Abstract
Adhesion interactions during hematogenous dissemination of Candida albicans likely involve a complex array of host and fungal factors. Possible C. albicans factors include changes in cell surface hydrophobicity and exposed antigens that have been shown in static adhesion assays to influence attachment events. We used a novel in vitro shear analysis system to investigate host-pathogen interactions and the role of fungal cell surface hydrophobicity in adhesion events with human endothelial cells under simulated physiologic shear. Endothelial monolayers were grown in capillary tubes and tested with and without interleukin-1β activation in buffered medium containing human serum. Hydrophobic and hydrophilic stationary-phase C. albicans yeast cells were infused into the system under shear flow and found to adhere with widely varying efficiencies. The average number of adherent foci was determined from multiple fields, sampled via video microscopy, between 8 and 12 min after infusion. Hydrophobic C. albicans cells demonstrated significantly more heterotypic binding events (Candida-endothelial cell) and greater homotypic binding events (Candida-Candida) than hydrophilic yeast cells. Cytokine activation of the endothelium significantly increased binding by hydrophobic C. albicans compared to unactivated host cells. Preincubation of hydrophobic yeast cells with a monoclonal antibody against hydrophobic cell wall proteins significantly blocked adhesion interactions with the endothelial monolayers. Because the antibody also blocks C. albicans binding to laminin and fibronectin, results suggest that vascular adhesion events with endothelial cells and exposed extracellular matrix may be blocked during C. albicans dissemination. Future studies will address the protective efficacy of blocking or redirecting blood-borne fungal cells to favor host defense mechanisms.
While multiple host and fungal factors contribute to development of disseminated candidiasis (reviewed in references 39 and 44), the capacity of Candida albicans to adhere to many different host tissues is broadly considered a virulence trait to initiate invasive activity (12, 29). The dissemination process likely begins by fungal cells gaining access to the bloodstream through gastrointestinal persorption, by seeding from a biofilm-fouled intramedic device, or through trauma-related inoculation (10, 23, 49). Exit from the vasculature is thought to occur by penetration through endothelial cells lining the vessels, except for possible direct attachment to extracellular matrix (ECM) components that are normally exposed in kidney glomerular regions or exposed during vascular damage or inflammation (35). Thus, successful attachment of C. albicans cells to vascular endothelial cells or exposed ECM during hematogenous distribution appears crucial for subsequent tissue invasion and development of organ pathologies (34).
Adhesion interactions between C. albicans and host vascular tissues may be very efficient, as murine models of dissemination indicate that intravenously administered yeast cells rapidly disappear from circulating blood (13). Static adhesion assays have described C. albicans (and Candida spp.) molecules that could facilitate binding during dissemination. They include integrin analogues (reviewed in references 22 and 29); ligands for CD11b and CD18 (17); mannosylated and nonmannosylated components that bind endothelial cells (14–16) or spleen and lymph node macrophages (32, 37); ALS gene products expressed in vivo (30) that promote adhesion to endothelial cells, epithelial cells, and ECM proteins (18, 19); ECM-binding mannoproteins (reviewed in reference 8), including an α5β1 integrin-like fibronectin receptor (50); and ECM-binding hydrophobic surface proteins (40, 52).
Based on the above list, Candida may follow an emerging theme for microbial pathogenesis in the utilization of host cell adhesion molecules and receptor ligands, either directly or through molecular mimicry, to anchor invasive activities (reviewed in references 38, 47, and 54). A major consideration for defining vascular adhesins of C. albicans is the variability of surface antigens (reviewed in reference 8) and the associated changes in cell surface hydrophobicity (CSH) that occur during normal growth and morphogenesis of C. albicans, both in vivo and in vitro (21, 24, 27). Adhesion studies show, either directly or by inference from the stated growth conditions, that hydrophobic C. albicans cells adhere better and with greater site diversity than hydrophilic cells to endothelial cells, epithelial cells, ECM proteins, and other host tissues (8, 11, 16, 20, 25). Thus, surface antigenic changes related to hydrophobicity may provide a fungal virulence strategy for evasion of immune responses and for selective adhesion interactions with host cells.
Our interest in defining the role of CSH on adhesion interactions with endothelial cells during vascular dissemination led us to use a novel assay system (ProteoFlow; LigoCyte Pharmaceuticals, Inc., Bozeman, Mont.) that was developed for studying leukocyte interactions with vascular endothelium under simulated physiological shear (3). With system adaptations for host-pathogen interactions, analysis of C. albicans binding showed rapid interactions with human endothelial cells under physiologic shear. Both C. albicans CSH status and the endothelial activation status influenced the number of host-pathogen interactions. Hydrophobic C. albicans binding in the vascular modeling system could be blocked by one of the same anti-hydrophobic protein monoclonal antibodies (MAbs) that blocked attachment to ECM proteins (40). These studies provide new information about the dynamics of C. albicans-host adherence phenomena that may well have important implications in design of novel therapeutic approaches against candidiasis.
MATERIALS AND METHODS
C. albicans isolates and culture conditions.
C. albicans A9 (55), LGH1095 (1), and ATCC 90029 were maintained as −80°C glycerol stocks and were subcultured aerobically at 23 and 37°C in 2% glucose–0.3% yeast extract–1% peptone broth (GYEP), in 0.055 M sodium phosphate (pH 7.2)-buffered yeast nitrogen base plus amino acids (Difco) containing 2% glucose (YNB2G), or in antibiotic medium 3 (Difco) with 2% glucose (AM3-2G). Stationary-phase cultures were harvested from a third subculture (1) and washed three times in cold, sterile distilled H2O, the concentration was determined, and CSH was assessed by the hydrophobic microsphere assay (28). Stationary-phase yeast cultures grown at 23°C were hydrophobic (typical CSH values ≥ 92%), while those grown at 37°C were hydrophilic (typical CSH values ≤ 8%). Harvested yeast aliquots were held on ice as pellets and used within 4 h. We also determined for each yeast population the sphere-to-cell unit (S:C) ratio, which is a measurement reflecting the abundance of singlet blastoconidia (24). For example, a mother-daughter combination would be two spheres but one contiguous cell unit. S:C ratios of ≤2:1 reflect stationary-phase yeast cultures and were important for establishing the amount of Candida-Candida adhesion observed in these assays.
MAb reagents.
The anti-Candida MAbs and control MAbs used in this study are listed in Table 1. The hydrophobicity and cell wall location of the Candida antigens are previously noted in work describing separations of cell wall components by hydrophobic interaction chromatography and generation of polyclonal antiserum against the hydrophobic protein fractions for tracking in vivo expression (21, 27). The MAbs to C. albicans hydrophobic proteins were produced at the University of Virginia Health System Lymphocyte Culture Center (9) as described elsewhere (40). Serum-free antibody preparations of MAbs 6C5, 5D8, and EL246 were produced by LigoCyte Pharmaceuticals from hybridoma cultures grown in serum-free tissue culture medium, followed by ammonium sulfate precipitation of supernatant fluids and exhaustive dialysis against phosphate-buffered saline. The control immunoglobulin G2a (IgG2a) antibody, UPC-10 (M9144; Sigma Chemical), was κ haplotype similar to MAbs 6C5 and 5D8.
TABLE 1.
MAb reagents used in this study
| Antibody | Isotype, prepn | Antigen specificity | Reference or source |
|---|---|---|---|
| MAb 6C5 | IgG2a, serum free | 38-kDa hydrophobic protein, C. albicans | 40 |
| MAb 5D8 | IgG2a, serum free | 37-kDa hydrophobic protein, C. albicans | 40 |
| EL246 | IgG1, serum free | E- and L-selectins | 3 |
| UPC-10 | IgG2a, purified from ascites fluid | β-2,6-Linked fructosan | Sigma Chemical Co. |
In vitro shear analysis of C. albicans adhesion to HUVECs.
An in vitro flow system (ProteoFlow; LigoCyte Pharmaceuticals) to analyze adhesion events under simulated physiologic shear across mammalian cells or tissue components was used as described previously (3), with modification for the Candida work as follows. Human umbilical vein endothelial cells (HUVECs) were harvested and prepared as previously described (3) or obtained commercially (Clonetics, San Diego, Calif.). HUVEC monolayers were grown on the lumenal surface of glass capillary tubes. Some monolayers were pre-activated for 1 h with interleukin-1β (IL-1β; 10 ng/ml; Genzyme Diagnostics, Cambridge, Mass.) to upregulate expression of cellular adhesion molecules like E-selectin (reviewed in reference 33), rinsed, and placed in fresh medium for 120 min prior to the assay. Each capillary tube was inspected, and only those having satisfactory monolayer development (>75%) along the tube length were used for the assays.
C. albicans yeast cells were suspended in medium alone (HEPES-buffered Hanks' balanced salts solution, plus Ca2+ and Mg2+, containing 5% human serum) or suspended in medium containing the test or control MAbs (60 μg/ml) and incubated in an ice-water slurry for 10 min. Antibody concentrations for the serum-free preparations were selected based on past inhibition studies involving ECM (40) and are expressed as total micrograms of protein per milliliter in the final shear assay volume. Yeast cell suspensions were infused into the ProteoFlow system under high flow rates (4 to 5 dynes/cm2) at a final concentration of 0.5 × 107 to 1.0 × 107 spheres/ml of medium (maintained at 37°C). The ProteoFlow system integrates an inverted microscope equipped with stage heater, Hoffman optics, and high-resolution video capture equipment. Video recording was initiated, and after 1 min, the flow rate was adjusted to 1 to 2 dynes/cm2, which is at the lower end of the 0- to 20-dyne/cm2 physiological range for vascular beds (reviewed in references 36 and 45). Introduction of test cells or compounds under the high rather than the lower flow rate guards against bolus-driven interactions occurring under physiologic shear and promotes even distribution of test cells in the system. Video data capture of the Candida binding was done between 8 and 12 min from the time of yeast cell infusion by recording multiple, nonoverlapping fields (n ≥ 10 fields per capillary) along the HUVEC monolayer to document numbers of attached yeast cells. For recording each field of view, the microscope was adjusted through multiple focal planes to ensure distinction of yeast bound to the HUVEC surface. Video records were analyzed offline, and the internal time stamp on each video frame was used to identify the respective fields of view.
Evaluation of C. albicans binding.
Two types of binding events were observed for C. albicans in the ProteoFlow system. Heterotypic binding events were defined as Candida-to-HUVEC adhesion interactions that produced focal attachment sites. The average number of foci per n fields of view was calculated for each assay. Homotypic binding events were defined as Candida-to-Candida interactions that occurred when a yeast cell from bulk fluid flow attached to an already bound yeast cell at a focal attachment site. To record the amount of homotypic binding events under physiologic shear, each focus in a field of view was evaluated for the number of blastoconidia attached at that site and recorded as ranked values of 1, 2, 3, 4, 5 to 9, 10 to 15, and ≥16 blastoconidia per focal site. The ranks with three or more attached blastoconidia were considered the result of homotypic binding based on the S:C ratios of the Candida populations used for these shear experiments. To compare the amounts of homotypic binding between treatment and control assays, the average number of foci in the ≥3 blastoconidial ranks for n fields was determined. In some assays, the average number of total blastoconidia per field was estimated from ranked data, using values of 7, 12.5, and 16 spheres for the number of bound blastoconidia in the three larger ranks.
SigmaPlot version 4.01 and SigmaStat version 2.0 (SPSS Inc., Chicago, Ill.) were used for most graphing (and exported to Excel 97 [Microsoft]) and all statistical analyses of experiments that were performed at least in duplicate. Student t tests (two tailed) were used for normally distributed data sets; those failing normality or equal variance were analyzed with the Mann-Whitney rank sum test, the Kruskal-Wallis analysis of variance on ranks, or the Tukey or Dunn method for multiple comparisons where noted in the text.
RESULTS
Adaptation of in vitro shear system for adhesion studies with Candida.
The ProteoFlow system was tested as a means of investigating host-pathogen adhesion interactions under simulated physiologic shear. Several methodological variables were addressed before the shear assay could be used to evaluate C. albicans binding to endothelial cells under shear flow.
Preliminary studies showed that the data capture could be performed either by monitoring a single field of view over time or by sampling multiple fields within a given period during the assay. The second data capture method was chosen to provide multiple sample fields for statistical evaluation of adhesion interactions. Shear assay tests performed with assay medium containing 5% serum, a typical level utilized in leukocyte studies (3), revealed similar Candida binding levels between certain human donor serum and fetal bovine serum. For all assays reported here (representative data from at least three replicates), a single human donor was selected based on immunoassay screens for low reactivity to hydrophobic and hydrophilic cell wall antigens (data not shown). Serial samples from the donor were monitored to ensure comparable, low anti-Candida reactivity.
The HUVEC monolayers were activated on a staggered schedule to ensure similar time intervals before use of the monolayers in adhesion studies. In experiments with HUVECs from single donors, differences in the average heterotypic binding supported by HUVECs were noted (data not shown). Such donor differences in adhesion interactions with C. albicans were anticipated, as observed in other endothelial adhesion studies (51). All data presented in this report were obtained in shear assays involving pooled donor HUVECs.
In general, the preliminary studies revealed that the binding of Candida cells out of bulk fluid flow to attachment sites on endothelial cells occurs very rapidly. Offline analysis demonstrated that yeast cells could be moving with fluid flow in two video frames and then be completely stopped in the next two frames (30 frames/s). For both hydrophobic and hydrophilic yeast cell populations, the attachment occurred rapidly. Bound Candida cells occasionally (estimated <5% of foci) detached and rejoined bulk fluid flow.
Influence of CSH status on attachment to activated HUVECs.
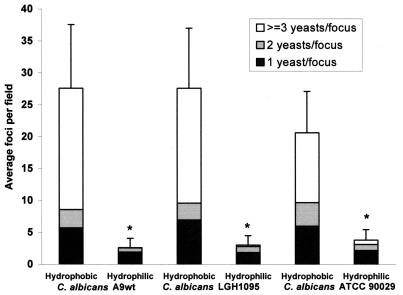
The impact of C. albicans CSH status on adhesion events with IL-1β-activated HUVECs was tested under simulated physiologic shear. Assay results show that hydrophobic cells bind significantly (P < 0.001) more than hydrophilic cells for C. albicans isolates grown in defined media (Fig. 1). The total height of each bar shows the average number of focal attachments per field (n > 10 fields per assay) produced by hydrophobic and hydrophilic yeast cells. This average foci value represents the average amount of heterotypic binding (Candida-HUVEC) observed in an assay, because each focus started with a heterotypic binding event. Results shown for the three Candida isolates in Fig. 1 typify overall findings that hydrophobic cells show significantly greater heterotypic binding than hydrophilic cells to endothelial monolayers.
FIG. 1.
In vitro shear analysis of hydrophobic and hydrophilic C. albicans adhesion to activated endothelial cell monolayers. HUVECs were activated with IL-1β as described in Materials and Methods. Hydrophobic and hydrophilic yeast cells of C. albicans A9wt, LGH1095, and ATCC 90029 were cultured in YNB2G. For each isolate, hydrophobic cells demonstrated significantly higher average foci per field than hydrophilic cells (∗, P < 0.001 for each comparison, Mann-Whitney rank sum test). Error bars show the SD for the average foci per field (total bar height), while the upper white portion of each bar corresponds to the amount of homotypic binding, as described in the text.
Greater heterotypic binding by hydrophobic cells was also observed when C. albicans isolates were grown in complex media. For example, hydrophobic C. albicans A9wt yeast grown in GYEP bound significantly better (P < 0.001, two-tailed Student t test) than hydrophilic cells (average foci per field of 26.1 ± 13.7 [standard deviation {SD}] compared to 5.4 ± 2.1 [SD], respectively). Hydrophobic C. albicans ATCC 90029 hydrophobic cells grown in AM3-2G also bound significantly better (P < 0.001, Mann-Whitney rank sum test) than hydrophilic yeast cells (average foci per field of 14.2 ± 6.2 [SD] compared to 4.3 ± 2.1 [SD], respectively). These values for the average binding of yeast grown in complex media compare favorably with the same isolate grown in defined medium (YNB2G) as presented in Fig. 1.
The stacked portions of each bar in Fig. 1 show the relative proportion of foci in the 1, 2, or ≥3 blastoconidial ranks, where the ≥3 blastoconidial rank indicates the extent of homotypic binding (Candida-Candida). The S:C ratios (described in Materials and Methods) for these cultures were 1.18:1 for hydrophobic and 1.09:1 for hydrophilic A9wt cells, 1.30:1 for hydrophobic and 1.16:1 for hydrophilic LGH1095 cells, and 1.50:1 for hydrophobic and 1.26:1 for hydrophilic ATCC 90029 cells. These low S:C ratios indicate that the yeast cell populations used for the adhesion assays were predominantly singlet blastoconidia with few mother-daughter combinations and rare, if any, contiguous triplet spheres as a cell unit. Results show that hydrophobic yeast cells support significantly more homotypic binding events than hydrophilic yeast cells under simulated physiologic shear (P < 0.001 for comparison of the average foci in the ≥3 blastoconidia ranks for hydrophobic versus hydrophilic cells for each C. albicans isolate shown Fig. 1 [Mann-Whitney rank sum Test]). The tendency of hydrophobic cells to show more homotypic binding than hydrophilic cells was consistent in numerous in vitro shear experiments (at least eight replicates) regardless of the growth medium chosen.
In some experiments with hydrophilic cells, we stopped the flow while viewing a field with no attached yeast at the end of the 8- to 12-min sampling period. Yeast cells from bulk flow were allowed to gravity settle onto the HUVEC monolayer for 5 to 10 min, but none remained in the field of view once flow was restored.
Effect of IL-1β activation of endothelial cells on C. albicans attachment.
Adhesion of hydrophobic C. albicans A9 yeast cells was tested on HUVEC monolayers that were unactivated or activated with the proinflammatory cytokine IL-1β. The average binding on activated HUVECs was 23.6 ± 6.9 (SD) foci per field (n = 14), compared to 12.0 ± 3.8 (SD) foci (n = 14 fields) for unactivated monolayers. The difference in heterotypic binding interactions was significant (P < 0.001, Student t test). Evaluation of the homotypic binding interactions indicated that activated HUVECs supported an average of 13.2 ± 4.6 (SD) foci in ≥3 blastoconidial ranks, whereas unactivated HUVECs showed 4.4 ± 1.4 (SD) foci. The difference in homotypic binding values was also significant (P < 0.001, Mann-Whitney rank sum test).
Effect of MAb treatment on C. albicans attachment under shear flow.
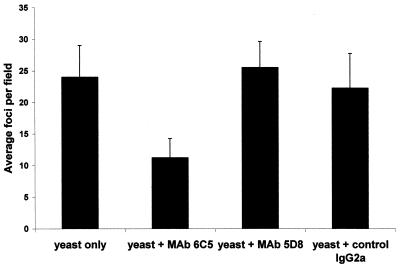
MAbs 6C5 and 5D8 recognize hydrophobic proteins of 38 and 37 kDa, respectively, and effectively inhibit C. albicans cell attachment to ECM proteins in static adhesion assays (40). To test whether these antibodies would inhibit adhesion to HUVEC monolayers under shear, we preincubated hydrophobic yeast cells with the anti-Candida MAbs or the control IgG2a antibody and infused the suspension into the system. Results (Fig. 2) of in vitro shear analysis show that MAb 6C5 caused significant inhibition of hydrophobic yeast cell binding to IL-1β-activated endothelial cells compared to both the control yeast-only and control IgG2a treatment groups (P < 0.001 for each pairwise multiple comparison, Tukey test). In contrast, pretreatment with MAb 5D8 did not significantly inhibit hydrophobic C. albicans yeast cell binding to activated HUVECs compared to control treatment groups.
FIG. 2.
Effects of MAb 6C5 and 5D8 on hydrophobic C. albicans attachment to activated HUVECs. Hydrophobic C. albicans LGH1095 yeast cells (YNB2G, 23°C stationary phase) were suspended in assay medium with or without 60 μg of MAb 6C5, MAb 5D8, or control IgG2a (UPC-10; Sigma product no. M9144) per ml, incubated in an ice water slurry for 10 min, and infused into the shear assay system harboring IL-1β-activated HUVECs. Pretreatment with MAb 6C5 significantly decreased binding compared to the yeast-only control and compared to the yeast plus control IgG2a (P < 0.001 for each in pairwise multiple comparison, Tukey test).
The effect of MAb 6C5 pretreatment on the amount of homotypic binding and the average number of total blastoconidia bound per field was evaluated in similar experiments, except that a different IgG antibody (MAb EL246) was used as a control for serum-free growth and processing as for the MAb 6C5 preparation. Results (Table 2) show that MAb 6C5 significantly decreased both the average number of foci with ≥3 blastoconidia and total bound blastoconidia compared to control yeast only (P < 0.001 for both t tests). Inhibition of homotypic binding by treatment with MAb 6C5 was also significantly different from that of control MAb EL246 treatment (P = 0.003 for average foci and P = 0.001 for total blastoconidia, t tests). The homotypic binding differences between yeast only and EL246 pretreatment for both average foci and total blastoconidia were not significant (P = 0.114 and P = 0.06, respectively, for t tests).
TABLE 2.
Effect of MAb 6C5 on homotypic binding events under simulated shear flow
| Treatment | No. of fields of view | Blastoconidia/fielda | Foci with ≥3 blastoconidia/fieldb |
|---|---|---|---|
| Yeast only | 12 | 85.7 ± 45.7 | 11.8 ± 5.5 |
| Yeast + MAb 6C5 | 15 | 22.5 ± 21.9c | 3.3 ± 2.5c |
| Yeast + MAb EL246 | 13 | 55.1 ± 30.4 | 8.3 ± 3.6 |
Means ± SD estimated from ranked data as described in Materials and Methods.
Mean ± SD. The S:C ratio was 1.14:1 for the C. albicans LGH1095 culture in the assay.
Significantly different (P ≤ 0.003) from values for the yeast-only control group and for the MAb EL246 treatment group as calculated by Student's t test.
DISCUSSION
Functional analysis of host-pathogen adhesion interactions occurring during vascular dissemination presents various limitations for in vitro modeling. Previous adhesion studies for Candida spp. or other microorganisms have generally been done either under static conditions with target host cells or under flow conditions with parallel plate chambers and no host cells (for examples, see references 6, 14 to 16, 41, and 48). Both types of assays miss reconstituting the interface between a host fluid and a host tissue with appropriate physiological shear forces, such as the high shear vascular interface in capillary beds or the peristaltic shear interface present across intestinal epithelial cells. To address these limitations, we used the ProteoFlow system to investigate adhesion interactions of C. albicans with endothelial monolayers under simulated physiologic shear. Prior application of the in vitro shear system has been instrumental in characterizing complex host-host adhesion interactions and in distinguishing shear-dependent and shear-independent interactions that happen during lymphocyte homing and leukocyte recruitment to endothelial surfaces (3, 31). The in vitro shear data on adhesion interactions and the effects of blocking compounds correlate well with in vivo experimental evidence for various host-host cell interactions (3).
C. albicans binding interactions with endothelial cells under shear flow revealed the capacity to form rapid, tight adhesions with host cells. This type of binding behavior by C. albicans is similar to the rapid adhesion interactions between leukocytes and activated α4 integrin receptors on endothelial cells (2, 4). Candida cells also acted like leukocytes in their ability to undergo homotypic binding events. Rolling adhesion behavior for C. albicans cells, analogous to selectin-mediated leukocyte rolling on activated HUVECs (3), was not observed in any of the assays.
Both a fungal variable and a host variable that influence vascular adhesion events under physiological shear forces, were identified. CSH status of the C. albicans yeast cells influenced adhesion to the human endothelial cells, with hydrophobic C. albicans cells showing greater heterotypic and homotypic binding capabilities than hydrophilic yeast cells. The relatively short assay times (<15 min in total) for the shear assays help ensure that the yeast cells maintain their original CSH values (24). Because stationary-phase 37°C yeast cultures are not 100% hydrophilic cells (typical CSH values ≤ 8%), the few yeast cells binding from hydrophilic cultures may actually be hydrophobic cells present in the cultures. For example, 143 total blastoconidia bound (Fig. 1, 18 fields of view) in the hydrophilic ATCC 90029 assay could be amply accounted for by hydrophobic cells in the hydrophilic culture (total adherent yeast much less than 8% of 2.1 × 107 yeast infused). With both hydrophilic and hydrophobic cell surfaces expressed in vivo (21), our study suggests that hydrophobic cells would have an advantage for broader distribution during vascular dissemination events. Ex vivo adhesion assay differences in binding by hydrophobic versus hydrophilic yeast cells to various tissues would also support broader distribution based on CSH status (25).
The host variable demonstrated by the results is that activation of HUVECs with the proinflammatory cytokineIL-1β supported significantly more binding by hydrophobic C. albicans cells. IL-1β activation of the HUVEC layer increases surface expression of cell adhesion molecules including selectins (e.g., E-selectin [3]), as well as integrin receptors such as intracellular adhesion molecule 1 (ICAM-1) and ICAM-2 (reviewed in references 7 and 53). Although ICAM-2 is expressed constitutively on the endothelial cells (33), cytokine activation increases levels of both ICAM-1 and ICAM-2 well above baseline. The increase in yeast cell binding observed here may be due to upregulation of these host adhesion molecules upon exposure to IL-1β. These results contrast with a report by Gustafson et al. where tumor necrosis factor activation of the HUVEC monolayer did not increase binding by Candida (22). The reason may be that static-versus-stimulated physiologic shear assays reveal a difference in shear-dependent and shear-independent host-pathogen interactions. Adhesion differences have been reported for yeast cells in static assays compared to ones performed under bulk liquid flow across various synthetic substrata (reviewed in reference 5, 6, 42, and 43).
The amount of homotypic binding shown for C. albicans correlates with expression of yeast cell hydrophobicity and with IL-1β activation of HUVECs. The later suggests that endothelial cell-derived components that increase during inflammation could provide a molecular bridge for the observed yeast cell-yeast cell binding rather than a direct yeast-to-yeast interaction. The Candida homotypic binding behavior revealed with the ProteoFlow system may be analogous to coadhesion events that others have reported, but preferred to limit, in static assays (41). The impact of homotypic binding events on Candida pathogenesis is unknown, but the formation of vascular microcolonies could amplify a local infectious burden and influence phagocytic clearance of fungal cells during dissemination.
Pretreatment of the hydrophobic C. albicans cells with MAb 6C5, but not MAb 5D8, decreased both heterotypic and homotypic binding events with activated endothelial cells. In previous studies, both antibodies inhibited hydrophobic C. albicans interactions with laminin and fibronectin (40). The differential capacity of MAb 6C5 to inhibit adhesion in these studies suggests that the 38-kDa hydrophobic protein antigen may play a direct role in mediating attachment to endothelial cells during dissemination. However, based on hydrophobicity theories describing forces acting through primary and secondary interaction distances (reviewed in reference 26), the blocking effect of an anti-hydrophobic protein MAb could result from direct blocking of an adhesin function, by steric hindrance of an adhesin, or by changing the hydrophobic interactive capacity of the yeast cell surface. The abundance and relative exposure of the 6C5 and 5D8 antigens on hydrophobic C. albicans surfaces require further characterization before an endothelial adhesin role for the 6C5 hydrophobic protein can be defined. Another approach in defining the inhibition mechanism would be to investigate the blocking effect of antibodies that recognize hydrophilic cell wall components displayed on hydrophobic C. albicans cells. This approach will be tested once such an anti-hydrophilic protein antibody is available.
Our current hypothesis is that the greater virulence of hydrophobic cells compared to hydrophilic cells is due to the prominent role of hydrophobic interactions in yeast cell attachment to vascular endothelium and exposed ECM. The evidence presented here based on the physiologic shear assay supports this hypothesis and further suggests that inhibition of hydrophobic interactions could affect pathogenesis by modulating the distribution of fungal cells during vascular dissemination and possibly favoring host defense mechanisms.
ACKNOWLEDGMENTS
This work was supported with funds from LigoCyte Pharmaceuticals, Inc., by National Institute of Allergy and Infectious Disease grants R01AI31048 (K.C.H.), RO1AI24912 (J.E.C.), and POIAI37194 (J.E.C.), and by Pfizer, Inc.
REFERENCES
- 1.Antley P P, Hazen K C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 1988;56:2884–2890. doi: 10.1128/iai.56.11.2884-2890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargatze R F, Jutila M A, Butcher E C. Distinct roles for L-selectin, α4 integrin, and LFA-1 in lymphocyte interactions with HEV in situ: the multi-step model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 3.Bargatze R F, Kurk S, Watts G, Kishimoto T K, Speer C A, Jutila M A. In vivo and in vitro functional examination of a conserved epitope of L- and E-selectin crucial for leukocyte-endothelial cell interactions. J Immunol. 1994;152:5814–5825. [PubMed] [Google Scholar]
- 4.Berlin C, Bargatze R F, Campbell J J, von Andrian U H, Szabo M C, Hasslen S R, Nelson R D, Berg E L, Erlandsen S L, Butcher E C. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 5.Busscher H J, Noordmans J, Meinders J, van der Mei H C. Analysis of the spatial arrangement of microorganisms adhering to solid surfaces—methods of presenting results. Biofouling. 1991;4:71–79. [Google Scholar]
- 6.Busscher H J, Van der Mei H C. Use of flow chamber devices and image analysis methods to study microbial adhesion. Methods Enzymol. 1995;253:455–477. doi: 10.1016/s0076-6879(95)53039-8. [DOI] [PubMed] [Google Scholar]
- 7.Carlos T M, Harlan J M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 8.Chaffin W L, Lopez-Ribot J L, Casanova M, Gozalbo D, Martínez J P. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J-H, Sutherland W M, Parsons S J. Monoclonal antibodies to oncoproteins. Methods Enzymol. 1994;254:430–435. doi: 10.1016/0076-6879(95)54029-6. [DOI] [PubMed] [Google Scholar]
- 10.Cole G T, Halawa A A, Anaissie E J. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis. 1996;22(Suppl. 2):S73–S78. doi: 10.1093/clinids/22.supplement_2.s73. [DOI] [PubMed] [Google Scholar]
- 11.Cutler J E, Brawner D L, Hazen K C, Jutila M A. Characteristics of Candida albicans adherence to mouse tissues. Infect Immun. 1990;58:1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutler J E, Han Y, Miller D. Fungal factors implicated in pathogenesis. In: Howard D H, editor. The Mycota. Berlin. Germany: Springer-Verlag; 1996. pp. 3–29. [Google Scholar]
- 13.Evans Z A, Mardon D N. Organ localization in mice challenged with a typical Candida albicans strain and a pseudohyphal variant. Proc Soc Exp Biol Med. 1977;155:234–238. doi: 10.3181/00379727-155-39780. [DOI] [PubMed] [Google Scholar]
- 14.Filler S G, Ibe B O, Ibrahim A S, Ghannoum M A, Raj J U, Edwards J E., Jr Mechanisms by which Candida albicans induces endothelial cell prostaglandin synthesis. Infect Immun. 1994;62:1064–1069. doi: 10.1128/iai.62.3.1064-1069.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filler S G, Ibe B O, Luckett P M, Raj J U, Edwards J E., Jr Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis. 1991;164:928–935. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- 16.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsyth C B, Plow E F, Zhang L. Interaction of the fungal pathogen Candida albicans with integrin CD11b/CD18: recognition by the I domain is modulated by the lectin-like domain and the CD18 subunit. J Immunol. 1998;161:6198–6205. [PubMed] [Google Scholar]
- 18.Fu Y, Rieg G, Fonzi W A, Belanger P H, Edwards J E, Jr, Filler S G. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect Immun. 1998;66:1783–1786. doi: 10.1128/iai.66.4.1783-1786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaur N K, Klotz S A, Henderson R L. Overexpression of the Candida albicans ALA1 gene in Saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of Candida albicans. Infect Immun. 1999;67:6040–6047. doi: 10.1128/iai.67.11.6040-6047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glee P M, Masuoka J, Ozier W T, Hazen K C. Presence of multiple laminin- and fibronectin-binding proteins in cell wall extract of Candida albicans: influence of dialysis. J Med Vet Mycol. 1996;34:57–61. doi: 10.1080/02681219680000091. [DOI] [PubMed] [Google Scholar]
- 21.Glee P M, Sundstrom P, Hazen K C. Expression of surface hydrophobic proteins by Candida albicans in vivo. Infect Immun. 1995;63:1373–1379. doi: 10.1128/iai.63.4.1373-1379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafson K S, Vercellotti G M, Bendel C M, Hostetter M K. Molecular mimicry in Candida albicans. J Clin Investig. 1991;87:1896–1902. doi: 10.1172/JCI115214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawser S P, Douglas L J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun. 1994;62:915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazen B W, Hazen K C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988;56:2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazen K C, Brawner D L, Riesselman M H, Cutler J E, Jutila M A. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazen K C, Glee P M. Cell surface hydrophobicity and medically important fungi. Curr Top Med Mycol. 1995;6:1–37. [PubMed] [Google Scholar]
- 27.Hazen K C, Hazen B W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 1992;60:1499–1508. doi: 10.1128/iai.60.4.1499-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazen K C, Hazen B W. A polystyrene microsphere assay for detecting surface hydrophobicity variations within Candida albicans populations. J Microbiol Methods. 1987;6:289–299. [Google Scholar]
- 29.Hostetter M K. Linkage of adhesion, morphogenesis, and virulence in Candida albicans. J Lab Clin Med. 1998;132:258–263. doi: 10.1016/s0022-2143(98)90038-5. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer L L, Clevenger J, Hecht J E, Ehrhart E J, Poulet F M. Detection of Als proteins on the cell wall of Candida albicans in murine tissues. Infect Immun. 1999;67:4251–4255. doi: 10.1128/iai.67.8.4251-4255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jutila M A, Bargatze R F, Kurk S, Warnock R A, Ehsani N, Watson S R, Walcheck B. Cell surface P- and E-selectin support shear-dependent rolling of bovine γ/δ T cells. J Immunol. 1994;153:3917. [PubMed] [Google Scholar]
- 32.Kanbe T, Cutler J E. Minimum chemical requirements for adhesin activity of the acid-stable part of Candida albicans cell wall phosphomannoprotein complex. Infect Immun. 1998;66:5812–5818. doi: 10.1128/iai.66.12.5812-5818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kansas G S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 34.Klotz S A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992;14:340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- 35.Klotz S A, Smith R L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991;163:604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- 36.Ley K, Cerrito M, Arfors K E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991;260:1667–1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- 37.Li R-K, Cutler J E. Chemical definition of an epitope/adhesin molecule on Candida albicans. J Biol Chem. 1993;268:18293–18299. [PubMed] [Google Scholar]
- 38.Marcantonio E E, Hynes R O. Antibodies to the conserved cytoplasmic domain of the integrin β1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988;106:1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maródi L. Local and systemic host defense mechanisms aainst Candida: immunopathology of candidal infections. Pediatr Infect Dis J. 1997;16:795–801. doi: 10.1097/00006454-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Masuoka J, Wu G, Glee P M, Hazen K C. Inhibition of Candida albicans attachment to extracellular matrix by antibodies which recognize hydrophobic cell wall proteins. FEMS Microbiol Immunol. 1999;24:421–429. doi: 10.1111/j.1574-695X.1999.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 41.Mayer C L, Filler S G, Edwards J E., Jr Candida adherence to endothelial cells. Microvasc Res. 1992;43:218–226. doi: 10.1016/0026-2862(92)90018-k. [DOI] [PubMed] [Google Scholar]
- 42.Millsap K W, Bos R, Busscher H J, Van der Mei H C. Surface aggregation of Candida albicans on glass in the absence and presence of adhering Streptococcus gordonii in a parallel-plate flow chamber: a surface thermodynamic analysis based on acid-base interactions. J Colloid Interface Sci. 1999;212:495–502. doi: 10.1006/jcis.1998.6054. [DOI] [PubMed] [Google Scholar]
- 43.Millsap K W, Bos R, Van der Mei H C, Busscher H J. Influence of aeration of Candida albicans during culturing on their surface aggregation in the presence of adhering Streptococcus gordonii. FEMS Immunol Med Microbiol. 1999;26:69–74. doi: 10.1111/j.1574-695X.1999.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 44.Odds F C. Pathogenesis of Candida infections. J Am Acad Dermatol. 1994;31:S2–S5. doi: 10.1016/s0190-9622(08)81257-1. [DOI] [PubMed] [Google Scholar]
- 45.Perry M A, Granger D N. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Investig. 1991;87:1798–1804. doi: 10.1172/JCI115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pincus S H, Smith M J, Jennings H J, Burritt J B, Glee P M. Peptides that mimic the group B streptococcal type III capsular polysaccharide antigen. J Immunol. 1998;160:293–298. [PubMed] [Google Scholar]
- 47.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozdzinski E, Toumanen E. Adhesion of microbial pathogens to leukocyte integrins: methods to study ligand mimicry. Methods Enzymol. 1995;253:3–26. doi: 10.1016/s0076-6879(95)53003-7. [DOI] [PubMed] [Google Scholar]
- 49.Samonis G, Anaissie E J, Bodey G P. Effects of broad-spectrum antimicrobial agents on yeast colonization of the gastrointestinal tracts of mice. Antimicrob Agents Chemother. 1990;34:2420–2422. doi: 10.1128/aac.34.12.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santoni G, Gismondi A, Liu J H, Punturieri A, Santoni A, Frati L, Piccoli M, Djeu J Y. Candida albicans expresses a fibronectin receptor antigenically related to α5β1 integrin. Microbiology. 1994;140:2971–2979. doi: 10.1099/13500872-140-11-2971. [DOI] [PubMed] [Google Scholar]
- 51.Segal E, Sandovsky-Losica H. Adhesion and interaction of Candida albicans with mammalian tissues in vitro and in vivo. Methods Enzymol. 1995;253:439–452. doi: 10.1016/s0076-6879(95)53038-x. [DOI] [PubMed] [Google Scholar]
- 52.Silva T M J, Glee P M, Hazen K C. Influence of cell surface hydrophobicity on attachment of Candida albicans to extracellular matrix proteins. J Med Vet Mycol. 1995;33:117–122. [PubMed] [Google Scholar]
- 53.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 54.Tuomanen E. Subversion of leukocyte adhesin systems by respiratory pathogens. ASM News. 1993;59:292–296. [Google Scholar]
- 55.Whelan W L, Delga J M, Wadsworth E, Walsh T J, Kwon-Chung K J, Calderone R, Lipke P N. Isolation and characterization of cell surface mutants of Candida albicans. Infect Immun. 1990;58:1552–1557. doi: 10.1128/iai.58.6.1552-1557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]