Abstract
Our previous observations showed that mannoprotein (MP) induces early and massive production of interleukin-12 (IL-12) in vitro. This study was designed to investigate whether this phenomenon could be applied in vivo and to determine the biological significance of MP in Cryptococcus neoformans infection. The results reported here show that MP treatment induces IL-12 secretion by splenic macrophages and IL-12 p40 mRNA in the brain. During C. neoformans infection, MP reinforced IL-12 and IFN-γ secretion that coincided with enhanced antifungal activity of natural effector cells, early resolution of the inflammatory process, and clearance of fungal load from the brain. These studies show that MP is a key inflammatory mediator that induces a protective immune response against C. neoformans infection. This information can be used to facilitate the design of a rational approach to manipulate the immune response to C. neoformans.
Cryptococcus neoformans is an opportunistic encapsulated yeast that causes systemic infections, including fatal meningoencephalitis, in normal, diabetic, and immunocompromised subjects particularly in patients with AIDS (9), lymphoreticular malignancy (14), chronic renal failure, and organ transplants (12). Immunocompromised individuals suffering from cryptococcosis remain on antifungal therapy for life because drugs do not completely eradicate the organism from the body (11). Morbidity, mortality, and relapse rates are very high despite antifungal therapy, and survivors may encounter visual loss, cranial nerve palsies, and dementia (29).
The major virulence factors of C. neoformans are its elaborate polysaccharide capsule, melanin, mannitol, and mating type α (15). The three main capsule components of C. neoformans are glucuronoxylomannan (GXM), galactoxylomannan, and mannoprotein (MP) (4, 30). The principal constituent of capsular material, GXM, has been implicated in multiple fungal mechanisms that evade or weaken host defense (31, 33). Immunosuppressive properties of the cellular and humoral immune response have been described for GXM. In particular, inhibition of the delayed-type hypersensitivity response has been shown (19, 22), as well as proinflammatory cytokine secretion by phagocytic cells (32), specific T-cell response (18, 28), inhibition of leukocyte accumulation (10), and suppression of the specific antibody response (20).
In contrast to GXM, MP, a minor component of C. neoformans capsular material, may be considered as an immunopotentiating antigen involved in the induction of the cell-mediated immune response (1, 2, 7, 8, 19, 26). MP also promotes T-cell activation (24, 26) and induction of tumor necrosis factor alpha (TNF-α) secretion by monocytes (1). Recently we demonstrated that MP induces strong and early production of interleukin (IL)-12 by human monocytes, resulting in early induction of gamma interferon (IFN-γ) secretion by T cells (25). Previous data showed that IL-12 plays a pivotal role in the induction of the Th1 response against C. neoformans infection in a murine model and that development of the Th1 response is essential for protection (6). However, the role of MP in modulation of the immune response to C. neoformans has been scarcely explored. Because the majority of data on inflammatory properties of MP are from in vitro studies and given that these results cannot always be applied to an in vivo system, this study was designed to examine whether augmentation of anticryptococcal immune responses may be achieved in vivo and whether this effect has biological significance influencing the course of C. neoformans infection.
MATERIALS AND METHODS
Mice.
Female CD1 mice 4 to 6 weeks of age, were purchased from Charles River Breeding Laboratories (Calco, Lecco, Italy).
Microorganism.
C. neoformans 6995, a thinly encapsulated serotype A strain from the Central Bureau Schimmel Cultures, Delft, The Netherlands (CBS 6995 = NIH 37), was used in this study. The cultures were maintained by serial passage on Sabouraud agar (BioMérieux, Lyon, France). Log-phase yeasts were harvested by suspending a single colony in saline, counted on a hemocytometer, and adjusted to the desired concentration. Yeasts were killed by heating at 60°C for 30 min.
MP preparation.
An acapsular mutant of C. neoformans (NIH B-4131) was cultured in medium containing 2% glucose for 5 days at 35°C as previously described (4). The culture supernatant, containing MPs, was concentrated by ultrafiltration. Purification of MP (fraction II) was performed by affinity chromatography (concanavalin A) and anion-exchange chromatography (DEAE) (2). Anion-exchange chromatography of the MPs yielded two fractions, MP1 (35.6 kDa) and MP2 (8.2 kDa) (24). In this study MP2, which consists of 13.3% protein and 43% carbohydrate (2), was used. MP2 did not contain endotoxin, since no 2-keto-3-deoxyoctulosonic acid, determined by the thiobarbituric acid method of (35) and by the semicarbazide method of McGee and Doudoroff (17), was detected in the sample.
Maintenance of endotoxin-free conditions.
Preparations of the various cryptococcal components were negative for endotoxin contamination using a Limulus assay (Coatest Endotoxin; Kabi Diagnostica, Mölndal, Sweden) with a sensitivity of 25 pg of Escherichia coli lipopolysaccharide (LPS). Nevertheless, all different types of in vitro experiments were carried out at least once in the presence of 10 μg of polymyxin B (Sigma) per ml in order to neutralize any undetected contamination with bacterial LPS
C. neoformans infection.
C. neoformans yeasts from a 2-day culture on Sabouraud agar were washed with sterile endotoxin-free physiological saline, counted on a hemocytometer, and adjusted to the desired concentration for intravenous (i.v.) injection. The number of viable yeast cells injected was confirmed by culturing dilutions of the inoculum on Sabouraud agar. Cells, in 0.5 ml of prewarmed yeast cell suspension (4 × 106 yeast cells/ml), were injected i.v. into the tail vein using a 27-gauge needle.
Treatment of mice with MP.
Groups of 7 to 10 mice were injected intraperitoneally with 10 μg of MP in saline 24 h and 6 h before challenge with C. neoformans. Mice injected with saline alone served as controls.
Clearance of cryptococci from brain.
At designated times after infection, mice were sacrificed and brains were removed and homogenized. Serial 10-fold dilutions of each sample were plated in duplicate on Sabouraud agar plates and incubated at room temperature for 2 to 3 days and CFU were determined.
Preparation and stimulation of spleen cells.
Spleens were removed aseptically and placed in 5 ml of RPMI 1640, and single-cell suspensions were prepared. One portion was cultured to examine cytokine production from total spleen cells; the second portion was cultured for 2 h in RPMI 1640 plus 10% fetal calf serum at 37°C. After incubation, nonadherent cells were removed and the adherent cells were cultured to evaluate cytokine production. Total cells or adherent cells (20 × 106 cells/ml) were cultured in the presence or absence of MP (2.5 μg/ml) and/or C. neoformans (20 × 106 cells/ml) for 18 h in RPMI 1640 plus 10% fetal calf serum. After incubation, supernatants were recovered and stored at −80°C. Anticryptcoccal activity of splenic macrophages was evaluated by CFU inhibition assay as previously described (31). In selected experiments, splenic macrophages were treated with MP (10 μg/ml) or LPS (Sigma) and/or goat polyclonal anti-mouse antibody to CD14 (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) or irrelevant monoclonal antibody (MAb; Sigma).
Murine sera.
Mice were treated with 10 μg of MP 24 and 6 h before challenge with 2 × 106 C. neoformans cells. Blood samples were obtained 2, 8, 16, and 30 days after infection. Sera were recovered and stored at −80°C.
Determination of total IL-12 and IFN-γ production.
Cytokine levels in sera or culture supernatant fluids were measured with an enzyme-linked immunosorbent assay (ELISA) kit for mouse IL-12 p70 and mouse IFN-γ (Endogen Inc., Woburn, Mass.).
Histological examination.
Mice were sacrificed 15 days after i.v. infection with C. neoformans. Brains were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned for histological examination. The sections were stained with hematoxylin and eosin and examined microscopically.
RNA extraction.
RNA isolation from the brain was performed with Trizol reagent (Life Technologies, Grand Island, N.Y.); quantities referred to extracts made from 100 mg of tissue. Samples were homogenized with 1 ml of Trizol reagent using a glass homogenizer and incubated for 5 min at room temperature to allow complete dissociation of nucleoprotein complexes, followed by addition of 0.2 ml of chloroform. Tubes were vigorously shaken by hand for 15 s and incubated for 2 to 3 min at room temperature. Samples were centrifuged for 15 min at 12,000 × g at 2 to 8°C. RNA in the aqueous phase was recovered in an RNase-free tube, and 0.5 ml of isopropyl alcohol was added. Samples were incubated at room temperature for 10 min and centrifuged for 15 min at 12,000 × g at 2 to 8°C. The RNA pellet was washed with 75% ethanol (1 ml), vortexed, centrifuged at 10,000 × g for 5 min at 2 to 8°C, and air dried. The residue was dissolved in RNase-free water (Eppendorf) and stored at −80°C. RNA concentration was determined spectrophotometrically (optical density of 260), and integrity was verified by running on a denaturating formaldehyde agarose gel. DNase I was used according to the manufacturer's directions to prevent genomic contamination (amplification grade; Life Technologies).
cDNA synthesis.
Complementary DNA was synthesized from RNA (3 μg) using antisense primers for IL-12 p40 and β-actin (housekeeping gene) and reverse transcriptase (RT; Life Technologies) according to the manufacturer's instructions. Genomic DNA contamination was checked by subjecting samples to PCR without adding RT.
PCR amplification.
After the RT reaction, aliquots of 1.0 μl of cDNA were amplified, using IL-12 p40 and β-actin primers (10 μM). The expected sizes of the RT-PCR products were 617 bp for IL-12 p40 and 514 bp for β-actin. PCR for IL-12 p40 was carried out with 0.25 μl of AmpliTaq DNA polymerase (5 U/μl; Perkin-Elmer, Branchburg, N.J.), 1.0 μl of deoxynucleoside triphosphates (10 mM; Pharmacia, Uppsala, Sweden), 5 μl of Taq buffer (10×), 2.0 μl of both IL-12 p40 primers (sense [GAG GTG GAC TGG ACT CCC G] and antisense [CAA GTT CTT GGG CGG GTC TG]), and RNase-free water to a final volume of 50 μl. Amplification was performed on GeneAmp PCR System 2400 (Perkin-Elmer) using the following program: 95°C for 10 min (hot start), 35 cycles at 94°C for 30 s (denaturation), 62°C for 30 s (annealing), and 72°C for 45 s (extension), followed by 72°C for 10 min (final extension).
β-Actin PCR mix was the same except that β-actin primers (10 μM; sense [TGT GAT GGT GGG AAT GGG TCA G] and antisense [TTT GAT GTC ACG CAC GAT TTC C]) were used. Amplification started after a predenaturating step at 94°C for 90 s, 30 cycles at 94°C for 90 s (denaturation), 65°C for 30 s (annealing), and 72°C for 45 s (extension), followed by a final extension at 72°C for 7 min. The β-actin gene was generally found to be expressed at higher levels than the target gene.
PCR products were analyzed by electrophoresis on 2% agarose gels stained with ethidium bromide to quantify the size of the banding pattern, and 0.5 μg of a 100-bp DNA ladder (M-Medical Genenco, Florence, Italy) was run in parallel. Gels were scanned on a densitometer and analyzed using Molecular Analyst.
Statistical analysis.
Statistical significance was calculated using Student's paired t test. Results are presented as means ± standard deviations (SD).
RESULTS
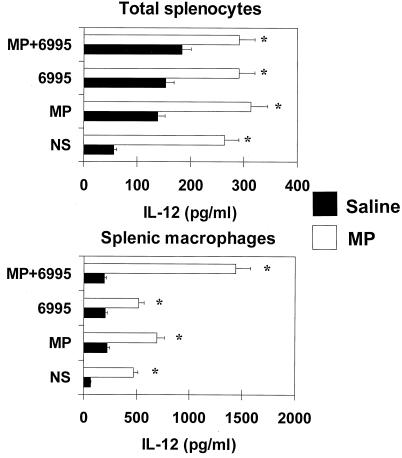
To evaluate whether MP was able to stimulate host defense against an encapsulated strain of C. neoformans (6995), MP (10 μg in saline) was injected i.p. into mice. Spleens were removed aseptically, and IL-12 p70 secretion was determined in supernatant fluids of splenocytes cultured for 18 h. The results reported in Fig. 1 show that 20 × 106 splenocytes from saline-treated mice produced IL-12 when stimulated in vitro with MP, 6995, or MP plus 6995. Inoculation of MP greatly increased IL-12 secretion by splenocytes with respect to saline. In addition, splenocytes from MP-treated mice produced significantly higher levels of IL-12 when restimulated in vitro with all stimuli. To verify whether macrophages were the major biological source of IL-12, 20 × 106 splenic macrophages were separated from total splenocytes. The results reported in Fig. 1 show an higher IL-12 levels in splenic macrophages than in unseparated cells. This enhancement was particularly evident after in vitro restimulation with MP plus 6995 (P <0.001, macrophages versus unseparated cells).
FIG. 1.
Effect of MP on IL-12 production by total splenocytes and splenic macrophages. Mice were treated with saline or 10 μg of MP and sacrificed 24 h later. Total splenocytes or splenic macrophages (20 × 106) were recovered and stimulated with MP (2.5 μg/ml) and/or C. neoformans 6995 (20 × 106 cells/ml) for 18 h. IL-12 levels in culture supernatants were evaluated by ELISA. The results are means ± SD of three separate experiments. NS, not stimulated. ∗, P < 0.001 (MP-treated versus saline-treated cells).
To verify whether the effect of MP was due to possible endotoxin contamination, experiments using splenic macrophages from MP-treated or untreated mice were carried out in the presence or absence of polymyxin B (10 μg/ml) added along with stimuli. The results showed that polymyxin B addition did not affect significantly MP-induced IL-12 secretion. In contrast, polymyxin B abrogated LPS-induced IL-12 secretion (data not shown). In addition, further experiments were performed to exclude that endotoxin contamination may participate in the MP-induced effect by saturating CD14 receptors, which are the natural ligands of LPS. To this end, splenic macrophages were treated with a MAb to CD14 (2 μg/ml) or irrelevant MAb (2 μg/ml), and then MP or LPS (100 ng/ml) was added. After 6 h or incubation, supernatants were harvested and IL-12 was determined. The results showed that the MAb to CD14, like the irrelevant MAb, did not affect MP-induced IL-12 p70 production (230 ± 14 pg/ml [MP-treated cells] versus 200 ± 15 pg/ml [anti-CD14 plus MP-treated cells]). In contrast, it abrogated the LPS-induced effect, while the irrelevant MAb did not (480 ± 30 pg/ml [LPS-treated cells] versus 80 ± 10 pg/ml [LPS plus MAb to CD14-treated cells]).
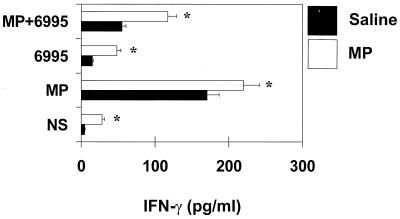
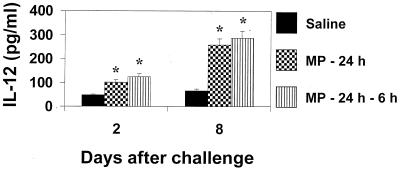
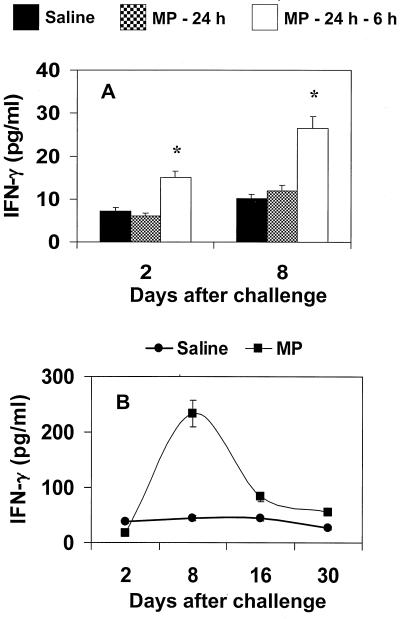
Previous studies in vitro showed that IL-12 induces early production of IFN-γ (25); thus, cytokine production by splenocytes from MP-treated mice was determined. Prompt secretion of IFN-γ was observed by splenocytes from MP-treated mice restimulated in vitro with MP or C. neoformans (Fig. 2). The combined treatment of MP plus C. neoformans produced a significant increase of IFN-γ secretion compared to stimulation with C. neoformans alone, although levels were significantly lower than those obtained with MP alone (P < 0.001). Having established that MP is able to induce IL-12 in vivo, the possibility that MP could promote a protective response against subsequent challenge with virulent C. neoformans was evaluated. Mice were pretreated with MP and then challenged with C. neoformans. After various days, splenocytes were cultured for 18 h without stimuli, and supernatant fluids were tested for IL-12 and IFN-γ production. The results reported in Fig. 3 show that splenocytes from C. neoformans-treated mice produced IL-12 spontaneously 2 days after challenge; however, more than a twofold increase in IL-12 levels was observed when mice were pretreated with MP. Kinetic studies showed that IL-12 was secreted in an early phase (day +2) in response to challenge, but greatest production was observed in the late phase 8 days after challenge. Maximum IL-12 production was observed in splenocytes from mice pretreated with MP. The increased production of IL-12 paralleled a strong enhancement of IFN-γ secretion. The kinetics of IFN-γ production by splenocytes showed greatest production 8 days-after challenge. This enhancement was maximum when MP was inoculated twice at 24 and 6 h before challenge with C. neoformans (Fig. 4A). IFN-γ determination in serum showed that, as observed for splenocytes, a maximum level was reached 8 days after challenge (Fig. 4B).
FIG. 2.
Effect of MP on IFN-γ production by total splenocytes. Mice were treated with saline or 10 μg of MP and sacrificed 24 h later. Splenocytes (20 × 106) were recovered and stimulated with MP (2.5 μg/ml) and/or C. neoformans 6995 (20 × 106 cells/ml) for 18 h. IFN-γ levels in culture supernatants were evaluated by ELISA. The results are means ± SD of three separate experiments. NS, not stimulated. ∗, P < 0.001 (MP-treated versus saline-treated cells).
FIG. 3.
Effect of MP on IL-12 production by total splenocytes. Mice were treated with saline or 10 μg of MP at 24 h or at 24 and 6 h before challenge with C. neoformans 6995 (2 × 106 cells/mouse). Two and 8 days after challenge, splenocytes (20 × 106 cells/ml) were recovered and incubated at 37°C in 5% CO2 for 18 h. IL-12 levels in culture supernatants were evaluated by ELISA. IL-12 levels from MP-treated (−24 h or −24 h −6 h) unchallenged mice were 90 ± 8 pg/ml 2 days after treatment and undetectable 8 days later. The results are means ± SD of three separate experiments. ∗, P < 0.001 (MP-treated versus saline-treated cells).
FIG. 4.
Effect of MP on IFN-γ production by total splenocytes (A) and in sera (B). (A) Mice were treated with saline or 10 μg of MP at 24 h or at 24 and 6 h before challenge with C. neoformans 6995 (2 × 106 cells/mouse). After 2 and 8 days, splenocytes (20 × 106 cells/ml) were recovered and incubated at 37°C in 5% CO2 for 18 h. ∗, P < 0.001 (MP-treated versus saline-treated cells). (B) Mice were treated with saline or 10 μg of MP at 24 and 6 h before challenge with C. neoformans 6995 (2 × 106 cells/mouse). IFN-γ levels in culture supernatants and in sera were evaluated by ELISA. The results are means ± SD of three separate experiments. ∗, P < 0.001 (MP-treated versus saline-treated mice).
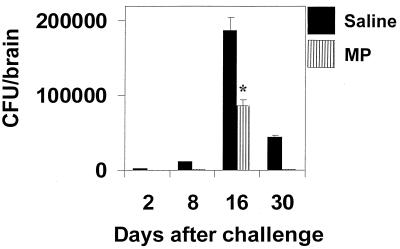
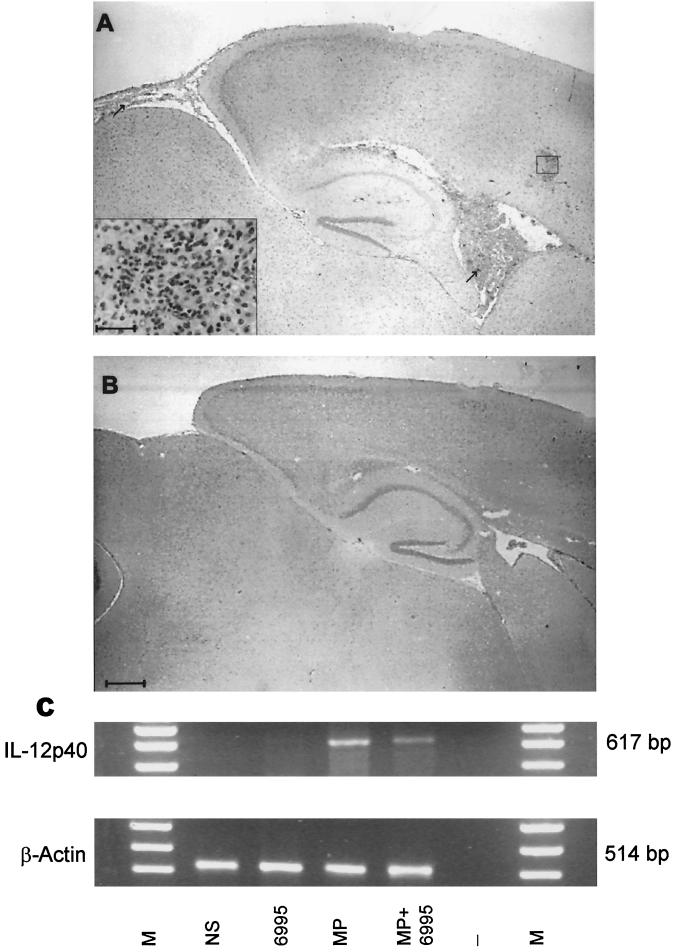
To evaluate whether the increased amount of IFN-γ reflected increased capability of macrophages to kill C. neoformans, antifungal activity of splenic macrophages from MP-treated mice challenged with C. neoformans was evaluated. A significant increase (P < 0.05) of macrophage anticryptococcal activity from MP-treated mice (percentage of killing activity, 66 ± 5) was detected with respect to saline-treated mice (percentage of killing activity, 45 ± 5). To evaluate whether these effects were related to induced clearance of C. neoformans from colonized tissue, CFU recovery from the whole brain, the target organ for C. neoformans, was determined. The results reported in Fig. 5 show that MP treatment drastically reduced brain colonization, favoring early clearance of the fungus. This is consistent with results from histological analysis, indicating that the inflammatory process in the brain of MP-treated mice is completely resolved after 20 days. Conversely, in mice not treated with MP, this process persisted. A representative portion of the brain from an untreated mouse 20 days after challenge shows an inflammatory reaction in the ventricular space as well as in the parenchymal and meningeal areas (Fig. 6A), whereas the brain section from an MP-treated mouse shows complete resolution of the inflammatory process (Fig. 6B). The facilitated clearance of C. neoformans and the prompt disappearance of the inflammatory process from the brain correlated with induction of IL-12 p40 mRNA at the cerebral level. IL-12 p40 mRNA was found in brain tissues of mice treated with MP once 24 h before or twice, 6 and 24 h, before mRNA detection. In contrast, no IL-12 p40 mRNA was detected in saline-treated mice (Fig. 6C).
FIG. 5.
Effect of MP on viable C. neoformans 6995 cells in the entire brain. Mice were treated with saline or 10 μg of MP at 24 and 6 h before challenge with C. neoformans 6995 (2 × 106 cells/mouse). At different times, mice were killed and viable yeasts in the brain (CFU) were counted by plating samples of homogenized tissue on Sabouraud agar. Data are means ± SD of four to five mice. ∗, P < 0.001 (MP-treated versus saline-treated mice).
FIG. 6.
Photomicrographs of parasagittal brain sections showing the effect of MP treatment on C. neoformans-challenged mice. Mice received MP treatment twice, 24 and 6 h before challenge. Twenty days after infection mice, were sacrificed and histopathological analysis was performed on periodic acid-Schiff-stained brain sections. Brain sections of challenged (A) and MP-treated and challenged (B) mice. Bar in panel B (applies also to panel A), 400 μm; bar in inset in panel A, 25 μm. Arrows (A) indicate the inflammatory reaction on ventricular and submeningeal spaces. (C) Analysis of IL-12 gene expression by brain homogenates from MP-treated or MP-treated and challenged mice. M, DNA markers; NS, not stimulated; 6995, mice challenged with strain 6995; MP, MP-treated mice; MP+6995, MP-treated and challenged mice; -, no DNA added to the amplification system. The results shown are from a representative experiment of three performed with similar results.
DISCUSSION
MP is a minor carbohydrate antigen contained in the cell envelope of C. neoformans. It has been recovered in soluble form in supernatant fluids of replicating C. neoformans cells (3) and is associated to the fungus cell wall (5, 26). Studies have emphasized the potential effect of MP as an inducer of proinflammatory cytokine secretion from peripheral blood mononuclear cells (7) or of the cell-mediated immune response (21). The present study describes two key features of MP immunoregulation: (i) induction of the Th1 response and (ii) its association with prompt recovery from C. neoformans infection.
The present study extends our prior study (25) on MP-induced IL-12 secretion by peripheral blood mononuclear cells and provides in vivo relevance in terms of IL-12 promotion, Th1 response, and anticryptococcal activity of macrophages. In addition, it shows that the onset of Th1 coincides with the appearance of IL-12 mRNA and reduction of C. neoformans load in the brain.
The analysis revealed two underlying aspects of C. neoformans immunity that had not been previously appreciated: (i) purified MP elicited a protective response to C. neoformans by promoting early release of IL-12 from a lymphoid organ (i.e., the spleen) and IL-12 mRNA from the brain; and (ii) early IL-12 production paralleled early induction of IFN-γ that coincided with activation of natural effector cells, resolution of the inflammatory process, and clearance of the fungus from the brain.
IL-12 was secreted to a greater extent by splenic macrophages after treatment with MP than total splenic cells (Fig. 1). This could be due to inhibitory factors such as IL-10 release by lymphocytes. A subsequent challenge with C. neoformans reinforced induction of IL-12. Indeed, splenic macrophages from C. neoformans-treated mice secreted IL-12 that reached a maximum 8 days after infection, although an increase of IL-12 production was observed in infected mice pretreated with MP. These data are consistent with our previous observations in vitro showing that IL-12 is produced in both a T-cell-independent and a T-cell-dependent manner and that the latter represents the maximum production (27). Our data show that the observed effect has in vivo relevance occurring during infection and provides evidence that encapsulated C. neoformans may diminish the MP effect. This is suggested by the fact that C. neoformans in combination with MP significantly reduces MP-induced IFN-γ secretion. Consistent with this inhibitory effect exerted by C. neoformans, brains of mice treated with MP and challenged with C. neoformans appear to express lower levels of IL-12 mRNA than brain of mice treated with MP alone. The inhibitory activity exerted by C. neoformans may be ascribed to an inhibitory factor such as IL-10 released by lymphocytes (28).
The fact that the greatest IL-12 production occurs when the level of IFN-γ is maximum, 7 days after infection, suggests a cytokine loop. It is conceivable that infection with C. neoformans induces per se a protective Th1 response in the immunocompetent host. This is consistent with the feeble ability of C. neoformans to induce infection in the immunocompetent host. However, pretreatment with MP reinforces onset of the Th1 response that is essential for protection (6, 13). The biological significance of this phenomenon was demonstrated by monitoring the kinetics of yeast clearance from the brain and examining inflammatory lesions within. The early reduction of yeast load was observed in the brains of MP-treated mice and corresponded with prompt resolution of the inflammatory process. A possible explanation may be ascribed to MP immune stimulation that favors increased clearance of C. neoformans likely by inducing activation of phagocytic cells via IFN-γ production. Alternatively, MP treatment induced activation directly in the brain compartment, producing a strong reduction of yeast load consistent with the presence of IL-12 mRNA in the brains of MP-treated mice. We cannot exclude the possibility that the two mechanisms work together to eliminate the fungus from the target organ.
In vitro studies have provided information on immunoregulation by MP. In particular, TNF-α induction has been shown in monocytes stimulated with MP; MP promotes TNF-α and IL-12 secretion by human monocytes in vitro (1, 25, 34). Activation of T cells induced by MP has been observed (24–26), while the effect of MP in the in vivo system has been scarcely explored. Murphy's group demonstrated MP-induced delayed-type hypersensitivity: an IFN-γ-dependent reactivity (19, 23) that may be a positive stimulation given that cell-mediated immunity is an essential defense mechanism against C. neoformans infection (16). This is the first demonstration that MP treatment (i) promotes a Th1 response showing the effect observed in vitro (25) and (ii) operates in vivo by inducing a host-protective response against C. neoformans infection.
These results indicate that MP antigen may counterbalance the immunosuppressive effect of C. neoformans capsular material and provide suggestions to design strategies evoking or reinforcing the protective response to prevent or cure cryptococcosis.
ACKNOWLEDGMENTS
Many thanks go to Eileen Mahoney Zannetti for dedicated editorial and secretarial support and to Gabriella Guelfi and Carla Barabani for technical assistance.
This study was supported by the National Research Program on AIDS, contract 50C.32, Rome, Italy.
REFERENCES
- 1.Chaka W, Verheul A F, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman A I. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 1997;159:2979–2985. [PubMed] [Google Scholar]
- 2.Chaka W, Verheul A F, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman A I. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect Immun. 1997;65:272–278. doi: 10.1128/iai.65.1.272-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherniak R. Soluble polysaccharides of Cryptococcus neoformans. Curr Top Med Mycol. 1988;2:40–54. doi: 10.1007/978-1-4612-3730-3_2. [DOI] [PubMed] [Google Scholar]
- 4.Cherniak R, Reiss E, Turner S H. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;125:343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- 5.Cherniak R, Sundstrom J B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delfino D, Cianci L, Lupis E, Celeste A, Petrelli M L, Curro F, Cusumano V, Teti G. Interleukin-6 production by human monocytes stimulated with Cryptococcus neoformans components. Infect Immun. 1997;65:2454–2456. doi: 10.1128/iai.65.6.2454-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delfino D, Cianci L, Migliardo M, Mancuso G, Cusumano V, Corradini C, Teti G. Tumor necrosis factor-inducing activities of Cryptococcus neoformans components. Infect Immun. 1996;64:5199–5204. doi: 10.1128/iai.64.12.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dismukes W E. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988;157:624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z M, Murphy J W. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect Immun. 1995;63:770–778. doi: 10.1128/iai.63.3.770-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay R J. Yeast infections. Dermatol Clin. 1996;14:113–124. doi: 10.1016/s0733-8635(05)70331-4. [DOI] [PubMed] [Google Scholar]
- 12.Howard R J, Simmons R L, Najarian J S. Fungal infections in renal transplant recipients. Ann Surg. 1978;188:598–605. doi: 10.1097/00000658-197811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huffnagle G B, Lipscomb M F. Cells and cytokines in pulmonary cryptococcosis. Res Immunol. 1998;149:387–396. doi: 10.1016/s0923-2494(98)80762-1. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan M H, Rosen P P, Armstrong D. Cryptococcosis in a cancer hospital: clinical and pathological correlates in forty-six patients. Cancer. 1977;39:2265–2274. doi: 10.1002/1097-0142(197705)39:5<2265::aid-cncr2820390546>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Kozel T R. Virulence factors of Cryptococcus neoformans. Trends Microbiol. 1995;3:295–299. doi: 10.1016/s0966-842x(00)88957-x. [DOI] [PubMed] [Google Scholar]
- 16.McGaha T, Murphy J W. CTLA-4 down-regulates the protective anticryptococcal cell-mediated immune response. Infect Immun. 2000;68:4624–4630. doi: 10.1128/iai.68.8.4624-4630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee J, Doudoroff M. A new phosphorylated intermediate in glucose oxidation. J Biol Chem. 1954;210:617–629. [PubMed] [Google Scholar]
- 18.Mody C H, Syme R M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–469. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy J W. Influence of cryptococcal antigens on cell-mediated immunity. Rev Infect Dis. 1988;10(Suppl. 2):432–435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- 20.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy J W, Mosley R L, Cherniak R, Reyes G H, Kozel T R, Reiss E. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect Immun. 1988;56:424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy J W, Pahlavan N. Cryptococcal culture filtrate antigen for detection of delayed-type hypersensitivity in cryptococcosis. Infect Immun. 1979;25:284–292. doi: 10.1128/iai.25.1.284-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy J W, Schafer F, Casadevall A, Adesina A. Antigen-induced protective and nonprotective cell-mediated immune components against Cryptococcus neoformans. Infect Immun. 1998;66:2632–2639. doi: 10.1128/iai.66.6.2632-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orendi J M, Verheul A F, De Vos N M, Visser M R, Snippe H, Cherniak R, Vaishnav V V, Rijkers G T, Verhoef J. Mannoproteins of Cryptococcus neoformans induce proliferative response in human peripheral blood mononuclear cells (PBMC) and enhance HIV-1 replication. Clin Exp Immunol. 1997;107:293–299. doi: 10.1111/j.1365-2249.1997.283-ce1169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitzurra L, Cherniak R, Giammarioli M, Perito S, Bistoni F, Vecchiarelli A. Early induction of interleukin-12 by human monocytes exposed to Cryptococcus neoformans mannoproteins. Infect Immun. 2000;68:558–563. doi: 10.1128/iai.68.2.558-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitzurra L, Vecchiarelli A, Peducci R, Cardinali A, Bistoni F. Identification of a 105 kilodalton Cryptococcus neoformans mannoprotein involved in human cell-mediated immune response. J Med Vet Mycol. 1997;35:299–303. [PubMed] [Google Scholar]
- 27.Retini C, Casadevall A, Pietrella D, Monari C, Palazzetti B, Vecchiarelli A. Specific activated T cells regulate IL-12 production by human monocytes stimulated with Cryptococcus neoformans. J Immunol. 1999;162:1618–1623. [PubMed] [Google Scholar]
- 28.Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel T R. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66:664–669. doi: 10.1128/iai.66.2.664-669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer E D, Spitzer S G, Freundlich L F, Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993;341:595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- 30.Turner S H, Cherniak R, Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr Res. 1984;125:343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- 31.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walenkamp A M, Chaka W S, Verheul A F, Vaishnav V V, Cherniak R, Coenjaerts F E, Hoepelman A M. Cryptococcus neoformans and its cell wall components induce similar cytokine profiles in human peripheral blood mononuclear cells despite differences in structure. FEMS Immunol Med Microbiol. 1999;26:309–318. doi: 10.1111/j.1574-695X.1999.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 35.Weissbach A, Hurwitz J. The formation of 2-keto-3-deoxyhepeptonic acid in extracts of Escherichia coli B. J Biol Chem. 1959;234:705–709. [PubMed] [Google Scholar]