Abstract
Previously, we had demonstrated the upregulation in the expression of several proteins, including the lipoproteins OspC and P35, of Borrelia burgdorferi in the stationary growth phase. Since the expression of OspC is also known to be affected by culture temperature and pH, we examined the effects of both variables on the expression of the remaining stationary-phase-upregulated proteins. Our study revealed that the expression of each of the remaining stationary-phase-upregulated proteins, P35 included, was also influenced by culture temperature; these proteins were selectively expressed at 34°C but not at 24°C. Significantly, the expression of a majority of these proteins was also affected by culture pH, since they were abundantly expressed at pH 7.0 (resembling the tick midgut pH of 6.8 during feeding) but only sparsely at pH 8.0 (a condition closer to that of the unfed tick midgut pH of 7.4). We propose that this group of B. burgdorferi proteins, which in culture is selectively expressed under conditions of 34°C and pH 7.0, may be induced in the tick midgut during the feeding event. Furthermore, the differential and coordinate expression of these proteins under different environmental conditions suggests that the encoding genes may be coregulated.
The relapsing fever and the Lyme disease borreliae, Borrelia hermsii and B. burgdorferi, respectively, exploit changes in their environmental temperature to shuffle surface proteins (8, 12, 19, 21, 22). The Lyme disease spirochetes, which are harbored in the midgut of an infected Ixodes scapularis nymphal tick, are transmitted to the mammalian host during the course of the tick blood meal. During feeding, the midgut spirochetes experience environmental changes such as an influx of fresh mammalian blood, an increase in temperature, and a decrease in pH from 7.4 to 6.8 (27), all of which promote conditions favorable for growth. Consequently, tick feeding results in a rapid multiplication of the spirochetes (4, 7, 16) and concomitant changes in the expression of their membrane proteins (9, 19). The synthesis of OspC (10, 19, 22), Bbk32 (9), and Bbk50 (9) proteins is induced specifically during the course of the tick blood meal. The shifts in temperature and pH probably are the relevant cues for spirochetal adaptation during tick feeding because the expression of many borrelial proteins in vitro is known to be affected by the temperature and the pH of the culture medium. Some of these proteins affected by culture temperature include OspC (19, 23, 27), decorin binding proteins A and B (6), OppA-5 (3), P6.6 (1), sigma-S (27), and members of the OspEF (23, 24) and Mlp (26) protein families. Additionally, OspC (5, 27), decorin binding protein A, OspF, Mlp-8, and sigma-S (27) are also expressed at pH <7.5 (resembling the midgut pH of the feeding tick) but not at pH 8.0 (closer to the midgut pH of the unfed tick). Most of these proteins are also expressed early during infection of the mice, as inferred from the presence in the serum of antibodies that are reactive to the purified recombinant versions of these proteins. These results suggest that the early expression of this group of proteins during the course of the disease may be important for colonization of the vertebrate host.
The expression of OspC, in addition to being temperature and pH dependent, varies in vitro with the growth phase (18) and the presence of tick cells in the culture medium (14) and varies in vivo with the density of spirochetes within a feeding tick (8). During growth in culture, the levels of OspC and several other proteins, including the lipoprotein P35, increased severalfold in stationary-phase spirochetes compared to their respective levels in logarithmic-phase spirochetes (13, 18). Since OspC appeared to be regulated by both temperature and pH, we set out to determine if the expression of any of the other proteins previously recognized as growth phase regulated was also responsive to changes in these two variables.
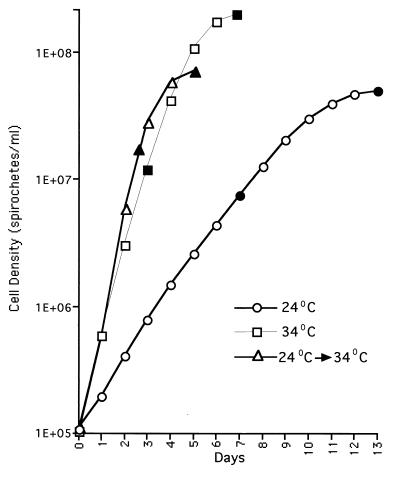
A low-passage (passage 3) variant of Sh-2-82 obtained from Denee Thomas, University of Texas Health Sciences Center, San Antonio, Tex., was inoculated into BSK-H medium (17) (Sigma Chemical Co., St. Louis, Mo.) at an initial density of 106 cells/ml and incubated for 2 weeks at 24°C for adaptation at the lower temperature, as described previously (23). This low-temperature-adapted culture was then used as the inoculum for two parallel cultures set up at 24 and 34°C (both at an initial density of 106 cells/ml). Cells from both cultures were harvested in the logarithmic (at a density of 8 × 106 cells/ml for the 24°C culture and 2 × 107 cells/ml for the 34°C culture) and stationary (5 × 107 cells/ml for the 24°C culture and 1 × 108 cells/ml for the 34°C culture) growth phases (Fig. 1). Spirochetal cultures in their logarithmic (1 × 107 cells/ml) and stationary (2 × 108 cells/ml) phases (Fig. 1) were also sampled from passage 3 Sh-2-82 cultures maintained at 34°C. The spirochetes were counted microscopically (15), and whole-cell lystates were prepared from the harvested cells and normalized to an optical density at 600 nm (OD600) of 3, as described previously (18). The cell lysates (3 μl/lane) were electrophoresed through a sodium dodecyl sulfate–14% polyacrylamide gel, and the proteins were transferred to nitrocellulose. Parallel nitrocellulose blots were then incubated individually with 1:100-diluted sera drawn from two mice (Q395 and Q396) 6 weeks following an infection with B. burgdorferi Sh-2-82. Each mouse had been experimentally infected intraperitoneally via a needle with 108 Sh-2-82 passage 3 organisms. Following incubation with the primary antibody, the blots were developed with a 1:200 dilution of biotinylated anti-mouse immunoglobulin G secondary antibody from Vector Laboratories, Burlingame, Calif. The biotinylated antibodies were probed with an avidin-biotinylated horseradish peroxidase complex (Vector) and developed using 4-chloro-1-naphthol (Sigma) as a chromogen, as described previously (2).
FIG. 1.
Growth curves of B. burgdorferi under different culture conditions. Cultures of B. burgdorferi Sh-2-82 passage 3 spirochetes were set up at an initial density of 105 organisms/ml and harvested in the logarithmic and stationary phases of growth under each of the three conditions indicated in the figure. The location of the sampling points in each case is denoted by solid symbols.
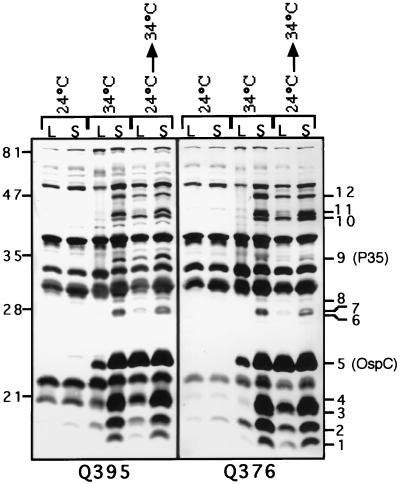
A total of 12 unique bands were apparent in the lanes containing the stationary-phase samples compared with the lanes in which the 24°C culture samples were loaded (Fig. 2, lanes 34°C-S and 24°C→34°C-S versus lanes 24°C-L and 24°C-S, bands 1 to 12). These 12 proteins will hereafter be referred to as temperature-regulated proteins because of their differential expression at the two culture temperatures. The levels of expression of each of these 12 proteins were higher in the stationary phase than in the logarithmic phase (Fig. 2, lane 34°C-S versus lane 34°C-L, and lane 24°C→34°C-S versus lane 24°C→34°C-L). Two of these proteins were identified using monoclonal antibodies. As expected, band 5, representing a 23-kDa protein (Fig. 2; Table 1), proved to be the lipoprotein, OspC (data not shown), and the 35-kDa band 9 protein (Fig. 2; Table 1) was confirmed to be the previously characterized lipoprotein P35 (11) (Fig. 3A). Finally, to further ensure that equivalent amounts of proteins were present in each of the three OD-normalized samples, the blot was additionally controlled for the constitutively expressed flagellin antigen (19, 23), using the flagellin-specific mouse monoclonal antibody H9724 (Symbicom, Umeå, Sweden) (Fig. 3A).
FIG. 2.
Western blots demonstrating the upregulation of temperature-regulated proteins of B. burgdorferi Sh-2-82 in the stationary phase. Whole-cell lysates prepared from Sh-2-82 passage 3 logarithmic-phase (lanes L) and stationary-phase (lanes S) spirochetes maintained at 24 or 34°C or shifted from 24 to 34°C were analyzed by Western blotting. The nitrocellulose blots were developed with sera from Sh-2-82-infected mice (panels Q395 and Q396), diluted 1:100 in phosphate-buffered saline containing 0.05% Tween 20. The 12 proteins that are upregulated in the stationary phase are indicated by number.
TABLE 1.
Relative molecular masses of the temperature-regulated bands identified in Fig 2
| Banda | Mol mass (kDa) |
|---|---|
| 1 | 16 |
| 2 | 17 |
| 3 | 18 |
| 4 | 21 |
| 5 (OspC) | 23 |
| 6 | 28 |
| 7 | 29 |
| 8 | 29.5 |
| 9 (P35) | 35 |
| 10 | 43.5 |
| 11 | 44 |
| 12 | 47 |
The band numbers correspond to the antigen bands enumerated in Fig. 2.
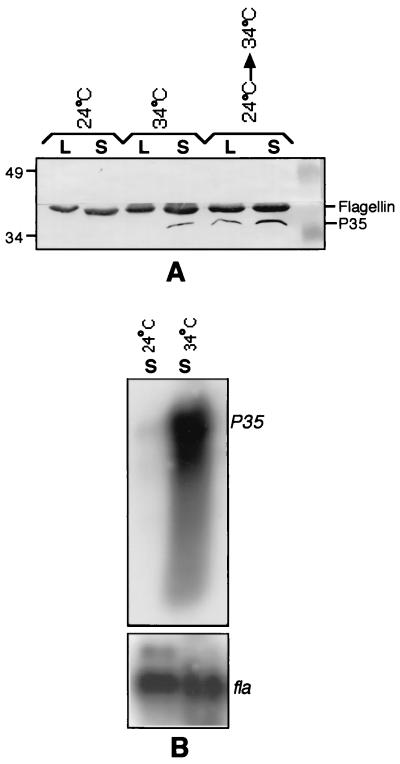
FIG. 3.
P35 is a temperature-regulated protein. (A) Western blot. The six samples described in Fig. 1 were analyzed by Western blotting for the differential expression of the P35 antigen. The blot was developed first with a monoclonal antibody specific for P35 (13) and then with the flagellin-specific antibody, H9724. (B) Northern blot. RNA isolated from spirochetes cultured at 24 and 34°C were electrophoresed (3 μg/lane) through a formaldehyde-agarose gel (18) and transferred overnight to a nitrocellulose filter. The filter was probed initially with the p35 coding sequence (13) and then with a flagellin coding sequence probe (18). The probes were radiolabeled randomly with [α-32P]dATP using the Prime-a-gene kit (Promega Corp., Madison, Wis.).
The temperature upshift-induced expression of ospC is probably controlled at the transcriptional level based on the relative levels of the ospC transcript found at the two temperatures (25). To investigate whether the temperature-inducible expression of P35 was similarly regulated at the transcriptional level, Northern blotting was carried out. RNA samples were isolated as described previously (18) from passage 3 Sh-2-82 24 and 34°C stationary-phase cultures (Fig. 1) and probed with the coding sequences of P35 and flagellin genes. The p35 transcript was found to be abundantly expressed in spirochetes cultured at the higher temperature, in sharp contrast to its nearly undetectable level of expression at the lower temperature (Fig. 3B, lane 34°C-S versus lane 24°C-S). The preferential hybridization of the p35 probe to the 34°C RNA sample was not a consequence of unequal loading of the two RNA samples, based on the similar hybridization intensities obtained for the constitutively expressed flagellin transcript in the same blot (Fig. 3B).
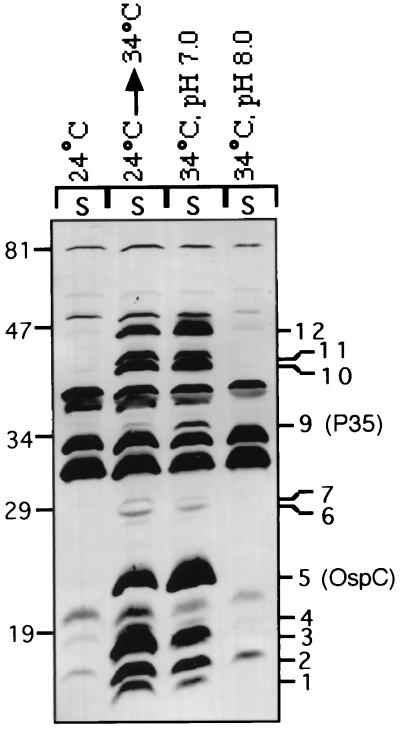
Next we examined the influence of external pH on the expression of the temperature-regulated proteins. Parallel cultures were set up in pH 7.0 and pH 8.0 BSK-H medium at an initial density of 5 × 105 spirochetes/ml and harvested after 7 to 8 days at a stationary-phase density of about 108 spirochetes/ml. In each case, the commercial BSK-H medium was supplemented with an additional 25 mM HEPES (50 mM final concentration) and adjusted to either pH 7.0 with HCl or pH 8.0 with NaOH, as previously described (5). The two stationary-phase samples were normalized to an OD600 of 3 and electrophoresed through a sodium dodecyl sulfate–14% polyacrylamide gel. Two samples, 24°C-S and 24°C→34°C-S, from the previous experiment (Fig. 2) were included as negative and positive control samples, respectively. The proteins were transferred to a nitrocellulose membrane, and the blot was probed with serum from infected mouse Q395. Although only 11 of the 12 antigens were detected in these experiments, they all appeared to be differentially expressed with respect to culture pH (Fig. 4, lane 34°C, pH 7.0 versus lane 34°C, pH 8.0). The weakly reacting 29.5-kDa antigen was not visible in this blot. In other experiments, only 7 of the other 11 antigens were found to be differentially expressed and the 17-, 28-, 29- and 47-kDa antigens were expressed at similar levels at pH 7.0 and 8.0. Although the reasons for these variations are not known, the variable expression of these four antigens may be related to their polymorphism in the uncloned spirochetal population or, alternatively, to a loss of the plasmids encoding the corresponding genes.
FIG. 4.
The expression of a majority of the temperature-regulated proteins is also affected by the pH of the culture medium. Parallel Sh-2-82 cultures set up in BSK-H medium at pH 7.0 and 8.0 were analyzed by Western blotting for the differential expression of the temperature-regulated proteins. The positions of the 12 temperature-regulated proteins including OspC and P35 are indicated.
In this study, we have demonstrated the effect of culture temperature, pH, and growth phase on the expression of a group of 12 proteins of B. burgdorferi. These proteins are abundantly expressed at 34°C and at pH 7.0 but very poorly expressed at 24°C and at pH 8.0. The expression of these proteins was also found to be considerably higher in the stationary phase than in the logarithmic phase. However, the increased expression of these proteins in the later stages of growth may be attributed to an effect of pH rather than some other distinct phenomenon because the later stages of growth at 34°C were associated with a decrease in the pH of the medium (data not shown). Therefore, the two key environmental conditions that are essential for the induction of expression of this group of proteins are (i) a temperature of about 34°C and (ii) a pH of about 7.0. These two factors also act simultaneously for the induction of these proteins. Therefore, this group of proteins, by definition, is consistent with the recently described group I proteins of B. burgdorferi, which include OspC, OspF, Mlp-8, sigma-S, and DbpA (27) and P35 (this study). It is interesting that this entire group of proteins was similarly affected by temperature and pH, raising the possibility that the regulation of these proteins may be tightly linked. It is entirely conceivable that the expression of these proteins involves the transcription factor sigma-S, because its expression mirrors that of these group I proteins (27).
We speculate that, like OspC, the proteins characterized in this study are also induced in vivo during tick feeding in response to the simultaneous upshift in temperature from ambient to ∼34°C or higher (20) and drop in pH from 7.4 to about 6.8 (27). These proteins may function to ensure transmission of the spirochete through the tick during feeding and/or facilitate the establishment of infection in the mammalian host. Further exploration of the effects of variables such as pH and temperature on spirochetal physiology is therefore paramount to understanding the adaptation of this microorganism to the environmental changes that accompany its journey through the tick and the vertebrate hosts.
Acknowledgments
We thank Denee Thomas (University of Texas Health Sciences Center, San Antonio, Tex.) for the gift of the low-passage Sh-2-82 strain, and we thank Murphy Dowouis for help with photography.
This work received financial support from NCRR-NIH (grant RR00164).
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aydintug M, Gu Y, Philipp M T. Borrelia burgdorferi antigens that are targeted by antibody-dependent, complement-mediated killing in the rhesus monkey. Infect Immun. 1994;62:4929–4937. doi: 10.1128/iai.62.11.4929-4937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bono J L, Tilly K, Stevenson B, Hogan D, Rosa P. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology. 1998;144:1033–1044. doi: 10.1099/00221287-144-4-1033. [DOI] [PubMed] [Google Scholar]
- 4.Burkot T R, Piesman J, Wirtz R A. Quantitation of the Borrelia burgdorferi outer surface protein A in Ixodes scapularis: fluctuations during the tick life cycle, doubling times, and loss while feeding. J Infect Dis. 1994;170:883–889. doi: 10.1093/infdis/170.4.883. [DOI] [PubMed] [Google Scholar]
- 5.Caroll J A, Garon C F, Schwan T G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassat D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.deSilva A M, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 8.deSilva A M, Zeidner N S, Zhang Y, Dolan M C, Peisman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fikrig E, Feng W, Barthold S W, Telford III S R, Flavell R A. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and inhibition of spirochete transmission. J Immunol. 2000;164:5344–5351. doi: 10.4049/jimmunol.164.10.5344. [DOI] [PubMed] [Google Scholar]
- 10.Fingerle V, Liegl G, Munderloh U, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus ticks removed from humans. Med Microbiol Immunol. 1998;187:121–126. doi: 10.1007/s004300050083. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore R D, Jr, Kappel K J, Johnson B J B. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore R D, Jr, Piesman J. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect Immun. 2000;68:411–414. doi: 10.1128/iai.68.1.411-414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Indest K J, Ramamoorthy R, Solé M, Gilmore R D, Johnson B J B, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obonyo M, Munderloh U G, Fingerle V, Wilske B, Kurtti T J. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J Clin Microbiol. 1999;37:2137–2141. doi: 10.1128/jcm.37.7.2137-2141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piesman J, Oliver J R, Sinsky R J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 17.Pollack R J, Telford III S R, Spielman A. Standarization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramamoorthy R, Philipp M T. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun. 1998;66:5119–5124. doi: 10.1128/iai.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 21.Schwan T G, Hinnebusch B J. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 22.Schwan T G, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–388. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilly K, Casjens S, Stevenson B, Bono J L, Samuels D S, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Popova T G, Hagman K E, Wikel S K, Schoeler G B, Caimano M J, Radolf J D, Norgard M V. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun. 1999;67:6008–6018. doi: 10.1128/iai.67.11.6008-6018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Goldberg M S, Popova T G, Schoeler G B, Wikel S K, Hagman K E, Norgard M V. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]