Abstract
Saccharomyces boulardii is a nonpathogenic yeast that protects against antibiotic-associated diarrhea and recurrent Clostridium difficile colitis. The administration of C. difficile toxoid A by gavage to S. boulardii-fed BALB/c mice caused a 1.8-fold increase in total small intestinal immunoglobulin A levels (P = 0.003) and a 4.4-fold increase in specific intestinal anti-toxin A levels (P < 0.001). Enhancing host intestinal immune responses may be an important mechanism for S. boulardii-mediated protection against diarrheal illnesses.
Clostridium difficile is the most common known cause of nosocomial infectious diarrhea in the developed world (18, 19, 27, 28, 37). Pathogenic strains of C. difficile produce two large protein exotoxins, toxin A and toxin B (41). Toxin A is a 308-kDa cytotoxin and enterotoxin that induces marked intestinal inflammation, fluid secretion, and mucosal injury (12, 23, 41). Toxin B, a 270-kDa protein, stimulates the release of inflammatory cytokines from monocytes and is cytotoxic to mammalian cells (2, 21, 22, 41). Toxin A appears to be the main cause of intestinal injury and inflammation in animal models of C. difficile ileocolitis (25, 41). However, toxin B may also cause injury to human colon (43).
Saccharomyces boulardii is a nonpathogenic yeast used to prevent or treat infectious diarrhea of many etiologies (14). In animal studies, S. boulardii protects against diarrhea and enterocolitis induced by a variety of enteric pathogens, including C. difficile (4, 5, 7–10, 13, 36, 42, 48, 49). In human studies, S. boulardii treatment significantly reduced the incidence of simple antibiotic-associated diarrhea (38, 46). S. boulardii also reduced the risk of subsequent relapse in patients with a history of multiple episodes of C. difficile diarrhea (20, 30, 39, 47).
The host's immune response to C. difficile toxins is now known to play a major role in determining disease expression (24, 26, 29, 31, 32, 33, 50). High titers of serum or intestinal antibodies against toxin A have been associated with asymptomatic carriage of toxigenic C. difficile and with shorter and less severe episodes of C. difficile diarrhea (32, 33, 34, 40, 45, 51, 52). Buts and colleagues found that S. boulardii significantly increased the secretion of immunoglobulin A (IgA) and secretory component in rat small intestine, but they did not study the specificity of the secretory IgA response (3). Based on these findings, we hypothesized that one mechanism whereby S. boulardii may protect against infection by C. difficile and other enteric pathogens is through a stimulation of the host's intestinal mucosal immune response (1, 14). The aim of this study was to examine this hypothesis by determining whether S. boulardii treatment altered serum or intestinal anti-toxin A antibody production in mice exposed to C. difficile toxin A.
C. difficile toxin A was purified from culture supernatants of strain VPI 10463 (American Type Culture Collection, Rockville, Md.) and inactivated by overnight incubation with 1% formaldehyde followed by ultrafiltration (5, 6, 42). For most experiments, BALB/c mice were immunized with formalin-inactivated toxoid A (100 μg) administered by gavage on days 0 and 7, and animals were sacrificed on day 21. S. boulardii (Biocodex Laboratories, Montrouge, France) was administered in the drinking water (3 × 108 CFU per ml) from the time of the first oral immunization until the time of sacrifice. In experiments that compared the mucosal adjuvant effects of S. boulardii to those of C. difficile toxoid A and/or cholera toxin (10 μg; Calbiochem, San Diego, Calif.) was administered on days 0, 7, 14, and 21, and the animals were sacrificed on day 35 (15–17, 53). After sacrificing the animals, the small intestine from the pylorus to the cecum was immediately excised and the intestinal contents were harvested by gently wrapping the small intestine around a Pasteur pipette. An equal volume of phosphate-buffered saline containing protease inhibitors was added (Protease Inhibitors-Complete; Boehringer, Mannheim, Germany), the samples were centrifuged, and the supernatants were collected and stored at −80°C.
Antibodies against C. difficile toxin A were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (29, 30, 34, 52). Briefly, microtiter plates (Polysorp; Nunc, Roskilde, Denmark) were coated with purified toxin A (0.5 μg/ml). Intestinal and serum samples were assayed at a 1:50 dilution. Peroxidase-labeled goat anti-mouse IgA (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was used to determine intestinal and serum IgA anti-toxin A. Peroxidase-labeled anti-mouse IgM (Kirkegaard and Perry) and biotinylated goat anti-mouse IgG (Sigma, St. Louis, Mo.) were used to determine serum IgM and IgG anti-toxin A, respectively. Antibody levels are reported as the mean optical density of triplicate samples. To measure total intestinal IgA, microtiter plates (Immunosorp; Nunc) were coated with purified anti-mouse IgA (0.5 μg/ml; Sigma) and intestinal samples were assayed at a 1:50,000 dilution. Purified mouse IgA (Pharmingen, San Diego, Calif.) was used as the standard.
Statistical analyses were performed using SigmaStat for Windows (version 2.0; Jandel Scientific Software, San Rafael, Calif.). Analysis of variance (ANOVA) on ranks and pairwise intergroup comparisons by Dunn's method were used. A P value of <0.05 was considered statistically significant.
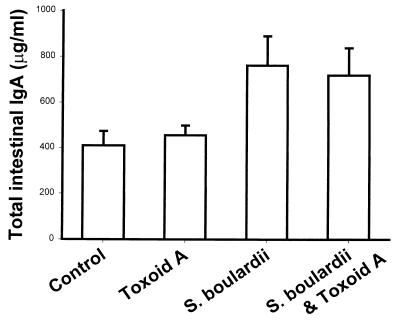
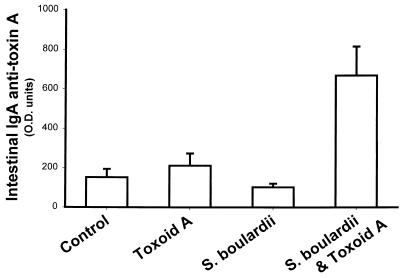
BALB/c mice treated with S. boulardii had a small but statistically significant increase (P = 0.003) in total IgA levels in their small intestine secretions (Fig. 1). In the S. boulardii-fed group, mean IgA levels were 1.9-fold higher than in controls. A similar, 1.8-fold, increase in mean total IgA levels was seen in mice who received both S. boulardii and toxoid A. We next examined whether S. boulardii treatment could stimulate an intestinal immune response to C. difficile toxin A. Small intestinal IgA anti-toxin A levels were low in control mice, in mice that were immunized orally with toxoid A in the absence of a mucosal adjuvant, and in mice that were treated with S. boulardii but not exposed to toxoid A (Fig. 2). However, when mice were immunized orally with toxoid A during treatment with S. boulardii, there was a marked (4.4-fold) and highly significant (P < 0.001) increase in specific small intestinal IgA anti-toxin A levels.
FIG. 1.
S. boulardii increases total IgA levels in small intestinal secretions. Mice were immunized with formalin-inactivated toxoid A administered by gavage on days 0 and 7 and were sacrificed on day 21. S. boulardii was administered in the drinking water (3 × 108 CFU per ml). Small intestinal total IgA levels were measured by ELISA. Data are shown as mean and standard error (control, n = 33; toxoid A, n = 13; S. boulardii alone, n = 15; S. boulardii plus toxoid A, n = 27). Total IgA levels were significantly higher in each of the two S. boulardii-fed groups versus the control (P = 0.003 by ANOVA).
FIG. 2.
S. boulardii increases small intestinal IgA anti-toxin A levels following oral immunization with C. difficile toxoid A. Mice were immunized with formalin-inactivated toxoid A administered by gavage on days 0 and 7 and were sacrificed on day 21. S. boulardii was administered in the drinking water (3 × 108 CFU per ml). Small intestinal IgA anti-toxin A antibody levels were measured by ELISA. Results are expressed as mean and standard error of optical density (O.D.) units (×1,000) (control, n = 32; toxoid A, n = 13; S. boulardii alone, n = 15; S. boulardii and toxoid A, n = 15). Small intestinal IgA anti-toxin A antibody levels were significantly higher in the S. boulardii plus toxoid A group compared to each of the other three treatment groups (P < 0.001 by ANOVA).
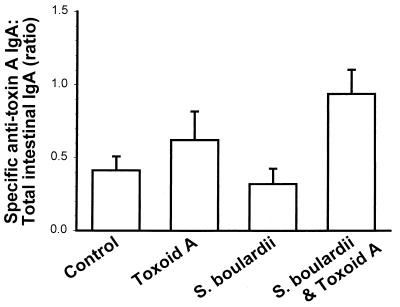
We next asked whether the increase in intestinal IgA anti-toxin A levels in S. boulardii-treated mice could be the result of a nonspecific stimulation of small intestinal IgA production. We therefore determined the ratio of specific IgA anti-toxin A to total IgA levels in the different groups of mice. As illustrated in Fig. 3, the median ratios of specific to total IgA were similar in the control (0.23) and toxoid A-treated (0.25) mice. The median ratio was somewhat lower (0.16; P > 0.05) in mice that were treated with S. boulardii but not immunized with toxoid A. This decrease in the ratio results from an increase in total IgA levels in this treatment group that is not associated with a corresponding increase in specific IgA anti-toxin A levels. In contrast, there was a 4.7-fold increase in the ratio of specific to total IgA (1.09; P = 0.01) in mice that were exposed to toxoid A during S. boulardii treatment. Thus, S. boulardii specifically increases intestinal IgA anti-toxin A levels during mucosal immunization against C. difficile toxin A.
FIG. 3.
S. boulardii specifically increases small intestinal IgA anti-toxin A levels following oral immunization. Mice were immunized with formalin-inactivated toxoid A administered by gavage on days 0 and 7 and were sacrificed on day 21. S. boulardii was administered in the drinking water (3 × 108 CFU per ml). The ratios of specific anti-toxin A IgA to total IgA in small intestine secretions (mean and standard error) are shown (control, n = 32; toxoid A, n = 13; S. boulardii alone, n = 15; S. boulardii and toxoid A, n = 15). The ratio of specific anti-toxin A IgA to total IgA was higher in the S. boulardii plus toxoid A group compared to the S. boulardii group (P = 0.01 by ANOVA).
These findings suggested that S. boulardii may act as a mucosal adjuvant. The mucosal adjuvant activities of cholera toxin are well recognized (15–17, 53). Therefore, we compared the intestinal IgA anti-toxin A responses following mucosal immunization with toxoid A during treatment with either S. boulardii or cholera toxin. As illustrated in Fig. 4, a significant increase in intestinal IgA anti-toxin A was observed only in the S. boulardii- and toxoid A-immunized group (3.0-fold increase; P = 0.003). In these experiments we also examined serum antibody levels against C. difficile toxin A. As shown in Fig. 5, serum IgA anti-toxin A was highest in the cholera toxin plus toxoid A group, but the differences were not statistically significant (P = 0.35). Serum IgG anti-toxin A was also highest in the cholera toxin plus toxoid A group, but again the differences did not reach statistical significance (P = 0.34). Serum IgM anti-toxin A was highest in the S. boulardii plus toxoid A group and in this instance was significantly higher than the control group. Serum IgM anti-toxin A was also somewhat increased in the cholera toxin and toxoid A group, but the increase did not reach statistical significance.
FIG. 4.
S. boulardii is more effective than cholera toxin in increasing small intestinal IgA anti-toxin A levels following oral immunization. Mice were immunized with formalin-inactivated toxoid A (TxA) administered by gavage on days 0, 7, 14, and 21 and were sacrificed on day 35. Other mice received cholera toxin (CT; 10 μg) administered by gavage either alone or with toxoid A. Other mice received toxoid A by gavage and S. boulardii (Sb) in their drinking water (3 × 108 CFU per ml). Small intestinal IgA anti-toxin A levels were measured by ELISA. Results are expressed as optical density (O.D.) units (×1,000) (mean and standard error; n = 9 for each treatment group). IgA anti-toxin A levels were significantly higher in the TxA plus Sb group compared to the control, CT, or TxA plus CT groups (P = 0.003 by ANOVA).
FIG. 5.
Serum anti-toxin A antibody levels following oral immunization against C. difficile toxoid A. Mice were immunized with formalin-inactivated toxoid A (TxA) administered by gavage on days 0, 7, 14, and 21 and were sacrificed on day 35. Some mice received cholera toxin (CT; 10 μg) administered by gavage with toxoid A. Other mice received toxoid A by gavage and S. boulardii (Sb) in their drinking water (3 × 108 CFU per ml). Serum IgA, IgG, and IgM anti-toxin A levels were measured by ELISA. Results are expressed as optical density (O.D.) units (×1,000 for IgA and ×100 for IgG and IgM) (mean and standard error; n = 9 for each treatment group). Serum IgM anti-toxin A levels were higher in the Sb plus TxA group compared to the control (P = 0.002 by ANOVA). There were no other statistically significant differences between any of the treatment groups.
The main finding of this study is that feeding with S. boulardii during oral immunization of BALB/c mice with C. difficile toxin A stimulates a specific immune response to toxin A. As reported previously, S. boulardii caused a twofold increase in IgA levels in small intestine secretions (3). However, previous studies did not examine the effect of S. boulardii treatment on specific immune responses to luminal antigens. We now find that S. boulardii induces a specific IgA immune response to toxin A when both are coadministered. The relative increase in specific IgA anti-toxin A production is greater than the observed increase in total IgA levels. This finding is consistent with antigen-specific stimulation of the intestinal mucosal immune system by S. boulardii. The mechanisms whereby S. boulardii can stimulate intestinal immune responses to coadministered antigens are poorly understood. However, stimulation of a more effective host mucosal immune response to prevalent antigens may be a general mechanism for the efficacy of S. boulardii in protecting against a wide variety of enteric disorders (4–11, 13, 35, 36, 42, 44, 48, 49).
There is increasing evidence that the immune response to C. difficile toxin A plays an important role in determining the clinical outcome of C. difficile infection in humans (24, 31). We recently reported a strong association between high levels of serum IgG anti-toxin A and asymptomatic carriage of C. difficile (32). Other clinical studies have shown that a low serum and/or intestinal antibody response to C. difficile toxin A is also associated with severe, prolonged, and recurrent C. difficile diarrhea (33, 34, 40, 45, 51, 52). Taken together, these studies indicate that an adequate antibody response to toxin A is an important element in asymptomatic carriage of C. difficile and in clinical recovery from C. difficile diarrhea. Thus, our finding of increased intestinal anti-toxin A levels in S. boulardii-treated mice may be directly relevant to the yeast's protective effects in recurrent C. difficile diarrhea and colitis.
Acknowledgments
This work was supported by a grant from Biocodex Laboratories, Montrouge, France.
REFERENCES
- 1.Arnheim K. Intestine-associated immune system stimulation. Saccharomyces boulardii—mechanism of action. Fortschr Med. 1994;112:52–53. . (In German.) [PubMed] [Google Scholar]
- 2.Barroso L A, Wang S Z, Phelps C J, Johnson J L, Wilkins T D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buts J P, Bernasconi P, Vaerman J P, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35:251–256. doi: 10.1007/BF01536771. [DOI] [PubMed] [Google Scholar]
- 4.Buts J P, Corthier G, Delmee M. Saccharomyces boulardii for Clostridium difficile-associated enteropathies in infants. J Pediatr Gastroenterol Nutr. 1993;16:419–425. doi: 10.1097/00005176-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Castagliuolo I, LaMont J T, Nikulasson S T, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225–5232. doi: 10.1128/iai.64.12.5225-5232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagliuolo I, Riegler M F, Valenick L, LaMont J T, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67:302–307. doi: 10.1128/iai.67.1.302-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castex F, Corthier G, Jouvert S, Elmer G W, Lucas F, Bastide M. Prevention of Clostridium difficile-induced experimental pseudomembranous colitis by Saccharomyces boulardii: a scanning electron microscopic and microbiological study. J Gen Microbiol. 1990;136:1085–1089. doi: 10.1099/00221287-136-6-1085. [DOI] [PubMed] [Google Scholar]
- 8.Corthier G, Dubos F, Ducluzeau R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can J Microbiol. 1986;32:894–896. doi: 10.1139/m86-164. [DOI] [PubMed] [Google Scholar]
- 9.Corthier G, Lucas F, Jouvert S, Castex F. Effect of oral Saccharomyces boulardii treatment on the activity of Clostridium difficile toxins in mouse digestive tract. Toxicon. 1992;30:1583–1589. doi: 10.1016/0041-0101(92)90030-9. [DOI] [PubMed] [Google Scholar]
- 10.Dias R S, Bambirra E A, Silva M E, Nicoli J R. Protective effect of Saccharomyces boulardii against the cholera toxin in rats. Can J Microbiol. 1991;37:315–317. [PubMed] [Google Scholar]
- 11.Dias R S, Bambirra E A, Silva M E, Nicoli J R. Protective effect of Saccharomyces boulardii against the cholera toxin in rats. Braz J Med Biol Res. 1995;28:323–325. [PubMed] [Google Scholar]
- 12.Dove C H, Wang S Z, Price S B, Phelps C J, Lyerly D M, Wilkins T D, Johnson J L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990;58:480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmer G W, McFarland L V. Suppression by Saccharomyces boulardii of toxigenic Clostridium difficile overgrowth after vancomycin treatment in hamsters. Antimicrob Agents Chemother. 1987;31:129–131. doi: 10.1128/aac.31.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmer G W, Surawicz C M, McFarland L V. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870–876. doi: 10.1001/jama.275.11.870. [DOI] [PubMed] [Google Scholar]
- 15.Elson C O, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2741. [PubMed] [Google Scholar]
- 16.Elson C O, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–2897. [PubMed] [Google Scholar]
- 17.Elson C O, Woogen S, Gaspari M, Ealding W. Induction of secretory IgA responses to protein antigens. Adv Exp Med Biol B. 1987;216:877–885. [PubMed] [Google Scholar]
- 18.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739–750. [PubMed] [Google Scholar]
- 19.Fekety R, Shah A B. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993;269:71–75. [PubMed] [Google Scholar]
- 20.Hassett J, Meyers S, McFarland L, Mulligan M E. Recurrent Clostridium difficile infection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii. Clin Infect Dis. 1995;20(Suppl. 2):S266–S268. doi: 10.1093/clinids/20.supplement_2.s266. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann F, Busch C, Prepens U, Just I, Aktories K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 22.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 23.Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 24.Kelly C P. Immune response to Clostridium difficile infection. Eur J Gastroenterol Hepatol. 1996;8:1048–1053. doi: 10.1097/00042737-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kelly C P, Becker S, Linevsky J K, Joshi M A, O'Keane J C, Dickey B F, LaMont J T, Pothoulakis C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Investig. 1994;93:1257–1265. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly C P, LaMont J T. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe M M, editor. Gastrointestinal pharmacotherapy. W. B. Philadelphia, Pa: Saunders; 1993. pp. 199–212. [Google Scholar]
- 27.Kelly C P, LaMont J T. Clostridium difficile infection. Annu Rev Med. 1998;49:375–390. doi: 10.1146/annurev.med.49.1.375. [DOI] [PubMed] [Google Scholar]
- 28.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 29.Kelly C P, Pothoulakis C, Orellana J, LaMont J T. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992;102:35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- 30.Kimmey M B, Elmer G W, Surawicz C M, McFarland L V. Prevention of further recurrences of Clostridium difficile colitis with Saccharomyces boulardii. Dig Dis Sci. 1990;35:897–901. doi: 10.1007/BF01536805. [DOI] [PubMed] [Google Scholar]
- 31.Kyne L, Kelly C P. Prospect for a vaccine for Clostridium difficile. BioDrugs. 1998;10:173–181. doi: 10.2165/00063030-199810030-00001. [DOI] [PubMed] [Google Scholar]
- 32.Kyne L, Warny M, Qamar A, Kelly C P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;340:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 33.Kyne L, Warny M, Qamar A, Kelly C P. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 34.Leung D Y, Kelly C P, Boguniewicz M, Pothoulakis C, LaMont J T, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:633–637. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 35.Massot J, Desconclois M, Astoin J. Protection by Saccharomyces boulardii against Escherichia coli diarrhea in newborn mice. Ann Pharm Fr. 1983;40:445–449. . (In French.) [PubMed] [Google Scholar]
- 36.Massot J, Sanchez O, Couchy R, Astoin J, Parodi A L. Bacterio-pharmacological activity of Saccharomyces boulardii in clindamycin-induced colitis in the hamster. Arzneim-Forsch. 1984;34:794–797. [PubMed] [Google Scholar]
- 37.McFarland L V, Mulligan M E, Kwok R Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 38.McFarland L V, Surawicz C M, Greenberg R N, Elmer G W, Moyer K A, Melcher S A, Bowen K E, Cox J L. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439–448. [PubMed] [Google Scholar]
- 39.McFarland L V, Surawicz C M, Greenberg R N, Fekety R, Elmer G W, Moyer K A, Melcher S A, Bowen K E, Cox J L, Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- 40.Mulligan M E, Miller S D, McFarland L V, Fung H C, Kwok R Y. Elevated levels of serum immunoglobulins in asymptomatic carriers of Clostridium difficile. Clin Infect Dis. 1993;16(Suppl. 4):S239–S244. doi: 10.1093/clinids/16.supplement_4.s239. [DOI] [PubMed] [Google Scholar]
- 41.Pothoulakis C. Pathogenesis of Clostridium difficile-associated diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:1041–1047. doi: 10.1097/00042737-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Pothoulakis C, Kelly C P, Joshi M A, Gao N, O'Keane C J, Castagliuolo I, LaMont J T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 43.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont J T. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues A C, Nardi R M, Bambirra E A, Vieira E C, Nicoli J R. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;81:251–256. doi: 10.1111/j.1365-2672.1996.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 45.Salcedo J, Keates S, Pothoulakis C, Castagliuolo I, LaMont J T, Kelly C P. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surawicz C M, Elmer G W, Speelman P, McFarland L V, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 47.Surawicz C M, McFarland L V, Elmer G, Chinn J. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am J Gastroenterol. 1989;84:1285–1287. [PubMed] [Google Scholar]
- 48.Toothaker R D, Elmer G W. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984;26:552–556. doi: 10.1128/aac.26.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidon N, Huchet B, Rambaud J C. Influence of Saccharomyces boulardii on jejunal secretion in rats induced by cholera toxin. Gastroenterol Clin Biol. 1986;10:13–16. . (In French.) [PubMed] [Google Scholar]
- 50.Viscidi R, Laughon B E, Yolken R, Bo-Linn P, Moench T, Ryder R W, Bartlett J G. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 51.Warny M, Denie C, Delmee M, Lefebvre C. Gamma globulin administration in relapsing Clostridium difficile-induced pseudomembranous colitis with a defective antibody response to toxin A. Acta Clin Belg. 1995;50:36–39. doi: 10.1080/17843286.1995.11718419. [DOI] [PubMed] [Google Scholar]
- 52.Warny M, Vaerman J P, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384–389. doi: 10.1128/iai.62.2.384-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson A D, Stokes C R, Bourne F J. Adjuvant effect of cholera toxin on the mucosal immune response to soluble proteins. Differences between mouse strains and protein antigens. Scand J Immunol. 1989;29:739–745. [PubMed] [Google Scholar]