Abstract
Background
Acute pancreatitis (AP) carries a significant morbidity and mortality worldwide. AP is a potential complication of hematopoietic stem cell transplantation (HSCT) although its incidence remains unclear. HSCT recipients are at increased risk of AP due to various factors but the effect of AP on mortality and resource utilization in the adult population has not been studied. We investigated the impact of AP on hospitalization outcomes among patients following HSCT.
Methods
We queried the National Inpatient Sample (NIS) database using the International Classification of Diseases (ICD)-10 codes. All adult patients with a diagnosis or procedure code of HSCT were included in the study. Patients were divided into those with a diagnosis of AP and those without. Sensitivity analysis was performed for patients with a length of stay greater than 28 days. The relationship between AP and mortality, length of stay, total hospitalization cost, and charges was assessed using univariate analysis followed by multivariate analysis.
Results
Of the 140,130 adult patients with HSCT, 855 (0.61%) patients developed AP. There was 1.74 times higher risk of mortality in patients with AP as compared to controls (adjusted odds ratio (aOR): 1.74, P = 0.0055). There was no statistically significant difference in the length of stay, hospitalization charge, or cost before sensitivity analysis. After sensitivity analysis, 13,240 patients were included, from which 125 (0.94%) had AP. There was 3.85 times higher risk of mortality in patients who developed AP as compared to controls (aOR: 3.85, P = 0.003). There was a statistically significant increase noted in the length of stay (adj coeff: 20.3 days, P = 0.002), hospital charges (+$346,616, P = 0.017), and cost (+$121,932.4, P = 0.001) in patients with AP as compared to those who did not develop AP.
Conclusion
Recipients of HSCT who develop AP have shown to have higher mortality on sensitivity analysis. This study highlights that AP in HSCT patients is associated with worse outcomes and higher resource utilization. Physicians should be aware of this association as the presence of pancreatitis portends a poor prognosis.
Keywords: Acute pancreatitis, Hematopoietic stem cell transplantation, Hospitalization outcomes
Introduction
Hematopoietic stem cell transplantation (HSCT) has been widely adopted as a treatment modality for hematological malignancies and non-malignant disorders. Acute pancreatitis (AP) is a less-known complication of HSCT, with the earliest cases dating back to the 1990s [1]. The presentation of AP can range from a self-limited course to multi-organ dysfunction.
There are limited data on the incidence and prevalence of AP in HSCT recipients. A recent single-center retrospective analysis on allogeneic HSCTs from 2009 to 2018 found the incidence of AP in recipients to be 0.76% [2]. Shore et al studied 68 patients with HSCT and found the incidence to be 4.4% [3]. Another study demonstrated an incidence of 3.5% in the pediatric HSCT population [4]. The prevalence rates of pancreatitis in the autopsies of HSCT recipients were reported to be as high as 27%, with AP-related death reported to be 10%, representing the widespread pancreatic involvement in this population [5].
There has been a substantial increase in the total number of adult HSCT recipients over the past few years [6, 7]. Despite the rapid availability and application of HSCT for various hemato-oncological indications, there is a scarcity of large-scale retrospective studies evaluating the incidence and outcomes of AP after HSCT. Our study analyzes the prevalence, clinical outcomes, and healthcare utilization in this patient population.
Materials and Methods
The data used in this study were obtained from National Inpatient Sample (NIS) which includes de-identified patient information. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as the Helsinki Declaration. IRB approval was not required for this study.
Data were collected from the Healthcare Cost and Utilization Project (HCUP) and Agency for Healthcare Research and Quality database, NIS [8]. This publicly available database contains unweighted discharge information of 7 million hospitalizations annually. However, by applying weights provided by HCUP, we can extrapolate the data to provide information on all hospitalizations in the United States. Data were collected from January 2016 to December 2019. Each record represents the hospital discharge encounter with up to 40 diagnostic and 25 procedure codes, categorized according to the International Classification of Diseases (ICD)-10 classification.
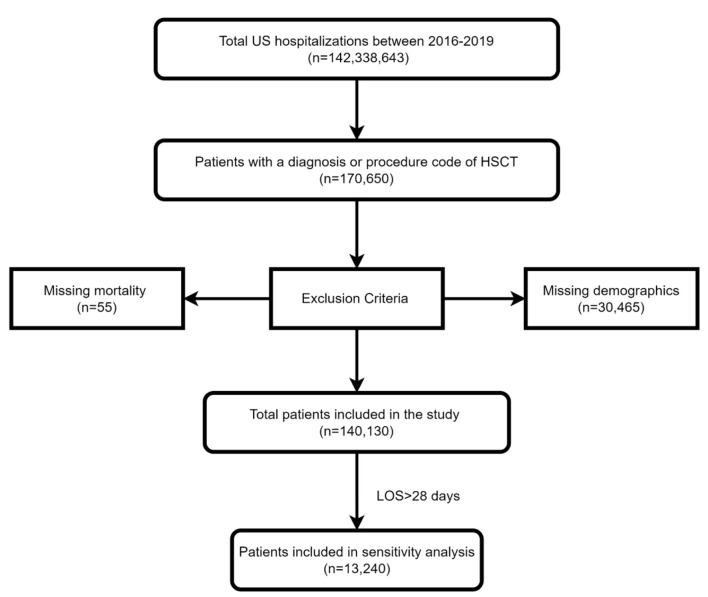
Using ICD-10 codes, we included all patients with a prior diagnosis or a procedure code for HSCT. The patients were divided into two groups, those diagnosed with AP and those without AP. Patients with missing mortality or patient demographics were excluded from the analysis. This can be seen in Figure 1. We classified the age into 18 - 44 years, 45 - 64 years, and > 65 years. We stratified the hospitals based on their teaching status into teaching and non-teaching hospitals. Hospitals were also stratified based on their location into rural and urban hospitals.
Figure 1.
Inclusion and exclusion flow diagram.
We also collected information on comorbidities using the Charlson comorbidity index [9]. In addition, we studied other comorbidities commonly associated with HSCT and the development of AP. Diseases requiring HSCT included myelodysplastic syndrome, lymphoid/myeloid leukemia, Hodgkin and non-Hodgkin lymphoma, other solid tumors, aplastic anemia, other bone marrow failure syndromes, hemophagocytic lymphohistiocytosis (HLH), thalassemia, inborn errors of metabolism, and primary immunodeficiency disorders. Comorbidities related to AP included hemolytic uremic syndrome, cholangitis, choledocholithiasis, hypertriglyceridemia, hypercalcemia, systemic lupus erythematosus, diabetic ketoacidosis, cystic fibrosis, alcohol ingestion, abdominal trauma, and graft-versus-host disease (GVHD). A similar approach was used by Thavamani et al to analyze the effect of AP in patients with HSCT in the pediatric population [4].
Outcome measures
Our primary outcome was in-hospital mortality. Secondary outcomes included sepsis, shock, ICU admission, respiratory failure, length of hospital stay, and total hospitalization charges, which were used as surrogates to measure healthcare resource use.
Sensitivity analysis was performed by including only those patients who had a hospitalization stay for at least 28 days. The arbitrary cutoff was set to eliminate the influence of short-duration hospitalizations, such as elective admissions for chemotherapy, procedure-related short stays, or evaluation of HSCT-related complications, such as febrile neutropenia or mucositis. As per the HCUP data user agreement, any category with fewer than 11 patients was not reported. All ICD-10 diagnosis and procedure codes are described in the Supplementary Material 1 (www.gastrores.org).
Statistical analyses
All the categorical variables are reported as frequencies and percentages. Pearson χ2 test was used to compare categorical variables. A multivariable logistic regression model was constructed using all the variables with a P-value of < 0.2 in univariate analysis, and results are reported as adjusted odds ratio (aOR) and 95% confidence interval (CI). Separate multivariable linear regression models were constructed for length of stay and total hospitalization charges as outcome variables. A P-value of < 0.05 was considered significant. All analyses were performed using weighted data in STATA 17.0.
Results
Analysis in the total population (n = 140,130 patients)
Patient characteristics
A total of 140,130 patients with a diagnosis of HSCT were included in the analysis. Of these, 855 patients (0.61%) developed pancreatitis. Patients who developed pancreatitis were younger, with 42.11% of patients in the 18 - 44 age group compared to 21.57% in patients without AP. There were no gender or racial differences between the two groups. Most patients (95.91%) who developed pancreatitis were at urban hospitals. Out of 855 patients diagnosed with AP, 125 (14.62%) patients had gallstone-related AP, 50 (5.85%) had alcohol-related AP and the remaining were idiopathic or unknown etiology. Furthermore, 25 (2.92%) patients were diagnosed with necrotizing pancreatitis, while 830 (97.08%) patients had interstitial pancreatitis. A complete list of patient demographics and hospital characteristics is presented in Table 1.
Table 1. Patient Characteristics and Hospital Demographics of Patients With Hematopoietic Stem Cell Transplantation, Stratified by the Presence of Acute Pancreatitis.
| Without pancreatitis | With pancreatitis | P-value | |
|---|---|---|---|
| Age category | < 0.001 | ||
| 18 - 45 | 30,045 (21.57) | 360 (42.11) | |
| 45 - 65 | 63,845 (45.84) | 330 (38.6) | |
| > 65 | 45,385 (32.59) | 165 (19.3) | |
| Sex | 0.2574 | ||
| Male | 79,465 (57.06) | 450 (52.6) | |
| Female | 59,810 (42.94) | 405 (47.4) | |
| Race | 0.3758 | ||
| White | 97,105 (69.72) | 540 (63.16) | |
| African American | 17,020 (12.22) | 140 (16.37) | |
| Hispanic | 14,695 (10.55) | 110 (12.87) | |
| Asian/Pacific islander | 4,405 (3.16) | 35 (4.09) | |
| Native American | 475 (0.34) | 0 (0) | |
| Other | 5,575 (4.00) | 30 (3.51) | |
| Insurance | 0.0491 | ||
| Medicare | 53,505 (38.42) | 260 (30.41) | |
| Medicaid | 15,735 (11.3) | 180 (21.05) | |
| Private | 64,080 (46.01) | 365 (42.69) | |
| Underinsured | 1,485 (1.07) | 25 (2.92) | |
| Income | 0.2693 | ||
| Lowest quartile | 28,760 (20.65) | 190 (22.22) | |
| Second quartile | 32,890 (23.62) | 235 (27.49) | |
| Third quartile | 37,755 (27.11) | 175 (20.47) | |
| Highest quartile | 39,870 (28.63) | 255 (29.82) | |
| Region | 0.4801 | ||
| Northeast | 29,355 (21.08) | 220 (25.73) | |
| Midwest | 34,535 (24.8) | 185 (21.64) | |
| South | 46,580 (33.44) | 285 (33.33) | |
| West | 28,805 (20.68) | 165 (19.3) | |
| Hospital location | 0.0575 | ||
| Rural | 2,960 (2.13) | 35 (4.09) | |
| Urban | 136,315 (97.85) | 820 (95.91) | |
| Hospital teaching status | < 0.001 | ||
| Non-teaching hospitals | 14,070 (10.1) | 170 (19.88) | |
| Teaching hospitals | 125,205 (89.9) | 685 (80.12) | |
| Hospital bed size | 0.007 | ||
| Small | 18,035 (12.95) | 120 (14.04) | |
| Medium | 24,680 (17.72) | 230 (26.9) | |
| Large | 96,560 (69.33) | 505 (59.06) | |
| Charlson comorbidities | 0.0001 | ||
| 0 | 12,480 (8.96) | 135 (15.79) | |
| 1 | 7,470 (5.36) | 85 (9.94) | |
| 2 | 56,840 (40.81) | 255 (29.82) | |
| > 3 | 62485 (44.86) | 380 (44.44) |
Complications related to HSCT and pancreatitis
The most common indication for HSCT in patients with AP was GVHD (21.64%), followed by myeloid leukemia (18.13%) and lymphoid leukemia (14.04%). No patients with a diagnosis of HLH, hemolytic uremic syndrome, thalassemia and bone marrow failure developed AP. Furthermore, there was a statistically significant higher proportion of patients with cholangitis, choledocholithiasis, other biliary conditions and hypertriglyceridemia in patients with AP. There was a greater proportion of alcohol use in patients who developed AP. Patients who developed AP had higher endoscopic retrograde cholangiopancreatography (ERCP) rates than those without. There was no statistically significant difference in the rates of hypercalcemia between the two groups. A complete list of complications related to HSCT and pancreatitis is presented in Table 2.
Table 2. Complications Related to HSCT and Acute Pancreatitis in HSCT Patients, Stratified by the Presence of AP.
| Without pancreatitis | With pancreatitis | P-value | |
|---|---|---|---|
| Etiologies/complications related to HSCT | |||
| Myelodysplastic syndrome | 9,375 (6.73) | 50 (5.85) | 0.641 |
| Lymphoid leukemia | 11,595 (8.33) | 120 (14.04) | 0.009 |
| Myeloid leukemia | 26,095 (18.74) | 155 (18.13) | 0.842 |
| Hodgkin’s lymphoma | 4,430 (3.18) | 20 (2.34) | 0.532 |
| Non-Hodgkin’s lymphoma | 3,505 (2.52) | 15 (1.75) | 0.526 |
| Bone marrow failure | 30 (0.02) | 0 (0) | 0.846 |
| HLH | 290 (0.21) | 0 (0) | 0.569 |
| Thalassemia | 290 (0.21) | 0 (0) | 0.57 |
| Sickle cell | 1,590 (1.14) | 25 (2.92) | 0.061 |
| Metabolism error | 210 (0.15) | 0 (0) | 0.639 |
| Primary immunodeficiency | 5,710 (4.1) | 35 (4.09) | 0.997 |
| Graft-versus-host disease | 19,390 (13.92) | 185 (21.64) | 0.005 |
| Hemolytic uremic syndrome | 130 (0.09) | 0 (0) | 0.726 |
| Conditions related to acute pancreatitis | |||
| Cholangitis | 660 (0.47) | 55 (6.43) | < 0.001 |
| Choledocholithiasis | 1,680 (1.21) | 135 (15.79) | < 0.001 |
| Other biliary disorders | 1,680 (1.21) | 55 (6.43) | < 0.001 |
| Hypertriglyceridemia | 1,025 (0.74) | 90 (10.53) | < 0.001 |
| Hypercalcemia | 2,025 (1.45) | 25 (2.92) | 0.112 |
| Systemic lupus erythematosus | 455 (0.33) | a | 0.057 |
| Diabetic ketoacidosis | 255 (0.18) | 25 (2.92) | < 0.001 |
| Cystic fibrosis | 50 (0.04) | 0 (0) | 0.843 |
| Alcohol use | 1,805 (1.3) | 50 (5.85) | < 0.001 |
| Abdominal trauma | 225 (0.16) | 0 (0) | 0.607 |
| Anomalies of the pancreas | 0 (0) | 20 (2.34) | < 0.001 |
| Percutaneous biliary procedures | 25 (0.02) | 0 (0) | 0.881 |
| ERCP | 285 (0.2) | 60 (7.02) | < 0.001 |
aValues less than 11, not to be reported as per HCUP data user agreement (DUA). AP: acute pancreatitis; HSCT: hematopoietic stem cell transplantation; HLH: hemophagocytic lymphohistiocytosis; ERCP: endoscopic retrograde cholangiopancreatography.
Results after sensitivity analysis (n = 13,240 patients)
A total of 13,240 patients with a diagnosis of HSCT were included in the analysis. Of these, 125 patients (0.94%) developed pancreatitis. Seventy patients were in the 18 - 44 years age group and 40 patients (32%) were male while the remaining were female. More than 50% of the patients who developed AP were white. The majority of admissions (> 90%) were at urban teaching hospitals. A complete list of patient demographics and hospital characteristics is presented in Table 3. After sensitivity analysis, the most common indication for HSCT in patients with AP was myeloid leukemia (32%), followed by GVHD (28%).
Table 3. Patient Characteristics and Hospital Demographics of Patients With Hematopoietic Stem Cell Transplantation, Stratified by the Presence of Acute Pancreatitis.
| Without pancreatitis | With pancreatitis | P-value | |
|---|---|---|---|
| Age category | 0.0089 | ||
| 18 - 45 | 3,795 (28.94) | 70 (56.00) | |
| 45 - 65 | 6,050 (46.13) | 35 (28.00) | |
| > 65 | 3,270 (24.93) | 20 (16.00) | |
| Sex | 0.139 | ||
| Male | 7,330 (55.89) | 40 (32) | |
| Female | 5785 (44.11) | 85 (68) | |
| Race | 0.5248 | ||
| White | 8,620 (65.73) | 65 (52.00) | |
| African American | 1,375 (10.48) | 25 (20.00) | |
| Hispanic | 1,660 (12.66) | 25 (20.00) | |
| Asian/Pacific islander | 645 (4.77) | a | |
| Native American | 40 (0.31) | 0 (0) | |
| Other | 795 (6.06) | a | |
| Insurance | 0.4607 | ||
| Medicare | 3,450 (26.31) | 30 (24.00) | |
| Medicaid | 1,810 (13.80) | 40 (32.00) | |
| Private | 7,215 (55.01) | 50 (40.00) | |
| Underinsured | 95 (0.72) | 0 (0) | |
| Income | 0.603 | ||
| Lowest quartile | 2,675 (20.40) | 30 (24.00) | |
| Second quartile | 2,875 (21.92) | 30 (24.00) | |
| Third quartile | 3,590 (27.37) | 20 (16.00) | |
| Highest quartile | 3,975 (30.31) | 45 (36.00) | |
| Region | 0.0765 | ||
| Northeast | 3,125 (23.83) | 55 (44.00) | |
| Midwest | 2,800 (21.35) | 20 (16.00) | |
| South | 3,930 (29.97) | 20 (16.00) | |
| West | 3,260 (24.86) | 30 (24.00) | |
| Hospital location | 0.6051 | ||
| Rural | a | 0 (0) | |
| Urban | 13,105 (99.92) | 125 (100.00) | |
| Hospital teaching status | 0.0003 | ||
| Non-teaching hospitals | 255 (1.94) | a | |
| Teaching hospitals | 12,860 (98.06) | 120 (96.00) | |
| Hospital bed size | 0.1696 | ||
| Small | 1,380 (10.52) | a | |
| Medium | 2,105 (16.05) | 30 (24.00) | |
| Large | 9,630 (73.43) | 90 (72.00) | |
| Charlson comorbidities | |||
| 0 | 1,540 (11.74) | a | |
| 1 | 580 (4.43) | a | |
| 2 | 5,540 (42.24) | 50 (40) | |
| > 3 | 5,455 (41.59) | 60 (48) |
aValues less than 11, not to be reported as per HCUP DUA. A complete table of complications related to HSCT and pancreatitis is not presented due to number of patients for the majority of the categories being less than 11.
Outcomes
Death
Mortality in the total population of patients with HSCT was noted to be 3.65%. The incidence of mortality in patients with AP was higher than in those without AP (7.01% vs. 3.63%, P = 0.0179). There was no statistically significant difference in the mortality between the two groups (aOR: 1.55, 95% CI: 0.99 - 3.54, P = 0.055) in the total population. Other factors associated with mortality were age, Charlson comorbidity index, HLH, GVHD and hypercalcemia. After sensitivity analysis, there was a statistically significant difference in mortality between the two groups (aOR: 3.85, 95% CI: 1.56 - 9.46, P = 0.003).
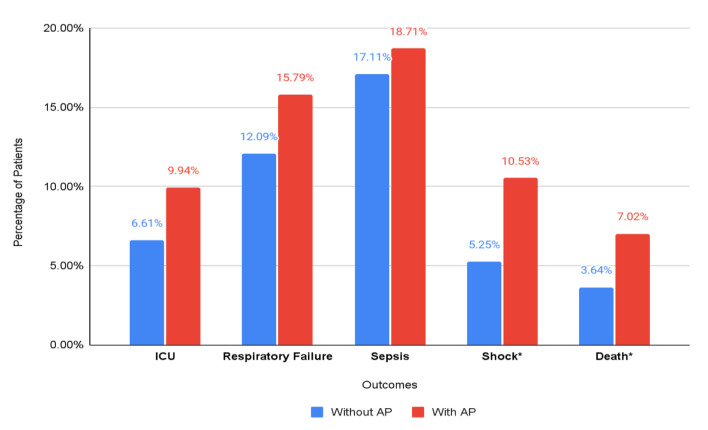
Shock
Total incidence of shock was noted to be 5.28%. The incidence of shock in patients with AP was higher than in those without AP (10.53% vs. 5.25%, P = 0.0019). On multivariate analysis, patients with AP had a statistically significant higher risk of shock (aOR: 1.74, 95% CI: 1.03 - 2.95, P = 0.038). This relationship was also significant after sensitivity analysis (aOR: 2.55, 95% CI: 1.05 - 6.19, P = 0.038).
Sepsis
Total incidence of sepsis was noted to be 17.12%. The incidence of shock in patients with AP was similar to patients without AP (17.11% vs. 18.71%, P = 0.59). On multivariate analysis, AP was not associated with sepsis (aOR: 0.93, 95% CI: 0.62 - 1.39, P = 0.723). The relationship continued to be statistically non-significant on sensitivity analysis (aOR: 1.51, 95% CI: 0.66 - 3.44, P = 0.329).
Respiratory failure
Total incidence of respiratory failure was 12.11%. There was no significant difference in the incidence of respiratory failure between the two groups (12.1% vs. 15.8%, P = 0.15). On multivariate analysis, AP was not an independent predictor of respiratory failure (aOR: 1.4, 95% CI: 0.88 - 2.33, P = 0.144). The relationship continued to be statistically non-significant on sensitivity analysis (aOR: 1.89, 95% CI: 0.78 - 4.58, P = 0.161).
ICU admission
Total ICU admissions in the study population were 6.63%. There was no difference in the incidence of ICU admissions between the two groups (9.94% vs. 6.61%, P = 0.08). On multivariate analysis, AP was not an independent predictor of ICU admissions (aOR: 1.36, 95% CI: 0.79 - 2.34, P = 0.265). There was no statistically significant relationship between AP and ICU admission on sensitivity analysis (aOR: 2.01, 95% CI: 0.80 - 5.05, P = 0.136). The results of categorical outcomes are depicted in Figure 2.
Figure 2.
Categorical outcomes in total hospitalized patients with HSCT, stratified by the presence of acute pancreatitis. *Statistically significant outcomes.
Length of stay
The mean length of stay in patients with AP was 15.24 days as compared to 14.17 in patients without AP. On multivariate analysis, there was no statistically significant difference between the two groups (+2.35 days, P = 0.211). On sensitivity analysis, the mean length of stay in patients with AP was 64.9 days compared to 43.6 days in patients without AP. After adjusting for confounding factors, AP was associated with an increased length of stay (+20.3 days, P = 0.002).
Total hospitalization cost
Mean hospitalization cost in patients with AP was $65,233.49 compared to $56,245.6 in patients without AP. On multivariate analysis, AP was not associated with hospitalization cost ($10,861.6, P = 0.287). On sensitivity analysis, the mean hospitalization cost was $333,921.1 in patients with AP compared to $206,015.9 in patients without AP. After adjusting for confounding factors, AP was associated with statistically significant increased hospitalization cost ($121,932.4, P = 0.001).
Total hospitalization charges
Mean hospitalization charge in patients with AP was $231,326.8 compared to $221,415 in patients without AP. On multivariate analysis, AP was not associated with hospitalization charges ($21,817.31, P = 0.56). On sensitivity analysis, the mean hospitalization charge was $1,163,798 in patients with AP compared to $801,135 in patients without AP. After adjusting for confounding factors, AP was associated with statistically significant increased hospitalization charges ($346,618, P = 0.017).
Discussion
The success of HSCT has resulted from continued advances in the field of oncology. HSCT has become a common treatment for hematological malignancies, solid tumors, and other benign diseases such as hematopoietic and inherited metabolic disorders. However, complications such as AP can develop after HSCT. A collection of factors play a role in the development of AP in HSCT recipients. The application of myeloablative or reduced-intensity chemotherapy regimens and total body irradiation prior to HSCT puts patients at risk for the development of acinar cell injury, leading to AP [5]. Iron overload from cytotoxic chemotherapy and pre-HSCT blood transfusions can further damage the pancreas [10]. Immunosuppressive medications such as thalidomide, calcineurin inhibitors, and steroids are frequently used in HSCT recipients and are common causes of AP [11]. Furthermore, the immunocompromised state can potentially lead to staggering infections such as cytomegalovirus (CMV) and adenovirus, which are well known to cause AP [12, 13]. Presence of GVHD and greater length of survival after HSCT are found to be independent risk factors for the development of AP [1]. Additionally, the use of radiation results in increased biliary sludge development along with biliary tree abnormalities leading to AP [14, 15].
Previous studies have reported an AP incidence rate of 0.76% to 4.4% in the HSCT population with a study on the autopsies of HSCT patients revealing this number to be high as 27% [2, 3, 5]. Our study revealed the incidence of AP in patients with HSCT to be 0.61%. After sensitivity analysis, the incidence rate was noted to be 0.96%. This is in contrast to the incidence of AP in general population, which has been estimated to be 4.9 - 35 per 100,000 population [16]. The mortality rate in patients with AP after HSCT was 7.01%, which is higher when compared to the general population with AP-related mortality ranging from 1% to 6% [17]. Furthermore, patients in the 18 - 45 age category had the highest rates of AP which differs from the general population. This was also demonstrated by Wang et al who documented the mortality rate to be 7.5% in HSCT patients with AP and higher rates in those 30 years old or younger [2]. Although the relationship between AP and mortality was statistically insignificant in the total population, AP was associated with 3.85 times higher risk of mortality after sensitivity analysis. We believe this is due to the inclusion of patients admitted electively for chemotherapy and procedure-related short stays, which might have affected the statistical analysis. The increased mortality can also be attributed to the increased incidence of shock observed in the AP population (10.53%) compared to controls (5.25%). Patients with pancreatitis are susceptible to hypovolemic shock secondary to the volume deficit created by fluid losses.
Our study found significant differences in the incidence of alcohol use (5.85% vs. 1.3%), hypertriglyceridemia (10.53% vs. 0.74%), and diabetic ketoacidosis (DKA) (2.92% vs. 0.18%) between AP and the control group. Alcohol intake, hypertriglyceridemia, and DKA are well-established risk factors for the development of AP and may explain their higher incidence. Alcohol use disorder has been found to be an independent risk factor for mortality in HSCT patients [18]. Despite adjusting for alcohol use disorder, our study revealed higher mortality in the AP group. Additionally, HSCT patients develop hypertriglyceridemia more frequently than the general population which is a common etiology of AP [19]. Patients are at a higher risk of gallstone formation due to increased biliary sludge formation after HSCT [14, 15]. Pre-existing gallstones are also recognized as an independent risk factor for the occurrence of post-HSCT AP [2]. In our retrospective analysis, patients with AP had a significantly greater incidence of biliary pathologies such as cholangitis (6.43%) and choledocholithiasis (15.79%) compared to controls (0.47% and 1.21%, respectively). Acknowledging the higher rates of pre-existing gallstones in patients with AP can result in the consideration of performing elective cholecystectomies in HSCT patients. This can lead to decreased healthcare utilization and improvement in mortality.
The most common indication for HSCT in the total population was GVHD, followed by myeloid leukemia. Wang et al reported GVHD of grades 2-4, in addition to younger age and history of donor lymphocyte infusion (DLI) to be independent risk factors for post-HSCT AP [2]. However, it is difficult to discern whether AP occurs due to the treatment administered for GVHD which includes steroids and immunosuppressants, or due to GVHD itself. This led to the generation of a risk score model classifying HSCT recipients into low, medium, and high-risk groups to forecast AP occurrence [2]. The classification allows the physicians to be wary of the high-risk group and assemble pre-emptive strategies to prevent AP development. Nevertheless, there is a scarcity of effective strategies to prevent, detect and treat this complication.
After sensitivity analysis, the results of our study clearly demonstrated a greater length of stay in the AP group by more than 20 days in comparison to the non-AP group. Longer hospital stays in HSCT recipients admitted for AP can complicate clinical outcomes due to the interruption of chemotherapy. The increased length of stay and mortality rates also contributed to higher hospital charges and cost in the AP group by $346,618 and $121,932, respectively.
We acknowledge the following limitations of this study. First, NIS lacks objective data limiting our ability to calculate common risk stratification scores such as APACHE-II or BISAP. Secondly, NIS does not provide patient identifiers therefore we are unable to track readmissions. As a result, it is difficult to ascertain if pancreatitis episodes were primary or recurrent. In addition, data from NIS only include acute hospitalization episodes therefore, the patients cannot be followed longitudinally. We were not able to exclude patients admitted electively or for procedures in the total population analysis and used sensitivity analysis to overcome this limitation. The results of our study rely on accurate coding and proper documentation which can be inconsistent at times. Coding for AP, HSCT, comorbidities and pertinent procedures was performed using distinct ICD-10 codes to reduce coding errors and to improve the reproducibility of the study. Furthermore, these codes have been validated by a prior study [4]. There is also a possibility that patients with mild pancreatitis were discharged early during the hospitalization and were not included in the sensitivity analysis. The study’s strength comes from the large population size and exclusion of sample bias from data collected from a single region or hospital. Our findings should be validated in a prospective cohort that captures more granular clinical data including information on time to pancreatitis from stem cell transplant and long-term mortality.
Despite these limitations, our study expands on the knowledge of AP as a complication post-HSCT. Our principle finding provides insight that AP development after HSCT is associated with significantly higher mortality. Patients who develop AP fare worse and have a higher healthcare burden. Gastroenterologists and hematologists should be aware of the association of AP in HSCT recipients. Developing predictive risk models can aid in improving clinical outcomes and reduce the healthcare burden of AP after HSCT.
Supplementary Material
All ICD-10 Diagnosis and Procedure Codes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Hunza Chaudhry, Armaan Dhaliwal, Kanwal Bains, Aalam Sohal, Gagan Gupta, Piyush Singla, Raghav Sharma, Dino Dukovic, and Isha Kohli made substantial contributions to the design of the study and reviewed the literature, drafted the manuscript, revised it for important intellectual content and were involved in the final approval of the version to be published. Devang Prajapati revised the manuscript for important intellectual content and was involved in the final approval of the version to be published.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Werlin SL, Casper J, Antonson D, Calabro C. Pancreatitis associated with bone marrow transplantation in children. Bone Marrow Transplant. 1992;10(1):65–69. [PubMed] [Google Scholar]
- 2.Wang XL, Han W, Zhao P, Liu X, Wang JZ, Wang FR, Yan CH. et al. Incidence, risk factors, outcomes, and risk score model of acute pancreatitis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(6):1171–1178. doi: 10.1016/j.bbmt.2019.12.721. [DOI] [PubMed] [Google Scholar]
- 3.Shore T, Bow E, Greenberg H, Persad R, Patterson D, Rubinger M, Schroeder ML. Pancreatitis post-bone marrow transplantation. Bone Marrow Transplant. 1996;17(6):1181–1184. [PubMed] [Google Scholar]
- 4.Thavamani A, Umapathi KK, Dalal J, Sferra TJ, Sankararaman S. Acute pancreatitis is associated with increased risk of in-hospital mortality and health care utilization among pediatric patients with hematopoietic stem cell transplantation. J Pediatr. 2022;246:110–115.e114. doi: 10.1016/j.jpeds.2022.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Ko CW, Gooley T, Schoch HG, Myerson D, Hackman RC, Shulman HM, Sale GE. et al. Acute pancreatitis in marrow transplant patients: prevalence at autopsy and risk factor analysis. Bone Marrow Transplant. 1997;20(12):1081–1086. doi: 10.1038/sj.bmt.1701024. [DOI] [PubMed] [Google Scholar]
- 6.Xu LP, Wu DP, Han MZ, Huang H, Liu QF, Liu DH, Sun ZM. et al. A review of hematopoietic cell transplantation in China: data and trends during 2008-2016. Bone Marrow Transplant. 2017;52(11):1512–1518. doi: 10.1038/bmt.2017.59. [DOI] [PubMed] [Google Scholar]
- 7.Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, Dolstra H. et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56(7):1651–1664. doi: 10.1038/s41409-021-01227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp.
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Maximova N, Gregori M, Simeone R, Sonzogni A, Zanon D, Boz G, D'Antiga L. Total body irradiation and iron chelation treatment are associated with pancreatic injury following pediatric hematopoietic stem cell transplantation. Oncotarget. 2018;9(28):19543–19554. doi: 10.18632/oncotarget.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung LW, Yeh SP, Hsieh CY, Liao YM, Huang HH, Lin CY, Chiu CF. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87(5):421–423. doi: 10.1007/s00277-007-0410-7. [DOI] [PubMed] [Google Scholar]
- 12.Niemann TH, Trigg ME, Winick N, Penick GD. Disseminated adenoviral infection presenting as acute pancreatitis. Hum Pathol. 1993;24(10):1145–1148. doi: 10.1016/0046-8177(93)90198-P. [DOI] [PubMed] [Google Scholar]
- 13.Tomonari A, Takahashi S, Takasugi K, Ooi J, Tsukada N, Konuma T, Iseki T. et al. Pancreatic hyperamylasemia and hyperlipasemia in association with cytomegalovirus infection following unrelated cord blood transplantation for acute myelogenous leukemia. Int J Hematol. 2006;84(5):438–440. doi: 10.1532/IJH97.06119. [DOI] [PubMed] [Google Scholar]
- 14.Frick MP, Snover DC, Feinberg SB, Salomonowitz E, Crass JR, Ramsay NK. Sonography of the gallbladder in bone marrow transplant patients. Am J Gastroenterol. 1984;79(2):122–127. [PubMed] [Google Scholar]
- 15.Teefey SA, Hollister MS, Lee SP, Jacobson AF, Higano CS, Bianco JA, Colacurcio CJ. Gallbladder sludge formation after bone marrow transplant: sonographic observations. Abdom Imaging. 1994;19(1):57–60. doi: 10.1007/BF02165864. [DOI] [PubMed] [Google Scholar]
- 16.Vege SS, Yadav D, Chari ST. In: GI Epidemiology, 1st ed. Talley NJ, Locke GR, Saito YA, editors. Blackwell Publishing; Malden, MA; 2007. Pancreatitis. [Google Scholar]
- 17.Gapp J, Hall AG, Walters RW, Jahann D, Kassim T, Reddymasu S. Trends and outcomes of hospitalizations related to acute pancreatitis: epidemiology from 2001 to 2014 in the United States. Pancreas. 2019;48(4):548–554. doi: 10.1097/MPA.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 18.Graf SA, Vaughn JE, Chauncey TR, Storer BE, Gopal AK, Holmberg LA, McCune JS. et al. Comorbidities, alcohol use disorder, and age predict outcomes after autologous hematopoietic cell transplantation for lymphoma. Biol Blood Marrow Transplant. 2016;22(9):1582–1587. doi: 10.1016/j.bbmt.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagoya Y, Seo S, Nannya Y, Kurokawa M. Hyperlipidemia after allogeneic stem cell transplantation: prevalence, risk factors, and impact on prognosis. Clin Transplant. 2012;26(2):E168–175. doi: 10.1111/j.1399-0012.2012.01628.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All ICD-10 Diagnosis and Procedure Codes.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.