Abstract
The mannose receptor (MR) plays an important role in the recognition of some pathogens in nonopsonic phagocytosis and in antigen presentation to T cells. We found that Borrelia burgdorferi, the agent of Lyme borreliosis, adheres to monocyte-derived macrophages and to rat MR-transfected cells but not to untransfected cells. Antibodies to MR and sugars such as mannose, mannan, fucose, and some lectins significantly lowered the adhesion, confirming participation of the MR in the binding.
The etiological agent of Lyme disease, Borrelia burgdorferi, is able to disseminate widely and avoid clearance by innate immunity and by the immune system, thereby establishing chronic infection. Spirochetes undertake complex interactions with a variety of mammalian cells, which contribute to the establishment of infection in the host. Such interactions consist of adhesion to cells and tissues: B. burgdorferi binds to platelets via the integrins αIIβ3 and to endothelial cells through the integrins αVβ3 and α5β1 (7, 8) and to a variety of cells which express glycosaminoglycans such as heparin, heparan sulfate, and dermatan sulfate (19, 2). Lyme borreliosis Borrelia also recognizes other substrates like fibronectin (17, 23) and decorins (12), which explains the attachment of B. burgdorferi to the extracellular matrix of the skin and other tissues. Binding to such receptors as integrins, which are true cell-associated receptors, not only provides adhesion to cells, allowing B. burgdorferi to attach itself firmly to the tissues, but also can induce effector mechanisms, normally triggered by the integrin under appropriate stimuli. This is the case with integrin αMβ2, a dynamic molecule also known as CR3 or CD11b/CD18 receptor; this molecule was shown to bind borrelias (4) and trigger phagocytosis by neutrophils in the absence of specific opsonization; such binding activates the molecule to up-regulation and increases adhesion to fibronectin (6). The binding seems to involve not only the I domain of the molecule, specific for the iC3b and the RGD motif, but also the lectin-like domain, which is known to recognize lipopolysaccharide, mannose, and other sugar residues (5).
Another cell receptor which is involved in microbe recognition and phagocytosis in the absence of specific opsonization is the mannose receptor (MR), which acts as a true lectin in the lectin phagocytosis of microorganisms (22). It is a type I transmembrane glycoprotein of 165 kDa containing as many as eight adjoining carbohydrate recognition domains, a fibronectin type II domain, and a cysteine-rich domain (2); it is expressed on tissue macrophages, dendritic cells (mostly on Langerhans cells), endothelium, and rat microglia. Besides acting as a scavenger of mannose-containing glycoconjugates on the surface of a wide spectrum of microorganisms, such as Escherichia coli, Klebsiella pneumoniae, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Candida albicans, Leishmania, and Pneumocystis carinii (21), it mediates their ingestion by macrophages. Moreover, it has been recently reported that dendritic cells expressing the MR exhibit a 100-fold increase in antigen presentation to T cells (10). Dendritic cells and Langerhans cells are the first line of phagocytosis, serving as antigen-processing cells in the dermis and epidermis which can internalize B. burgdorferi in the tissues, in the absence of specific antibodies (11). It has recently been demonstrated that the principal immunogens of B. burgdorferi, the lipoproteins, are rapidly processed by human dendritic cells and presented to CD8+ T cells (2). Since the MR is the main molecule involved in antigen recognition and the binding process in antigen-presenting cells (9, 10), this study was undertaken to establish whether B. burgdorferi recognizes and binds this receptor. We used three cellular models; one was a culture of monocyte-derived macrophages (MDMs), which naturally expresses the MR, while the other two were cell line MRF-61 (a rat fibroblast line transfected with the human MR) and its wild-type, untransfected cell parent.
Microorganisms, cell cultures, and labeling.
The borreliae used were Borrelia garinii strain BITS, isolated from Ixodes ricinus, and the low-passage-number. B. garinii strain M3/5, isolated from a mouse. Culture conditions and counting were as previously reported (3). When required, borreliae were labeled with fluorescein isothiocyanate (FITC) as previously described (3). C. albicans (ATCC 3153) was grown in Sabouraud medium and fluorescein labeled as for borreliae.
MDM monolayers were prepared from blood monocytes as described elsewhere (25). In brief, buffy coats obtained from the blood bank of the Ospedale Maggiore (Trieste, Italy) were diluted with an equal volume of Ca2+- and Mg2+-free phosphate-buffered saline, pH 7.4 (PBS), containing 1 mM EDTA and 5 mM glucose and then centrifuged at 250 × g for 10 min at 4°C to remove platelets. The pellet containing erythrocytes and leukocytes was suspended in PBS-EDTA-glucose solution. Thirty-five milliliters of this suspension was layered over 15 ml of Lymphoprep and centrifuged at 800 × g for 25 min at 4°C. The band at the PBS-Lymphoprep interface containing the lymphocytes and monocytes was collected, centrifuged at 250 × g for 10 min at 4°C, and washed twice in PBS-EDTA-glucose solution; the cells were resuspended in RPMI 1640 with 25 mM HEPES (pH 7.4) and counted electronically (Coulter Counter model ZBI; Coulter Counter Electronics Ltd., Luton, United Kingdom). Differential counts were then made on Diff-Quick-stained cytospin preparations, and the cells were finally diluted to a concentration of 1.5 × 106 monocytes/ml. Aliquots (3 ml) of the cell suspension were seeded in sterile 24-well plates and incubated for 90 min at 37°C; adherent monocytes were cultured in RPMI 1640 with 25 mM HEPES (pH 7.4) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mM glutamine, and 10% human serum. The cells were kept in culture for 3 to 9 days before being used for the experiments; during this incubation, the decrease in CD14 expression was checked as a marker of monocyte differentiation into macrophages, by reaction with anti-CD14 My4 antibody (Coulter clone; Coulter, Zurich, Switzerland). The MDMs were then tested for MR expression by reaction with the goat anti-MR (G-anti-HuManRec, TNO-PG; Gaubius Laboratory) polyclonal antibody and the isotype-matched control immunoglobulin G2a (Dako SpA Milano) by immunofluorescence. Cells were resuspended in PBS–0.5% paraformaldehyde for cytometric reading. MRF-61 rat cells which had been transfected with the human MR (27) were a generous gift from Philip Stahl (Department of Cell Biology and Physiology, Washington University, St. Louis, Mo.); they were cultured as monolayers in Dulbecco modified Eagle medium (DMEM)–HEPES–25-mM medium and checked for MR expression as described for macrophages. The untransfected parental rat fibroblasts (clone Rat-6H) were used as the control and cultivated in the same medium as MRF-61 cells.
Adhesion experiments.
In adhesion experiments, MDMs were detached from the plates with 5 mM EDTA–PBS, washed, and then resuspended in minimal essential medium (MEM) plus 2 mM glutamine and 2% synthetic serum (Ultraser HY; Gibco BRL) at 106 cells/ml. The MRF-61 MR-expressing cells and Rat-H6 cells were washed and suspended in 100 μl of MEM–0.1% bovine serum albumin at 106 cells/ml. The macrophages and cultured cells were incubated with 20 μl of FITC-labeled B. burgdorferi (5 × 108/ml) for 45 min at 37°C with shaking. The final cell spirochete ratio was 1:100. When FITC-labeled C. albicans was used, the Candida /cell ratio was 2:1, 10:1, or 20:1. Adhesion experiments were performed in MEM solution containing 0.1% bovine serum albumin for the purpose of reducing nonspecific binding; PBS–0.5% paraformaldehyde was added to each suspension to a final volume of 0.5 ml and analyzed by cytometry.
As potential inhibitors of MR-mediated adhesion, the following reagents were added to the cells and incubated for 15 min at 37°C prior to addition of the microorganisms: sugars such as mannan (2 mg/ml) or d-mannose, l-fucose, d-galactose, and N-acetyl-d-glucosamine (NAGD) (each at 100 mM), and the lectins asparagus pea, garden pea, soybean, and Maackia (whose sugar specificity is reported in Table 1) at 100 μg/ml. The anti-MR polyclonal antibody, at a dilution of 1:200 (corresponding to 5 μg/ml), was preincubated with the cells at 4°C for 15 min. Readings of fluorescence were performed with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) equipped with an air-cooled argon ion laser fitted at 488 nm and with a filter setting for FITC (530 nm). Events were acquired in list mode and analyzed with CellQuest software (Becton Dickinson). Acquisition of 10,000 events was dependent on a gating on MRF-61 or macrophage cell morphology (forward and side scatter) to exclude cells debris or abnormally large cells or aggregate from analysis. Detector gain and voltage adjustment and scatter gating had been set and saved in preliminary experiments with untreated cells, and the same protocol was used for all subsequent studies. Single green fluorescence emission of FITC-labeled borreliae bound to MRF-61 cells or MDMs was measured and expressed as a percentage of positive cells. To select the binding of spirochetes to MDMs and cells, the specific background fluorescence of macrophages alone was used as the threshold level, and a marker was set. MDMs or MRF-61 cells, or Rat-H6 cells with a fluorescence intensity higher than the threshold level, were considered to represent significant adhesion. The expression of MR antigen on the cells was quantified as green fluorescence signal. For each experiment, unstained cells or cells stained with isotype-matched monoclonal antibodies were used as negative controls to set the threshold for positivity. Data from repeated experiments were analyzed by the unpaired Student t test, using GraphPad Prism (GraphPad Software, San Diego, Calif.). We first verified that the MR was expressed during the differentiation of human monocytes into macrophages and in the MR-transfected MRF-61 cell cultures. Detection of the receptor by the anti-MR antibody was evident by day 3 and increased thereafter to day 6 in about 80% of the MDMs; MR-associated fluorescence was detected on about 70% of the MRF-61 fibroblasts, and no fluorescence was obtained on non-MR-transfected Rat-H6 cells (data not shown).
TABLE 1.
Lectins used in inhibition binding studies
| Lectin source | Common name | Oligosaccharide recognition |
|---|---|---|
| Tetragololobus purpureas | Asparagus pea | α-l-Fucosyl |
| Pisum sativum | Garden pea | α-d-Mannosyl α-Glucosyl |
| Glycine max | Soybean | N-Acetylgalactosamyl |
| Maackia amurensis | Maackia | Sialic acids |
Adhesion on MDMs.
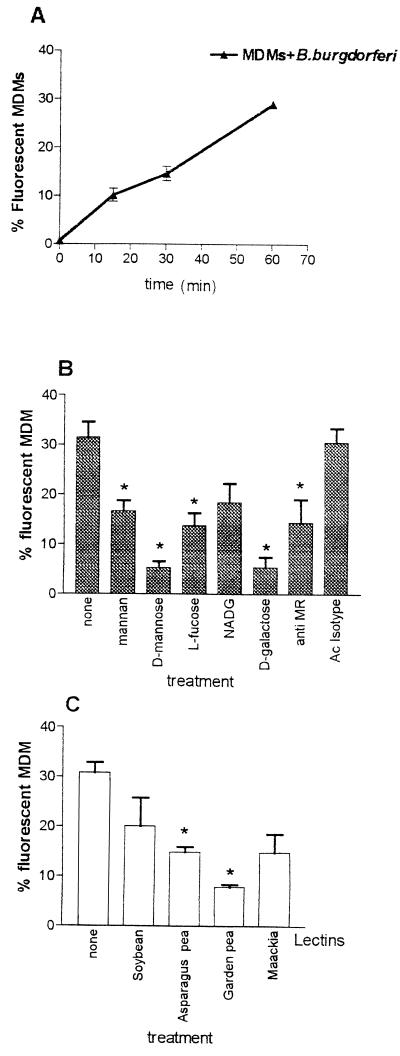
Fluorescent B. burgdorferi was added to MDM suspensions in the presence or absence of different reagents, and binding of the spirochetes to phagocytes was quantified by flow cytometry. Preliminary time course experiments were performed to investigate the kinetics of adhesion to MDMs; as reported in Fig. 1A, the rate of adhesion of FITC-stained borreliae to the cells increased with time during the 60 min of incubation, involving up to 30% MDMs, and was expected to increase more; we chose the 45-min time interval to perform the subsequent adhesion experiments. MDMs preincubated for 15 min with the anti-MR antibody (Fig. 1B) exhibited a 70% inhibition of adhesion which was not detected with the isotype antibody. Although the use of whole antibody G-anti-HuManRec can cause a potential cross-linking and capping of the MR, this finding suggests that the MR is involved in the binding. Further inhibition was observed after preincubation with saccharides d-mannose (about 83%), d-galactose (about 82%), l-fucose (56%), mannan (about 46%), and NADG (about 41% [not significant]). Increasing the concentration of soluble ligand failed to further inhibit adhesion (data not shown). Further evidence of probable involvement of the MR in MDM adhesion comes from the inhibition experiments with lectins (Fig. 1C): asparagus pea and garden pea, recognizing the alpha-l-fucosyl and alpha-d-mannosyl residues, respectively, strongly inhibited binding of the spirochetes; minor and less significant inhibition was observed in the presence of lectins Maackia and soybean, which are specific for sialic acids and N-acetylgalactosamyl residues.
FIG. 1.
Adhesion of FITC-labeled B. burgdorferi to MDMs in different experimental conditions. (A) Time course of B. burgdorferi adhesion. (B) Inhibitory effect on adhesion by pretreatment with sugars and with anti-MR serum. Isotype control serum was used as control. (C) Inhibitory effect on adhesion by different lectins. Adhesion was measured as the percentage of cells becoming fluorescent after binding of FITC-labeled B. burgdorferi. Bars express the means ± standard deviations of three independent determinations. P ≤ 0.05.
Adhesion on MR-transfected cells.
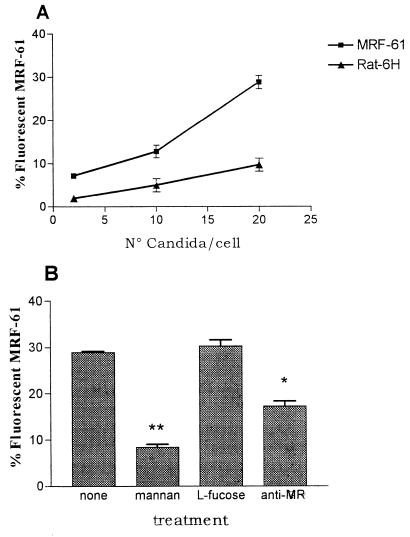
Adhesion assays were also carried out on MRF-61 cells, a cell line used to avoid the participation of CR3 or other unknown macrophage receptors which might recognize B. burgdorferi in nonopsonic conditions; untransfected clone Rat-H6 cells were used as a control. Besides testing the level of MR expression, we also checked binding of the MR in adhesion experiments with C. albicans, which is known to be recognized by this receptor (18). As reported in Fig. 2A, there was a clear dose-dependent adhesion of Candida on MRF-61 cells and a little adhesion on untransfected Rat-H6 fibroblasts. Involvement of the MR in binding is indicated by a strong decrease of adhesion in the presence of mannan, the classic antagonist of MR binding, and by the anti-MR antibody; a nonsignificant level of inhibition was exerted by l-fucose, as already reported (15) for Candida. Taken together, these data confirm the expression of a functional MR on the cultured cells.
FIG. 2.
Adhesion of FITC-labeled C. albicans on MRF-61 cells. (A) Rate of adhesion on MRF-61 fibroblasts at different concentrations of C. albicans. (B) Effect on adhesion by pretreatment with mannan, l-fucose, and anti-MR serum. Adhesion was measured as the percentage of cells becoming fluorescent after binding of FITC-labeled C. albicans. Bars express the means ± standard deviations of three independent determinations. P ≤ 0.01.
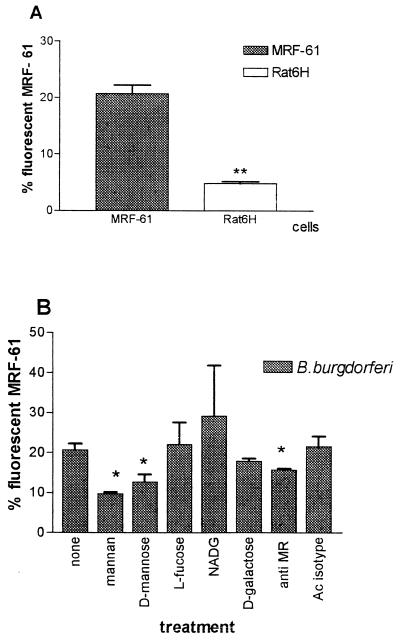
Parallel experiments carried out with B. burgdorferi (Fig. 3A) showed that the rates of adhesion of the organism were in the order of 22% on MR-transfected cells but only 6% on nontransfected cells; this finding in itself seems to indicate that the MR was involved in the greater MRF-61 adhesion. When MRF-61 cells were preincubated with potential antagonists of MR binding (Fig. 3B), the anti-MR antibody decreased adhesion 20%; among the saccharides, significant inhibition was exerted by mannan (52%) and d-mannose (38.35%) but not by l-fucose and d-galactose. The pattern of inhibition by saccharides partly confirmed the results obtained with MDMs. In fact, mannan and in particular mannose behaved as strong antagonists of binding in both MDMs and MRF-61 cells; l-fucose and d-galactose inhibited adhesion only on MDMs. The failure of l-fucose to inhibit adhesion on MRF-61 cells had already been observed with C. albicans (see above). The stronger antagonism of mannose on MDM adhesion and the antagonist effect of d-galactose on MDM binding may be due to participation in the binding of integrin CR3 (recognized by mannose) and of the 42-kDa lectin (26) which has recently been found to specifically bind d-galactose on macrophages. Both of these receptors act as lectins on MDMs and are absent on fibroblasts.
FIG. 3.
Adhesion of FITC-labeled B. burgdorferi to MRF-61 cells in different experimental conditions. (A) Adhesion on MR transfected MRF-61 cells and untransfected Rat-6H cells P ≤ 0.01. (B) Effect of different saccharides and anti-MR antibody on adhesion, determined as for Fig. 2. P ≤ 0.05.
Taken together, these findings suggest that adhesion on MDMs is the result of multiple binding of lectin-functioning receptors such as CR3 and MR; also, we cannot exclude involvement of the 42-kDa galactose-specific lectin and of the β-glucan receptor (26, 20). Further evidence of participation of the MR in binding is provided by the higher adhesion of B. burgdorferi on MR-transfected fibroblasts than on untransfected clone Rat-H6 fibroblasts; these cells gave a low background adhesion, due to unknown naive receptors for B. burgdorferi (16).
Since the MR recognizes saccharides and glycosylated proteins, the exact counter receptor present on the B. burgdorferi outer membrane is unclear; in fact, there is general agreement that these spirochetes mainly expose lipoproteins, although there are interesting reports on the presence of glycoconjugates on B. burgdorferi (13, 24) containing at least four sugars, mainly NADG and mannose; these saccharides probably take part in adhesion to the lectin-like domain of CR3 (4, 5) and maybe to the MR CDR1 epitope.
However, the interaction with the MR is peculiar: it seems that binding to the MR, which has multiple binding sites (27), involves either multiple binding pathways or one single promiscuous pathway, as happens in the binding to different proteoglycans. Thus, since it is demonstrated that B. burgdorferi avidly binds to fibronectin (17, 23) and the MR exposes a fibronectin type II repeat, we can hypothesize that the adhesion is first mediated by this domain, followed by the participation and clustering of the other sugar-specific domains in a complex multiple binding.
The consequence of MR-B. burgdorferi recognition may influence the host immune reactions at the beginning of the Borrelia infection, when the spirochetes are inoculated into the epidermis and dermis and become phagocytosed and processed by Langerhans (14) and dendritic (11) cells. It has been recently demonstrated that dendritic cells play a crucial role in the Borrelia immune response because they process and present B. burgdorferi antigens associated with major histocompatibility complex class I and class II molecules, resulting in the activation of both humoral and cellular immunity. Given the extensive literature (9, 10) on the role of the MR on dendritic and Langerhans cells in processing and presenting antigens, following a pathway and subcellular fractionation resembling that observed in dendritic cells for B. burgdorferi lipoproteins (1, 2), we are inclined to think that this receptor plays an important role in the development of macrophage phagocytosis and early immunity to B. burgdorferi. Both of these mechanisms affect the innate response of the host.
Acknowledgments
We thank Philip Stahl, Department of Cell Biology and Physiology, Washington University, St. Louis, Mo., for sending the MRF-61 cells and anti-MR antibody and for critical reading of the text.
Work in our laboratory was supported by the MURST 40% funding.
REFERENCES
- 1.Beerman C, Lochnit G, Geyer R, Groscurth P, Filgueira L. The lipid component of lipoproteins from Borrelia burgdorferi: structural analysis, antigenicity and presentation via human dendritic cells. Biochem Biophys Res Commun. 2000;267:897–905. doi: 10.1006/bbrc.1999.2057. [DOI] [PubMed] [Google Scholar]
- 2.Beermann C, Allenspach H W, Groscurth P, Filgueira L. Lipoproteins from Borrelia burgdorferi applied in liposomes and presented by dendritic cells induce CD8+ T-lymphocytes in vitro. Cell Immunol. 2000;201:124–131. doi: 10.1006/cimm.2000.1640. [DOI] [PubMed] [Google Scholar]
- 3.Cinco M, Murgia R, Presani G, Perticarari S. Simultaneous measurement by flow cytometry of phagocytosis and metabolic burst induced in phagocytic cells in whole blood by Borrelia burgdorferi. FEMS Microbiol Lett. 1994;122:187–194. doi: 10.1111/j.1574-6968.1994.tb07163.x. [DOI] [PubMed] [Google Scholar]
- 4.Cinco M, Murgia R, Presani G, Perticarari S. Integrin CR3 mediates the binding of nonspecifically osponized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect Immun. 1997;65:4784–4789. doi: 10.1128/iai.65.11.4784-4789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinco M, Murgia R, Perticarari S, Presani G. Surface receptors of neutrophils towards B. burgdorferi. Middle Eur J Med. 1998;110:866–869. [PubMed] [Google Scholar]
- 6.Cinco M, Panfili E, Presani G, Perticarari S. Interaction with Borrelia burgdorferi causes increased expression of the CR3 integrin and increased binding affinity to fibronectin via CR3. J Mol Microbiol Biotechnol. 2000;2:575–579. [PubMed] [Google Scholar]
- 7.Coburn J, Barthold S W, Leong J M. Diverse Lyme disease spirochetes bind integrin αIIβ3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coburn J, Magoun L, Bodary S C, Leong J. Integrin αVβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condaminet B, Peguet-Navarro J, Stahl P D, Dalbiez-Gauthier C, Schmitt D, Berthier-Vergnes O. Human epidermal Langerhans cells express the mannose-fucose binding receptor. Eur J Immunol. 1998;28:3541–51. doi: 10.1002/(SICI)1521-4141(199811)28:11<3541::AID-IMMU3541>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Engering A J, Cella M, Fluitsma D. The mannose receptor functions as high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 11.Filgueira L, Nestle F, Rittig M, Joller H I, Groscurt P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 12.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulinska D, Volf P, Grubhoffer L. Characterisation of Borrelia burgdorferi glycoconjugates and surface carbohydrates. Zentbl Bakteriol. 1992;276:473–480. doi: 10.1016/s0934-8840(11)80672-9. [DOI] [PubMed] [Google Scholar]
- 14.Hulinska D, Bartak P, Hercogova J, Hancil J, Basta J, Schramlova J. Electron microscopy of Langerhans cells and Borrelia burgdorferi in Lyme disease patients. Zentbl Bakteriol. 1994;280:348–359. doi: 10.1016/s0934-8840(11)80597-9. [DOI] [PubMed] [Google Scholar]
- 15.Karbassi A, Becker M J, Foster S J, Moore R N. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J Immunol. 1987;139:417–421. [PubMed] [Google Scholar]
- 16.Klempner M S, Noring R, Rogers R A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1993;167:1074–1081. doi: 10.1093/infdis/167.5.1074. [DOI] [PubMed] [Google Scholar]
- 17.Kopp P A, Schmitt M, Wellensiek H J, Blobel H. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect Immun. 1995;63:3804–3808. doi: 10.1128/iai.63.10.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazlo M, Korchak H M, Johnston R B. Mechanism of host defense against Candida species. J Immunol. 1991;146:2783–2789. [PubMed] [Google Scholar]
- 19.Leong J M, Morissey P E, Ortega-Barria E, Pereira M E, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linehan S A, Martinez-Pomares L, Gordon S. Macrophage lectins in host defence. Microb Infect. 2000;2:279–288. doi: 10.1016/s1286-4579(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 21.Mosser D M. Receptors on phagocytic cells involved in microbial recognition. Immunol Ser. 1994;60:99–114. [PubMed] [Google Scholar]
- 22.Ofek I, Goldhar J, Keisari Y. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1992;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 23.Probert W S, Johnson B J. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 24.Sambri V, Massaria F, Ardizzoni M, Stefanelli C, Cevenini R. Glycoprotein patterns in Borrelia spp. Zentbl Bakteriol. 1993;279:330–335. doi: 10.1016/s0934-8840(11)80365-8. [DOI] [PubMed] [Google Scholar]
- 25.Spessotto P, Dri P, Bulla R, Zabucchi G, Patriarca P. Human eosinophil peroxidase enhances tumor necrosis factor and hydrogen peroxide release by human monocyte-derived macrophages. Eur J Immunol. 1995;25:1366–1373. doi: 10.1002/eji.1830250535. [DOI] [PubMed] [Google Scholar]
- 26.Stahl P D. The mannose receptor and other macrophage lectins. Curr Opin Immunol. 1992;4:49–52. doi: 10.1016/0952-7915(92)90123-v. [DOI] [PubMed] [Google Scholar]
- 27.Taylor M E, Conary J T, Lennartz M R, Stahl P D, Drickamer K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. Biol Chem. 1990;265:12156–12162. [PubMed] [Google Scholar]