Abstract
Background
Immunocompromised individuals with hematological malignancy have increased risk for poor outcomes and death from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This special population may mount a suboptimal response to vaccination. We assessed the effectiveness of tixagevimab and cilgavimab (Evusheld), a monoclonal antibody combination against SARS-CoV-2, in conjunction with standard preventative measures, at preventing symptomatic incident infection.
Methods
Patients aged 18 years and older with hematological malignancy consented to receive Evusheld. Patients were followed longitudinally for development of symptomatic incident SARS-CoV-2 infections. Adverse events were monitored.
Results
Two hundred and three patients (94 female) with hematological malignancies and mean age 72 ± 10 years were included. Of the patients, 99.5% had received at least one mRNA vaccination against SARS-CoV-2. Average time of follow-up was 151 ± 50 days. Nineteen patients (9.3%) developed incident symptomatic SARS-CoV-2 infection, with only one (0.5%) requiring hospitalization. During the same follow-up period, local incident rate of infection was 84,123 cases (11.3% of population). Of those, 3,386 cases (4%) of SARS-CoV-2 required hospital admission. The incidence rate ratio was 0.79. No serious adverse events occurred following administration of Evusheld.
Conclusion
Patients with hematological malignancy who received Evusheld infrequently developed symptomatic infections or require hospitalization. The high-risk cohort incidence was at least as comparable to the average risk general population. Evusheld appears effective and is well tolerated, and may be administered in conjunction with vaccination and standard prevention measures, at decreasing incident SARS-Co-V2 cases in this high-risk population.
Keywords: Hematological malignancy, SARS-CoV-2, Evusheld
Introduction
The global pandemic with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continues to evolve, yet as of September 2022, an average of 358 individuals continue to die on a daily basis in the United States [1]. Of these, a large percentage are immunocompromised individuals, including those with hematological malignancy, who have been shown to have increased risk of poor outcomes and death from SARS-CoV-2 [2]. Despite vaccination being the primary strategy for risk mitigation against SARS-CoV-2, individuals with hematological malignancy are known to have a suboptimal response to vaccination [3, 4]. In the PROVENT study, the monoclonal antibody combination tixagevimab and cilgavimab (Evusheld) exhibited a 76.7% relative risk reduction of symptomatic infection with SARS-CoV-2 compared to placebo in individuals at high risk of poor outcomes, including 0.5% with immunosuppressive diseases [5]. Evusheld offers passive antibody delivery, in contrast to vaccine administration, which requires an active immune response, in order to achieve protection. Under the United States Food and Drug Administration (FDA) emergency use authorization (EUA), Evusheld may be administered to high-risk patients, including those with hematological malignancy [6]. However, there are minimal data on real-world experience with Evusheld in patients with hematological malignancy. Thus, we longitudinally followed patients with hematological malignancy who received Evusheld to determine real-world effectiveness at preventing symptomatic infection with SARS-CoV-2.
Materials and Methods
Patients with hematological malignancies aged 18 years and older and who consented to receive Evusheld per FDA EUA were followed longitudinally in a single-center study. Administration of the original EUA 150 mg/150 mg dose or the revised 300 mg/300 mg dose occurred in all patients [7, 8]. Symptomatic SARS-CoV-2 infection required a positive home antigen test or laboratory confirmation with a positive polymerase chain reaction test. This was determined via patient report, chart review, and querying patients via telephone. The community incidence of SARS-CoV-2 infection was calculated by dividing the number of weekly cases reported to the Monroe County Department of Health by the population of Monroe country (743,000 people). Local community SARS-CoV-2 incidence and hospitalization rates were publicly available from New York State online [9]. Adverse events were evaluated on the date of Evusheld administration and post administration via chart review and telephone interview.
The institutional review board at Rochester Regional Health approved this study. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Results are reported in mean ± standard deviation unless specified.
Results
Two hundred and three patients were included, with 94 female (46%) and 109 male (54%). Mean age was 72 ± 10 years. One hundred eighty-two (90%) were identified as white/Caucasian, 10 (5%) as African American, four (2%) as Latino/Hispanic, and seven (3%) were not identified with any race. Table 1 lists the primary diagnosis for each patient, with the majority having chronic lymphocytic leukemia (CLL), multiple myeloma (MM), or lymphoma. Table 2 includes the frequency of chemotherapy medications used during the study period, with rituximab, cyclophosphamide, and ibrutinib being the most common.
Table 1. Hematological Malignancy Diagnoses.
| Hematological malignancy | Number of cases (%) | Median age (range) | Number of female | Number of receiving treatment |
|---|---|---|---|---|
| Chronic lymphocytic leukemia | 93 (46) | 73 (36 - 94) | 40 | 55 |
| Multiple myeloma | 36 (18) | 70 (54 - 91) | 17 | 35 |
| Follicular lymphoma | 17 (8) | 66 (56 - 85) | 11 | 14 |
| Waldenstrom macroglobulinemia | 16 (8) | 73 (43 - 93) | 7 | 16 |
| Diffuse large B-cell lymphoma | 15 (7) | 76 (53 - 88) | 7 | 14 |
| Marginal zone lymphoma | 6 (3) | 69 (59 - 70) | 4 | 6 |
| Mantle cell lymphoma | 5 (2) | 80 (62 - 86) | 0 | 5 |
| Acute myeloid leukemia | 4 (2) | 72 (67 - 82) | 0 | 4 |
| Large granular lymphocytic leukemia | 3 (1) | 71 (62 - 92) | 2 | 3 |
| Acute lymphocytic leukemia | 2 (1) | 61 (53 - 68) | 1 | 2 |
| Monoclonal gammopathy of unknown significance | 2 (1) | 78 (78) | 1 | 1 |
| B-cell prolymphocytic leukemia | 1 (0.5) | 70 (70) | 0 | 1 |
| Chronic myeloid leukemia | 1 (0.5) | 63 (63) | 1 | 1 |
| Hairy cell leukemia | 1 (0.5) | 62 (62) | 0 | 1 |
| Peripheral T-cell lymphoma | 1 (0.5) | 88 (88) | 0 | 1 |
Table 2. Chemotherapy Agents.
| Chemotherapy medication | Frequency of use (% of total patients) |
|---|---|
| Rituximab | 89 (44) |
| Cyclophosphamide | 42 (21) |
| Ibrutinib | 40 (20) |
| Bendamustine | 39 (19) |
| Prednisone | 39 (19) |
| Lenalidomide | 32 (16) |
| Dexamethasone | 29 (14) |
| Vincristine | 25 (12) |
| Bortezomib | 24 (12) |
| Daratumumab | 22 (11) |
| Fludarabine | 20 (10) |
| Adriamycin | 17 (8) |
| Venetoclax | 15 (7) |
| Chlorambucil | 10 (5) |
| Acalabrutinib | 7 (3) |
| Methotrexate | 5 (2) |
| Pomalidomide | 5 (2) |
| Cytarabine | 4 (2) |
| Decitabine | 4 (2) |
| Doxorubicin | 4 (2) |
| Melphalan | 4 (2) |
| Daunorubicin | 3 (1) |
| Obinutuzumab | 3 (1) |
| 6-mercaptopurine | 2 (1) |
| Asparaginase | 2 (1) |
| Cladribine | 2 (1) |
| Elotuzumab | 2 (1) |
| Oxaliplatin | 2 (1) |
| Bosutinib | 1 (0.5) |
| Carfilzomib | 1 (0.5) |
| Cisplatin | 1 (0.5) |
| Dasatinib | 1 (0.5) |
| Docetaxel | 1 (0.5) |
| Epirubicin | 1 (0.5) |
| Fluorouracil | 1 (0.5) |
| Imexon | 1 (0.5) |
| Interferon alpha | 1 (0.5) |
| Ivosidenib | 1 (0.5) |
| Nelarabine | 1 (0.5) |
| Ofatumumab | 1 (0.5) |
| Pembrolizumab | 1 (0.5) |
| Pertuzumab | 1 (0.5) |
| Thalidomide | 1 (0.5) |
Forty-one (20%) patients reported a history of SARS-CoV-2 prior to receiving Evusheld. The average time between contracting SARS-CoV-2 and receiving the first dose of Evusheld was 171 ± 166 days (range 26 - 779 days). All patients had recovered from previous infection prior to receiving Evusheld. At the time of receiving Evusheld, 70 (34%) patients received four previous doses of mRNA SARS-CoV-2 vaccinations, 101 (50%) patients received three doses of mRNA vaccinations, 23 (11%) patients received two doses of mRNA vaccinations, five (2%) patients received one dose of mRNA vaccination, and one (0.5%) patient did not receive any mRNA vaccination. One hundred ninety-seven patients (97%) received the 300 mg/300 mg dose, with six (3%) patients receiving the 150 mg/150 mg dose. These six patients received the lower dose before the revised FDA dose recommendation and declined repeat dosing.
Following administration of Evusheld, patients were followed from January 15, 2022 through August 24, 2022. The average time of follow-up since the first Evusheld dose was 151 ± 50 days (median 158 days, range 29 - 221 days). During follow-up, 19 patients (9.3%) developed incident symptomatic SARS-CoV-2 infections. Of those who developed incident SARS-Co-V-2 infections, the mean age was 72 ± 6 years, six (32%) were female, 13 (68%) were receiving active treatment, and four (21%) had prior SARS-Co-V-2 infections. Thus, 10% of those with prior SARS-Co-V-2 infection before Evusheld administration developed a second acute infection. When assessing the two most common malignancy diagnoses (CLL and MM), 10 patients with CLL (11% of total CLL) and two patients with MM (6% of total MM) developed SARS-CoV-2 infections. Overall, these accounted for 63% of all incident cases and 64% of total patients. Only one patient (0.5%), who had a diagnosis of CLL on active chemotherapy, required hospitalization due to hypoxia with a 3-day length of stay, without intubation or intensive care. Ten cases received anti-SARS-CoV-2 medication: seven received nirmatrelvir/ritonavir, one received bebtelovimab, one received sotrovimab, and one received remdesivir.
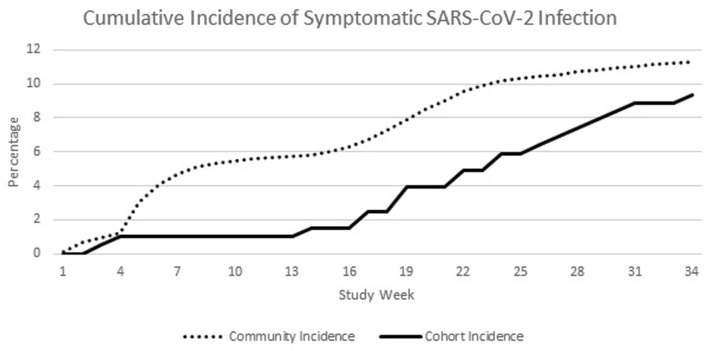
During the same follow-up period, 84,123 cases of symptomatic SARS-CoV-2 infection were reported in Monroe County, New York, USA, for a local cumulative incident rate of 11.3% (Fig. 1). During that period, a total of 3,386 new hospital admissions for SARS-CoV-2 occurred for a cumulative hospitalization rate of 0.46% of the general population or 4% of reported cases.
Figure 1.
Longitudinal incidence of new SARS-CoV-2 infections in the cohort of patients with hematological malignancy who received Evusheld versus the local community. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Incidence rates for the hematological malignancy patients was 0.15 cases per patient-year compared to 0.19 cases per patient-year for the local population. Incidence rate ratio was 0.79.
Following administration of Evusheld, reported adverse events included one case of epistaxis, one case of transient fever that resolved within 3 h, six cases of injection site pain, two reports of fatigue with myalgia, one report of flu-like symptoms that lasted about 7 days. No patients experienced serious adverse events requiring medical attention. No type 1 hypersensitivity reactions occurred, and no cardiac events were observed during the study period.
Discussion
We present our real-world experience showing individuals with hematological malignancy who received Evusheld infrequently experienced incident symptomatic infection with SARS-CoV-2 or required hospitalization during the Omicron wave and subsequent variants. Compared to the general population, this high-risk immunocompromised cohort was at least comparable. Importantly, it is highly likely that the local incidence rate of symptomatic SARS-CoV-2 infection is underreported, as many cases were diagnosed via home antigen testing and likely not reported to the department of health. Conversely, patients with hematological malignancy were all counseled to immediately notify their healthcare team if they tested positive for symptomatic SARS-CoV-2 infection. Thus, the hematological malignancy cohort receiving Evusheld may have had less overall incidence of infection.
Although Evusheld was proven to be effective in a high-risk population [4], special populations at particularly high risk for suboptimal response to vaccination and poor outcomes from SARS-CoV-2 were underrepresented in the pivotal PROVENT study. Recently published reports have demonstrated safety and likely efficacy in patients with solid organ transplant and inflammatory conditions treated with B-cell depletion, as well as inborn errors of humoral immunity [10-12]. There was also a recent study of 52 patients with hematological malignancy completed prior to the Omicron wave, who were followed for 79 days [13]. Additionally, Al-Obaidi et al described effectiveness of Evusheld in a high-risk cohort including hematological malignancy patients [14]. Our study is unique in that it enrolled a large number of patients focusing solely on those with hematological malignancy and followed them for nearly 6 months during the Omicron wave and subsequent Omicron variants through summer 2022. Along with decreased risk of infection, it is reassuring that those who experienced symptomatic infection were largely managed as outpatients, with only one patient requiring inpatient hospitalization. In addition to added risk mitigation along with vaccination and non-pharmaceutical considerations such as hand hygiene, social distancing, etc., Evusheld was well tolerated by our patients, with rare side effects and no serious concerns. Therefore, given the favorable safety profile along with potential benefit, in conjunction with the Center for Disease Control and Prevention (CDC) and Advisory Committee on Immunization Practices vaccination guidelines, we advocate that all patients with hematological malignancy should receive Evusheld in addition to a complete vaccination series against SARS-CoV-2 [15].
Given its duration of action of 6 months, a single administration of Evusheld may be expected to provide protection from a number of viral variants. To date, given our data and no evidence of increasing symptomatic SARS-CoV-2 infections, Evusheld appears to maintain adequate clinical activity against variants during the study period, ranging from the Omicron wave through the predominant strain of BA.5. With that being said, antiviral activity for Evusheld may have been greatest against the early Omicron lineages, as Figure 1 demonstrates the greatest separation during this time period. Given that Evusheld’s duration is approximately 6 months, repeat dosing is reasonable at the 6-month mark in high-risk populations.
Our study has notable limitations. Most importantly, we did not have a control group, and compared our cohort to the background rate of reported infection in the region. Our group felt withholding this potentially life-saving medication from this high-risk group would not have been ethical. The local background rate is based on the general population infection patterns, and confounding variables exist when comparing the general population and patients with hematological malignancies. However, given that we did not have another hematological malignancy group who did not receive Evusheld for a matched-pair analysis, we decided to use the local background rate as a “surrogate” comparison with assumed “average risk” overall given the large local population. Future prospective studies may consider a matched-pair study design. Additionally, our sample size was small, with a heterogeneous group of patients with varied hematological malignancy. Given that Evusheld should be used in combination with vaccination and other strategies for risk mitigation, we cannot comment on effectiveness of Evusheld alone in preventing symptomatic infection with SARS-CoV-2. Two malignancies, CLL and MM, accounted for over 60% of both incident cases and total patients. Thus, this does make generalization to all malignancies limited. Further sub-analysis by type of malignancy is limited by the small number of patients. Studies focusing on specific malignancy types could be conducted in the future. We did not assess neutropenia or lymphopenia status. Future studies could assess whether these are independent risk factors for developing acute SARS-CoV-2 infection following vaccination and Evusheld administration.
We surveyed for acute infection based on serological testing. While acute cases could be missed due to a patient not testing, our cohort generally received close primary care and hematological follow-up, and patients were advised to test for acute disease upon onset of symptoms. We were unable to assess titers of neutralizing antibodies in our cohort or whether patients who had prior SARS-CoV-2 infections had additional humoral or adaptive immunity. We were unable to assess pharmacokinetics of Evusheld in our cohort given that it was an observational longitudinal study. Additional studies are necessary to determine whether pharmacokinetics of Evusheld are alternated in high-risk populations. We do not suggest that Evusheld be used as acute treatment for COVID-19 or in place of primary vaccination but rather as a supplement in risk migration strategies during the pandemic.
Despite these limitations, our study is one of the first to report real-world effectiveness of Evusheld in patients with hematological malignancies. We believe our data suggest that Evusheld should be used in combination with vaccination as part of a risk mitigation strategy in this high-risk population, thus increasing national awareness and administration of Evusheld in patients with hematological malignancy.
Acknowledgments
We would like to thank Holly Blue, LPN, and Dawn Sheflin, RN, of our clinical research staff for assistance with IRB review, data collection, and patient telephone contact.
Financial Disclosure
None to declare.
Conflict of Interest
AJO, KEO, JB, SKL, NN, HS have no financial conflict of interest. SSM reports speaker’s bureau: Genentech, Regeneron/Sanofi, GSK, AstraZeneca, CSL Behring, Aimmune. SJ reports speaker’s bureau for Takeda and received honoraria from Bristol Meyers Squibb.
Informed Consent
Patients gave written consent to receive Evusheld.
Author Contributions
AJO and SSM participated in design, literature review, data collection, writing, and reviewing the manuscript. KEO and SJ participated in literature review, writing, and reviewing the manuscript. JB, SKL, NN, and HS participated in data collection, writing, and reviewing the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- FDA
Food and Drug Administration
- EUA
emergency use authorization
- CLL
chronic lymphocytic leukemia
- MM
multiple myeloma
References
- 1. Center for Disease Control and Prevention. COVID data tracker weekly review online. 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html.
- 2.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martin-Moro F. et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog Tzarfati K, Gutwein O, Apel A, Rahimi-Levene N, Sadovnik M, Harel L, Benveniste-Levkovitz P. et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, Yuan Y. et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of COVID-19. N Engl J Med. 2022;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tixagevimab and Cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327(4):384–385. doi: 10.1001/jama.2021.24931. [DOI] [PubMed] [Google Scholar]
- 7. Astra Zenica Pharmaceuticals. Fact sheet for healthcare providers: emergency use authorization for Evusheld. Online: United States Food and Drug Administration. 2022.
- 8. United States Food and Drug Administration. FDA authorizes revisions to Evusheld dosing online. 2022 [cited 2022]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing.
- 9. https://coronavirus.health.ny.gov .
- 10.Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant. 2022 doi: 10.1101/2022.05.17.22274980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese C, Kirchner E, Villa-Forte A, Hajj-Ali RA, Moss BP, Fernandez JP, Calabrese L. Early experience with tixagevimab/cilgavimab pre-exposure prophylaxis in patients with immune-mediated inflammatory disease undergoing B cell depleting therapy and those with inborn errors of humoral immunity. RMD Open. 2022;8(2):e002557. doi: 10.1136/rmdopen-2022-002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocon AJ, Mustafa SS. Real-World Experience of Tixagevimab and Cilgavimab (Evusheld) in Rheumatologic Patients on Rituximab. J Clin Rheumatol. 2022 doi: 10.1097/RHU.0000000000001907. [DOI] [PubMed] [Google Scholar]
- 13.Stuver R, Shah GL, Korde NS, Roeker LE, Mato AR, Batlevi CL, Chung DJ. et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell. 2022;40(6):590–591. doi: 10.1016/j.ccell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Obaidi MM, Gungor AB, Kurtin SE, Mathias AE, Tanriover B, Zangeneh TT. The prevention of COVID-19 in high-risk patients using Tixagevimab-Cilgavimab (Evusheld): real-world experience at a large academic center. Am J Med. 2022 doi: 10.1016/j.amjmed.2022.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Center for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2022. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.