Abstract
In this study, we investigated the potential of a tuberculosis subunit vaccine based on fusion proteins of the immunodominant antigens ESAT-6 and antigen 85B. When the fusion proteins were administered to mice in the adjuvant combination dimethyl dioctadecylammonium bromide-monophosphoryl lipid A, a strong dose-dependent immune response was induced to both single components as well as to the fusion proteins. The immune response induced was accompanied by high levels of protective immunity and reached the level of Mycobacterium bovis BCG-induced protection over a broad dose range. The vaccine induced efficient immunological memory, which remained stable 30 weeks postvaccination.
Tuberculosis (TB) is the leading infectious disease in the developing world, and the World Health Organization estimates 80 million new cases of tuberculosis in this decade (8). The current vaccine against Mycobacterium tuberculosis, M. bovis Bacillus Calmette-Gúerin (BCG), has been extensively evaluated and demonstrated variable protective efficacies ranging from 0 to 85% in different field trials (13). An improved second-generation vaccine is therefore urgently needed. Alternative strategies in TB vaccine development such as subunit vaccines (2, 16, 23), genetic immunization (17, 27), and attenuated strains of M. tuberculosis (14) are currently being explored in many laboratories. Due to the complexity of the host immune response against tuberculosis and the genetic restriction imposed by major histocompatibility complex molecules, it has become clear that an effective subunit vaccine containing multiple epitopes may be required to ensure a broad coverage of a genetically heterogeneous population. We and others have previously demonstrated that vaccines based on a mixture of culture filtrate antigens can induce levels of protection similar to BCG in mice (2, 16, 23), but so far only a few experimental vaccines based on a single antigen have proved successful in animal models (6, 17, 27).
The strategy being explored in our laboratory is the molecular engineering of recombinant fusion proteins. Compared to mixtures of proteins extracted from cultures or cell lysates, the fusion protein approach offers at least two substantial advantages: (i) it is a more defined product and (ii) it reduces the number of recombinant expression and purification steps. The purpose of our study was to evaluate the potential of a subunit vaccine based on a fusion protein between two immunodominant antigens, Ag85B and the 6-kDa early secretory antigenic target (ESAT-6). In this study, we show that this approach is very promising and promotes an efficient immune response which is highly protective against TB in the mouse model.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female C57BL/6J (H-2b) and B6CBAF1 (H-2b,k) mice were purchased from Bomholtgaard (Ry, Denmark). All mice used were 6- to 12 weeks of age and were housed in cages contained within a BL-3 laminar flow safety enclosure. Animals were allowed free access to water and standard mouse chow.
Bacteria.
M. tuberculosis Erdman and H37Rv were grown at 37°C in modified Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose. BCG Danish 1331 was obtained as a freeze-dried vaccine and was rehydrated with phosphate-buffered saline (PBS).
Mycobacterial antigens.
Short-term culture filtrate (ST-CF) and recombinant ESAT-6 were produced as described previously (3, 15). Recombinant Ag85B and the fusion molecules were produced as follows. The coding regions of ag85B and esat6 were amplified by PCR from M. tuberculosis H37Rv chromosomal DNA with the primers shown in Table 1.
TABLE 1.
Primers used
| Primer | Sequence |
|---|---|
| ag85B-F1 | GGCAACCGCGAGATCTTTCTCCCGGCCGGGC |
| BglII | |
| ag85B-R1 | GGCAAGCTTGCCGGCGCCTAACGAACT |
| HindIII | |
| esat6-F1 | GGCGCCGGCAAGCTTGCCATGACAGAGCAGCAGTGG |
| HindIII | |
| esat6-R1 | CGAACTCGCCGGATCCCGTGTTTCGC |
| BamHI | |
| ag85B-F2 | GTTCGCAAAGCTTTTCTCCCGGCCGGGGCTGCCGGTCGAGTACC |
| HindIII | |
| ag85B-R2 | CCTTCGGTGGATCCCGTCAG |
| BamHI | |
| esat6-F2 | GGACCCAGATCTATGACAGAGCAGCAGTGG |
| BglII | |
| esat6-R2 | CGGCAGCCCCGGCCGGGAGAAAAGCTTTGCGAACATCCCAGTGACG |
| HindIII |
For the production of recombinant Ag85B, the coding region (without the secretory signal sequence) of Ag85B was PCR amplified from M. tuberculosis H37Rv chromosomal DNA using primers ag85B-F1 and ag85B-R2. A unique BamHI site was introduced by primer ag85B-R2. The PCR product was digested by BglII and BamHI and cloned into pMCT6 (15). DNA sequences of the inserts were confirmed by sequencing. The His-tagged protein was expressed in Escherichia coli XL-1 Blue and purified on a Talon column followed by protein anion-exchange chromatography using a HiTrap Q column (Pharmacia, Uppsala, Sweden). The sample was dialyzed against 25 mM HEPES buffer (pH 8.0)–0.15 M NaCl–10% glycerol–0.01% Tween 20 and stored at −20°C. For construction of the ag85B-esat6 fusion molecule, the PCR fragments of each gene obtained by using the F1-R1 set of primers were joined at the unique HindIII site, introduced by primers ag85B-R1 and esat6-F1. The resulting fusion molecule was cloned into the BglII/BamHI sites on pMCT6, in frame with eight N-terminal histidine residues. The esat6-ag85B chimeric plasmid was constructed in a similar way except that the PCR products obtained by the F2-R2 set of primers were used. The recombinant His-tagged fusion proteins were purified by the same procedure as the recombinant His-tagged Ag85B.
Vaccine preparation and immunization procedure.
Mice were immunized with experimental vaccines in doses from 0.01 to 50 μg emulsified with 250 μg of dimethyl dioctadecylammonium bromide (DDA; Eastman Kodak, Rochester, N.Y.) adjuvanted with 25 μg of monophosphoryl lipid A (MPL; RIBI ImmunoChem Research Inc., Hamilton, Mont.). The vaccines (0.2 ml/mice) were injected three times subcutaneously (s.c.) on the back with 2-week interval. A single dose of BCG Danish 1331 (5 × 104 bacilli/mouse) was injected s.c. at the base of the tail at the same time as the first subunit vaccination; no booster injections were administered. The prechallenge immunity was evaluated with blood lymphocytes 5 weeks after the first vaccination.
Lymphocyte cultures.
Blood lymphocytes were purified on a density gradient. Cells were pooled from eight mice in each group and cultured in triplicate in round-bottomed microtiter wells (96 well; Nunc, Roskilde, Denmark) containing 2 × 105 cells in a volume of 200 μl of RPMI 1640 medium supplemented with 5 × 10−5 M 2-mercaptoethanol, 1 mM glutamine, penicillin-streptomycin, and 5% (vol/vol) fetal calf serum. The mycobacterial antigens were used in concentrations ranging from 5 to 1.3 μg/ml. Culture supernatants were harvested from parallel cultures after 72 h of incubation, and the amount of gamma interferon (IFN-γ) was determined by enzyme-linked immunosorbent assay as described previously (7).
Experimental infections and bacterial enumeration in organs.
To evaluate the level of protection, mice were challenged 10 or 30 weeks after the first immunization either by the aerosol route in a Glas-Col inhalation exposure system, calibrated to deliver approximately 100 CFU of M. tuberculosis Erdman per lung, or by the intravenous (i.v.) route with an inoculum of 5 × 104 CFU of M. tuberculosis H37Rv suspended in PBS in a volume of 0.2 ml. Mice were sacrificed 6 weeks (aerosol route) or 2 weeks (i.v. route) later, and lungs and spleens were removed for bacterial enumeration. The organs were homogenized separately in sterile saline, and serial dilutions were plated onto Middlebrook 7H11 agar supplemented with 2 μg of 2-thiophene-carboxylic acid hydrazide per ml to selectively inhibit the growth of residual BCG in the test organs. Colonies were counted after 2 to 3 weeks of incubation at 37°C.
Statistical methods.
Assessment of experiments was carried out using analysis of variance. Differences between means were assessed by Tukey's test. A P value of <0.05 was considered significant.
RESULTS
Experimental vaccines based on recombinant fusion proteins between Ag85B and ESAT-6.
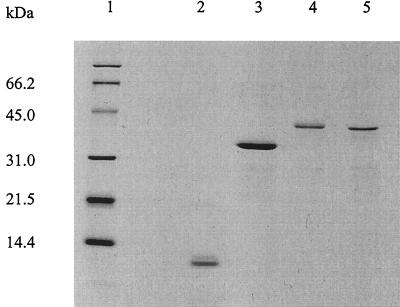
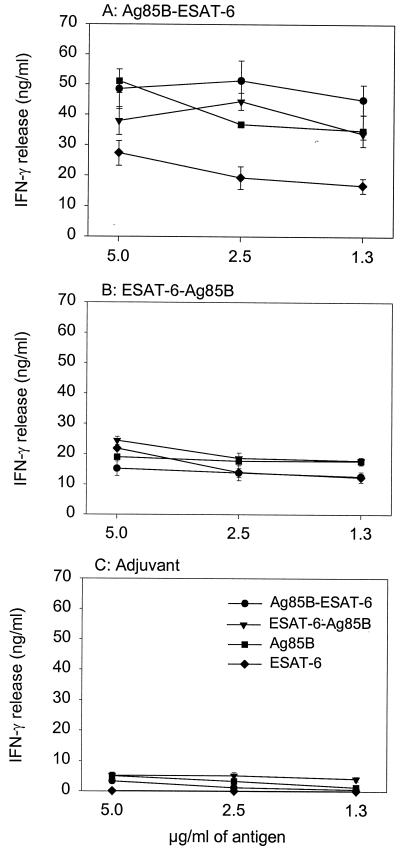
Two fusion proteins, Ag85B-ESAT-6 and ESAT-6-Ag85B, were recombinantly produced in E. coli using the His-tag cloning system, pMCT6. The two fusion proteins were affinity purified, subjected to ion exchange chromatography, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Coomassie blue-stained gel) together with the individual proteins Ag85B and ESAT-6 (Fig. 1). Western blotting demonstrated that the proteins retained the ability to bind antibodies against either component (HYT27 and HYB76.8) (result not shown). The initial immunological investigations were done to compare the immunogenicities of the two proteins and to clarify whether both components of the fusion proteins were recognized by the immune system after processing. Groups of C57BL/6J mice were immunized with 10 μg of each fusion protein emulsified in MPL and DDA, an adjuvant combination which has recently been shown to induce a highly efficient Th1 response protective against TB (6). As a negative control, a group of mice received the adjuvant combination alone. One week after the last injection, the mice were bled, peripheral blood mononuclear cells (PBMC) were purified, and the IFN-γ release was evaluated after in vitro stimulation with different concentrations of Ag85B, ESAT-6, and fusion proteins (all at 5, 2.5, and 1.3 μg/ml) (Fig. 2). Immunization with both Ag85B-ESAT-6 (Fig. 2A) and ESAT-6-Ag85B (Fig. 2B) fusion proteins induced strong IFN-γ release in response to restimulation with either fusion protein or Ag85B or ESAT-6. Immunization with the Ag85B-ESAT-6 fusion protein gave rise to the highest responses, with IFN-γ levels in the range of 45 to 50 ng/ml. This level of IFN-γ did not titrate out in the concentration range investigated in this experiment. In another experiment, the concentration interval 5 to 0.08 μg/ml was investigated; even with the lowest concentration (0.08 μg/ml), a significant (though lower) amount of IFN-γ (10 ng/ml) was released compared to the highest concentration (result not shown). The Ag85B-ESAT-6 fusion protein was selected for subsequent studies.
FIG. 1.
SDS-PAGE analysis of purified recombinant M. tuberculosis antigens. One microgram of protein was loaded in each lane. Lane 1, molecular weight standard; lane 2, recombinant ESAT-6; lane 3, recombinant Ag85B; lane 4, Ag85B-ESAT-6 fusion protein; lane 5, ESAT-6-Ag85B fusion protein. Protein bands were visualized by Coomassie blue staining.
FIG. 2.
Antigen-specific responses by PBMC 1 week after the last immunization with the fusion proteins between Ag85B and ESAT-6. C57BL/6J mice were immunized three times with either Ag85B-ESAT-6 (A) or ESAT-6-Ag85B (B) emulsified in MPL-DDA. As a negative control, a group of mice received the adjuvant combination alone (C). The IFN-γ responses were measured in cell cultures pooled from eight animals in each group. Each point represents the mean of triplicate values ± standard error of the mean. The experiment was performed twice with similar results.
Protective efficacy of the fusion protein vaccine in the mouse model.
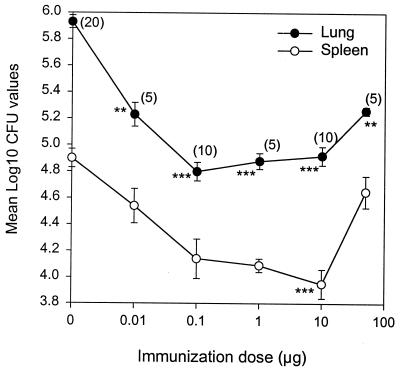
Mice were immunized with Ag85B-ESAT-6 in doses ranging from 0.01 to 50 μg. A group of mice receiving the adjuvant combination alone and a group of naive mice were included as controls. Ten weeks after the first immunization, the mice received an aerosol challenge with M. tuberculosis Erdman. Figure 3 shows the number of bacteria in lungs and spleens expressed as mean log10 CFU. Even with a dose as low as 0.01 μg, a statistical reduction in the number of bacteria was seen in the lungs (P < 0.01) compared to naive controls. This was followed by a range of doses (0.1 to 10 μg) inducing a higher level of protection (P < 0.001). There was no statistical difference between these three doses. Immunization with a dose of 50 μg was accompanied by reduced levels of protection in both organs. An immunization dose of 10 μg was the only one giving a significant level of protection in the spleen (P < 0.001) and was used for subsequent studies.
FIG. 3.
Efficacy of different doses of a subunit vaccine based on Ag85B-ESAT-6. C57BL/6J mice were immunized s.c. three times with different doses of Ag85B-ESAT-6 emulsified in MPL-DDA. Mice immunized with the adjuvant alone were included. Ten weeks after the first vaccination, the mice received an aerosol challenge with M. tuberculosis Erdman, and the numbers of bacteria (CFU) were quantified in the lungs and spleens 6 weeks later. The values are shown as log10 CFU in the lung and spleen. All data represent the mean of 5 to 20 individual mice ± standard error of the mean. Numbers in parentheses indicate the number of animals in each group. CFU counts in naive mice were 5.74 ± 0.04 and 4.65 ± 0.22 (n = 10) in the lung and spleen, respectively. ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared to naive mice.
We compared the protective efficacy of the fusion protein with that of a simple mix of Ag85B and ESAT-6, the single components, an ST-CF vaccine, or BCG in two different strains of mice, C57BL/6J and B6CBAF1. The molar concentrations of Ag85B and ESAT-6 in the mixture were adjusted to be the same level as the concentrations of the two components in the fusion protein. Ten weeks after the first vaccination, the mice were challenged by the aerosol (experiments 1 and 2) or the i.v. (experiment 3) route with virulent M. tuberculosis. Six (experiments 1 and 2) or two (experiment 3) weeks postchallenge, the mice were killed and the bacterial numbers were determined in the lungs and spleens. The vaccine-induced protection is shown in Table 2. In all three experiments, the fusion protein induced high levels of protection comparable to that induced by BCG. Slightly lower levels were obtained after immunizing with the mixture (experiments 1 and 3). The protective efficacy of the fusion molecule was also superior to that of ESAT-6 and Ag85B administered as single components, reducing the number of bacteria by 0.3 to 0.4 log more than the single components in both lungs and spleens. Although the tendency was the same in all three experiments, a statistically significant difference was found only between Ag85B and the fusion protein in the lung in experiment 2 (P = 0.039).
TABLE 2.
Vaccine-induced protection in the mouse model
| Vaccine groupa | Mean log10 CFU of M. tuberculosis ± SEM (n = 5)b
|
|||||
|---|---|---|---|---|---|---|
| Exp 1
|
Exp 2
|
Exp 3
|
||||
| Lung | Spleen | Lung | Spleen | Lung | Spleen | |
| Naive | 5.80 ± 0.05 | 4.69 ± 0.14 | 5.74 ± 0.04 | 4.65 ± 0.22 | 5.44 ± 0.05 | 6.39 ± 0.03 |
| MPL-DDA | 5.91 ± 0.08 | 4.94 ± 0.07 | 5.96 ± 0.06 | 4.65 ± 0.13 | 5.13 ± 0.03 | 6.26 ± 0.09 |
| ESAT-6 | 5.44 ± 0.10 | 4.68 ± 0.18 | 5.34 ± 0.06* | 4.17 ± 0.22 | 4.48 ± 0.21*** | 6.20 ± 0.08 |
| Ag85B | 5.49 ± 0.10 | 4.43 ± 0.07 | 5.41 ± 0.06 | 4.38 ± 0.12 | 4.41 ± 0.09∗∗∗ | 5.65 ± 0.11∗∗∗ |
| Ag85B-ESAT-6 | 5.07 ± 0.06*** | 4.20 ± 0.09 | 5.03 ± 0.12*** | 3.72 ± 0.15** | 4.11 ± 0.15*** | 5.42 ± 0.10*** |
| Ag85B + ESAT-6 | 5.38− ± 0.08 | 4.40 ± 0.20 | ND | ND | 4.43 ± 0.12*** | 5.76 ± 0.10** |
| ST-CF | 5.02 ± 0.11*** | 4.18 ± 0.11 | 4.90 ± 0.13*** | 4.09 ± 0.08 | 4.15 ± 0.11*** | 5.75 ± 0.09** |
| BCG | 5.31 ± 0.08* | 4.04 ± 0.21 | 5.18 ± 0.05** | 3.92 ± 0.12* | 4.16 ± 0.08*** | 5.39 ± 0.14*** |
Mice were immunized once s.c. with BCG (5 × 104 CFU) or injected three times with the experimental vaccines emulsified in MPL-DDA.
Number of bacteria isolated from the spleen and lung 6 weeks after aerosol challenge {experiments 1 and 2 (C57BL/6J[H-2b])} and 2 weeks after i.v. challenge {experiment 3 (B6CBAF1 [H-2b,k])}. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared to naive controls. ND, not determined.
Immunological memory induced by the fusion protein vaccine.
We next investigated whether the fusion protein vaccine induced stable immunological memory. Other groups included naive mice, BCG-vaccinated mice, and a group of mice receiving the adjuvant alone. Mice were aerosol challenged with M. tuberculosis Erdman 10 and 30 weeks after the first vaccination. Both the fusion protein and BCG induced significant and similar levels of protection at 10 weeks (P < 0.05) compared to naive controls (Table 3). The efficacy levels were similar to those found in the previous experiment (Table 2). The same pattern was observed after a longer rest period (30 weeks), and both vaccines induced long-lived memory immunity, which protected efficiently against TB. However, whereas the subunit vaccine promoted a stable level of protective immunity over the observation period, the efficacy of BCG had waned and induced significantly lower levels of protection in the lung than the subunit vaccine (experiment 1, P = 0.028; experiment 2, P = 0.013). There was no significant difference between the fusion protein and BCG in the spleen (experiment 1, P = 0.956; experiment 2, P = 0.243).
TABLE 3.
Vaccine-induced long-term protection in the mouse model
| Vaccine groupa | Mean log10 CFU of M. tuberculosis ± SEM (n = 4–5)b
|
|||||
|---|---|---|---|---|---|---|
| 10 wk
|
30 wk
|
|||||
| Lung | Spleen | Exp 1
|
Exp 2c
|
|||
| Lung | Spleen | Lung | Spleen | |||
| Naive | 5.72 ± 0.07 | 4.91 ± 0.07 | 5.86 ± 0.16 | 5.05 ± 0.10 | 5.94 ± 0.08 | 5.07 ± 0.10 |
| MPL-DDA | 5.64 ± 0.05 | 5.16 ± 0.08 | 6.11 ± 0.06 | 5.30 ± 0.14 | 5.98 ± 0.10 | 4.92 ± 0.08 |
| Ag85B-ESAT-6 | 4.80 ± 0.08** | 3.89 ± 0.16** | 4.82 ± 0.09** | 4.01 ± 0.36 (P = 0.051) | 5.08 ± 0.09** | 4.38 ± 0.09* |
| BCG | 4.81 ± 0.12** | 3.74 ± 0.18** | 5.36 ± 0.10* | 4.18 ± 0.27 (P = 0.115) | 5.59 ± 0.10 | 3.97 ± 0.27** |
C57BL/6J mice were immunized once s.c. with BCG (5 × 104 CFU) or injected three times with the experimental vaccines emulsified in MPL-DDA.
Number of bacteria isolated from the spleen and lung 6 weeks after aerosol challenge. ∗, P < 0.05; ∗∗, P < 0.001 compared to naive controls.
ESAT-6-Ag85B was used for immunization.
DISCUSSION
Ag85B and ESAT-6 are both very promising vaccine candidate molecules for several reasons: (i) they are strongly recognized T-cell antigens in the first phase of infection (7, 22, 28); (ii) they have demonstrated protective efficacy in animal models (6, 16, 27); and (iii) they contain numerous well-characterized epitopes recognized in TB patients (22, 24, 28). In the present study, we demonstrate that a subunit vaccine based on a fusion protein of these molecules and the recently developed adjuvant for cell-mediated immunity responses, DDA-MPL (6), induce levels of protective immunity similar to BCG in the mouse model of TB infection. One note of caution is that the level of BCG protection monitored after the aerosol infection in this study (Table 2, experiments 1 and 2) is lower than that reported before (4, 10, 18). This difference may, however, be related to the route of challenge, because in the present study high levels of protection were obtained with BCG given by the i.v. route (Table 2, exp 3). Of interest in this regard, also in this experiment the protection induced by the fusion molecule was at the same level as that induced by BCG.
Recent international focus on TB vaccine research and the sequencing of the M. tuberculosis genome (9) have resulted in the accelerated identification of novel mycobacterial proteins. Culture filtrates have attracted particular interest as a source of antigens which elicit protective immune responses in various animal models of TB (2, 4, 16, 23). Many of the recently identified proteins, such as ESAT-6 (26), TB 10.4 and CFP10 (25), MTB12 (29), MTB39 (11), and the APA (45/47-kDa) antigen (12), originate from culture filtrate. Human T-cell responses to most of these antigens have been studied and compared to responses to complex antigens such as tuberculin purified protein derivative and ST-CF (5, 25, 28). The data generated in these studies collectively demonstrate that even for the most immunodominant antigens described to date, a significant proportion of nonresponders exist among donors responsive to purified protein derivative in vitro (22, 25). To ensure the necessary coverage of human populations with strongly recognized T-cell epitopes, multicomponent vaccines will therefore be necessary. Such vaccines will not necessarily have to contain a large number of different components, as candidate antigens which are recognized by a very high proportion of donors already exist. In this regard, most of the analyses of human T-cell recognition conducted so far have been based on PBMC cultures; more sensitive analyses will increase the percentage of responders as exemplified by the recent enzyme-linked immunospot-based evaluation of ESAT-6 recognition in TB patients, where responses could be detected in more than 90% of the individuals tested (21).
In addition to being more cost-effective and less time-consuming, the delivery of these selected molecules as a single fusion protein has the potential advantage of inducing amplified responses to molecules with a low inherent immunogenicity. We have previously shown that ESAT-6 has a low inherent immunogenicity and requires a strong adjuvant such as DDA-MPL, whereas no response to this molecule is found if ESAT-6 is provided in DDA alone (6). In this regard, a recent evaluation of the efficacy of immunization with the fusion protein in DDA demonstrated that even this mild adjuvant induced a very strong response to both ESAT-6 and Ag85B (results not shown), indicating that the fusion to Ag85B may amplify the immune responses to a low-immunogenicity molecule like ESAT-6.
A precondition for the successful implementation of any subunit vaccine as a possible replacement for BCG is the generation of long-term immunological memory. This point has been a particular cause of concern in TB subunit vaccine development and has been debated for years (20). This concern arose from the original observations that subunit vaccines based on killed mycobacterial cell wall preparations could induce high levels of immunity immediately after vaccination but that resistance waned rapidly over time (1). This was later demonstrated to be a consequence of the nonspecific inflammatory response induced by these preparations (19). More recently a similar observation was made with the finding of a rapid waning of specific immunity after vaccination with experimental vaccines based on culture filtrate proteins and Freund's incomplete adjuvant (23). In this study the resistance to TB was almost at prevaccination levels 150 days postvaccination. Based on such findings, it has been thought that continuous antigen exposure provided by a live vaccine such as BCG would be necessary for the maintenance of efficient immunological memory. In contrast to the findings described above, the subunit vaccine described in the present study induced high levels of protection throughout the observation period; somewhat surprisingly, the level of immunity tended to be higher at day 210 than at day 70. At this late time point, the subunit vaccine even exceeded the immunity expressed in the lung after BCG vaccination. Although the reason for this high activity is not clear, the DDA component of our adjuvant, in addition to being highly stimulatory, may act as a depot for antigen by the formation of micelles with a slow but sustained release of antigen. That DDA may have this activity would be in agreement with our original observation of high levels of specific protective T cells which could adoptively transfer immunity to recipient mice as late as 22 weeks after vaccination with a mixture of DDA and M. tuberculosis culture filtrate (2).
In conclusion, our study clearly demonstrates that a subunit vaccine based on a fusion protein between Ag85B and ESAT-6 is able to induce efficient long-term memory immunity highly protective against TB in the mouse model. Together with results from ongoing work in guinea pigs and primates, these promising results may lay the groundwork for introducing such vaccines as a realistic alternative to BCG in the near future.
ACKNOWLEDGMENTS
This study was supported by the European Commission (18CT970254: Development of a tuberculosis vaccine with consistent efficacy in different region of the world).
We thank Lene Rasmussen and Tina Lerche for excellent technical assistance.
REFERENCES
- 1.Anacker R L, Barclay W R, Brehmer W, Larson C L, Ribi E. Duration of immunity to tuberculosis in mice vaccinated intravenously with oil-treated cell walls of Mycobacterium bovis strain BCG. J Immunol. 1967;98:1265–1273. [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D N, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt L, Elhay M J, Rosenkrands I, Lindblad E B, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–795. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt L, Oettinger T, Holm A, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Development of new vaccines for tuberculosis. Recommendations of the Advisory Council for the Elimination of Tuberculosis (ACET) Morb Mortal Wkly Rep. 1998;47:1–6. [PubMed] [Google Scholar]
- 9.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, Mclean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrel B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 10.Delogu G, Howard A, Collins F M, Morris S L. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect Immun. 2000;68:3097–3102. doi: 10.1128/iai.68.6.3097-3102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon D C, Alderson M R, Day C H, Lewinsohn D M, Coler R, Bement T, Campos-Neto A, Skeiky Y A, Orme I M, Roberts A, Steen S, Dalemans W, Badaro R, Reed S G. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect Immun. 1999;67:2941–2950. doi: 10.1128/iai.67.6.2941-2950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobos K M, Khoo K H, Swiderek K M, Brennan P J, Belisle J T. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine P E. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 14.Guleria I, Teitelbaum R, McAdam R A, Kalpana G, Jacobs W R, Bloom B R. Auxotrophic vaccines for tuberculosis. Nat Med. 1996;2:334–337. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- 15.Harboe M, Wiker H G, Ulvund G, Malin A S, Dockrell H, Holm A, Jorgensen M C, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van-Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 21.Pathan A, Brookes R, Pritchard H, Wilkinson R, Pasvol G, Hill A, Lalvani A. Human T cell responses to the antigen ESAT-6 characterize a vaccine candidate and potential diagnostic test for tuberculosis. Immunology. 1998;95(Suppl.):90. [Google Scholar]
- 22.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy H, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J, von-Reyn C F, Andersen P. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 23.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 24.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skjøt R L V, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. Comparative evaluation of low molecular mass T-cell antigens from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant. Infect Immun. 2000;68:214–220. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen A L, Nagai S, Houen G, Andersen P, Andersen A B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 28.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Webb J R, Vedvick T S, Aldersen M R, Guderian J A, Jen S S, Ovendale P J, Johnson S M, Reed S G, Skeiky Y A. Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1998;66:4208–4214. doi: 10.1128/iai.66.9.4208-4214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]