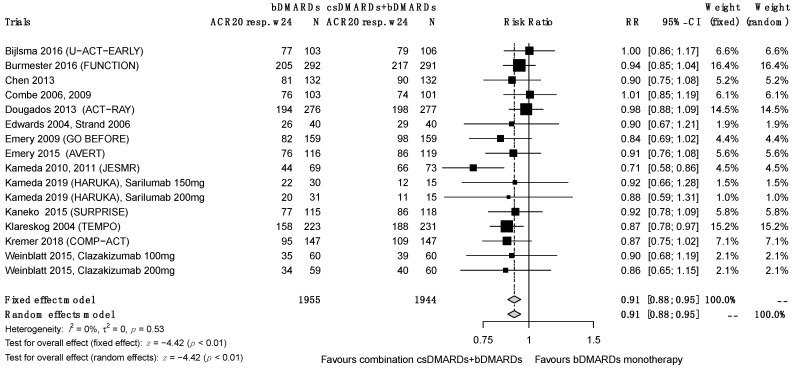
The ACR 20 results at 24 weeks were reported for 16 studies. Using a random effects model, we found that the therapeutic combination performed significantly better for this endpoint, RR = 0.88 (0.83; 0.93), despite significant heterogeneity between studies (I2 = 46%, τ2 = 0.0068, p = 0.02) (Figure 3). We made the results more consistent by performing a sensitivity analysis and a funnel plot asymmetry test. The remaining 14 studies showed that significantly better results for this endpoint were obtained with the combination treatment (RR = 0.91 (0.88; 0.95); I2 = 0%, τ2 = 0, p = 0.53). We decided a priori to include open-label studies in the quantitative analysis and to use the qualitative analysis to check whether this decision had any effect on the results. Consistent with the τau2 test results, we can conclude that the inclusion of SUPRISE, ADORE and JESMR with an open-label design, despite their lower grade of recommendation, had no effect on the results of the analysis.
Figure 3.
ACR 20 responses at week 24.
3.5. Other Endpoints
3.5.1. ACR Reponses
With fourteen studies providing ACR 20 results at 52 weeks, the overall result obtained with the random effects model was significantly in favor of the therapeutic combination (RR= 0.90 (0.84; 0.97)). However, it was not possible to have confidence in the results, due to the degree of heterogeneity (I2 = 64%, τ2 = 0.0116, p < 0.01). After controlling for heterogeneity, we selected 11 studies. The results were also in favor of the combination treatment (RR = 0.94 (0.90; 0.98), I2 = 7%, τ2 = 0.0004, p = 0.38). The ACR 20 results at 104 weeks were available for eight studies. The results were significantly in favor of the combination treatment both before (RR = 0.89 (0.84; 0.94), I2 = 66%, τ2 = 0.0129, p < 0.01) and after the sensitivity analysis (RR = 0.92 (0.87; 0.98)) (I² = 0%, τ² = 0, p = 0.42). The ACR 50 scores were significantly in favor of the combination treatment and were not affected by sensitivity testing (RR = 0.81 (0.76; 0.87), I2 = 0%, τ2 = 0, p = 0.77) at 24 weeks, (RR = 0.89 (0.82; 0.97), I2 = 10%, τ2 = 0.0019, p = 0.35) at 52 weeks and (RR = 0.84 (0.77; 0.93), I2 = 14%, τ2 = 0.0022, p = 0.32) at 104 weeks. The same was true for the ACR 70 score at weeks 24 and 52 (RR = 0.76 (0.68; 0.85), I2 = 10%, τ2 = 0.0055, p = 0.33) at 24 weeks, (RR = 0.81 (0.73; 0.90), I2 = 16%, τ2 = 0.0063, p = 0.28) at 52 weeks. At 104 weeks, the ACR 70 data were significantly in favor of the combination treatment in the analysis (RR = 0.77 (0.64; 0.93), I2 = 61%, τ2 = 0.00393, p = 0.01), but were not significant after sensitivity analysis (RR = 0.89 (0.78; 1.01), I2 = 0%, τ2 = 0, p = 0.84). Overall, five studies reported ACR 90 scores at 24 weeks, showing these results to be significantly in favor of the combination treatment (RR = 0.64 (0.44; 0.93), heterogeneity: I2 = 0%, τ2 = 0, p = 0.70). At 52 weeks, the ACR 90 data were not significantly in favor of either therapeutic strategy, even after sensitivity analysis (RR = 0.88 (0.65; 1.19), I2 = 0%, τ2 = 0, p = 0.84). Only two studies reported ACR 90 scores at 104 weeks; the lack of data did not allow for reliable analyses.
3.5.2. Remission According to DAS 28 (Using ESR or CRP)
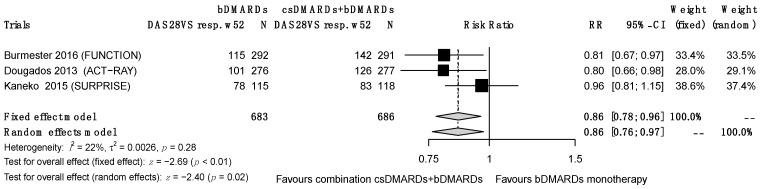
At 24 weeks, remission according to DAS 28 (<2.6) was reported, based on CRP in six RCTs and ESR in eight RCTs. For the DAS 28–CRP remission scores, the objective was achieved significantly more frequently for the combined treatment group at weeks 24 and 52 (RR = 0.66 (0.56–0.77), I2 = 0%, τ² = 0.00, p = 0.70) at 24 weeks and (RR = 0.73 (0.63–0.85), I2 = 0%, τ2 = 0.00, p = 0.92) at 52 weeks. Only two studies provided results at 104 weeks. For the DAS 28–ESR remission scores, the initial data at 24 weeks showed an I2 value of 0% for heterogeneity, but the funnel plot asymmetry test revealed a publication bias for the GO-FORWARD study, so we excluded this study from the final analysis. The objective was significantly more frequently achieved in the association group at weeks 24 and 52 (at 24 weeks, RR = 0.87 (0.80–0.95), I2 = 0%, τ2 = 0, p = 0.90 (Figure 4); at 52 weeks, RR = 0.86 (0.76–0.97), I2 = 22%, τ2 = 0.0026, p = 0.28), but the results ceased to be significant at 104 weeks (RR = 0.83 (0.83–1.05), I2 = 0%, τ2 = 0.00, p = 0.89). Note that the results at 52 weeks were initially non-significant but became so after sensitivity analysis.
Figure 4.
DAS 28–ESR remission at 52 weeks.
3.5.3. HAQ, CDAI and SDAI Scores
In total, nine RCTs reported an improvement in HAQ ≥ 0.22 and showed an absence of significance for either arm of the study at 24 weeks (RR = 0.90 (0.80; 1.01), I2 = 0%, τ2 = 0.00, p = 0.49), but a significant difference emerged from 52 weeks onwards in favor of the combination therapy (RR= 0.83 (0.75; 0.92), I² = 4%, τ2 = 0.0005, p = 0.37 at 52 weeks; RR= 0.89 (0.83; 0.96), I2 = 17%, τ2 = 0.0012, p = 0.31 at 104 weeks). Similar results were obtained for CDAI remission (<2.8) at 24, 52 and 104 weeks and SDAI remission (<3.3) at 24 and 52 weeks after sensitivity analysis (no results available at 104 weeks).
3.5.4. Subgroup Meta-Analysis
All our results show an RR close to 1, meaning that the combination therapy was not much more effective than the biological agent used as a monotherapy. We decided to perform a subgroup analysis by successively removing etanercept, tocilizumab and then both molecules from the analysis to try to explain these results. We found that removing tocilizumab resulted in a decrease in RR, whereas removing etanercept did not appear to change the results. This sub-analysis suggests that tocilizumab appears to be more effective as a single agent than other available biotherapies.
3.5.5. Structural Progression
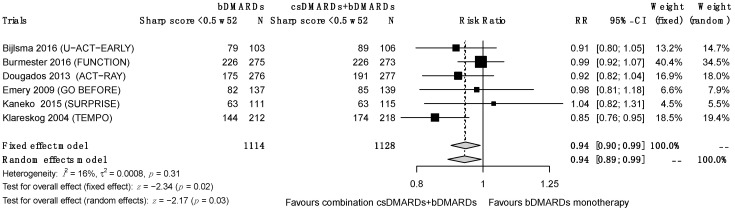
A total of eleven RCTs reported X-ray progression based on mTSS <0 or <0.5 at weeks 24, 52 and/or 104. A significantly lower progression was observed for the combination treatment from 52 weeks (Figure 5) and remained at 104 weeks before and after sensitivity analysis; the results at 24 weeks were not significant (RR = 0.98 (0.91; 1.04), I2 = 6%, τ2 = 0.0003, p = 0.35 at 24 weeks; RR = 0.94 (0.89; 0.99), I2 = 16%, τ2 = 0.0008, p = 0.31 at 52 weeks; RR = 0.92 (0.87; 0.98), I2 = 12%, τ2 = 0.0008, p = 0.34).
Figure 5.
Sharp remission at 52 weeks.
3.5.6. Discontinuation Due to a Lack of Efficacy
The rate of discontinuation due to lack of efficacy did not differ between groups up to 52 weeks (RR = 1.74 (0.92; 3.29) with I2 = 0%, τ2 = 0, p = 0.59 at 24 weeks; RR = 1.39 (0.88; 2.18) with I2 = 6%, τ2 = 0.0241, p = 0.39 at 52 weeks). However, an increase in the number of discontinuations due to a lack of efficacy was reported at 104 weeks for the biotherapy monotherapy group (RR= 2.83 (1.82; 4.41) with I2 = 19%, τ2 = 0.0398, p = 0.30 at 104 weeks).
3.5.7. Toxicity
The heterogeneity of the toxicity data made it impossible to perform a reliable statistical analysis. Nevertheless, we were able to compare study outputs for discontinuation due to adverse events. We found no advantage for either group in terms of the number of discontinuations: RR = 0.73 (0.53; 1.01) with I2 = 0% and τ2 = 0, p = 0.70 at 24 weeks; RR= 0.95 (0.70; 1.31) with I² = 40%, τ2 = 0.1151, p = 0.07 at 52 weeks; and RR = 0.84 (0.67; 1.05) with I2 = 0%, τ2 = 0, p = 0.53 at 104 weeks. The I² value for heterogeneity obtained at 52 weeks was >30%. We decided to retain this result because significance did not differ before and after sensitivity analysis, and a cutoff of 40% has been reported to be acceptable [54].
4. Discussion
Through this systematic review and meta-analysis, we aimed to compare the use of biological agents in monotherapy and in association with a CsDMARD. Many meta-analyses have compared studies of different therapeutic combinations (for example, biotherapies in monotherapy versus CsDMARDs or placebo), making it difficult to extrapolate results to our arms of interest [55,56,57,58,59].
This is the second systematic review comparing the value of adding MTX to bDMARD treatment with bDMARD monotherapy. Our work confirms the work of Tarp and al. [11], with the difference that our study is more recent, which allowed us to include a larger number of randomized trials and, therefore, patients.
We found that the combination treatment was more effective than monotherapy, as shown by the main endpoint, ACR 20 at 24 weeks. The results are similar for the other endpoints, with, for some, a loss of efficacy at 104 weeks, possibly with a loss of power of the study. The modified Sharp’s score was significantly in favor of the combined treatment from 52 weeks onwards. At 24 weeks, the duration of exposure may not have been sufficiently long to distinguish between the two study arms considered. It is interesting to note that the PREMIER study [24] was a source of great heterogeneity for several of the variables studied with no obvious cause found. Our results are comparable with French and international recommendations [1,2].
The purpose of this meta-analysis was not to compare the different bDMARDs between each other. Nevertheless, we could observe that all our results showed an RR close to 1, showing little difference in clinical efficacy between groups. The subgroup analysis showed that excluding tocilizumab from the analysis decreased this RR, suggesting that tocilizumab is probably the most effective single-agent biologic. These results are consistent with the literature. Tarp et al. [60] have shown that most biological agents are effective in monotherapy, with an advantage for etanercept and tocilizumab supported by other network meta-analyses [57,59,61]. Some studies about IL-6 receptor blockers in monotherapy have shown that tocilizumab monotherapy yields response rates close to those obtained in combination with MTX in randomized studies and cohorts [62,63]. The ADACTA and MONARCH studies have shown tocilizumab and sarilumab to be superior to adalimumab in monotherapy [64,65]. In the TOCERRA registry [63], the therapeutic efficacy and maintenance of tocilizumab monotherapy are similar to those of the anti-TNF agents associated with MTX.
Finally, structural damage was not studied for all the biological treatments included in our meta-analysis. Tarp et al. [11] obtained identical results to those reported here and, after a subgroup analysis, no structural differences were found.
We found no difference in terms of safety between the two treatment arms, essentially due to the heterogeneity of the data collection. However, we were able to show that there was no difference between the study arms in terms of the rate of treatment discontinuation due to adverse events. With regard to the risk of infection, Singh et al. [66] showed that, in patients treated with CsDMARDs, the median annual absolute risk of infection was 2%, or 20 per 1000 treated patients per year, whereas there was an increase to 6 per 1000 patients treated with bDMARDs in combination with a CsDMARD, with a significant difference. Ramiro et al. [67] confirmed that patients on bDMARDs (both anti-TNF and no anti-TNF agents) had a higher risk of serious infections than patients on CsDMARDs, and that there was generally no difference between bDMARDs. They also investigated the occurrence of different cancers after exposure to biologics. Relative to both the general population and patients on CsDMARDs, patients on bDMARDs had no higher risk of individual solid cancers or of lymphoma. By contrast, non-melanoma skin cancer may occur more frequently in patients on bDMARDs than in the general population (HR 1.7), but the risk in these patients is no higher than that in patients treated with CsDMARDs. One study with a low risk of bias showed that patients on bDMARDs may have a higher risk of melanoma than patients on CsDMARDs (HR 1.5, 95% CI 1.0 to 2.2) [68].
Nevertheless, our study has several limitations. First, as with all systematic literature reviews, this study is subject to certain publication and selection biases. Second, there was some heterogeneity between these studies. The variables tested with the sensitivity analysis did not significantly influence the results of the meta-analysis. In addition, few studies have used CsDMARDs other than methotrexate, limiting the extrapolation of results for leflunomide or sulfasalazine. We also chose to consult the data collected at weeks 24, 52 and 104; when data for these time points were not available, other time points were used. Comparisons at different time points may limit the interpretation of our results, as may not having been able to contact the authors to recover missing data.
5. Conclusions
Our meta-analysis confirms the results of a previous one, but with updated research and a larger number of studies included. The results indicate that the combination therapy of a biological agent with CsDMARDs is more effective than monotherapy and should be preferred in uncontrolled RA, in accordance with the usual guidelines. MTX should be switched to another CsDMARD in the case of contraindication or intolerance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12010286/s1. Figure S1: Original RCTs.
Author Contributions
Conceptualization, P.H.; methodology, P.H., C.D. and F.-X.L.; software, F.-X.L.; validation, C.D., F.-X.L. and P.H.; formal analysis, F.-X.L.; investigation, C.D.; data curation, C.D.; writing—original draft preparation, C.D.; writing—review and editing, P.H.; supervision, P.H.; project administration, P.H. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
For the unpublished data, we collected data on the EULAR response criteria, as well as the variation over time of ACR-N, DAS 28-ESR and DAS28-CRP, total sharp score, erosions and joint-space scores, HAQ-DI score, as well as the variation of ESR, CRP, number of painful and swollen joints, VAS pain, patient global VAS and physician global VAS. The information is available on request by e-mail from Célia Delpech.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gabriel S.E., Crowson C.S., O’Fallon W.M. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42:415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Daien C., Hua C., Gaujoux-Viala C., Cantagrel A., Dubremetz M., Dougados M., Fautrel B., Mariette X., Nayral N., Richez C., et al. Update of the Recommendations of the French Society of Rheumatology for the 340 Management of Rheumatoid Arthritis. Jt. Bone Spine. 2019;86:135–150. doi: 10.1016/j.jbspin.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A., McInnes I.B., Sepriano A., van Vollenhoven R.F., de Wit W., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 4.Smolen J.S., Landewé R., Bijlsma J., Burmester G., Chatzidionysiou K., Dougados M., Nam J., Ramiro S., Voshaar M., van Vollenhoven R., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 5.Soliman M.M., Ashcroft D.M., Watson K.D., Lunt M., Symmons D.P., Hyrich K.L. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 2011;70:583–589. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Listing J., Strangfeld A., Rau R., Kekow J., Gromnica-Ihle E., Klopsch T., Demary W., Burmester G.R., Zink A. Clinical and functional remission: Even though biologics are superior to conventional DMARDs overall success rates remain low—Results from RABBIT, the German biologics register. Arthritis Res. Ther. 2006;8:R66. doi: 10.1186/ar1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariette X., Gottenberg J.-E., Ravaud P., Combe B. Registries in rheumatoid arthritis and autoimmune diseases: Data from the French registries. Rheumatol. Oxf. Engl. 2011;50:222–229. doi: 10.1093/rheumatology/keq368. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.J., Chang H., Yazici Y., Greenberg J.D., Kremer J.M., Kavanaugh A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J. Rheumatol. 2009;36:1611–1617. doi: 10.3899/jrheum.080889. [DOI] [PubMed] [Google Scholar]
- 9.Curtis J.R., Bykerk V.P., Aassi M., Schiff M. Adherence and Persistence with Methotrexate in Rheumatoid Arthritis: A Systematic Review. J. Rheumatol. 2016;43:1997–2009. doi: 10.3899/jrheum.151212. [DOI] [PubMed] [Google Scholar]
- 10.Emery P., Sebba A., Huizinga T.W.J. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann. Rheum. Dis. 2013;72:1897–1904. doi: 10.1136/annrheumdis-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliddal H., Eriksen S.A., Christensen R., Lorenzen T., Hansen M.S., Østergaard M., Dreyer L., Luta G., Vestergaard P. Adherence to Methotrexate in Rheumatoid Arthritis: A Danish Nationwide Cohort Study. Arthritis. 2015;2015:915142. doi: 10.1155/2015/915142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarp S., Jørgensen T.S., Furst D.E. Added value of combining methotrexate with a biological agent compared to biological monotherapy in rheumatoid arthritis patients: A systematic review and meta-analysis of randomised trials. Semin. Arthritis Rheum. 2019;48:958–966. doi: 10.1016/j.semarthrit.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drevon D., Fursa S.R., Malcolm A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017;41:323–339. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 16.Flower A., McKenna J.W., Upreti G. Validity and Reliability of GraphClick and DataThief III for Data Extraction. Behav. Modif. 2016;40:396–413. doi: 10.1177/0145445515616105. [DOI] [PubMed] [Google Scholar]
- 17.Rakap S., Rakap S., Evran D., Cig O. Comparative evaluation of the reliability and validity of three data extraction programs: UnGraph, GraphClick, and DigitizeIt. Comput. Hum. Behav. 2016;55:159–166. doi: 10.1016/j.chb.2015.09.008. [DOI] [Google Scholar]
- 18.Shadish W.R., Brasil I.C.C., Illingworth D.A., White K.D., Galindo R., Nagler E.D., Rindskopf D.M. Using UnGraph to extract data from image files: Verification of reliability and validity. Behav. Res. Methods. 2009;41:177–183. doi: 10.3758/BRM.41.1.177. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleiss J. Review papers: The statistical basis of meta-analysis. Stat. Methods Med. Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 24.Emery P., Burmester G.R., Bykerk V.P., Combe B.G., Furst D.E., Barré E., Karyekar C.S., Wong D.A., Huizinga T.W. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: Results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann. Rheum. Dis. 2015;74:19–26. doi: 10.1136/annrheumdis-2014-206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breedveld F.C., Weisman M.H., Kavanaugh A.F., Cohen S.B., Pavelka K., van Vollenhoven R., Sharp J., Perez J.L., Spencer-Green G.T. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 26.Combe B., Codreanu C., Fiocco U., Gaubitz M., Geusens P.P., Kvien T.K., Pavelka K., Sambrook P.N., Smolen J.S., Wajdula J., et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: A double-blind comparison. Ann. Rheum. Dis. 2006;68:1146–1152. doi: 10.1136/ard.2007.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Combe B., Codreanu C., Fiocco U., Gaubitz M., Geusens P.P., Kvien T.K., Pavelka K., Sambrook P.N., Smolen J.S., Khandker R., et al. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: A double-blind randomised 2-year study. Ann. Rheum. Dis. 2009;68:1146–1152. doi: 10.1136/ard.2007.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klareskog L., van der Heijde D., de Jager J.P., Gough A., Kalden J., Malaise M., Martín Mola E., Pavelka K., Sany J., Settas L., et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: Double-blind randomised controlled trial. Lancet. 2004;363:675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 29.Emery P., Breedveld F.C., Hall S., Durez P., Chang D.J., Robertson D., Singh A., Pedersen R.D., Koenig A.S., Freundlich B. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): A randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 30.Pope J.E., Haraoui B., Thorne J.C., Vieira A., Poulin-Costello M., Keystone E.C. The Canadian Methotrexate and Etanercept Outcome Study: A randomised trial of discontinuing versus continuing methotrexate after 6 months of etanercept and methotrexate therapy in rheumatoid arthritis. Ann. Rheum. Dis. 2014;73:2144–2151. doi: 10.1136/annrheumdis-2013-203684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kameda H., Kanbe K., Sato E., Ueki Y., Saito K., Nagaoka S., Hidaka T., Atsumi T., Tsukano M., Kasama T., et al. Continuation of Methotrexate Resulted in Better Clinical and Radiographic Outcomes Than Discontinuation upon Starting Etanercept in Patients with Rheumatoid Arthritis: 52-week Results from the JESMR Study. J. Rheumatol. 2011;38:1585–1592. doi: 10.3899/jrheum.110014. [DOI] [PubMed] [Google Scholar]
- 32.Kameda H., Ueki Y., Saito K., Nagaoka S., Hidaka T., Atsumi T., Tsukano M., Kasama T., Shiozawa S., Tanaka Y., et al. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: A randomized trial. Mod. Rheumatol. 2010;20:531–538. doi: 10.3109/s10165-010-0324-4. [DOI] [PubMed] [Google Scholar]
- 33.van Riel P.L., Taggart A.J., Sany J., Gaubitz M., Nab H.W., Pedersen R., Freundlich B., MacPeek D. Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: The ADORE study. Ann. Rheum. Dis. 2006;65:1478–1483. doi: 10.1136/ard.2005.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X.X., Dai Q., Huang A.B., Wu H.X., Zhao D.B., Li X.F., Hu S.X., Yang N.P., Tao Y., Xu J.H., et al. A multicenter, randomized, double-blind clinical trial of combination therapy with Anbainuo, a novel recombinant human TNFRII:Fc fusion protein, plus methotrexate versus methotrexate alone or Anbainuo alone in Chinese patients with moderate to severe rheumatoid arthritis. Clin. Rheumatol. 2013;32:99–108. doi: 10.1007/s10067-012-2096-z. [DOI] [PubMed] [Google Scholar]
- 35.Emery P., Fleischmann R., Van der Heijde D., Keystone E.C., Genovese M.C., Conaghan P.G., Hisa E.C., Xu W., Baratelle A., Beutler A., et al. The Effects of Golimumab on Radiographic Progression in Rheumatoid Arthritis. Arthritis Rheum. 2011;63:1200–1210. doi: 10.1002/art.30263. [DOI] [PubMed] [Google Scholar]
- 36.Emery P., Fleischmann R.M., Moreland L.W., Hsia E.C., Strusberg I., Durez P., Nash P., Amante E.J., Churchill M., Park W., et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: Twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–2283. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]
- 37.Emery P., Fleischmann R.M., Doyle M.K. Golimumab, a Human Anti–Tumor Necrosis Factor Monoclonal Antibody, Injected Subcutaneously Every 4 Weeks in Patients with Active Rheumatoid Arthritis Who Had Never Taken Methotrexate: 1-Year and 2-Year Clinical, Radiologic, and Physical Function Findings of a Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Care Res. 2013;65:1732–1742. doi: 10.1002/acr.22072. [DOI] [PubMed] [Google Scholar]
- 38.Kremer J., Ritchlin C., Mendelsohn A., Baker D., Kim L., Xu Z., Han J., Taylor P. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2010;62:917–928. doi: 10.1002/art.27348. [DOI] [PubMed] [Google Scholar]
- 39.Keystone E. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann. Rheum. Dis. 2010;69:1129–1135. doi: 10.1136/ard.2009.116319. [DOI] [PubMed] [Google Scholar]
- 40.Keystone E.C., Genovese M.C., Klareskog L., Hsia E.C., Hall S.T., Miranda P.C., Pazdur J., Bae S.C., Palmer W., Zrubek J., et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: The GO-FORWARD Study. Ann. Rheum. Dis. 2009;68:789–796. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards J.C., Szczepanski L., Szechinski J., Filipowicz-Sosnowska A., Emery P., Close D.R., Stevens R.M., Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 42.Strand V., Balbir-Gurman A., Pavelka K., Emery P., Li N., Yin M., Lehane P.B., Agarwal S. Sustained benefit in rheumatoid arthritis following one course of rituximab: Improvements in physical function over 2 years. Rheumatology. 2006;45:1505–1513. doi: 10.1093/rheumatology/kel358. [DOI] [PubMed] [Google Scholar]
- 43.Dougados M., Kissel K., Sheeran T., Tak P.P., Conaghan P.G., Mola E.M., Schett G., Amital H., Navarro-Sarabia F., Hou A., et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY) Ann. Rheum. Dis. 2013;72:43–50. doi: 10.1136/annrheumdis-2011-201282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dougados M., Kissel K., Conaghan P.G., Mola E.M., Schett G., Gerli R., Hansen M.S., Amital H., Xavier R.M., Troum O., et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: The ACT-RAY study. Ann. Rheum. Dis. 2014;73:803–809. doi: 10.1136/annrheumdis-2013-204761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maini R.N., Taylor P.C., Szechinski J., Pavelka K., Bröll J., Balint G., Emery P., Raemen F., Petersen J., Smolen J., et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 46.Burmester G.R., Rigby W.F., Vollenhoven R.F., Kay J., Rubbert-Roth A., Kelman A., Dimonaco S., Mitchell N. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann. Rheum. Dis. 2016;5:1081–1091. doi: 10.1136/annrheumdis-2015-207628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bijlsma J.W.J., Welsing P.M.J., Woodworth T.G., Middelink L.M., Pethö-Schramm A., Bernasconi C., Borm M.E.A., Wortel C.H., Ter Borg E.J., Jahangier Z.N., et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): A multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343–355. doi: 10.1016/S0140-6736(16)30363-4. [DOI] [PubMed] [Google Scholar]
- 48.Teitsma X.M., Jacobs J.W.G., Welsing P.M.J., Pethö-Schramm A., Borm M.E.A., van Laar J.M., Lafeber F.P.J.G., Bijlsma J.W.J. Radiographic joint damage in early rheumatoid arthritis patients: Comparing tocilizumab- and methotrexate-based treat-to-target strategies. Rheumatol. Oxf. Engl. 2018;57:309–317. doi: 10.1093/rheumatology/kex386. [DOI] [PubMed] [Google Scholar]
- 49.Pablos J.L., Navarro F., Blanco J.F. Efficacy of tocilizumab monotherapy after response to combined tocilizumab and methotrexate in patients with rheumatoid arthritis: The randomised JUST-ACT study. Clin. Exp. Rheumatol. 2019;37:437–444. [PubMed] [Google Scholar]
- 50.Kremer J.M., Rigby W., Singer N.G. Sustained Response Following Discontinuation of Methotrexate in Patients with Rheumatoid Arthritis Treated with Subcutaneous Tocilizumab Results from a Randomized, Controlled Trial. Arthritis Rheumatol. 2018;70:1200–1208. doi: 10.1002/art.40493. [DOI] [PubMed] [Google Scholar]
- 51.Edwards C.J., Ostor A.J.K., Naisbett-Groet B., Kiely P. Tapering versus steady-state methotrexate in combination with tocilizumab for rheumatoid arthritis: A randomized, double-blind trial. Rheumatology. 2018;57:84–91. doi: 10.1093/rheumatology/kex358. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko Y., Atsumi T., Tanaka Y., Inoo M., Kobayashi-Haraoka H., Amano K., Miyata M., Murakawa Y., Yasuoka H., Hirata S., et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study) Ann. Rheum. Dis. 2016;75:1917–1923. doi: 10.1136/annrheumdis-2015-208426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kameda H., Wada K., Takahashi Y., Hagino O., van Hoogstraten H., Graham N., Tanaka Y. Sarilumab monotherapy or in combination with non-methotrexate disease-modifying antirheumatic drugs in active rheumatoid arthritis: A Japan phase 3 trial (HARUKA) Mod Rheumatol. 2020;30:239–248. doi: 10.1080/14397595.2019.1639939. [DOI] [PubMed] [Google Scholar]
- 54.Weinblatt M.E., Mease P., Mysler E., Takeuchi T., Drescher E., Berman A., Xing J., Zilberstein M., Banerjee S., Emery P. The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: Results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol. 2015;67:2591–2600. doi: 10.1002/art.39249. [DOI] [PubMed] [Google Scholar]
- 55.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh J.A., Christensen R., Wells G.A., Suarez-Almazor M.E., Buchbinder R., Lopez-Olivo M.A., Ghogomu E.T., Tugwell P. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: A Cochrane overview. Can. Med. Assoc. J. 2009;181:787–796. doi: 10.1503/cmaj.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alfonso-Cristancho R., Armstrong N., Arjunji R., Riemsma R., Worthy G., Ganguly R., Kleijnen J. Comparative effectiveness of biologics for the management of rheumatoid arthritis: Systematic review and network meta-analysis. Clin. Rheumatol. 2017;36:25–34. doi: 10.1007/s10067-016-3435-2. [DOI] [PubMed] [Google Scholar]
- 58.Buckley F., Finckh A., Huizinga T.W.J., Dejonckheere F., Jansen J.P. Comparative Efficacy of Novel DMARDs as Monotherapy and in Combination with Methotrexate in Rheumatoid Arthritis Patients with Inadequate Response to Conventional DMARDs: A Network Meta-Analysis. J. Manag. Care Spec. Pharm. 2015;21:409–423. doi: 10.18553/jmcp.2015.21.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donahue K.E., Schulman E.R., Gartlehner G., Jonas B.L., Coker-Schwimmer E., Patel S.V., Weber R.P., Bann C.M., Viswanathan M. Comparative Effectiveness of Combining MTX with Biologic Drug Therapy Versus Either MTX or Biologics Alone for Early Rheumatoid Arthritis in Adults: A Systematic Review and Network Meta-analysis. J. Gen. Intern. Med. 2019;34:2232–2245. doi: 10.1007/s11606-019-05230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Migliore A., Bizzi E., Egan C.G., Bernardi M., Petrella L. Efficacy of biological agents administered as monotherapy in rheumatoid arthritis: A Bayesian mixed-treatment comparison analysis. Ther. Clin. Risk Manag. 2015;11:1325–1335. doi: 10.2147/TCRM.S89678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarp S., Furst D.E., Dossing A., Østergaard M., Lorenzen T., Hansen M.S., Singh J.A., Choy E.H., Boers M., Suarez-Almazor M.E., et al. Defining the optimal biological monotherapy in rheumatoid arthritis: A systematic review and meta-analysis of randomised trials. Semin. Arthritis Rheum. 2017;46:699–708. doi: 10.1016/j.semarthrit.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Orme M.E., Macgilchrist K.S., Mitchell S., Spurden D., Bird A. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: Analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics. 2012;6:429–464. doi: 10.2147/BTT.S36707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gabay C., Riek M., Hetland M.L., Hauge E.M., Pavelka K., Tomšič M., Canhao H., Chatzidionysiou K., Lukina G., Nordström D.C., et al. Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: Results from a European collaborative study. Ann. Rheum. Dis. 2016;75:1336–1342. doi: 10.1136/annrheumdis-2015-207760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lauper K., Nordström D.C., Pavelka K., Hernández M.V., Kvien T.K., Kristianslund E.K., Santos M.J., Rotar Ž., Iannone F., Codreanu C., et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: Analyses from the pan-European TOCERRA register collaboration. Ann. Rheum. Dis. 2018;77:1276–1282. doi: 10.1136/annrheumdis-2017-212845. [DOI] [PubMed] [Google Scholar]
- 65.Gabay C., Emery P., van Vollenhoven R., Dikranian A., Alten R., Pavelka K., Klearman M., Musselman D., Agarwal S., Green J., et al. ADACTA Study Investigators. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): A randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 66.Burmester G.R., Lin Y., Patel R., van Adelsberg J., Mangan E.K., Graham N.M., van Hoogstraten H., Bauer D., Ignacio Vargas J., Lee E.B. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): A randomised, double-blind, parallel-group phase III trial. Ann. Rheum. Dis. 2017;76:840–847. doi: 10.1136/annrheumdis-2016-210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramiro S., Sepriano A., Chatzidionysiou K., Nam J.L., Smolen J.S., van der Heijde D., Dougados M., van Vollenhoven R., Bijlsma J.W., Burmester G., et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2017;76:1101–1136. doi: 10.1136/annrheumdis-2016-210708. [DOI] [PubMed] [Google Scholar]
- 68.Raaschou P., Simard J.F., Holmqvist M., Askling J. ARTIS Study Group. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: Nationwide population based prospective cohort study from Sweden. BMJ. 2013;346:f1939. doi: 10.1136/bmj.f1939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For the unpublished data, we collected data on the EULAR response criteria, as well as the variation over time of ACR-N, DAS 28-ESR and DAS28-CRP, total sharp score, erosions and joint-space scores, HAQ-DI score, as well as the variation of ESR, CRP, number of painful and swollen joints, VAS pain, patient global VAS and physician global VAS. The information is available on request by e-mail from Célia Delpech.