Abstract
A trypsin-like serine peptidase activity, levels of which correlate with blood parasitemia levels, is present in the plasma of rats acutely infected with Trypanosoma brucei brucei. Antibodies to a trypanosome peptidase with a trypsin-like substrate specificity (oligopeptidase B [OP-Tb]) cross-reacted with a protein in the plasma of trypanosome-infected rats on a Western blot. These antibodies also abolished 80% of the activity in the plasma of trypanosome-infected rats, suggesting that the activity may be attributable to a parasite-derived peptidase. We purified the enzyme responsible for the bulk of this activity from parasite-free T. b. brucei-infected rat plasma and confirmed its identity by protein sequencing. We show that live trypanosomes do not release OP-Tb in vitro and propose that disrupted parasites release it into the host circulation, where it is unregulated and retains full catalytic activity and may thus play a role in the pathogenesis of African trypanosomiasis.
African trypanosomes, genus Trypanosoma, are protozoan parasites that cause widespread disease in livestock and humans. Africa is currently experiencing a resurgence in the incidence of both forms of this disease, and in some areas the mortality related to human African trypanosomiasis is estimated to be the same as that caused by AIDS (3). The reemergence of African trypanosomiasis as a public health and agricultural threat has prompted renewed interest in the identification of novel virulence factors to expand the understanding of the pathogenesis of this group of diseases and to facilitate drug and vaccine development.
Peptidases are widely implicated as virulence factors and chemotherapeutic targets in parasitic diseases (13). Although peptidase activities in the plasma of Trypanosoma brucei brucei-infected rodents have been reported (5, 11), and the extracellular release of cysteine peptidase by T. b. brucei in vitro has been demonstrated (1, 12), no parasite-derived peptidase that is released into the host has ever been identified. We report here the identification of the enzyme responsible for up to 80% of the trypsin-like hydrolytic activity observed in the plasma of rats infected with T. b. brucei. Based on partial amino acid sequencing and kinetic and immunochemical characterization of the peptidase, we have identified it as T. b. brucei oligopeptidase B (OP-Tb) (the opdB gene product [8]).
A serine oligopeptidase activity is present in T. b. brucei-infected rat plasma.
Adult male Sprague-Dawley rats (n = 3) were infected intraperitoneally with T. b. brucei ILTat1.1 (106 trypanosomes per rat). At peak bloodstream parasitemia (∼3 × 108 trypanosomes · ml−1), rats were euthanatized by ether asphyxiation. Blood was harvested by cardiac puncture, diluted 1:1 with 57 mM Na2HPO4–3 mM NaH2PO4–44 mM NaCl–56 mM d-(+)-glucose–0.1 mM hypoxanthine–2% (m/vol) sodium citrate, pH 7.4 (PSGT), and centrifuged (1,500 × g, 5 min, 25°C), and supernatants were confirmed to be trypanosome free by light microscopy. An Nα-carbobenzyloxy-l- arginyl-l-arginyl-7-amido-4-methylcoumarin (Cbz-Arg-Arg-AMC)-hydrolyzing activity that was minimal in healthy, uninfected rats was detected in the plasma of infected rats (Table 1). This activity was sensitive to the low-molecular-mass serine peptidase inhibitors 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) and 3,4-dichloroisocoumarin and insensitive to the cysteine peptidase inhibitor E-64, implicating a serine peptidase. This activity was also insensitive to the high-molecular-mass serine peptidase inhibitors soybean trypsin inhibitor and turkey ovomucoid, suggesting that the enzyme was an oligopeptidase. Purified trypanosomes subjected to the same centrifugation steps in a variety of media did not liberate Cbz-Arg-Arg-AMC-hydrolyzing activity, nor did they liberate acetylesterase or acid phosphatase activity, two intracellular markers (15) which would be expected if trypanosomes were damaged during the process (results not shown). This suggests that the processing of the plasma did not cause artifactual release of peptidase activity from the trypanosomes. The kinetic profile of the activity identified in T. b. brucei-infected rat plasma (Table 1) was not unlike that of a trypanosome serine oligopeptidase (OP-Tb) that has been cloned and characterized from T. b. brucei (8). Since chicken egg yolk (IgY) antibodies to OP-Tb were available, we then probed infected rat plasma for OP-Tb by Western blotting.
TABLE 1.
Detection of OP-Tb activity in the bloodstream of infected rodents
| Inhibitora | Mean activityb (AFU · min−1 · 25 μl of serum−1) ± SD
|
|
|---|---|---|
| Uninfected | Infected | |
| None | 220 ± 28 | 2,247 ± 88 |
| E-64 (10 μM) | 188 ± 17 | 2,355 ± 109 |
| Soybean trypsin inhibitor (100 μg · ml−1) | 169 ± 21 | 2,219 ± 199 |
| Turkey ovomucoid (100 μg · ml−1) | 166 ± 12 | 2,303 ± 202 |
| AEBSF (1 mM) | 145 ± 12 | 166 ± 7 |
| 3,4-Dichloroisocoumarin (1 mM) | 161 ± 11 | 154 ± 9 |
Inhibitor concentrations reflect the concentrations required for maximum inhibition of Cbz-Arg-Arg-AMC-hydrolytic activity in the sample. Increasing the inhibitor concentrations by up to double those reported here did not increase the degree of inhibition observed.
Trypanosome-free plasma from acutely infected rats and plasma from healthy rats was assayed for activity against Cbz-Arg-Arg-AMC (5 μM) in 50 mM Tris-HCl–1 mM DTT, pH 8, at 37°C.
Anti-OP-Tb antibodies cross-react with an 80-kDa protein in T. b. brucei-infected rat plasma on a Western blot.
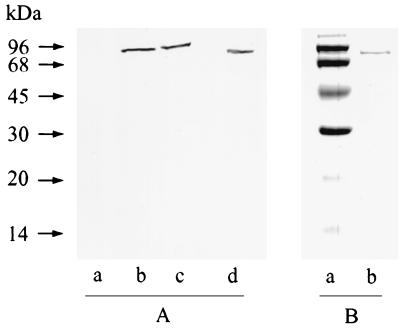
Plasma was partially fractionated by three-phase partitioning (TPP) by dilution to 100 ml with 0.1% (m/vol) Brij 35 and addition of t-butanol (44 ml) to 30% (vol/vol) in 144 ml. Solid (NH4)2SO4 (14.4 g) was added and the suspension was centrifuged (10,000 × g, 10 min, 25°C) to yield a 0 to 10% (NH4)2SO4 TPP fraction. The interfacial pellet was discarded, and a 10 to 25% (NH4)2SO4 TPP fraction was prepared by adding solid (NH4)2SO4 (21.6 g) to the aqueous phase-organic phase mixture and recentrifuging. The 10 to 25% (NH4)2SO4 TPP fraction was resuspended in 50 mM Tris-HCl–1 mM dithiothreitol (DTT), pH 8 (buffer A; 40 ml), and applied to a para-aminobenzamidine–Sepharose column (120 by 15 mm, 1 ml · min−1) equilibrated in buffer A. Bound protein, eluted with 250 mM NaCl in buffer A, was resolved by Tris-Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and probed for immunoreactive OP-Tb by Western blotting with anti-OP-Tb IgY as described in reference 7. Fractionated plasma from T. b. brucei-infected rats yielded a single strong band on a Western blot (Fig. 1A, lanes b and c), whereas no band was evident in similarly treated plasma from a healthy rat (Fig. 1A, lane a). These bands correspond to those seen for purified OP-Tb (Fig. 1A, lane d). These data illustrate that immunoreactive OP-Tb is present in the plasma of T. b. brucei-infected rats and not in the plasma of uninfected rats. We now attempted to address whether the OP-Tb we had detected by Western blotting in infected rat plasma was responsible for the Cbz-Arg-Arg-AMC-hydrolytic activity.
FIG. 1.
Detection of OP-Tb in the bloodstream of T. b. brucei-infected rats. (A) Detection of OP-Tb in infected rat plasma by Western blotting. Lanes: a, noninfected control plasma; b and c, plasma from two different experimentally infected rats; d, OP-Tb isolated from T. b. brucei (50 ng). (B) OP-Tb isolated from the plasma of infected rodents was resolved by Tris-Tricine-SDS-PAGE. Lanes: a, molecular mass markers; b, OP-Tb isolated from infected rat plasma (1 μg).
Anti-OP-Tb antibodies neutralize Cbz-Arg-Arg-AMC-hydrolytic activity in T. b. brucei-infected rat plasma.
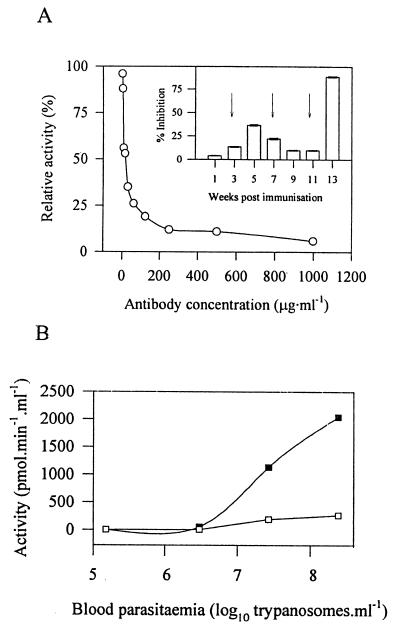
Although we detected OP-Tb in T. b. brucei-infected rat plasma by Western blotting, it was not known whether the OP-Tb was catalytically active and thus responsible for the observed Cbz-Arg-Arg-AMC-hydrolytic activity. Several host-derived plasma peptidases (including members of the coagulation and complement systems) can also hydrolyze Cbz-Arg-Arg-AMC, as can mast cell tryptase, which would probably be produced during the inflammatory response to an infection. We thus required a tool that could selectively neutralize OP-Tb activity in whole plasma. Since polyclonal antibodies to enzymes can abrogate enzyme activity, we evaluated IgY fractions, obtained over the OP-Tb immunization protocol described in reference 7, for their ability to inhibit the activity of OP-Tb (25 ng · ml−1, purified as described in reference 8) against 5 μM Cbz-Arg-Arg-AMC in 50 mM Tris-HCl, pH 8. IgY preparations inhibited OP-Tb activity against Cbz-Arg-Arg-AMC to various degrees (Fig. 2A, inset). Week 13 IgY produced 92% inhibition of OP-Tb activity at 250 μg · ml−1, and the inhibitory activity of week 13 IgY was titrated out with half-maximal inhibition at 7.5 μg · ml−1 (Fig. 2A). This represents some of the most potent activity-neutralizing antibodies described to date. Week 13 anti-OP-Tb IgY had no effect (at 250 μg · ml−1) on the hydrolytic activity of several mammalian serine peptidases, including neutrophil elastase, plasmin, thrombin, factor Xa, plasma kallikrein, trypsin, chymotrypsin, and mast cell tryptase (results not shown). The selective inhibition of OP-Tb by these antibodies provided us with a tool with which to characterize the potential OP-Tb activity in T. b. brucei-infected rat plasma.
FIG. 2.
Characterization of plasma OP-Tb activity with anti-OP-Tb IgY. (A) OP-Tb activity-neutralizing activity of anti-OP-Tb IgY. Week 13 anti-OP-Tb IgY was tested at various concentrations for inhibitory activity against the OP-Tb-catalyzed hydrolysis of Cbz-Arg-Arg-AMC (5 μM) in 50 mM Tris-HCl, pH 8, at 37°C. (Inset) Generation of inhibitory antibodies over the immunization period. Antibodies harvested at various times after the primary immunization were tested for their ability to inhibit OP-Tb activity against Cbz-Arg-Arg-AMC. Antibodies were tested at an assay concentration of 250 μg · ml−1, and their effect was compared to the effect of preimmune (control) antibodies at the same assay concentration. Arrows indicate booster immunizations. Values are means ± standard deviations (n = 3). (B) Kinetics of OP-Tb release into the plasma of infected rodents. Blood from T. b. brucei-infected rats was drawn daily and evaluated for Cbz-Arg-Arg-AMC-hydrolyzing activity and parasitemia. Activity is represented as picomoles of AMC released per minute per milliliter of whole blood in the presence of preimmune control (■) and week 13 anti-OP-Tb IgY (□) at 250 μg · ml−1. While the data here are depicted for a single animal, they are representative of the trend observed in two other animals.
Rats (n = 3) were infected as described above. Blood (∼125 μl) was drawn daily from the tail vein into an equal volume of PSGT and centrifuged (1,500 × g, 5 min, 25°C). Hydrolytic activity against Cbz-Arg-Arg-AMC was observed in trypanosome-free plasma of infected rats on day 4, at ∼4 × 106 trypanosomes · ml−1 (Fig. 2B), followed by a steady increase in parasitemia and plasma Cbz-Arg-Arg-AMC-hydrolyzing activity. Activity was completely inhibited by 1 mM AEBSF (results not shown) and was abolished by up to 80% in the presence of anti-OP-Tb IgY (250 μg · ml−1) relative to activity determined in the presence of preimmune IgY at the same concentration (Fig. 2B). These data illustrate that the bulk of the Cbz-Arg-Arg-AMC hydrolytic activity in T. b. brucei-infected rat plasma is most likely attributable to trypanosome-derived OP-Tb. To unequivocally identify the responsible peptidase, we then sought to purify the Cbz-Arg-Arg-AMC-hydrolyzing factor from T. b. brucei-infected rat plasma.
Purification and sequencing of the peptidase from the plasma of T. b. brucei-infected rats.
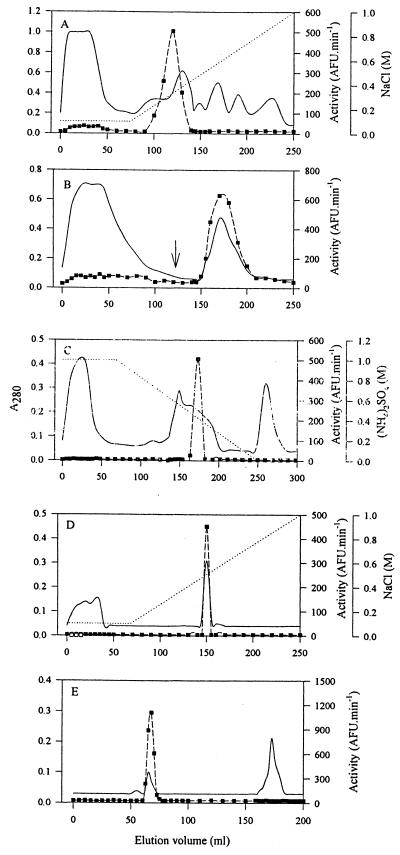
The peptidase was purified 16,336-fold from parasite-free T. b. brucei-infected rat plasma, with a 34% yield (Table 2). Plasma (47 ml) from six rats was diluted to 100 ml with 0.1% (m/vol) Brij 35, and a 10 to 25% (NH4)2SO4 TPP fraction was prepared as described above. The fraction was resuspended in 30 mM NaH2PO4–1 mM DTT, pH 6.8 (30 ml), and dialyzed against the same buffer (2 × 4,000 ml, 16 h, 4°C). During dialysis, some protein precipitated. This was removed by centrifugation (15,000 × g, 30 min, 4°C), and the resultant supernatant (46 ml) was loaded onto a Cibacron blue F3GA-Sepharose column (200 by 25 mm, 0.8 ml · min−1) equilibrated in 30 mM NaH2PO4, pH 6.8. The Cibacron blue F3GA-Sepharose flowthrough fraction, containing all the Cbz-Arg-Arg-AMC-hydrolyzing activity, was dialyzed against 50 mM Tris-HCl–100 mM NaCl–1 mM DTT (buffer B; 2,000 ml, 4 h, 4°C) and applied to a Q-Sepharose column (26 by 100 mm, 1 ml · min−1) equilibrated in buffer B. Bound material was eluted with a linear gradient of 0.1 to 1 M NaCl in buffer B, over five column volumes (Fig. 3A). Eluted fractions displaying activity against Cbz-Arg-Arg-AMC were pooled, dialyzed (2,000 ml, 4 h, 4°C) against buffer A, and loaded onto a para-aminobenzamidine–Sepharose column (120 by 15 mm, 1 ml · min−1) equilibrated in buffer A. Bound protein was eluted in a single step with 250 mM NaCl in buffer A (Fig. 3B). The active fraction from para-aminobenzamidine–Sepharose (44 ml) was made to 1 M (NH4)2SO4 and loaded onto a phenyl-Sepharose column (100 by 25 mm, 1 ml · min−1) equilibrated in 1 M (NH4)2SO4 in buffer A. Bound protein was eluted with a linear gradient of 1.0 to 0 M (NH4)2SO4 in buffer A over 10 column volumes (Fig. 3C), and the eluted active fractions were pooled (30 ml). The sample was dialyzed against buffer B (2,000 ml, 4 h, 4°C) and applied to a poly-l-lysine–Sepharose column (90 by 15 mm, 1 ml · min−1) equilibrated in buffer B. Bound protein was eluted with a linear gradient of 0.1 to 1.0 M NaCl in buffer B (Fig. 3D). The active fractions eluted from poly-l-lysine–Sepharose were pooled (20 ml), concentrated by ultrafiltration in polysulfone concentrators to 2 ml, and applied to a Sephacryl S-100 HR column (900 by 15 mm, 0.32 ml · min−1) equilibrated in buffer A (Fig. 3E). The pooled active fractions yielded a single 80-kDa band by reducing Tris-Tricine-SDS-PAGE (Fig. 1B, lane b). Two peptides yielded by endoproteinase Lys-C digests of the purified protein were purified by high-pressure liquid chromatography, and subjected to N-terminal sequence analysis. The sequences of these two peptides, TPGEGEDEEIVLD and MDLESGHFSASDR, match exactly residues 124 to 136 and 677 to 689, respectively, of the deduced amino acid sequence of the T. b. brucei opdB gene (8).
TABLE 2.
Purification of OP-Tb from T. b. brucei-infected rat plasma
| Step | Protein (mg) | Total activitya (pmol · s−1) | Sp act (pmol · s−1 · mg−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Plasma | 2,688 | 1,986 | 0.74 | 1 | 100 |
| 10–25% TPP fraction | 1,506 | 1,109 | 0.74 | 1 | 56 |
| Cibacron blue F3GA- Sepharose | 442 | 1,071 | 2.42 | 3 | 54 |
| Q-Sepharose | 38 | 774 | 20.37 | 28 | 39 |
| para-Aminobenzamidine– Sepharose | 1.211 | 700 | 578.04 | 781 | 35 |
| Phenyl-Sepharose | 0.138 | 712 | 5,159.42 | 6,972 | 36 |
| Poly-l-lysine–Sepharose | 0.096 | 700 | 7,291.67 | 9,854 | 35 |
| Sephacryl S-100 HR | 0.056 | 677 | 12,089.29 | 16,336 | 34 |
Activity was determined against Cbz-Arg-Arg-AMC (5 μM) in 50 mM Tris-HCl–1 mM DTT, pH 8, at 37°C.
FIG. 3.
Purification of OP-Tb from T. b. brucei-infected rat plasma. After processing by TPP and Cibacron blue F3GA-Sepharose chromatography, plasma was subjected to chromatography on Q-Sepharose at pH 8 (A), para-aminobenzamidine–Sepharose (B), phenyl-Sepharose (C), poly-l-lysine–Sepharose (D), and Sephacryl S-100 HR (E). —, A280; ■, activity against Cbz-Arg-Arg-AMC; ⋯, salt gradient. Application of a step gradient is indicated by an arrow.
Why does OP-Tb remain catalytically active in the host bloodstream, despite an abundance of serine peptidase inhibitors? It has previously been shown that OP-Tb isolated directly from trypanosomes was not inhibited by mammalian plasma serpins (17). OP-Tb activity is therefore unregulated after release into the bloodstream. Furthermore, OP-Tb neither binds to nor is inhibited by α2-macroglobulin (17), and therefore it is not subject to the same clearance mechanisms as other peptidases when released into the blood (4). OP-Tb would thus be cleared more slowly from the host bloodstream than would other exogenous peptidases, and this may account for its persistence in the host circulation.
T. b. brucei does not release OP-Tb activity in vitro.
How does OP-Tb get out of the trypanosomes and into the host bloodstream? To address this question, we incubated trypomastigote-form T. b. brucei (108 cells · ml−1, 37°C) as described in reference 9. Aliquots (1 ml) were removed at 30-min intervals for 4 h and centrifuged (1,500 × g, 2 min, 25°C). No Cbz-Arg-Arg-AMC-hydrolyzing activity was identified in cell-free culture supernatants. However, Cbz-Arg-Arg-AMC-hydrolyzing activity was detected in the Triton X-100-solubilized pellet fraction from the same cultures (results not shown). These results indicate that there was no observable release of OP-Tb by live trypanosomes over the time frame of the experiments. This is consistent with observations that the deduced amino acid sequence of the opdB gene does not contain any known secretion sequences (8) and that the OP-Tb homologue in Trypanosoma cruzi appears, by electron microscopy, to be cytosolic (2). It seems most likely that OP-Tb is released in vivo by dead or dying trypanosomes that are lysed in the host circulation by the various antimicrobial host defense mechanisms (e.g., the alternative pathway of complement activation).
OP-Tb retains full catalytic activity after release into the host plasma.
Kinetic analysis of OP-Tb isolated from infected rat plasma was undertaken as described in reference 8. Km and kcat values for the hydrolysis of Cbz-Arg-Arg-AMC (Km = 250 nM, kcat = 121 s−1), t-butoxycarbonyl-Leu-Arg-Arg-AMC (Km = 0.97 μM, kcat = 77.1 s−1), Cbz-Gly-Gly-Arg-AMC (Km = 1.11 μM, kcat = 150 s−1), and Cbz-Phe-Arg-AMC (Km = 1.3 μM, kcat = 80.8 s−1) approximated (within 25%) values reported for OP-Tb isolated from trypanosomes (8). Thus, after release into the host plasma, neither the substrate specificity (as reflected by the Km) nor the catalytic power (as reflected by the kcat) of OP-Tb was altered. OP-Tb released into the host plasma had a pH optimum of 9 and remained 75% active at physiological pH, compared to activity at pH 9 (results not shown). Furthermore, it has been shown that OP-Tb isolated from trypanosomes is maximally stable at pH 7.5 (8), indicating that the host bloodstream is an ideal catalytic environment for OP-Tb.
Implications of the extracellular release of OP-Tb.
We illustrate here that OP-Tb is released by T. b. brucei into the plasma of infected rats, where it persists and retains full catalytic activity. One implication of a foreign, highly catalytic (kcat > 120 s−1) peptidase accumulating in the host plasma is that it is in an ideal position to influence the dynamics of biologically active peptides in the bloodstream. The anomalous degradation of peptide hormones in host tissues would seriously impair host metabolic homeostasis. The potential importance of this is underscored by the observed reduction in the levels of one peptide hormone, atrial natriuretic factor, in the plasma of T. b. brucei-infected dogs (10). Atrial natriuretic factor is a substrate for OP-Tb in vitro (17), but it is not known whether the observed depletion in the levels of this hormone in plasma are due to OP-Tb activity. Support for this idea is also lent by reports of the unusual cleavage of peptide hormones added exogenously to the serum of T. b. brucei-infected rats (16), where investigators concluded that the activity was due to a trypanosome-derived serine peptidase. OP-Tb has similar properties; however, the activity reported in reference 16 was partially inhibited by 3.5 mM EDTA and 5 mM α-dl-difluoromethylornithine. Since OP-Tb is not inhibited by EDTA (8) or α-dl-difluoromethylornithine (6) it is possible that the effects of multiple peptidases were being observed.
Indirect support for a role for OP-Tb in the pathology of trypanosomiasis comes from the recent report that administration of OP-Tb inhibitors improved the survival rate of mice infected with T. b. brucei (9). However, there was no significant correlation between OP-Tb-inhibitory potency and in vitro antitrypanosomal efficacy of these inhibitors. Considering the data presented in this report, these inhibitors may have been acting on OP-Tb released into the plasma of the infected rodents, rather than on OP-Tb in the parasite cytosol, and, in doing so, they influenced the course of the infection.
This study represents the first report of the conclusive identification of any parasite-derived peptidase that is released into the host system, and as such, it lends critical support to the emergence of peptidases as important virulence factors and chemotherapeutic targets in trypanosome infections.
Acknowledgments
This study received financial support from the South African National Research Foundation (grant 2034170), the University of Natal Research Fund, and the Deutsche Bundesministerium für Bildung und Forschung (grant 39.6.60B.6.B).
Amino acid sequencing work was carried out (by R.M.) in the laboratory of Freiderich Lottspeich (Max Planck Institute for Biochemistry, Martinsreid, Germany). We thank Lawrence B. Schwartz (Virginia Commonwealth University, Richmond) for the gift of human mast cell tryptase and Norma W. Andrews (Yale University School of Medicine, New Haven, Conn.), Barbara A. Burleigh (Harvard School of Public Health, Boston, Mass.), Elaine Del Nery (Institute Curie, Paris, France), and Denis J. Grab (Kurume University School of Medicine, Kurume, Japan) for critical reading of the manuscript.
REFERENCES
- 1.Boutignon F, Huet-Duvillier G, Demeyer D, Richet C, Degand P. Studies of proteolytic activities released by incubation of trypanosomes (Trypanosoma brucei brucei) in pH 5.0 and pH 7.0 phosphate/glucose buffers. Biochim Biophys Acta. 1990;1035:369–377. doi: 10.1016/0304-4165(90)90102-3. [DOI] [PubMed] [Google Scholar]
- 2.Burleigh B A, Caler E V, Webster P, Andrews N W. A cytosolic serine endopeptidase from Trypanosoma cruzi is involved in the generation of Ca2+ signalling in mammalian cells. J Cell Biol. 1997;136:609–620. doi: 10.1083/jcb.136.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekwanzala M, Pépin J, Khonde N, Molisho S, Bruneel H, De Wals P. In the heart of darkness: sleeping sickness in Zaïre. Lancet. 1996;348:1427–1430. doi: 10.1016/S0140-6736(96)06088-6. [DOI] [PubMed] [Google Scholar]
- 4.Gliemann J, Larsen T R, Sottrup-Jensen L. Cell-association and degradation of α2M-trypsin complexes in hepatocytes. Biochim Biophys Acta. 1983;756:230–237. doi: 10.1016/0304-4165(83)90096-x. [DOI] [PubMed] [Google Scholar]
- 5.Knowles G, Black S J, Whitelaw D D. Peptidase in the plasma of mice infected with Trypanosoma brucei brucei. Parasitology. 1987;95:291–300. doi: 10.1017/s0031182000057747. [DOI] [PubMed] [Google Scholar]
- 6.Morty R E, Troeberg L, Pike R N, Jones R, Nickel P, Lonsdale-Eccles J D, Coetzer T H T. A trypanosome oligopeptidase as a target for the trypanocidal agents pentamidine, diminazene and suramin. FEBS Lett. 1998;433:251–256. doi: 10.1016/s0014-5793(98)00914-4. [DOI] [PubMed] [Google Scholar]
- 7.Morty R E, Authié E, Troeberg L, Lonsdale-Eccles J D, Coetzer T H T. Purification and characterisation of a trypsin-like serine oligopeptidase from Trypanosoma congolense. Mol Biochem Parasitol. 1999;102:145–155. doi: 10.1016/s0166-6851(99)00097-3. [DOI] [PubMed] [Google Scholar]
- 8.Morty R E, Lonsdale-Eccles J D, Morehead J, Caler E V, Mentele R, Auerswald E A, Coetzer T H T, Andrews N W, Burleigh B A. Oligopeptidase B from Trypanosoma brucei brucei: a new member of an emerging subgroup of serine oligopeptidases. J Biol Chem. 1999;274:26149–26156. doi: 10.1074/jbc.274.37.26149. [DOI] [PubMed] [Google Scholar]
- 9.Morty R E, Troeberg L, Powers J C, Ono S, Lonsdale-Eccles J D, Coetzer T H T. Characterisation of the antitrypanosomal activity of peptidyl α-aminoalkyl phosphonate diphenyl esters. Biochem Pharmacol. 2000;60:1497–1504. doi: 10.1016/s0006-2952(00)00459-7. [DOI] [PubMed] [Google Scholar]
- 10.Ndung'u J M, Wright N, Jennings F, Murray M. Changes in atrial natriuretic factor in dogs infected with Trypanosoma brucei brucei. Parasitol Res. 1992;78:553–558. doi: 10.1007/BF00936451. [DOI] [PubMed] [Google Scholar]
- 11.Nwagwu M, Okenu D M N, Olusi T A, Molokwu R I. Trypanosoma brucei releases proteases extracellularly. Trans R Soc Trop Med Hyg. 1988;82:577. doi: 10.1016/0035-9203(88)90515-9. [DOI] [PubMed] [Google Scholar]
- 12.Okenu D M N, Opara K N, Nwuba R I, Nwagwu M. Purification and characterisation of an extracellularly released protease of Trypanosoma brucei. Parasitol Res. 1999;85:424–428. doi: 10.1007/s004360050571. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal P J. Proteases of protozoan parasites. Adv Parasitol. 1999;43:105–159. doi: 10.1016/s0065-308x(08)60242-0. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz L B, Bradford T R. Regulation of tryptase from human lung mast cells by heparin: stabilisation of the active tetramer. J Biol Chem. 1986;261:7372–7379. [PubMed] [Google Scholar]
- 15.Steiger R, Opperdoes F R, Bontemps J. Subcellular fractionation of Trypanosoma brucei bloodstream-forms. Eur J Biochem. 1980;105:163–175. doi: 10.1111/j.1432-1033.1980.tb04486.x. [DOI] [PubMed] [Google Scholar]
- 16.Tetaert D, Soudan B, Huet-Duvillier G, Degand P, Boersma P. Unusual cleavage of peptidic hormones generated by trypanosome enzymes released in infested rat serum. Int J Pept Protein Res. 1993;41:147–152. doi: 10.1111/j.1399-3011.1993.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 17.Troeberg L, Pike R N, Morty R E, Berry R K, Coetzer T H T, Lonsdale-Eccles J D. Proteases from Trypanosoma brucei brucei: purification, characterisation and interactions with host regulatory molecules. Eur J Biochem. 1996;238:728–736. doi: 10.1111/j.1432-1033.1996.0728w.x. [DOI] [PubMed] [Google Scholar]