Abstract
Cannabidiol (CBD) is a biologically active compound present in the plants of the Cannabis family, used as anticonvulsant, anti-inflammatory, anti-anxiety, and more recently, anticancer drug. In this work, its use as a new self-assembly inducer in the formation of nanoparticles is validated. The target conjugates are characterized by the presence of different anticancer drugs (namely N-desacetyl thiocolchicine, podophyllotoxin, and paclitaxel) connected to CBD through a linker able to improve drug release. These nanoparticles are formed via solvent displacement method, resulting in monodisperse and stable structures having hydrodynamic diameters ranging from 160 to 400 nm. Their biological activity is evaluated on three human tumor cell lines (MSTO-211H, HT-29, and HepG2), obtaining GI50 values in the low micromolar range. Further biological assays were carried out on MSTO-211H cells for the most effective NP 8B, confirming the involvement of paclitaxel in cytotoxicity and cell death mechanism
Keywords: self-assembled nanoparticles, cannabidiol, anticancer drug
1. Introduction
Nanotechnology is an increasingly interesting approach in medicinal chemistry, allowing to improve the bioavailability and, thus, the delivery of drugs to their site of action. Among all types of nanoparticles (NPs), we have been primarily interested in lipidic, self-assembled NPs, formed by the spontaneous aggregation in water of compounds made by the conjugation of the drug of choice to a self-assembly inducer [1]. These nanostructures, in which the drug moiety is already contained in its building blocks instead of being loaded on inert carriers, present several advantages: (1) A high and precisely tunable drug-loading capacity; (2) a simple adjustment of the physicochemical features of the NPs by optimizing the molecular design; (3) an easy preparation; and (4) an increased biocompatibility as there is no potential carrier-induced cytotoxicity and immunogenicity [2].
For several years we have been interested in using this kind of NPs to improve the properties of both anticancer and neuroprotective drugs [3,4,5,6,7,8,9,10,11,12,13,14]. We designed conjugates able to form NPs that can release the drug in cellular media, [3,8,11,14] hetero-NPs bearing two different drugs [6,9] and fluorescent NPs obtained mixing drug- and fluorophore-based conjugates [4,5]. In our previous works, squalene, [3,4,5,6,11,12,13] 4-(1,2-diphenylbut-1-en-1-yl)aniline, [7,8,14] 20-hydroxyecdisone [9], or betulinic acid [10] was used. The goal of the present work is to identify a molecule that, besides being able to induce the aggregation, would also present some biological activity to further improve the NPs pharmacological properties.
Cannabidiol (CBD, 1, Figure 1) is a nonpsychoactive phytocannabinoid compound extracted from flowers or leaves of the plants of the Cannabis genus and, in particular, from Cannabis sativa. It was first isolated between 1930 and 1940, but its chemical structure was only clarified in 1963. The biological effects of this compound were investigated in the following years and included anticonvulsant, anti-inflammatory, anti-anxiety, and anti-cancer activities, along with beneficial effects for the immune system [15,16]. In particular, CBD, alone or in combination with other agents, has been shown to successfully induce cell death, inhibit cell migration and invasion in vitro, decrease tumor size, vascularization, growth, and weight, and increase survival and induce tumor regression in vivo [17,18,19].
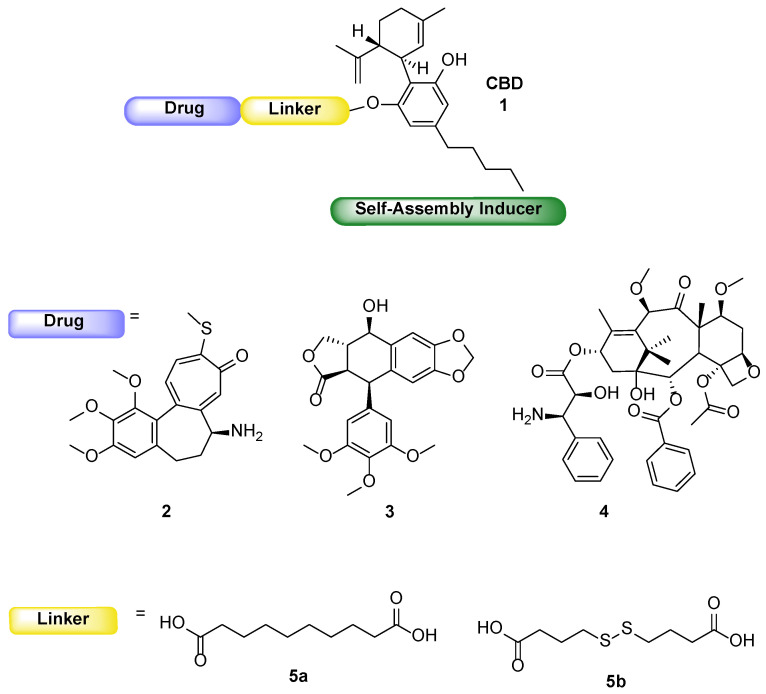
Figure 1.
Schematic structure of the drug conjugates and building blocks.
Based on the above statements, we considered CBD for its potential dual activity (cytotoxic compound and self-assembly inducer) and to conjugate it to well-known tubulin binder drugs, N-desacetyl thiocolchicine (2), podophyllotoxin (3), and paclitaxel (4), through two different linkers, 5a and 5b (Figure 1). The synthesis and characterization of the planned conjugates and their ability to form self-assembled NPs are here reported. The ability to induce an antiproliferative effect was assayed on three human tumor cell lines (MSTO-211H, HT-29 and HepG2) and the maintenance of the cell target was assessed by confocal microscopy.
2. Results and Discussion
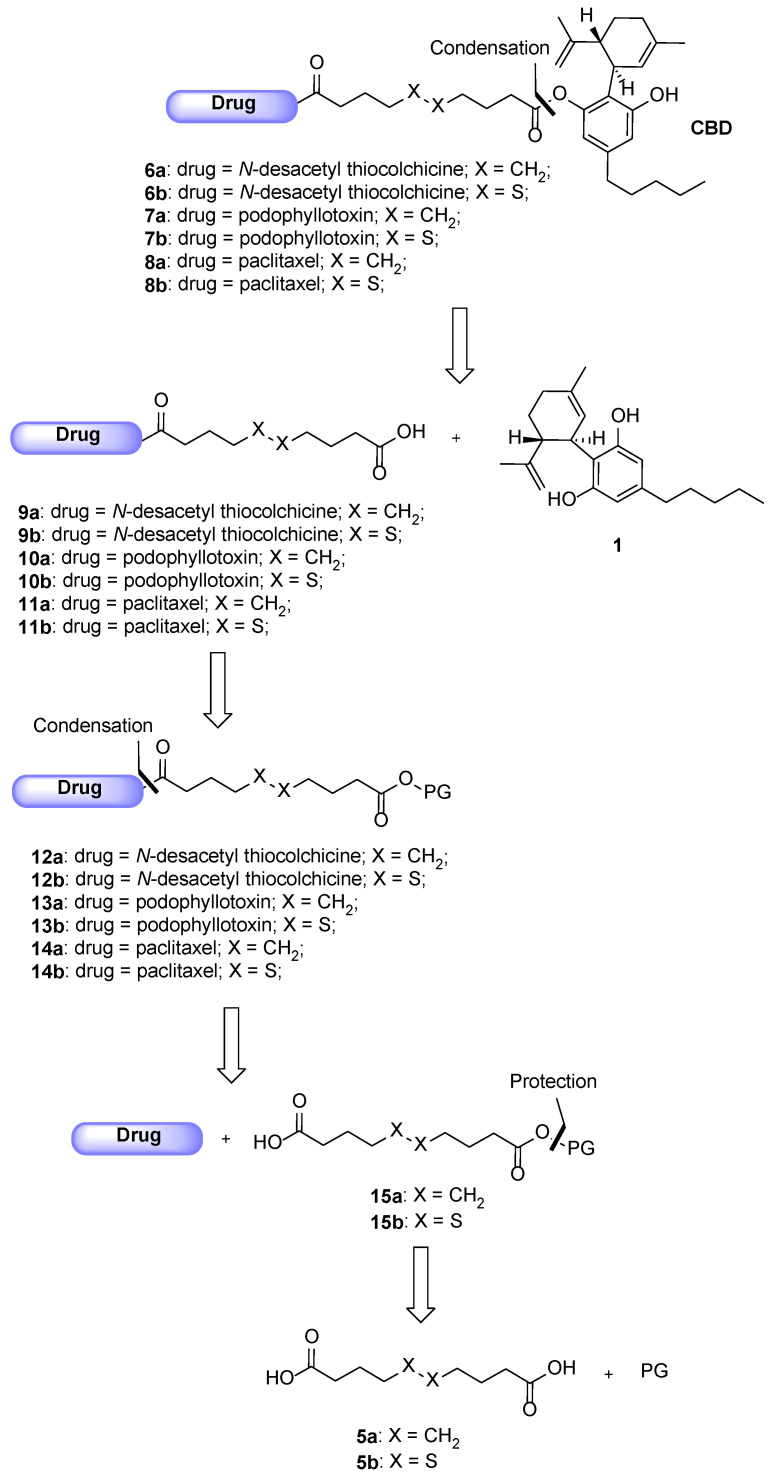
The following Scheme 1 shows the retrosynthetic approach for the preparation of the CBD-based conjugates 6–8a,b.
Scheme 1.
Retrosynthetic pathway for the conjugates 6–8a,b.
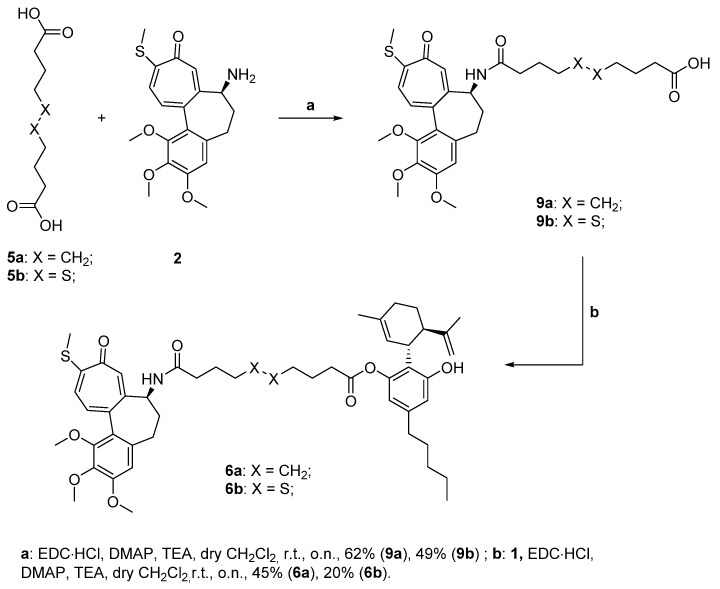
Said conjugates should be obtained through a condensation reaction between one among the cytotoxic drug-linker intermediates 9–11a,b and CBD 1. Compounds 9–11a,b could derive from the deprotection of carboxy-protected intermediates 12–14a,b, which could be formed as the result of another condensation reaction between the selected drugs and the mono carboxy-protected esters 15a,b. Eventually, said compounds could be protected by reacting the linkers 5a,b with the proper protecting group under esterification conditions. Starting with the description of the conjugates obtained involving N-desacetyl thiocolchicine 2 as the drug, the pathway depicting their synthesis is shown below in Scheme 2. As the conjugates differ only by the linker (being either sebacic or 4,4′-dithiodibutyric acid), their synthesis was carried out following the same protocol: the initial protection of the dicarboxylic acids was avoided and the direct reaction was tried leading to the 9a,b intermediates in good enough yields (step a, Scheme 2). The latter were coupled with our potential self-assembly inducer 1 under Steglich esterification conditions leading to final products 6a,b (step b, Scheme 2).
Scheme 2.
Synthesis of the N-desacetyl thiocolchicines-CBD conjugates 6a,b.
In both syntheses, diamides and diesters were the principal generated by-products, even though all the condensation reactions were performed with excess of the moiety presenting the double functionality.
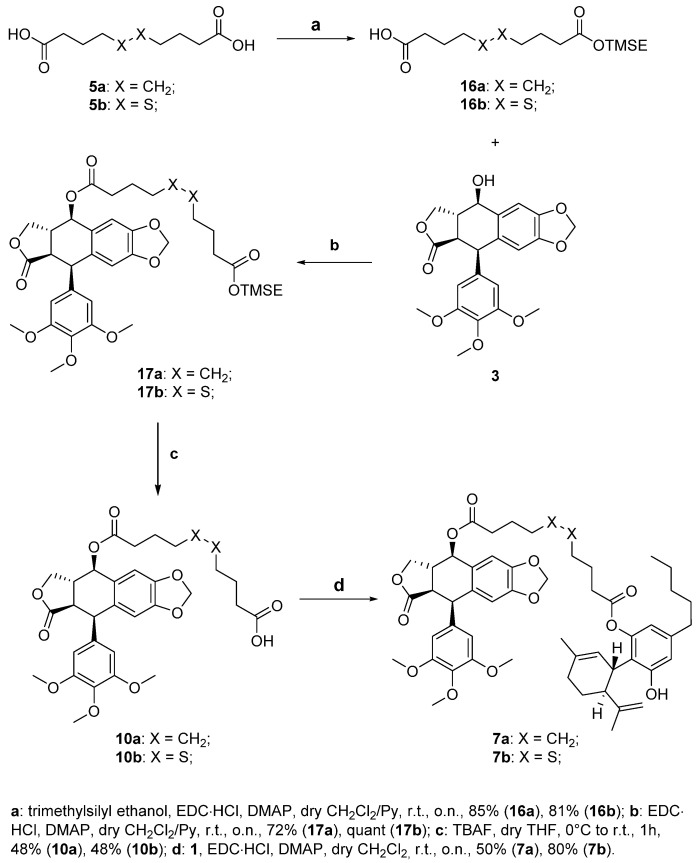
Then, we will consider the synthesis of the two compounds 7a,b, presenting podophyllotoxin as the drug, as shown in Scheme 3.
Scheme 3.
Synthesis of the podophyllotoxin-CBD conjugates 7a,b.
The first step of the synthesis is the selective protection of one of two carboxylic acids of sebacic acid 5a and 4,4′-dithiodibutyric acid 5b (step a, Scheme 3). This reaction was needed as it was seen that, in this case, the use of dicarboxylic acids under condensation conditions led to the formation of significant amounts of dimeric side products, losing precious quantities of drugs and complicating the purification of target compounds. We decided to use 2-(trimethylsilyl)ethanol (0.3 eq.) as the protective group in a Steglich esterification. It is to be noted that even using low amounts of esterifying agent to reduce the diesterification that can occur, we obtained both target monoesters 16a,b and minoritary diester by-products. We then proceeded with the coupling of these monoprotected linkers with the drug podophyllotoxin 3, using Steglich esterification, obtaining TMSE-protected linker-drug conjugates 17a,b in good yields (step b, Scheme 3). Conjugates 17a,b were then deprotected with TBAF to obtain the corresponding free carboxylic acids 10a,b with acceptable yields (step c, Scheme 3). The final step of this synthetic pathway is the coupling of CBD, the self-assembly inducer, with the conjugates 10a,b. The esterification reaction is again performed under Steglich conditions in the presence of EDC·HCl and DMAP, leading to the obtainment of target compounds 7a,b (step d, Scheme 3). For both conjugates the diesterification by-products were not detected, probably due to the restricted rotation of the CBD cyclohexenyl moiety that could shield the second free hydroxy group.
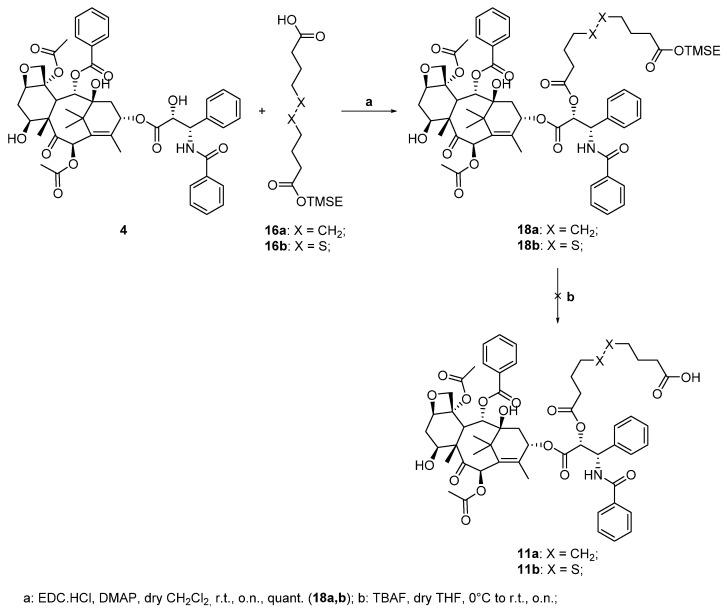
The last conjugates to be synthetized were the two paclitaxel-based derivatives. At first, we followed the strategy used to synthesize conjugates 7a,b: the TMSE-protected esters 16a,b were reacted with 4 under Steglich esterification conditions to obtain intermediates 18a,b quantitatively (step a, Scheme 4). We then studied the cleavage of the silylated protecting group to obtain the corresponding carboxylic acids 11a,b (step b, Scheme 4). The deprotection was performed with different experimental conditions. Unfortunately, all the studied conditions led to the degradation of the products. This result is probably generated by the instability of paclitaxel in the presence of TBAF. As the previous synthetic strategy failed, we decided to change the protecting group of the linker into another one that could be removed in different conditions.
Scheme 4.
First attempts on the synthesis of paclitaxel-CBD conjugates 8a,b.
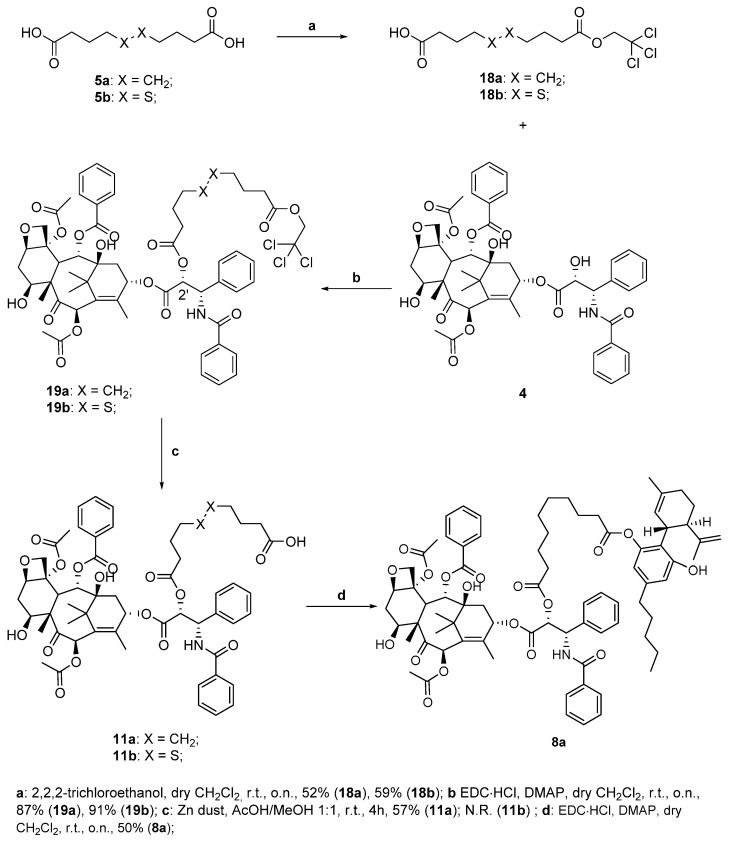
We then protected one of the two carboxylic acids of sebacic acid 5a and of 4,4′-dithiodibutyric acid 5b as 2,2,2-trichloroethyl esters (TCEs), which are cleavable in reductive conditions, with metallic zinc at mildly acidic pH (step a, Scheme 5). We proceeded again with the coupling to the drug, performing once again a Steglich esterification in the presence of 4 with the usual conditions, obtaining the derivatives 19a,b with high yields (step b, Scheme 5). At this point, we tried the reductive cleavage of the previously synthesized conjugates 19a,b to obtain the corresponding carboxylic acids 11a,b (step c, Scheme 5). For what regards the paclitaxel-sebacic-TCE 19a, it was dissolved in a 1:1 mixture of glacial acetic acid/methanol at room temperature, and after adding a large excess of zinc dust and waiting four hours, we could successfully isolate the target carboxylic acid 11a with a 57% yield (step c, Scheme 5).
Scheme 5.
Synthesis of paclitaxel-CBD conjugate 8a.
Unfortunately, performing the same reaction on protected ester 19b did not lead to the same result, as we assisted at the degradation of the starting material with no formation of desired compound 11b. This may be due to the presence of the disulfide bond, which could be particularly sensitive in the presence of zinc, which shows a great tendency to form Zn-S bonds (soft-soft interactions). However, we were able to complete the synthesis with conjugate 11a, performing its conjugation with cannabidiol 1 and obtaining target compound 8a in moderate yield (step d, Scheme 5).
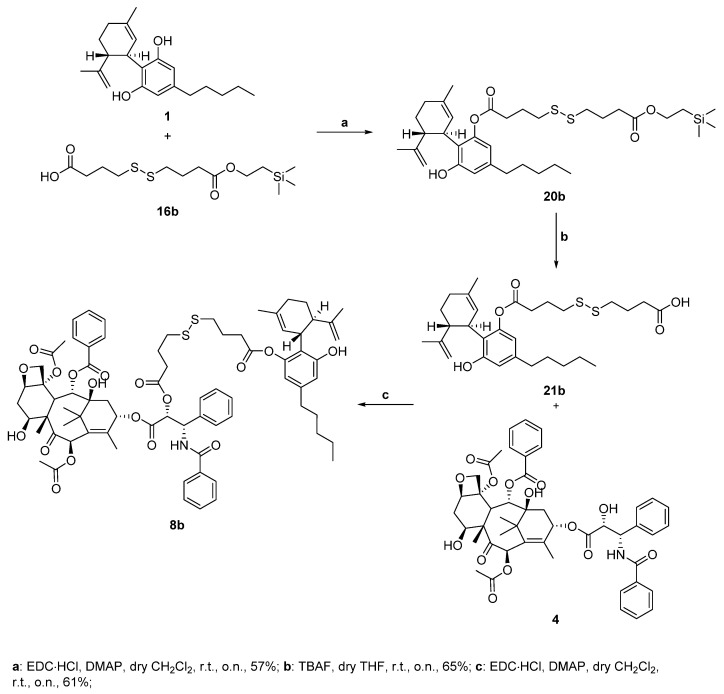
Since all the previous attempts failed when we tried to synthesize the target conjugate 8b, as the concomitant presence of the paclitaxel and disulfide moiety makes the molecule more sensitive and delicate under various conditions, we decided to change the synthetic strategy. In Scheme 6, the second synthetic strategy is reported. Here the order of the condensation steps is reversed, as we first reacted cannabidiol with the protected linker and only then with paclitaxel. We started this different synthetic approach with the monoesterification under Steglich conditions between two equivalents of 1 and the already prepared mono-protected TMSE-carboxylic acid 16b (step a, Scheme 6), trying to limit the diesterification by-product formation. The desired conjugate 20b was successfully obtained with a 57% yield, as despite the cannabidiol excess, we also obtained 11% of the undesired diesterification by-product. The following step was the deprotection of intermediate 20b using TBAF to achieve the carboxylic acid 21b (step b, Scheme 6). Having the linker-self-assembly inducer conjugate 21b in our hands, we could perform the last reaction step to obtain the target compound 8b, which consisted once again in a Steglich coupling between paclitaxel and the carboxylic acid 21b. Although the CBD conjugate contains another free hydroxy group, we could obtain the target derivative 7–4c with a good yield (step c, Scheme 6).
Scheme 6.
Synthesis of paclitaxel-CBD conjugate 8b.
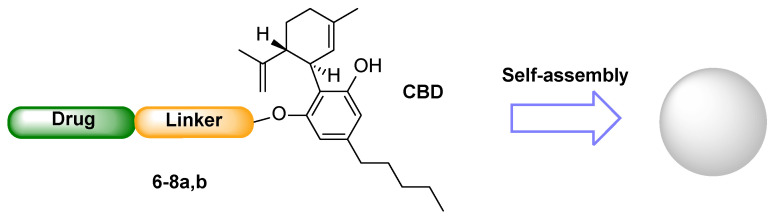
To test the self-assembly ability imparted by cannabidiol to the obtained conjugates 6–8a,b, we proceeded with the preparation and characterization of their nanoparticles (Figure 2).
Figure 2.
Self-assembly of NPs 6–8A,B from CBD-linker-drug conjugates 6–8a,b.
The six nanosuspensions—one for each 6–8a,b conjugate—were prepared in accordance with standard solvent evaporation protocols [20]. The NP suspensions 6–8A,B obtained were then characterized for what regards their bio-physical properties. DLS and Z-potential measurements were carried out on each NP after 10 minutes’ sonication, giving the following results (Table 1).
Table 1.
Hydrodynamic diameter and Z-potential of nanoformulations 6–8A,B.
| Drug | NP | Hydrodynamic Diameter (nm) |
Z-Potential (mV) |
Polydispersity Index (PI) |
|---|---|---|---|---|
| 2 | 6A | 163.1 ± 4.1 | −38.9 ± 9.3 | 0.051 ± 0.012 |
| 6B | 175.2 ± 6.2 | −33.0 ± 7.8 | 0.100 ± 0.027 | |
| 3 | 7A | 229.4 ± 5.0 | −38.32 ± 0.75 | 0.137 ± 0.031 |
| 7B | 393.3 ± 5.1 | −39.95 ± 1.32 | 0.173 ± 0.018 | |
| 4 | 8A | 396.0 ± 9.2 | −37.5 ± 0.6 | 0.207 ± 0.059 |
| 8B | 275.0 ± 4.5 | −45.60 ± 0.28 | 0.103 ± 0.024 |
DLS confirmed the formation of nanoassemblies in aqueous medium. Namely, the low polydispersity index values (PI < 0.2) indicated that each CBD-linker-drug conjugate 6–8a,b was able to give monodisperse suspensions of NPs, with hydrodynamic diameters (HDs) in the 160–400 nm range. Even though the dimension of some of them is around the higher end of NPs’ definition (500 nm), we expect them to be able to exert their action and be internalized in cells. The zeta potential was negative (<−25 mV) for all the nanoassemblies, suggesting that electrostatic repulsion contributes to the colloidal stability of each suspension.
The antiproliferative effect of the obtained NPs was evaluated on three human tumor cell lines, MSTO-211H (biphasic mesothelioma), HT-29 (colorectal adenocarcinoma), and HepG2 (hepatocellular carcinoma). The cytotoxicity exerted by CBD (1), N-desacetyl thiocolchicine (2), podophyllotoxin (3), and paclitaxel (4) was also determined in all cell lines. The results are expressed as GI50 values, that is the concentration inducing a 50% reduction in cell number with respect to a control culture, and are shown in Table 2.
Table 2.
Antiproliferative effect of NPs and the corresponding reference drugs, CBD (1), N-desacetyl thiocolchicine (2), podophyllotoxin (3), and paclitaxel (4).
| GI50 (μM) a | |||
|---|---|---|---|
| MSTO-211H | HT-29 | HepG2 | |
| 1 | 10.1 ± 1.8 | 10.1 ± 0.5 | 13.3 ± 1.7 |
| 2 | 0.014 ± 0.001 | 0.021 ± 0.003 | 0.021 ± 0.003 |
| 6A | 12.1 ± 1.9 | 7.2 ± 0.3 | >20 |
| 6B | 1.1 ± 0.2 | 1.4 ± 0.5 | 3.9 ± 0.7 |
| 3 | 0.012 ± 0.001 | 0.013 ± 0.002 | 0.018 ± 0.003 |
| 7A | 4.8 ± 0.8 | 6.1 ± 0.9 | 14.5 ± 1.7 |
| 7B | 2.5 ± 0.7 | 2.6 ± 0.1 | 2.6 ± 0.6 |
| 4 | 0.0035 ± 0.0002 | 0.0035 ± 0.0005 | 0.025 ± 0.006 |
| 8A | 9.5 ± 0.5 | >20 | >20 |
| 8B | 0.43 ± 0.01 | 2.5 ± 0.2 | 4.8 ± 0.7 |
a GI50 values are the mean ± SD of at least three independent experiments in duplicate.
According to the literature data [21], the self-assembly inducer CBD (1) shows an antiproliferative effect on human tumor cell lines with GI50 values in the micromolar range, while the well-known antiproliferative agents, n-desacetylthiocolchicine (2), podophyllotoxin (3) and paclitaxel (4), provoke a strong cytotoxicity, assessed by GI50 values in the nanomolar range.
The treatment with NPs 6A,B, 7A,B, and 8A,B induced a cytotoxicity that appears less pronounced with respect to that of the corresponding free drugs, 2, 3, and 4, respectively, with GI50 values ranging from 0.43 μM to 14.5 μM. Because the cell effect of NPs depends on the release of the cytotoxic agent, it is possible that the hydrolysis of the linker and the kinetics of such release account for a lower intracellular content with respect to the incubation with free drugs, leading to NPs that are not so effective as pure drugs. Moreover, the antiproliferative activity exerted by the NPs depends on the cell line, with MSTO211-H generally the most sensitive and in particular, the lowest GI50 value, 0.43 μM, was obtained by treating this cell line with 8B. This result appears in agreement with the literature data demonstrating that the treatment with paclitaxel-loaded NPs prolonged the survival of a murine model of malignant pleural mesothelioma, obtained by intrathoracic injection of MSTO-211H cells [22]. Overall, these results suggest that the poor clinical response of mesothelioma to paclitaxel could be attributable to an ineffective delivery kinetics of the systemic administration rather than to the mechanism of drug itself, and allow to consider the use of NPs extremely interesting for the development of effective antitumor therapy. The antiproliferative effect appears to depend also on the type of linker. In this connection, it is to note that NPs prepared with conjugates carrying 4,4′-dithiodibutyric acid as the linker and then characterized by the presence of a disulfide bond (6B, 7B and 8B) are remarkably more effective in inducing cell death with respect to the corresponding NPs where the linker of the conjugates is the sebacic acid (6A, 7A and 8A). The more pronounced difference in activity emerges by comparing 6A vs. 6B and 8A vs. 8B, and indeed, in these NP pairs the presence of the disulfide bond induces a decrease in GI50 values of about 11 and 22 times, respectively.
Furthermore, the treatment of MeT-5A, human mesothelial cells, with paclitaxel allowed to obtain a GI50 value of 3.2 ± 0.6 nM, similar to that of human mesothelioma MSTO-211H (3.5 ± 0.2 nM, Table 2), confirming the absence of tumor selectivity by the drug. Otherwise, the incubation of MeT-5A with 8B showed a GI50 value of 2.1 ± 0.5 µM, that is about four times higher than that obtained for mesothelioma tumor cells (0.43 ± 0.01 µM, Table 2), indicating a lower effect in normal cells. These data are in agreement with the observation that intracellular levels of reduced glutathione are upregulated in a number of human cancers [23] and support the rationale of the synthetic approach, in accordance with the ability of the disulfide-containing bivalent conjugates to release the active drug inside cells, as already reported [3,11].
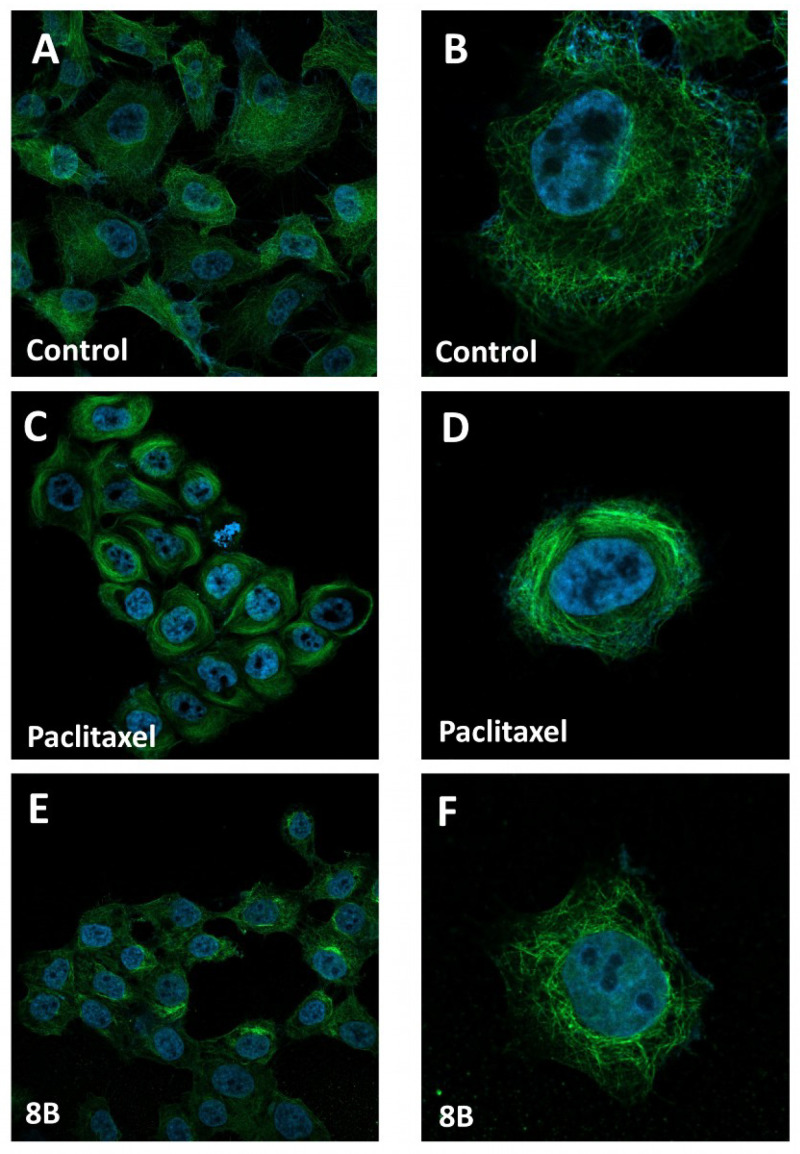
The interesting cell effect of 8B suggested us to investigate its possible mechanism of action, taking into account that the major intracellular effect of paclitaxel is the kinetic suppression of microtubule dynamics and then their stabilization [24]. For this purpose, confocal microscopy experiments were performed in the most sensitive MSTO-211H cells treated with 8B. Figure 3 shows the results obtained by incubating cells in standard conditions (A,B), in the presence of free paclitaxel (C,D) or 8B (E,F) for 4 h at 1 μM and 100 μM, respectively, that is a difference in concentration comparable to that observed between the GI50 values (see Table 2).
Figure 3.
Effect of paclitaxel and 8B on microtubules in MSTO-211H cells. Microtubules in cells were visualized by confocal microscopy after treatment for 4h with vehicle (control, A,B), 1 μM paclitaxel (C,D) and 100 μM 8B (E,F). Cells were stained with the antibody conjugate Alexa Fluor 488 mouse anti-β-tubulin (green) and DAPI (blue) to detect microtubules and nucleus, respectively.
As shown by confocal microscope imaging, the cells in control condition (Figure 3A,B) exhibited a normal microtubule array with filamentous microtubules distributed in the cytoplasm. As expected, treatment with paclitaxel (Figure 3C,D), induced the formation of a highly organized network of microtubules with the formation of long microtubule bundles, mainly surrounding the nucleus. In the presence of 8B, a partial displacement of the microtubule network present in non-treated cells is observed, with the onset of a concurrent microtubule aggregation (Figure 3E,F).
It is well-known that cell death induced by paclitaxel occurs through multiple mechanisms that depend on cell type, concentrations, and cell cycle stage [25,26,27].
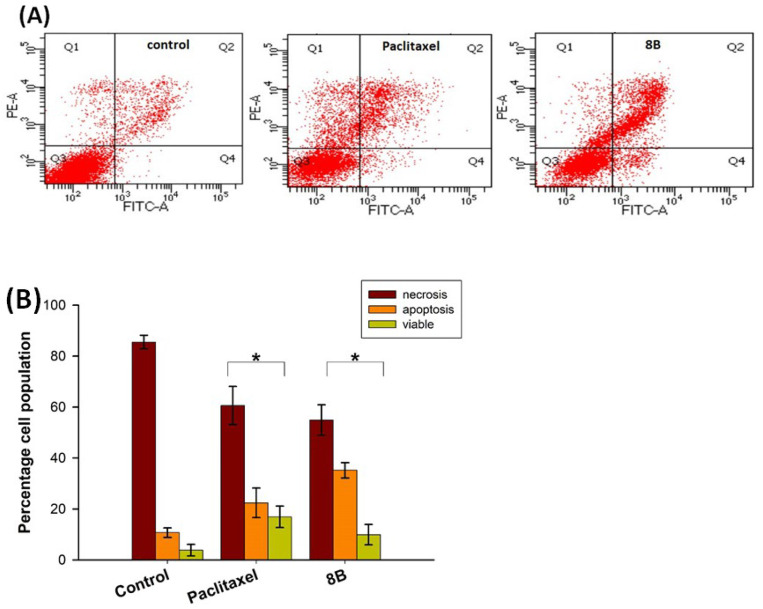
Starting from these considerations and based on the interesting cytotoxic effect of 8B, we investigated the cell death mediated by the NP, in comparison with paclitaxel, to assess the mechanism of cell death. In detail, the most sensitive MSTO-211H were incubated with 8B or paclitaxel for 24 h at a concentration about three times higher with respect to the GI50 value, stained with Annexin V-FITC/propidium iodide and analyzed by flow cytometry. Annexin V stains apoptotic cells by binding to phosphatidylserine, a marker of apoptosis, while propidium iodide stains late apoptotic and necrotic cells because it is internalized only in cells undergoing the loss of plasma and nuclear membrane integrity. Figure 4 shows the dot plots of a representative experiment (A) and the percentages of viable, apoptotic, and necrotic cells (B).
Figure 4.
Flow cytometric analysis of cell death in MSTO-211H cells treated for 24 h with 12 nM paclitaxel and 1.5 μM 8B, and stained with FITC-conjugated Annexin V and PI. (A) Dot plots of a representative experiment for untreated cells (control) and cells treated with paclitaxel, or 8B. (B) Percentages of viable (Q3), apoptotic (Q2 + Q4), and necrotic (Q1) cells. Values are the mean ± SD of three independent experiments in duplicate. * p < 0.05, significant difference in comparison to the control sample.
The treatment with paclitaxel provokes a clear decrease in cell viability, from about 85% in control condition to about 60%, accompanied by a significant increase in both apoptotic and necrotic cells, more than 20% and 15%, respectively. A similar behavior is also observed in cells incubated in the presence of 8B. In this experimental condition, the decrease in viability is pronounced, with about 50% of cell death, which occurs through both apoptosis (35%) and necrosis (10%) pathway.
These results confirm the involvement of paclitaxel in cytotoxicity and cell death mechanism mediated by 8B, supporting the rationale of the approach and confirming the ability of the NP to address the cytotoxic drug inside the cell, allowing the related effect.
3. Materials and Methods
All reactions were carried out in oven-dried glassware and dry solvents under nitrogen atmosphere. Unless otherwise stated, all solvent and reagents were purchased from Sigma-Aldrich (Milan, Italy), Fluorochem (Hadfield, UK), or TCI (Zwijndrecht, Belgium) and used without further purification. CBD was extracted through dynamic maceration (DM) of Cannabis sativa inflorescences using EtOH, following a published protocol [28]. HPLC analysis was performed to assess its purity (>95%) on an ASCENTIS RP-C18 column (5 μm × 4.6 × 150 mm). The pressure was set at about 101 bar, and the temperature was maintained at 40 °C, with a constant flow rate of 0.95 mL/min. UV spectra were recorded at 228 nm using a gradient elution method. The mobile phase consisted of a mixture of A (0.1% v/v HCOOH in H2O) and B (0.1% v/v HCOOH in MeCN). The gradient elution program was adapted to a 30 min duration to obtain RRT 1.00 for CBD, following a published protocol [29]. Thin layer chromatography (TLC) was performed on Merck precoated 60F254 plates. Reactions were monitored by TLC on silica gel, with detection by UV light (254 nm) or by staining with p-anisaldehyde or potassium permanganate solutions with heating. Purification of intermediates and final products was mostly carried out by flash chromatography using as stationary phase high purity grade silica gel (Merck Grade, pore size 60 Å, 230–400 mesh particle size, Sigma Aldrich). Alternatively, purification was performed by a Biotage® system in normal phase using Biotage® Sfär Silica D cartridges (10/25 g). Intermediates and final products were structurally characterized by 1H NMR and 13C NMR spectroscopy at 400/500 MHz, using a Bruker AC 400/500 spectrometer. Chemical shifts (δ) for proton and carbon resonances are quoted in parts per million (ppm) relative to tetramethylsilane (TMS), which was used as an internal standard. 1H and 13C spectra of all synthetized compounds can be found in Supplementary Materials. HRMS spectra were recorded using electrospray ionization (ESI) technique on a FT-ICR APEXII (Bruker Daltonics, Bremen, Germany). Specific rotations were measured with a P-1030-Jasco polarimeter with 10 cm optical path cells and 1 mL capacity (Na lamp, λ = 589 nm).
3.1. Synthetic Procedures
General Procedure for Steglich Coupling
To a stirred solution of the corresponding carboxylic acid (1.0–1.2 eq) in dry dichloromethane (CH2Cl2) (0.02–0.05 M), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC∙HCl) (1.5 eq), and N,N-dimethyl-4-aminopyridine (DMAP) (0.5 eq) are added under nitrogen atmosphere and at r.t. The reaction is stirred for 15 min, and the corresponding alcohol (1.0–1.2 eq) is added. The reaction mixture is then left stirring overnight at r.t. After reaction completion (TLC monitoring), 1M HCl solution is added, and the mixture is extracted with CH2Cl2. The collected organic phases are then dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude reaction mixtures are eventually purified by flash column chromatography or Biotage®, when needed.
4-((4-oxo-4-(2-(trimethylsilyl)ethoxy)butyl)disulfaneyl)butanoic acid (16b)
Following the general procedure for the Steglich coupling, to a solution of 4,4′-dithiodibutyric acid 5b (1000 mg, 4.20 mmol), EDC∙HCl (472 mg, 2.46 mmol), DMAP (75 mg, 0.62 mmol) in dry CH2Cl2 (25 mL, 0.1 M) and pyridine (2.5 mL), trimethylsilyl ethanol (0.26 mL, 1.84 mmol) is added to obtain 503 mg of product 16b with 81% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.17 (m, 2H), 2.73 (m, 4H), 2.52 (t, J = 7.3 Hz, 2H), 2.42 (t, J = 7.3 Hz, 2H), 2.03 (m, 4H), 0.98 (m, 2H), 0.04 (s, 9H).
HRMS (ESI+): m/z [M + Na]+ calcd. for C13H26O4S2SiNa, 361.0939; found, 361.0940.
Spectroscopic data are consistent with those described in the literature [9].
10-oxo-10-(2-(trimethylsilyl)ethoxy)decanoic acid (16a)
Following the general procedure for the Steglich coupling, to a solution of sebacic acid 5a (1000 mg, 4.94 mmol), EDC∙HCl (559 mg, 2.92 mmol), DMAP (89 mg, 0.73 mmol) in dry CH2Cl2 (25 mL, 0.1 M) and pyridine (2.5 mL), trimethylsilyl ethanol (0.31 mL, 2.18 mmol) were added to obtain 560 mg of product 16a with 85% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.20–4.11 (m, 2H), 2.34 (t, J = 7.5 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.69–1.55 (m, 4H), 1.37–1.25 (m, 8H), 1.03–0.93 (m, 2H), 0.04 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 180.2, 174.2, 62.5, 34.6, 34.2, 29.2, 29.1, 29.1, 25.0, 24.7, 17.4, −1.4.
HRMS (ESI+): m/z [M + Na]+ calcd. for C15H30O4SiNa, 325.1811; found, 325.1815.
4-((4-oxo-4-(2,2,2-trichloroethoxy)butyl)disulfaneyl)butanoic acid (18b)
Following the general procedure for the Steglich coupling, to a solution of 4,4′-dithiodibutyric acid 5b (1.56 g, 6.54 mmol), EDC∙HCl (751 mg, 3.92 mmol), DMAP (478 mg, 3.91 mmol) in dry CH2Cl2 (52 mL, 0,05 M), 2,2,2-trichloro ethanol (0.25 mL, 2.61 mmol) is added to obtain 567 mg of product 18b with 59% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.75 (s, 2H), 2.74 (q, J = 7.2 Hz, 4H), 2.61 (t, J = 7.2 Hz, 2H), 2.50 (t, J = 7.2 Hz, 2H), 2.16–1.98 (m, 4H).
13C NMR (100 MHz, CDCl3) δ 179.4, 171.4, 95.0, 74.0, 37.6, 37.6, 32.4, 32.3, 24.0, 23.9.
HRMS (ESI+): m/z [M + Na]+ calcd. for C10H15Cl3O4S2Na, 390.9375; found, 390.9377.
10-oxo-10-(2,2,2-trichloroethoxy)decanoic acid (18a)
Following the general procedure for the Steglich coupling, to a solution of sebacic acid 5a (1.32 g, 6.53 mmol), EDC∙HCl (750 mg, 3.91 mmol), DMAP (480 mg, 3.93 mmol) in dry CH2Cl2 (52 mL, 0.05 M), 2,2,2-trichloro ethanol (0.25 mL, 2.61 mmol) is added to obtain 472 mg of product 18a with 52% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.74 (s, 2H), 2.46 (t, J = 7.5 Hz, 2H), 2.35 (t, J = 7.5 Hz, 2H), 1.66 (m, 4H), 1.40–1.26 (m, 8H).
13C NMR (100 MHz, CDCl3) δ 180.4, 172.2, 95.2, 73.9, 34.1, 34.0, 29.1, 29.0, 29.0, 29.0, 24.8, 24.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C12H19Cl3O4Na, 355.0247; found, 355.0248
(S)-10-oxo-10-((1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)amino)decanoic acid (9a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 5a (135 mg, 0.67 mmol), EDC∙HCl (56 mg, 0.29 mmol), DMAP (4 mg, 0.03 mmol), TEA (0.30 mL, 2.14 mmol) in dry CH2Cl2 (3.0 mL, 0.09 M), 2 (100 mg, 0.27 mmol) is added to obtain 93 mg of product 9a with 62% yield. Reaction is monitored by TLC (CH2Cl2/MeOH 95:5) and purified by flash chromatography (CH2Cl2/MeOH 95:5 eluent mixture).
1H NMR (400 MHz, CDCl3) δ 7.60 (s, 1H), 7.44 (s, 1H), 7.34 (d, J = 10.5 Hz, 1H), 7.11 (d, J = 10.5 Hz, 1H), 6.53 (s, 1H), 4.71 (dt, J = 12.8, 6.7 Hz, 1H), 3.93 (s, 3H), 3.89 (s, 3H), 3.65 (s, 3H), 2.51 (dd, J = 13.2, 5.9 Hz, 1H), 2.43 (s, 3H), 2.41–2.25 (m, 3H), 2.26–2.17 (m, 3H), 1.95–1.84 (m, 1H), 1.67–1.49 (m, 4H), 1.34–1.21 (m, 8H).
HRMS (ESI+): m/z [M + Na]+ calcd. for C30H39NO7SNa, 580.2345; found, 580.2348.
Spectroscopic data are consistent to the ones reported in the literature [30].
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 10-oxo-10-(((S)-1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)amino)decanoate (6a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 9a (50 mg, 0.09 mmol), EDC∙HCl (19 mg, 0.98 mmol), DMAP (1 mg, 0.01 mmol), TEA (0.01 mL, 0.98 mmol) in dry CH2Cl2 (2 mL, 0.04 M), 1 (28 mg, 0.09 mmol) are added to obtain 36 mg of product 6a with 45% yield. Reaction is monitored by TLC (n-hex/EtOAc 1:1) and purified by flash chromatography (n-hex/EtOAc 1:1).
1H NMR (400 MHz, CDCl3) δ 7.36–7.27 (m, 2H), 7.08 (d, J = 10.5 Hz, 1H), 6.53 (s, 2H), 6.45 (d, J = 7.4 Hz, 1H), 6.37 (s, 1H), 5.95 (bs, 1H), 5.51 (bs, 1H), 4.67 (dt, J = 13.2, 6.8 Hz, 1H), 4.62–4.57 (m, 1H), 4.44 (bs, 1H), 3.94 (s, 3H), 3.90 (s, 3H), 3.66 (s, 3H), 3.49 (bs, 1H), 2.59–2.32 (m, 9H), 2.31–2.13 (m, 4H), 2.11–1.64 (m, 12H), 1.65–1.50 (m, 3H), 1.39–1.22 (m, 14H), 0.88 (t, J = 7.7 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 182.2, 173.0, 172.2, 158.4, 153.8, 152.0, 151.3, 141.8, 138.8, 135.8, 135.1, 134.5, 129.9, 128.6, 127.8, 127.0, 125.8, 123.5, 114.5, 111.4, 107.5, 61.8, 61.5, 56.2, 52.0, 45.7, 37.9, 37.0, 36.5, 35.5, 34.4, 31.6, 30.6, 30.3, 30.1, 29.8, 29.4, 29.2, 28.1, 27.0, 25.6, 24.9, 23.7, 22.6, 19.9, 15.3, 14.1.
HRMS (ESI+):m/z [M + Na]+ calcd. for C51H67NO8SNa, 876.4485; found, 876.4491.
: -72.8 (c 1 in CHCl3).
(S)-4-((4-oxo-4-((1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a] heptalen-7-yl)amino)butyl)disulfaneyl)butanoic acid (9b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 5b (238 mg, 1.0 mmol), EDC∙HCl (84 mg, 0.44 mmol), DMAP (5 mg, 0.04 mmol), TEA (0.45 mL, 3.2 mmol) in dry CH2Cl2 (4.5 mL, 0.01 M), 2 (150 mg, 0.40 mmol) is added to obtain 117 mg of product 9b with 49% yield. Reaction is monitored by TLC (CH2Cl2/MeOH 98:2 + 1% HCOOH) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H), 7.56 (d, J = 10.7 Hz, 1H), 7.33 (d, J = 10.7 Hz, 1H), 6.56 (s, 1H), 4.85–4.72 (m, 1H), 3.97–3.89 (m, 6H), 3.76–3.59 (m, 3H), 2.88–2.67 (m, 5H), 2.63–2.41 (m, 9H), 2.43–2.26 (m, 2H), 2.13–1.88 (m, 4H).
HRMS (ESI+): m/z [M + Na]+ calcd. for C28H35NO7S3Na, 616.1473; found, 616.1478.
Spectroscopic data are consistent to the ones reported in literature [31].
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 4-((4-oxo-4-(((S)-1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7 yl)amino)butyl)disulfaneyl)butanoate (6b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 9b (70 mg, 0.18 mmol), EDC∙HCl (38 mg, 0.20 mmol), DMAP (3 mg, 0.02 mmol), TEA (0.2 mL, 1.44 mmol) in dry CH2Cl2 (4 mL, 0.01 M), 1 (57 mg, 0.18 mmol) is added to obtain 32 mg of product 6b with 20% yield. Reaction is monitored by TLC (n-hex/EtOAc 3:7) and purified by flash chromatography (n-hex/EtOAc 3:7 + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 7.37–7.27 (m, 2H), 7.07 (d, J = 10.5 Hz, 1H), 6.99–6.83 (m, 1H), 6.76–6.65 (m, 1H), 6.53 (bs, 2H), 6.37 (s, 1H), 5.97 (bs, 1H), 5.47 (bs, 1H), 4.64 (dt, J = 11.9, 6.6 Hz, 1H), 4.61–4.56 (m, 1H), 4.44 (s, 1H), 3.93 (s, 3H), 3.89 (s, 3H), 3.66 (s, 3H), 2.83–2.72 (m, 3H), 2.72–2.60 (m, 4H), 2.55–2.41 (m, 7H), 2.42–2.29 (m, 2H), 2.27–2.05 (m, 4H), 1.99 (q, J = 7.3 Hz, 2H), 1.85–1.68 (m, 5H), 1.62–1.47 (m, 3H), 1.36–1.18 (m, 8H), 0.97–0.77 (m, 3H).
13C NMR (100 MHz, CDCl3) δ 182.4, 171.8, 171.7, 158.5, 155.7, 153.8, 151.4, 142.9, 141.8, 138.5, 134.9, 134.5, 128.6, 126.8, 125.8, 124.5, 114.7, 111.5, 107.6, 61.8, 61.5, 56.2, 52.1, 45.8, 38.4, 38.0, 37.6, 36.9, 35.5, 35.3, 34.6, 32.5, 31.6, 30.6, 30.5, 30.3, 30.1, 29.8, 24.7, 24.7, 24.3, 23.8, 22.6, 20.0, 15.3, 14.2.
HRMS (ESI+):m/z [M + Na]+ calcd. for C49H63NO8S3Na, 912.3613; found, 912.3619.
: -117.1 (c 0.96 in CHCl3).
(5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7] naphtho[2,3-d][1,3] dioxol-5-yl 4-((4-oxo-4-(2-(trimethylsilyl)ethoxy)butyl)disulfaneyl) butanoate (17b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16b (74.0 mg, 0.22 mmol), EDC∙HCl (41.7 mg, 0.22 mmol), DMAP (11.0 mg, 0.09 mmol) in dry CH2Cl2 (9 mL, 0.02 M), 3 (75 mg, 0.18 mmol) is added to obtain 1 mg of product 17b quantitatively. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.74 (s, 1H), 6.51 (s, 1H), 6.36 (s, 2H), 5.95 (d, J = 5.4 Hz, 2H), 5.86 (d, J = 9.0 Hz, 1H), 4.57 (d, J = 3.9 Hz, 1H), 4.39–4.30 (m, 1H), 4.22–4.09 (m, 3H), 3.77 (s, 3H), 3.73 (s, 6H), 2.90 (dd, J = 14.5, 4.5 Hz, 1H), 2.87–2.75 (m, 1H), 2.77–2.65 (m, 4H), 2.65–2.45 (m, 2H), 2.38 (t, J = 7.4 Hz, 2H), 2.12–1.93 (m, 4H), 1.00–0.91 (m, 2H), 0.01 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 173.6, 173.3, 172.9, 152.5 (2C), 148.1, 147.5, 137.0, 134.8, 132.3, 128.2, 109.6, 108.0 (2C), 106.9, 101.6, 73.6, 71.3, 62.6, 60.6, 56.1 (2C), 45.4, 43.6, 38.6, 37.7, 37.6, 32.7, 32.5, 24.2, 24.0, 17.3, −1.5 (3C).
HRMS (ESI+):m/z [M + Na]+ calcd. for C35H46O11S2SiNa, 757.2148; found, 757.2150.
4-((4-oxo-4-(((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl)oxy)butyl)disulfaneyl)butanoic acid (10b)
To a well-stirred solution of compound 17b (141 mg, 0.192 mmol) in dry THF (3.9 mL, 0.05M), 1M TBAF in THF (1.92 mL, 1.92 mmol) is added at 0 °C under nitrogen atmosphere. The reaction is stirred at r.t. and monitored by TLC (n-hex/EtOAc 1:1 + 1% HCOOH). After 1 h, the reaction is quenched with saturated NH4Cl aqueous solution and extracted with abundant CH2Cl2. The organic phase is then dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is then purified by Biotage® (gradient with n-hex/EtOAc + 1% HCOOH eluent mixture) to obtain 59 mg of the desired product 10b (48% yield).
1H NMR (400 MHz, CDCl3) δ 6.74 (d, J = 5.0 Hz, 1H), 6.54 (s, 1H), 6.38 (s, 2H), 5.99–5.92 (m, 2H), 5.73 (d, J = 4.9 Hz, 1H), 4.47–4.33 (m, 2H), 4.28 (dd, J = 9.6, 3.0 Hz, 1H), 3.82 (s, 3H), 3.80 (s, 6H), 3.29 (dd, J = 9.2, 3.6 Hz, 1H), 3.03–2.92 (m, 1H), 2.79–2.62 (m, 4H), 2.52–2.45 (m, 2H), 2.43–2.25 (m, 2H), 2.08–1.90 (m, 4H).
13C NMR (100 MHz, CDCl3) δ 178.5, 177.5, 172.7, 153.3 (2C), 148.5, 147.3, 139.0, 136.9, 131.3, 126.2, 109.9, 108.4, 105.5 (2C), 101.5, 72.6, 70.9, 61.0, 56.3 (2C), 45.5, 44.3, 39.8, 37.8, 37.6, 32.5, 32.3, 24.0, 24.0.
HRMS (ESI+): m/z [M + Na]+ calcd. for C30H34O11S2Na, 657.1440; found, 657.1443.
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 4-((4-oxo-4-(((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6, 8,8a,9-hexahydrofuro[3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl)oxy)butyl)disulfaneyl)butanoate (7b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 10b (59 mg, 0.093 mmol), EDC∙HCl (22 mg, 0.112 mmol), DMAP (6 mg, 0.047 mmol) in dry CH2Cl2 (2 mL, 0.05 M), 1 (44 mg, 0.140 mmol) is added to obtain 69 mg of product 7b with 80% yield. Reaction is monitored by TLC (1:1 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.73 (s, 1), 6.54 (s, 2H), 6.40–6.37 (m, 3H), 5.95 (dd, J = 10.4, 1.4 Hz, 2H), 5.74 (d, J = 5.0 Hz, 1H), 5.52 (s, 1H), 4.63–4.57 (m, 1H), 4.48–4.35 (m, 3H), 4.28 (dd, J = 9.6, 2.9 Hz, 1H), 3.83 (s, 3H), 3.80 (s, 6H), 3.47 (s, 1H), 3.28 (dd, J = 9.2, 3.6 Hz, 1H), 3.02–2.92 (m, 1H), 2.83–2.72 (m, 2H), 2.68 (t, J = 7.0 Hz, 2H), 2.64 (s, 2H), 2.58–2.44 (m, 3H), 2.44–2.27 (m, 2H), 2.28–2.16 (m, 1H), 2.17–2.05 (m, 3H), 1.98 (p, J = 7.1 Hz, 2H), 1.87–1.69 (m, 5H), 1.63–1.50 (m, 5H), 1.36–1.24 (m, 4H), 0.87 (t, J = 6.8 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 177.3, 172.7, 171.4, 153.4 (2C), 148.6, 147.4, 143.0, 142.2, 139.0, 133.0, 131.4, 126.3, 123.3, 114.8, 114.1, 111.5, 111.2, 110.0, 108.3, 105.6 (2C), 101.6, 72.6, 70.9, 61.0, 56.4 (2C), 53.6, 45.7, 45.6, 44.3, 39.9, 38.1, 37.6 (2C), 35.5, 32.5 (2C), 31.6, 30.6, 30.3, 29.8, 28.0, 24.1, 24.0, 23.7, 22.6, 14.1.
HRMS (ESI+): m/z [M + Na]+ calcd. for C51H62O12S2Na, 953.3580; found, 953.3582.
: -39.5 (c 0.32 in CHCl3).
1-((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro [3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl) 10-(2-(trimethylsilyl)ethyl) decanedioate (17a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16a (67 mg, 0.22 mmol), EDC∙HCl (42 mg, 0.22 mmol), DMAP (11 mg, 0.09 mmol) in dry CH2Cl2 (9 mL, 0.02 M), 3 (75 mg, 0.18 mmol) is added to obtain 91 mg of product 17a with 72% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.74 (s, 1H), 6.53 (s, 1H), 6.39 (s, 2H5.98 (dd, J = 6.5, 1.3 Hz, 2H), 5.88 (d, J = 9.1 Hz, 1H), 4.60 (d, J = 4.4 Hz, 1H), 4.36 (dd, J = 9.3, 7.0 Hz, 1H), 4.24–4.11 (m, 3H), 3.81 (s, 3H), 3.75 (s, 6H), 2.92 (dd, J = 14.5, 4.4 Hz, 1H), 2.88–2.74 (m, 1H), 2.49–2.33 (m, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.73–1.54 (m, 4H), 1.36–1.26 (m, 8H), 1.03–0.92 (m, 2H), 0.03 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 174.2, 174.0, 173.7, 152.7 (2C), 148.1, 147.6, 137.2, 134.9, 132.4, 128.5, 109.7, 108.1 (2C), 107.0, 101.6, 73.4, 71.4, 62.4, 60.8, 56.2 (2C), 45.6, 43.8, 38.8, 34.5, 34.4, 29.1, 29.1, 29.1, 29.1, 25.0, 24.9, 17.3, −1.5 (3C).
HRMS (ESI+):m/z [M + Na]+ calcd. for C37H50O11SiNa, 721.3020; found, 721.3023.
10-oxo-10-(((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl)oxy)decanoic acid (10a)
To a well-stirred solution of compound 17a (139 mg, 0.199 mmol) in dry THF (4.0 mL, 0.05M), 1M TBAF in THF (2.00 mL, 2.00 mmol) is added at 0 °C under nitrogen atmosphere. The reaction is stirred at r.t. and monitored by TLC (n-hex/EtOAc 1:1 + 1% HCOOH). After 1 h, the reaction is quenched with saturated NH4Cl aqueous solution and extracted with abundant CH2Cl2. The organic phase is then dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is then purified by Biotage® (gradient with n-hex/EtOAc + 1% HCOOH eluent mixture) to obtain 57 mg of the desired product 10a (48% yield).
1H NMR (400 MHz, CDCl3) δ 6.71 (s, 1H), 6.52 (s, 1H), 6.39 (s, 2H), 5.94 (dd, J = 10.8, 1.4 Hz, 2H), 5.72 (d, J = 5.0 Hz, 1H), 4.41 (dd, J = 9.6, 6.8 Hz, 1H), 4.36 (d, J = 3.7 Hz, 1H), 4.29 (dd, J = 9.7, 2.9 Hz, 1H), 3.81 (s, 2H), 3.78 (s, 6H), 3.26 (dd, J = 9.2, 3.7 Hz, 1H), 2.99–2.88 (m, 1H), 2.33 (t, J = 7.5 Hz, 2H), 2.21 (td, J = 7.5, 4.6 Hz, 2H), 1.66–1.49 (m, 4H), 1.35–1.22 (m, 9H).
13C NMR (100 MHz, CDCl3) δ 179.6, 177.4, 173.5, 153.3 (2C), 148.4, 147.3, 138.9, 136.9, 131.3, 126.4, 109.8, 108.2, 105.5 (2C), 101.5, 72.3, 71.0, 60.9, 56.2 (2C), 45.6, 44.2, 39.9, 34.3, 34.0, 29.0 (2C), 29.0, 29.0, 24.8, 24.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C32H38O11Na, 621.2312; found, 621.2313.
1-((1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl) 10-((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7] naphtho[2,3-d][1,3]dioxol-5-yl) decanedioate (7a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 10a (59 mg, 0.093 mmol), EDC∙HCl (22 mg, 0.112 mmol), DMAP (6 mg, 0.047 mmol) in dry CH2Cl2 (2 mL, 0.05 M), 1 (44 mg, 0.140 mmol) is added to obtain 69 mg of product 7a with 80% yield. Reaction is monitored by TLC (1:1 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.72 (s, 1H), 6.56–6.51 (m, 2H), 6.40 (s, 2H), 6.37 (d, J = 1.8 Hz, 1H), 5.95 (dd, J = 11.0, 1.4 Hz, 2H), 5.74 (d, J = 5.1 Hz, 1H), 5.53 (s, 1H), 4.62–4.57 (m, 1H), 4.47–4.35 (m, 3H), 4.31 (dd, J = 9.6, 2.8 Hz, 1H), 3.83 (s, 3H), 3.80 (s, 6H), 3.47 (s, 1H), 3.26 (dd, J = 9.2, 3.7 Hz, 1H), 2.99–2.89 (m, 1H), 2.59–2.37 (m, 5H), 2.30–2.14 (m, 3H), 2.11–2.01 (m, 1H), 1.86–1.61 (m, 7H), 1.60 (s, 3H), 1.59–1.50 (m, 4H), 1.42–1.21 (m, 12H), 0.87 (t, J = 6.9 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 177.3, 173.5, 153.4, 148.5, 147.3, 139.0, 137.0, 131.3, 126.5, 109.9, 108.2, 105.6 (2C), 101.5, 72.3, 71.0, 68.1, 60.9, 56.3 (2C), 45.7 (2C), 44.3, 39.9, 38.0, 35.5 (2C), 34.4, 34.3, 31.6, 30.6, 30.3, 29.2, 29.2, 29.2, 29.2, 28.2, 25.7, 24.9, 24.9, 23.7, 22.6, 20.0, 14.1.
HRMS (ESI+): m/z [M + Na]+ calcd. for C53H66O12Na, 917.4452; found, 917.4457.
: -24.5 (c 1.07 in CHCl3).
(2aR,4S,4aS,6R,9S,11S,12S,12bS)-9-(((R)-17-((S)-benzamido(phenyl)methyl)-2,2-dimethyl-6,15-dioxo-5,16-dioxa-10,11-dithia-2-silaoctadecan-18-oyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4] benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate (18b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16b (36 mg, 0.11 mmol), EDC∙HCl (21 mg, 0.11 mmol), DMAP (6 mg, 0.045 mmol) in dry CH2Cl2 (1.5 mL, 0.05 M), 4 (75 mg, 0.09 mmol) is added to obtain 100 mg of product 18b quantitatively. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.17–8.10 (m, 2H), 7.77–7.70 (m, 2H), 7.65–7.56 (m, 1H), 7.55–7.46 (m, 3H), 7.46–7.30 (m, 7H), 6.94 (d, J = 9.2 Hz, 1H), 6.32–6.21 (m, 2H), 5.98 (dd, J = 9.2, 3.1 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.1 Hz, 1H), 4.97 (dd, J = 9.6, 2.3 Hz, 1H), 4.44 (dd, J = 10.9, 6.6 Hz, 1H), 4.31 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 4.18–4.08 (m, 2H), 3.82 (d, J = 7.0 Hz, 1H), 2.75–2.61 (m, 4H), 2.61–2.48 (m, 4H), 2.46 (s, 3H), 2.42–2.32 (m, 3H), 2.24–2.12 (m, 4H), 2.09–1.96 (m, 4H), 1.94 (s, 3H), 1.92–1.80 (m, 2H), 1.68 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H), 1.03–0.91 (m, 2H), 0.03 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 203.9, 171.2, 169.9, 168.1, 167.3, 167.0, 142.7, 137.0, 133.7, 132.1, 130.3 (2C), 129.2 (2C), 128.8 (2C), 128.6 (2C), 127.2 (2C), 126.6 (2C), 84.5, 76.5, 75.7, 75.2, 74.1, 72.1, 71.9, 62.8, 58.5, 52.8, 45.7, 37.7, 37.1, 35.7, 35.6, 32.8, 32.0, 26.9, 24.3, 24.0, 22.8, 22.2, 17.4, 14.9, 9.7, −1.4 (3C).
HRMS (ESI+): m/z [M + Na]+ calcd. for C60H75NO17S2SiNa, 1196.4143; found, 1196.4149.
1-((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl) 10-(2-(trimethylsilyl)ethyl) decanedioate (18a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16a (71 mg, 0.071 mmol), EDC∙HCl (14.0 mg, 0.071 mmol), DMAP (4.0 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0,05 M), 4 (50 mg, 0.059 mmol) is added to obtain 59.9 mg of product 18a with 87% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 7.4 Hz, 2H), 7.73 (d, J = 7.4 Hz, 2H), 7.58 (t, J = 7.4 Hz, 1H), 7.53–7.44 (m, 3H), 7.44–7.34 (m, 6H), 7.34–7.27 (m, 1H), 7.00 (d, J = 9.1 Hz, 1H), 6.28 (s, 1H), 6.27–6.16 (m, 1H), 5.93 (dd, J = 9.2, 3.5 Hz, 1H), 5.65 (d, J = 7.1 Hz, 1H), 5.50 (d, J = 3.5 Hz, 1H), 4.94 (dd, J = 9.6, 2.3 Hz, 1H), 4.42 (dd, J = 10.9, 6.6 Hz, 1H), 4.28 (d, J = 8.5 Hz, 1H), 4.21–4.09 (m, 3H), 3.79 (d, J = 7.0 Hz, 1H), 2.60–2.46 (m, 1H), 2.43 (s, 3H), 2.42–2.28 (m, 3H), 2.24 (t, J = 7.5 Hz, 3H), 2.19 (s, 3H), 2.17–2.07 (m, 1H), 1.92 (s, 3H), 1.90–1.76 (m, 1H), 1.65 (s, 3H), 1.63–1.50 (m, 4H), 1.32–1.18 (m, 11H), 1.11 (s, 3H), 1.01–0.91 (m, 2H), 0.02 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 203.9, 174.0, 172.8, 171.2, 169.8, 168.2, 167.2, 167.0, 142.8, 137.1, 133.8, 133.7, 132.8, 132.0, 130.2 (2C), 129.3, 129.1 (2C), 128.8 (2C), 128.7 (2C), 128.5, 127.2 (2C), 126.7 (2C), 84.5, 81.1, 79.1, 76.5, 75.6, 75.2, 73.9, 72.1, 71.8, 62.4, 58.5, 53.0, 45.7, 43.2, 35.6, 34.5 (2C), 33.8, 29.1, 29.1, 29.0, 28.9, 26.8, 24.9, 24.7, 22.7, 22.2, 20.9, 17.3, 14.8, 9.7, −1.4 (3C).
HRMS (ESI+): m/z [M + Na]+ calcd. for C62H79NO17SiNa, 1160.5015; found, 1160.5019.
1-((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl) 10-(2,2,2-trichloroethyl) decanedioate (19a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 18a (24 mg, 0.071 mmol), EDC∙HCl (14 mg, 0.071 mmol), DMAP (4 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0.05 M), 4 (50 mg, 0.059 mmol) is added to obtain 60 mg of product 19a with 87% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.18–8.10 (m, 2H), 7.78–7.70 (m, 2), 7.65–7.57 (m, 1H), 7.56–7.47 (m, 3H), 7.46–7.30 (m, 7H), 6.87 (d, J = 9.2 Hz, 1H), 6.32–6.21 (m, 2H), 5.95 (dd, J = 9.2, 3.2 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.50 (d, J = 3.2 Hz, 1H), 4.98 (dd, J = 9.8, 2.3 Hz, 1H), 4.74 (s, 2H), 4.45 (dd, J = 10.9, 6.6 Hz, 1H), 4.32 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.82 (d, J = 7.0 Hz, 1H), 2.56 (ddd, J = 14.7, 9.8, 6.6 Hz, 1H), 2.49–2.28 (m, 8H), 2.23 (s, 3H), 2.21–2.10 (m, 1H), 1.94 (d, J = 1.4 Hz, 3H), 1.89 (ddd, J = 14.6, 11.0, 2.4 Hz, 1H), 1.79–1.51 (m, 7H), 1.35–1.21 (m, 11H), 1.13 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 172.8 (2C), 171.3, 169.9, 168.2, 167.2, 167.1, 142.9, 137.1, 133.8, 133.8, 132.9, 132.1, 130.3, 129.3 (4C), 129.1 (4C), 128.8, 128.6, 127.2 (2C), 126.6 (2C), 95.2, 84.6, 81.2, 79.2, 76.5, 75.7, 75.2, 74.0, 73.9, 72.2, 71.9, 58.6, 52.9, 45.7, 43.3, 35.7, 35.6, 34.0, 33.8, 29.1, 29.0 (2C), 28.9, 26.9, 24.8, 22.8, 22.2, 20.9, 14.9, 14.3, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C59H68Cl3NO17Na, 1190.3451; found, 1190.3454.
1-((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl) 10-(2,2,2-trichloroethyl) decanedioate (19b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 18b (26.0 mg, 0.071 mmol), EDC∙HCl (14.0 mg, 0.071 mmol), DMAP (4.0 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0,05 M), 4 (50.0 mg, 0.059 mmol) is added to obtain 77.7 mg of product 19b with 91% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.17–8.10 (m, 2H), 7.78–7.69 (m, 2H), 7.65–7.55 (m, 1H), 7.56–7.46 (m, 3H), 7.47–7.31 (m, 7H), 6.90 (d, J = 9.2 Hz, 1H), 6.32–6.21 (m, 2H), 5.97 (dd, J = 9.2, 3.1 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.1 Hz, 1H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.74 (s, 2H), 4.45 (dd, J = 10.9, 6.6 Hz, 1H), 4.32 (d, J = 8.4 Hz, 1H), 4.23–4.17 (m, 1H), 3.82 (d, J = 7.0 Hz, 1H), 2.74–2.46 (m, 9H), 2.46 (s, 3H), 2.38 (dd, J = 15.4, 9.3 Hz, 1H), 2.23 (s, 3H), 2.22–2.12 (m, 1H), 2.12–1.95 (m, 4H), 1.95 (s, 3H), 1.94–1.83 (m, 1H), 1.68 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 172.8 (2C), 171.3, 169.9, 168.2, 167.2, 167.1, 142.9, 137.1, 133.8, 133.8, 132.9, 132.1, 130.3, 129.3 (4C), 129.1 (4C), 128.8, 128.6, 127.2 (2C), 126.6 (2C), 95.2, 84.6, 81.2, 79.2, 76.5, 75.7, 75.2, 74.0, 73.9, 72.2, 71.9, 58.6, 52.9, 45.7, 43.3, 35.7, 35.6, 34.0, 33.8, 28.9, 26.9, 24.8, 22.8, 22.2, 20.9, 14.9, 14.3, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C57H64Cl3NO17S2Na, 1226.2579; found, 1226.2583.
10-(((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl)oxy)-10-oxodecanoic acid (11a)
Following the reported procedure described by Negretti et al. [32], to a solution of the trichloroethyl ester 19a (59.9 mg, 0.051 mmol) in AcOH/MeOH 1:1 (2 mL, 0.03 M), zinc dust (83.4 mg, 1.275 mmol) is added at r.t. under vigorous stirring. The reaction is monitored by TLC (n-hex/EtOAc 4:6 + 1% HCOOH) and, after 4 h, is filtered over celite washing with MeOH. The organic phase is then washed with H2O extracting with abundant EtOAc, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is purified by Biotage® (gradient with n-hex/EtOAc + 1% HCOOH as eluent mixture), obtaining 30.1 mg of product 11a with 57% yield.
1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 7.6 Hz, 2H), 7.72 (d, J = 7.6 Hz, 2H), 7.60 (t, J = 7.3 Hz, 1H), 7.50 (dd, J = 8.7, 6.6 Hz, 3H), 7.45–7.29 (m, 7H), 6.97 (d, J = 9.2 Hz, 1H), 6.30 (s, 1H), 6.25 (t, J = 9.1 Hz, 1H), 5.96 (dd, J = 9.2, 3.3 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.3 Hz, 1H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.43 (dd, J = 10.9, 6.7 Hz, 1H), 4.30 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 3.81 (d, J = 7.0 Hz, 1H), 2.63–2.49 (m, 1H), 2.49–2.30 (m, 6H), 2.30–2.11 (m, 6H), 1.93 (s, 3H), 1.92–1.82 (m, 1H), 1.67 (s, 3H), 1.62–1.49 (m, 4H), 1.33–1.18 (m, 11H), 1.13 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 178.5, 172.9, 171.4, 170.0, 168.3, 167.6, 167.1, 142.8, 137.0, 133.8, 133.7, 133.0, 132.2, 130.3, 129.4 (4C), 129.2 (2C), 128.9, 128.8, 128.6, 127.2 (2C), 126.7 (2C), 84.6, 81.2, 79.1, 76.6, 75.8, 75.3, 73.9, 72.2, 71.9, 58.6, 53.0, 45.7, 43.3, 35.7, 33.9, 33.8, 29.0, 28.8, 26.9, 24.7, 24.7, 22.8, 22.2, 20.9, 14.9, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C57H67NO17Na, 1060.4307; found, 1060.4311.
10-(((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl)oxy)-10-oxodecanoic acid (8a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 11a (30.1 mg, 0.029 mmol), EDC∙HCl (7.0 mg, 0.044 mmol), DMAP (2.0 mg, 0.015 mmol) in dry CH2Cl2 (1 mL, 0.05 M), 1 (14 mg, 0.044 mmol) is added to obtain 34.1 mg of product 8a with 88% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.7 Hz, 2H), 7.73 (d, J = 7.6 Hz, 2H), 7.61 (t, J = 7.4 Hz, 1H), 7.56–7.46 (m, 3H), 7.45–7.30 (m, 7H), 6.88 (d, J = 9.2 Hz, 1H), 6.54 (s, 1H), 6.38 (d, J = 1.7 Hz, 1H), 6.32–6.20 (m, 2H), 5.96 (dd, J = 9.2, 3.3 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.3 Hz, 2H), 4.97 (dd, J = 9.6, 2.3 Hz, 1H), 4.60 (s, 1H), 4.49–4.40 (m, 2H), 4.31 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 3.82 (d, J = 7.0 Hz, 1H), 3.48 (s, 1H), 2.64–2.31 (m, 11H), 2.22 (s, 3H), 2.20–1.98 (m, 3H), 1.95 (s, 3H), 1.93–1.70 (m, 6H), 1.68 (s, 3H), 1.63–1.52 (m, 7H), 1.42–1.24 (m, 12H), 1.23 (s, 3H), 1.13 (s, 3H), 0.87 (t, J = 6.7 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 204.0, 175.6, 172.8, 171.4, 171.3, 170.9, 169.9, 168.2, 167.2, 167.2, 142.9, 137.1, 135.1, 133.8, 132.9, 132.2, 130.4, 129.3 (4C), 129.2 (4C), 128.9, 128.6, 127.2 (2C), 126.6 (2C), 84.6, 81.2, 79.3, 76.6, 75.7, 75.2, 73.9, 72.3, 71.9, 58.6, 52.9, 45.7, 43.3, 38.0, 35.7, 35.6, 35.5, 34.4, 33.9, 31.6, 30.8, 30.6, 29.3, 29.2, 29.0, 26.9, 24.9, 24.8, 23.7, 22.8, 22.6, 22.3, 20.9, 14.9, 14.1, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C78H95NO18Na, 1356.6447; found, 1356.6452.
: -65.9 (c 0.69 in CHCl3).
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 4-((4-oxo-4-(2-(trimethylsilyl)ethoxy)butyl)disulfaneyl)butanoate (20b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16b (150 mg, 0.44 mmol), EDC∙HCl (93 mg, 0.049 mmol), DMAP (71 mg, 0.58 mmol) in dry CH2Cl2 (9 mL, 0.05 M), 1 (279 mg, 0.89 mmol) is added to obtain 159 mg of product 20b with 57% yield. Reaction is monitored by TLC (9:1 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) 6.54 (bs, 1H), 6.38 (s, 1H), 5.98 (bs, 1H), 5.52 (bs, 1H), 4.65–4.55 (m, 1H), 4.44 (bs, 1H), 4.24–4.10 (m, 2H), 3.48 (bs, 1H), 2.78 (t, J = 7.1 Hz, 2H), 2.73 (t, J = 7.1 Hz, 2H), 2.68–2.58 (m, 2H), 2.53–2.38 (m, 5H), 2.29–2.07 (m, 4H), 2.07–1.98 (m, 2H), 1.87–1.67 (m, 5H), 1.61–1.51 (m, 5H), 1.38–1.21 (m, 5H), 1.03–0.95 (m, 2H), 0.87 (t, J = 6.8 Hz, 3H), 0.04 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 172.9, 171.4, 156.8, 149.2, 143.1, 140.7, 139.1, 123.3, 114.8, 114.1, 111.6, 62.6, 45.7, 38.1, 37.6, 35.5, 32.5, 31.6, 30.6, 30.3, 29.8, 28.0, 24.1, 23.8, 22.6, 20.1, 17.3, 14.2, −1.5.
HRMS (ESI+): m/z [M + Na]+ calcd. for C34H54O5S2SiNa, 657.3080; found, 657.3084.
4-((4-(((1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl)oxy)-4-oxobutyl)disulfaneyl)butanoic acid (21b)
To a well-stirred solution of compound 20b (155.0 mg, 0.244 mmol) in dry THF (5.0 mL, 0.05M), 1M TBAF in THF (2.44 mL, 2.44 mmol) is added at 0 °C under nitrogen atmosphere. The reaction is stirred at r.t. and monitored by TLC (n-hex/EtOAc 7:3 + 1% HCOOH). The reaction is quenched with saturated NH4Cl aqueous solution and extracted with abundant EtOAc. The organic phase is then dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is then purified by flash chromatography (n-hex/EtOAc 8:2 + 1% HCOOH eluent mixture) to obtain 78 mg of the desired product 21b with 65% yield.
1H NMR (400 MHz, CDCl3) δ 6.54 (bs, 1H), 6.38 (s, 1H), 5.98 (bs, 1H), 5.52 (bs, 1H), 4.60 (s, 1H), 4.44 (s, 1H), 3.48 (bs, 1H), 2.78 (t, J = 7.1 Hz, 2H), 2.73 (t, J = 7.1 Hz, 2H), 2.68–2.59 (m, 2H), 2.53–2.38 (m, 4H), 2.23–2.07 (m, 3H), 2.03 (p, J = 7.2 Hz, 2H), 1.86–1.66 (m, 5H), 1.62–1.52 (m, 5H), 1.35–1.24 (m, 5H), 0.93–0.82 (m, 3H).
13C NMR (100 MHz, CDCl3) δ 176.9, 171.4, 155.8, 149.2, 143.1, 140.7, 139.1, 123.3, 114.8, 114.1, 111.6, 45.7, 38.1, 37.6, 35.5, 32.5, 31.6, 30.6, 30.3, 29.8, 28.0, 24.1, 23.8, 22.6, 20.1, 14.2.
HRMS (ESI+): m/z [M + Na]+ calcd. for C29H42O5S2Na, 557.2371; found, 557.2377.
(2aR,4S,4aS,6R,9S,11S,12S,12bS)-9-(((2R,3S)-3-benzamido-2-((4-((4-(((1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl)oxy)-4-oxobutyl)disulfaneyl)butanoyl)oxy)-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca [3,4]benzo [1,2-b]oxete-6,12b(2aH)-diyl diacetate (8b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 21b (32 mg, 0.059 mmol), EDC∙HCl (11 mg, 0.059 mmol), DMAP (4 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0.05 M), 4 (50 mg, 0.059 mmol) is added to obtain 49 mg of product 8b with 61% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.6 Hz, 2H), 7.76–7.70 (m, 1H), 7.64–7.55 (m, 1H), 7.55–7.45 (m, 3H), 7.45–7.30 (m, 7H), 6.93 (d, J = 9.2 Hz, 1H), 6.54 (s, 1H), 6.37 (d, J = 1.6 Hz, 1H), 6.32–6.20 (m, 2H), 5.97 (dd, J = 9.2, 3.2 Hz, 1H), 5.68 (d, J = 7.0 Hz, 1H), 5.51 (d, J = 3.2 Hz, 1H), 4.97 (d, J = 9.8 Hz, 1H), 4.61–4.56 (m, 1H), 4.48–4.39 (m, 2H), 4.31 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.81 (d, J = 7.0 Hz, 1H), 3.46 (bs, 1H), 2.76–2.43 (m, 15H), 2.37 (dd, J = 15.3, 9.4 Hz, 1H), 2.21 (s, 3H), 2.20–1.95 (m, 8H), 1.96–1.92 (m, 3H), 1.92–1.69 (m, 8H), 1.67 (s, 3H), 1.62–1.50 (m, 6H), 1.35–1.25 (m, 4H), 1.22 (s, 3H), 1.13 (s, 3H), 0.86 (t, J = 6.6 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 172.0, 171.3, 169.9, 168.1, 167.2, 167.1, 142.8, 137.0, 133.8, 132.9, 132.1, 130.3, 129.3, 129.2, 128.8, 128.6, 127.2, 126.6, 117.2, 114.8, 84.6, 81.2, 79.2, 76.5, 75.7, 75.2, 74.2, 72.2, 72.0, 60.5, 58.6, 52.8, 45.7, 43.3, 38.1, 37.5, 37.1, 35.6, 35.5, 32.5, 32.0, 31.6, 30.6, 24.1, 24.0, 23.7, 22.8, 22.6, 22.2, 21.1, 20.9, 20.1, 14.9, 14.3, 14.1, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C76H91NO18S2Na, 1392.5575; found, 1392.5579.
3.2. Dynamic Light Scattering (DLS)
DLS measurements were carried out by a 90 plus particle size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA) equipped with a solid state He−Ne laser (wavelength = 661 nm). Experiments were carried out at a scattering angle of 90° on samples at 298 K. For both DLS and ζ-potential analysis, the purified samples were diluted in distilled water to a concentration of 200 µg/mL and briefly sonicated prior to the analysis. The results were expressed as mean ± standard deviation (SD) of three measurements.
3.3. Nanoparticle Preparation
Nanoparticle suspensions were prepared by a solvent displacement method [33]. Briefly, CBD containing analogs were dissolved in either ethanol (6a,b) or tetrahydrofuran (7a,b and 8a,b) (4 mg/mL) and the solution was added dropwise to ultrapure water under stirring in order to have a final aqueous suspension 2 mg/mL. Finally, the organic solvent was evaporated under reduced pressure.
3.4. Cell Cultures
MSTO-211H (human biphasic mesothelioma) and MeT-5A (human mesothelial) cells were grown in RPMI-1640 (R6504, Sigma Chemical Co.) modified by the addition of 2.38 g/L Hepes, 0.11 g/L pyruvate sodium and 2.5 g/L glucose. HT-29 (human colorectal adenocarcinoma) and HepG2 (human hepatocellular carcinoma) cell lines were grown in RPMI-1640 (R6504, Sigma Chemical Co.) and MEM (M0894, Sigma Chemical Co.), respectively. 10% heat-inactivated fetal calf serum (Biowest), 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B (Sigma Chemical Co.) were added to both media. The cells were cultured at 37 °C in a moist atmosphere of 5% carbon dioxide in air.
3.5. Inhibition Growth Assay
Cells (2.5–4 × 104) were seeded into each well of a 24-well cell culture plate. After incubation for 24 h in standard conditions, various concentrations of the test nanoparticles or reference drug were added, and cells were then incubated for a further 72 h. Cells reached about 80% confluence in control condition. Untreated cells and cells treated with vehicle alone were also taken into consideration as controls. The trypan blue exclusion assay was performed to determine cell viability. Cytotoxicity data were expressed as GI50 values, that is, the concentration of the test agent inducing 50% reduction in cell number compared with control cultures.
3.6. Evaluation of Cell Death by Annexin V-FITC and Propidium Iodide Staining
The cell death was detected by a FITC Annexin V Apoptosis Detection Kit I (BD Pharmigen). MSTO-211H cells (2.5 × 105) were seeded into each cell culture plate in complete growth medium. After incubation for 24 h, cells were treated with the test nanoparticle or the reference drug for a further 24 h so that in control condition about 50% confluence was reached. After treatment, cells were collected and resuspended in the supplied Binding Buffer at a density of at least 106 cells/mL. Cell suspensions (500 μL) were added with Annexin V-FITC and propidium iodide (PI) and incubated for 15 min at room temperature in the dark, as indicated by the supplier’s instructions. The viable (Annexin V-negative/PI-negative), early apoptotic (Annexin V-positive/PI-negative), late apoptotic (Annexin V-positive/PI-positive) and necrotic (Annexin V-negative/PI-positive) cells were analyzed by FACSAria III flow cytometer and evaluated by FACSDiva software (BectonDickinson, Mountain View, CA, USA).
3.7. Confocal Microscopy Analysis
MSTO-211H cells (2 × 104) were seeded on glass coverslips in 24-well plates and cultured until reached approximately 50% confluence. Cells were then incubated for a further 4 h in the presence of 100 μM tested nanoparticle or 1 μM paclitaxel, as reference drug. At the end of the experimental protocols, cells were washed twice with PBS, fixed with 4% formaldehyde for 15 min at room temperature, and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. Then, cells were blocked by the incubation in 3% fetal bovine serum in PBS for 30 min at room temperature, and stained with the antibody conjugate Alexa Fluor 488 mouse anti-β-tubulin (BD Pharmingen) for 1 h at room temperature. The coverslips were mounted on glass slides by using Mowiol 40–88 (Sigma, St Louis, MO, USA) added with 1 μg/mL DAPI (Sigma-Aldrich). Images were acquired through a ×60 CFI Plan Apochromat Nikon objectives with a Nikon C1 confocal microscope and finally analyzed using NIS Elements software (Nikon Instruments, Florence, Italy), NIH Image J and Adobe Photoshop CS4 version 11.
4. Conclusions
A series of conjugates (6–8A,B) where CBD was used as a self-assembly inducer coupled with three different anticancer drugs was synthesized and characterized. These conjugates are able to self-assemble forming NPs, confirming the ability of CBD to induce this aggregation. The obtained NPs were characterized both for their physico-chemical properties and their biological activity. The ability to exert an antiproliferative effect was evaluated on three human tumor cell lines (MSTO-211H, HT-29, and HepG2), obtaining GI50 values in the low micromolar range. In particular, all the NPs containing 4,4′-dithiodibutyric acid as the linker, characterized by the presence of a disulfide bond (6B, 7B, and 8B), are remarkably more effective in inducing cell death with respect to the corresponding NPs presenting sebacic acid as linker, as predictable due to their easier intracellular cleavage. Further biological assays were carried out on MSTO-211H cells for the most effective NP 8B, containing paclitaxel as the drug and 4,4′-dithiodibutyric acid as the linker, confirming the involvement of paclitaxel in cytotoxicity and cell death mechanism. This result supports the rationale of the approach, confirming the ability of the NP to address the drug inside the cell allowing its cytotoxic effect. In conclusion, these data further demonstrate the easy obtainment of self-assembled NPs by chemical functionalization of known anticancer drugs with a suitable self-assembly inducer, and of the possible modulation of their activity by varying the nature of the linker.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010112/s1, 1H and 13C spectra of all synthetized compounds.
Author Contributions
Conceptualization, D.P. and L.D.V.; methodology, L.D.V. and E.C.; formal analysis, L.P. and E.C.; investigation, E.C., D.A.C., M.H., M.L.D.P. and G.N.; resources, U.C. and G.P.; data curation, E.C, D.A.C. and L.D.V.; writing—original draft preparation, E.C., D.A.C. and L.D.V.; writing—review and editing, D.P., E.C. and D.A.C.; supervision, D.P. and L.D.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available contacting the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all compounds are available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fumagalli G., Marucci C., Christodoulou M.S., Stella B., Dosio F., Passarella D. Self-Assembly Drug Conjugates for Anticancer Treatment. Drug. Discov. Today. 2016;21:1321–1329. doi: 10.1016/j.drudis.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Fang F., Li L., Zhang J. Self-Assembled Organic Nanomaterials for Drug Delivery, Bioimaging, and Cancer Therapy. ACS Biomater. Sci. Eng. 2020;6:4816–4833. doi: 10.1021/acsbiomaterials.0c00883. [DOI] [PubMed] [Google Scholar]
- 3.Borrelli S., Christodoulou M.S., Ficarra I., Silvani A., Cappelletti G., Cartelli D., Damia G., Ricci F., Zucchetti M., Dosio F., et al. New Class of Squalene-Based Releasable Nanoassemblies of Paclitaxel, Podophyllotoxin, Camptothecin and Epothilone A. Eur. J. Med. Chem. 2014;85:179–190. doi: 10.1016/j.ejmech.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Borrelli S., Cartelli D., Secundo F., Fumagalli G., Christodoulou M.S., Borroni A., Perdicchia D., Dosio F., Milla P., Cappelletti G., et al. Self-Assembled Squalene-Based Fluorescent Heteronanoparticles. ChemPlusChem. 2015;80:47–49. doi: 10.1002/cplu.201402239. [DOI] [Google Scholar]
- 5.Fumagalli G., Mazza D., Christodoulou M.S., Damia G., Ricci F., Perdicchia D., Stella B., Dosio F., Sotiropoulou P.A., Passarella D. Cyclopamine-Paclitaxel-Containing Nanoparticles: Internalization in Cells Detected by Confocal and Super-Resolution Microscopy. ChemPlusChem. 2015;80:1380–1383. doi: 10.1002/cplu.201500156. [DOI] [PubMed] [Google Scholar]
- 6.Fumagalli G., Stella B., Pastushenko I., Ricci F., Christodoulou M.S., Damia G., Mazza D., Arpicco S., Giannini C., Morosi L., et al. Heteronanoparticles by Self-Assembly of Doxorubicin and Cyclopamine Conjugates. ACS Med. Chem. Lett. 2017;8:953–957. doi: 10.1021/acsmedchemlett.7b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fumagalli G., Christodoulou M.S., Riva B., Revuelta I., Marucci C., Collico V., Prosperi D., Riva S., Perdicchia D., Bassanini I., et al. Self-Assembled 4-(1,2-Diphenylbut-1-En-1-Yl)Aniline Based Nanoparticles: Podophyllotoxin and Aloin as Building Blocks. Org. Biomol. Chem. 2017;15:1106–1109. doi: 10.1039/C6OB02591A. [DOI] [PubMed] [Google Scholar]
- 8.Fumagalli G., Polito L., Colombo E., Foschi F., Christodoulou M.S., Galeotti F., Perdicchia D., Bassanini I., Riva S., Seneci P., et al. Self-Assembling Releasable Thiocolchicine-Diphenylbutenylaniline Conjugates. ACS Med. Chem. Lett. 2019;10:611–614. doi: 10.1021/acsmedchemlett.8b00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fumagalli G., Giorgi G., Vágvölgyi M., Colombo E., Christodoulou M.S., Collico V., Prosperi D., Dosio F., Hunyadi A., Montopoli M., et al. Heteronanoparticles by Self-Assembly of Ecdysteroid and Doxorubicin Conjugates to Overcome Cancer Resistance. ACS Med. Chem. Lett. 2018;9:468–471. doi: 10.1021/acsmedchemlett.8b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo E., Polito L., Biocotino M., Marzullo P., Hyeraci M., Dalla Via L., Passarella D. New Class of Betulinic Acid-Based Nanoassemblies of Cabazitaxel, Podophyllotoxin, and Thiocolchicine. ACS Med. Chem. Lett. 2020;11:895–898. doi: 10.1021/acsmedchemlett.9b00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frapporti G., Colombo E., Ahmed H., Assoni G., Polito L., Randazzo P., Arosio D., Seneci P., Piccoli G. Squalene-Based Nano-Assemblies Improve the Pro-Autophagic Activity of Trehalose. Pharmaceutics. 2022;14:862. doi: 10.3390/pharmaceutics14040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo E., Biocotino M., Frapporti G., Randazzo P., Christodoulou M.S., Piccoli G., Polito L., Seneci P., Passarella D. Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers. Pharmaceutics. 2019;11:422. doi: 10.3390/pharmaceutics11080422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ntungwe E., Domínguez-Martín E.M., Bangay G., Garcia C., Guerreiro I., Colombo E., Saraiva L., Díaz-Lanza A.M., Rosatella A., Alves M.M., et al. Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes. Int. J. Mol. Sci. 2021;22:10210. doi: 10.3390/ijms221910210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo E., Coppini D.A., Maculan S., Seneci P., Santini B., Testa F., Salvioni L., Vanacore G.M., Colombo M., Passarella D. Folic acid functionalization for targeting self-assembled paclitaxel-based nanoparticles. RSC Adv. 2022;12:35484. doi: 10.1039/D2RA06306A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mechoulam R., Parker L.A., Gallily R. Cannabidiol: An Overview of Some Pharmacological Aspects. J. Clin. Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 16.Thakkar K., Ruan C.H., Ruan K.H. Recent Advances of Cannabidiol Studies in Medicinal Chemistry, Pharmacology and Therapeutics. Future Med. Chem. 2021;13:1935–1937. doi: 10.4155/fmc-2021-0125. [DOI] [PubMed] [Google Scholar]
- 17.Seltzer E.S., Watters A.K., Mackenzie D., Granat L.M., Zhang D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers. 2020;12:3203. doi: 10.3390/cancers12113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solinas M., Massi P., Cinquina V., Valenti M., Bolognini D., Gariboldi M., Monti E., Rubino T., Parolaro D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE. 2013;8:e76918. doi: 10.1371/journal.pone.0076918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massi P., Vaccani A., Ceruti S., Colombo A., Abbracchio M.P., Parolaro D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. ASPET. 2004;308:838–845. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia L., Gallarate M. Lipid Nanoparticles: State of the Art, New Preparation Methods and Challenges in Drug Delivery. Expert. Opin. Drug. Deliv. 2012;9:497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 21.Chan J.Z., Duncan R.E. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells. 2021;10:1251. doi: 10.3390/cells10051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu N.-Q., Liu R., Colby A., de Forcrand C., Padera R.F., Grinstaff M.W., Colson Y.L. Paclitaxel-Loaded Expansile Nanoparticles Improve Survival Following Cytoreductive Surgery in Pleural Mesothelioma Xenografts. J. Thorac. Cardiovasc. Surg. 2020;160:e159–e168. doi: 10.1016/j.jtcvs.2019.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalzoppo D., Di Paolo V., Calderan L., Pasut G., Rosato A., Caccuri A.M., Quintieri L. Thiol-Activated Anticancer Agents: The State of the Art. Anticancer Agents Med. Chem. 2017;17:4–20. doi: 10.2174/1871520616666160817110310. [DOI] [PubMed] [Google Scholar]
- 24.Gallego-Jara J., Lozano-Terol G., Sola-Martínez R.A., Cánovas-Díaz M., Puente T.d.D. A Compressive Review about Taxol®: History and Future Challenges. Molecules. 2020;25:5986. doi: 10.3390/molecules25245986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao P.C., Lieu C.H. Cell Cycle Specific Induction of Apoptosis and Necrosis by Paclitaxel in the Leukemic U937 Cells. Life Sci. 2005;76:1623–1639. doi: 10.1016/j.lfs.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Wang T.-H., Wang H.-S., Soong Y.-K. Paclitaxel-Induced Cell Death. Cancer. 2000;88:2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::AID-CNCR26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Khing T.M., Choi W.S., Kim D.M., Po W.W., Thein W., Shin C.Y., Sohn U.D. The Effect of Paclitaxel on Apoptosis, Autophagy and Mitotic Catastrophe in AGS Cells. Sci. Rep. 2021;11:23490. doi: 10.1038/s41598-021-02503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brighenti V., Pellati F., Steinbach M., Maran D., Benvenuti S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis Sativa L. (Hemp) J. Pharm. Biomed. Anal. 2017;143:228–236. doi: 10.1016/j.jpba.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Marzullo P., Foschi F., Coppini D.A., Fanchini F., Magnani L., Rusconi S., Luzzani M., Passarella D. Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization. J. Nat. Prod. 2020;83:2894–2901. doi: 10.1021/acs.jnatprod.0c00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonandi E., Foschi F., Marucci C., Dapiaggi F., Sironi M., Pieraccini S., Christodoulou M.S., de Asís Balaguer F., Díaz J.F., Zidar N., et al. Synthesis of Thicolchicine-Based Conjugates: Investigation towards Bivalent Tubulin/Microtubules Binders. ChemPlusChem. 2019;84:98–102. doi: 10.1002/cplu.201800497. [DOI] [PubMed] [Google Scholar]
- 31.Passarella D., Comi D., Vanossi A., Paganini G., Colombo F., Ferrante L., Zuco V., Danieli B., Zunino F. Histone Deacetylase and Microtubules as Targets for the Synthesis of Releasable Conjugate Compounds. Bioorg. Med. Chem. Lett. 2009;19:6358–6363. doi: 10.1016/j.bmcl.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 32.Negretti S., Cohen C.M., Chang J.J., Guptill D.M., Davies H.M.L. Enantioselective Dirhodium(II)-Catalyzed Cyclopropanations with Trimethylsilylethyl and Trichloroethyl Aryldiazoacetates. Tetrahedron. 2015;71:7415–7420. doi: 10.1016/j.tet.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolas J., Mura S., Brambilla D., Mackiewicz N., Couvreur P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/Biocompatible Polymer -Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013;42:1147–1235. doi: 10.1039/C2CS35265F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available contacting the corresponding authors upon reasonable request.