Abstract
Here, we report the identification of a novel CD8+ cytotoxic T-lymphocyte epitope on the Plasmodium falciparum circumsporozoite protein (3D7; amino acids 310 to 319 [EPSDKHIKEY]) that is restricted by HLA-A*01 and is recognized by human volunteers immunized with irradiated P. falciparum sporozoites. HLA-A*01 is the second most common HLA allele among Caucasians.
Sterile protective immunity against malaria in humans is induced by immunization with irradiated Plasmodium falciparum sporozoites (1, 2, 7, 9, 19), is dependent on CD8+ T cells (5, 20, 27), and is presumed to be directed against antigens expressed by irradiated sporozoites in infected hepatocytes (10, 11). Accordingly, a major approach to developing a malaria vaccine that duplicates the excellent protection induced by the irradiated sporozoite vaccine is to identify CD8+ T-cell epitopes on parasite proteins expressed by irradiated sporozoites in hepatocytes (10, 17). To date, 32 P. falciparum CD8+ T-cell epitopes derived from five proteins known to be expressed in infected hepatocytes have been reported (6; Table 1).
TABLE 1.
CD8+ CTL epitopes on P. falciparum preerythrocytic-stage proteins recognized by T cells from volunteers immunized with radiation-attenuated P. falciparum sporozoites
| Peptide no. | Protein | HLA | Residues | Sequence | Reference |
|---|---|---|---|---|---|
| 1 | CSP | 368–390 | KPKDELDYENDIEKKICKMEKCS | 16 | |
| 2 | SSP2a | A2 | 1–15 | MNHLGNVKYLVIVFL | 28 |
| 3 | SSP2 | A2 | 46–60 | EVDLYLLMDCSGSIR | 28 |
| 4 | SSP2 | A2 | 121–135 | LLSTNLPYGKTNLTD | 28 |
| 5 | SSP2 | A2 | 126–140 | LPYGKTNLTDALLQV | 28 |
| 6 | SSP2 | A2 | 131–145 | TNLTDALLQVRKHLN | 28 |
| 7 | SSP2 | A2 | 136–150 | ALLQVRKHLNDRINR | 28 |
| 8 | SSP2 | A2 | 221–235 | ENVKNVIGPFMKAVC | 28 |
| 9 | SSP2 | A2 | 281–295 | CEEERCLPKREPLDV | 28 |
| 10 | SSP2 | A2 | 286–300 | CLPKREPLDVPDEPE | 28 |
| 11 | SSP2 | A2 | 521–535 | ALLACAGLAYKFVVP | 28 |
| 12 | SSP2 | A2 | 546–560 | APFDETLGEEDKDLD | 28 |
| 13 | SSP2 | A2 | 551–565 | TLGEEDKDLDEPEQF | 28 |
| 14 | SSP2 | B8 | 107–115 | ASKNKEKAL | 29 |
| 15 | SSP2 | B8 | 109–117 | KNKEKALII | 29 |
| 16 | SSP2 | A2 | 14–23 | FLIFFDLFLV | 4 |
| 17 | EXP1 | A2 | 80–88 | VLAGLLGNV | 4 |
| 18 | CSP | A2 | 394–402 | GLIMVLSFL | 4 |
| 19 | EXP1 | A2 | 2–10 | KILSVFFLA | 4 |
| 20 | EXP1 | A2 | 83–91 | GLLGNVSTV | 4 |
| 21 | EXP1 | A2 | 91–100 | VLLGGVGLVL | 4 |
| 22 | CSP | A2 | 7–6 | ILSVSSFLFV | 4 |
| 23 | LSA1 | A3 | 94–102 | QTNFKSLLR | 4 |
| 24 | SSP2 | A3 | 523–531 | LACAGLAYK | 4 |
| 25 | CSP | A3 | 344–353 | VTCGNGIQVR | 4 |
| 26 | EXP1 | A3 | 10–18 | ALFFIIFNK | 4 |
| 27 | SSP2 | A3 | 522–531 | LLACAGLAYK | 4 |
| 28 | LSA1 | A3 | 105–113 | GVSENIFLK | 4 |
| 29 | LSA1 | A3 | 59–68 | HVLSHNSYEK | 4 |
| 30 | LSA1 | A3 | 11–20 | FILVNLLIFH | 4 |
| 31 | PfS16 | B7 | 77–85 | MPLETQLAI | 4 |
| 32 | SSP2 | B7 | 539–548 | TPYAGEPAPF | 4 |
Some epitopes reported on P. falciparum sporozoite surface protein 2 (SSP2) were identified as overlapping epitopes (28).
One potential challenge to the development of epitope-based vaccines is the polymorphism of major histocompatibility complex (MHC) class I molecules. Many HLA-A molecules can be grouped into different HLA supertypes which are characterized by largely overlapping peptide-binding repertoires and are present in high frequencies, irrespective of the particular ethnicity considered. Focusing on the major HLA supertypes generally simplifies the process of development of epitope-based vaccines (21, 22, 24). HLA-A1 is one of five HLA antigens (A1, A2, A3, A11, and A24) expressed in a high proportion of different populations (12, 24) and is the second most common antigen expressed by Caucasians (26 to 36.5%), after HLA-A2 (42 to 64%) (6; Table 2). Recent data also suggest that HLA-A*01 might represent a prototype allele of an HLA-A1 supertype composed of several alleles with similar peptide-binding motifs (22). Thus, HLA-A*01-restricted epitopes should be considered for inclusion in multiepitope peptide-based vaccines.
TABLE 2.
Prevalence of HLA-A∗01 allele in various populationsa
| Population | Frequency (%) |
|---|---|
| U.S. Whites | 30.94 |
| Australians | 36.48 |
| British | 27.75 |
| Swedish | 34.55 |
| Cornish | 31.61 |
| Germans | 32.76 |
| Greeks | 17.55 |
| Italians | 26.04 |
| North American Blacks | 10.32 |
| North American Amerinds | 9.56 |
| North African Blacks | 7.07 |
| South African Blacks | 4.74 |
| West African Blacks | 10.32 |
| Japanese | 1.40 |
| Northern Han | 9.18 |
| Southern Han | 0.80 |
| Thai-Chinese | 5.52 |
| Javanese | 3.17 |
| Papua New Guinea highlanders | 0 |
Reproduced from Doolan et al. (6).
Accordingly, we searched the P. falciparum circumsporozoite protein (PfCSP) 3D7 sequence for sequences containing potential HLA-A*01 binding motifs, specifically a threonine (T), serine (S), or methionine (M) at position 2 or an aspartic acid (D), glutamic acid (E), serine (S), or threonine (T) at position 3 and a tyrosine (Y) at the C terminus (3, 13–15). Two peptides, amino acids (aa) 31 to 40 (NTRVLNELNY) and aa 310 to 319 (EPSDKHIKEY), predicted to contain an HLA-A*01 peptide-binding motif were synthesized and analyzed for their affinity of binding (23, 25) to HLA-A*01. Peptides which bound to HLA-A*01 with high affinity (50% inhibitory concentration [IC50], 500 nM) were then assessed for their capacity to induce peptide-specific recall cytotoxic T-lymphocyte (CTL) responses from peripheral blood mononuclear cells (PBMC) from three volunteers expressing the HLA-A*01 molecule (Table 3) (7) and immunized with P. falciparum irradiated sporozoites.
TABLE 3.
HLA phenotypes of volunteers immunized with P. falciparum irradiated sporozoites (NF54 or 3D7)
| Volunteer no. | HLA type
|
|
|---|---|---|
| Class I | Class II | |
| 4 | A1, A28, B44, Bw57, Cw6 | DR7, DRw11, DQw2, DQw7, DRw52, DRw53 |
| 16 | A1, A24, B8, B38, Bw4 | DR11, DR5, DQw2, DRw52, DRw53 |
| 17 | A1, A3, B7, B8, Bw6 | DR17, DR3, DQw2, DQw3, DRw52, DRw53 |
Generation of effector cells.
PBMC from irradiated sporozoite-immunized volunteers were infected with recombinant vaccinia virus expressing the entire PfCSP 3D7 sequence at 10 PFU/cell (16) or stimulated with 10 μg of synthetic PfCSP peptide (aa 310 to 319 [EPSDKHIKEY]) per ml for 6 days as described previously (16). In some experiments effector cell populations were depleted of CD8+ T cells using anti-CD8+-coated Dynabeads.
CTL assay.
CTL assays and transient transfection of autologous and HLA-mismatched lymphoblastoid B-cell lines (B-LCL) were performed as described previously (16) using plasmid DNA expressing the 3D7 PfCSP gene (VR2510) or the same plasmid without the PfCSP insert (VR1020) (8) or autologous and HLA-mismatched B-LCL pulsed at 10 μg/ml with peptide PfCSP 3D7 (aa 310 to 319) or control peptide from P. falciparum sporozoite surface protein 2 (PfSSP2 [RRHNWVNHA]) (28).
The studies reported herein were conducted in accordance with U.S. Navy regulations governing the protection of human subjects in medical research. The research protocols employing human subjects in this study were reviewed and approved by the Naval Medical Research Institute's Committee for the Protection of Human Subjects and the Walter Reed Army Institute of Research Human Use Committee.
Antigen-specific CD8+ CTL against endogenously synthesized PfCSP are induced in HLA-A*01-irradiated sporozoite-immunized volunteers.
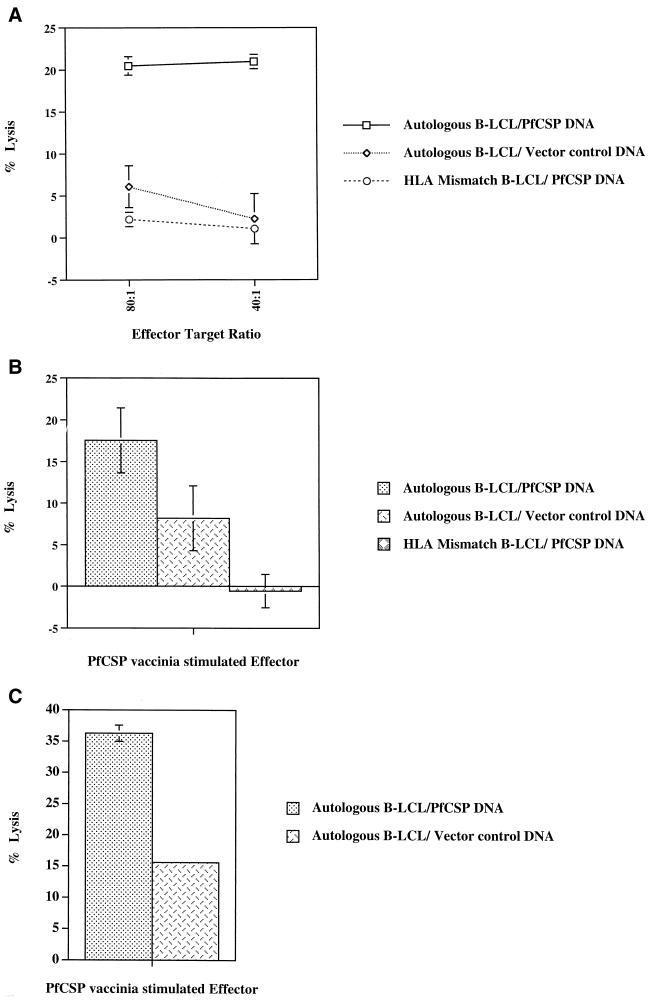
To determine whether immunization with radiation-attenuated P. falciparum sporozoites could generate anti-PfCSP CTL responses in HLA-A*01 individuals (Table 3), and if so, whether such CTL would recognize endogenously synthesized antigen, immune PBMC from irradiated sporozoite-immunized volunteers (4, 16, 17) were stimulated in vitro with autologous PBMC infected with recombinant PfCSP-expressing vaccinia virus. Cytolytic activity was assessed against autologous or HLA-mismatched B-LCL transiently transfected with PfCSP-encoding DNA (VR2510) or control DNA (VR1020). Effector cells from all three volunteers lysed autologous B-LCL transiently transfected with PfCSP-encoding DNA (Fig. 1) but not autologous B-LCL transfected with control DNA or HLA-mismatched B-LCL targets transfected with PfCSP-encoding DNA. These data demonstrated that an antigen-specific, HLA-restricted CTL response against endogenously presented PfCSP was induced in all three HLA-A*01-positive irradiated sporozoite-immunized volunteers.
FIG. 1.
CTL lysis of target cells expressing endogenously synthesized PfCSP. Immune PBMC from volunteer 16 (A), volunteer 4 (B), and volunteer 17 (C) were stimulated with autologous PBMC infected with recombinant vaccinia virus expressing the entire gene of PfCSP 3D7 (PfCSP vaccinia). Cytotoxicity was assessed in a CTL assay against autologous and HLA-mismatched B-LCL transiently transfected with PfCSP-encoding plasmid DNA (2510) or vector control DNA (1020) at an 80:1 effector/target ratio.
Identification of an HLA-A*01-restricted epitope on PfCSP 3D7.
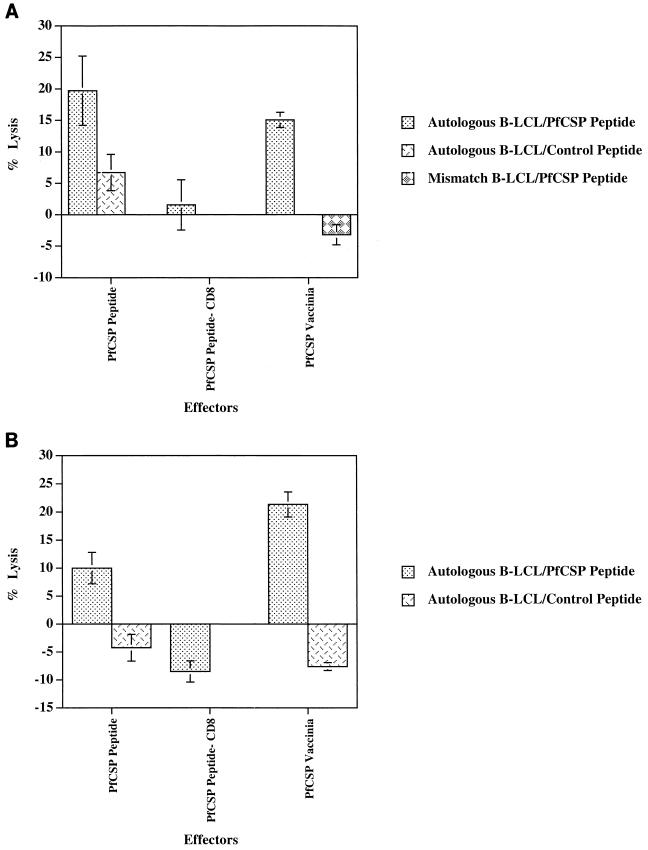
We next determined whether these effector cells could recognize either of two predicted HLA-A*01 epitopes from PfCSP (aa 31 to 40 [NTRVLNELNY] and aa 310 to 319 [EPSDKHIKEY]). Two peptides containing these epitopes were synthesized, and their HLA-A*01 binding capacity was estimated to be 2,604 and 147 nM, respectively, as described previously (14, 23). Only one peptide, PfCSP 310-319, bound with an IC50 of less than 500 nM. Based on our previous work we consider a peptide that binds with an affinity between 50 and 500 nM to be a high binder (23), and studies have established a correlation between high binding affinity and immunogenicity (23). Accordingly, PBMC from the three HLA-A*01 volunteers were stimulated with peptide PfCSP 3D7 310-319. In parallel, PBMC cultures were also stimulated with PBMC infected with recombinant PfCSP-expressing vaccinia virus. Autologous B-LCL were pulsed with either the PfCSP 3D7 310-319 peptide or with a control peptide or were transiently transfected with PfCSP-encoding DNA (VR2510) or control DNA (VR1020). The CTL response elicited by peptide PfCSP 3D7 310-319 was peptide specific, since effectors stimulated with peptide PfCSP 3D7 310-319 lysed targets pulsed with peptide PfCSP 3D7 310-319 but only minimally lysed or failed to lyse targets pulsed with the control peptide (Fig. 2). Furthermore, the CTL response was genetically restricted, since no significant CTL activity was detected when HLA-mismatched targets were pulsed with peptide PfCSP 3D7 310-319 (Fig. 2A and 3). The CTL response was dependent on CD8+ T cells, since depletion of CD8+ T cells from the effector cells eliminated the CTL activity (Fig. 2).
FIG. 2.
Antigen-specific, MHC-restricted, CD8+ T-cell-dependent CTL activity in HLA-A*01 volunteers. Immune PBMC from volunteer 4 (A) and volunteer 17 (B) were stimulated with peptide PfCSP 3D7 310–319 (EPSDKHIKEY) (PfCSP peptide) or PfCSP-expressing vaccinia virus (PfCSP vaccine). Cytotoxicity was assessed in a CTL assay at an 80:1 effector/target ratio. Autologous or HLA-mismatched B-LCL were pulsed with peptide PfCSP 3D7 310–319 or a control peptide from the PfSSP2 sequence. Where indicated, cells were depleted of CD8+ T cells using Dynabeads (PfCSP peptide-CD8).
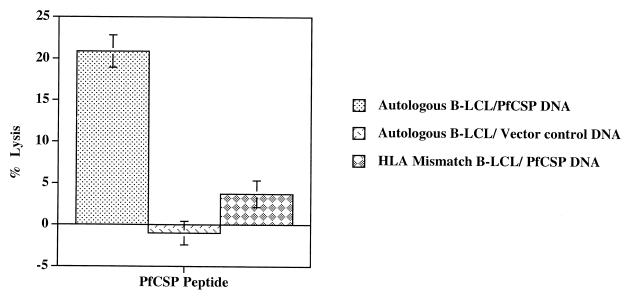
FIG. 3.
Antigen-specific, MHC-restricted CTL response in volunteer 16 (HLA-A*01). Immune PBMC from volunteer 16 were stimulated with peptide PfCSP 3D7 310-319. Cytolytic activity was then assessed in a CTL assay at an 80:1 effector/target ratio using autologous and HLA-mismatched B-LCL transiently transfected targets with a plasmid expressing PfCSP-encoding DNA (2510) or vector control DNA (1020) as a negative control.
Recognition of endogenously synthesized PfCSP by peptide PfCSP 3D7 310-319 stimulated immune effectors.
Next, we wanted to assess whether effector cells induced by the PfCSP 3D7 310-319 peptide could recognize target cells that endogenously synthesized the PfCSP antigen. Accordingly, PBMC from volunteer 16 were stimulated with the PfCSP 3D7 310-319 peptide, and autologous B-LCL and HLA-mismatched B-LCL were transiently transfected with PfCSPs-encoding DNA (VR2510) or control DNA (VR1020). Data presented in Fig. 3 establish that peptide-stimulated effectors could recognize endogenously processed PfCSP, since they lysed autologous B-LCL targets transfected with the PfCSP gene but not autologous B-LCL transfected with control vector VR1020. Furthermore, this CTL response was genetically restricted, since CTL activity could not be detected using HLA-mismatched B-LCL transfected with the PfCSP gene (Fig. 3).
In summary, our data demonstrate for the first time that HLA-A*01 volunteers immunized with P. falciparum irradiated sporozoites generate a specific CD8+ CTL response which recognizes a 10-amino-acid peptide, PfCSP 3D7 310-319 (EPSDKHIKEY). The peptide contains an HLA-A*01 motif and binds to the purified HLA-A*01 molecule with high affinity. This is the first HLA-A*01-restricted CTL epitope identified on PfCSP or on any malaria protein.
It has been reported that CD4+T cells from malaria-exposed individuals may respond to stimulation with synthetic peptides but not to native parasite (18), suggesting that endogenously produced protein may be processed differently than synthetic peptides. Our data demonstrate that the PfCSP 3D7 310-319 epitope is recognized by irradiated sporozoite-immunized volunteers following stimulation with endogenously synthesized PfCSP as a result of infection with recombinant vaccinia or with PfCSP presented on the surface of the target cells by transient transfection. Thus, our data indicate that the PfCSP 3D7 310-319 epitope is generated by natural processing of the PfCSP antigen. Recently, it has also been shown that CD8+ T cells from volunteers immunized with a plasmid DNA vaccine expressing PfCSP (26) recognize this epitope.
Given the high prevalence of HLA-A*01 and related alleles (HLA-A1 supertype) in the Caucasian population (26 to 36.5%; Table 2) and in other ethnic groups worldwide (22) and the fact that this epitope is the only reported HLA-A*01-restricted epitope identified on PfCSP or on any malaria protein, we believe that this epitope should be included in all PfCSP-containing vaccines. The fact that the HLA-A*01 allele is found in low prevalence in Africa (4 to 10%) does not undermine the importance of this epitope from a vaccine perspective. Identification and incorporation of such epitopes in a multiepitope-based vaccine are necessary to protect tens of thousands of individuals bearing this particular HLA type who annually visit areas where malaria is endemic. Furthermore, the peptide containing this epitope will be important for the routine evaluation of the efficacy of experimental PfCSP malaria vaccines in immunized volunteers.
Acknowledgments
We thank the volunteers who participated in this study and Denise Doolan for critically reviewing the manuscript.
This work was funded by NMRC work unit numbers 61102A.00101-BFX.1431 and 6287A00101EFX.1432 and in part by a grant from the Belgian Walloon Region to SmithKline Beecham Biologicals.
REFERENCES
- 1.Clyde D F, Most H, McCarthy V C, Vanderburg J P. Immunization of man against sporozoite induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Clyde D F, McCarthy V C, Miller R M, Hornick R B. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci. 1974;266:398–403. doi: 10.1097/00000441-197312000-00001. [DOI] [PubMed] [Google Scholar]
- 3.DiBrino M, Tsuchida T, Turner R V, Parker K C, Colligan J E, Biddison W E. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J Immunol. 1993;151:5930–5935. [PubMed] [Google Scholar]
- 4.Doolan D L, Hoffman S L, Southwood S, Wentworth P A, Chesnut R W, Keogh E, Appella E, Nutman T B, Lal A A, Gordon D M, Oloo A, Sette S. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA supertype alleles. Immunity. 1997;7:97–112. doi: 10.1016/s1074-7613(00)80513-0. [DOI] [PubMed] [Google Scholar]
- 5.Doolan D L, Hoffman S L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 6.Doolan D L, Wizel B, Hoffman S L. Class I HLA restricted cytotoxic T lymphocyte responses against malaria-elucidation on the basis of HLA peptide binding motifs. Immunol Res. 1996;15:280–305. doi: 10.1007/BF02935313. [DOI] [PubMed] [Google Scholar]
- 7.Egan J E, Hoffman S L, Haynes J D, Sadoff J D, Schneider I, Grau J E, Hollingdale M R, Ballou W R, Gordon D M. Humoral immune responses in volunteers immunized with irradiated Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 1993;49:166–173. doi: 10.4269/ajtmh.1993.49.166. [DOI] [PubMed] [Google Scholar]
- 8.Hedstrom R C, Doolan D, Wang R, Kumar A, Gardner M J, Aguiar J C, Charoenvit Y, Sacci J B, Sedegah M, Tine J, Margalith M, Hobart P, Hoffman S L. Expression and immunogenicity of DNA vaccines against pre-erythrocytic Plasmodium falciparum malaria. Int J Mol Med. 1998;2:29–38. doi: 10.3892/ijmm.2.1.29. [DOI] [PubMed] [Google Scholar]
- 9.Herrington D A, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, Nussenzweig R S, Clyde D, Edelman R. Successful immunization of humans with irradiated sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman S L, Franke E D, Hollingdale M R, Druilhe P. Attacking the infected hepatocyte. In: Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C.: ASM Press; 1996. pp. 35–75. [Google Scholar]
- 11.Hoffman S L, Rogers W O, Carucci D J, Venter J C. From genomics to vaccines: malaria as a model system. Nat Med. 1998;4:1351–1353. doi: 10.1038/3934. [DOI] [PubMed] [Google Scholar]
- 12.Imanshi T, Akai T, Kimura A, Tokunaga K, Gojobori T. Allele and haplotype frequencies for HLA and complement loci in various ethnic groups. In: Tsuji K, Aizawa M, Sasazuki T, editors. HLA 1991. Vol. 1. Oxford, United Kingdom: Oxford Press; 1992. pp. 1065–1220. [Google Scholar]
- 13.Kast W M, Brandt R M, Sidney J, Drijfhout J W, Kubo R T, Grey H M, Melief C J, Sette A. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J Immunol. 1994;152:3904–3912. [PubMed] [Google Scholar]
- 14.Kondo A, Sidney J, Southwood S, del Guercio M F, Appella E, Sakamoto H, Grey H M, Celis E, Chesnut R W, Kubo R T, Sette A. Two distinct HLA-A*0101-specific submotifs illustrate alternative peptide binding modes. Immunogenetics. 1997;45:249–258. doi: 10.1007/s002510050200. [DOI] [PubMed] [Google Scholar]
- 15.Kubo R T, Sette A, Grey H M, Appella E, Sakaguchi K, Zhu N Z, Arnott D, Sherman N, Shabanowitz J, Michel H, et al. Definition of specific peptide motifs for four major alleles. J Immunol. 1994;152:3913–3924. [PubMed] [Google Scholar]
- 16.Malik A, Egan J E, Houghton R A, Sadoff J C, Hoffman S L. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci USA. 1991;88:3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller L H, Hoffman S L. Research toward vaccines against malaria. Nat Med. 1998;4:520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 18.Quakyi I A, Currier J, Fell A, Taylor D W, Roberts T, Houghten R A, Berzofsky J A, Miller L H, Good M F. Analysis of human T cell clones specific for conserved peptide sequences within malaria proteins. Paucity of clones responsive to intact parasites. J Immunol. 1994;153:2082–2092. [PubMed] [Google Scholar]
- 19.Rieckmann K H, Carson P E, Beaudoin R L, Cassells J S, Sell K W. Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med. 1974;68:258–259. doi: 10.1016/0035-9203(74)90129-1. [DOI] [PubMed] [Google Scholar]
- 20.Schofield L J, Villaquiran A, Farreira H, Schellekens R S, Nussenzweig R S, Nussenzweig V. Gamma-interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 21.Sette A, Sidney J. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr Opin Immunol. 1998;10:478–482. doi: 10.1016/s0952-7915(98)80124-6. [DOI] [PubMed] [Google Scholar]
- 22.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 23.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J M, Oseroff C, Yuan L, Ruppert J, del Guerocio M-F, Southwood S, Kubo R T, Chesnut R W, Grey H M, Chisari F M. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 24.Sidney J, Grey H M, Kubo T, Sette A. Practical, biochemical and evolutionary implications of the discovery of HLA class I supermotifs. Immunol Today. 1996;17:261–266. doi: 10.1016/0167-5699(96)80542-1. [DOI] [PubMed] [Google Scholar]
- 25.Sidney J, Southwood S, Oseroff C, del Guercio M F, Sette A, Grey H M. The measurement of MHC/peptide interactions by gel filtration. Curr Protocols Immunol. 1998;18:18.3.1–18.3.19. doi: 10.1002/0471142735.im1803s31. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Doolan D L, Thong T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith J, Weiss W R, Sedegah M, Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 27.Weiss W R, Sedegah M, Beaudoin R L, Miller L H, Good M F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wizel B, Houghton R, Church P, Tine J A, Lenar D E, Gordon D M, Ballou W R, Sette A, Hoffman S L. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J Immunol. 1995;155:766–775. [PubMed] [Google Scholar]
- 29.Wizel B, Houghton R, Parker K, Coligan J E, Church P, Gordon D M, Ballou W R, Hoffman S L. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2. J Exp Med. 1995;182:1435–1445. doi: 10.1084/jem.182.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]