Abstract
Objectives
To evaluate the efficacy and safety of regdanvimab, a neutralizing antibody, in patients with mild-to-moderate SARS-CoV-2 including against the Delta variant.
Methods
A single-center, retrospective, observational cohort study in adults with confirmed COVID-19. The primary end point was the proportion of patients deteriorating with peripheral oxygen saturation <90% in room air, requiring supplemental oxygen therapy above high flow, or experiencing mortality due to COVID-19 up to day 28.
Results
A total of 722 patients were eligible; 418 received regdanvimab and 304 received standard of care (SoC), of whom 71.1% (297/418, regdanvimab) and 37.8% (115/304, SoC) were infected with the Delta variant. The proportion of patients with a primary end point event was significantly lower with regdanvimab than SoC (3.1% vs 9.9%; difference: -6.8 [95% confidence interval: -10.9, -2.8]; P = 0.0002). A similar trend was observed in the Delta variant subgroup (regdanvimab, 2.7% vs SoC, 7.0%; difference -4.3 [95% confidence interval: -10.8, 0.2]; P = 0.0827). The secondary efficacy end points supported the primary analysis findings in the overall cohort and Delta variant subgroup. No new safety signals were identified.
Conclusion
Regdanvimab demonstrated clinical efficacy in the overall cohort and may provide a clinical benefit for patients with mild-to-moderate COVID-19 infected with the Delta variant.
Keywords: Observational study, Monoclonal antibody, SARS-CoV-2, CT-P59, Regdanvimab
Introduction
Since SARS-CoV-2 was identified in 2019 [1], the evolution of the virus has resulted in the emergence of variants with increased transmissibility and virulence.
The Delta variant was first identified in India in November 2020 [2] and was estimated to be around 60% more transmissible than the Alpha variant [3]. The Delta variant rapidly became the dominant circulating variant in many countries [4]. Moreover, the risk of hospitalization is markedly increased by the Delta variant [5,6].
By the end of 2021, the worldwide COVID-19 vaccination programs administered 8.6 billion vaccine doses, but 40% of the global population remained unvaccinated [7]. A systematic review of vaccine effectiveness against infection with the Delta variant found that vaccines were 10-20% less effective at preventing symptomatic infection with the Delta variant than with the Alpha variant, but prevention of hospitalizations is similar for the two variants [8]. Analysis of COVID-19 postvaccine breakthrough infections in India showed that the majority of infections were of the Delta variant [9].
As a large proportion of the global population remains unvaccinated, and because individuals can develop COVID-19 despite being vaccinated, this highlights the need for effective drug treatments to complement the vaccination program [10,11]. In addition to repurposing existing treatments, new approaches include the development of COVID-19 monoclonal antibody therapies that target SARS-CoV-2 and interrupt the viral life cycle [11], [12], [13]. Regdanvimab is a neutralizing antibody that prevents SARS-CoV-2 from interacting with the angiotensin-converting enzyme 2 receptor and gaining entry to epithelial and endothelial cells [14]. In vivo mouse challenge studies have demonstrated antiviral activity of regdanvimab against the Delta variant, with reduced symptoms and viral loads in regdanvimab-treated animals compared with those given placebo [15]. In patients with mild COVID-19, larger reductions in viral load and shorter mean times to recovery were observed with regdanvimab than with placebo [16]. Significant reductions in the proportion of patients requiring hospitalization, oxygen therapy, or experiencing mortality and time to clinical recovery were observed in a phase II/III study of regdanvimab versus placebo in patients with mild-to-moderate COVID-19 [17]. However, the timing of the latter study makes it unlikely that the patients would have been infected with the Delta variant.
Regdanvimab was approved for emergency use in the Republic of Korea in February 2021 [18]. We conducted a retrospective cohort study of regdanvimab in patients with mild-to-moderate COVID-19, with a subgroup analysis of patients with the Delta variant. An additional subgroup analysis evaluated the effectiveness of regdanvimab according to vaccination status.
Methods
Study design and participants
We conducted a single-center, retrospective cohort study of patients with mild or moderate COVID-19 between September 2020 and October 2021 in Incheon Medical Center in the Republic of Korea. During this time, the national management guidelines for COVID-19 issued by the Korean Central Disease Control Headquarters dictated that patients with moderate COVID-19 and those with mild disease and at least one high-risk factor for progression (age >50 years, body mass index >30 kg/m², cardiovascular disease, chronic lung, kidney or liver disease, type 1 or type 2 diabetes mellitus, or immunosuppressed status) were to be hospitalized and treated by medical professionals. The study protocol was reviewed and approved by the local institutional review board.
Medical records (including electronic records) of all patients, who were admitted to the study center with COVID-19 and treated with regdanvimab (40 mg/kg administered as an intravenous infusion over 90 minutes) or standard of care (SoC; e.g., antibiotics, antipyretics), were reviewed and further assessed for eligibility. Data from the medical records were anonymized, and statistically analyzed in accordance with the study protocol. During the COVID-19 pandemic, the Korea Disease Control and Prevention Agency (KDCA) performed genotyping on a portion of confirmed COVID-19 samples (approximately 15% during May 2021, when the Delta variant was first detected rising to 30% during August 2021 to enhance monitoring of the Delta variant). Genotyping results were provided at the study site and recorded in the patient case report form.
Eligible patients were adults aged ≥19 years with mild COVID-19 with at least one high-risk factor or moderate COVID-19 (confirmed by reverse transcription-polymerase chain reaction), peripheral oxygen saturation (SpO2) >94% on room air, no requirement for supplemental oxygen, and with one or more mild or moderate symptoms of COVID-19 before receiving regdanvimab (for patients treated with regdanvimab) or at admission (for those receiving SoC), which included fever >38°C, shortness of breath, cough, diarrhea, sputum, sore throat, headache, myalgia, and loss of taste or smell.
The key exclusion criteria were severe conditions related to COVID-19 within 7 days before receiving any treatments for COVID-19 (in the opinion of the investigator) or participation in clinical studies of any other investigational medical products for the treatment of COVID-19 (including convalescent plasma, remdesivir, and hydroxychloroquine).
Hospitalized patients with mild-to-moderate COVID-19 who met the severity and symptom eligibility criteria before the conditional approval of regdanvimab in February 2021 were assigned to the SoC group. After conditional approval of regdanvimab, eligible patients who received regdanvimab within 7 days of symptom onset, according to regdanvimab's indication, were assigned to the regdanvimab group or were otherwise allocated to the SoC group.
Study objectives
The primary study objective was to evaluate the clinical efficacy of regdanvimab, as determined by the proportion of patients deteriorating with SpO2 <90% in room air, requiring supplemental oxygen therapy above high flow or experiencing mortality due to COVID-19 up to day 28 in the overall cohort and in those infected with the Delta variant. The secondary objectives were to evaluate additional measures of efficacy and safety for regdanvimab.
Study end points
The primary efficacy end point was the proportion of patients with SpO2 deterioration to <90% in room air, requiring supplemental oxygen therapy above high flow, or experiencing mortality due to COVID-19 up to day 28.
The secondary efficacy end points were time to sustained recovery of fever due to COVID-19 (tympanic temperature <38°C), the proportion of patients with SpO2 deterioration to <90%/<94% in room air up to day 28, the proportion of patients requiring supplemental low-flow/high-flow oxygen therapy due to COVID-19 up to day 28, the duration of supplemental low-flow/high-flow oxygen therapy due to COVID-19, the proportion of patients requiring mechanical ventilation due to COVID-19 up to day 28, the duration of mechanical ventilation due to COVID-19, the duration of hospitalization due to COVID-19, the proportion of patients with intensive care unit transfer due to COVID-19 up to day 28, the proportion of patients discharged up to day 11 and day 14, the proportion of patients requiring remdesivir due to COVID-19 up to day 28, the proportion of patients requiring tocilizumab due to COVID-19 up to day 28, and the proportion of patients with all-cause mortality up to day 28.
The safety end point analyzed adverse events (AEs) up to day 28 in the regdanvimab cohort only.
Statistical analysis
The proposed sample size of approximately 800 patients was based on the estimated number of patients with COVID-19 admitted at the study center during the data collection period, rather than on a formal statistical hypothesis.
All statistical analyses were conducted using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA). Analysis populations comprised the efficacy set (all patients who received a full dose of regdanvimab or SoC and had at least one postadmission efficacy result) and the safety set (all patients who received a full or partial dose of regdanvimab).
Efficacy end points were analyzed based on the efficacy set, unless otherwise indicated. The secondary efficacy end point “duration of hospitalization due to COVID-19” was analyzed for patients with hospital discharge in the efficacy set. Efficacy results were summarized by regdanvimab and SoC cohorts for the total population of patients with COVID-19 and for the subgroup of patients with the Delta variant using descriptive statistics or frequency tables. Subgroup analyses were also conducted in unvaccinated patients. For categorical data, Fisher's exact test or chi-squared test was used. A sensitivity analysis was performed on the primary efficacy end point using a logistic regression model with treatment as a fixed effect. To exclude the influence of factors that differed at baseline, age, and vaccination status were included as covariates. Body mass index and high-risk factors were also included as covariates due to their status as known risk factors for progression to severe disease. For continuous data, Student's t-test was used. Results were considered statistically significant if the P-value was <0.05.
For the safety analysis, AEs were listed for the safety set (regdanvimab cohort). AEs were coded according to the Medical Dictionary for Regulatory Activities and graded according to the Common Terminology Criteria for AEs, Version 5.0.
Results
Patient population and baseline characteristics
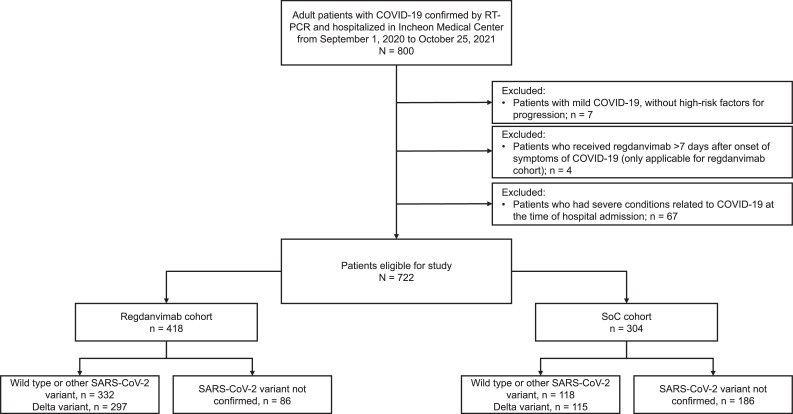
Patient disposition is presented in Figure 1 . Between September 2020, and October 2021, 800 patients were admitted to the study center with COVID-19; 180 patients were recruited before February 05, 2021 (date of conditional approval of regdanvimab in Korea), and 620 patients were recruited afterward (Table S1). In total, 722 patients were considered eligible for inclusion in this study; 418 patients in regdanvimab cohort and 304 patients in the SoC cohort. Before February 5, 2021, none of the patients received regdanvimab and 180 patients were assigned to the SoC cohort. After February 5, 2021, 418 patients met the eligibility criteria and received regdanvimab. A further 124 patients were included in the SoC cohort after conditional approval of regdanvimab because they were either hospitalized >7 days after symptom onset (n = 57) or refused regdanvimab treatment (n = 67).
Figure 1.
Patient disposition.
RT-PCR, reverse transcription-polymerase chain reaction; SoC, standard of care.
In total, 412 patients were confirmed as infected with the Delta variant (regdanvimab, n = 297 [71.1%]; SoC, n = 115 [37.8%]).
Baseline characteristics were generally well-balanced between the two cohorts (Table 1 ). The proportion of males was similar in the regdanvimab and SoC cohorts (47.4% and 48.4%, respectively), as was the mean body mass index (25.0, Standard deviation (SD): 4.3 and 24.8, SD: 3.6 kg/m2, respectively). The median patient age was slightly lower in the regdanvimab cohort than in the SoC cohort (52 [range: 18-90] vs 60 [18-91] years), as was the frequency of patients with the high-risk factor of advanced age (>50 years; 52.9% vs 65.1%).
Table 1.
Baseline demographics and characteristics.
| Overall regdanvimab n = 418 |
Overall SoC n = 304 |
Delta variant subgroup regdanvimab n = 297 |
Delta variant subgroup SoC n = 115 |
|
|---|---|---|---|---|
| Age, years | ||||
| Median (range) | 52 (18, 90) | 60 (18, 91) | 46 (18, 88) | 42 (18, 82) |
| Male, n (%) | 198 (47.4) | 147 (48.4) | 148 (49.8) | 68 (59.1) |
| Female, n (%) | 220 (52.6) | 157 (51.6) | 149 (50.2) | 47 (40.9) |
| BMI, mean (SD), kg/m2 | 25.0 (4.3) | 24.8 (3.6) | 25.2 (4.4) | 24.4 (3.9) |
| At least one high-risk factor | 288 (68.9) | 236 (77.6) | 177 (59.6) | 57 (49.6) |
| Age >50 years | 221 (52.9) | 198 (65.1) | 121 (40.7) | 34 (29.6) |
| BMI >30 kg/m² | 46 (11.0) | 25 (8.2) | 39 (13.1) | 11 (9.6) |
| Cardiovascular disease, including hypertension | 145 (34.7) | 105 (34.5) | 77 (25.9) | 23 (20.0) |
| Chronic lung disease, including asthma | 25 (6.0) | 15 (4.9) | 18 (6.1) | 4 (3.5) |
| Type 1 or type 2 diabetes mellitus | 63 (15.1) | 66 (21.7) | 35 (11.8) | 8 (7.0) |
| Chronic kidney disease, including dialysis | 2 (0.5) | 4 (1.3) | 2 (0.7) | 1 (0.9) |
| Chronic liver disease | 7 (1.7) | 10 (3.3) | 5 (1.7) | 4 (3.5) |
| Immunosuppressed status | 9 (2.2)a | 4 (1.3)b | 5 (1.7) | 0 |
| Baseline COVID-19 symptoms, n (%) | ||||
| Fever | 208 (49.8) | 125 (41.1) | 151 (50.8) | 40 (34.8) |
| Shortness of breath | 32 (7.7) | 12 (3.9) | 28 (9.4) | 8 (7.0) |
| Cough | 227 (54.3) | 158 (52.0) | 168 (56.6) | 75 (65.2) |
| Diarrhea | 24 (5.7) | 13 (4.3) | 18 (6.1) | 7 (6.1) |
| Sputum | 108 (25.8) | 62 (20.4) | 83 (27.9) | 32 (27.8) |
| Sore throat | 104 (24.9) | 55 (18.1) | 67 (22.6) | 23 (20.0) |
| Headache | 122 (29.2) | 72 (23.7) | 90 (30.3) | 35 (30.4) |
| Myalgia | 92 (22.0) | 62 (20.4) | 60 (20.2) | 15 (13.0) |
| Lack of taste or smell | 38 (9.1) | 22 (7.2) | 33 (11.1) | 11 (9.6) |
| Disease severity, n (%) | ||||
| Mild | 20 (4.8) | 53 (17.4) | 5 (1.7) | 5 (4.3) |
| Moderate | 398 (95.2) | 251 (82.6) | 292 (98.3) | 110 (95.7) |
| Time since symptom onsetc, median (range), days | 2.0 (−2.0, 7.0) | 3.0 (0.0, 26.0) | 3.0 (−1.0, 7.0) | 7.0 (0.0, 26.0) |
| Baseline peripheral oxygen saturation level in room air, median (range), % | 97 (94, 100) | 98 (94, 100) | 97 (94, 100) | 97 (94, 99) |
| Unvaccinated, n (%) | 297 (71.1) | 275 (90.5) | 177 (59.6) | 86 (74.8) |
Neoplasm, malignant (n = 6), systemic lupus erythematosus (n = 2) and acquired immunodeficiency syndrome (n = 1)
Neoplasm, malignant (n = 2), rheumatoid arthritis (n = 1) and psoriasis (n = 1)
Date of admission (day 1) - date of earliest symptom start.
BMI, body mass index; SoC, standard of care.
Most patients had moderate disease with a higher proportion recorded in the regdanvimab cohort (95.2%) than the SoC cohort (82.6%); no patients had asymptomatic or critical disease. In the Delta variant subgroup, 98.3% of patients in the regdanvimab cohort and 95.7% in the SoC cohort had moderate disease. Of the patients in the regdanvimab and SoC cohorts, 297 (71.1%) and 275 (90.5%), respectively had not received a COVID-19 vaccination.
Median baseline SpO2 levels were 97-98% (with ranges between 94% and 100%) in both treatment cohorts within the overall population and the Delta variant subgroup. This was also true for unvaccinated individuals in the regdanvimab cohort (97% [range 94-100%]) and SoC cohort (98% [94-100%]).
Efficacy
The proportion of patients who deteriorated to SpO2 <90% in room air, required supplemental oxygen therapy above high flow, or experienced mortality due to COVID-19 up to day 28 (i.e., primary end point) was significantly lower in the regdanvimab cohort than in the SoC cohort (3.1% vs 9.9%; difference: -6.8 [95% confidence interval (CI): -10.9, -2.8]; P = 0.0002; Table 2 ). A similar trend was observed in the Delta variant subgroup (regdanvimab, 2.7%; SoC, 7.0%); although, the difference of -4.3 (95% CI: -10.8, 0.2) was not statistically significant (P = 0.0827; Table 2). Estimated odds ratio from logistic regression model with baseline covariates showed a significant regdanvimab treatment effect in the overall population (0.4241 [95% CI: 0.2108, 0.8532]; P = 0.0162) and in those with the Delta variant (0.3395 [95% CI: 0.1168, 0.9873]; P = 0.0473; Table 2). In the unvaccinated population, 12 patients (4.0%) in the regdanvimab cohort and 30 patients (10.9%) in the SoC cohort (difference: -6.9, P = 0.002) deteriorated to SpO2 <90%, required supplemental oxygen therapy above high flow, or experienced mortality due to COVID-19; of these, seven (4.0%) patients in the regdanvimab group and eight (9.3%) patients in the SoC group (difference: -5.3, P = 0.0929) had the Delta variant (Table S2). Although there were few patients in the regdanvimab group with known variant data other than Delta (n = 35), a subgroup analysis for the primary end point was performed that compared patients with Delta variant versus patients with any other identified variant (other variant + wild type; Table S3).
Table 2.
Proportion of patients who deteriorated to peripheral oxygen saturation <90% in room air, required supplemental oxygen therapy above high flow, or experienced mortality due to COVID-19 up to Day 28 (primary efficacy endpoint) and logistic regression model with treatment as a fixed effect and age, body mass index, at least one high-risk factor and at least one COVID-19 vaccine as covariates
| Primary efficacy endpoint | Logistic regression model | |||
|---|---|---|---|---|
| Regdanvimab | SoC | % difference (95% CI) [P-value]a,b | Odds ratio (95% CI) [P-value]c | |
| Overall study cohort | 13/418 (3.1) | 30/304 (9.9) | -6.8 (-10.9, -2.8) [0.0002] | 0.4241 (0.2108, 0.8532) [0.0162] |
| Delta variant subgroup | 8/297 (2.7) | 8/115 (7.0) | -4.3 (-10.8, 0.2) [0.0827] | 0.3395 (0.1168, 0.9873) [0.0473] |
Data are n (%), unless otherwise specified.
Farrington and Manning method used to calculate the 95% exact CI for the proportion difference between regdanvimab and SoC cohort in each group
P-values were derived from Fisher exact test
P-value calculated as a treatment effect from logistic model.
CI, confidence interval; SoC, standard of care.
Because patients with unknown variant data (n = 86) may have included Delta variant or others, they have been excluded from this analysis.
The secondary end point data for the overall population and subgroup of patients with the Delta variant are summarized by cohort in Table 3 (corresponding data for the unvaccinated subgroup are presented in Table S4).
Table 3.
Summary of secondary efficacy endpoint data up to day 28, by cohort.
| Overall regdanvimab n = 418 |
Overall SoC n = 304 |
P-valuea | Delta variant subgroup regdanvimab n = 297 |
Delta variant subgroup SoC n = 115 |
P-valuea | |
|---|---|---|---|---|---|---|
| Patients with SpO2 deterioration to <94% in room air, n (%) | 32 (7.7) | 87 (28.6) | <0.0001 | 19 (6.4) | 13 (11.3) | 0.0951 |
| Patients with SpO2 deterioration to <90% in room air, n (%) | 5 (1.2) | 24 (7.9) | <0.0001 | 3 (1.0) | 4 (3.5) | 0.0987b |
| Patients requiring low-flow oxygen therapy, n (%) | 92 (22.0) | 130 (42.8) | <0.0001 | 66 (22.2) | 32 (27.8) | 0.2308 |
| Patients requiring high-flow oxygen therapy, n (%) | 10 (2.4) | 13 (4.3) | 0.1547 | 6 (2.0) | 6 (5.2) | 0.1029b |
| Patients requiring mechanical ventilation, n (%) | 0 | 0 | NA | 0 | 0 | NA |
| Duration of hospitalization due to COVID-19, mean (SD), days | 10.27 (2.94) | 11.69 (4.71) | <0.0001 | 9.93 (2.53) | 8.55 (4.01) | 0.0009 |
| Patients discharged, n (%) Up to day 14 |
387 (92.6) | 234 (77.0) | <0.0001 | 281 (94.6) | 102 (88.7) | 0.0352 |
| Patients requiring therapy, n (%) Remdesivir |
62 (14.8) | 110 (36.2) | <0.0001 | 48 (16.2) | 29 (25.2) | 0.0344 |
| Patients with all-cause mortality, n (%) | 0 | 1 (0.3) | 0.4211b | 0 | 1 (0.9) | 0.2791b |
P-values were derived from Student's t-test for continuous variables and chi-squared test or Fisher exact test for categorical variables
P-value derived from Fisher exact test; chi-squared test may not be valid because 25% or 50% of the cells have expected counts <5.
NA, not applicable; SoC, standard of care; SpO2, peripheral oxygen saturation.
The proportion of patients with COVID-19 who deteriorated to SpO2 <94% in room air was significantly lower in the regdanvimab cohort than in the SoC cohort (7.7% vs 28.6%), with a corresponding trend in the Delta variant subgroup for these cohorts (6.4% vs 11.3%). Fewer patients in the regdanvimab cohort than in the SoC cohort had deterioration of SpO2 to <90% on room air (1.2% vs 7.9%). A similar trend was observed in the Delta variant subgroup (1.0% vs 3.5%).
Low-flow supplemental oxygen was required by a significantly lower proportion of patients in the regdanvimab cohort than in the SoC cohort (22.0% vs 42.8%). High-flow supplemental oxygen was required by 2.4% of patients in the regdanvimab cohort and by 4.3% in the SoC cohort, but the difference was not statistically significant. No patients in either cohort required mechanical ventilation. In the subgroup of patients with the Delta variant, the proportions of patients requiring low- or high-flow oxygen therapy were lower for the regdanvimab cohort than the SoC cohort.
Compared with SoC, regdanvimab treatment resulted in a higher proportion of patients being discharged up to day 14 (92.6% vs 77.0%) and a shorter duration of hospitalization. In the Delta variant subgroup, a higher proportion of patients were discharged up to day 14 in the regdanvimab cohort than in the SoC cohort (94.6% vs 88.7%) even though mean (SD) duration of hospitalization was significantly longer in the regdanvimab cohort than in the SoC cohort (9.93 [2.53] vs 8.55 [4.01] days).
The mean (SD) duration of fever was significantly shorter in patients who received regdanvimab (3.72 [2.44] days) than in those on SoC (5.25 [3.64] days). A numerical trend was evident in the subgroup of patients with the Delta variant (3.56 [2.19] and 3.97 [3.94] days, respectively).
A significantly lower proportion of patients required treatment with remdesivir in the regdanvimab cohort than in the SoC cohort in both the overall population (14.8% vs 36.2%) and the Delta variant subgroup (16.2% vs 25.2%).
One (0.3%) patient who was infected with the Delta variant died due to COVID-19 (SoC cohort).
The trends observed in the secondary efficacy results for a clinically beneficial effect with regdanvimab compared with SoC in the overall population were generally also evident for the subset of patients who were unvaccinated (Table 3, Table S4).
Safety
A total of 11 individuals in the regdanvimab cohort reported 15 treatment-emergent AEs (TEAEs). TEAEs reported by more than one participant included dizziness (n = 6), itching (n = 2), and rash (n = 2). The severity was primarily grade I and no serious AEs were reported. All of the TEAEs were resolved.
Discussion
This retrospective cohort study of Korean patients admitted to hospital with mild-to-moderate COVID-19 supports the clinical efficacy of regdanvimab observed in other clinical studies [16,17,19,20].
The primary end point in our study was SpO2 <90% in room air, requirement of supplemental oxygen above high flow, or death due to COVID-19. This is different from the currently approved monoclonal antibody therapies, where a reduction in hospitalization or death was used as the end point in the phase III studies, which enrolled outpatients with mild-to-moderate disease [21], [22], [23]. In Korea, patients with mild-to-moderate COVID-19 are hospitalized, providing a unique position to closely monitor these patients for progression to severe disease. The primary end point encompassed the definition of severe disease as per COVID-19 guidelines issued by the KDCA (high-flow oxygen, mechanical ventilation, extracorporeal membrane oxygenation, or continuous renal replacement therapy) and World Health Organization (SpO2 <90% on room air) [24,25]. The proportion of patients who experienced deterioration of SpO2 levels to less than 90% in room air, required supplemental oxygen therapy above high flow, or died from COVID-19 (i.e., the primary end point) was significantly lower in the regdanvimab cohort than in the SoC cohort for the overall population. Similar results were observed for secondary end points: the proportion of patients with COVID-19 who deteriorated to SpO2 <90% and <94% in room air and low-flow and high-flow supplemental oxygen was lower in the regdanvimab cohort than in the SoC cohort; compared with SoC, the regdanvimab treatment resulted in a higher proportion of patients being discharged up to day 14 (92.6% vs 77.0%) and a significantly lower proportion of patients required treatment with remdesivir in the regdanvimab cohort than in the SoC cohort. A shorter duration of hospitalization was observed in the regdanvimab group than in SoC; however, at the time of the study, a 10-day quarantine period was required according to Korean national policy, starting from the onset of symptoms. Depending on the date of hospitalization compared with symptom onset, the duration of hospitalization could vary by 1-2 days; so, this measurement may not be clinically relevant. No new safety concerns were observed.
In the Delta subgroup, the proportions of patients with a deterioration of SpO2 <90% or requiring supplemental oxygen therapy above high flow were numerically lower in the regdanvimab cohort than in the SoC cohort. Although the primary end point did not reach statistical significance, the application of a logistic regression model that controlled for varying covariates at baseline did show statistical significance (P = 0.0473). In addition, compared with the SoC cohort, a significantly higher proportion of patients were discharged by day 14, and a significantly lower proportion of patients required treatment with remdesivir, which was indicated for severe COVID-19 in the study period. The duration of hospitalization in the regdanvimab group appeared slightly longer than SoC for the Delta subgroup, but as described previously, the variability in discharge dates means this is unlikely to be clinically relevant.
Although the end point measurement method was different in the CT-P59 3.2 study (clinical symptoms requiring hospitalization, oxygen therapy, or mortality due to SARS-CoV-2 infection up to day 28), regdanvimab showed a 72% reduction of deterioration rate (3.1% vs 11.1%) in patients with high-risk of progressing to severe disease [17]. The results of our study showed similar reduction rates in the patient population overall and the Delta variant subgroup. Given these findings, although the primary end point did not reach statistical significance in the Delta variant cohort, regdanvimab showed clinical effectiveness against the Delta variant by preventing progression to severe COVID-19 and the requirement of oxygen therapy or rescue treatment. Because individuals with the Delta variant are at greater risk of deteriorating and needing emergency care than those infected with the Alpha variant and are twice as likely to be admitted to hospital [26], this may affect the evaluation of therapeutic efficacy of regdanvimab against the Delta variant.
Given the rapid evolution of SARS-CoV-2, relatively few published data are available on the effectiveness of other COVID-19 therapies against the Delta variant. In vitro studies with the Delta variant and monoclonal antibodies showed that bamlanivimab lost antiviral activity against the Delta variant, whereas etesevimab, casirivimab, and imdevimab retained activity [27]. The REGEN-COV antibody cocktail of casirivimab and imdevimab was more effective against the Delta variant than remdesivir, with a reduction in symptomatic individuals and decreased viral load and inflammatory markers on day 7 [28]. However, we interpret these results with caution due to small patient numbers (REGEN-COV n = 115, remdesivir n = 25), particularly in the remdesivir arm. A retrospective observational study at the Mayo Clinic (USA) at the time of the SARS-CoV-2 Delta surge reported significantly lower rates of hospitalization in patients who received casirivimab-imdevimab than patients who did not receive antibody therapy. This was true for vaccinated and unvaccinated individuals. Although routine sequencing was not performed (hence, it could not be confirmed that all cases were linked to the Delta variant), the Centers for Disease Control reported that the Delta variant was the predominant variant circulating in the area at the time of the study [29]. A retrospective analysis of monoclonal antibody therapy (bamlanivimab, bamlanivimab/etesevimab, or casirivimab-imdevimab) during two time periods covering an Alpha- and Beta-predominant period and a Delta-dominant period found that the odds of severe infection after treatment in the Delta cohort (4.9%) were greater than for the Alpha/Beta cohort (3.0%). The higher odds of severe infection in Delta variant could be due to a more virulent Delta variant and the insufficient number of patients compared with the total population; although, the possibility of decreased antispike monoclonal antibody effectiveness in the clinical setting cannot be excluded [30]. This study was conducted before the emergence and worldwide dominance of the Omicron variant. The efficacy and safety of regdanvimab will be evaluated in the future in patients infected with Omicron variants, including the recently prevalent new Omicron subtype Centaurus (BA.2.75), recently designated as an Omicron subvariant under monitoring by the World Health Organization [31].
In the current study, in the overall unvaccinated population, a significantly lower proportion of patients in the regdanvimab cohort deteriorated to SpO2 <90%, required supplemental oxygen therapy above high flow, or died due to COVID-19 than in the SoC cohort; this trend was also observed in the subgroup of patients with the Delta variant but did not reach statistical significance. Fully vaccinated individuals were not analyzed as a cohort because they are less likely to deteriorate and develop serious illness, even with the Delta variant [32]. Although, there are limited data available on the safety and effectiveness of COVID-19 vaccines in individuals who received passive antibody products, the latest guidance suggests no requirement to delay vaccination after receiving such therapies [33]. Our results confirmed that there was no safety issue when monoclonal antibody treatments were administered to patients with COVID-19 after vaccination.
The limitations of our study include its retrospective design and specific patient population (Korean patients admitted to a single center for treatment of mild-to-moderate COVID-19 over a 13-month period), with a relatively small number of patients infected with the Delta variant. In addition, data were reported up to 28 days only. However, given the disease course, most patients would be expected to have either recovered or progressed to severe or critical disease by this time point. The primary end point of the study was an unadjusted comparison of SpO2 <90% in room air, requirement of supplemental oxygen above high flow, or death due to COVID-19 between treatment groups. Such an analysis does not assess the impact of patient factors on these outcomes so a logistic regression analysis with baseline covariates was performed, the results of which supported a clinical benefit of regdanvimab versus SoC. The genotyping of infected patients by the KDCA was not routinely performed when this study was started so the variant type of 186 patients in the SoC group and 86 patients in the regdanvimab treatment group were unconfirmed. Consequently, the proportions of COVID-19 variants within the two treatment groups may have been different, which in turn could have affected the analyses. The strengths of the current study include the large number of patients analyzed, comprehensive set of outcome measures, and inclusion of subgroup analysis by vaccination status. In addition, to the best of our knowledge, this is the first published study to examine the impact of the Delta variant on regdanvimab effectiveness in a population of patients with objective evidence of Delta variant infection.
In conclusion, our results demonstrate that regdanvimab treatment provides clinical efficacy compared with SoC for patients hospitalized with mild-to-moderate COVID-19 in the overall cohort and may provide a clinical benefit for patients infected with the Delta variant of SARS-CoV-2.
Declaration of competing interest
Y.R. Jang and J.Y. Kim have been investigators for COVID-19 clinical trials sponsored by Bukwang Pharmaceutical, Daewoong Pharmaceuticals, Enzychem Lifesciences, GC Pharma, and Pfizer, outside the scope of the submitted work, and for Celltrion, Inc., within the scope of the submitted work. Y.J. Oh has no competing interests to declare.
Funding
Celltrion Inc., the manufacturers of regdanvimab, provided funding to Incheon Medical Center for the conduct of this study and contributed to performing the statistical analyses. The study investigators were responsible for study design, conduct, data collection, interpretation, and manuscript preparation. All authors reviewed and approved the final version of the manuscript. Celltrion Inc. reviewed the manuscript throughout development for scientific accuracy.
Ethical approval
The protocol was reviewed and approved by the local institutional review board. Formal informed consent was not required for this retrospective analysis, in accordance with Article 16 of the Bioethics and Safety Act of the Republic of Korea [34].
Acknowledgments
The authors thank all patients and investigators involved in the study (Jae Kap Lee [Division of Pulmonary Diseases, Department of Internal Medicine], Hyun Jin Kim [Division of Nephrology, Department of Internal Medicine], Jae Bum So [Department of Internal Medicine], Boram Han [Department of Neurosurgery], Sung Won Park [Division of Colorectal Surgery, Department of Surgery], Chang Yong Yoon [Department of Anesthesiology and Pain Medicine], and Yun Ok Park [Department of Anesthesiology and Pain Medicine]). Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) was provided by Duncan Campbell at Aspire Scientific (Bollington, UK) and funded by the manufacturer of regdanvimab, Celltrion Inc. (Incheon, Republic of Korea).
Author contributions
YRJ, YJO, and JYK were jointly responsible for the acquisition/interpretation of data, conception, and design of the study. Statistical analysis was conducted by Celltrion, Inc. All authors contributed to manuscript development and approved the final version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.12.035.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y, Huang J, Zhang L, Chen S, Gao J, Jiao H. The global transmission of new coronavirus variants. Environ Res. 2022;206 doi: 10.1016/j.envres.2021.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Delta variant: what is happening with transmission, hospital admissions, and restrictions? BMJ. 2021;373:n1513. doi: 10.1136/bmj.n1513. [DOI] [PubMed] [Google Scholar]
- 4.Tian D, Sun Y, Zhou J, Ye Q. The global epidemic of the SARS-CoV-2 delta variant, key spike mutations and immune escape. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.751778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CA, Patel K, Pham H, Whitaker M, Anglin O, Kambhampati AK, et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance - COVID-NET, 14 states, January–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1513–1519. doi: 10.15585/mmwr.mm7043e1. https://www.ncbi.nlm.nih.gov/pubmed/34710076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Weekly epidemiological update on COVID-19 –26 October 2021, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—26-october-2021; 2021 [accessed 01 November 2021].
- 8.Harder T, Külper-Schiek W, Reda S, Treskova-Schwarzbach M, Koch J, Vygen-Bonnet S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.41.2100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta N, Kaur H, Yadav PD, Mukhopadhyay L, Sahay RR, Kumar A, et al. Clinical characterization and genomic analysis of samples from COVID-19 breakthrough infections during the second wave among the various states of India. Viruses. 2021;13:1782. doi: 10.3390/v13091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Huuskonen S, Laitinen T, Redchuk T, Bogacheva M, Salokas K, et al. SARS-CoV-2-host proteome interactions for antiviral drug discovery. Mol Syst Biol. 2021;17:e10396. doi: 10.15252/msb.202110396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesan P. Repurposing drugs for treatment of COVID-19. Lancet Respir Med. 2021;9:e63. doi: 10.1016/S2213-2600(21)00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baral PK, Yin J, James MNG. Treatment and prevention strategies for the COVID 19 pandemic: a review of immunotherapeutic approaches for neutralizing SARS-CoV-2. Int J Biol Macromol. 2021;186:490–500. doi: 10.1016/j.ijbiomac.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184:3086–3108. doi: 10.1016/j.cell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C, Ryu DK, Lee J, Kim YI, Seo JM, Kim YG, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12:288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu DK, Kang B, Noh H, Woo SJ, Lee MH, Nuijten PM, et al. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–96. doi: 10.1016/j.bbrc.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Jang YR, Hong JH, Jung JG, Park JH, Streinu-Cercel A, et al. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection. Clin Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency. Regkirona: EPAR - Public assessment report, https://www.ema.europa.eu/documents/product-information/regkirona-epar-product-information_en.pdf; 2021 [accessed 31 January 2022].
- 18.Syed YY. Regdanvimab: first approval. Drugs. 2021;81:2133–2137. doi: 10.1007/s40265-021-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Lee SO, Lee JE, Kim KH, Lee SH, Hwang S, et al. Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: a propensity score–matched retrospective cohort study. Int Immunopharmacol. 2022;106 doi: 10.1016/j.intimp.2022.108570. https://www.sciencedirect.com/science/article/pii/S1567576922000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim JY, Kim YS, Cheon S, et al. Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019. Open Forum Infect Dis. 2022;9:ofac053. doi: 10.1093/ofid/ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 23.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Clinical management of COVID-19 patients: living guidance, https://app.magicapp.org/#/guideline/j1WBYn/section/L0bmdE; 2021 [accessed 01 February 2022].
- 25.Park AKK, I-H., Kim J-M, Lee N-J, Rhee JE, Kim E-J, Kim J, Kim JY, Gwak J, Kim E-K, Kim Y-M, Lee S-E, Park YJ. [COVID-19 special report]. Update 2021 status and characteristics of COVID-19 variant virus outbreak in the Republic of Korea, https://nih.go.kr/filepath/boardDownload.es?bid=0034&list_no=713840&seq=1; 2021 [accessed 14 February 2022].
- 26.Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Péré H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Lorière E, Rey FA, Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 28.Kumar VJ, Banu S, Sasikala M, Parsa KVL, Sowpati DT, Yadav R, et al. Effectiveness of REGEN-COV antibody cocktail against the B.1.617.2 (delta) variant of SARS-CoV-2: a cohort study. J Intern Med. 2021;291:380–383. doi: 10.1111/joim.13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierle DM, Ganesh R, Razonable RR. Breakthrough COVID-19 and casirivimab-imdevimab treatment during a SARS-CoV-2 B1.617.2 (Delta) surge. J Clin Virol. 2021;145 doi: 10.1016/j.jcv.2021.105026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Horo JC, Challener DW, Speicher L, Bosch W, Seville MT, Bierle DM, et al. Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant. Mayo Clin Proc. 2022;97:327–332. doi: 10.1016/j.mayocp.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Tracking SARS-CoV-2 variants, https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/; 2022 [accessed 02 August 2022].
- 32.Centers for Disease Control and Prevention. The possibility of COVID-19 after vaccination: breakthrough infections, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html; 2021 [accessed 01 February 2022].
- 33.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States, https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html; 2022 [accessed 14 February 2022].
- 34.Statutes of the Republic of Korea . 2014. Bioethics and Safety Act.https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=33442&type=part&key=36 [accessed 11 June 2021] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.