Abstract
The insertion of pedicle screws in the lateral position without a position change has been reported. We completed a retrospective comparison of the radiologic and clinical outcomes of 36 patients who underwent either single-position oblique lateral lumbar interbody fusion (SP-OLIF) using the O-arm (36 cases) or conventional OLIF (C-OLIF) using the C-arm (20 cases) for L2–5 single-level lumbar degenerative diseases. Radiological parameters were analyzed, including screw accuracy (Gertzbein-Robbins classification system; GRS), segmental instability, and fusion status. Screw misplacement was defined as a discrepancy of ≥2 mm. Clinical outcomes, including visual analog scale, Oswestry Disability Index (ODI), 36-Item Short Form Health Survey (SF-36), and postoperative complications, were assessed. The spinal fusion rate was not different between the SP-OLIF and C-OLIF groups one year after surgery (p = 0.536). The ODI score was lower (p = 0.015) in the SP-OLIF than the C-OLIF group. Physical (p = 0.000) and mental component summaries (p = 0.000) of the SF-36 were significantly higher in the SP-OLIF group. Overall complication rates, including revision, surgical site infection, ipsilateral weakness, and radicular pain/numbness, were not significantly different. SP-OLIF using the O-arm procedure is feasible, with acceptable accuracy, fusion rate, and complication rate. This may be an alternative to conventional two-stage operations.
Keywords: oblique lateral lumbar interbody fusion, minimally invasive surgery, O-arm navigation, C-arm, spinal fusion
1. Introduction
The oblique lumbar interbody fusion (OLIF) has gained popularity as a minimally invasive spinal fusion technique for the treatment of degenerative lumbar diseases including lumbar stenosis, spondylolisthesis, degenerative disc diseases, spinal instability, and spinal deformity. Compared to traditional posterior approaches, such as posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF), OLIF provides favorable fusion by enabling a large fusion bed, and facilitates early recovery with less muscle damage, blood loss, and wound infection [1,2,3,4,5]. Unlike PLIF and TLIF, OLIF traditionally requires repositioning the patient from supine to prone for supplemental pedicle screw fixation.
Recently, the O-arm and intraoperative navigation techniques have become increasingly important in spinal surgery [6,7]. These techniques increase the accuracy of pedicle screw placement and cage insertion, and reduce malposition compared with freehand and conventional fluoroscopy techniques [8,9,10]. Fluoroscopy in the lateral position during pedicle screw insertion is awkward and inconvenient for surgeons. Because the O-arm system is removed from the operating field during virtual navigation, pedicle screws can be inserted from the lateral position without machine intervention. In addition, lower radiation exposure to surgeons and surgical teams is an advantage of the O-arm compared to the C-arm [11,12].
Previous studies on pedicle screw insertion in the lateral position without positional change have recently been reported [13,14,15,16,17]. Therefore, we considered that performing OLIF in a single position by taking advantage of the O-arm would be very efficient in various clinical aspects and can be helpful for surgeons who are just starting out with single-segment OLIF. We aimed to evaluate the accuracy and efficiency of the single-position OLIF (SP-OLIF) using the O-arm procedure and to obtain clinical evidence supporting SP-OLIF by comparing two surgical methods: SP-OLIF using the O-arm and conventional OLIF (C-OLIF) using the C-arm.
2. Materials and Methods
2.1. Patient Population
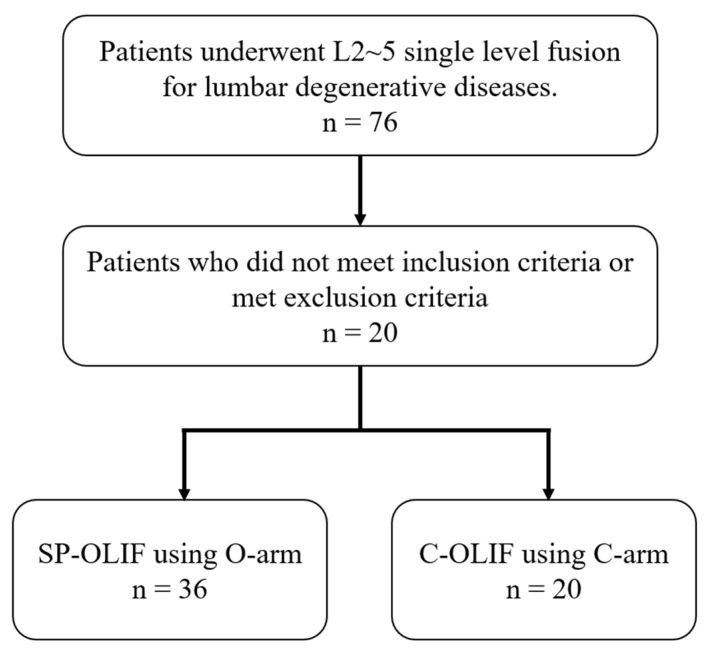
Between June 2017 and September 2020, 76 patients who underwent either SP-OLIF using the O-arm (49 cases) or C-OLIF using the C-arm (27 cases) for L2–5 single-level fusion for lumbar degenerative diseases were enrolled in this study. All patients provided written informed consent and the relevant Institutional Review Board of the Yonsei University Health System, Severance Hospital approved this study (4-2021-1528). Among the 76 patients who underwent L2-5 single level lumbar fusion surgeries, 56 patients (SP-OLIF: 36 cases vs. C-OLIF: 20 cases) who met the inclusion criteria or did not meet the exclusion criteria were analyzed retrospectively (Figure 1). There were 16 males and 20 females in the SP-OLIF group and six males and 14 females in the C-OLIF group. The inclusion criteria were as follows: age > 18 years, patients who underwent surgery at the level of L3–4 or L4–5, spinal stenosis with typical symptoms, degenerative or spondylolytic spondylolisthesis of Meyerding grade 1 or 2, failure of conservative treatment for more than six months, and available follow-up data for at least 12 months. The exclusion criteria were as follows: spinal infection, systemic malignancy, spinal trauma, previous spinal surgery, history of abdominal surgery, diagnosed osteoporosis, incomplete follow-up, or missing medical records. The average age at surgery was 61 years in both groups. The mean body mass index in each group was 25.6 and 25.0, respectively. The selection of surgery was not only based on the image findings, surgical period and requirement of direct neural decompression, but also the surgeon also decided on the surgical procedure according to the patient’s request and the surgeon’s discretion after explaining the pros and cons of each surgical procedure.
Figure 1.
Patient enrollment.
2.2. Data Collection
Variables, including demographic characteristics, disease-related data, parameters related to the operation, and outcomes, were investigated. Demographic parameters included sex, age, body mass index (BMI), bone mineral density (BMD), and the American Society of Anesthesiologists class. A T-score < −2.5 on dual-energy X-ray absorptiometry was defined as osteoporosis. Disease parameters included diagnosis, index levels, and Meyerding grade [18]. Clinical outcomes were routinely assessed using the visual analog scale (VAS), Oswestry Disability Index (ODI), and the 36-Item Short Form Health Survey (SF-36). All patients received follow-up X-rays at one, three, six, and 12 months, and computed tomography (CT) at 12 months. Radiographs were used to evaluate segmental instability. CT scans were used to determine the fusion status one year postoperatively. Information on the operation time, estimated blood loss (EBL), American Society of Anesthesiologists (ASA) score, and hospital stay was obtained from chart reviews.
2.3. Surgical Technique
The surgeries were performed by two spine surgeons with more than 20 years of surgical experience at our institution using the same protocol.
2.3.1. SP-OLIF
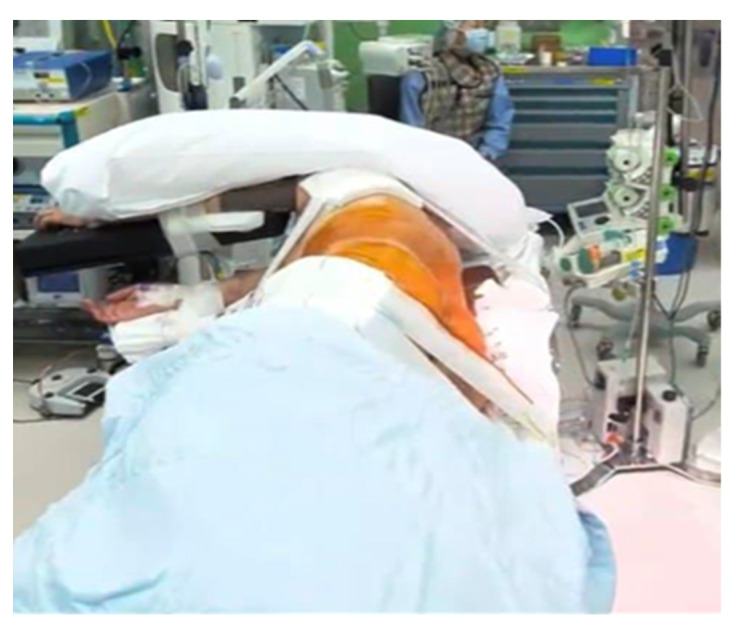
Patients were placed in the 70° right lateral decubitus position on a radiolucent table to facilitate percutaneous pedicle screw fixation (PPSF) (Figure 2).
Figure 2.
Intraoperative positioning of the patient showing 70-degree right lateral decubitus (left side up).
After preoperative administration of skin antiseptics, the surgical field was covered with an iodine-impregnated incision drape. A navigation reference arc was then placed over the iliac crest approximately 2 inches superolateral to the posterior superior iliac spine. Reference arrays were registered for real-time navigation (Stealth Station, Medtronic, Memphis, TN) based on the first CT scan (O-Arm, Medtronic, Memphis, TN). An oblique incision was made 3 cm anterior to the mid-portion of the index disc space. Blunt dissection was gently performed with sequential exposure of the external oblique, internal oblique, and transversus abdominis fascia. The retroperitoneal fat and space were then identified by visualizing the psoas muscle. The adventitial layers were mobilized at the anterior aspect of the psoas muscle with gentle dissection to allow a wider surgical corridor and to avoid injuring the psoas muscle and lumbar plexus. Navigation was used to identify the correct disc space for entry anterior to the psoas. Sequential dilators were inserted, and a tubular retractor and a light source were placed. A shaver, curette, trial implant, and cage (Clydesdale, Medtronic, Memphis, TN, USA) were used for navigation. The intervertebral cage was filled with the graft material (Grafton, Medtronic, Memphis, TN, USA). The cage was placed using an orthogonal maneuver to achieve a 90° angle during the cage placement. To confirm cage position and obtain a preoperative image for PPSF, a second CT scan was performed. The retractor was removed and the abdominal wound was closed in layers.
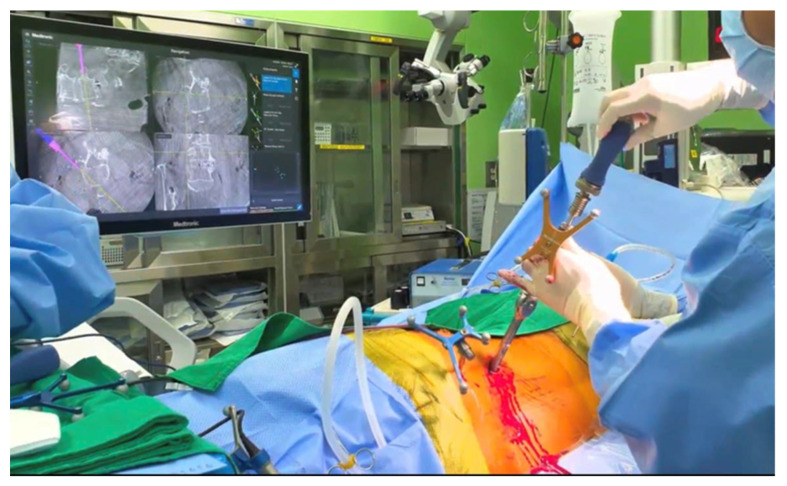
Before PPSF, the patients were positioned in the 70° right decubitus position to facilitate downside screwing (right side). Percutaneous screws (Legacy, Medtronic, Memphis, TN) were placed using the CD Horizon Sextant system and Stealth Navigation, without changing the patient’s position (Figure 3). Finally, a third CT scan was performed to confirm ideal pedicle screw and rod positioning.
Figure 3.
Intraoperative images demonstrating placement of percutaneous pedicle screws under O-arm navigation system.
2.3.2. C-OLIF
In the C-OLIF procedure, the patient was placed in the right lateral decubitus position and a 2-inch skin incision was made in the same manner as SP-OLIF. Fluoroscopy using the C-arm (OEC 9900 Elite, General Electric Company, Boston, MA, USA) was performed to confirm the proper level and successful cage insertion. After cage placement, the patients were placed in the prone position for PPSF using the C-arm.
2.4. Radiologic Outcomes
A postoperative CT scan was used to analyze the accuracy of screw placement using the Gertzbein-Robbins classification system (GRS) immediately after surgery [19]. Plain radiographs, including anteroposterior, lateral, flexion, and extension views, were obtained before surgery and at six months and one year postoperatively to compare the segmental stability and fusion status. We defined a mobility of <5° at the index level as solid fusion. Conventional CT scans were obtained postoperatively at 12 months to assess bony fusion by confirming bridging trabecular bone (BTB) in sagittal and coronal cuts. To minimize inter- and intra-observer errors, two independent spine surgeons evaluated the plain radiographs and CT scans.
2.5. Clinical Outcomes
VAS and ODI were assessed preoperatively and at 12 months postoperatively. Pain intensity was reported from 0 to 10 using a subjective VAS (0 = no pain; 10 = worst pain imaginable). The ODI scores ranged from 0 to 100 (0 = no disability; 100 = maximum disability). All patients also completed the 36-Item Short Form Survey (SF-36), which consisted of a physical component summary (PCS) and a mental component summary (MCS). All clinical outcome scales were surveyed preoperatively and at one, three, six, and 12 months after surgery by a pain-specialist nurse who was blinded to the type of surgery. Postoperative complications, including wound revision, wound infection, postoperative pain, acquired weakness, and sensory changes, were investigated.
2.6. Statistical Analysis
The results are expressed as mean ± standard deviation. Categorical data are presented as numbers (%). A repeated measures analysis of variance was used to evaluate differences in the VAS and ODI scores between the SP-OLIF cage and C-OLIF groups before surgery and at one year after surgery. The fusion rate was calculated for each group, and between-group differences in the rates of fusion and postoperative complications were compared using the chi-square test or Fisher’s exact test, as appropriate for data distribution. All statistical analyses were performed using SPSS version 23 for Windows (IBM, Armonk, NY); p < 0.05 was considered statistically significant.
3. Results
3.1. Patient Demographics
The demographic characteristics of patients are presented in Table 1. There were no significant differences between the two groups regarding age, sex, BMI, BMD, operative time, ASA class, preoperative diagnosis, and instrumented level distribution. However, the hospital stays were significantly higher in the SP-OLIF group than that of C-OLIF group (7.97 ± 2.43 vs. 5.40 ± 1.00, p = 0.000). In the SP-OLIF group, the EBL was significantly less than in the C-OLIF group (131.94 ± 95.40 vs. 270.00 ± 238.64, p = 0.003).
Table 1.
Demographic characteristics of the patients.
| SP-OLIF (O-Arm) | C-OLIF (C-Arm) | p-Value | 95% C.I of the Difference | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Total number | 36 | 20 | - | ||
| Sex (male:female) | 16:20 | 6:14 | 0.255 | ||
| Mean age (years) | 61.78 ± 8.33 | 64.30 ± 7.01 | 0.258 | −6.950 | 1.906 |
| BMI (kg/m2) | 25.67 ± 5.02 | 25.00 ± 2.67 | 0.579 | −1.752 | 3.103 |
| BMD (T-score; mean ± SD) | −0.98 ± 1.03 | −1.43 ± 1.38 | 0.169 | −0.199 | 1.105 |
| ASA class | 2.50 ± 0.66 | 2.20 ± 0.52 | 0.084 | −0.042 | 0.642 |
| Operative time (mins) | 185.00 ± 36.46 | 198.30 ± 41.75 | 0.220 | −34.773 | 8.173 |
| Estimated blood loss (mL) | 131.94 ± 95.40 | 270.00 ± 238.64 | 0.003 | −228.103 | −48.008 |
| Hospital stays (days) | 7.97 ± 2.43 | 5.40 ± 1.00 | 0.000 | 1.429 | 3.716 |
| Pre-operative diagnosis, n (%) | 0.963 | ||||
| Spinal stenosis | 21 (58.3%) | 11 | - | - | - |
| Degenerative SPL | 12 (33.3%) | 7 | - | - | - |
| Spondylolytic SPL | 3 (8.3%) | 2 | - | - | - |
| Distribution of instrumented levels, n (%) | 0.362 | ||||
| L3/4 | 4 (11.1%) | 4 (20.0%) | - | - | - |
| L4/5 | 32 (88.9%) | 16 (80.0%) | - | - | - |
Values are presented as mean ± standard deviation. SD, standard deviation; BMI, Body mass index; BMD, Bone Mineral Density; ASA, American society of anesthesiologists; SPL, spondylolisthesis.
3.2. Radiologic Outcomes
3.2.1. Pedicle Screw Accuracy
Table 2 shows the pedicle screw placement accuracy measured using GRS. In the SP-OLIF group, 144 pedicle screws were inserted, while 80 pedicle screws were inserted in the C-OLIF group. There were no significant differences between the groups in distribution of each GRS grade. According to the GRS of SP-OLIF group, 139 screws (96.5%) were classified as grade A and five screws (3.5%) as grade B. There were no cases of grades C, D, or E. In the C-OLIF group, 77 screws (96.3%) were classified as grade A, three screws (3.8%) as grade B, one screw (1.3%) as grade C, and there were no cases of grade D or E (Table 3).
Table 2.
Gertzbein-Robbins classification system of pedicle screw accuracy.
| Grade | Breach Distance (mm) |
|---|---|
| A | 0 |
| B | <2 |
| C | <4 |
| D | <6 |
| E | >6 |
Table 3.
Lumbar pedicle screw placement accuracy grades according to the Gertzbein-Robbins classification system.
| GRS Grade | SP-OLIF (O-Arm) | C-OLIF (C-Arm) | p-Value |
|---|---|---|---|
| Grade A | 139/144 (96.5%) | 77/80 (96.3%) | 0.915 |
| Grade B | 5/144 (3.5%) | 3/80 (3.8%) | 0.915 |
| Grade C | 0/144 (0%) | 1/80 (1.3%) | 0.179 |
| Grade D, E | 0/144 (0%) | 0/80 (0%) | 1.000 |
GRS, Gertzbein-Robbins classification system of pedicle screw accuracy.
3.2.2. Radiologic Parameters and Fusion Rates
Bone fusion and one-year postoperative Cobb angles measured using lateral flexion/extension plain films of the instrumented levels are summarized in Table 4. There were no differences in the fusion rates between the two groups at one year after surgery (94.4% vs. 90.0%, p = 0.536). Furthermore, the number of BTB formations was not significantly different between the groups (33/36 segments [91.7%] vs. 17/20 segments [85.0%], p = 0.930). There was no significant difference in radiologic parameters (the mean Cobb angle of the instrumented level at flexion, extension, and the amount of motion [flexion minus extension]) between the groups at one year after surgery.
Table 4.
Radiologic outcomes.
| SP-OLIF (O-Arm) | C-OLIF (C-Arm) | p-Value | |
|---|---|---|---|
| Flexion (°) | 9.94 ± 5.64 | 8.05 ± 3.11 | 0.171 |
| Extension (°) | 11.39 ± 5.96 | 9.98 ± 3.01 | 0.324 |
| Dynamic (flexion minus extension, °) | 1.45 ± 3.98 | 1.95 ± 1.53 | 0.598 |
| The number of BTB formation (by CT) | 33/36 (91.7%) | 17/20 (85.0%) | 0.930 |
| Fusion rates | 94.4% (34/36) | 90.0% (18/20) | 0.536 |
Postop, postoperative; BTB, bridging trabecular bone.
3.3. Clinical Outcomes
Preoperative clinical outcomes and those one year after surgery are summarized in Table 5. The mean ODI at one year after surgery was lower (showing an improved outcome) for the SP-OLIF group than for the C-OLIF group (18.81 ± 10.99 vs. 28.20 ± 17.06, p = 0.015). Preoperative pain decreased significantly at one year after surgery (7.31 ± 1.31 vs. 2.56 ± 2.04, p = 0.002). In the SF-36 survey, the PCS (62.22 ± 14.09 vs. 47.63 ± 15.03, p = 0.000) and MCS (74.17 ± 13.82 vs. 52.89 ± 15.68, p = 0.000) of SF-36 were significantly higher in the SP-OLIF group than in the C-OLIF group.
Table 5.
Clinical outcomes.
| SP-OLIF (O-Arm) | C-OLIF (C-Arm) | p-Value | |
|---|---|---|---|
| Pre_VAS | 7.31 ± 1.31 | 7.25 ± 1.94 | 0.899 |
| Pre_ODI | 45.69 ± 14.60 | 46.55 ± 18.77 | 0.850 |
| Pre_PCS of SF-36 | 34.53 ± 16.69 | 41.15 ± 16.61 | 0.160 |
| Pre_MCS of SF-36 | 52.50 ± 18.35 | 51.46 ± 21.20 | 0.848 |
| Post_VAS | 2.56 ± 2.04 | 3.30 ± 2.27 | 0.214 |
| Post_ODI | 18.81 ± 10.99 | 28.20 ± 17.06 | 0.015 |
| Post_PCS of SF-36 | 62.22 ± 14.09 | 47.63 ± 15.03 | 0.000 |
| Post_MCS of SF-36 | 74.17 ± 13.82 | 52.89 ± 15.68 | 0.000 |
Values are presented as mean ± standard deviation. Preop, preoperative; Postop, postoperative; VAS, Visual analogue scale; ODI, Oswestry disability index; PCS, Physical component summary; SF-36, 36-Item short form survey; MCS, Mental component summary.
3.4. Surgical Complications
Postoperative complications, including revision surgery, are summarized in Table 6. There were no cases of wound revision or infection in the SP-OLIF group. However, in the C-OLIF group, there was one revision case due to screw malposition.
Table 6.
Surgery-related complications.
| Type of Complication | SP-OLIF (O-Arm) | C-OLIF (C-Arm) | p-Value |
|---|---|---|---|
| Revision | None (0%) | 1 (5%) | 0.176 |
| Surgical site infection | None (0%) | None (0%) | 1.000 |
| Ipsilateral weakness | 1 (2.8%) | None (0%) | 0.452 |
| Radicular pain or numbness | 3 (8.3%) | 2 (10%) | 0.788 |
| Overall complication rate | 4 (11.1%) | 3 (15%) | 0.673 |
There was one case (2.8%) of motor weakness in the SP-OLIF group, but there were no cases of postoperative weakness in the C-OLIF group. Foot drop occurred immediately after surgery but completely recovered two weeks later. The number of patients complaining of temporary radicular pain and numbness was three (8.3%) in the SP-OLIF group, and two (10%) in the C-OLIF group, but all these patients recovered after three months. All of the above-mentioned complications resolved after conservative treatment.
4. Discussion
Since Amiot et al. first reported the use of a computer-assisted navigation system for pedicle screw fixation in 1995 [20], navigation technology has been used in various spinal surgical procedures worldwide [21,22,23]. O-arm navigation produces high-quality images comparable to those of conventional C-arm scans and provides the surgeon with clear intraoperative guidance. Three-dimensional (3D) real-time navigation, performed using an intraoperative O-arm system, can reveal 3D anatomic structures. The more intuitive 3D-position guidance provides a significant advantage in complex spine surgery [24,25].
Since Mayer first reported OLIF in 1997, it has become one of the most popular minimally invasive surgical procedures worldwide [26]. OLIF spares the psoas and provides direct visualization of key structures, while minimizing the risk of injury to the lumbar plexus, ureter, and great vessels [27]. However, conventional OLIF requires the repositioning of the patient from the lateral position to the prone position during surgery. Repositioning is time-consuming and has the potential to increase perioperative risk [28]. To overcome this issue, several surgeons have attempted pedicle screw fixation in the lateral position, that is, SP-OLIF with PPSF [29]. Favorable outcomes have been reported with clinical feasibility. Blizzard reported that the pedicle screw breach rate was 5.1% and the fusion rate at six months postoperatively was 87.5% in SP-OLIF with PPSF under C-arm fluoroscopy [13]. Drazin et al. reported that the operation time was shorter in single-position lateral interbody fusion and PPSF than in repositioned patients (130 min vs. 190 min, p = 0.009) [30]. In our study, a shorter operation time and less EBL were observed in the SP-OLIF group than in the C-OLIF group, although the EBL was significantly different.
Pedicle screw placement accuracy was also high in this study, similar to the results of previous studies. Xi et al. reported that a total of 350 levels were operated upon using a navigation system, and 94.86% of cages were placed within the acceptable range [12]. Tian and Xu reported that CT-based navigation systems had higher accuracy rates for pedicle screw placement than fluoroscopic guided screws (90.76% vs. 85.48%) [31]. In a meta-analysis by Feng et al., O-arm navigation had significant advantages in terms of accuracy over conventional C-arm fluoroscopy [32]. In addition, several studies have evaluated the accuracy of PPSF under navigation guidance in the lateral position [12,33,34]. Ouchida et al. reported that the rate of screw misplacement in single-position OLIF using O-arm navigation is only 1.8% [14,33].
In contrast, Hiyama et al. raised the criticism that inserting screws while viewing fluoroscopy in a lateral position is unfamiliar to surgeons, and that a working space between the patient and fluoroscope cannot be secured [29]. In addition, Mills et al. reported that pedicle screws placed in the lateral position had a higher rate of complications than those placed in the prone position [16]. The oblique angle can also be disorienting for surgeons, and navigation may be a method to mitigate this disorientation and solve these problems [35]. To offset this disorientation, we reduced the angle by 20° in the right lateral position and set it to 70°. Spatial perception can be secured using real-time position tracking, even in the awkward lateral position. Furthermore, the O-arm was retrieved after scanning; therefore, the working space was much wider. In our study, no screw placements were outside the clinically acceptable range. We believe that the use of the O-arm provides not only a precise view of the anatomy in an unfamiliar position but also high screw insertion accuracy.
In our study, which used SP-OLIF, there was one case (2.8%) of motor weakness, and three patients (8.3%) showed temporary radicular pain and numbness, but all recovered after three months. Lateral cage misplacement has been reported to range from 0.26 to 3.8% [36]. However, our patient did not experience radicular pain due to cage misplacement. Radicular pain is thought to be caused by genitofemoral nerve irritation or nerve stretch caused by the elevation of the intervertebral space. Unlike lateral lumbar interbody fusion (LLIF), OLIF does not manipulate the psoas muscle, but orthogonal maneuvers may temporarily stretch the genitofemoral nerve and psoas muscle. However, these complications are very rare compared to LLIF [37,38]. In our study, there were no cases of wound revision or infection in the SP-OLIF group; however, there were also no cases of infection or revision in the C-OLIF group. The reasons for this may be as follows: less damage to the paraspinal muscles and facet joints [39]; little stimulation of the nerve roots due to no laminectomy [37]; and the increased accuracy of pedicle screw insertion using the navigation system.
The SP-OLIF group showed a higher fusion rate at one year after surgery than the C-OLIF group (94.4% vs. 90.0%, p = 0.536), which was not significantly different but is consistent compared with previous studies. A meta-analysis by Tai-bang et al. revealed that postoperative fusion rates were similar between the OLIF and TLIF groups, with no statistical difference (mean difference = 1.55, 95% CI: -0.47 to 5.1, p = 0.09) [40]. Woods et al. reported that fusion rates of OLIF of L2–5 based on CT at six months was 95.3%, and there was successful fusion at 97.9% of surgical levels [38]. Kotani reported a fusion rate of 96.8% (non-fusion was detected in three patients) in single-position OLIF with PPSF [15]. Several studies report a slightly lower fusion rate of around 90%, and cage sinking and screw loosening often lead to pseudarthrosis [41,42,43]. We believe that correct endplate preparation and proper cage placement can prevent these complications.
This study has some limitations, mainly due to its retrospective design, small sample size, and risk for confounding. A large multicenter randomized controlled trial is needed to obtain higher-level evidence. Our study was performed on L2–5 with single-level OLIF, excluding L5/S1; therefore, there may be differences from previous studies of multi-level OLIF, including L5/S1 or multi-level OLIF. We have considered including these factors in future studies.
5. Conclusions
SP-OLIF using the O-arm combined with PPSF serves as an accurate, safe, and effective surgical procedure without the need for a position change compared to C-OLIF using the C-arm. It provides not only a precise view of the anatomy in the unfamiliar position, but also comparable clinical and radiologic outcomes, including fusion rate.
Acknowledgments
This research was supported by the ‘2022 Joint Research Project of Institutes of Science and Technology’ and ‘CGBIO’.
Author Contributions
Conceptualization, H.C.K. and D.A.S.; methodology, H.C.K. and D.A.S.; software, H.C.K.; validation, Y.H.J. and S.H.O.; formal analysis, H.C.K. and D.A.S.; investigation, H.C.K., Y.H.J., S.H.O. and C.K.L.; resources, H.C.K. and J.M.L.; data curation, H.C.K.; writing—original draft preparation, H.C.K.; writing—review and editing, H.C.K. and D.A.S.; visualization, H.C.K.; supervision, C.K.L., D.A.S., S.Y., Y.H. and K.N.K.; project administration, D.A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board Board of NAME OF INSTITUTE (date of approval: 21 November 2021; IRB number: 2021-3604-001).
Informed Consent Statement
Written informed consent was obtained from all the participants.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Nomura H., Yamashita A., Watanabe T., Shirasawa K. Quantitative analysis of indirect decompression in extreme lateral interbody fusion and posterior spinal fusion with a percutaneous pedicle screw system for lumbar spinal stenosis. J. Spine Surg. 2019;5:266–272. doi: 10.21037/jss.2019.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellvi A.E., Nienke T.W., Marulanda G.A., Murtagh R.D., Santoni B.G. Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin. Orthop. Relat. Res. 2014;472:1784–1791. doi: 10.1007/s11999-014-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soegaard R., Bünger C.E., Christiansen T., Høy K., Eiskjaer S.P., Christensen F.B. Circumferential fusion is dominant over posterolateral fusion in a long-term perspective: Cost-utility evaluation of a randomized controlled trial in severe, chronic low back pain. Spine. 2007;32:2405–2414. doi: 10.1097/BRS.0b013e3181573b2d. [DOI] [PubMed] [Google Scholar]
- 4.Bassani R., Morselli C., Querenghi A.M., Nuara A., Sconfienza L.M., Peretti G.M. Functional and radiological outcome of anterior retroperitoneal versus posterior transforaminal interbody fusion in the management of single-level lumbar degenerative disease. Neurosurg. Focus. 2020;49:E2. doi: 10.3171/2020.6.FOCUS20374. [DOI] [PubMed] [Google Scholar]
- 5.Fritzell P., Hägg O., Wessberg P., Nordwall A. Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the swedish lumbar spine study group. Spine. 2002;27:1131–1141. doi: 10.1097/00007632-200206010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Holly L.T., Foley K.T. Image guidance in spine surgery. Orthop. Clin. N. A. 2007;38:451–461. doi: 10.1016/j.ocl.2007.04.001. abstract viii. [DOI] [PubMed] [Google Scholar]
- 7.Costa F., Cardia A., Ortolina A., Fabio G., Zerbi A., Fornari M. Spinal navigation: Standard preoperative versus intraoperative computed tomography data set acquisition for computer-guidance system: Radiological and clinical study in 100 consecutive patients. Spine. 2011;36:2094–2098. doi: 10.1097/BRS.0b013e318201129d. [DOI] [PubMed] [Google Scholar]
- 8.Mason A., Paulsen R., Babuska J.M., Rajpal S., Burneikiene S., Nelson E.L., Villavicencio A.T. The accuracy of pedicle screw placement using intraoperative image guidance systems. J. Neurosurg. Spine. 2014;20:196–203. doi: 10.3171/2013.11.SPINE13413. [DOI] [PubMed] [Google Scholar]
- 9.Gelalis I.D., Paschos N.K., Pakos E.E., Politis A.N., Arnaoutoglou C.M., Karageorgos A.C., Ploumis A., Xenakis T.A. Accuracy of pedicle screw placement: A systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur. Spine J. 2012;21:247–255. doi: 10.1007/s00586-011-2011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J., Zhu Z., Sui T., Kong D., Cao X. Position and complications of pedicle screw insertion with or without image-navigation techniques in the thoracolumbar spine: A meta-analysis of comparative studies. J. Biomed. Res. 2014;28:228–239. doi: 10.7555/JBR.28.20130159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y.H., White I., Potts E., Mobasser J.P., Chou D. Comparison perioperative factors during minimally invasive pre-psoas lateral interbody fusion of the lumbar spine using either navigation or conventional fluoroscopy. Glob. Spine J. 2017;7:657–663. doi: 10.1177/2192568217716149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi Z., Chou D., Mummaneni P.V., Burch S. The navigated oblique lumbar interbody fusion: Accuracy rate, effect on surgical time, and complications. Neurospine. 2020;17:260–267. doi: 10.14245/ns.1938358.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blizzard D.J., Thomas J.A. Mis single-position lateral and oblique lateral lumbar interbody fusion and bilateral pedicle screw fixation: Feasibility and perioperative results. Spine. 2018;43:440–446. doi: 10.1097/BRS.0000000000002330. [DOI] [PubMed] [Google Scholar]
- 14.Ouchida J., Kanemura T., Satake K., Nakashima H., Ishikawa Y., Imagama S. Simultaneous single-position lateral interbody fusion and percutaneous pedicle screw fixation using o-arm-based navigation reduces the occupancy time of the operating room. Eur. Spine J. 2020;29:1277–1286. doi: 10.1007/s00586-020-06388-6. [DOI] [PubMed] [Google Scholar]
- 15.Kotani Y., Koike Y., Ikeura A., Tokunaga H., Saito T. Clinical and radiologic comparison of anterior-posterior single-position lateral surgery versus mis-tlif for degenerative lumbar spondylolisthesis. J. Orthop. Sci. 2021;26:992–998. doi: 10.1016/j.jos.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Mills E.S., Treloar J., Idowu O., Shelby T., Alluri R.K., Hah R.J. Single position lumbar fusion: A systematic review and meta-analysis. Spine J. 2022;22:429–443. doi: 10.1016/j.spinee.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Hiyama A., Katoh H., Sakai D., Sato M., Tanaka M., Watanabe M. Comparison of radiological changes after single- position versus dual-position for lateral interbody fusion and pedicle screw fixation. BMC Musculoskelet. Disord. 2019;20:601. doi: 10.1186/s12891-019-2992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyerding H.W. Low backache and sciatic pain associated with spondylolisthesis and protruded intervertebral disc: Incidence, significance, and treatment. JBJS. 1941;23:461–470. [Google Scholar]
- 19.Gertzbein S.D., Robbins S.E. Accuracy of pedicular screw placement in vivo. Spine. 1990;15:11–14. doi: 10.1097/00007632-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Amiot L.P., Labelle H., DeGuise J.A., Sati M., Brodeur P., Rivard C.H. Computer-assisted pedicle screw fixation. A feasibility study. Spine. 1995;20:1208–1212. doi: 10.1097/00007632-199505150-00019. [DOI] [PubMed] [Google Scholar]
- 21.Kalfas I.H. Machine vision navigation in spine surgery. Front. Surg. 2021;8:640554. doi: 10.3389/fsurg.2021.640554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham M.H., Diaz-Aguilar L.D., Shah V., Brandel M., Loya J., Lehman R.A. Simultaneous robotic single position oblique lumbar interbody fusion with bilateral sacropelvic fixation in lateral decubitus. Neurospine. 2021;18:406–412. doi: 10.14245/ns.2040774.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T.Y., Park C., Dalton T., Rajkumar S., McCray E., Owolo E., Than K.D., Abd-El-Barr M.M. Robotic navigation in spine surgery: Where are we now and where are we going? J. Clin. Neurosci. 2021;94:298–304. doi: 10.1016/j.jocn.2021.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Sielatycki J.A., Mitchell K., Leung E., Lehman R.A. State of the art review of new technologies in spine deformity surgery-robotics and navigation. Spine Deform. 2022;10:5–17. doi: 10.1007/s43390-021-00403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J., Wu D., Wang Q., Wei Y., Yuan F. Pedicle screw insertion: Is o-arm-based navigation superior to the conventional freehand technique? A systematic review and meta-analysis. World Neurosurg. 2020;144:e87–e99. doi: 10.1016/j.wneu.2020.07.205. [DOI] [PubMed] [Google Scholar]
- 26.Mayer H.M. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine. 1997;22:691–699. doi: 10.1097/00007632-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 27.Choy W., Mayer R.R., Mummaneni P.V., Chou D. Oblique lumbar interbody fusion with stereotactic navigation: Technical note. Glob. Spine J. 2020;10:94S–100S. doi: 10.1177/2192568220910181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce K.E., Kapadia B.H., Bortz C., Brown A., Alas H., Naessig S., Ahmad W., Vasquez-Montes D., Manning J., Wang E., et al. Operative fusion of patients with metabolic syndrome increases risk for perioperative complications. J. Clin. Neurosci. 2020;72:142–145. doi: 10.1016/j.jocn.2019.12.043. [DOI] [PubMed] [Google Scholar]
- 29.Hiyama A., Sakai D., Sato M., Watanabe M. The analysis of percutaneous pedicle screw technique with guide wire-less in lateral decubitus position following extreme lateral interbody fusion. J. Orthop. Surg. Res. 2019;14:304. doi: 10.1186/s13018-019-1354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drazin D., Kim T.T., Johnson J.P. Simultaneous lateral interbody fusion and posterior percutaneous instrumentation: Early experience and technical considerations. Biomed. Res. Int. 2015;2015:458284. doi: 10.1155/2015/458284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian N.F., Xu H.Z. Image-guided pedicle screw insertion accuracy: A meta-analysis. Int. Orthop. 2009;33:895–903. doi: 10.1007/s00264-009-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng W., Wang W., Chen S., Wu K., Wang H. O-arm navigation versus c-arm guidance for pedicle screw placement in spine surgery: A systematic review and meta-analysis. Int. Orthop. 2020;44:919–926. doi: 10.1007/s00264-019-04470-3. [DOI] [PubMed] [Google Scholar]
- 33.Hiyama A., Katoh H., Nomura S., Sakai D., Watanabe M. Intraoperative computed tomography-guided navigation versus fluoroscopy for single-position surgery after lateral lumbar interbody fusion. J. Clin. Neurosci. 2021;93:75–81. doi: 10.1016/j.jocn.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Park P. Impact of spinal navigation on the oblique lumbar interbody fusion. Neurospine. 2020;17:268–269. doi: 10.14245/ns.2040518.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiGiorgio A.M., Edwards C.S., Virk M.S., Mummaneni P.V., Chou D. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: Technical note and case series. Neurosurg. Focus. 2017;43:E14. doi: 10.3171/2017.5.FOCUS17168. [DOI] [PubMed] [Google Scholar]
- 36.Mehren C., Mayer H.M., Zandanell C., Siepe C.J., Korge A. The oblique anterolateral approach to the lumbar spine provides access to the lumbar spine with few early complications. Clin. Orthop. Relat. Res. 2016;474:2020–2027. doi: 10.1007/s11999-016-4883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mobbs R.J., Phan K., Malham G., Seex K., Rao P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including plif, tlif, mi-tlif, olif/atp, llif and alif. J. Spine Surg. 2015;1:2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods K.R., Billys J.B., Hynes R.A. Technical description of oblique lateral interbody fusion at l1-l5 (olif25) and at l5-s1 (olif51) and evaluation of complication and fusion rates. Spine J. 2017;17:545–553. doi: 10.1016/j.spinee.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 39.He W., He D., Sun Y., Xing Y., Liu M., Wen J., Wang W., Xi Y., Tian W., Ye X. Quantitative analysis of paraspinal muscle atrophy after oblique lateral interbody fusion alone vs. Combined with percutaneous pedicle screw fixation in patients with spondylolisthesis. BMC Musculoskelet. Disord. 2020;21:30. doi: 10.1186/s12891-020-3051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tai-bang C., Xiao-qing H.E., Liang J.-l. Comparison of oblique lateral interbody fusion and transforaminal lumbar interbody fusion for degenerative lumbar disease: A meta-analysis. Res. Sq. 2021 doi: 10.21203/rs.3.rs-70799/v1. [DOI] [Google Scholar]
- 41.Teng I., Han J., Phan K., Mobbs R. A meta-analysis comparing alif, plif, tlif and llif. J. Clin. Neurosci. 2017;44:11–17. doi: 10.1016/j.jocn.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Parajón A., Alimi M., Navarro-Ramirez R., Christos P., Torres-Campa J.M., Moriguchi Y., Lang G., Härtl R. Minimally invasive transforaminal lumbar interbody fusion: Meta-analysis of the fusion rates. What is the optimal graft material? Neurosurgery. 2017;81:958–971. doi: 10.1093/neuros/nyx141. [DOI] [PubMed] [Google Scholar]
- 43.Lowe T.G., Tahernia A.D., O’Brien M.F., Smith D.A. Unilateral transforaminal posterior lumbar interbody fusion (tlif): Indications, technique, and 2-year results. J. Spinal. Disord. Tech. 2002;15:31–38. doi: 10.1097/00024720-200202000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.