Abstract
In bacterial endocarditis (BE), intravascular infection with Staphylococcus aureus, Streptococcus sanguis, or Staphylococcus epidermidis can lead to formation of a fibrin clot on the inner surface of the heart and cause heart dysfunction. The events that start the coagulation in the early stage of the disease are largely unknown. We have recently shown that human endothelial cells (EC) upon binding and internalization of S. aureus, but not S. sanguis or S. epidermidis, express tissue factor (TF)-dependent procoagulant activity (TFA). The present study shows that infection of EC with these three pathogens induces surface expression of intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) and monocyte adhesion. Subsequent coculture of these cells synergistically enhanced TFA, which was exclusively dependent on TF molecules that were expressed on EC during coculture. TFA induction required direct contact between monocytes and bacterium-infected EC, but the signals for this response were not generated by the binding of monocytes through their β2- or α4-integrins to ICAM-1 or VCAM-1, respectively, on infected EC. The mechanism by which monocytes induce TFA in bacterium-infected EC was partly mediated by the proinflammatory cytokine interleukin-1 produced by the cells during coculture. Endogenous tumor necrosis factor alpha was not involved. This modulating effect of monocytes on species- and strain-dependent TFA of bacterium-infected EC supports our hypothesis that in an early stage in the pathogenesis of BE, as well as other intravascular infections that lead to detrimental fibrin formation, the coagulation cascade can be activated on the surfaces of EC as a consequence of specific interactions between pathogenic bacteria, EC, and monocytes.
Attachment of pathogenic microorganisms to vascular endothelial cells (EC) or sites of vascular injury is considered a critical initiating event for many types of intravascular infections. In bacterial endocarditis (BE), the microbial infection is localized on the endocardial surface of the heart and, depending on the bacterial species, may cause an inflammatory reaction that in most cases affects the mural endocardium and the mitral and aortic valves (4). In humans, virulent bacteria, such as Staphylococcus aureus, and less virulent bacteria, such as Streptococcus sanguis and Staphylococcus epidermidis, are the primary organisms that cause BE (4, 13, 26, 38). Despite significant improvements in medical and surgical therapies, BE remains a severe disease, with an overall incidence of 17 to 40 per million persons in the general population and a mortality of about 19% in The Netherlands (4, 42).
Cardinal processes in the pathogenesis of BE are initiation of a local inflammatory reaction and activation of the extrinsic pathway of the clotting system (4, 12). This reaction results in the formation of vegetations on the surface of the heart valve, which cause heart dysfunction. These endocardial vegetations consist of a clot of fibrin and platelets, in which the causative microorganisms are embedded and multiply. Many clinical and experimental studies on the pathogenesis of BE gave clear insight into the processes in vegetations that are already formed on mechanically damaged valvular endocardium. In this stage of the disease the procoagulant molecule tissue factor (TF) is required to activate the clotting system and maintain fibrin deposition (2, 3, 44). TF is a 47-kDa transmembrane glycoprotein that in complex with coagulation factor VIIa (FVIIa) acts as a serine protease and cleaves factor X (FX). The resulting factor Xa (FXa) triggers downstream coagulation pathways, ultimately leading to the generation of fibrin (12). Subsequent in vivo experiments with the rabbit model of BE revealed that TF needed to maintain fibrin formation was generated by monocytes from the circulatory system settling on the fibrin of growing (infected) vegetations (2, 3, 44). Also, in vitro, human monocytes generated increased levels of TF-dependent procoagulant activity (TFA) upon binding to fibrin containing bacteria, in particular S. aureus, S. sanguis, or S. epidermidis (1–3, 44).
However, these studies of established vegetations left unexplained the events that induce fibrin formation on intact endothelial surfaces of still undamaged heart valves in the initial phase of BE. In an in vitro model of endovascular infection, we recently showed that activation of the coagulation system may start as a consequence of immediate damage or activation of endothelial cells (EC) by bacteria that cause BE (43). This finding was predisposed by the type, number, and virulence of the infecting microorganisms as well as their ability to interact with vascular EC. Adding to the results of studies by other investigators (5, 25, 32), we found that S. aureus, a pathogen with a high propensity to colonize endocardial tissue and to cause infections of intact heart valves, binds to and is actively internalized by cultured human EC (9, 43, 45). As a result, virulent S. aureus strains caused direct EC damage but less virulent strains induced EC activation that resulted in the production of a variety of proinflammatory mediators, including interleukin-1 (IL-1), IL-6, IL-8, monocyte chemotactic protein 1 (MCP-1), and RANTES (9, 40, 48, 49), surface membrane expression of intercellular adhesion molecule 1 (ICAM-1; CD54) and vascular cell adhesion molecule 1 (VCAM-1; CD106), and monocyte adhesion. S. aureus-infected EC also expressed TFA, which was controlled at the level of TF gene transcription and TF protein synthesis and was synergistically enhanced by IL-1, a representative cytokine released during bacteremia (43). By contrast, S. sanguis and S. epidermidis, bacteria with a low propensity to cause infection on undamaged endocardial tissue (13, 38), did bind to EC, but these organisms alone did not induce endothelial TFA but did so in combination with IL-1 (43).
So far these investigations indicated that coagulation may start as a consequence of the interaction between bacteria and EC, a response that is governed by specific properties of the infecting microoganisms as well as the inflammatory status of EC. The present study was undertaken to assess whether monocytes that bind to the surface of bacterium-infected endothelium play a role in the amplification of coagulation and the extent to which amplification is influenced by the type of infecting microorganism. In particular, our aim was to find arguments for the hypothesis that prior to destruction of the endocardium by bacteria, resulting in exposure of the thrombogenic subendothelial matrix, the coagulation cascade is activated on the surfaces of EC as a consequence of the interaction between pathogenic bacteria, EC, and monocytes.
MATERIALS AND METHODS
Reagents.
Fetal calf serum (FCS), culture medium 199 (M199), and RPMI 1640 were purchased from GIBCO Laboratories (Grand Island, N.Y.). Penicillin was obtained from Brocades Pharma B.V. (Leiderdorp, The Netherlands), streptomycin was obtained from Gist-brocades N.V. (Delft, The Netherlands), amphotericin B was obtained from Squibb B.V. (Rijswijk, The Netherlands), and l-glutamine was obtained from Flow Laboratories (Irvine, United Kingdom). EC growth factor was prepared from bovine hypothalamus as described previously (8). Lysostaphin and agarose were obtained from Sigma Chemical Co. (St. Louis, Mo.); gelatin and trypsin were obtained from Difco Laboratories (Detroit, Mich.). EDTA was purchased from Boerhinger (Mannheim, Germany). Human serum (HuS) samples were collected from healthy donors and inactivated (HuSi) at 56°C for 30 min. Clotting factor VII (FVII) was prepared from human plasma as described previously (1); factor X (FX) and the chromogenic substrate PefachromeFXa were purchased from Kordia (Leiden, The Netherlands). FVII-depleted human plasma was generously given by the Department of Hematology (Leiden University Medical Center, Leiden, The Netherlands). Acetic acid, CaCl2, and Tris base were obtained from Merck (Darmstadt, Germany). Rusel's Viper Venom was obtained from Chromogenix (Mölndal, Sweden).
MAbs and cytokines.
The following monoclonal antibodies (MAbs) against surface molecules on human EC or blood leukocytes were used: MAb ENA-1 (immunoglobulin G1 [IgG1]; Monosan, Uden, The Netherlands) against E-selectin (CD62E) (22); MAb RUU-SP 2.17 (IgG1) against P-selectin (CD62P) (28), a gift from K. Nieuwenhuizen (Department of Hematology, University Hospital, Utrecht, The Netherlands); MAb RR1/1 (IgG1) against ICAM-1 (CD54) (39), kindly donated by T. A. Springer (Dana Farber Cancer Institute, Boston, Mass.); MAb CBR-IC2/2 (IgG2a; Endogen, Inc., Boston, Mass.) against ICAM-2 (CD102) (17); MAb E1/6 (IgG1; Becton Dickinson Benelux S.A., Aalst, Belgium) against VCAM-1 (CD106) (37); MAb L133.1 (IgG1; Becton Dickinson) against platelet-endothelial cell adhesion molecule-1 (PECAM-1; CD31) (31); MAb IB4 (IgG2a) against CD18 (β2-integrin) (47), obtained as a supernatant of the hybridoma cell line (American Type Culture Collection, Manassas, Va.); MAb 15A8 (IgG1; Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands) against VLA-4α (CD49d) (21); MAb 2LPM19C (IgG1; Dako, Glostrup, Denmark) against the I-domain of CR3α (CD11b) (18); and TF9-10H10 (IgG1; OMNILabo International B.V., Breda, The Netherlands) against TF (CD142) (29). Neutralizing MAb against human tumor necrosis factor alpha (TNF-α) was purchased from R&D Systems. Human recombinant IL-1α (hereafter referred to as rIL-1) and human recombinant IL-1 receptor antagonist (rIL-1ra) were provided by P. Lomedico (Hoffmann-La Roche, Nutley, N.J.) and ITK Diagnostics BV (Uithoorn, The Netherlands), respectively.
Cells.
EC were isolated from human umbilical veins after collagenase digestion, according to the protocol described previously (8). The cells were cultured in culture medium (M199 supplemented with 100 U of penicillin G/ml, 0.1 mg of streptomycin/ml, 100 U of amphotericin B/ml, 0.1 mg of EC growth factor/ml, 5 U of heparin/ml, 1 mM l-glutamine, and 10% HuSi in a 5% CO2 incubator at 37°C). The cells were grown to confluence in plastic tissue culture dishes (Falcon, Becton Dickinson Europe, Le Pont de Claix, France) coated with 0.75% gelatin in pyrogen-free water. Primary cultures of EC were harvested with 0.05% (wt/vol) trypsin–0.01% (wt/vol) EDTA and subsequently subcultured in culture medium in tissue culture dishes (6- or 24-well tissue culture plates; Corning Costar Europe, Badhoevedorp, The Netherlands). In most experiments confluent monolayers of secondary cultures of EC, i.e., cultured after one passage, were used. In some experiments secondary cultures of EC were grown to confluence on 0.75% gelatin-coated glass coverslips (6) in 24-well tissue culture plates.
As a source for human monocytes, cells from the human monocytic cell line THP-1, which for this study possesses the relevant characteristics of monocytes, were used in the experiments (34, 41). In an extensive pilot study the ability of THP-1 cells to express surface adherence molecules enabling adherence to EC and to produce TFA upon lipopolysaccharide (LPS)-stimulation was confirmed (data not shown). THP-1 cells were cultured in monocyte culture medium (RPMI 1640 supplemented with 100 U of penicillin G/ml, 0.1 mg of streptomycin/ml, 1 mM l-glutamin, and 10% heat-inactivated FCS [FCSi] in a 5% CO2 incubator at 37°C). Prior to their use in the adhesion and coculture assay, the cells were suspended in M199 plus 10% HuSi at the desired concentration. Henceforth, THP-1 cells will be referred to as monocytes.
Granulocytes, used in some experiments, were obtained from human peripheral blood by differential centrifugation on a Ficoll-amidotrizoate gradient as described elsewhere (9).
Bacteria and infection of EC.
The bacteria used in this study were S. aureus 42D, S. epidermidis ATCC 149900, and S. sanguis NCTC 7864 and have been used extensively in our previous studies of BE (2, 3, 40, 43). Bacteria suspensions were stored at −70°C and prepared for use as described previously (43). Briefly, S. aureus and S. epidermidis were routinely grown overnight in brain heart infusion broth and S. sanguis was grown in Todd-Hewitt broth at 37°C. The bacteria were harvested by centrifugation, resuspended in M199 plus 0.1% (wt/vol) gelatin and 10% (vol/vol) fresh human serum, and incubated for 30 min with rotation at 4 rpm for opsonization. The bacteria were then diluted in M199 with 10% HuSi at the desired concentration prior to use in the infection assay. Confluent monolayers of EC were washed once with culture medium without antibiotics and incubated for 24 h with opsonized bacteria in M199 with 10% HuSi at 37°C in a 5% CO2 incubator as described previously (9). The number of bacteria used in the infection assay was determined by colony counting after serial dilutions were plated on blood agar plates and incubated overnight at 37°C.
Monocyte adherence and coculture conditions.
Monolayers of EC grown on gelatin-coated glass coverslips in 24-well cell culture plates were incubated for 24 h with bacteria as described above. After a wash in warm phosphate-buffered saline (PBS), about 2.5 × 105 monocytes (≈1 monocyte per single EC) in M199 with 10% HuSi were added. Monocytes were allowed to bind to these EC for different periods of time at 37°C in a 5% CO2 incubator. The coverslips were then removed from the wells, washed five times in warm PBS to eliminate nonadherent monocytes, fixed with methanol for 15 min, and stained with Giemsa stain. The number of adherent monocytes was determined by counting under a light microscope according to our method described elsewhere (6), and the data were expressed as the percentage of EC-bound monocytes of the total number added. To assess the contribution of cell surface adhesion molecules in the binding of monocytes to EC and induction of procoagulant activity (PCA), coculture experiments were performed in the presence of 5 to 10 μg of a relevant MAb/ml in M199 plus 10% HuSi. For coculture without direct cell contact, a two-compartment culture system was used. Monocytes in the upper compartment of an inner well insert and (infected) endothelial cell monolayers in the lower compartment were cocultured physically separated by a porous membrane (pore size, 0.45 μm; Falcon cell culture insert; BD Labware, Lincoln Park, N.J.). After incubation the insert with monocytes was transferred to an empty well, and monocytes were spun down onto the filter by centrifugation. The EC in the lower compartment were washed once with warm PBS. From both cell types TFA was determined as described below.
TFA assay.
TFA assays were conducted using confluent secondary cultures of EC on gelatin-coated glass coverslips in 24-well culture plates (43). Following infection and/or incubation with monocytes as described above, the coverslips were washed once with warm PBS and transferred to a new 24-well cell culture plate. The level of TFA was measured as described by Bancsi et al. (1). Briefly, the coverslips were incubated with 125 μl of buffer containing 0.125 pmol of purified FVII and 0.125 nmol of CaCl2 for 20 min at 37°C with rotation at 200 rpm, to allow formation of a TF-FVII-Ca complex. Next, 20 μl of FX (10 U/ml) was added. After 5 min at 37°C 100 μl of the mixture was removed and added to 100 μl of cold (4°C) buffer containing EDTA, to stop FXa formation. Then, the sample mixture was warmed to 37°C. Subsequently, 25 μl of a 1-mg/ml concentration of PefachromeFXa, a chromogenic substrate for FXa, was added. After 20 min at 37°C the conversion of the substrate was stopped by the addition of 200 μl of 50% (vol/vol) acetic acid. The optical density at 405 nm was measured and converted into FXa concentrations. For this calculation a calibration curve was made from purified FX that was fully activated with Rusel's Viper Venom. Data are expressed as milliunits of FXa/well containing about 2 ×105 EC and variable numbers of EC-bound monocytes.
Flow cytometric analysis of endothelial adhesion molecule expression.
For fluorescence-activated cell sorting (FACS) analysis, EC were collected and prepared as described previously (9, 43). Briefly, EC were grown to confluence in gelatin-coated tissue culture flasks and incubated shortly before use in culture medium without antibiotics. These cultures were then incubated with approximately 5 × 108 CFU of opsonized bacteria (about 200 bacteria per EC) in 10 ml of M199 with 10% HuSi or culture medium alone for 24 h at 37°C in a 5% CO2 incubator. Cells were washed with PBS. Then, they were harvested by mild trypsinization, collected, and washed in cold PBS with 1% FCSi (wash buffer). Subsequently, these cells were incubated for 30 min in cold PBS supplemented with 1% goat serum and 1% HuSi. All further incubations were done on ice. The cells were washed twice in wash buffer and incubated with 1 μg of the appropriate MAb/ml for 30 min. After two washes the cells were incubated for 30 min with phycoerythrin-conjugated goat anti-mouse Ig (Southern Biotechnology Associates Inc., Birmingham, Ala.) according to the supplier's manual, washed once, and then analyzed by flow cytometry with a FACScan (Becton Dickinson). In each sample 10,000 cells were analyzed. EC treated with conjugated antibody alone served as a control to set background fluorescence.
Analysis of TF antigen expression in monocyte-EC cocultures.
Monocytes were allowed to adhere to bacterium-infected EC as described above. After 5 h of incubation, nonadherent monocytes were removed by washes in warm PBS, and EC with bound monocytes were brought into suspension by mild trypsinization. These mixed-cell suspensions were analyzed for surface expression of TF antigen in the FACScan by using the TF9-10H10 MAb. The procedures for cell labeling and FACS analysis were similar to those described above. Monocyte and EC populations were distinguished on the basis of their distinct forward and side scatter profiles. In addition, the anti-CD18 MAb IB4 was used to identify the monocyte population in these mixed cell suspensions. In this way monocytes and EC could be analyzed separately for TF antigen expression.
Statistical analysis.
Differences between the results of the various experiments were evaluated by means of the Wilcoxon signed ranks test.
RESULTS
Induction of PCA in cocultures of monocytes and EC after bacterial challenge.
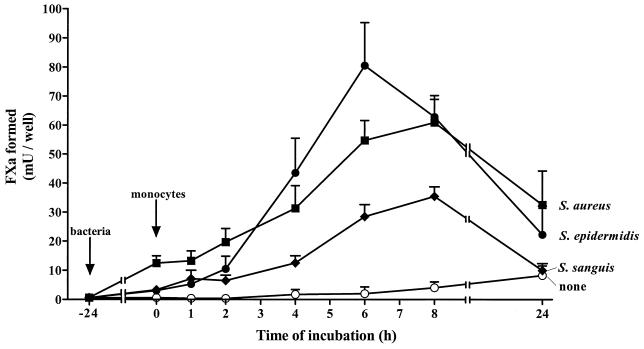
Unstimulated EC expressed very little TF-dependent PCA (TFA), i.e., mean ± standard deviation of 2.33 ± 3.7 mU of FXa/well of 2 × 105 EC (n = 56). At 24 h of bacterial exposure, the value for TFA of S. aureus-infected EC was 21.37 ± 13.9 mU of FXa/well (n = 39) (Fig. 1; TFA values for EC infected with bacteria for 24 h are depicted at time zero). This result confirmed our previous finding (11). Next, PCA of bacterium-infected EC in the presence of monocytes was evaluated. Monocytes in suspension expressed little PCA, i.e., approximately 5 mU of FXa per 2 × 105 cells. The addition of 2 × 105 monocytes to the control, noninfected, EC monolayers induced a small increase in the total level of PCA during subsequent coculture. Time course experiments showed that the amount of FXa generated in these cells gradually increased to (8.2 ± 3.3) mU of FXa/well at 24 h of coculture (Fig. 1). A more pronounced increase in PCA was found during incubation of the monocytes with monolayers of S. aureus-infected EC. The level of PCA was significantly increased after 4 h of culture in the presence of monocytes, reached a high level of 60.9 ± 8.0 mU of FXa/well after 8 h, and then declined to 32.5 ± 11.7 mU of FXa/well after 24 h of coculture. With S. sanguis-infected EC induction of PCA during coculture with monocytes followed a similar time course, but at each time values for PCA were significantly lower (P < 0.05; n = 6) than those observed for cocultures with S. aureus-infected EC (Fig. 1). The time course of PCA induction and the level of PCA of cocultures of S. epidermidis-infected EC and monocytes were somewhat different from those observed for EC infected with the two other bacteria. With S. epidermidis-infected EC, a threefold increase (P = 0.018; n = 5) in the PCA level was detectable after 2 h of coculture. The highest level was reached at 6 h. From this time onward PCA gradually declined to 22.2 ± 9.9 mU of FXa/well (n = 3) after 24 h (Fig. 1).
FIG. 1.
Bacterial species- and strain-dependent PCA during coculture of monocytes and infected EC. Monolayers of 2 × 105 EC/well were infected for 24 h with 4 × 107 to 5 × 107 of the indicated bacterium (closed symbols) or incubated in culture medium (open circles). These cultures were then washed, and 2.5 × 105 monocytes were added. Time zero represents the time at which monocytes were added. At the indicated times during subsequent coculture, PCA was determined by FVIIa-dependent FX activation as described in Materials and Methods. Values represent means ± standard deviations of six separate experiments with EC from different donors.
Additional experiments showed that incubation of bacterium-infected EC with a 10-fold-higher number of monocytes did not further increase PCA of cocultures (data not shown). Also, values for PCA of bacterium-infected EC after 6 h of coculture in the continuous presence of monocytes, as depicted in Fig. 1, were not different from those of cocultures from which all nonadherent monocytes had been removed after 1 h of incubation (Table 1), suggesting that PCA occurs in the presence of EC-bound monocytes.
TABLE 1.
Effect of removal of nonadherent monocytes on PCA of cocultures of monocytes and bacterium-infected ECa
| Time of culture (h)b | PCA (mU of FXa/well) in cocultures of monocytes and EC infected with:
|
||
|---|---|---|---|
| S. aureus | S. sanguis | S. epidermidis | |
| 0 (no monocytes) | 21.37 ± 14.0 | 1.67 ± 0.5 | 1.57 ± 0.8 |
| 1 | 24.28 ± 4.6 | 13.48 ± 2.9 | 23.45 ± 4.7 |
| 4 | 53.42 ± 18.5 | 27.31 ± 7.8 | 50.18 ± 15.5 |
| 6 | 76.68 ± 13.8 | 57.89 ± 7.1 | 91.99 ± 16.9 |
Monolayers of 2 × 105 EC/well were incubated for 24 h with 4 × 107 to 5 × 107 organisms of the indicated bacterium, washed, and incubated for 1 h with 2.5 × 105 monocytes. Then, nonadherent monocytes were removed by washing, EC with membrane-bound monocytes were cocultured for an additional period of time; and PCA was determined. Values represent the means ± standard deviations for three individual experiments.
At time zero, the levels of PCA were determined 24 h after bacterial infection just before the addition of monocytes. At 1 h of culture, nonadherent monocytes were removed and PCA was assessed 1 h after continuous incubation of bacterium-infected EC with monocytes. At 4 and 6 h after the addition of monocytes (i.e., 3 and 5 h, respectively, after the removal of nonadherent monocytes from the coculture, PCA was determined.
To further determine whether direct cell-to-cell contact is essential for PCA enhancement, the coculture experiments were repeated in a two-compartment culture system. Separation of monocytes in the upper compartment from monolayers of bacterium-infected EC in the lower compartment by a microporous filter membrane that only allowed free diffusion of soluble mediators did not enhance PCA in either monocytes or the EC (data not shown). Thus, PCA induction requires physical contact between monocytes and bacterium-infected EC.
Coculture experiments in which monocytes were replaced by pure suspensions of human granulocytes demonstrated that the ability to induce PCA was not exclusively restricted to monocytes. In cocultures of noninfected EC and granulocytes, a small increase in PCA was detected. At 6 h of coculture PCA values had increased to 6.3 ± 1.1 mU of FXa/well (n = 3). With bacterium-infected EC these values were significantly higher, namely, 80.4 ± 10.4 mU of FXa/well (n = 3) for S. aureus-infected EC, 53.1 ± 5.4 mU of FXa/well (n = 3) for S. sanguis-infected EC, and 39.3 ± 7.4 mU of FXa/well (n = 3) for S. epidermidis-infected EC.
Monocyte adhesion to EC after bacterial challenge.
In order to explain species- and strain-dependent PCA induction in cocultures of monocytes and infected EC (Fig. 1), experiments were performed to investigate whether the three types of bacteria differ in their abilities to modulate monocyte binding to EC.
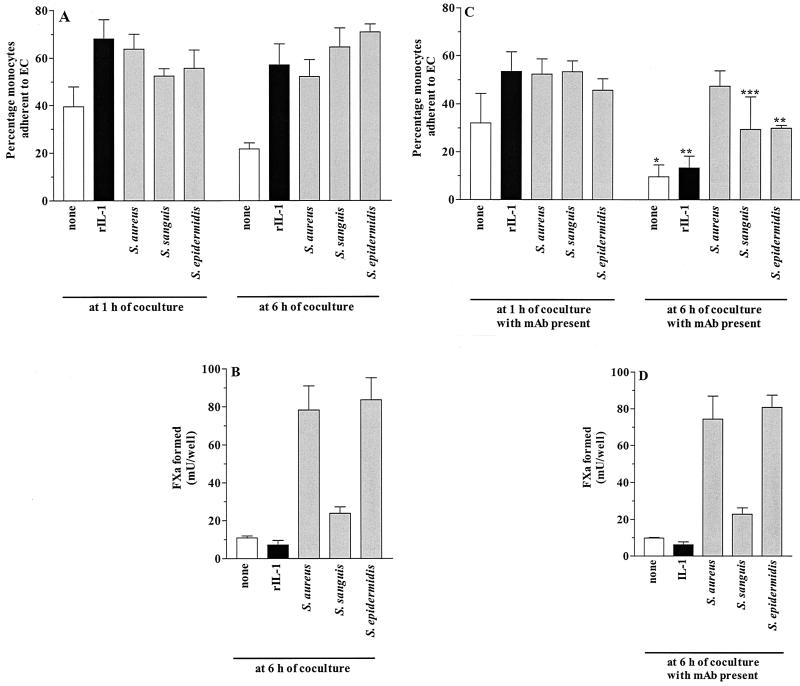
In our previous study we observed that monolayers of EC upon binding and internalization of S. aureus were induced to bind monocytes isolated from fresh human blood (9). In the present study using THP-1 monocytic cells, this observation was confirmed. THP-1 binding to EC was determined at 1 and 6 h of coincubation, the times between which the PCA of cocultures increased to a high level (Fig. 1). The mean percentage of THP-1 cells (hereafter referred to as monocytes) that adhered to monolayers of control, noninfected EC within 1 h of incubation was 39.5% ± 8.5% (n = 4). During the next 5 h of coculture, monocyte binding was reduced to 21.7% ± 2.6% (P < 0.035 versus monocyte binding after 1 h; n = 4) (Fig. 2A). Infection of EC with S. aureus, S. sanguis, or S. epidermidis resulted in an increased number of bound monocytes, which occurred when the adhesion assay was performed 24 h after the addition of bacteria to the EC (Fig. 2A). The number of monocytes that were bound to S. aureus-infected EC after 1 h of coculture was slightly, but not significantly, higher than the number that were bound to S. sanguis- or S. epidermidis-infected EC. For all three types of bacteria the number of EC-bound monocytes after 6 h of coculture was similar to that observed after 1 h, as was also the case with monocyte binding to control rIL-1-stimulated EC (Fig. 2A). Accurate microscopic assessment of the number of monocytes bound to EC at times later than 6 to 8 h of coculture was not possible, mainly because after binding to EC an increasing number of monocytes lose their spherical appearance and adopt a flattened morphology, an observation similar to that previously reported by us (6, 7).
FIG. 2.
Effect of MAb directed against VLA-4 or β2− integrins on monocytes on adherence of monocytes to EC (A and C) and induction of PCA (B and D) during coculture. Monolayers of 2 × 105 EC/well were incubated for 24 h in medium alone (white bars), with 5 ng of rIL-1/ml (black bars), or with the indicated bacterium (gray bars). After a wash, coculture experiments were initiated by the addition of 2.5 × 105 monocytes. Prior to their addition to EC, monocytes were incubated with 10 μg of anti-CD49d MAb/ml and 10 μg of anti-CD18 MAb/ml in combination (C and D) or in medium alone (A and B). At the indicated times during coculture, the percentage of EC-bound monocytes of the total number of added monocytes was determined as well as the level of PCA generated by the cells. Data are given as the means ± standard deviations for four separate experiments with EC from different donors. (C) P values were 0.007 (∗), 0.003 (∗∗), and 0.017 (∗∗∗) versus the same stimulus at 6 h without MAb (A).
Species- and strain-dependent expression of adhesion molecules on bacterium-infected EC and their role in PCA induction.
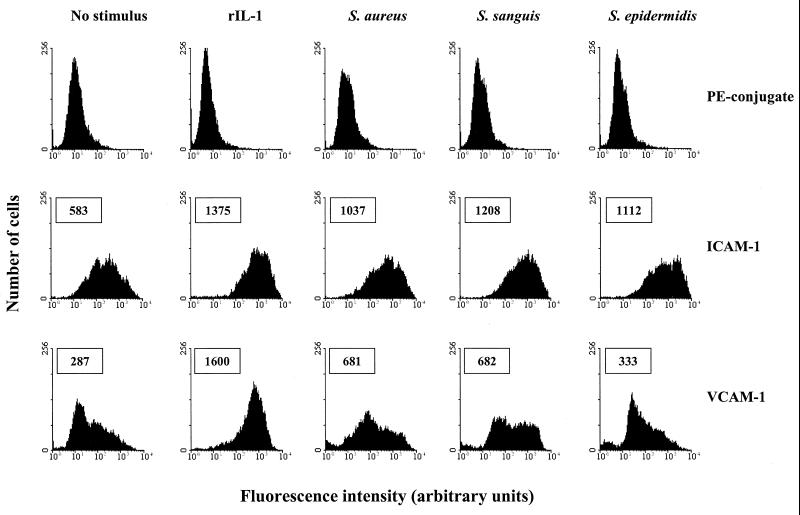
The mechanism by which monocytes bind to bacterium-infected EC and induce PCA was further explored. Monolayers of EC infected with the three types of bacteria were prepared for FACS analysis, and membrane expression of a variety of adhesion molecules involved in leukocyte binding to these cells was evaluated. Control, noninfected EC expressed relatively high levels of CD31 (PECAM-1) and CD102 (ICAM-2) (data not shown) and moderate levels of CD54 (ICAM-1) (Fig. 3) and did not express CD106 (VCAM-1) (Fig. 3), CD62E (E-selectin) (data not shown), or CD62P (P-selectin) (data not shown). These expression profiles did not change during the first 4 h of incubation with S. aureus, S. sanguis, or S. epidermidis (data not shown). However, after 24 h of bacterial exposure, EC expressed both VCAM-1 and an elevated level of ICAM-1 molecules on their cell membranes (Fig. 3); surface expression of the other above-mentioned adhesion molecules remained unchanged (data not shown). Increased endothelial expression of ICAM-1 during bacterial infection was observed on each single cell, whereas VCAM-1 expression was induced in only a proportion of the cells. This latter finding appeared to be dependent on the type of the infecting bacteria. Incubation of EC with S. aureus for 24 h resulted in VCAM-1 expression by 53.8% ± 11.5% (n = 3) of the cells, whereas incubation with the same number of S. sanguis or S. epidermidis organisms resulted in VCAM-1 expression by 44.1% ± 9.1% (n = 3) or 27.0 ± 3.1% (n = 3) of the cells, respectively. As a control, monolayers of EC were incubated with rIL-1 for 24 h. This resulted in markedly increased expression of ICAM-1 on all cells and VCAM-1 on almost all cells (91.3% ± 2.3%; n = 3) (Fig. 3), demonstrating the capability of each individual EC to express VCAM-1 in response to an appropriate stimulus.
FIG. 3.
Flow cytometric analysis of the species- and strain-dependent expression of surface adhesion molecules on bacterium-infected EC. Monolayers of EC were incubated for 24 h with culture medium alone (no stimulus), with 5 ng of rIL-1/ ml (positive control), or with the indicated bacteria and then analyzed for surface expression of ICAM-1 and VCAM-1 molecules. Data from one representative experiment are shown. Boxed numbers represent the mean fluorescence intensities expressed in arbitrary units of all analyzed EC. Mean background fluorescence, established by incubation of EC with the phycoerythrin-conjugated MAb only (PE-conjugate), ranged from 13 to 19 arbitrary units.
Engagement of integrin receptors on monocytes can initiate intracellular signals leading to expression of TF (16, 20). To determine whether the enhancement of PCA in cocultures of monocytes and bacterium-infected EC requires adhesion of monocytes to endothelial ICAM-1 and VCAM-1, coculture experiments were performed in the presence of anti-CD18 and anti-CD49d MAbs directed against the integrin-β2 subunit and VLA-4 integrin-α4 subunit, respectively, on monocytes. These antibodies block binding of monocytes to either endothelial ICAM-1 or VCAM-1 (7, 21, 47). The number of monocytes bound to bacterium-infected EC was assessed after 1 h as well as after 6 h of coculture, the latter being the time at which PCA was also determined. For comparison, monocyte binding and PCA values were evaluated in cocultures of these cells with noninfected or rIL-1-stimulated EC. The experiments with bacterium-infected EC showed that the values of PCA after 6 h of coculture with monocytes were not affected by the presence of a combination of anti-CD18 and anti-CD49d MAbs (Fig. 2B versus D). At 6 h of coculture, however, the binding of monocytes to S. sanguis- or S. epidermidis-infected EC but not to S. aureus-infected EC was inhibited 55 to 60% by these MAbs (Fig. 2A versus C). Likewise in the experiments with noninfected or rIL-1-stimulated EC these MAbs also did not affect PCA in the cells in coculture but markedly reduced monocyte binding, i.e., 58.2% inhibition of binding to noninfected EC and 76.7% inhibition of binding to rIL-1-stimulated EC (Fig. 2). However, under each experimental condition, the inhibitory effect of these MAbs on monocyte-EC interaction was not observed at 1 h of coculture (Fig. 2A versus C). Apparently, monocytes initially bind to EC independently of or not solely via their CD18 and CD49d integrin molecules.
TF dependency of PCA and surface expression of TF on cells in coculture.
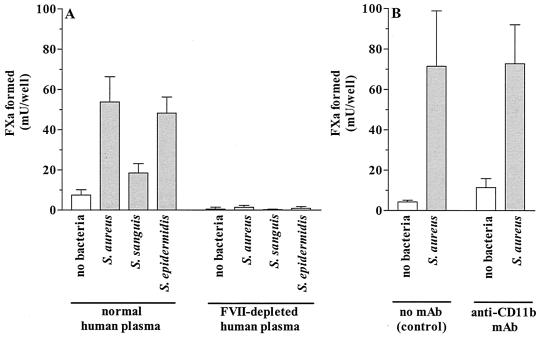
In our experiments PCA was assayed by the conversion of factor X to Xa, most likely through cleavage by the membrane-bound TF-FVII/Ca2+ complex. To confirm the involvement of FVII and thereby TF as the PCA effector molecule, coculture experiments were performed by using FVII-depleted pooled human plasma instead of normal human plasma, and PCA was determined in the absence of FVII. The capacity of the cells in coculture to generate PCA in the absence of FVII was completely abolished, revealing the TF dependency of the observed PCA (Fig. 4A). The alternative procoagulant response of monocytes to activate conversion of factor X to Xa independent of TF antigen via their CR3 (CD11b/CD18; αMβ2) integrin molecules, as suggested by others (27, 33), was excluded by the use of a neutralizing MAb against CD11b (MAb 2LPM19C). The presence of 10 μg of this MAb/ml during coculture of monocytes and EC infected with S. aureus (Fig. 4B) or S. sanguis or S. epidermidis (data not shown) did not abolish the induction of TFA.
FIG. 4.
TF dependency of PCA of cocultures of monocytes and bacterium-infected EC. Monolayers of ≈2 × 105 S. aureus-, S. sanguis-, or S. epidermidis-infected EC or noninfected EC/well were cocultured for 6 h with 2.5 × 105 monocytes in normal or FVII-depleted human plasma (A) and in the absence (no MAb) or presence of 10 μg of anti-CD11b MAb 2LPM19C/ml. Then, PCA was determined. Values are the means ± standard deviations for three (A) and six (B) separate experiments with EC from different donors.
To determine the cellular source of TFA, expression of TF antigen on the surfaces of monocytes and EC after coculture was analyzed by flow cytometry. As described in our previous study, stimulation of EC with rIL-1 as well as exposure of these cells to S. aureus, but not to S. sanguis or S. epidermidis, resulted in time-dependent TF mRNA and TF antigen expression (43). As a result of this, rIL-1-stimulated EC and S. aureus-infected EC still express elevated numbers of TF molecules on their cell membranes at 24 h after stimulation or bacterial infection. This is the time at which in the present study the coculture with monocytes was started. The values for endothelial TF expression at the time of the addition of monocytes are included in Fig. 5. When S. aureus-infected EC were then cocultured with monocytes for 5 h the surface expression of TF remained unchanged. Similarly, monocyte binding to rIL-1-stimulated EC did not further increase endothelial TF expression. On the other hand, the low basal level of expression of TF antigen on S. sanguis- or S. epidermidis-infected EC increased about fourfold upon incubation with monocytes (Fig. 5). Also, TF expression on noninfected EC was slightly increased upon coculture with monocytes. Changes in the basal expression level of TF antigen on monocytes were not found in either coculture experiment described above (Fig. 5).
FIG. 5.
Flow cytometric analysis of TF antigen expression on the surfaces of monocytes or EC after coculture. Monolayers of EC were incubated for 24 h in culture medium alone, with 5 ng of rIL-1/ml, or with the indicated bacteria. These monolayers were subsequently washed and cultured for 5 h in the presence of monocytes. Then, EC and EC-adherent monocytes were analyzed separately for membrane expression of TF molecules as described in Materials and Methods. Open profiles represent the cells before coculture. Closed profiles represent the cells after 5 h of coculture. Data from one representative experiment are shown.
Role of endogenous IL-1α, IL-1β, or TNF-α in induction of TFA in coculture of monocytes with bacterium-infected EC.
Although induction of TFA appeared to require cell-cell contact, we explored the possibility that soluble factors that are released by the cells during coculture might also be involved. The most likely candidates are the proinflammatory cytokines IL-1 and TNF-α, since these cytokines are produced by monocytes and/or EC upon activation and induce TFA in EC (11). To test possible auto- or paracrine stimulation of TFA by endogenous IL-1α, IL-1β, or TNF-α, coculture experiments were performed in the presence of rIL-1ra or neutralizing concentrations of anti-human TNF-α MAb. The results show that anti-human TNF-α MAb did not prevent the increase in TFA in cocultures of monocytes and bacterium-infected EC (Fig. 6). In contrast, generation of TFA was markedly inhibited in the presence of 30 ng of rIL-ra/ml. The mean value for TFA inhibition was about 40%; no significant differences were found between S. aureus-, S. sanguis-, or S. epidermidis-infected EC. Also, no further inhibition was obtained when both blocking compounds were used in combination (P > 0.05 versus rIL-1ra alone; n = 8) (Fig. 6). Control experiments demonstrated that similar concentrations of these blocking compounds completely abolished the increase in TFA in EC upon exposure to rIL-1 (43) or rTNF-α (data not shown).
FIG. 6.
Contribution of endogenous IL-1 or TNF-α to enhanced TFA of monocyte-EC cocultures. Monolayers of 2 × 105 EC/well were incubated for 24 h with culture medium alone (no bacteria) or with ∼4 × 107 cells of the indicated bacteria, washed, and incubated for about 15 min with culture medium with (+) or without (−) 1 μg of anti-human TNF-α MAb/ml and/or 30 ng of rIL-1ra/ml. Then, these cells were cocultured for 6 h with 2 × 105 monocytes in the absence (−) or presence (+) of anti-human TNF-α MAb and/or rIL-1ra. TFA was determined as described in Materials and Methods. Values are the means ± standard deviations for eight separate experiments with EC from different donors. P values were <0.03 (∗)and <0.02 (∗∗) versus the same stimulus in the absense of rIL-1ra and anti-human TNF-α MAb (open bars). All mean values for TFA determined in the presence of both blocking compounds were not significantly different (P > 0.05; n = 8) from values measured in the presence of rIL-1ra alone.
DISCUSSION
The focus of this study was to understand more fully some fundamental aspects of the initiation of coagulation in an early stage of the pathogenesis of BE. In particular, the roles of EC and monocytes were explored because these cell types can generate TFA (12). The use of a coculture system allowed us to establish contacts between EC, pathogenic bacteria, and monocytes that may more closely resemble interactions as they occur in vivo. The results from this investigation show that monocytes bind to the surfaces of bacterium-infected EC and as a result of their interaction promote, in a synergistic manner, an increase in PCA that depends entirely on TF molecules. This finding was observed for cocultures of monocytes and S. aureus-infected EC, i.e., after infection EC expressed an elevated level of TFA, and also in cocultures with EC infected with S. sanguis or S. epidermidis, each a bacterial species unable to induce TFA in EC by itself (reference 43 and this study). The mechanism by which monocytes increase TFA in bacterium-infected EC is partly mediated by the proinflammatory cytokine IL-1 produced endogenously by the cells during coculture. This modulating effect of monocytes on species- and strain-dependent TFA of bacterium-infected EC supports our hypothesis that specific interactions between bacteria, monocytes, and vascular EC coordinate the early activation of the coagulation system in BE or other intravascular infections that lead to detrimental fibrin formation.
Our previous studies demonstrated that virulent as well as less virulent S. aureus organisms and to a lesser extent S. sanguis and S. epidermidis infect and colonize EC in culture (9, 40, 43). As a result of infection with S. aureus, but not S. sanguis or S. epidermidis, EC are activated to express TF mRNA, TF surface antigen, and TFA (43). In addition to bacterium-dependent activation of EC, the results of the present study show that infection with each of these three types of bacteria is accompanied by increased ability of EC to bind to monocytes and an enhancement of TFA a few hours later. Incubation of monocytes with EC infected with S. epidermidis appears to be the most effective condition for initiation of TFA. The level of TFA in these cocultures was more pronounced and TFA could be detected at an earlier time, i.e., at 2 h of coculture, than in experiments with EC infected with each of the two other microorganisms. The lowest values for TFA were observed in the experiments with S. sanguis-infected EC. With regard to these differences some interesting conclusions can be drawn. First, the amount of TFA induced during coculture of monocytes with bacterium-infected EC most likely is not regulated simply by the number of EC-associated bacteria. This, for example, becomes clear when previous (43) and present findings with S. epidermidis-infected EC are combined. Using the same experimental procedure, we recently showed that incubation of EC with S. epidermidis results in a consistently lower degree of infection than with S. aureus and S. sanguis (43). Yet, values for TFA are relatively high in cocultures of monocytes and S. epidermidis-infected EC. Second, TFA levels are not directly related to the number of monocytes that bind to the surfaces of bacterium-infected EC. Despite differences in TFA levels and kinetics, we found that for all three types of bacteria the numbers of EC-adherent monocytes were similar. Together, these findings point out that certain bacterial factors or bacterium-induced processes can, at least to some extent, modulate TFA induction during monocyte-EC interaction. This, however, awaits further investigation.
The ability of monocytes to induce PCA during incubation with cultured human EC has been documented previously by several research groups (14, 23, 24, 30, 46). These studies have been performed according to different experimental procedures with confluent or subconfluent cultures of either cytokine-activated or quiescent human EC. These differences in the activation status of EC, and consequently that of the monocytes bound to these EC, most likely account for the discrepancies in the amount of TFA, the kinetics of TFA induction, and the cell type(s) expressing this TFA that are described in these reports. Consistent, however, with observations by Napoleone et al. (30) and Collin et al. (14), our present data show that values for TFA expressed by nonstimulated EC or monocytes before coculture were almost negligible but became slightly elevated during 24 h of coculture. In addition, we show that increased adhesion of monocytes to EC stimulated with rIL-1 for 24 h did not generate an increase in TFA during subsequent coculture. Analogous to experiments with 24-h TNF-α-stimulated EC (24), this lack of TFA induction could be explained, at least in part, by the absence of E-selectin (CD62E) molecules on the surfaces of rIL-1-stimulated EC (10). These molecules augment monocyte-EC interaction and were shown to mediate TFA induction during subsequent coculture of these cells (24).
However, the most essential and novel finding of the present study is that the PCA is markedly enhanced in cocultures of monocytes and EC infected with pathogenic bacteria that cause BE. From the experiment using FVII-depleted plasma and anti-CD11b MAb, this activity was identified as being exclusively dependent on TF glycoprotein molecules that become expressed and functionally active on bacterium-infected EC during coculture with monocytes. The observation that the amount of TFA is not proportionate to the number of monocytes present in the coculture, as discussed above, also points towards EC as the sole cellular source of the increased TFA. This is further supported by the experiments with human granulocytes. These leukocytes are unable to express TF antigen by themselves (12) but increase TFA in bacterium-infected EC during coculture.
The increased endothelial surface expression of TF was most obvious from the coculture experiments of monocytes with S. epidermidis- or S. sanguis-infected EC. The results with S. aureus-infected EC reveal that an increase in TFA is not always accompanied by an increase in the number of TF molecules on the cell surfaces. This finding argues for the existence of an alternative mechanism, possibly TF deencryption, a mechanism whereby TF surface molecules acquire their full functional activity by qualitative changes after cell activation (36).
Our study using the two-compartment coculture system suggests that direct contact between monocytes and bacterium-infected EC is mandatory for induction of TFA, indicating the involvement of cellular adhesion receptors. We previously reported that S. aureus upon internalization by EC induces monocyte adhesion and chemotaxis by increasing EC surface expression of ICAM-1 and VCAM-1 molecules (9) and secretion of MCP-1 (40). In extension to this, the present study shows a similar response of EC after infection with S. epidermidis or S. sanguis. Since these latter two types of bacteria were unable to induce TFA in EC by themselves (reference 43 and this study), these findings once more indicate that processes regulating the induction of a proinflammatory activity that promotes leukocyte binding to bacterium-infected EC differ from those regulating endothelial PCA. However, the generation of TFA in the cocultures of monocytes and bacterium-infected EC seems not to be a consequence of the interaction of ICAM-1 and VCAM-1 molecules on EC with their respective β2- or β1-integrin receptors on monocytes. The data clearly show that antibodies against β2-integrins and the β1-integrin VLA-4 partially but significantly reduced the number of monocytes that were bound to bacterium-infected EC after 6 h of coculture without influencing the elevated level of TFA. Also, the ICAM-1- and VCAM-1-dependent binding of monocytes to rIL-1-stimulated EC did not coincide with an increase in TFA during coculture of the cells. Based on these observations we surmise that other adhesive molecules involved in the initial contacts between monocytes and bacterium-infected EC provide the signals required for the expression of TFA by EC a few hours later. The identity of these costimulatory adhesive molecules remains to be determined. However, a possible contribution of E-selectin molecules that are rapidly expressed on EC after monocyte-EC interaction (24, 35) cannot be excluded. It is also plausible that the synergistic induction of TFA in cocultures of monocytes and bacterium-infected EC is achieved by a cooperation of adhesion molecules and EC-bound bacteria. The credibility of these potential mechanisms is presently under investigation.
Cellular interactions between monocytes and EC induce early release of proinflammatory cytokines, such as TNF-α and IL-1β (15, 19, 30, 50). Considering the effects of these cytokines on EC TFA (reference 11 and this study) and the synergistic effect of rIL-1 on TFA of bacterium-infected EC (43), we investigated whether these cytokines mediate TFA induction in our coculture studies. The experiments with rIL-1ra and/or neutralizing anti-human TNF-α MAb revealed a significant contribution by IL-1 and possibly other, unknown cell-derived factors. This suggests that IL-1 is produced by monocytes or EC during coculture and acts in concert with the adhesive cellular interactions, in an auto- or paracrine fashion, to augment the production of TFA in bacterium-infected EC. Alternatively, in vivo, IL-1 could also have a profound stimulatory effect on EC distal to the site of infection.
In summary, our findings suggest an important synergistic relationship between EC, bacteria, and monocytes in the initiation of fibrin formation at sites of intravascular infections. With regard to the early events in the pathogenesis of BE, the present results and those of previous studies (9, 40, 43) have prompted us to consider the following sequence of events that can start fibrin formation on structurally normal heart valves. The contact of bacteria with quiescent or preconditioned EC and/or the persistence of the bacteria in these cells causes direct injury to EC or induces EC activation. This mechanism is dependent on the strain and/or the virulence of the bacterium. Activation of bacterium-infected EC is accompanied by binding of monocytes (and granulocytes) to the surfaces of EC and, with S. aureus, by the initiation of endothelial PCA. The contact of infected EC with monocytes elicits the release of cytokines as well as expression of TF molecules, which are needed to start fibrin formation at the site of endothelial colonization. We surmise that these processes further increase the (leukocyte-mediated) damage to the infected endothelium (M. H. A. M. Veltrop and H. Beekhuizen, unpublished data). Ultimately, monocytes, which settle from the circulatory system onto these infected endocardial lesions, are activated to express TFA (1–3, 44). This result will intensify the deposit of fibrin clots on the endocardial surface (2, 3, 44), ultimately causing heart dysfunction.
ACKNOWLEDGMENTS
We thank coworkers of the Department of Gynecology, Leiden University Medical Center, Leiden, The Netherlands, for providing human umbilical cords and especially J. S. van de Gevel for assistance in the preparation of EC cultures.
Part of this study was supported by grants 3.2.13 and 3.2.15 from the Institute for Radiopathology and Radiation Protection, J. A. Cohen Institute, Leiden, The Netherlands.
REFERENCES
- 1.Bancsi M J L F M, Thompson J, Bertina R M. Stimulation of monocyte tissue factor expression in an in vitro model of bacterial endocarditis. Infect Immun. 1994;62:5669–5672. doi: 10.1128/iai.62.12.5669-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancsi M J L F M, Veltrop M H A M, Bertina R M, Thompson J. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetations in rabbits infected with Streptococcus sanguis. Infect Immun. 1996;64:448–451. doi: 10.1128/iai.64.2.448-451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancsi M J L F M, Veltrop M H A M, Bertina R M, Thompson J. Role of monocytes and bacteria in Staphylococcus epidermidis endocarditis. Infect Immun. 1998;66:448–450. doi: 10.1128/iai.66.2.448-450.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer A S, Scheld W M. Endocarditis and intravascular infections. In: Mandell G L, Bennett J E, Dolin M, editors. Mandell, Douglas, and Bennett's principals and practices of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 857–902. [Google Scholar]
- 5.Becker R C, DiBello P M, Lucas F V. Bacterial tissue tropism: an in vitro model for infective endocarditis. Cardiovasc Res. 1987;21:813–820. doi: 10.1093/cvr/21.11.813. [DOI] [PubMed] [Google Scholar]
- 6.Beekhuizen H, Corsèl-van Tilburg A J, van Furth R. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J Immunol. 1990;145:510–518. [PubMed] [Google Scholar]
- 7.Beekhuizen H, van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993;54:363–378. [PubMed] [Google Scholar]
- 8.Beekhuizen H, van Furth R. Growth characteristics of cultured human macrovascular venous and arterial and microvascular endothelial cells. J Vasc Res. 1994;31:230–239. doi: 10.1159/000159048. [DOI] [PubMed] [Google Scholar]
- 9.Beekhuizen H, van de Gevel J S, Olsson B, van Benten I J, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 10.Beekhuizen H, van de Gevel J S. Endothelial cell adhesion molecules in inflammation and postischemic reperfusion injury. Transplant Proc. 1998;30:4251–4256. doi: 10.1016/s0041-1345(98)01405-5. [DOI] [PubMed] [Google Scholar]
- 11.Bevilacqua P M, Pober J S, Majeau G R, Fiers W, Cotran R S, Gimbrone M A. Recombinant tumor necrosis factor induces procoagulant activity in cultured vascular endothelium: characterization and comparison with the actions of interleukin-1. Proc Natl Acad Sci USA. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camerer E, Kolsto A-B, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 13.Chastre J, Trouillet J L. Early infective endocarditis on prosthetic valves. Eur Heart J. 1995;16:32–38. doi: 10.1093/eurheartj/16.suppl_b.32. [DOI] [PubMed] [Google Scholar]
- 14.Collins P W, Noble K E, Reittie J R, Hoffbrand A V, Pasi K J, Yong K L. Induction of tissue factor expression in human monocyte/endothelium cocultures. Br J Haematol. 1995;91:963–970. doi: 10.1111/j.1365-2141.1995.tb05420.x. [DOI] [PubMed] [Google Scholar]
- 15.Combe C, Duplàa C, Couffinhal T, Moreau C, Bonnet J. Induction of intercellular adhesion molecule-1 by monocyte adhesion to endothelial cells in human culture system. J Cell Physiol. 1995;164:295–303. doi: 10.1002/jcp.1041640210. [DOI] [PubMed] [Google Scholar]
- 16.Dackiw A P B, Nathens A B, Marshall J C, Rotstein O D. Integrin engagement induces monocyte procoagulant activity and tumor necrosis factor production via induction of tyrosine phosphorylation. J Surg Res. 1996;64:210–215. doi: 10.1006/jsre.1996.0330. [DOI] [PubMed] [Google Scholar]
- 17.De Fougerolles A R, Stacker S A, Schwarting R, Springer T A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991;174:253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond S M, Garcia-Aguilar J, Bickford J K, Corbi A L, Springer T A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eissner G, Lindner, H. H, Konur A, Kreutz M, Andreesen R, Holler E. Naïve monocytes can trigger transendothelial migration of peripheral blood cells through the induction of endothelial tumour necrosis factor-alpha. Scand J Immunol. 2000;51:251–261. doi: 10.1046/j.1365-3083.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- 20.Fan S-T, Mackman N, Cui M-Z, Edgington T S. Integrin regulation of an inflammatory effector gene. Direct induction of tissue factor promotor by engagement of β1 or α4 integrin chains. J Immunol. 1995;154:3266–3274. [PubMed] [Google Scholar]
- 21.Humphries M J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990;97:585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- 22.Leeuwenberg J F M, Jeunhomme T M A A, Buurman W A. Characterization of two monoclonal antibodies directed against an adhesion molecule on human endothelial cells. Transplant Proc. 1990;22:1991–1993. [PubMed] [Google Scholar]
- 23.Lewis J C, Jones N L, Hermanns M I, Rohrig O, Klein C L, Kirkpatrick C J. Tissue factor expression during coculture of endothelial cells and monocytes. Exp Mol Pathol. 1995;62:207–218. doi: 10.1006/exmp.1995.1023. [DOI] [PubMed] [Google Scholar]
- 24.Lo S K, Cheung A, Zheng Q, Silverstein R L. Induction of tissue factor on monocytes by adhesion to endothelial cells. J Immunol. 1995;154:4768–4777. [PubMed] [Google Scholar]
- 25.Lowy F D, Fant J, Higgins L L, Ogawa S K, Hatcher V B. Staphylococcus aureus-human endothelial cell interactions. J Ultrastruct Mol Struct Res. 1988;98:137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- 26.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 27.Mesri M, Plescia J, Altieri D C. Dual regulation of ligand binding by CD11b I domain. Inhibition of intercellular adhesion and monocyte procoagulant activity by a factor X-derived peptide. J Biol Chem. 1998;273:744–748. doi: 10.1074/jbc.273.2.744. [DOI] [PubMed] [Google Scholar]
- 28.Metzelaar M J, Sixma J J, Nieuwenhuis H K. Activation-dependent mAb recognizing a 140-kDa platelet α-granule membrane protein, expressed after activation. In: Knapp W, Dörken B, Gilks W R, Rieber E P, Schmidt R E, Stein H, von dem Borne A E G K, editors. Leucocyte typing IV. White cell differentiation antigens. Oxford, United Kingdom: Oxford University Press; 1989. pp. 1039–1040. [Google Scholar]
- 29.Morrissey J H, Fair D S, Edgington T S. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988;52:247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 30.Napoleone E, Di Santos A, Lorenzet R. Monocytes upregulate endothelial cell expression of tissue factor: a role for cell-cell contact and cross-talk. Blood. 1997;89:541–549. [PubMed] [Google Scholar]
- 31.Newman P J, Paddock C. CD31 cluster workshop report. In: Schlossman S F, Boumsell L, Gilks W, Harlan J M, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, et al., editors. Leucocyte typing V. White cell differentiation antigens. Oxford, United Kingdom: Oxford University Press; 1995. pp. 1259–1260. [Google Scholar]
- 32.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plescia J, Altieri D C. Activation of Mac-1 (CD11b/CD18)-bound factor X by released cathepsin G defines an alternative pathway of leucocytes initiation of coagulation. Biochem J. 1996;319:873–879. doi: 10.1042/bj3190873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto J, Eklund A, Pattaroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 35.Rainger G E, Wautier M-P, Nash G B, Wautier J-L. Prolonged E-selectin induction by monocytes potentiates the adhesion of flowing neutrophils to cultured endothelial cells. Br J Haematol. 1996;92:192–199. doi: 10.1046/j.1365-2141.1996.00308.x. [DOI] [PubMed] [Google Scholar]
- 36.Rao L V M, Pendurthi U R. Tissue factor on cells. Blood Coagul Fibrinolysis. 1998;9:S27–S35. [PubMed] [Google Scholar]
- 37.Rice G E, Bevilacqua M P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989;246:1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 38.Roberts R B. Streptococcal endocarditis. In: Kaye D, editor. Infective endocarditis. New York. N.Y: Raven Press; 1995. pp. 191–208. [Google Scholar]
- 39.Rothlein R, Dustin M L, Marlin S D, Springer T A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 40.Tekstra J, Beekhuizen H, van de Gevel J S, van Benten I J, Tuk C W, Beelen R H J. Infection of human endothelial cells with Staphylococcus aureus induces the production of monocyte chemotactic protein-1 and monocyte chemotaxis. Clin Exp Immunol. 1999;117:489–495. doi: 10.1046/j.1365-2249.1999.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchiya S, Yamabe M, Yamayuchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 42.Van der Meer J T, Thompson J, Valkenburg H A, Michel M F. Epidemiology of bacterial endocarditis in The Netherlands. Part I. Patient characteristics. Arch Intern Med. 1992;152:1863–1868. doi: 10.1001/archinte.152.9.1863. [DOI] [PubMed] [Google Scholar]
- 43.Veltrop M H A M, Beekhuizen H, Thompson J. Bacterial species- and strain-dependent induction of tissue factor in human vascular endothelial cells. Infect Immun. 1999;67:6130–6138. doi: 10.1128/iai.67.11.6130-6138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veltrop M H A M, Bancsi M J L M F, Bertina R M, Thompson J. Role of monocytes in experimental Staphylococcus aureus endocarditis. Infect Immun. 2000;68:4818–4821. doi: 10.1128/iai.68.8.4818-4821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vriesema A J M, Beekhuizen H, Hamdi M, Soufan A, Lammers A, Willekens B, Bakker O, Welten A G A, Veltrop M H A M, van de Gevel J S, Dankert J, Zaat S A J. Altered gene expression in Staphylococcus aureus upon interaction with human endothelial cells. Infect Immun. 2000;68:1765–1772. doi: 10.1128/iai.68.4.1765-1772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wharram B L, Fitting K, Kunkel S L, Remick D G, Merritt S E, Wiggins R C. Tissue factor expression in endothelial cell/monocyte cocultures stimulated by lipopolysaccharide and/or aggregated IgG: mechanisms of cell:cell communication. J Immunol. 1991;146:1437–1445. [PubMed] [Google Scholar]
- 47.Wright S D, Rao P E, Voorhis W C. Identification of the C3bi-receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80:5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao L, Bengualid V, Lowy F D, Gibbons J J, Hatcher V B, Berman J W. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao L, Lowy F D, Berman J W. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect Immun. 1996;64:3407–3409. doi: 10.1128/iai.64.8.3407-3409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zohlnhofer D, Brand K, Schipek K, Pogatsa-Murray G, Schomig A, Neumann F J. Adhesion of monocyte very late antigen-4 to endothelial vascular cell adhesion molecule-1 induces interleukin-1beta-dependent expression of interleukin-6 in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:353–359. doi: 10.1161/01.atv.20.2.353. [DOI] [PubMed] [Google Scholar]