Abstract
Genus Angelica is one of the widely distributed and well-known genera of family Umbelliferae. It is utilized mainly by Chinese and Korean populations especially in their folk medicine. Angelica comprises a lot of medicinally important phytoconstituents such as coumarins, furanocoumarins, flavonoids, essential oils, verbascosides, polysaccharides, etc. Members of this genus play important roles, namely antioxidant, anti-inflammatory, anti-microbial, anti-diabetic, skin-whitening, cytotoxic, hepatoprotective, and many others. This review draws attention to many species of genus Angelica with much focus on A. dahurica being one of the highly medicinally used species within this genus.
Keywords: Angelica, Umbellifereae, coumarins, biological activity, phytochemistry, traditional uses
1. Introduction
Family Apiaceae (Umbelliferae), also known as the carrot family, is one of the largest plant families. It is composed of 466 genera and about 3800 species that are distributed worldwide (Figure 1). Many members of this family are well-known by people from different cultures due to their odors, flavors, or toxicity. The most famous members are anise, fennel, cumin, caraway, dill, parsley, coriander, etc. The root parts of the family members play an important role in the medical field, and the most popular among them are: carrots (Daucus carota), celeriac (Apium graveolens), parsnips (Pastinaca sativa), angelica (especially Angelica dahurica), parsley root (Petroselinum crispum), alexanders (Smyrnium olusatrum), pignut (Conopodium majus), and skirret (Sium sisarum) [1].
Figure 1.
Diagram showing different Angelica species.
Genus Angelica is a member of family Apiaceae (Umbelliferae), which is composed of 60 to 90 species of biennial perennial herbs that are widely distributed in Asia, Europe, and North America. Forty-five species out of 90 are located in China (about 32 endemic species) [2]. Plants of the genus Angelica are known as “women’s ginseng” in Southwest Asia and are used to treat amenorrhea and dysmenorrhea, menopausal disorders, hypertonia, anemia, and vascular dystonia; in many countries, these plants are accepted as officinal [3,4]. Angelica dahurica is considered one of the most important species of genus Angelica, especially its roots. Various phytoconstituents had been isolated from A. dahurica roots viz. coumarins, furanocoumarins, phthalides, polysaccharides, benzofurans, alkaloids, phenols, and sterols.
The dried root of A. dahurica is known as ‘Bai Zhi’ (Angelica dahurica; named Chuan Baizhi, Yu Baizhi, and Qi Baizhi), an important herbal medicine documented in the Chinese pharmacopoeia. It was reported that the pharmacological activities of this natural herb include protection against dexamethasone-induced ailments, hepatoprotection, antimicrobial effect, and anti-inflammatory and cytotoxic activities [5]. Several coumarins isolated from A. dahurica have been extensively studied for their chemical structure [6,7] and pharmacological effects [8,9].
Angelica dahurica root has been widely used for the treatment of acne, erythema, sinusitis, cold, headache (especially for migraine), toothache, and even cancer, for decades in Asia [10,11]. The A. dahurica root is also used for leukorrhea and arthralgia due to wind-dampness in Korean traditional medicine [12]. The root of the same species helps also in the protection against sepsis [13], and has anti-staphylococcal activity [10,14].
Northern Angelica (Angelica archangelica or Angelica officinalis) is widely used nowadays, both in the folk medicine and officinal medicine of many countries. In addition to A. archangelica, A. dahurica, Angelica ursina, Angelica sylvestris, and a range of other species are used for the preparation of medicines [15].
Huato Boluses containing Angelica sinensis and Angelica dahurica roots have gained great popularity during the recent years. The high antioxidant activity of ethereal oil from the roots of plants belonging to the genus Angelica has been reported [16,17,18].
This review work aimed at providing comprehensive and updated literature data on genus Angelica and its common species A. dahurica, based on published research articles, emphasizing the phytochemistry, biological activities, folk medicinal uses, potential toxicity, and side effects. In addition to that, this review may help researchers around the world find more information on genus Angelica, which will provide new research opportunities regarding the understudied phytochemical constituents and biological activities of this genus. This review covers the reported literature dating back to 2000, until 2022. The data collected in this review were summarized from different research databases viz. Scopus, Google Scholar, and PubMed.
1.1. Distribution
Today, A. sinensis is cultivated widely in the Gansu, Yunnan, Sichuan, Shaanxi, and Hubei provinces of China. It is also cultivated in Vietnam as a medicinal plant [19].
In most cultivation areas, A. sinensis seeds are placed in June and harvested in October of the next year. However, in Hubei Province and Zhanyi county, Yunnan Province, where the climate is much warmer and the altitude of the cultivated regions is much lower, growth is faster than in other regions, so it is sown in January and harvested in December of the same year. Post-harvesting, the soil is then rinsed off, and the rootlets and stalk are trimmed, after that, the roots are air-dried, then grouped into 0.5–1 kg flat bundles and baked dry over a slow fire [20]. A. gigas Nakai is located in Korea and usually used as Angelica roots in Southeast Asia [21].
1.2. Morphology
Genus Angelica carries the same general morphological characteristics of family Umbelliferae. The members of family Umbelliferae are usually annual or perennial herbs and rarely woody at their bases. The stems are caulescent or acaulescent, and either solid or hollow. The leaves are alternate, compound, pinnate, petiolated with sheath at the base of the petiole, and exstipulate. Leaflets are lanceolate in shape. Flowers are epigynous, small with long pedicels. The fruits are dry with two mericarps that unite to form one cremocarp, and each mericarp is one-seeded (Table 1) [1,22].
Table 1.
Botanical characteristics of genus Angelica.
| Plant Organ | Botanical Characteristics |
|---|---|
| Stem | Caulescent or acaulescent |
| Solid or hollow | |
| Leaf | Alternate |
| Compound | |
| Pinnate | |
| Lanceolate shape | |
| Petiolate | |
| Exstipulate | |
| Flower | Epigynous |
| Small | |
| Long pedicels | |
| Fruit | Dry |
| Cremocarp (two mericarps) | |
| Mericarp is one-seeded |
Angelica dahurica produces white flowers that bloom in umbrella-like clusters in June and July. The plant typically grows to a height of approximately 2 m (Table 1). The dried root is valued for its therapeutic properties. Its flavor is a distinct blend of bitter, sweet, and pungent, and its overall effect is warming in nature [23].
Angelica sinensis (Oliv.) Diels is a perennial plant, 0.4–1 m tall. The root is cylindrical, branched, succulent, strong aromatic, with many rootlets. The stem is ribbed, and is branched from above. The leaflets are ovate or ovate-lanceolate, with serrate margins. The flowers have 13–36 umbellules. The petals are white in color, and sometimes purplish-red in color. The fruits are ellipsoid or suborbicular (4–6.9, 3–4 mm) (Table 1) [24,25].
Angelica archangelica, also known as garden angelica, is a biennial herbaceous species of genus Angelica. It has a hollow stem carrying compound, alternate, yellowish-green leaves. It blooms during the summer, the seeds ripen in late summer, and then the plant dies. The flowers are greenish-white in color and small in size [26].
A. gigas is a stout plant that is 1 to 2 m high, with deep thick roots, and a purplish, ribbed stem. It has deeply dissected, very big, broad, pointy leaves. The plant is a biennial that flowers in the months of July to August in dark purple umbels, and self-seeds abundantly when the seeds have ripened [27].
A. glauca is an erect perennial plant of the temperate and alpine regions of the Himalayas. It is herb, about 1–2.5 m tall, glabrous, and having a stout stem. The roots are tuberous, thick, and aromatic. The leaves are petiolate, compound, and showing 1–2-ternate-pinnate venation; the leaflets are oval to ovate, mucronate-serrate, and glaucous. The flowers are usually bisexual, pedicellate, epigynous, actinomorphic, with pentamerous whorls and compound umbel inflorescence. The flower petals are free, five in number, white colored, and obovate in shape. The stamens are also five in number, green-coloured, bilobed, dorsifixed, exerted, and alternate to petals. The fruits are oblong-ellipsoid with prominent dorsal ribs [28].
A. acutiloba grows to about 0.3–1 m high. The color of the stems ranges from reddish to purplish. The stems are erect, glabrous, and thinly ribbed. The leaves are deep green, and alternately arranged, often with a leathery or fleshy texture. In most cases, the lower and basal leaves are petiolate or perfoliate. The petioles attached to them are about 10–30 cm in length. The mature blades are one- or two-pinnatifid. Young blades are usually three-pinnatifid. The leaves are of variable sizes. The upper leaves are simplified to oblong, with lanceolate and dentate incised blades. The leaf lobes are about 2–9 cm long and 1–3 cm wide. Most leaves are sessile, but sometimes they bear short stalks [29].
1.3. Traditional Uses
Angelica dahurica Radix (ADR; ‘Baek-ji’ in Korean, ‘Bai-zhi’ in Chinese) is commonly used in the traditional Korean and Chinese pharmacopoeias, such as Gumiganghwal-tang and Oyaksungi-san. In traditional Oriental medicine, Angelica dahurica was reported to be used as an anti-inflammatory agent for respiratory diseases (e.g., common cold and nasal congestion), dermal disorders (e.g., acne, ulcer, and carbuncle), pain (e.g., headache, toothache, and rheumatism), and intestinal disorders (e.g., diarrhea, dysentery, and chronic ulcerative colitis) [30,31,32].
In traditional Chinese medicine (TCM), A. sinensis was reported for the treatment of various diseases such as gynecological diseases, apoplexia, constipation, malaria, chills, fever, and hemorrhoids. The plant has also been used as a supplement in anemia as a haematopiotic agent, to regulate menstrual cycles, and to relax the bowels in constipation [33,34].
In traditional Korean medicine, A. gigas roots have been used for anemia, gynecological disorders, cardiovascular diseases, arthritis, sedative, analgesic, and tonic agents [11,35,36,37]. A. acutiloba is traditionally used to treat gynecological diseases and anemia [38].
Nevertheless, A. archangelica was traditionally valued in curing nervousness, insomnia, stomach and intestinal disturbances, skin diseases, respiratory problems, and arthritis [39], while A. glauca was used to treat bilious complaints, infantile atrophy, and constipation [40]. Moreover, A. dahurica was used to treat headaches, rhinitis, toothaches, rheumatism, and sore throat [41], and A. pubescentis was used to treat rheumatoid arthritis, headache, paralysis, and insomnia [42,43].
“Dang-Gui” refers to a raw drug belonging to the genus Angelica (Umbelliferae) that has been widely used as a traditional medicinal plant throughout Korea, China, and Japan. Dang-Gui carried different botanical names in three pharmacopoeias (Korean, Chinese, and Japanese), namely Angelica gigas Nakai, Angelica sinensis (Oliv.) Diels, and Angelica acutiloba Kitagawa, respectively. In the Korean pharmacopoeia, the roots of A. gigas are (“Dang-Gui”), while those of A. acutiloba are (“Il-Dang-Gui”) or the Japanese Dang-Gui [44,45].
1.4. Toxicity and Side Effects of Genus Angelica
Although many members of family Umbelliferae are edible fruits, condiments, and flavouring agents, members of genus Angelica may present certain side effects and toxicity from their use. Furanocoumarins from A. archangelica, especially the linear furanocoumarin, 8-methoxypsorlan, may present serious skin reactions and phototoxicity, especially in the presence of UV light [46]. Moreover, abdominal pain, convulsions, elevated bilirubin level, diarrhea, dystonia, and GIT hemorrhage were associated with A. sinensis root use [47]. Concerning A. dahurica, fewer reports were found on its potential toxicity; however, one report mentioned an LD50 value of (55–89 g/Kg) in mice, suggesting less toxicity [48].
2. Effects of Drying Methods on Contents of Bioactive Compounds and Antioxidant Activities of Angelica dahurica
Liang et al. (2018) aimed to analyze the quality (antioxidant and furanocoumarin content) of Bai Zhi roots after freeze-drying (the control), and in-the-shade, 40, and 70 °C drying. Antioxidant activity was evaluated through DPPH and iron-chelating assays. Six furanocoumarin compounds viz. xanthotoxin, bergapten, oxypeucedanin, imperatorin, phellopterin, and isoimperatorin were detected by HPLC. Shade-dried roots showed higher antioxidant activity compared to (40 and 70 °C) drying and freeze-drying. The furanocoumarin content was similar for both 70 °C drying and freeze-drying. Thus, it was concluded that A. dahurica roots dried at 70 °C may be an alternative method for maintaining high quality [49,50].
3. Phytochemical Constituents
Different phytochemical constituents belonging to diverse chemical classes had been reported from genus Angelica. The reported phytochemicals included mainly essential oils, coumarins, furanocoumarins, phthalides, polysaccharides, benzofurans, polyacetylenes, and many others. In this context, the aforementioned chemical classes will be detailed with more insights on Angelica dahurica. Herein, the different isolated components were arranged according to the chemical class to which they belong.
3.1. Essential Oil (Table 2)
Many essential oil components had been reported from members of genus Angelica, especially their roots. The main reported essential oil components were α-pinene, limonene, α- and β-phellandrene, p-cymene, β-ocimene, trans-carveol, and many others. They carry vast biological activities, presented mainly by the anti-oxidant and antimicrobial activities (see Section 4. Pharmacological activities). Thus, the essential oil components isolated from genus Angelica can be detailed as followed.
Shchipitsyna and Efremov (2011) evaluated the chemical profile of the essential oil of Angelica archangelica (roots, flower heads, and seeds) from Kemerovo Oblast. The essential oil was analyzed by GC/MS, and it contained 20 major components viz. α-pinene, camphene, β-pinene, β-myrcene, α-phellandrene, Δ3-carene, β-phellandrene, β-cis-ocimene, β-transocimene, β-copaene, β-bourbonene, β-elemene, α-cedrene, -α-bergamotene, β-cedrene, cis-γelemene, β-(Z)-farnesene, β-humulene, germacrene D, bicyclogermacrene, (E,E)-α-farnesene, γ-cadinene, δ-cadinene, α-cadinene, and α-cadinol [51]. In another piece of research, the A. dahurica essential oil was found to be rich in α-pinene (46%), followed by sabinene (9%), myrcene (5%), 1-dodecanol (5%), and terpinen-4-ol (4%), while the root essential oil of A. pubescentis showed α-pinene (37%), p-cymene (11%), limonene (8%), and cryptone (6%) [42,52]. The A. sinensis essential oil held 3-N-butylphthalide, butylidene phthalide, ligustilide, and di-iso-octyl phthalate as its major ingredients [53,54].
The essential oil content of A. sinensis Radix was (0.4–0.7%) rich with n-butylidenephthalide, ligustilide, n-butyl-phthalide, ferulic acid, nicotinic acid, and succinic acid as the main constituents [55]. Nivinskienë et al., 2005, found that the essential oil of A. archangelica seed was rich in β-phellandrene (33–63%) and α-pinene (4–12%) [56]. The A. archangelica roots essential oil carried α-pinene (21%), δ-3-carene (16%), limonene (16%), and α-phellandrene (8%) as its main components [57]. Nivinskienë et al., 2005, studied the essential oil of A. archangelica roots collected from three regions during the period of 1995–2002. The main constituents were α-Pinene (15–20%) from Svencionys and Prienai, while β-phellandrene (13–18%) and α-pinene (11–15%) were the major components from Vilnius. From Lithuania, the essential oil contained (67–79%) monoterpenes, (9–19%) sesquiterpenes, and (3–6%) macrocyclic lactones [56]. Chauhan et al., 2016, found that dillapiole (35–91%) and nothoapiole (0.1–62%) were the main essential oil components isolated from the rhizomes of A. archangelica collected from the Western Himalayan region [39]. On the other side, Pasqua et al., 2003, studied the effect of the development stage on the essential oil formation in A. archangelica subsp. archangelica roots, where a high content of α- and β-phellandrene was found only in taproots larger 5 mm in diameter [58].
Irshad, et al., 2011, studied the essential oil content of the A. glauca whole plant from Jammu and Kashmir. The essential oil contained α-phellandrene (18%), trans-carveol (16%), β-pinene (14%), β-caryophyllene (8%), and β-caryophyllene oxide (8%) as its major compounds [59]. Similarily, Agnihotri et al., 2004, analyzed the essential oil composition of the A.glauca aerial parts from the Kashmir Valley, Himalaya, India. The essential oil was rich in α-phellandrene (13%), trans-carveol (12%), and β-pinene (11%) [40]. In another similar study, the A. glauca roots’ essential oil from two alpine Himalayan locations, Uttarakhand, India showed the presence of (Z)-ligustilide (40–53%) and (Z)-butylidene phthalide (20–32%) [60].
The essential oil A. gigas, A. sinensis, and A. acutiloba rhizomes were analyzed by a solvent-free solid injector method. The major components were coumarin derivatives viz. decursinol angelate (16%) and decursin (29%) succeeded by lomatin (10%), and marmesin (9%) in A. gigas, while the main constituents in A. sinensis were butylidene dihydro-phthalide, (15%), butylidene phthalide (14 %), furfural (16%), and camphene (10%), while butylidenephthalide (17%) and furfural (13%) represented the major components in A. acutiloba [61]. Sowndhararajan et al., 2017, detected the essential oil of the A. gigas root by steam distillation and supercritical carbon dioxide extract (SC-CO2). It was found that the essential oil mainly composed of monoterpene hydrocarbons (52%), followed by oxygenated sesquiterpenes (25%). The main monoterpenes were α-pinene (28%), β-eudesmol (14%), nonane (8%), and γ-eudesmol (5%); these were the major components in the essential oil of the A. gigas root [36]. On the other hand, Seo et al., 2007, identified decursin (40%) and decursinol angelate (28%) as the major components using SC-CO2, while α-pinene (30%) was also the main component identified from A. gigas using simultaneous steam distillation and extraction methods [62].
Chen et al., 2014, compared the essential oil content of A. acutiloba isolated from different organs (roots, stems, and leaves) through steam distillation and headspace solid-phase micro extraction (HS-SPME). In the steam distillation, n-butyl phthalide, γ-terpinene, p-cymene, and cis-β-ocimene represented the main components. Using HS-SPME, γ-terpinene and p-cymene were the main constituents [63]. Cavaleiro et al., 2015, found that the essential oil of the A. major root contained α-pinene (21%) and cis-β-ocimene (30%) as its major components [64].
Mohammadi et al., 2010, found that the essential oil content of the A. urumiensis leaves showed α-cadinol (20%), hexahydrofarnesyl acetone (10%), and 1-dodecanol (7%) as its major components, while α-cadinol (9%) and δ-cadenine (6%) were the main identified components from the stem essential oil of the same plant [65].
Simonović et al., 2014, identified β-phellandrene, α-pinene, and α-phellandrene as major components from the A. pancicii aerial parts’ essential oil [66]. Similarily, caryophyllene oxide (61%) and α-pinene (67%) were the main components from the A. viridiflora and A. cincta aerial parts’ essential oils, respectively [67].
Two hundred and thirty nine compounds were identified from the GC/MS analysis of three Angelica species (A. sinensis, A. dahurica, and A. pubescens). The main identified constituents were osthole (44.61%), obepin (0.59–86.58%), undecanol (8.58%), α-muurolene (7.95%), cis-anethol (9.11%), E-ligustilide (0.14–81.14%), (-)-spathulenol (0.08–1.21%), (-)-terpinen-4-ol (4.91%), 2-butylthiolane (5.76%), and α-bisabolol (3.80%) [68].
Table 2.
Essential oil composition of selected species from genus Angelica.
| Angelica Species | Part Used | Essential Oil Components | Reference(s) |
|---|---|---|---|
| A. archangelica | roots, flower heads, and seeds | α-pinene, camphene, β-pinene, β-myrcene, α-phellandrene, Δ3-carene, β-phellandrene, β-cis-ocimene, β-transocimene, β-copaene, β-bourbonene, β-elemene, α-cedrene, -α-bergamotene, β-cedrene, cis-γelemene, β-(Z)-farnesene, β-humulene, germacrene D, bicyclogermacrene, (E,E)-α-farnesene, γ-cadinene, δ-cadinene, α-cadinene, and α-cadinol | [51,56,57] |
| rhizome | Dillapiole and nothoapiole | [39] | |
| A. archangelica subsp. archangelica | root | α- and β-phellandrene | [58] |
| A. dahurica | root | α-pinene, sabinene, myrcene, 1-dodecanol, and terpinen-4-ol. | [42,52] |
| root | cis-anethol, undecanol, α-muurolene, and (2E)-2-decenal | [68] | |
| A. pubescentis | root | α-pinene, p-cymene, limonene and cryptone. | [42,52] |
| A. sinensis | rhizome and root | 3-N-butylphthalide, butylidene phthalide, ligustilide, di-iso-octyl phthalate, ferulic acid, nicotinic acid, and succinic acid | [53,54,55] |
| rhizome | Butylidene dihydro-phthalide, butylidene phthalide, furfural, and camphene | [61] | |
| root | E-ligustilide and (-)-spathulenol | [68] | |
| A. glauca | whole plant | α-phellandrene, trans-carveol, β-pinene, β-caryophyllene, and β-caryophyllene oxide | [59] |
| aerial parts | α-phellandrene, trans-carveol, and β-pinene | [40] | |
| root | (Z)-ligustilide and (Z)-butylidene phthalide | [60] | |
| A. gigas | rhizome | Decursinol angelate, decursin, lomatin, and marmesin | [61] |
| root | α-pinene, β-eudesmol, nonane, γ-eudesmol, decursin, and decursinol angelate | [36,62] | |
| A. acutiloba | rhizome | Butylidenephthalide and furfural | [61] |
| roots, stems, and leaves |
n-butyl phthalide, γ-terpinene, p-cymene, and cis-β-ocimene (steam distillation) γ-terpinene and p-cymene (HS-SPME) |
[63] | |
| A. major | root | α-pinene and cis-β-ocimene | [64] |
| A. urumiensis | leaves | α-cadinol, hexahydrofarnesyl acetone, and 1-dodecanol | [65] |
| stem | α-cadinol and δ-cadenine | [65] | |
| A. pancicii | aerial parts | β-phellandrene, α-pinene, and α-phellandrene | [66] |
|
A. viridiflora
A. cincta |
aerial parts | Caryophyllene oxide and α-pinene | |
| A. pubescens | root | Osthole, obepin, undecanol, α-muurolene, cis-anethol, E-ligustilide, (-)-spathulenol, (-)-terpinen-4-ol, 2-butylthiolane, and α-bisabolol | [68] |
3.2. Coumarins and Furanocoumarins (Table 3)
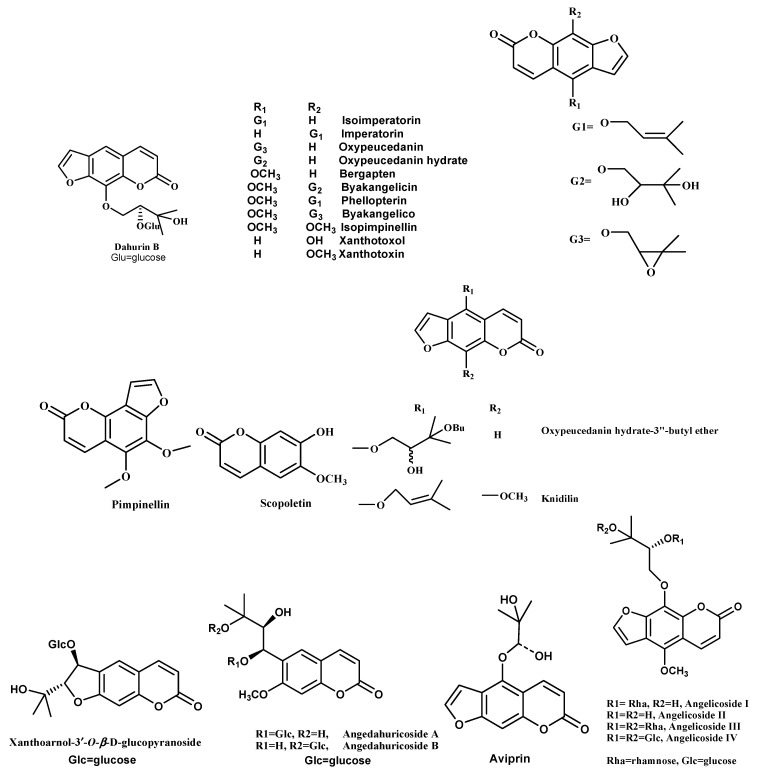
Earlier research on the A. dahurica plant showed the isolation of about 20 coumarins and 3 coumarin glycosides [69]. Zhao et al. (2007) focused on the water-soluble constituents from the fresh material of this plant. Here, the isolation and structure elucidation of the new coumarin glycoside named dahurin B were described. It was obtained as an optically active, yellowish amorphous powder. Dahurin B (C22H26O11) (Figure 2) was determined on the basis of its ESI-MS (489 [M+Na]+), and confirmed by 1H NMR and 13C NMR data. Detailed analysis of its 1H NMR, 13C NMR, COSY, HSQC, and HMBC spectra indicated the presence of a linear furanocoumarin glucoside and a 2-methylbutane structural unit [70].
Figure 2.
Structures of coumarins and furanocoumarins isolated from genus Angelica.
Li and Wu (2017) investigated the chemical constituents of A. dahurica. Fifteen compounds have been identified as isoimperatorin (1), imperatorin (2), oxypeucedanin (3), oxypeucedanin hydrate (4), bergapten (5), byakangelicin (6), phellopterin (7), byakangelicol (8), isopimpinellin (9), xanthotoxol (10), xanthotoxin (11), pimpinellin (12), scopoletin (13) (Figure 2), β-sitosterol (14), and daucosterol (15) [71,72].
Kim et al. (2002) found that the A. dahurica roots’ methanol extract fractionation led to the isolation of three furanocoumarins, namely isoimperatorin, imperatorin, and oxypeucedanin (Figure 2). The isolated compounds inhibited AChE activity in a dose-dependent manner (IC50 63.7 to 89.1 µM) [73]. In another study, five furanocoumarins (isoimperatorin, oxypeucedanin hydrate-3”-butyl ether, imperatorin, knidilin, and oxypeucedanin hydrate) (Figure 2) were isolated from the root of A. dahurica by repeated silica-gel column chromatography [74].
Yang et al. (2020) utilized a quantitative 1HNMR method (1H-qNMR) for the determination of the imperatorin, akangelicin, and oxypeucedanin content in A. dahurica, which are part of traditional Chinese medicine (TCM). The extraction was performed using an ultrasonication-assisted extraction method. The quantitative proton NMR measurements were executed on a 600-MHz spectrometer with hydroquinone as the internal standard reference in a deuterated dimethyl sulfoxide (DMSO-d6) solvent. Quantification was carried out using the 1H resonance signals for hydroquinone (6.55 ppm) and for imperatorin, byakangelicin, and oxypeucedanin, (7.68, 7.38–7.39 and 6.38–6.39 ppm), respectively. The linearity, limit of quantitation, precision, reproducibility, stability, limit of detection, and methodology were evaluated, and the results were good. The newly developed method has been applied to determine the three coumarins in A. dahurica [75].
Zhao et al. (2018) isolated ten compounds from the roots of A. dahurica, which were namely xanthoarnol-3′-O-β-D-glucopyranoside, angedahuricoside A, angedahuricoside B (Figure 2), isofraxidin-7-O-β-D-glucopyranoside, fraxidin-8-O-β-D-glucopyranoside, (-)-marmesinin, (2′S,3′R)-3′-hydroxymarmesinin, hyuganoside V, daucosterol, and sucrose [76]. In another study, six furocoumarins were isolated from the methanol extract of A. dahurica Benth. The isolated compounds were imperatorin, isoimperatorin, (±)-byakangelicol, (+)-oxypeucedanin, (+)-byakangelicin, and (+)-aviprin. [77].
Shua et al. (2020) investigated the phytochemicals present in A. dahurica roots using different chromatographic techniques viz. UV, IR, NMR, and HR-ESI-MS, together with acid hydrolysis and enzymatic hydrolysis. Four furanocoumarins were isolated, namely angelicosides I–III and angelicoside IV (Figure 2). [78].
Matsuo et al. (2020) performed a phytochemical investigation of the root of A. dahurica using 2D NMR data, hydrolysis, and different solvent solubility, followed by either physicochemical and spectroscopic data or X-ray crystallographic analysis. The investigation resulted in the isolation of a combination of benzofuran and coumarin derivatives. [79].
Isoimperatorin is a novel compound isolated from A. dahurica with wide biological applications [80]. Psoralen, isopsoralen, imperatorin, isoimperatorin, phellopterin, and cnidilin represent new furanocoumarins isolated from the root of A. dahurica [81]. Similarly, xanthotoxol was isolated from A. dahurica, and it carried potential anticancer activity [82]. Through NMR, IR, and LC/MS analysis, the structure of a new coumarin named angedahurin A was determined. This coumarin was isolated from the A. dahurica roots [83,84,85].
Table 3.
Coumarins and furanocoumarins isolated from Angelica dahurica.
| No. | Compound Name | Reference(s) |
|---|---|---|
| 1 | Dahurin B | [70] |
| 2 | Isoimperatorin | [71,73,74,77,80,81] |
| 3 | Imperatorin | [71,73,75,77,81] |
| 4 | Oxypeucedanin | [71,73,75,77] |
| 5 | Oxypeucedanin hydrate | [71,74] |
| 6 | Bergapten | [71] |
| 7 | Byakangelicin | [71,77] |
| 8 | Phellopterin | [71] |
| 9 | Byakangelicol | [71,77] |
| 10 | Isopimpinellin | [71] |
| 11 | Xanthotoxol | [71,82] |
| 12 | Xanthotoxin | [71] |
| 13 | Pimpinellin | [71] |
| 14 | Scopoletin | [71] |
| 15 | Oxypeucedanin hydrate-3”-butyl ether | [74] |
| 16 | Knidilin | [74] |
| 17 | Akangelicin | [75] |
| 18 | Xanthoarnol-3′-O-β-D-glucopyranoside | [76] |
| 19 | Angedahuricoside A | [76] |
| 20 | Angedahuricoside B | [76] |
| 21 | Isofraxidin-7-O-β-D-glucopyranoside | [76] |
| 22 | Fraxidin-8-O-β-D-glucopyranoside | [76] |
| 23 | (-)-Marmesinin | [76] |
| 24 | (2′S,3′R)-3′-Hydroxymarmesinin | [76] |
| 25 | Hyuganoside V | [76] |
| 26 | (+)-Aviprin | [77] |
| 27 | Angelicosides I–IV | [78] |
| 28 | Psoralen | [81] |
| 29 | Isopsoralen | [81] |
| 30 | Phellopterin | [81] |
| 31 | Cnidilin | [81] |
| 32 | Angedahurin A | [83] |
3.3. Phthalides
Zhang et al. (2013) evaluated the chemical and biological profiles of different Angelica roots collected from different geographical origins. The roots of A. sinensis presented ferulic acid, Z-ligustilide, and senkyunolide A, while butylphthalide and Z-butylenephthalide were isolated from A. gigas roots. [86]. Chao and Lin (2011) isolated about 70 compounds from A. sinensis (Oliv.) Diels roots. Ferulic acid represented the major component, followed by butylidenephthalide and other polysaccharides. [87]. Novel phthalide derivatives, namely oxaspiroangelioic acids A, B, and C, were isolated from the root extract of A. sinensis [88,89] (Table 4).
Table 4.
Phthalides isolated from genus Angelica.
3.4. Polysaccharides
A glucoarabinan polysaccharide was isolated from A. dahurica, formed mainly of arabinose and traces of glucose. It was named (ADP80-2), and its monomeric structure is composed of →5)-α-L-Araf-(1→, →3, 5)-α-L-Araf-(1→, →6)-α-D-Glcp-(1→, with a terminal branch α-L-Araf-(1 →residue [90]. Similarly, another polysaccharide was isolated from A. dahurica, with its monosaccharide content formed of D-mannose, D-glucose, D-galactose, and L-arabinose, and its first isomer was formed of D-mannose, D-glucose, D-galactose, and L-arabinose, while its second isomer was composed of D-mannose, D-galacturonic acid, D-glucose, D-galactose, and L-arabinose, and the third isomer was mainly constituted by D-mannose, L-rhamnose, D-galacturonic acid, D-galactoe, and L-arabinose [91]. A new acidic polysaccharide was isolated from A. dahurica with a sugar and uronic acid content of 91.04% and 12.69%. This polysaccharide was composed of rhamnose, arabinose, galactose, glucose, mannose, glucuronic acid, and galacturonicacid, with the following ratios (0.09: 0.61: 1.88: 1: 0.14: 0.63: 0.03). Its chemical skeleton showed the presence of this bonding manner: →3)-Manp-(1→, →4, 6)-Galp-(1→, →4)-Galp-(1→, →3)-Glcp-(1→, →5)-Araf-(1→, →2)-Galp-(1→ (0.32:0.57:0.29:0.95:0.71:0.26 molar ratios) [92].
3.5. Benzofurans
Bezofurans comprise an important class of phytoconstituents belonging to the heterocyclic compounds. From A. dahurica roots, six benzofurans were isolated including 3-[6,7-furano-9-hydroxy4-(2″,3″-dihydroxy-3″-methylbutyloxy)]-phenyl propionic acid, 3-[6,7-furano-9-(β-D-glucopyranosyloxy)-4-(2″,3″-dihydroxy-3″- methy butyloxy)]-phenyl propionic acid, 3-[6,7-furano-9-(β-D-glucopyranosyloxy)-4-(2″,3″-dihydroxy-3″-methylbutyloxy)]-phenyl propionic acid methyl ester, cnidioside A, methylcnidioside A, and methylpicraquassioside [79].
3.6. Polyacetylenes
Only two polyacetylenes were reported from genus Angelica, namely falcarindiol and octadeca-1,9-dien-4,6-diyn-3,8,18-triol [93].
4. Pharmacological Activities
4.1. Analgesic and Anti-Inflammatory Activity
An isolate from A. dahurica named byakangelicol was tested against IL-1-induced COX-2 expression and PGE2 release in the human pulmonary epithelial cell line (A549). The isolated compound decreased the expression of IL-1 and PGE2 release at a dose of (10–50 M) in a dose dependent manner. Byakangelicol when used at a dose of up to 200 M showed no effect on the expression of the COX-1 enzyme. The compound also showed no effect on IL-1-induced p44/42 mitogen-activated protein kinase (MAPK) activation. Treatment of cells with byakangelicol (50 M) or pyrrolidine dithiocarbamate (PDTC; 50 M) partially inhibited the IL-1-induced degradation of I B- in the cytosol, translocation of p 65NF- B from the cytosol to the nucleus, and the NF- B-specific DNA–protein complex formation. The mechanism of inhibition by which byakangelicol could be explained was by suppression of NFB activity [94].
Choi et al., 2008, evaluated the analgesic and anti-inflammatory activities of A. dahurica, using acetic acid and carrageenan to induce pain and edema in rats, respectively. In the analgesic model, the rats were injected with acetic acid, and visceral pain was tested through the writhing reflex. In the inflammation model, rats were injected with carrageenan in their paws, and the volume of edema was measured. Groups treated with A. dahurica showed a lower writhing reflex in a dose-dependent manner, while in the inflammation model, the paw edema was reduced significantly by treatment with the extract [95].
In this study, 5-Methoxy-8-(2-hydroxy-3-buthoxy-3-methylbutyloxy)-psoralen (MP) isolated from A. dahurica acted as an inhibitor of the COX-2-dependent phase of prostaglandin D2 (PGD2) generation in bone-marrow-derived mast cells (IC50 23.5 µM). This was further confirmed through a Western blot with specific anti-COX-2 antibodies, which caused a reduction in the production of PGD2, and also, in COX-2 protein levels. Moreover, MP further inhibited the production of leukotriene C4 (IC50 2.5 µM, depending on dose). Moreover, MP helps in the degranulation reaction inhibition (IC50 4.1 µM) [96].
Lee et al. (2011) descried the effects of the ethanol extract of A. dahurica on airway inflammation in an ovalbumin-induced airway inflammation model in mice. The group of mice treated with the extract showed significant lower airway eosinophilia, cytokine levels, including IL-4, IL-5, and TNF-α levels, mucus production, and IgE compared with OVA-induced mice. The extract acts by reducing airway inflammation and decreasing oxidative radical levels through the activation of heme oxygenase (HO)-1; thus the ethanol extract of A. dahurica can act as an allergic inflammatory modulator for asthmatic patients [97].
The essential oil of A. dahurica (dose 100 mg/kg) had an anti-inflammatory activity against xylene-induced ear swelling and carrageenan-induced paw edema in a mice model. The essential oil also significantly alleviated Freund’s complete adjuvant-induced arthritis in rats by improving hind-paw swelling and reducing the serum levels of nitric oxide, TNF-α, prostaglandin E2, and serum nitric oxide synthase activity [41]. Zhang et al. (2015) studied the relation between the GC/MS-identified metabolomics of the A. sinensis essential oil and their activity in rats with acute inflammation. In the carrageenan-injected rats, treatment with the essential oil of A. sinensis significantly rectified the levels of PGE2, histamine, and 5-hydroxytryptamine in the inflammatory fluid [98].
In another study, the essential oil of A. sinensis was tested against inflammation in carrageenan-induced acute inflammation in rats. Different processed essential oil samples were prepared for the study including: stir-fried A. sinensis, fried A. sinensis with alcohol, cooked A. sinensis with soil, and fried A. sinensis with sesame oil. All of the essential oil samples significantly inhibited PGE2, histamine, 5-hydroxytryptamine, and TNF-α release [99]. Similarly, the essential oil from A. sinensis showed an anti-inflammatory activity against the lipopolysaccharide (LPS)-induced inflammation in rats. The mechanism behind such activity is due to the regulation of the Krebs cycle, improving the glucose content, and thus restoring the fatty acid metabolism [100]. Li et al. (2016) also analyzed the anti-inflammatory activity of the A. sinensis essential oil on the LPS-induced acute inflammation in rats. The essential-oil-treated groups had a decrease in the levels of the inflammatory markers viz. cytokines (TNF-α, IL-1β, and IL-6), mediators (histamine, 5-hydroxytryptamine, PGE2, and nitric oxide), enzymes (nitric oxide synthase and cyclooxygenase 2), thus inducing anti-inflammatory and hepatoprotective activities compared to the control groups [101].
In this current study, Angelica polysaccharide (AP) was tested as an anti-inflammatory agent against mast cells and their molecular mechanism. AP was tested at doses of (50, 100, and 200 μg/mL), and the polysaccharide significantly reduced histamine, β-hexosaminidase, leukotrienes C4 (LTC4), IL-1, IL-4, TNF-α, IL-6, and human monocyte chemotactic protein-1 release; in addition, it inhibited Ca2+ entry to mast cells. Moreover, it downregulated the protein expressions of p-Fyn, p-Akt, p-P38, IL-4, TNF-α, and NF-κB p65 [102].
Li and Wu (2017) reported the isolation of fifteen compounds from A. dahurica. The isolated compounds inhibited the secretion of inflammatory mediators such as TNF-α, IL-1β, and IL-4. The anti-inflammatory activity was related to their inhibitory activity against the expression of the cytokine-producing genes, and, thus, the inhibition of nuclear factor-κB activation [71].
Isoimperatorin, isolated from A. dahurica roots, possessed potent anti-inflammatory activity in vitro. The anti-inflammatory activity was assayed through an MTT assay, real-time PCR, ELISA, and western blot, together with molecular docking, to assess the binding of isoimperatorin and myeloid differentiation protein-2 (MD-2), and elucidate the possible anti-inflammatory mechanism. Isoimperatorin significantly inhibited the release of NO, TNF-α, IL-6, and IL-1β usually secreted in inflammation. Real-time PCR resulted in the observation that isoimperatorin reduced the mRNA expressions of iNOs, COX-2, TNF-α, IL-6, and IL-1β. Isoimperatorin inhibited the formation of the proteins associated with the LPS-TLR4/MD-2-NF-κB signaling pathway, as shown in the Western blot. In addition to that, molecular docking showed the binding between isoimperatorin and MD-2 [103].
A traditional Chinese medicine named Huoxiangzhengqi oral liquid (HXZQ-OL) and is constituted of A. dahurica was evaluated for its potential anti-inflammatory and anti-allergic activity. The anti-allergic activity was tested using IgE/Ag-mediated RBL-2H3 cells with doses of (43.97, 439.7, and 4397 μg/mL) in vitro. The release of cytokines and eicosanoids were quantified using ELISA. RT-qPCR was utilized to assess cytokine gene expression. Immunoblotting analysis showed the mechanism of action of this traditional formula. The formula was also tested in vivo in mice through the passive cutaneous anaphylaxis (PCA) assay, where mice were orally administrated with the formula using doses of (263.8, 527.6, and 1055 mg/kg/d) for seven consecutive days. The formula successfully inhibited mast cell degranulation with an IC50 value of 123 μg/mL, and also prevented both the generation and secretion of IL-4 (IC50 171.4 μg/mL), TNF-α (IC50 88.4 μg/mL), LTC4 (IC50 52.9 μg/mL), and PGD2 (IC50 195.8 μg/mL). The formula also inhibited IL-4 and TNF-α mRNA expression. Moreover, the formula helped in the attenuation of the IgE-mediated PCA, with a 55% suppression of Evans blue exudation in mice (527.5 mg/kg) [104].
The essential oils isolated from different Angelica species, namely A. sinensis, A. dahurica, and A. pubescens, were evaluated for their anti-inflammatory activity using an ear edema model caused by 12-O-tetracycline-propylphenol-13-acetic acid (TPA) in mice, compared to Ibuprofen. Levels of the inflammatory markers and mediators such as TNF-α, COX-2, IL-6, and RelA (p65) were recorded by the immune-histochemical method. The tested essential oils significantly decreased the levels of TNF-α, COX-2, IL-6, and p65 [68].
Three polysaccharide isomers were purified from A. dahurica, and they showed scavenging activity against both DPPH free radical and hydroxyl free radical, along with ferric reducing power, which explains their potential use as natural antioxidants [91].
The A. dahurica extracts’ anti-inflammatory activity was evaluated using a complete Freund’s adjuvant-induced inflammatory pain mice model. The extract successfully reduced the mechanical and thermal hypersensitivities in a CFA-induced inflammatory pain model in mice; thus, the extract could play a role in chronic inflammation management [105].
The anti-inflammatory potential of the furanocoumarins (imperatorin and byakangelicin) isolated from the A. dahurica root extract was analyzed in order to pinpoint which furanocoumarin induces such activity in hepatocytes. The methanol root extract was fractionated using ethyl acetate, butanol, and water. Rat hepatocytes were grouped into two categories: the first treated with I)-1β, the second with each fraction (ethyl acetate, butanol, and aqueous fractions) for 8 h. Levels of both NO production and lactate dehydrogenase were recorded. The ethyl acetate fraction markedly suppressed NO production without showing cytotoxicity and decreased iNOS expression in hepatocytes. Five furanocoumarins were isolated from the ethyl acetate fraction, namely isoimperatorin, imperatorin, phellopterin, oxypeucedanin, and oxypeucedanin methanolate. Phellopterin and oxypeucedanin methanolate significantly suppressed NO production and reduced the mRNA expression of iNOs and TNF-α. A comparison of their chemical structures suggests that a methoxy group at carbon 5 and a side chain at carbon 8 in the furanocoumarin skeleton may be essential for NO production suppression. [106].
4.2. Cytotoxic Activity
Lee et al. (2020) utilized the chloroform fraction of the roots of A. dahurica to isolate eight furanocoumarins. Psoralen, xanthotoxin, and bergapten showed potent inhibitory activity against IR-induced migration at a non-cytotoxic concentration (50 μM) in human NSCLC A549 cells, and thus played an important role in cancer metastasis treatment [107].
The anti-cancer activity of the A. dahurica extract against the HT-29 colon cancer cell line was evaluated. The measured parameters included: cell viability, apoptotic, and necrotic activities. The non-polar extract of A. dahurica significantly reduced the gene expression of p53, Bcl, and Bax, and enhanced apoptosis through caspase cascade and cell cycle arrest. The polar fractions (ethanol-ethyl acetate) had cytotoxic activity in HT-29 cancer cells. Imperatorin and isoimperatorin were the main causes of such cytotoxic activity [108]. Isoimperatorin, isolated from A. dahurica, had a pronounced activity on the signaling pathway involved in cancer cell metastasis in colon and hepatic cancers when compared to its isomer, imperatorin [80]. A novel compound isolated from A. dahurica called xanthotoxol showed promising cytotoxic activity against non-small cell lung cancer development via the inhibition of cell viability, colony formation capacity, DNA replication, cell cycle transition, migration and invasion, and induction of apoptosis in NSCLC cells. It also suppresses NSCLC xenograft growth in vivo without toxicity [82].
A new acidic polysaccharide was isolated from A. dahurica, which showed significant cytotoxicity in an in vivo model of tumors in mice via enhancing the activities of spleen lymphocytes and natural killer (NK) cells and increasing the levels of IL-2 and TNF-α. Tumor cell apoptosis ranged from 7.54% to 19.32% (dose of 100 and 200 mg/kg) and was confirmed by pathological samples [92].
A new coumarin named angedahurin A was isolated from A. dahurica roots. Its potential cytotoxic activity against MG-63 human osteosarcoma cell lines was evaluated, where it showed marked cytotoxicity with IC50 7.2 μM, compared to 5-florouracil (IC50 32.4 μM) [83].
Cholecystokinin octapeptide was used to screen the cytotoxicity of alloimperatorin on HeLa, SiHa, and MS-751 cancer cell lines, and flow cytometry was used to record apoptosis. Apoptosis was confirmed through mitochondrial membrane potential, Western blot, and fluorescent PCR. Alloimperatorin showed potent cytotoxic activity against Hela cells (IC50 116.9 μM), by accelerating the apoptotic rate of HeLa cells and decreasing its mitochondrial membrane potential. Alloimperatorin also enhanced the expression of caspases 3, 8, and 9 in the Western blot [109].
4.3. Anti-Oxidant Activity
Pervin et al. (2014) evaluated the anti-oxidant activity of the aqueous and ethanol extracts of A. dahurica root. Antioxidant activity was tested using DPPH, ABTS assays, hydroxide radical scavenging activity, and lipid peroxidation (dose 0.12–2.0 mg/mL). The fractions scavenged the DPPH and ABTS radicals (IC50 0.32 and 0.20 mg/mL), respectively, for the aqueous extract, and (0.24 and 0.13 mg/mL), respectively, for the ethanol extract. The two extracts had potent reducing power and inhibited superoxide dismutase, catalase and DNA damage. It also inhibited the production of NO in a dose-dependent manner in lipopolysaccharide-treated RAW264.7 cells [110].
4.4. Antimicrobial Activity
Yang et al., (2020) evaluated the antibacterial activity of A. dahurica. Thirty Sprague–Dawley rats were divided into three groups: normal saline (NS), extract-treated, and biomycin-ointment-treated (BO). S. aureus was used to induce infection in dorsal excisions made on each rat, and each group received treatment once daily for 7 days. Treatment with the extract led to a smaller wound area compared to the two other groups, and the total bacterial count was lower as well. Body temperature and inflammatory markers viz. TNF-α and IL-6 levels markedly decreased in the extract-treated group. In wound tissue samples, the extract-treated group showed faster scab formation, denser granulation tissue, thicker epidermis, and more angiogenesis markers than the other groups [111,112].
The essential oil isolated from the Korean Angelica, especially sabinene and m-cresol, had strong antifungal activity against different species of Aspergillus and Trichophyton (MIC 125–1000 μg/mL). Moreover, the essential oil showed synergistic activity with itraconazole [113].
Irshad et al., 2011, tested the antimicrobial activity of the A. glauca essential oil against different bacterial (Staph. aureus, B. subtilis, E. coli, and Past. multocida) and fungal (C. albicans, M. canis, A. flavus, and F. solani) strains. The extract was active against E. coli and Staph. aureus (MIC 141.3 and 159.3 µg/mL), respectively, and M. canis (MIC178.1 µg/mL) [59].
Fraternale et al., 2014 & 2016, proved that the essential oil of the A. archangelica root had antimicrobial activity against C. difficile, C. perfringens, Ent. faecalis, E. limosum, P. anaerobius, and C. albicans. Moreover, the essential oil showed weaker activity against bifido bacteria and lactobacilli. In another study, the same species’ essential oil showed antifungal activity against some species of the Fusarium genus, B. cinerea and A. solani [57,114]. A combination of equal proportions of the A. archangelica essential oil, phenyl ethyl alcohol, and α-terpineol inhibited A. flavus NKDW-7 (afla toxigenic strain) and afla toxin B1 production (2.25 and 2.0 μL/mL), respectively [115].
Cavaleiro et al., 2015, tested the A. major essential oil (α-pinene and cis-β-ocimene) against different strains of yeasts and molds. The essential oil showed a broad spectrum of activity against all tested fungi (animal and human pathogenic species or spoilage fungi) viz. Candida spp., C. neoformans, Aspergillus spp. and dermatophytes. α-pinene was more potent compared to cis-β-ocimene [64]. Similarily, A. sinensis and A. dahurica essential oils had significant antibacterial activity against mastitis-causing pathogens (Staph. aureus, Staph. Chromogenes, and S. uberis) [116]. In addition to that, the essential oil of A. pubescentis exhibited weak antifungal activity against Colletotrichum acutatum, C. fragariae, and C. gloeosporioides. On the other hand, the A.dahurica root essential oil showed no activity against the same tested fungal strains [42].
In a recent study, the gold and copper nanoparticles prepared from the leaf extract of A. keiski were evaluated for their antibacterial activity, where they showed interaction with the bacterial cell wall of some tested Gram-negative bacteria, leading to cell wall rupture. The copper nanoparticles were more effective as antibacterial agents, showing wider zones of inhibition against E. coli, S. typhimurium, and Staph. aureus [117].
A. dahurica inhibited the ability of P. aeruginosa to form biofilm, thus reducing its resistant power and limiting its growth. In this context, coumarins such as imperatorin and isoimperatorin isolated from the extract of A. dahurica, combined with antibiotics such as ampicillin and ceftazidime, were evaluated against P. aeruginosa. The combination treatment showed superior antibacterial activity and reduced the biofilm formation in P. aeruginosa [118]. Four furanocoumarins were isolated through bioactivity-guided isolation from the 70% ethanol extract of A. dahurica roots. The isolated compounds were evaluated against the influenza virus. They acted by inhibiting cytopathic effects, which acted as a marker for their antiviral activity against the Chik influenza A (H1N1) and swine flu (H9N2) viruses. The most active compound was subjected to in-depth mechanistic studies viz. viral protein synthesis inhibition, cytopathic inhibition in different phases of the viral replication cycle, neuraminidase (NA) inhibition, antiapoptotic activity using flow cytometry, and immunofluorescence. The active compound showed anti-influenza-A activity through the inhibition of the early phase of the viral replication cycle, and not via direct neutralization of surface proteins, such as hemagglutinin and NA, and abnormal apoptosis [119].
4.5. Effects on Cardio- and Cerebrovascular Systems
Lee et al. (2015) studied the pharmacological mechanism behind the anti-hypertensive effect of A. dahurica, leading to its vasorelaxant effect. The 70% methanol extract of A. dahurica root was evaluated on the vasorelaxation of the rat thoracic aorta. Isolated rat aortic rings were suspended in organ chambers containing 10 mL Krebs–Henseleit (K–H) solution, and placed between two tungsten stirrups and connected to an isometric force transducer. Differences in tension were documented through isometric transducers connected to a data acquisition system. The extract had a dose-dependent relaxation in both the endothelium-intact and endothelium-denuded aortic rings precontracted with phenylephrine (PE; 1 μM) or potassium (KCl; 60 mM) in the K–H solution. Pre-treatment of the rings with the extract (1 mg/mL) successfully inhibited the Ca-induced vasocontraction of the aortic rings. Thus, the Angelica extract had a vasorelaxant effect, and this effect was mediated through an endothelium-independent pathway, which involves extracellular calcium influx inhibition via the receptor-operated Ca2+ channel and voltage-dependent calcium channel pathways [30].
The pharmacological activities of the extracts from A. sinensis and its active compounds on cardio- and cerebrovascular systems have been introduced in Modern Research and Application of Chinese Medicinal Plants, published in 2000 [120]. The water extract of the roots of A. sinensis and ferulic acid (FA) were able to inhibit rat platelet aggregation induced by adenosine diphosphate (ADP) and collagen in vitro. At the dose of 0.4–0.6 mg/kg (iv), sodium ferulate inhibited aggregation induced by ADP and collagen in rats [121]. Neither platelet aggregation nor arterial PGI2-like substance release was observed following the IV administration of sodium ferulate at a dose of 300 mg/kg. In a combined sodium ferulate and acetylsalicylic acid regimen, platelet aggregation and production of the TXA2-like substance were inhibited by 65% and 84%, respectively, while the production of the arterial PGI2-like substance remained unchanged. The combined treatment using sodium ferulate and acetylsalicylic acid could potentiate the antiplatelet action without inhibiting arterial PGI2-like substance release, suggesting that they may be valuable for the treatment of thromboembolic diseases [122].
Coumarin and its derivatives, natural anti-coagulants in Angelica spp., have been associated with both the bioactivity and toxicity of the plants, although A. sinensis contains a lower coumarin content compared to other closely related species [123]. FA, one of the constituents of A. sinensis, could inhibit the polymerization of platelets in blood circulation. It retards platelet release of 5-hydroxy-tryptamine (5-HT) and ADP [55]. Both FA and an aqueous extract of A. sinensis were found to inhibit platelet aggregation and serotonin release [55]. Due to the untold number of constituents, several pharmacological actions might be attributed to A. sinensis. Such characteristics include anticoagulation and antiplatelet activities [55], as well as hematopoiesis [124].
In an in vitro study of the preovulation follicles on chicken blood vessels, the extract of A. sinensis potentiated angiogenic activity, by enhancing the endothelial blood vessel growth through promoting the phosphorylation reaction involved in the activation of the endothelial growth factor receptor II [125].
4.6. Neuroprotective Action
LIG has been shown to reduce ischemic brain injury via anti-apoptotic pathways in a study by Chen et al., 2011, who investigated the neuroprotective potential of LIG after experimental subarachnoid hemorrhage (SAH) in rats. Rats with SAH, induced using the established double hemorrhage model, were studied with and without LIG treatment. Mortality, neurobehavioral evaluation, brain water content, blood–brain barrier (BBB) permeability, and vasospasm assessment of the basilar artery were measured on days 3 and 7 after injury. Additional testing was done to evaluate apoptosis using TdT-mediated dUTP-biotin nick end-labeling staining, as well as immunohistochemistry and Western blotting, to identify key proapoptotic/survival proteins such as p53, Bax, Bcl-2, and cleaved caspase-3. LIG treatment reduced mortality, neurobehavioral deficits, brain edema, BBB permeability, and cerebral vasospasm. In addition, treatment reduced the number of apoptotic cells in the surrounding brain injury site, which accompanied a marked downregulation of proapoptotic proteins, p53, and cleaved caspase-3 [126,127].
The extract of A. sinensis was orally administered to different groups of rats (0.5–1 g/Kg) to test its neuroprotective activity. The tested extract showed upregulation of several proteins in the hippocampus that are directly linked to the neuronal survival in this area, giving hope for treatment in cerebral ischemia patients [128]. Different Angelica sinensis extracts were evaluated against chronic unpredictable mild stress (CUMS)-induced depression in rats. A CUMS-inducing procedure was performed in male rats to induce depression. They were exposed to it for 5 weeks, which led to depressive behaviors in rats, involving a reduction in sucrose consumption and reduced sucrose preference ratios in a sucrose preference test, lengthened immobility times, lesser struggling time in a forced-swim test, and decreased locomotor activity in an open field test. Moreover, the expression of brain-derived neurotrophic factor (BDNF), and the phosphorylation of cAMP-response element binding protein and extracellular signal-regulated protein kinase (ERK 1/2) were significantly decreased in the hippocampus in depressed rats. Treating the depressed rats with Angelica sinensis extracts (1 g/kg) normalized their depression-related behaviors and molecular profiles [129].
4.7. Immune Support and Hematopoiesis
FA-induced anti-immobility was prevented by pretreatment with PCPA, WAY-100635, ketanserin, sulpiride, SCH233390, haloperidol, and yohimbine, independently. CRH, ACTH, and 5-HT were significantly decreased, but ghrelin was apparently increased compared with vehicle. In summary, FA induced anti-depression and prokinetics via inhibiting 5-HT, norepinephrine and dopamine reuptakes, regulating HPA axis, increasing ghrelin, and stimulating jejunal contraction simultaneously [130]. Lymphocyte proliferation assays indicate that A. sinensis consistently exerts an immune-stimulatory effect [131,132]. A high-molecular-weight polysaccharide found in A. sinensis has demonstrated immune-stimulating activity and a blood-tonifying effect by inducing hematopoiesis in the bone marrow. This is accomplished, in part, by either the direct or indirect stimulation of macrophages, fibro-blasts, erythrocytes, granulocytes, and lymphocytes, and could induce an increased secretion of human growth factors from muscle tissue. The evidence of hematopoiesis is further supported by the presence of significant amounts of vitamin B12, folinic acid, and biotin in A. sinensis [124].
4.8. Antifibrotic Action
A mixture of Angelica and Astragalus demonstrated antifibrotic activity in a recent animal study. Rat models with chronic puromycin-induced nephrosis were treated with either an Angelica and Astragalus mixture (3 mL/d), or Enalapril (10 mg/kg). The normal control group received saline, and another group received puromycin with no treatment [133]. After 12 weeks, the untreated rats showed marked renal fibrosis. However, the Angelica and Astragalus mixture significantly retarded the progression of renal fibrosis and deterioration of renal histological damage, with effects comparable to Enalapril [133].
Geng et al., 2017, found that the ethanol and aqueous extracts from either ASR or AR led to a reduction in the area of collagen fibers and the extent of alveolus inflammation, and also the content of Hyp in lung tissue and lung index. The above-mentioned extracts may protect against rat pulmonary fibrosis induced by bleomycin and showed a protective effect on pulmonary fibrosis in rats in the early period [134].
4.9. Antispasmodic Activity
LIG, butylidenephthalide, and butylphthalide were found to have antispasmodic activity against rat uterine contractions and in other smooth muscle systems. The components were characterized as non-specific anti-spasmodic, with a mechanism different from papaverine [123,135].
4.10. Hepatoprotective Activity
Cao et al. (2018) investigated the hepatoprotective effects of Angelica sinensis polysaccharide (ASP), an active constituent derived from the aqueous extract of Angelica sinensis, in rats exposed to an APAP overdose. The mechanisms underlying the activity of this compound were also considered. Specifically, serum and hepatic biochemical parameters including alanine aminotransferase (ALT), aspartate transaminase (AST), glutathione (GSH), malondialdehyde (MDA), and superoxide dismutase (SOD) were evaluated, and key proteins involved in hepatic apoptosis, including cleaved caspase-3, Bax, and Bcl-2 were quantified. In vivo, H&E staining reveals that ASP reduces the degeneration of hepatocytes and the amount of cytoplasmic vacuolation in rats exposed to an overdose of APAP. ASP markedly alleviated liver injury via an increase in GSH levels and the inhibition of hepatic apoptosis. In vitro, ASP significantly elevated the survival rate of rat primary hepatocytes exposed to an overdose of APAP. The beneficial effect might be, at least in part, due to the amelioration of lipid peroxidation and oxidative stress, along with the inhibition of apoptosis. Taken together, their findings reveal that ASP has the potential to be used as a hepatoprotective agent for the management of APAP-induced liver injury [136].
4.11. Antidiabetic
Park et al. (2016) found that phellopterin, isolated from Angelica dahurica, can act as a powerful anti-diabetic agent. This compound can elevate insulin secretion and enhance glucose tolerance in vivo via the activation of GPR119. Mice treated with this Angelica extract showed an enhanced glucose tolerance and increased insulin secretion, while giving them repeated doses of the Angelica extract or the n-hexane fraction led to an improvement in the glucose tolerance in diabetic mice. Imperatorin, phellopterin, and isoimperatorin were isolated from the active fraction of the extract from which phellopterin caused the activation of GPR119 and GLP-1, increased insulin secretion in vitro, and enhanced glucose tolerance in normal and diabetic mice. Thus, this pure compound could play an important role in type II diabetes treatment [137,138,139].
4.12. Skin Permeation Enhancer
Essential oils, being lipid soluble, could help in overcoming the skin permeation of drugs through bypassing the stratum corneum barrier. In this context, [140] evaluated the potential activity of five essential oil samples (clove, Angelica, Chuanxiong, Cyperus, and cinnamon) as permeation enhancers to help in drug delivery through the transdermal route. Ibuprofen was selected and applied using dysmenorrheal model mice. Chuanxiong and Angelica essential oils effectively enhanced the transdermal drug delivery of ibuprofen.
Similarly, Jiang et al. (2017) studied different essential oils viz. turpentine, Angelica, Chuanxiong, Cyperus, cinnamon, and clove oils (concentration 3% w/v) as skin permeation enhancers for ibuprofen in rats. The studied essential oil samples caused a significant increase in skin penetration by the drug effect, with less skin irritation [141].
4.13. Estrogenic Activity
Piao et al. (2006) isolated eleven furanocoumarins as new and effective phytoestrogens from A. dahurica. They were effective in treating menopausal symptoms, showing estrogenic activity on the Ishikawa cell line. 9-hydroxy-4-methoxypsoralen and alloisoim-peratofin significantly induced alkaline phosphatase (EC50 1.1 and 0.8 µLg/mL), respectively, compared to no or weak activity for the rest of the isolated furanocoumarins [142]. The effect of A. sinensis on many gynecological disorders is well-known; however, this effect is usually imparted through the hormonal-like activity of this herb, which raised many concerns for its use in breast cancer patients by enhancing tumor growth [143].
4.14. Skin Whitening
The root extract of A. dahurica was investigated on NK-1R and Wnt/β-catenin signaling, and evaluated against NK-1R on melanogenesis in B16F0 cells. The extract showed a potent reduction in Neurokinin-1 receptor and Wnt/β-catenin signaling activities via reducing the expression of β-catenin, MITF, LEF-1, TYR, TRP1, and TRP2, and enhancing the expression of GSK3β [144].
4.15. Immunomodulatory Activity
A polysaccharide isolated from A. dahurica exhibited an immunoregulatory activity in a Zebra fish model. It activated phagocytosis, enhanced the production of (NO), and promoted the secretion of (IL-6, IL-1β, and TNF-α) [90].
4.16. Effect on Gut Flora
The aqueous extract of A. dahurica containing novel polysaccharides was evaluated for its interaction with gut flora. The pure polysaccharide was administered at a dose of 200 mg/kg daily to mice for 21 days. The microbial composition was evaluated in fecal samples using high-throughput sequencing. The polysaccharide showed an effect on the composition and structure of gut microbiota, and can potentially regulate the intestinal flora as prebiotics [145].
4.17. Insecticidal Activity
Essential oil isolated from A. dahurica and A. pubescentis roots were studied as pest management prospectives. When compared with A. pubescentis, A. dahurica showed better biting inhibition and insecticidal effects against Ae. egypti and Stephanitis pyrioides. In mosquito bioassays, components of the A. dahurica essential oil, 1-dodecanol and 1-tridecanol, showed antibiting activity against Ae. egypti [42].
Chung et al., 2012, investigated the immune toxicity effect of the essential oil from the leaves of A. anomala, A. cartilagino-marginata var. distans, A. czernevia, A. dahurica, A. decursiva, A. fallax, A. gigas, and A. japonica. Among them, the essential oil of A. dahurica showed a significant toxic effect against early fourth-stage larvae of Ae. aegypti (LC50 43.12 ppm) [146]. In another study, out of 33 plant species tested, Champakaew et al., 2015, found that A.sinensis essential oil showed the best repellent activity against Ae. egypti with a complete protection time of 7.0 h [53].
5. Discussion
In this review work, genus Angelica’s various phytochemical constituents, folk medicinal uses, adverse effects, potential toxicity, and reported biological activities have been summarized, with a special emphasis on Angelica dahurica. The A. dahurica species was selected for more detailed literature reviewing, as it is one of the most important and popular members of genus Angelica, being rich in coumarins and furanocoumarins, as one of the highly important class of compounds accounting for a wide range of biological activities viz. analgesic, anti-inflammatory, anti-coagulant, and cytotoxic activities. Angelica dahurica is commonly reported in folk medicinal practices, such as its use against skin diseases, pruritus, common cold, headache, and toothache; hepatoprotection; its antimicrobial effect; and its anti-inflammatory and cytotoxic activities. Different phytoconstituents were summarized covering different chemical classes, including coumarins, furanocoumarins, phtalides, polysaccharides, benzofurans, polyacetylenes, and essential oils. A total of 64 essential oil and other volatile components were reported from different Angelica species, together with 32 coumarins and furanocoumarins, six phthalides, six polyacetylenes, two benzofurans, and two polysaccharides. Essential oil components were traced from different Angelica species viz. A. archangelica, A. archangelica subsp. Archangelica, A. dahurica, A. pubescentis, A. sinensis, A. glauca, A. gigas, A. acutiloba, A. major, A. urumiensis, A. pancicii, A. viridiflora, A. cincta, and A. pubescens, while coumarins, furanocoumarins, benzofurans, and polyacetylenes accumulated mainly in Angelica dahurica. Moreover, phthalides were reported from A. gigas and A. sinensis. Different biological activities were summarized and directly linked to the reported phytoconstituents. The analgesic, anti-inflammatory, anti-histaminic, anti-oxidant, and cytotoxic activities were mainly related to the richness of genus Angelica with coumarins and furanocoumarins. Furanocoumarins, especially isoimperatorin, imperatorin, and oxypeucedanin, showed strong acetylcholine esterase inhibitory activity, which explains their activity in CNS-related disorders and, furthermore, Alzheimer’s and dementia. Many oxygenated terpenoids had been reported from the essential oils of different organs of genus Angelica, thus carrying strong anti-oxidant and anti-microbial activities against a wide range of bacteria, viruses, and fungi.
6. Conclusions
In conclusion, this review summarizes the reported literature on the phytochemical and biological activities of genus Angelica, focusing on A. dahurica as one of the most important plant genera, with many folk medicinal uses. Genus Angelica is rich mainly in coumarins and furanocoumarins, with about 32 of them reported only from A. dahurica, followed by phthalides and polysaccharides. Such phytoconstituents showed many biological activities, including, mainly: antimicrobial, antioxidant, anti-inflammatory, hepatoprotective, insecticidal, antidiabetic, etc. This updated review provides valuable references regarding genus Angelica, thus enriching the scientific development and future research on genus Angelica.
Author Contributions
Conceptualization, G.E.-S.B.; data validation, G.E.-S.B., H.M.S., E.A.E. and N.M.M.; data analysis, H.M.S., E.A.E. and N.M.M.; writing—original draft preparation, G.E.-S.B. and H.M.S.; writing—review and editing, E.A.E., N.M.M., O.A.E. and J.-M.S.; supervision, G.E.-S.B., N.M.M., O.A.E. and J.-M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Reduron J.-P. Carrots and Related Apiaceae Crops. CABI; Wallingford, UK: 2020. Taxonomy, origin and importance of the Apiaceae family; pp. 1–8. [Google Scholar]
- 2.Feng T., Downie S.R., Yu Y., Zhang X., Chen W., He X., Liu S. Molecular systematics of Angelica and allied genera (Apiaceae) from the Hengduan mountains of China based on nrDNA its sequences: Phylogenetic affinities and biogeographic implications. J. Plant Res. 2009;122:403–414. doi: 10.1007/s10265-009-0238-4. [DOI] [PubMed] [Google Scholar]
- 3.Trineeva O. Features quality assessment and prospects standartization fatty oils and oil extracts for pharmaceutical purposes. Drug Dev. Regist. 2016;2:114–134. [Google Scholar]
- 4.Younis I.Y., El-Hawary S.S., Eldahshan O.A., Abdel-Aziz M.M., Ali Z.Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 2021;11:16868. doi: 10.1038/s41598-021-96377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J.-h., Zhang Z.-l., Wang Y.-q., Yang M., Wang C.-h., Li X.-w., Guo Y.-w. Chemical constituents from mycelia and spores of fungus Cordyceps cicadae. Chin. Herb. Med. 2017;9:188–192. doi: 10.1016/S1674-6384(17)60094-7. [DOI] [Google Scholar]
- 6.Saiki Y., Morinaga K., Okegawa O., Sakai S., Amaya Y. On the coumarins of the roots of Angelica dahurica Benth. et Hook. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1971;91:1313–1317. doi: 10.1248/yakushi1947.91.12_1313. [DOI] [PubMed] [Google Scholar]
- 7.Wang N.-H., Yoshizaki K., Baba K. Seven new bifuranocoumarins, dahuribirin AG, from Japanese Bai Zhi. Chem. Pharm. Bull. 2001;49:1085–1088. doi: 10.1248/cpb.49.1085. [DOI] [PubMed] [Google Scholar]
- 8.Kimura Y., Ohminami H., Arichi H., Okuda H., Baba K., Kozawa M., Arichi S. Effects of various coumarins from roots of Angelica dahurica on actions of adrenaline, ACTH and insulin in fat cells. Planta Med. 1982;45:183–187. doi: 10.1055/s-2007-971370. [DOI] [PubMed] [Google Scholar]
- 9.Kwon Y.-S., Kobayashi A., Kajiyama S.-I., Kawazu K., Kanzaki H., Kim C.-M. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry. 1997;44:887–889. doi: 10.1016/S0031-9422(96)00634-6. [DOI] [PubMed] [Google Scholar]
- 10.Lechner D., Stavri M., Oluwatuyi M., Pereda-Miranda R., Gibbons S. The anti-staphylococcal activity of Angelica dahurica (Bai Zhi) Phytochemistry. 2004;65:331–335. doi: 10.1016/j.phytochem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.K., Kim Y.S., Ryu S.Y. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res. 2007;21:288–290. doi: 10.1002/ptr.2043. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.-O., Lee G.-D., Kwon J.-H., Kim K.-S. Clinical Traditional Herbalogy, 99-689, 1997. Biol. Pharm. Bull. 2009;32:421–426. doi: 10.1248/bpb.32.421. [DOI] [PubMed] [Google Scholar]
- 13.Song D.-K., Kim J.-Y., Li G., Lee K.-S., Seo C.-S., Yan J.-J., Jung J.-S., Kim H.-J., Chang H.-W., Son J.-K. Agents protecting against sepsis from the roots of Angelica dahurica. Biol. Pharm. Bull. 2005;28:380–382. doi: 10.1248/bpb.28.380. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi-Rad J., Quispe C., Durazzo A., Lucarini M., Souto E.B., Santini A., Imran M., Moussa A.Y., Mostafa N.M., El-Shazly M. Resveratrol’biotechnological applications: Enlightening its antimicrobial and antioxidant properties. J. Herb. Med. 2022;32:100550. doi: 10.1016/j.hermed.2022.100550. [DOI] [Google Scholar]
- 15.Germann I., Hagelauer D., Kelber O., Vinson B., Laufer S., Weiser D., Heinle H. Antioxidative properties of the gastrointestinal phytopharmaceutical remedy STW 5 (Iberogast®) Phytomedicine. 2006;13:45–50. doi: 10.1016/j.phymed.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Wei A., Shibamoto T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007;55:1737–1742. doi: 10.1021/jf062959x. [DOI] [PubMed] [Google Scholar]
- 17.Ka M.-H., Choi E.H., Chun H.-S., Lee K.-G. Antioxidative activity of volatile extracts isolated from Angelica tenuissimae roots, peppermint leaves, pine needles, and sweet flag leaves. J. Agric. Food Chem. 2005;53:4124–4129. doi: 10.1021/jf047932x. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah S.H., Mostafa N.M., Mohamed M.A.E.H., Nada A.S., Singab A.N.B. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat. Prod. Res. 2021;36:1–7. doi: 10.1080/14786419.2021.2015594. [DOI] [PubMed] [Google Scholar]
- 19.Hanelt P., Institute of Plant Genetics And Crop Plant Research, editor. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops. Volume 1. Springer; Berlin, Germany: 2017. p. 3716. [Google Scholar]
- 20.Bai Y.-J., Kong M., Xu J.-D., Zhang X.-L., Zhou S.-S., Wang X.-N., Liu L.-F., Li S.-L. Effect of different drying methods on the quality of Angelicae sinensis radix evaluated through simultaneously determining four types of major bioactive components by high performance liquid chromatography photodiode array detector and ultra-high performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2014;94:77–83. doi: 10.1016/j.jpba.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe A., Araki S., Kobari S., Sudo H., Tsuchida T., Uno T., Kosaka N., Shimomura K., Yamazaki M., Saito K. In vitro propagation, restriction fragment length polymorphism, and random amplified polymorphic DNA analyses of Angelica plants. Plant Cell Rep. 1998;18:187–192. doi: 10.1007/s002990050554. [DOI] [PubMed] [Google Scholar]
- 22.Menglan S., Fading P., Zehui P., Watson M.F., Cannon J.F., Holmes-Smith I., Kljuykov E.V., Phillippe L.R., Pimenov M.G. Apiaceae (Umbelliferae) Flora China. 2005;14:1–205. [Google Scholar]
- 23.Wei W., Gong S., Zhang T., Hu J. Research progress on the compositions of Angelica polysaccharide and their pharmacological action. Drug Eval. Res. 2009;32:130–134. [Google Scholar]
- 24.Ma J., Clemants S. A history and overview of the Flora Reipublicae Popularis Sinicae (FRPS, Flora of China, Chinese edition, 1959–2004) Taxon. 2006;55:451–460. doi: 10.2307/25065592. [DOI] [Google Scholar]
- 25.Zhang H., Luo L., Yu Y., Qiu J., Hu Z., Liao W. The study of the germplasm survey of Angelica sinensis. J. Chin. Med. Mat. 2009;32:335–337. [Google Scholar]
- 26.Maurya A., Verma S.C., Gupta V., Shankar M. Angelica archangelica L.-A phytochemical and pharmacological review. Asian J. Res. Chem. 2017;10:852–856. doi: 10.5958/0974-4150.2017.00142.0. [DOI] [Google Scholar]
- 27.Park Y., Park P.S., Jeong D.H., Sim S., Kim N., Park H., Jeon K.S., Um Y., Kim M.-J. The characteristics of the growth and the active compounds of Angelica gigas Nakai in cultivation sites. Plants. 2020;9:823. doi: 10.3390/plants9070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar P., Rana V., Singh A.N. Angelica glauca Edgew—A comprehensive review. J. Appl. Res. Med. Aromat. Plants. 2022;31:100397. doi: 10.1016/j.jarmap.2022.100397. [DOI] [Google Scholar]
- 29.Matsubara K., Shindo S., Watanabe H., Ikegami F. Identification of Angelica acutiloba and related species by analysis of inter-and intra-specific sequence variations in chloroplast and nuclear DNA sequences. Am. J. Plant Sci. 2012;3:1260–1265. doi: 10.4236/ajps.2012.39152. [DOI] [Google Scholar]
- 30.Lee K., Shin M.S., Ham I., Choi H.-Y. Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC Complement. Altern. Med. 2015;15:1–8. doi: 10.1186/s12906-015-0889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C., Tseng A., Yang S. Chinese Herbal Medicine: Modern Applications of Traditional Formulas. CRC Press; Boca Raton, FL, USA: 2004. [Google Scholar]
- 32.Mostafa N.M., Edmond M.P., El-Shazly M., Fahmy H.A., Sherif N.H., Singab A.N.B. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat. Prod. Res. 2022;36:4187–4192. doi: 10.1080/14786419.2021.1961252. [DOI] [PubMed] [Google Scholar]
- 33.Chen J., Li H., Wang D., Yang C., Liu Y. Determination of sucrose in radix Angelicae sinensis by HPLC-ELSD. Mod. Chin. Med. 2008;10:19–20. [Google Scholar]
- 34.Hook I.L. Danggui to Angelica sinensis root: Are potential benefits to European women lost in translation? A review. J. Ethnopharmacol. 2014;152:1–13. doi: 10.1016/j.jep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Sowndhararajan K., Kim S. Neuroprotective and cognitive enhancement potentials of Angelica gigas Nakai root: A review. Sci. Pharm. 2017;85:21. doi: 10.3390/scipharm85020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowndhararajan K., Seo M., Kim M., Kim H., Kim S. Effect of essential oil and supercritical carbon dioxide extract from the root of Angelica gigas on human EEG activity. Complement. Ther. Clin. Pract. 2017;28:161–168. doi: 10.1016/j.ctcp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Abdelghffar E.A., El-Nashar H.A., Al-Mohammadi A.G., Eldahshan O.A. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 2021;12:9443–9455. doi: 10.1039/D1FO01905H. [DOI] [PubMed] [Google Scholar]
- 38.Hatano R., Takano F., Fushiya S., Michimata M., Tanaka T., Kazama I., Suzuki M., Matsubara M. Water-soluble extracts from Angelica acutiloba Kitagawa enhance hematopoiesis by activating immature erythroid cells in mice with 5-fluorouracil-induced anemia. Exp. Hematol. 2004;32:918–924. doi: 10.1016/j.exphem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan R.S., Nautiyal M.C., Cecotti R., Mella M., Tava A. Variation in the essential oil composition of Angelica archangelica from three different altitudes in Western Himalaya, India. Ind. Crops Prod. 2016;94:401–404. doi: 10.1016/j.indcrop.2016.08.044. [DOI] [Google Scholar]
- 40.Agnihotri V.K., Thappa R.K., Meena B., Kapahi B.K., Saxena R.K., Qazi G.N., Agarwal S.G. Essential oil composition of aerial parts of Angelica glauca growing wild in North-West Himalaya (India) Phytochemistry. 2004;65:2411–2413. doi: 10.1016/j.phytochem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang C., Sun J., Li H., Yang X., Liu H., Chen J. In vivo anti-inflammatory activities of the essential oil from radix Angelicae dahuricae. J. Nat. Med. 2016;70:563–570. doi: 10.1007/s11418-016-0978-0. [DOI] [PubMed] [Google Scholar]
- 42.Tabanca N., Gao Z., Demirci B., Techen N., Wedge D.E., Ali A., Sampson B.J., Werle C., Bernier U.R., Khan I.A. Molecular and phytochemical investigation of Angelica dahurica and Angelica pubescentis essential oils and their biological activity against Aedes aegypti, Stephanitis pyrioides, and Colletotrichum species. J. Agric. Food Chem. 2014;62:8848–8857. doi: 10.1021/jf5024752. [DOI] [PubMed] [Google Scholar]
- 43.Gamal El-Din M.I., Youssef F.S., Ashour M.L., Eldahshan O.A., Singab A.N.B. Comparative analysis of volatile constituents of Pachira aquatica Aubl. and Pachira glabra Pasq., their anti-Mycobacterial and anti-Helicobacter pylori activities and their metabolic discrimination using chemometrics. J. Essent. Oil Bear. Plants. 2018;21:1550–1567. doi: 10.1080/0972060X.2019.1571950. [DOI] [Google Scholar]
- 44.Ahn J., Ahn M.-J., Chin Y.-W., Kim J. Pharmaceutical Studies on “Dang-Gui” in Korean Journals. Nat. Prod. Sci. 2019;25:285–292. doi: 10.20307/nps.2019.25.4.285. [DOI] [Google Scholar]
- 45.China P.C. Pharmacopoeia of the People’s Republic of China. Chemical Industry Press; Beijing, China: 2000. [Google Scholar]
- 46.Schmidt M. Recent developments in risk assessments of herbal medicinal products: Unlimited limitation? Planta Med. 2007;73:1006. doi: 10.1055/s-2007-987370. [DOI] [Google Scholar]
- 47.Dymowski W. Assessment Report on Angelica Sinensis (Oliv.) Diels, Radix. Committee on Herbal Medicinal Products; London, UK: 2013. EMA/HMPC/614586/2012. [Google Scholar]
- 48.Zhao H., Feng Y.-L., Wang M., Wang J.-J., Liu T., Yu J. The Angelica dahurica: A review of traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 2022:2367. doi: 10.3389/fphar.2022.896637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang W.-H., Chang T.-W., Charng Y.-C. Effects of drying methods on contents of bioactive compounds and antioxidant activities of Angelica dahurica. Food Sci. Biotechnol. 2018;27:1085–1092. doi: 10.1007/s10068-018-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarker U., Rabbani M., Oba S., Eldehna W.M., Al-Rashood S.T., Mostafa N.M., Eldahshan O.A. Phytonutrients, colorant pigments, phytochemicals, and antioxidant potential of orphan leafy Amaranthus species. Molecules. 2022;27:2899. doi: 10.3390/molecules27092899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shchipitsyna O., Efremov A. Composition of ethereal oil isolated from various vegetative parts of Angelica from the Siberian region. Russ. J. Bioorganic Chem. 2011;37:888–892. doi: 10.1134/S1068162011070223. [DOI] [Google Scholar]
- 52.Mostafa N.M., Mostafa A.M., Ashour M.L., Elhady S.S. Neuroprotective effects of black pepper cold-pressed oil on scopolamine-induced oxidative stress and memory impairment in rats. Antioxidants. 2021;10:1993. doi: 10.3390/antiox10121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Champakaew D., Junkum A., Chaithong U., Jitpakdi A., Riyong D., Sanghong R., Intirach J., Muangmoon R., Chansang A., Tuetun B. Angelica sinensis (Umbelliferae) with proven repellent properties against Aedes aegypti, the primary dengue fever vector in Thailand. Parasitol. Res. 2015;114:2187–2198. doi: 10.1007/s00436-015-4409-z. [DOI] [PubMed] [Google Scholar]
- 54.Ashmawy A., Mostafa N., Eldahshan O. GC/MS analysis and molecular profiling of lemon volatile oil against breast cancer. J. Essent. Oil Bear. Plants. 2019;22:903–916. doi: 10.1080/0972060X.2019.1667877. [DOI] [Google Scholar]
- 55.Vibrans H. Principals and practice of phytotherapy. Modern herbal medicine-Simon Mills, Kerry Bone. Churchill Livingstone, London. 2000. 643+ xx p. ISBN 0-443-060169. US $79.00. J. Ethnopharmacol. 2002;1:140–141. doi: 10.1016/S0378-8741(02)00032-6. [DOI] [Google Scholar]
- 56.Nivinskienë O., Butkienë R., Mockutë D. Chemical composition of seed (fruit) essential oils of Angelica archangelica L. growing wild in Lithuania. Chemija. 2005;16:51–54. [Google Scholar]
- 57.Fraternale D., Flamini G., Ricci D. Essential oil composition and antimicrobial activity of Angelica archangelica L.(Apiaceae) roots. J. Med. Food. 2014;17:1043–1047. doi: 10.1089/jmf.2013.0012. [DOI] [PubMed] [Google Scholar]
- 58.Pasqua G., Monacelli B., Silvestrini A. Accumulation of essential oils in relation to root differentiation in Angelica archangelica L. Eur. J. Histochem. 2003;47:87–90. doi: 10.4081/811. [DOI] [PubMed] [Google Scholar]
- 59.Irshad M., Shahid M., Aziz S., Ghous T. Antioxidant, antimicrobial and phytotoxic activities of essential oil of Angelica glauca. Asian J. Chem. 2011;23:1947. [Google Scholar]
- 60.Purohit V.K., Andola H.C., Haider S.Z., Tiwari D., Bahuguna Y.M., Gairola K.C., Arunachalam K. Essential oil constituents of Angelica glauca Edgew. Roots: An endangered species from Uttarakhand Himalaya (India) Natl. Acad. Sci. Lett. 2015;38:445–447. doi: 10.1007/s40009-015-0395-z. [DOI] [Google Scholar]
- 61.Kim M., Abd El-Aty A., Kim I., Shim J. Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic–mass spectrometric analysis. J. Chromatogr. A. 2006;1116:259–264. doi: 10.1016/j.chroma.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 62.Seo H.-Y., Yang S.-H., Shim S.-L., No K.-M., Park K.-S., Song K.-D., Kim K.-S. Volatile organic compounds of Angelica gigas Nakai, Korean medicinal herb. Nat. Prod. Res. 2007;21:265–273. doi: 10.1080/14786410601129598. [DOI] [PubMed] [Google Scholar]
- 63.Chen H.-C., Tsai Y.-J., Lin L.-Y., Wu C.-S., Tai S.-P., Chen Y.-C., Chiang H.-M. Volatile compounds from roots, stems and leaves of Angelica acutiloba growing in Taiwan. Nat. Prod. Commun. 2014;9:1934578X1400900441. doi: 10.1177/1934578X1400900441. [DOI] [PubMed] [Google Scholar]
- 64.Cavaleiro C., Salgueiro L., Gonçalves M.-J., Hrimpeng K., Pinto J., Pinto E. Antifungal activity of the essential oil of Angelica major against Candida, Cryptococcus, Aspergillus and dermatophyte species. J. Nat. Med. 2015;69:241–248. doi: 10.1007/s11418-014-0884-2. [DOI] [PubMed] [Google Scholar]
- 65.Mohammadi M., Yousefi M., Habibi Z. Essential oils from stem and leaves of Angelica urumiensis (Mozaffarian) from Iran. Nat. Prod. Res. 2010;24:1347–1351. doi: 10.1080/14786410903421438. [DOI] [PubMed] [Google Scholar]
- 66.Simonović S.R., Stankov-Jovanović V.P., Mitić V.D., Ilić M.D., Petrović G.M., Stojanović G.S. Chemical composition of Angelica pancicii essential oil determined by liquid and headspace GC-MS techniques. Nat. Prod. Commun. 2014;9:1934578X1400900235. doi: 10.1177/1934578X1400900235. [DOI] [PubMed] [Google Scholar]
- 67.Suleimen E., Iskakova Z.B., Dudkin R., Gorovoi P., Wang M., Khan I., Ross S., Martins C. Composition and biological activity of essential oils from East-Asian species Angelica viridiflora, A. cincta, and Coelopleurum gmelinii. Chem. Nat. Compd. 2014;50:1136–1139. doi: 10.1007/s10600-014-1184-5. [DOI] [Google Scholar]
- 68.Li C., Cai Q., Wu X., Tan Z., Yao L., Huang S., Zhang W., Hong Z., Chen Z., Zhang L. Anti-inflammatory Study on the Constituents of Angelica sinensis (Oliv.) Diels, Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav., Angelica pubescence Maxim and Foeniculum vulgare Mill. essential oils. J. Oleo Sci. 2022;71:1207–1219. doi: 10.5650/jos.ess22031. [DOI] [PubMed] [Google Scholar]
- 69.Zhao X.Z., Feng X., Jia X.D., Dong Y.F., Wang M. Neolignan glycoside from Angelica dahurica. Chin. Chem. Lett. 2007;18:168–170. doi: 10.1016/j.cclet.2006.12.011. [DOI] [Google Scholar]
- 70.Zhao X., Feng X., Jia X., Wang M., Shan Y., Dong Y. New coumarin glucoside from Angelica dahurica. Chem. Nat. Compd. 2007;43:399–401. doi: 10.1007/s10600-007-0148-4. [DOI] [Google Scholar]
- 71.Li D., Wu L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp. Ther. Med. 2017;14:874–880. doi: 10.3892/etm.2017.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edmond M.P., Mostafa N.M., El-Shazly M., Singab A.N.B. Two clerodane diterpenes isolated from Polyalthia longifolia leaves: Comparative structural features, anti-histaminic and anti-Helicobacter pylori activities. Nat. Prod. Res. 2021;35:5282–5286. doi: 10.1080/14786419.2020.1753048. [DOI] [PubMed] [Google Scholar]
- 73.Kim D.K., Lim J.P., Yang J.H., Eom D.O., Eun J.S., Leem K.H. Acetylcholinesterase inhibitors from the roots of Angelica dahurica. Arch. Pharmacal Res. 2002;25:856–859. doi: 10.1007/BF02977004. [DOI] [PubMed] [Google Scholar]
- 74.Baek N.-I., Ahn E.-M., Kim H.-Y., Park Y.-D. Furanocoumarins from the root of Angelica dahurica. Arch. Pharmacal Res. 2000;23:467–470. doi: 10.1007/BF02976574. [DOI] [PubMed] [Google Scholar]
- 75.Yang L., Li Q., Feng Y., Qiu D. Simultaneous determination of three coumarins in Angelica dahurica by 1H-qNMR method: A fast and validated method for crude drug quality control. J. Anal. Methods Chem. 2020;2020:8987560. doi: 10.1155/2020/8987560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao A.-h., Yang X.-w. New coumarin glucopyranosides from roots of Angelica dahurica. Chin. Herb. Med. 2018;10:103–106. doi: 10.1016/j.chmed.2018.01.008. [DOI] [Google Scholar]
- 77.Oh H., Lee H.-S., Kim T., Chai K.-Y., Chung H.-T., Kwon T.-O., Jun J.-Y., Jeong O.-S., Kim Y.-C., Yun Y.-G. Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med. 2002;68:463–464. doi: 10.1055/s-2002-32075. [DOI] [PubMed] [Google Scholar]
- 78.Shu P., Li J., Fei Y., Zhu H., Zhang L., Niu H., Li Y., Liu H., Ju Z., Wei X. Angelicosides I-IV, four undescribed furanocoumarin glycosides from Angelica dahurica roots and their tyrosinase inhibitory activities. Phytochem. Lett. 2020;36:32–36. doi: 10.1016/j.phytol.2020.01.006. [DOI] [Google Scholar]
- 79.Matsuo Y., Yamaguchi E., Hakamata R., Ootomo K., Takatori K., Fukaya H., Mimaki Y. Benzofuran and coumarin derivatives from the root of Angelica dahurica and their PPAR-γ ligand-binding activity. Phytochemistry. 2020;173:112301. doi: 10.1016/j.phytochem.2020.112301. [DOI] [PubMed] [Google Scholar]
- 80.Kim N.Y., Jung Y.Y., Yang M.H., Um J.-Y., Sethi G., Ahn K.S. Isoimperatorin down-regulates epithelial mesenchymal transition through modulating NF-κB signaling and CXCR4 expression in colorectal and hepatocellular carcinoma cells. Cell. Signal. 2022;99:110433. doi: 10.1016/j.cellsig.2022.110433. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y., Hou M., Yu Y., Xie W., Chang R., Zhang G., Zhang H., Yu H., Chen A. Simultaneous separation and determination of six furanocoumarins in radix Angelicae dahuricae by CZE with dual CDs system. Anal. Biochem. 2022;655:114869. doi: 10.1016/j.ab.2022.114869. [DOI] [PubMed] [Google Scholar]
- 82.Lin X., Liu J., Zou Y., Tao C., Chen J. Xanthotoxol suppresses non-small cell lung cancer progression and might improve patients’ prognosis. Phytomedicine. 2022;105:154364. doi: 10.1016/j.phymed.2022.154364. [DOI] [PubMed] [Google Scholar]
- 83.Chen W., Wang G., Mei K., Zhu J. Coumarins from Angelica dahurica and their antitumor activities in human MG-63 osteosarcoma cells. Rec. Nat. Prod. 2021;15:356–362. doi: 10.25135/rnp.225.21.01.1935. [DOI] [Google Scholar]
- 84.Al-Madhagy S.A., Mostafa N.M., Youssef F.S., Awad G.E., Eldahshan O.A., Singab A.N.B. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019;10:6267–6275. doi: 10.1039/C9FO01532A. [DOI] [PubMed] [Google Scholar]
- 85.Elhawary E.A., Mostafa N.M., Labib R.M., Singab A.N. Metabolomic profiles of essential oils from selected Rosa varieties and their antimicrobial activities. Plants. 2021;10:1721. doi: 10.3390/plants10081721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang W.L., Zheng K.Y., Zhu K.Y., Zhan J.Y., Bi C.W., Chen J., Dong T.T., Choi R.C., Lau D.T., Tsim K.W. Chemical and biological assessment of Angelica roots from different cultivated regions in a chinese herbal decoction danggui buxue tang. Evid.-Based Complement. Altern. Med. 2013;2013:483286. doi: 10.1155/2013/483286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chao W.-W., Lin B.-F. Bioactivities of major constituents isolated from Angelica sinensis (Danggui) Chin. Med. 2011;6:1–7. doi: 10.1186/1749-8546-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y., Xu C., Wang W., Wang X., Guo Q., Shi J. Phthalide-derived oxaspiroangelioic acids A–C with an unprecedented carbon skeleton from an aqueous extract of the Angelica sinensis root head. Chin. Chem. Lett. 2021;32:3257–3260. doi: 10.1016/j.cclet.2021.03.004. [DOI] [Google Scholar]
- 89.Moussa A.Y., Mostafa N.M., Singab A.N.B. Pulchranin A: First report of isolation from an endophytic fungus and its inhibitory activity on cyclin dependent kinases. Nat. Prod. Res. 2020;34:2715–2722. doi: 10.1080/14786419.2019.1585846. [DOI] [PubMed] [Google Scholar]
- 90.Wang H., Wang X., Zhou L., Zhang S., An L., Bao J., Li Z., Sun Y., Li Y., Cui J. Structural characteristics and in vitro and in vivo immunoregulatory properties of a gluco-arabinan from Angelica dahurica. Int. J. Biol. Macromol. 2021;183:90–100. doi: 10.1016/j.ijbiomac.2021.04.077. [DOI] [PubMed] [Google Scholar]
- 91.Pang X., Jing Y., Li P., Qiu X., Zheng Y., Wang Q., Wu L. Structural characterization and antioxidant activities of polysaccharides from Angelica dahurica as extracted by optimized ultrasonic-assisted method. Int. J. Food Prop. 2022;25:1635–1649. doi: 10.1080/10942912.2022.2096066. [DOI] [Google Scholar]
- 92.Dong X.-d., Liu Y.-n., Zhao Y., Liu A.-j., Ji H.-y., Yu J. Structural characterization of a water-soluble polysaccharide from Angelica dahurica and its antitumor activity in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021;193:219–227. doi: 10.1016/j.ijbiomac.2021.10.110. [DOI] [PubMed] [Google Scholar]
- 93.Choi S.Y., Ahn E.M., Song M.C., Kim D.W., Kang J.H., Kwon O.S., Kang T.C., Baek N.I. In vitro GABA-transaminase inhibitory compounds from the root of Angelica dahurica. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005;19:839–845. doi: 10.1002/ptr.1424. [DOI] [PubMed] [Google Scholar]
- 94.Lin C., Chang C., Wang C., Chang M., Yang L. Byakangelicol, isolated from Angelica dahurica, inhibits both the activity and induction of cyclooxygenase-2 in human pulmonary epithelial cells. J. Pharm. Pharmacol. 2002;54:1271–1278. doi: 10.1211/002235702320402125. [DOI] [PubMed] [Google Scholar]
- 95.Choi I.-H., Song Y.-k., Lim H.-H. Analgesic and anti-inflammatory effect of the aqueous extract of Angelica dahurica. J. Korean Med. 2008;29:32–40. doi: 10.3742/OPEM.2008.7.5.527. [DOI] [Google Scholar]
- 96.Hua J.M., Moon T.C., Hong T.G., Park K.M., Son J.K., Chang H.W. 5-Methoxy-8-(2-hydroxy-3-buthoxy-3-methylbutyloxy)-psoralen isolated from Angelica dahurica inhibits cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharmacal Res. 2008;31:617–621. doi: 10.1007/s12272-001-1202-9. [DOI] [PubMed] [Google Scholar]
- 97.Lee M.-Y., Seo C.-S., Lee J.-A., Lee N.-H., Kim J.-H., Ha H., Zheng M.-S., Son J.-K., Shin H.-K. Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem. Toxicol. 2011;49:829–837. doi: 10.1016/j.fct.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Zhang W.q., Hua Y.l., Zhang M., Ji P., Li J.x., Zhang L., Li P.l., Wei Y.m. Metabonomic analysis of the anti-inflammatory effects of volatile oils of Angelica sinensis on rat model of acute inflammation. Biomed. Chromatogr. 2015;29:902–910. doi: 10.1002/bmc.3372. [DOI] [PubMed] [Google Scholar]
- 99.Zhong L.-J., Hua Y.-L., Ji P., Yao W.-L., Zhang W.-Q., Li J., Wei Y.-M. Evaluation of the anti-inflammatory effects of volatile oils from processed products of Angelica sinensis radix by GC–MS-based metabolomics. J. Ethnopharmacol. 2016;191:195–205. doi: 10.1016/j.jep.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 100.Hua Y.-l., Ji P., Xue Z.-y., Wei Y.-m. Construction and analysis of correlation networks based on gas chromatography-mass spectrometry metabonomics data for lipopolysaccharide-induced inflammation and intervention with volatile oil from Angelica sinensis in rats. Mol. BioSystems. 2015;11:3174–3187. doi: 10.1039/C5MB00405E. [DOI] [PubMed] [Google Scholar]
- 101.Li J., Hua Y., Ji P., Yao W., Zhao H., Zhong L., Wei Y. Effects of volatile oils of Angelica sinensis on an acute inflammation rat model. Pharm. Biol. 2016;54:1881–1890. doi: 10.3109/13880209.2015.1133660. [DOI] [PubMed] [Google Scholar]
- 102.Mao W.-A., Sun Y.-Y., Mao J.-Y., Wang L., Zhang J., Zhou J., Rahman K., Ye Y. Inhibitory effects of Angelica polysaccharide on activation of mast cells. Evid.-Based Complement. Altern. Med. 2016;2016:6063475. doi: 10.1155/2016/6063475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen G., Liu Y., Xu Y., Zhang M., Guo S., Zhang G. Isoimperatorin exerts anti-inflammatory activity by targeting the LPS-TLR4/MD-2-NF-κB pathway. Eur. J. Inflamm. 2021;19:20587392211000573. doi: 10.1177/20587392211000573. [DOI] [Google Scholar]
- 104.Sun J., Huang S., Qin Y., Zhang P., Li Z., Zhang L., Wang X., Wu R., Qin S., Huo J. Anti-allergic actions of a Chinese patent medicine, huoxiangzhengqi oral liquid, in RBL-2H3 cells and in mice. Pharm. Biol. 2021;59:670–680. doi: 10.1080/13880209.2021.1928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu C., Wang M., Guo J., Su S.L., Yu G., Yang Y., Zhou Y., Tang Z. Angelica dahurica extracts attenuate CFA-induced inflammatory pain via TRPV1 in mice. Evid.-Based Complement. Altern. Med. 2022;2022:4684830. doi: 10.1155/2022/4684830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okada R., Abe H., Okuyama T., Nishidono Y., Ishii T., Sato T., Shirako S., Tanaka K., Ikeya Y., Nishizawa M. Comparison of the anti-inflammatory activities of furanocoumarins from the roots of Angelica dahurica. Bioact. Compd. Health Dis. 2021;4:287–300. doi: 10.31989/bchd.v4i12.866. [DOI] [Google Scholar]
- 107.Lee S.H., Han A.-R., Kang U., Kim J.-B., Seo E.K., Jung C.-H. Inhibitory effects of furanocoumarins from the roots of Angelica dahurica on ionizing radiation-induced migration of A549 human non-small cell lung cancer cells. Nat. Prod. Commun. 2020;15:1934578X20915036. [Google Scholar]
- 108.Zheng Y.M., Shen J.Z., Wang Y., Lu A.X., Ho W.S. Anti-oxidant and anti-cancer activities of Angelica dahurica extract via induction of apoptosis in colon cancer cells. Phytomedicine. 2016;23:1267–1274. doi: 10.1016/j.phymed.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 109.Bai Y., Yang L., Zhang C., Yang Y. Studies on the mechanism of alloimperatorin on the proliferation and apoptosis of Hela cells. J. Oncol. 2021;2021:6617312. doi: 10.1155/2021/6617312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pervin M., Hasnat M.A., Debnath T., Park S.R., Kim D.H., Lim B.O. Antioxidant, Anti-Inflammatory and antiproliferative activity of Angelica dahurica root extracts. J. Food Biochem. 2014;38:281–292. doi: 10.1111/jfbc.12046. [DOI] [Google Scholar]
- 111.Yang W.-T., Ke C.-Y., Wu W.-T., Tseng Y.-H., Lee R.-P. Antimicrobial and anti-inflammatory potential of Angelica dahurica and Rheum officinale extract accelerates wound healing in Staphylococcus aureus-infected wounds. Sci. Rep. 2020;10:5596. doi: 10.1038/s41598-020-62581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El-Nashar H.A., Mostafa N.M., El-Badry M.A., Eldahshan O.A., Singab A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2021;35:5369–5372. doi: 10.1080/14786419.2020.1765343. [DOI] [PubMed] [Google Scholar]
- 113.Roh J., Shin S. Antifungal and antioxidant activities of the essential oil from Angelica koreana Nakai. Evid.-Based Complement. Altern. Med. 2014;2014:398503. doi: 10.1155/2014/398503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fraternale D., Flamini G., Ricci D. Essential oil composition of Angelica archangelica L.(Apiaceae) roots and its antifungal activity against plant pathogenic fungi. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2016;150:558–563. doi: 10.1080/11263504.2014.988190. [DOI] [Google Scholar]
- 115.Prakash B., Singh P., Goni R., Raina A.K.P., Dubey N. Efficacy of Angelica archangelica essential oil, phenyl ethyl alcohol and α-terpineol against isolated molds from walnut and their antiaflatoxigenic and antioxidant activity. J. Food Sci. Technol. 2015;52:2220–2228. doi: 10.1007/s13197-014-1278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mullen K., Lee A., Lyman R., Mason S., Washburn S., Anderson K. An in vitro assessment of the antibacterial activity of plant-derived oils. J. Dairy Sci. 2014;97:5587–5591. doi: 10.3168/jds.2013-7806. [DOI] [PubMed] [Google Scholar]
- 117.Krishnaraj C., Young G.M., Yun S.-I. In vitro embryotoxicity and mode of antibacterial mechanistic study of gold and copper nanoparticles synthesized from Angelica keiskei (Miq.) Koidz. leaves extract. Saudi J. Biol. Sci. 2022;29:2552–2563. doi: 10.1016/j.sjbs.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zou J., Liu Y., Guo R., Tang Y., Shi Z., Zhang M., Wu W., Chen Y., Hou K. An in vitro coumarin-antibiotic combination treatment of Pseudomonas aeruginosa biofilms. Nat. Prod. Commun. 2021;16:1934578X20987744. doi: 10.1177/1934578X20987744. [DOI] [Google Scholar]
- 119.Lee B.W., Ha T.K.Q., Cho H.M., An J.-P., Kim S.K., Kim C.-S., Kim E., Oh W.K. Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J. Ethnopharmacol. 2020;259:112945. doi: 10.1016/j.jep.2020.112945. [DOI] [PubMed] [Google Scholar]
- 120.Liu C.-X., Pei-Gen X., Da-Peng L. Modern Research and Application of Chinese Medicinal Plants. Hong Kong Medical Publ.; Hong Kong, China: 2000. [Google Scholar]
- 121.Yin Z., Zhang L., Xu L. The effect of Dang-Gui (Angelica sinensis) and its ingredient ferulic acid on rat platelet aggregation and release of 5-HT (author’s transl) Yao Xue Xue Bao Acta Pharm. Sin. 1980;15:321–326. [PubMed] [Google Scholar]
- 122.Xu L., Wang R., Xu D. Effects of sodium ferulate combined with acetylsalicylic acid on rat platelet aggregation and on modulation of PGI2-TXA2 balance. Yao Xue Xue Bao Acta Pharm. Sin. 1985;20:5–9. [PubMed] [Google Scholar]
- 123.DerMarderosian A., Beuther J. The Review of Natural Products. Facts and comparisons; Wolters Kluwer Health Inc.; St. Louis, MO, USA: 2005. [Google Scholar]
- 124.Huang K.C. The Pharmacology of Chinese Herbs. CRC Press; Boca Raton, FL, USA: 1998. [Google Scholar]
- 125.Chen H., Chen X., Ping Z., Jiang X., Ge M., Ma J., Yu W. Promotion effect of Angelica sinensis extract on angiogenesis of chicken preovulatory follicles in vitro. Poult. Sci. 2022;101:101938. doi: 10.1016/j.psj.2022.101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen D., Tang J., Khatibi N.H., Zhu M., Li Y., Wang C., Jiang R., Tu L., Wang S. Treatment with Z-ligustilide, a component of Angelica sinensis, reduces brain injury after a subarachnoid hemorrhage in rats. J. Pharmacol. Exp. Ther. 2011;337:663–672. doi: 10.1124/jpet.110.177055. [DOI] [PubMed] [Google Scholar]
- 127.Mostafa N.M. β-Amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. Rec. Nat. Prod. 2018;12:480. doi: 10.25135/rnp.47.17.10.062. [DOI] [Google Scholar]
- 128.Cheng C.-Y., Huang H.-C., Kao S.-T., Lee Y.-C. Angelica sinensis extract promotes neuronal survival by enhancing p38 MAPK–mediated hippocampal neurogenesis and dendritic growth in the chronic phase of transient global cerebral ischemia in rats. J. Ethnopharmacol. 2021;278:114301. doi: 10.1016/j.jep.2021.114301. [DOI] [PubMed] [Google Scholar]
- 129.Shen J., Zhang J., Deng M., Liu Y., Hu Y., Zhang L. The antidepressant effect of Angelica sinensis extracts on chronic unpredictable mild stress-induced depression is mediated via the upregulation of the BDNF signaling pathway in rats. Evid.-Based Complement. Altern. Med. 2016;2016:7434692. doi: 10.1155/2016/7434692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y.-j., Huang X., Wang Y., Xie Y., Qiu X.-j., Ren P., Gao L.-c., Zhou H.-h., Zhang H.-y., Qiao M.-q. Ferulic acid-induced anti-depression and prokinetics similar to Chaihu–Shugan–San via polypharmacology. Brain Res. Bull. 2011;86:222–228. doi: 10.1016/j.brainresbull.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 131.Wilasrusmee C., Siddiqui J., Bruch D., Wilasrusmee S. In vitro immunomodulatory effects of herbal products. Am. Surg. 2002;68:860. doi: 10.1177/000313480206801005. [DOI] [PubMed] [Google Scholar]
- 132.Wilasrusmee C., Kittur S., Siddiqui J., Bruch D., Wilasrusmee S., Kittur D.S. In vitro immunomodulatory effects of ten commonly used herbs on murine lymphocytes. J. Altern. Complement. Med. 2002;8:467–475. doi: 10.1089/107555302760253667. [DOI] [PubMed] [Google Scholar]
- 133.Wang H., Li J., Yu L., Zhao Y., Ding W. Antifibrotic effect of the Chinese herbs, Astragalus mongholicus and Angelica sinensis, in a rat model of chronic puromycin aminonucleoside nephrosis. Life Sci. 2004;74:1645–1658. doi: 10.1016/j.lfs.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 134.Geng Q., Zhao H., Zong C., LI L., Wang S., Gao Y., Dong R. Effects of optimized formulas of radix Astragali and radix Angelicae sinensis extracts on survival status of idiopathic pulmonary fibrosis mice and on expression of cytogenesis-related factors in lung tissues. J. Guangzhou Univ. Tradit. Chin. Med. 2017;6:408–412. [Google Scholar]
- 135.Ko W.-C. A newly isolated antispasmodic-butylidenephthahde. Jpn. J. Pharmacol. 1980;30:85–91. doi: 10.1254/jjp.30.85. [DOI] [PubMed] [Google Scholar]
- 136.Cao P., Sun J., Sullivan M.A., Huang X., Wang H., Zhang Y., Wang N., Wang K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018;111:1133–1139. doi: 10.1016/j.ijbiomac.2018.01.139. [DOI] [PubMed] [Google Scholar]
- 137.Park E.-Y., Kim E.-H., Kim C.-Y., Kim M.-H., Choung J.-S., Oh Y.-S., Moon H.-S., Jun H.-S. Angelica dahurica extracts improve glucose tolerance through the activation of GPR119. PLoS ONE. 2016;11:e0158796. doi: 10.1371/journal.pone.0158796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.El-Nashar H.A., Mostafa N.M., Eldahshan O.A., Singab A.N.B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat. Prod. Res. 2022;36:1182–1190. doi: 10.1080/14786419.2020.1864365. [DOI] [PubMed] [Google Scholar]
- 139.El-Nashar H.A., Mostafa N.M., El-Shazly M., Eldahshan O.A. The role of plant-derived compounds in managing diabetes mellitus: A review of literature from 2014 To 2019. Curr. Med. Chem. 2021;28:4694–4730. doi: 10.2174/0929867328999201123194510. [DOI] [PubMed] [Google Scholar]
- 140.Chen J., Jiang Q.-D., Wu Y.-M., Liu P., Yao J.-H., Lu Q., Zhang H., Duan J.-A. Potential of essential oils as penetration enhancers for transdermal administration of ibuprofen to treat dysmenorrhoea. Molecules. 2015;20:18219–18236. doi: 10.3390/molecules201018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang Q., Wu Y., Zhang H., Liu P., Yao J., Yao P., Chen J., Duan J. Development of essential oils as skin permeation enhancers: Penetration enhancement effect and mechanism of action. Pharm. Biol. 2017;55:1592–1600. doi: 10.1080/13880209.2017.1312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Piao X.L., Yoo H.H., Kim H.Y., Kang T.L., Hwang G.S., Park J.H. Estrogenic activity of furanocoumarins isolated from Angelica dahurica. Arch. Pharmacal Res. 2006;29:741–745. doi: 10.1007/BF02974073. [DOI] [PubMed] [Google Scholar]
- 143.Zhu H., You J., Wen Y., Jia L., Gao F., Ganesan K., Chen J. Tumorigenic risk of Angelica sinensis on ER-positive breast cancer growth through ER-induced stemness in vitro and in vivo. J. Ethnopharmacol. 2021;280:114415. doi: 10.1016/j.jep.2021.114415. [DOI] [PubMed] [Google Scholar]
- 144.Fang C.-L., Goswami D., Kuo C.-H., Day C.H., Lin M.-Y., Ho T.-J., Yang L.-Y., Hsieh D.J.-Y., Lin T.-K., Huang C.-Y. Angelica dahurica attenuates melanogenesis in B16F0 cells by repressing Wnt/β-catenin signaling. Mol. Cell. Toxicol. 2022:1–9. doi: 10.1007/s13273-022-00250-0. [DOI] [Google Scholar]
- 145.Luo Y., Fang Q., Lai Y., Niu H., Wang R., Song C. High-throughput sequencing technology reveals polysaccharides from Angelica dahurica that affect gut microbiota in mice. Biotechnol. Biotechnol. Equip. 2021;35:1934–1940. doi: 10.1080/13102818.2022.2045216. [DOI] [Google Scholar]
- 146.Chung I.-M., Kim E.-H., Lee J.-H., Lee Y.-C., Moon H.-I. Immunotoxicity activity from various essential oils of Angelica genus from South Korea against Aedes aegypti L. Immunopharmacol. Immunotoxicol. 2012;34:42–45. doi: 10.3109/08923973.2011.572891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the authors.