Abstract
Toll-like receptors 2 and 4 (TLR2 and TLR4) have been found to transduce signals of peptidoglycan (PGN) and lipopolysaccharide (LPS), respectively, for NF-κB activation. However, little is known about the expression and regulation of the TLR2 gene in monocytes/macrophages in response to the two typical bacterial products. We show in the present study that both PGN and a high concentration of LPS increase TLR2 gene expression in macrophage-like cells, 1α,25-dihydroxyvitamin D3-differentiated human HL60 and mouse RAW264.7 cells, and human monocytes in a dose- and time-dependent manner. Actinomycin D and pyrrolidine dithiocarbamate inhibition of gene transcription and NF-κB activation, respectively, blocks LPS- and PGN-elevated TLR2 mRNA in monocytic cells. The LPS-induced increase in TLR2 mRNA in monocytic cells is abolished by polymyxin B pretreatment and is observed in peripheral blood mononuclear cells from pigs subjected to endotoxic shock. Further, high concentrations of LPS and synthetic lipid A increase TLR2 mRNA expression in peritoneal macrophages from both TLR4-deficient C3H/HeJ mice and normal C3H/HeN mice, a process that constitutes induction of TLR4-independent TLR2 expression. These findings demonstrate that TLR2 gene expression is upregulated in macrophage responses to PGN and to high concentrations of LPS in vitro and in vivo and correlates with NF-κB activation.
Several observed clinical and immunological similarities between lipopolysaccharide (LPS), an integral component of the outer cell membranes of gram-negative bacteria, and peptidoglycan (PGN), a component of the cell walls of gram-positive bacteria, suggest that the two bacterial components initiate analogous signaling pathways after being recognized by monocytes/macrophages (8, 12, 15, 33, 49). These signaling pathways are thought to be cascades for NF-κB activation. Activated NF-κB controls the transcription of numerous genes including those involved in cell proliferation, transformation, inflammation, and survival (2, 3, 32). Besides activating NF-κB, LPS and PGN similarly activate other transcription factors, such as CREB/ATF and AP-1, in a membrane CD14 (mCD14)-dependent or -independent manner (13, 14, 18). The real dissimilarity between their signal pathways was revealed recently, and their initial discrimination by different Drosophila melanogaster Toll proteins, evolutionarily conserved from fruit flies to humans, is under way (4, 21). To date, six human Toll-like receptors (TLRs) have been cloned (40, 44). Of these, TLR2 and TLR4 have been extensively investigated and are thought to be signal transducers of PGN and LPS, respectively (7, 24, 32, 35, 50).
TLR2, predominantly distributed in monocytes/macrophages and polymorphonuclear cells (PMNs) (34), had been thought to be a signal transducer of LPS. Conclusive evidence mainly came from the following data. First, the overexpression of TLR2 in human embryonic 293 cells conferred LPS responsiveness on the cells, resulting in NF-κB activation and interleukin-8 (IL-8) mRNA expression. Second, the cotransfection of TLR2 with mCD14 synergistically enhanced LPS signaling (24, 50). Third, the putative complex of mCD14-TLR2 after LPS exposure to the cotransfected cells was found to initiate an IL-1 receptor-like NF-κB signaling cascade (51). Fourth, the LPS-elevated expression of the TLR2 gene, as measured by reverse transcription-PCR (RT-PCR), was observed in human monocytes and in mouse peritoneal macrophages (31, 50). Like the transcriptional expression of TLR2, the transcriptional expression of mCD14 in human monocytes is upregulated by LPS (30). The elevated expression of the TLR2 and mCD14 genes undoubtedly contributes to the resensitization of macrophages to invasive pathogens. However, the finding that TLR4 but not TLR2 senses LPS has challenged the above conclusions.
TLR4 is predominantly present in monocytes/macrophages, PMNs, dermal microvessel and umbilical vein endothelial cells, and intestinal epithelial cells (6, 9, 34). Several studies have shown that a TLR4 transfectant initiates a series of events for NF-κB activation, as does TLR2 (7, 32). Moreover, the macrophages and B cells from mice (C3H/HeJ) that have a TLR4 mutation in the intracellular domain consisting of the replacement of a conserved proline residue by histone are hyporesponsive to LPS in vitro and in vivo (22, 36, 38). Further evidence from TLR2 and TLR4 knockout mice has confirmed that TLR4 is a signal transducer of LPS (27, 37, 43, 47), whereas TLR2 is a common transducer of diverse microbial products but not of LPS (28). This conclusion is clearly supported by the finding that a human TLR4 mutation in the extracellular domain consisting of the replacement of a conserved aspartic acid residue by glycine is present in individuals who showed hyporesponsiveness to inhaled LPS (1). Also, the observation of upregulation of TLR4 gene expression in human monocytes exposed to a low concentration of LPS favors the conclusion (34).
Indeed, LPSd mice with function-deficient TLR4 are hyporesponsive rather than unresponsive to LPS and can die from an injection with a high dose of LPS or with a normal dose of LPS after being infected with Mycobacterium bovis BCG or primed with gamma interferon (23, 48). Purification of LPS by the removal of the contaminant lipoprotein eliminated the responsiveness of the LPSd mice to the form of Ra-LPS from Salmonella enterica serovar Minnesota but not to the LPS from Prevotella intermedia and Porphyromonas gingivalis (23). Moreover, an LPS challenge results in different levels of expression of TLR4 mRNA in different species, i.e., its expression is upregulated in human monocytes but is downregulated in mouse peritoneal macrophages and macrophage-like cells (34). By contrast, TLR2 transcriptional expression is elevated by LPS challenges in human monocytes, PMNs, and mouse macrophages (31, 50), although one report showed that the downregulation of TLR2 expression was observed in human monocytes exposed to LPS (34). The upregulation of TLR expression after a pathogen challenge appears to be consistent with the function of a Drosophila Toll protein whose expression is upregulated by an immune challenge (26). Based on an analysis of the above issues concerning TLR2 and TLR4, we hypothesized that TLR2 may play a role in native macrophages in response to a severe invasion by gram-positive bacteria and large numbers of gram-negative bacteria. In the present study, we demonstrate that TLR2 gene expression is quickly upregulated in macrophages exposed to PGN or a high concentration of LPS in vitro and in peripheral blood mononuclear cells (PBMCs) from pigs with lethal endotoxic shock by means of a common mechanism involved in NF-κB activation.
MATERIALS AND METHODS
Materials.
B5-LPS (Escherichia coli strain O55:B5), B4-LPS (E. coli strain O111:B4), 1α,25-dihydroxyvitamin D3 (VitD3), polymyxin B, pyrrolidine dithiocarbamate (PDTC), actinomycin D, and cycloheximide were purchased from Sigma (St. Louis, Mo.). Re-LPS from S. enterica serovar Minnesota R595 was from List Biochemicals. Synthetic E. coli-type lipid A (LA-15-PP, 506) was purchased from Dai-ich Chemicals (Tokyo, Japan). Antibodies against NF-κB components p50 and p65, NF-κB consensus oligonucleotide, and RT-PCR reagents were from Promega (Madison, Wis.). Insoluble PGN from Staphylococcus aureus was from Fluka Biochemica (Steinheim, Switzerland), and Leu-M3 fluorescein isothiocyanate-labeled mouse immunoglobulin G M13 against human mCD14 was from Becton Dickinson (Fullerton, Calif.). Anti-TLR4 monoclonal antibody HTA125 was a gift from K. Miyake (Saga Medical School, Saga, Japan). [γ-32P]ATP and [α-32P]dCTP were from Amersham (Buckinghamshire, United Kingdom).
Cell preparation.
Human promyelocytic leukemia HL60 cells and murine macrophage-like RAW264.7 cells were obtained from the American Type Culture Collection (Manassas, Va.). HL60 cells were maintained in an RPMI 1640 medium with 10% fetal bovine serum (FBS) and were then differentiated into monocytes by being treated with 50 nM VitD3 for 4 days. The differentiated promyelocytic HI60 cells were designated the monocytic HL60 cells. RAW264.7 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS. Circulating human monocytes were separated from the blood of healthy donors by means of Percoll centrifugation (Pharmacia, Uppsala, Sweden) according to the manufacturer's instructions and were treated in vitro with Re-LPS. The porcine PBMCs were isolated from the blood of specific-pathogen-free pigs (male, weighing approximately 23 to 34 kg) after a 2-h infusion of B4-LPS (10 μg/kg/h) by means of Lymphoprep (Nycomed Parmaas, Oslo, Norway).
Murine peritoneal macrophages from C3H/HeN mice obtained from the Division of Research, National Institutes of Health (Bethesda, Md.) and C3H/HeJ mice obtained from Jackson Laboratories (Bar Harbor, Maine) were prepared as follows. The mice, used at 6 to 8 weeks of age and fed standard laboratory chow and water, were injected intraperitoneally with 2 ml of 4% sterile thioglycolate broth, and the resulting peritoneal exudate was harvested by lavage of the peritoneal cavities of mice with endotoxin-free Hanks' solution (Sigma) 4 to 5 days later. The peritoneal cells were cultured in 10% FBS–DMEM (with high glucose; Gibco BRL) in a 100-mm-diameter plastic dish for 2 h, and the dish was then washed four times with warm FBS-free DMEM to remove nonadherent cells. The adherent cells were continuousely cultured in the complete medium and after 16 h were washed twice with warm FBS-free DMEM. The adherent macrophages, more than 95% of which appeared to be typical macrophages by light microscopy, were used for each experiment.
Electrophoresis mobility shift assay (EMSA).
Nuclear extracts were prepared as previously described (25). Briefly, treated and untreated cells were lysed with buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol [DTT], 1 mM phenylmethyl sulfonyl fluoride [PMSF], 0.6% NP-40). Nuclear lysis was performed using buffer B (20 mM HEPES [pH 7.9], 550 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 1 mM PMSF, 10 μg of each proteinase inhibitor [aprotinin, pepstatin, and leupeptin]/ml). The nuclear extracts were obtained by centrifugation at 17,500 × g for 15 min and subjected to protein determination. Double-stranded deoxyoligonucleotides containing the NF-κB consensus recognition site (5′-AGTTGAGGGGACTTTCCCAGG-3′) were end labeled with [γ-32P]ATP using T4 polynucleotide kinase (Pharmacia, Piscataway, N.J.). A 7.5-μg sample of the nuclear proteins was incubated with 0.2 mg of poly(dI-dC)/ml in a binding buffer (5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris-HCl [pH 7.5], 20% glycerol) for 20 min at room temperature after the addition of a labeled probe. Competition assays were conducted with a 50-fold molar excess of an unlabeled probe. For the supershift assay, specific antibodies against NF-κB components p50 and p65 were used. The intensity of the DNA-protein complex bands was measured using a PhosphorImager system (FUJIX BAS 1000; Fujifilm, Tokyo, Japan).
Preparation of TLR probes.
A 478-bp cDNA fragment of human TLR2 was amplified by means of RT-PCR using primers (sense: 5′-ATGAAAATGATGTGGGCCTG-3′; antisense: 5′-TTACCCAAAATCCTTCCCGC-3′) corresponding to positions 1981 to 2001 and 2458 to 2438 in the TLR2 cDNA sequence with GenBank accession no. U88878 (40). PCR amplification was performed under conditions of 94°C denaturation, 60°C annealing, and 72°C extension; each step was carried out for 1 min, and 30 cycles were performed. An additional denaturation step (94°C for 5 min) and extension step (72°C for 5 min), each for one cycle, were required. The human TLR4 cDNA with a length of 507 bp was amplified by means of RT-PCR using primers (sense: 5′-TGGATACGTTTCCTTATAAG-3′; antisense: 5′-GAAATGGAGGCACCCCTTC-3′) corresponding to positions 1778 to 1798 and 2266 to 2285 in the TLR4 cDNA sequence with GenBank accession no. U88880 (40). The PCR amplification conditions for TLR4 were the same as those used for TLR2, except for the annealing temperature, which was 58°C instead of 60°C. PCR products were excised from agarose gel and purified by means of the QIAquick gel extraction kit (250) (Qiagen GmbH, Hilden, Germany). Each amplified cDNA fragment was ligated into a PGEM-T vector (Promega Corp.) The TLR cDNA fragments and their T-vector ligation products were sequenced to confirm their fidelity.
RNA isolation and Northern blot analysis.
Total cellular RNA was isolated from treated and untreated cells using TRI-Reagent (Gibco BRL) according to the manufacturer's instructions. An aliquot (20 μg) of total RNA was resolved on a 1% agarose gel and then transferred onto a Hybond-N+ membrane (Amersham Pharmacia Biotech) by a capillary transfer method. After a 30-min prehybridization at 65°C, the membranes were hybridized with 106 cpm of the labeled probe/ml for 3 h at 65°C in Rapid hyb buffer (Amersham Pharmacia Biotech). Following hybridization, the membranes were rinsed at room temperature with 2 × SSC (1 × SSC is 0.15 M NaOH plus 0.015 M sodium citrate) (two times) and then washed sequentially with 1 × SSC–0.1% sodium dodecyl sulfate (SDS) (two times for 3 min at 65°C) and 0.1 × SSC–0.1% SDS (two times for 3 min at 65°C). The intensities of the bands were measured by the PhosphorImager system (FUJIX BAS 1000). Autoradiography was carried out at −70°C using BioMax light film (Eastman Kodak). The membrane was reprobed by a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) human cDNA probe (Cayman Chemical, Ann Arbor, Mich.) for an internal control after the membrane was stripped by being washed in 2× SSC containing 50% formamide for 1 h at 65°C.
RESULTS
The monocytic HL60 cells responded to Re-LPS and to PGN with a strong activation of NF-κB.
It is known that promyelocytic HL60 cells do not express a detectable level of mCD14, which is necessary for a monocyte/macrophage response to low concentrations of LPS. However, when promyelocytic HL60 cells were differentiated into monocytes by being treated with 50 nM VitD3 for 4 days, the percentage of mCD14-positive cells reached more than 80%, as measured by a flow cytometry (data not shown). The differentiated HL60 cells strongly responded to both Re-LPS and PGN as judged by activated NF-κB (Fig. 1A). Re-LPS, at 0.2 ng/ml, markedly activated NF-κB, and 5 ng of Re-LPS/ml gave rise to the highest activation of NF-κB (data not shown). The PGN tested in this study showed the highest activation of NF-κB at a dose of 240 μg/ml (data not shown). Based on the supershift assay of NF-κB subunits, we confirmed that the NF-κB activated by Re-LPS or PGN was a heterodimer composed of p65 (RelA) and p50. In contrast, the undifferentiated promyelocytic HL60 cells did not respond to Re-LPS or PGN, as indicated by inactivated NF-κB in the gel shift assay. These results suggest that NF-κB activation by Re-LPS or PGN at the doses tested in this study is specific to monocytes/macrophages.
FIG. 1.
Re-LPS and PGN activate the DNA binding activity of NF-κB in monocytic HL60 cells. The promyelocytic HL60 cells were differentiated into monocytes by treatment with 50 nM VitD3 for 4 days at a cell density of 2 × 105/ml in a serum-free RPMI 1640 medium. The monocytic HL60 cells showed a strong activation of NF-κB after being treated with either Re-LPS (0.2 or 2 ng/ml) for 30 min or with PGN (60 or 120 μg/ml, dissolved in PBS by sonication) for 4 h. A supershift assay (lanes 8 to 13) using antibodies against p65 and p50 of NF-κB confirmed that the activated NF-κB was a heterodimer composed of p50 and p65. Lane 1, competition assay (Com) using a 50-fold molar excess of the NF-κB consensus. The result is representative of three separate experiments.
Re-LPS elevated TLR2 transcriptional expression in monocytic HL60 cells.
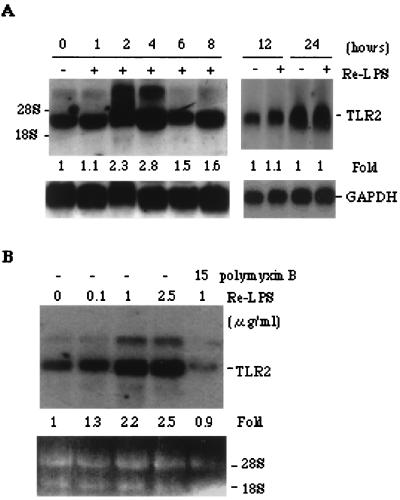
It has been reported that monocyte-like cells THP-1(53) and U937(6) constitutively express TLR2 and TLR4 as measured by RT-PCR. In contrast, we did not find that the promyelocytic HL60 cells expressed a detectable level of TLR2 based on RT-PCR detection. However, the differentiated HL60 cells expressed TLR2 and TLR4, as measured by RT-PCR (data not shown). We observed that the TLR2 gene expression was elevated by stimulation with a high dose of Re-LPS, as measured by RT-PCR (data not shown) and by Northern blot analysis (Fig. 2A and B). The RT-PCR product corresponding to TLR2 was excised for sequence identification, and the resulting sequence was identical to that with GenBank accession no. U88878 (40). Re-LPS elevation of TLR2 mRNA was time and dose dependent (Fig. 2A and B). The expression of TLR2 was highest at 2 to 4 h, quickly declined at 6 h, and remained unchanged at 12 or 24 h after the monocytic HL60 cells were exposed to 1 μg of LPS/ml. The optimal dose of Re-LPS for elevating the expression of TLR2 mRNA was 1 μg/ml (Fig. 2B), which is approximately 200-fold higher than the optimal dose (5 ng/ml) needed for NF-κB activation. Less than 100 ng of Re-LPS/ml was ineffective in stimulating the transcriptional expression of TLR2, but at this dose NF-κB was still largely activated. This result indicated that a low dose of Re-LPS activated NF-κB by means of a TLR2-independent signal pathway, whereas a high dose of Re-LPS activated NF-κB probably by means of a TLR2-dependent pathway. The elevation of TLR2 mRNA was not due to a trace contaminant that may be contained in commercial Re-LPS, since polymyxin B eliminated the LPS-induced TLR2 mRNA augmentation (Fig. 2B).
FIG. 2.
A high concentration of Re-LPS elevates the expression of TLR2 mRNA in monocytic HL60 cells. Monocytic HL60 cells were treated with either 1 μg of Re-LPS/ml for the indicated times (A) or various concentrations of Re-LPS for 2 h in the absence or presence of 15 μg of polymyxin B/ml (B). The TLR2 mRNA level was detected by Northern blot analysis using its cDNA probe. The fold increases shown were representative of four independent experiments after normalization to GAPDH. The TLR2 mRNA level in the control group was defined as 1 arbitrary unit.
PGN augmented the transcriptional expression of TLR2 in monocytic HL60 cells.
It has been reported that PGN at a dose of 240 μg/ml activated to the largest extent the NF-κB of macrophages (13). At this dose, PGN greatly increased the transcriptional expression of TLR2 in monocytic HL60 cells (Fig. 3A). The PGN-elevated TLR2 mRNA was observed after the cells were exposed to 240 μg of PGN/ml for 2 and 4 h, as measured by Northern blotting (Fig. 3B). The optimal condition of PGN required for elevating TLR2 mRNA was consistent with that needed for the activation of NF-κB, suggesting that TLR2 transduces the PGN signal for NF-κB activation.
FIG. 3.
PGN increased the expression of TLR2 mRNA in the monocytic HL60 cells. The monocytic HL60 cells were stimulated with either 240 μg of PGN/ml for 2 or 4 h (A) or with the indicated concentrations of PGN for 4 h in the absence or presence of 15 μg of polymyxin B/ml (B). The expression of TLR2 mRNA was detected by Northern blot analysis. The fold increases relative to the vehicle from one representative of three separate experiments after normalization to GAPDH are shown (A).
Upregulated expression of TLR2 in mouse macrophage-like RAW264.7 cells in response to B5-LPS and PGN.
To extend the above observations to mouse macrophages, we employed the cDNA probe of human TLR2 (hTLR2) to investigate the transcriptional expression of mouse TLR2 (mTLR2). The hTLR2 probe encompasses the region of the intracellular functional domain of hTLR2 in which there is a more than 85% homology between hTLR2 and mTLR2 (accession no. AF124741) (19). As expected, the increased expression of mTLR2 mRNA occurred in the RAW264.7 cells in response to a high dose of B5-LPS (Fig. 4A). In this assay, B5-LPS was substituted for Re-LPS merely because B5-LPS is a stronger stimulator of NF-κB activation in RAW264.7 cells than Re-LPS. Elevated expression of mTLR2 mRNA was also observed in RAW264.7 cells exposed to 240 μg of PGN/ml for 2 and 4 h (Fig. 4B). As in monocytic HL60 cells, B5-LPS elevated mTLR2 mRNA expression at an optimal dose which was as much as about 200-fold more than that needed for NF-κB activation.
FIG. 4.
B5-LPS and PGN elevate the expression of mTLR2 mRNA in mouse macrophage-like RAW264.7 cells. RAW264.7 cells were passaged into fresh DMEM containing 10% FBS for 24 h prior to treatment with the indicated dose of B5-LPS for 2 h (A) or with 240 μg of PGN/ml for the indicated periods of time (B). The levels of mTLR2 mRNA were detected by Northern blot analysis using as the probe hTLR2 cDNA. The fold increases of TLR2 mRNA relative to the vehicle after normalization to GAPDH are shown. The results are representative of three experiments.
LPS elevated TLR2 gene expression in human monocytes and in PBMCs from pigs with endotoxic shock.
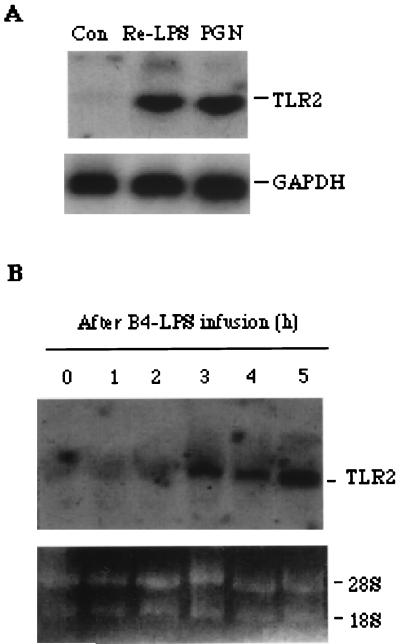
The transcriptional expression of TLR2 in human monocytes was examined and found to be upregulated by in vitro stimulation with either 1 μg of Re-LPS/ml or 240 μg of PGN/ml (Fig. 5A). To further confirm the role played by TLR2 in macrophage defense against infection with a large amount of LPS, we examined the expression of TLR2 mRNA in the PMBCs from pigs subjected to lethal endotoxic shock by using the hTLR2 probe. As expected, TLR2 mRNA was elevated after LPS infusion for 3 h and the elevation was sustained for 5 h (Fig. 5B). This in vivo result strongly supported the data obtained from monocytes/macrophages treated in vitro with a high concentration of LPS.
FIG. 5.
Upregulation of TLR2 gene expression was observed in human monocytes exposed to PGN or Re-LPS and in the PBMCs from pigs with endotoxic shock. Human monocytes were isolated and treated in vitro with 1 μg of Re-LPS/ml or with 240 μg of PGN/ml (A), each for 4 h. The PBMCs from the blood of pigs after infusion of B4-LPS were isolated at the indicated times (B). The total mRNA of the monocytes and PBMCs was extracted and detected by Northern blot analysis as described in Materials and Methods. One representative from three (monocyte) or five (PBMC) separate experiments is shown. Con, control.
The involvement of TLR2 gene expression in the activation of NF-κB.
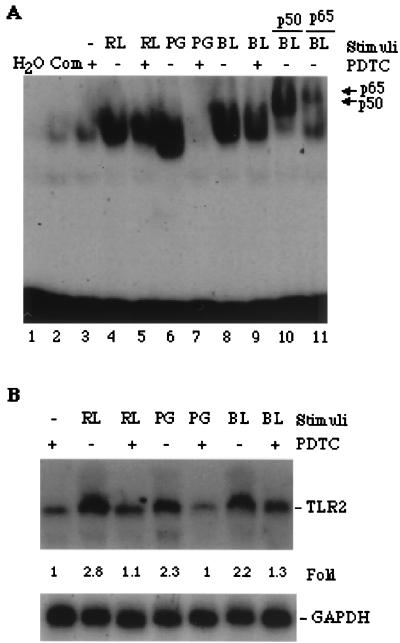
PDTC, an antioxidant, has been demonstrated to inhibit the activation of NF-κB in human monocyte-like U937 cells (29). Its inhibition of NF-κB activation is also effective in monocytic HL60 cells exposed to two kinds of LPS, Re-LPS and B5-LPS, and to PGN (Fig. 6A). Supershift by using an antibody against the p50 or p65 component of NF-κB confirmed that the NF-κB activated by B5-LPS is also a heterodimer composed of p50 and p65 (Fig. 6A, lanes 9 and 10). Monocytic HL60 cells like those used for the NF-κB assay were subjected to Northern blot analysis of the expression of TLR2 mRNA. Consequently, the expression of TLR2 mRNA in cells induced by Re-LPS, B5-LPS, and PGN was blocked by pretreatment with PDTC (Fig. 6B). These results demonstrated that NF-κB activation may be necessary for the expression of TLR2 mRNA.
FIG. 6.
The elevation of TLR2 mRNA expression is involved in NF-κB activation. (A) PDTC blocked NF-κB activation. Monocytic HL60 cells were pretreated with 100 μM PDTC for 30 min (lanes 3, 5, 7, and 9) and then continuously stimulated with 10 ng of Re-LPS (RL) and B5-LPS (BL)/ml, each for 30 min, or with 240 μg of PGN (PG)/ml for 3 h. Nuclear proteins were extracted and then subjected to EMSA analysis. Lanes 10 and 11 are the supershifts of lane 8 using antibodies against p50 and p65 of NF-κB, respectively. Com, competition assay (lane 4) using a 50-fold molar excess of the NF-κB consensus. (B) Northern blot analysis showed that PDTC blocked the expression of TLR2 mRNA in the monocytic HL60 cells. Cells like those used for EMSA were employed for detecting the expression of TLR2 mRNA. The results are representative of three different experiments. The fold increase relative to the vehicle (PDTC alone) was normalized to GAPDH and is shown at the bottom.
The augmentation of TLR2 mRNA expression occurred at the level of transcription.
The monocytic HL60 cells were pretreated with either actinomycin D or cycloheximide and then treated with Re-LPS. As a result, the Re-LPS-induced elevation of TLR2 mRNA was inhibited by 60% by cycloheximide and was completely blocked by actinomycin D (Fig. 7A). These results suggest that the elevated TLR2 mRNA in the monocytic HL60 cells responsive to Re-LPS may be due to the transcriptional activation of TLR2. The results of repeated experiments are shown in Fig. 7B.
FIG. 7.
Actinomycin D blocked the upregulation of TLR2 mRNA in monocytic HL60 cells. Monocytic HL60 cells were pretreated with 100 μM PDTC, 50 μg of cycloheximide (CHX)/ml, and 2 μg of actinomycin D (ActD)/ml, each for 30 min, and then treated with 1 μg of Re-LPS/ml for 2 h in the presence of the respective inhibitor. Northern blotting was subsequently performed as described in Materials and Methods. One representative from three separate experiments is shown in panel A. The fold increases relative to the control (Con) after normalization to GAPDH are presented as the means ± standard errors of three separate experiments in panel B.
High concentrations of LPS and synthetic E. coli-type lipid A increase TLR mRNA expression in peritoneal macrophages from normal and TLR4-deficient mice through a TLR4-independent mechanism.
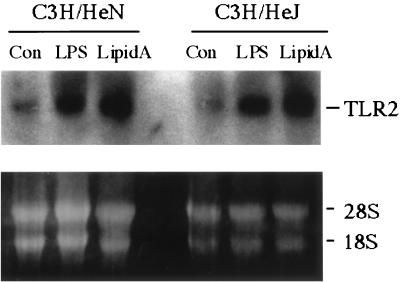
To further confirm that the increased TLR2 mRNA expression induced by high concentrations of LPS is not due to the trace contamination of endotoxic proteins contained in the commercial LPS used, we employed synthetic E. coli-type lipid A to stimulate in vitro primary cultured peritoneal macrophages from LPSd C3H/HeJ mice and control C3H/HeN mice. As shown in Fig. 8, B5-LPS and lipid A at a dose of 2.5 μg/ml augment TLR2 mRNA expression and lipid A shows a stronger effect than B5-LPS in the two types of mice. This result excludes the possibility that TLR2 gene expression induced by high doses of LPS results from the trace contamination of an endotoxic protein present in the LPS. This result also demonstrates that the increased TLR2 expression induced by LPS or lipid A is TLR4 independent. Since low doses of LPS, less than 100 ng/ml, do not elevate TLR2 mRNA expression in monocytes/macrophages from humans pigs, or BALB/c mice or in the monocyte/macrophage-like cell lines used here, we conclude that only high concentrations of LPS cause cells to respond by increasing TLR2 mRNA expression.
FIG. 8.
High concentrations of LPS and synthetic lipid A increase TLR2 mRNA expression in peritoneal macrophages from normal C3H/HeN mice and LPSd C3H/HeJ mice. The peritoneal macrophages separated as described in Materials and Methods were treated with B5-LPS or synthetic lipid A, each at 2.5 μg/ml, for 3 h. Subsequently, the total RNA was extracted and subjected to Northen blot analysis using the hTLR2 probe as described in Materials and Methods. The results are representative of two separate experiments, each done in duplicate. Con, control.
DISCUSSION
Since TLR2 and TLR4 are the best candidates for bridging from the innate immunity of macrophages to the adaptive immunity of T and B lymphocytes, an understanding of their transcriptional expression and regulation in native macrophages in response to gram-negative and gram-positive bacteria is important. However, little is known about these aspects, although two recent reports based on RT-PCR results demonstrated that TLR2 gene expression was upregulated both in human monocytes and in mouse peritoneal macrophages in response to Re-LPS and another kind of LPS (E. coli strain K253), respectively (31, 50). One report, however, demonstrated that TLR2 transcriptional expression was downregulated in human monocytes exposed to LPS. It is unclear why the transcriptional expression of TLR in monocytes/macrophages in response to LPS stimulation is divergent in humans and mice. Nevertheless, it is noteworthy that TLR2 expression is changeable in native monocytes/macrophages facing an LPS challenge. Convincing genetic evidence has definitely demonstrated that TLR4 is a signal transducer for LPS (27, 37, 43, 47), whereas TLR2 is a common transducer for all products of microbial pathogens except for LPS (28). However, in a circumstance in which monocytes/macrophages face the challenge of a large amount of LPS, what role is played by TLR2? In order to answer this question, we examined TLR2 gene expression in monocytes and macrophage-like cells in response to LPS and PGN in vitro and in PBMCs from pigs subjected to lethal endotoxic shock.
Human monocytic HL60 cells derived from vitamin D3-differentiated promyelocytic HL60 cells and mouse macrophage-like RAW264.7 cells were found to predominantly express both TLR2 and TLR4 and to exhibit activation of the p50-p65 heterodimer of NF-κB induced by PGN or LPS. We found that both LPS and PGN promote the transcription of the TLR2 gene in an early phase following NF-κB activation. However, the optimal dose required for NF-κB activation in cells responsive to PGN but not to LPS is identical to that required for TLR2 mRNA elevation. In the cell response to LPS, the optimal dose (5 ng/ml) required for NF-κB activation is approximately 200-fold less than that (1,000 ng/ml) required for TLR2 expression. Indeed, this phenomenon was seen by Yang et al., who reported that TLR2 mRNA is elevated in human monocytes exposed to 1,000 ng of Re-LPS/ml (50). In contrast, Muzio et al. reported that 0.1 ng of LPS/ml sufficed to elevate TLR4 gene expression in human monocytes and that the optimal dose is 1 ng/ml (34), which is identical to the optimal dose required for NF-κB activation induced by LPS. These findings, together with our data showing the dose-inconsistent effect of LPS on NF-κB activation and TLR2 mRNA elevation, strongly support the hypothesis that TLR4 but not TLR2 is sensitive to LPS stimulation. Thus, TLR2 may not participate in LPS signaling unless macrophages face the challenge of large amounts of LPS. The consequence of TLR2 participation is likely to be that TLR2 gene expression is elevated in order to compensate for the consumption of TLR2. This process may account for why TLR2-transfected cells also respond to LPS stimulation (5, 10, 24, 50).
The elevation of TLR2 gene expression induced by high concentrations of LPS could be attributed to the fact that large amounts of LPS, after aggregation by an LPS binding protein, could be preferentially discriminated by TLR2 with the help of mCD14 whereas an LPS monomer or its smaller aggregator could be preferentially discriminated by TLR4. We tend to believe that the elevation of LPS-induced TLR2 gene expression is due to the consumptive compensation of TLR2 and have shown that the elevation of TLR2 expression is not TLR4 dependent. The reason for this belief is that we found that high concentrations of LPS were able to elevate TLR2 mRNA expression in TLR4-deficient C3H/HeJ mice and that we did not detect a blockage of LPS-induced TLR2 mRNA elevation in RAW264.7 cells or monocytic HL60 cells by TLR4 antibody pretreatment (data not shown). In addition, the previous data showing that the macrophages from TLR4-deficient mice can respond to large amounts of LPS or to a normal dose of LPS after being primed by gamma interferon or by chronic infection of the mice with BCG imply that a TLR4-deficient macrophage is still able to sense LPS but lacks sensitivity and requires priming. This fact supports our above belief that the participation of TLR2 in LPS signaling occurs via a mechanism independent of TLR4.
To further confirm the elevation of TLR2 expression by a high concentration of LPS, we employed an hTLR2 probe to search for the in vivo expression of TLR2 mRNA in PBMCs from pigs with endotoxic shock. The hTLR2 probe was used merely because TLR2 is highly homologous among species, as opposed to TLR4, for which homology among species is relatively low. We found that elevated expression of TLR2 occurred in the PBMCs from pigs subjected to lethal endotoxic shock by infusion of a high dose of LPS. This in vivo result clearly supports the in vitro one showing LPS-increased TLR2 mRNA. We showed that LPS-elevated TLR2 expression was not a consequence of contamination with trace endotoxic proteins contained in the commercial Re-LPS, which had been determined to contain less than 1% protein. A reasonable explanation is that a high concentration of synthetic lipid A elevates, to a greater extent than LPS does, TLR2 mRNA expression in macrophages from normal C3H/HeN mice and TLR4-deficient C3H/HeJ mice and that polymyxin B, a neutralizer of LPS, eliminated the increase in TLR2 mRNA due to Re-LPS but not that due to PGN. The elimination of increased TLR2 expression due to Re-LPS by polymyxin B is consistent with the finding showing that polymyxin B abolished the responsiveness of interferon-primed macrophages from TLR4-deficient mice to nonrepurified Ra-LPS, another form of LPS from S. enterica serovar Minnesota (23). Undoubtedly, the upregulated expression of TLR2 mRNA is consistent with evidence demonstrating that TLR2 confers responsiveness to LPS (24, 50), PGN and lipoteichoic acid (42), lipoprotein (5, 20), mycobacterial cell wall component lipoarabinomannan (46), zymosan (45), and heat-killed gram-positive bacteria (52). Hence, TLR2 has been thought to be a common transducer of diverse microbial components for NF-κB activation (28). This notion is most likely true, but, in terms of LPS, TLR2 could be an insensitive transducer unless challenged by large amounts of LPS.
A recent report has demonstrated that TLR4 transcriptional expression is controlled by two transcriptional factors, PU.1 and interferon consensus sequence-binding protein (39). We found that TLR2 expression in the monocytic HL60 cells depended on NF-κB activation since the inhibition of NF-κB activation by PDTC blocked the increase of TLR2 mRNA in the cells exposed to Re-LPS, B5-LPS, and PGN. The elevation of TLR2-mRNA induced by LPS could be controlled at the transcription level because actinomycin D, a transcription inhibitor, blocked the LPS effect.
For confirmation of the above preliminary conclusion, a genetic approach is required. In most mammalian cells, NF-κB is a heterodimer composed of p50 and p65 and is sequestered in cytoplasm by IκB, an inhibitor of NF-κB activation. The heterodimeric generation of NF-κB requires the proteasome-mediated degradation of IκB (11). Proteasomes in eukaryotes are composed of seven different α and β subunits (41). Of them, three constitutive β subunits, β1 (d), β5 (MB1), and β2 (Z), can be replaced in higher eukaryotes by facultative subunits β1i (Lmp2), β5i (Lmp7), and β2i (Mecl1), which are expressed in response to external stimuli (41). Lpm2 has been identified as essential for NF-κB activation since Lpm2-deficient mice display impaired NF-κB p65 activation due to a deficiency in the proteasome-mediated IκB degradation process (16, 17). By using Lpm2-deficient mice, we discovered that the LPS-elevated transcriptional expression of TLR2 disappeared in the embryonic macrophages from the Lpm2-deficient mice (data not shown). The transcriptional expression of TLR2 controlled by NF-κB will be further defined by methods of NF-κB-driven transcription of the TLR2 promoter after its sequence is discovered. The result of a promoter assay for confirming the role of NF-κB in TLR2 gene expression will highlight the pharmaceutical strategy for therapy of LPS- and PGN-induced septic shock.
In summary, we found, for the first time, that TLR2 transcriptional expression is upregulated in monocytes/macrophages exposed in vitro to PGN or to large amounts of LPS and in PBMCs from pigs with lethal endotoxic shock, as measured by Northern blot analysis. We also found that the upregulation of TLR2 expression is involved in NF-κB activation and that it is probably controlled by an autocycle of TLR2–NF-κB–TLR2. These observations offer a new insight for understanding the role of TLR2 in the macrophage defence against gram-positive bacteria and large numbers of gram-negative bacteria.
REFERENCES
- 1.Arbour N C, Lorenz E, Schutte B C, Zabner J, Kline J N, Jones M, Frees K, Watt J L, Schwartz D A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Barkett M, Gilmore T D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 4.Belvin M P, Anderson K V. A conserved signalling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 5.Brightbill H D, Libraty D H, Krutzik S R, Yang R B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 6.Cario E, Rosenberg I M, Brandwein S L, Bedk P L, Reinecker H C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 7.Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 8.De Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from staphylococcus aureus act in synergy to cause shock and multiple organ failture. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faure E, Equils O, Sieling P A, Thomas L, Zhang F X, Kirschning C J, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 10.Flo T H, Halaas O, Lien E, Ryan L, Teti G, Golenbock D T, Sundan A. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 11.Gerondarkis S, Grossmann M, Nakamura Y, Pohl T, Grumont R. Genetic approaches in mice to understand Rel/NF-κB and IκB function: transgenics and knockouts. Oncogene. 1999;18:6888–6895. doi: 10.1038/sj.onc.1203236. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Jin Y P, Dziarski R. Peptidoglycan induces transcription and secretion of TNF-alpha and activation of lyn, extracellular signal-regulated kinase, and rsk signal transduction proteins in mouse macrophages. J Immunol. 1995;155:2620–2630. [PubMed] [Google Scholar]
- 13.Gupta D, Kirkland T N, Viriyakosol S, Dzearski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 14.Gupta D, Wang Q, Vinson C, Dziarski R. Bacterial peptidoglycan induces CD14-dependent activation of transcription factors CREB/ATF and AP-1. J Biol Chem. 1999;274:14012–14020. doi: 10.1074/jbc.274.20.14012. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez-Ramos J C, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Faustman D. NOD mice are defective in proteasome production and activation of NF-κB. Mol Cell Biol. 1999;19:8646–8659. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T, Faustman D. Essential role of human leukocyte antigen-encoded proteasome subunits in NF-kappaB activation and prevention of tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2000;275:5238–5247. doi: 10.1074/jbc.275.7.5238. [DOI] [PubMed] [Google Scholar]
- 18.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 19.Heine H, Kirschning C J, Lien E, Monks B G, Rothe M, Golenbock D T. Cutting edge: cells that carry a null allele for Toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 20.Hirschfeld M, Kirschning C J, Schwandner R, Wesche H, Weis J H, Wooten R W, Weis J J. Cutting edge: inflammatory signaling by borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 21.Hoffmann J A, Kafatos F C, Janeway C A, Jr, Ezekowitz R A B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Tadeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 23.Kirikae T, Nitta T, Kirikae F, Suda Y, Kusumoto S, Qureshi N, Nakano M. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect Immun. 1999;67:1736–1742. doi: 10.1128/iai.67.4.1736-1742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirschning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitajima I, Shinohara T, Bilakovics J, Brown D A, Xu X, Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-κB. Science. 1992;258:1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- 26.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 27.Lien E, Means T K, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton M J, Oikawa M, Qureshi N, Monks B, Finberg R W, Ingalls R R, Golenbock D T. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Investig. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 29.Manna S K, Aggarwal B B. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-κB activation and reactive oxygen intermediates. J Immunol. 1999;162:1510–1518. [PubMed] [Google Scholar]
- 30.Matsura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medvedev A E, Kopydlowski K M, Vogel S N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 32.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 33.Morrison W, Ryan J L. Endotoxin and disease mechanism. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 34.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, Van't Veer C, Penton-Rol G, Ruco L P, Allavena P, Mantovani A. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 35.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human Toll signaling pathway: divergence of nuclear factor-κB and JNF SAPK activation upstream of tumor necrosis factor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poltorak A, He X, Smirnova I, Liu M Y, van Huffelv C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Castagnoli P R, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr 4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–2167. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutation in Toll-like receptor 4 (tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehli M, Poltorak A, Schwarzfischer L, Krause S W, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773–9781. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- 40.Rock F L, Hardiman G, Timans J C, Kastelein R A, Bazan J F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt M, Kloetzel P E. Biogenesis of eukaryotic 20S proteasomes: the complex maturation pathway of a complex enzyme. FASEB J. 1997;11:1235–1243. doi: 10.1096/fasebj.11.14.9409542. [DOI] [PubMed] [Google Scholar]
- 42.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor-2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi O, Kawai T, Sanjo H, Gopeland N G, Gilbert D J, Jenkins N A, Takeda K, Akira S. TLR6: a novel member of an expanding Toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 45.Underhill D M, Ozinsky O, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–814. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 46.Underhill D M, Ozinsky A, Smith K D, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel S N, Johnson D, Perera P Y, Medvedev A, Lariviere L, Qureshi S T, Malo D. Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack a LPSn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- 48.Vogel S N, Moore R N, Sipe J D, Rosenstreich D L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980;124:2004–2009. [PubMed] [Google Scholar]
- 49.Warren H S. Strategies for the treatment of sepsis. N Engl J Med. 1997;336:952–953. doi: 10.1056/NEJM199703273361311. [DOI] [PubMed] [Google Scholar]
- 50.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 51.Yang R B, Mark M R, Gurney A L, Godowski P J. Signaling events induced by lipopolysaccharide-activated Toll-like receptor 2. J Immunol. 1999;163:639–643. [PubMed] [Google Scholar]
- 52.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 53.Zhang F X, Kirschning J, Mancinelli R, Xu X P, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signalling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]