Abstract
Anorectal malformations (ARM) represent a spectrum of rare malformations originating from a perturbated development of the embryonic hindgut. Approximately 60% occur as a part of a defined genetic syndrome or within the spectrum of additional congenital anomalies. Rare copy number variations (CNVs) have been associated with both syndromic and non-syndromic forms. The present study represents the largest study to date to explore the contribution of CNVs to the expression of ARMs. SNP-array-based molecular karyotyping was applied in 450 individuals with ARM and 4392 healthy controls. CNVs were identified from raw intensity data using PennCNV. Overlapping CNVs between cases and controls were discarded. Remaining CNVs were filtered using a stringent filter algorithm of nine filter steps. Prioritized CNVs were confirmed using qPCR. Filtering prioritized and qPCR confirmed four microscopic chromosomal anomalies and nine submicroscopic CNVs comprising seven microdeletions (del2p13.2, del4p16.2, del7q31.33, del9p24.1, del16q12.1, del18q32, del22q11.21) and two microduplications (dup2p13.2, dup17q12) in 14 individuals (12 singletons and one affected sib-pair). Within these CNVs, based on their embryonic expression data and function, we suggest FOXK2, LPP, and SALL3 as putative candidate genes. Overall, our CNV analysis identified putative microscopic and submicroscopic chromosomal rearrangements in 3% of cases. Functional characterization and re-sequencing of suggested candidate genes is warranted.
Subject terms: Medical research, Pathogenesis, Risk factors
Introduction
Anorectal malformations (ARM) represent a spectrum of rare malformations originating from a perturbated development of the embryonic hindgut. ARM present with a male to female ratio of 1.7, the overall birth prevalence has been estimated with 1 in 2500–3000 live births [1]. Approximately 60% occur as a part of a defined genetic syndrome or within the spectrum of additional congenital anomalies, the remaining 40% present isolated non-syndromic [2]. The current knowledge of the underlying molecular mechanisms of isolated non-syndromic ARM is still limited. Based on observations such as reduced reproduction rate one can assume a significant fraction of underlying de novo events. The experience in medical genetics suggests that such mutational events will comprise genomic alterations of different size ranging from small changes affecting single nucleotides to large alterations resulting in losses or gains of several thousand to millions of base-pairs. To test this hypothesis, we previously, between 2011 and 2017, systematically employed array-based molecular karyotyping in altogether 224 individuals with syndromic and non-syndromic ARM [3–10]. In total, we identified a pathogenic de novo CNV in 5 % of cases. 11 cases presented with syndromic ARM according to Rasmussen et al. [11]. Accordingly, Wong et al. using molecular karyotyping in 363 Han Chinese with sporadic ARM and 4006 controls found an enrichment of rare long duplications among individuals with syndromic ARM [12]. The aims of our present study were (i) to explore the overall contribution of CNVs to the expression of ARMs, (ii) to identify novel disease-causing CNVs, and (iii) to identify novel candidate genes.
Materials (subjects) and methods
Cases and controls
Affected individuals and their families were recruited within the framework of the German “Network for Congenital Uro-REctal malformations” (www.cure-net.de). All experiments involving human materials adhered to the recommendations of the World Medical Association, as stated in the revised Declaration of Helsinki from Seoul 2008. The proposed project has been approved by the Ethics Commission of the University of Bonn. The study sample encompassed 450 individuals with ARM. A total of 212 individuals presented with a syndromic, 238 with a non-syndromic form. Furthermore, we included 4392 healthy controls [13]. For 351 affected individuals, the DNA of both parents was available, for 57 affected individuals, only maternal, and for 5 affected individuals, only paternal DNA was available. For 37 affected individuals no parental sample was available.
DNA isolation and array-based molecular karyotyping
Genomic DNA of patients and their parents was isolated from the blood samples by using the Chemagic Magnetic Separation Module I (Chemagen, Baesweiler, Germany), or from saliva samples using the Oragene DNA Kit (DNA Genotek Inc., ON, Canada). Cases and Controls were genotyped using the single nucleotide polymorphism (SNP)-based array “Infinium Global Screening Array-24 v2.0”. This array contains >600.000 SNPs that are evenly distributed across the genome. All chromosomal positions are given in human genome build hg19 (GRCh37 Genome Reference Consortium Human Build 37 (GRCh37)).
CNV filtering
CNV calling was accomplished using PennCNV. The algorithm of PennCNV uses a Hidden-Markov model to estimate putative CNVs from log R ratio (normalized intensity data) and B allele frequency (allele frequency data) of each SNP. Quality criteria used to exclude individuals due to failed genotyping were (I) a gender mismatch between assumed and called gender; (II) a call rate <98%; (III) a number of CNVs per affected individual that exceeded double of the standard deviation of the mean number of CNVs called per individual. Subsequent criteria to exclude single CNVs were (IV) maximum log Bayes factor below 30; and (V) if the CNV comprised less than 3 consecutive called SNPs. These steps were followed by filtering of the remaining CNVs for prioritization of potential disease-causing CNVs. Filter steps included (VI) comparison of CNVs in cases and inhouse controls. All CNVs identified in cases that were overlapped by one or more CNV form controls were not further considered potential disease causing. Beforehand, all 4394 inhouse controls had been quality checked accordingly using criteria (I)–(V). For the remaining CNVs the information for each CNV from PennCNV was combined with the information from the annotation and ranking tool AnnotSV, and filtered out in a manual filter step (VII) [14]. Here, CNVs comprising deletions were discarded when overlapping with deletion CNVs annotated in the Database of Genomic Variants (DGV) and CNVs comprising duplications were discarded when overlapping with duplication CNVs annotated in the Database of Genomic Variants (DGV). Furthermore, (VIII) CNVs that did not reside within coding or promotor regions were filtered out. (IX) Using GenomeStudio Genotyping Module v2.0 and the Illumina Genome Viewer version 2.0.4 the remaining CNVs were visually inspected. (X) Final priorization of the remaining CNV regions was performed by gathered information from publicly available databases: Gnomad (https://gnomad.broadinstitute.org), OMIM (https://www.omim.org), UCSC (https://genome.ucsc.edu), MGI (http://www.informatics.jax.org), and PubMed (https://pubmed.ncbi.nlm.nih.gov). Genome build hg19/GRCh37 was used for all data.
CNV confirmation
To confirm all prioritized CNVs and to identify de novo events when parental DNA was available, quantitative polymerase chain reaction (qPCR) was carried out. SYBR Green (Applied Biosystems, Foster City, CA) was used for qPCR reactions. The evaluation of the copy numbers was performed using the comparative CT method (ΔΔCt method). Three housekeeping genes were used to compare relative copy counts (CFTR, BNC1, RNA). The primer sequences are shown in Supplementary Table 1.
Results
CNV analysis
After the application of quality filter step I (gender mismatch), two patients were excluded. Through step II (call rate <98%), 40 patients were discarded and after step III (exceeded double of standard deviation), 12 more patients were excluded. In the remaining 396 individuals with ARM, a total of 6316 CNVs, were called. Within the remaining 4066 controls, a total of 43,323 CNVs were called. Regarding the quality filter for each CNV, filter steps (IV) and (V) led to the exclusion of 5026 CNVs among ARM individuals and the exclusion of 32,075 CNVs among healthy control individuals. Comparison of frequencies in cases and controls (VI) excluded additional 887 CNVs. In this filter step, we identified three individuals with trisomy 21 (not further mentioned). Application of filter steps VII and VIII filtered out 117 microdeletions and 35 microduplications. Filter step IX yielded a final count of 82 microdeletions and 49 microduplications. According to the annotation of the respective CNVs in gnomAD (https://gnomad.broadinstitute.org), OMIM (https://www.omim.org), UCSC (https://genome.ucsc.edu), MGI (http://www.informatics.jax.org), and PubMed (https://pubmed.ncbi.nlm.nih.gov), we ultimately prioritized and qPCR confirmed four microscopic chromosomal anomalies and nine submicroscopic CNVs comprising seven microdeletions (del2p13.2, del4p16.2, del7q31.33, del9p24.1, del16q12.1, del18q32, del22q11.21) and two microduplications (dup2p13.2, dup17q12).
Unbalanced chromosomal translocations
Two affected individuals carried unbalanced terminal translocations including 46,XX,der(11)t(11;16)(q24.2;q22.2) and 46,XX,der(22)t(22;9)(q11.21;pter) (Table 1).
Table 1.
Unbalanced chromosomal translocations and microscopic chromosomal de novo duplications.
| Individual 1 | Individual 2 | Individual 3 | Individual 4 | |
|---|---|---|---|---|
| CNV | 46,XX,der(11)t(11;16) (q24.2;q22.2) | 46,XX,der(22)t(22;9) (q11.21;p) | 46,XY,dup(3) (q26.31-q29) | 46,XY,dup(17) (q25.3-qter) |
| Type of ARM | Perineal fistula | Perineal fistula | – | Perineal fistula |
| Phenotypes | ||||
| Heart | – | – | – | – |
| CNS | Intraventricular hemorrhage of the newborn | – | – | – |
| Esophagus | – | – | – | – |
| Extremities | Bilateral clubfoot | – | – | – |
| Vertebral bodies | – | – | – | – |
| Hips | – | – | – | – |
| Kidney | Dysplastic pelvic kidney (left) | Kidney hypoplasia (left). Bilateral hydronephrosis | – | – |
| Urinary tract | – | Persistent urogenital sinus | – | – |
| Genital organs | – | – | – | – |
| Other | Bilateral atresia of submandibular gland. Left sided aplasia of thyroid. Choanal atresia. Hearing disability. Macular delocalization | Premature infant (36 + 0), small for gestation age (2090 g) | – | – |
ARM anorectal malformation, CNS central nervous system.
Individual 1 presented with an ARM in form of a perineal fistula, congenital intraventricular hemorrhage with hydrocephalus, bilateral clubfoot, dysplastic pelvic kidney (left), bilateral atresia of submandibular gland, left sided aplasia of the thyroid gland, choanal atresia, hearing disability, and macular delocalization. Her chromosomal anomaly comprised an unbalanced translocation 46,XX,der(11)t(11;16)(q24.2;q22.2) with terminal deletion 11q24.2-qter. The first deleted SNP is located at chromosomal position chr11:127.078.525 and the last deleted SNP at chr11:134.923.456. Deletion of 11q23.3-qter has been associated with Jacobsen syndrome (JBS, MIM #147791). JBS is a contiguous gene deletion syndrome involving terminal chromosome 11q. Key phenotypic features are pre- and postnatal growth retardation, intellectual disability (ID), characteristic facial dysmorphism, thrombo- or pancytopenia. Further clinical features are congenital heart defects (CHD), congenital anomalies of the kidneys and urinary tract (CAKUT), gastrointestinal tract, genitalia, central nervous system (CNS) anomalies and/or skeleton. Previously, Mattina et al. reported gastrointestinal tract malformations in 18–25% of individuals with JBS including pyloric stenosis, ARM, and less frequently, duodenal atresia, annular pancreas, or gut malrotation [15]. Besides terminal deletion 11q24.2-qter Individual 1 carried also an 18 Mb duplication of chromosomal region 16q22.2-qter. The first duplicated SNP is located at chromosomal position chr16:71.956.505, the last duplicated SNP at chromosomal position chr16:90.161.959. Key features described for partial trisomy 16qter (ORPHA:96106) include low birth weight, failure to thrive, hypotonia, ID, CHD, limb anomalies and joint contractures, facial dysmorphism with a high prominent forehead, down slanting and small palpebral fissures, hypertelorism, periorbital edema, low-set and abnormal ears, prominent nose, micrognathia, CAKUT, genital anomalies, and ARM. Interestingly, several reports in the literature associated dup16pterqter with ARM [16].
Individual 2 presented with ARM in form of a perineal fistula, kidney hypoplasia (left), bilateral hydronephrosis, persistent urogenital sinus, and small for gestational age. Her chromosomal anomaly comprised an unbalanced translocation with a 2.5 Mb deletion of chromosomal region 22q11.21. The first deleted SNP was located at chromosomal position chr22:18.875.445 and the last deleted SNP was located at chromosomal position chr22:21.461.607. The 22q11.2 deletion syndrome is the most common microdeletion syndrome. It is not surprising that several cases of ARM have been described among patients with this deletion [16] (see below Individual 14). Individual 2 also carried a 38.8 Mb duplication of 9p. The first duplicated SNP was located at chromosomal position chr9:209.325, the last duplicated SNP was located at chromosomal position chr9:39.021.035. Common features of a 9p trisomy include ID, craniofacial dysmorphisms, skeletal alterations, CNS anomalies, CHD, and less common CAKUT. While ARM has not been associated with trisomy 9p, several reports in the literature mention ARM in association with mosaic or non-mosaic complete trisomy 9 [16].
Microscopic chromosomal de novo duplications
Two affected individuals carried each a terminal duplication including 46,XXdup(3)(q26.31-q29) and 46,XXdup(17)(q25.3-qter) (Table 1).
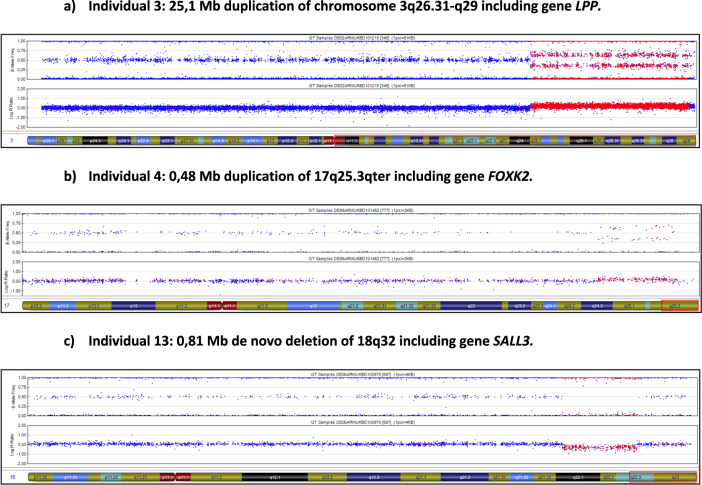
Individual 3 presented with an isolated ARM. It is uncertain if he presented with additional minor stigmata. His chromosomal anomaly comprised a 25.1 Mb duplication of chromosome 3q26.31-q29 (Fig. 1a). The first duplicated SNP was located at chromosomal position chr3:171.615.261, the last at chromosomal position chr3:196.805.528. Common features of 3q duplication syndrome are a hirsutism, synophrys, broad nasal root, anteverted nares, downturned corners of the mouth, malformed ears, CHD, CAKUT and genital anomalies, ID, and growth retardation. To the best of our knowledge, this is the first report of duplication 3q26.31-q29 in an individual with ARM.
Fig. 1.
CNVs harboring putative ARM candidate genes.
Individual 4 presented with an isolated ARM in form of a perineal fistula. He did not present with any other congenital anomaly or stigmata. His chromosomal anomaly comprised an 0.48 Mb duplication of 17q25.3-qter (Fig. 1b). The first duplicated SNP was located at chromosomal position chr17:80.545.076, the last at chromosomal position chr17:81.033.874. To the best of our knowledge, this is the first report of duplication of 17q25.3-qter in an individual with ARM.
Rare inherited CNVs and CNVs of unknown inheritance
Six affected individuals carried very rare inherited submicroscopic CNVs (Table 2). All of these CNVs were absent in 4066 healthy inhouse controls (CNV frequency <0.0003) and DGV.
Table 2.
Rare inherited CNVs and CNVs of unknown inheritance.
| Individuals | Inherited CNVs | First and last deleted SNP | Size | Type | Genes within CNV |
|---|---|---|---|---|---|
| Individual 5 | del2p13.2 | Chr2:72.623.204-72.939.279 | 316,076 bp | Deletion | EXOC6B |
| Individuals 6 and 7 | dup2p13.2 | Chr2:72.882.934-73.015.587 | 132,654 bp | Duplication | EXOC6B |
| Individual 8 | del4p16.2 | Chr4:5.162.593-5.427.691 | 265,099 bp | Deletion | STK32B |
| Individual 9 | del7q31.33 | Chr7:126.392.369-126.439.453 | 47,085 bp | Deletion | GRM8 |
| Individual 10 | dup17q12 | Chr17:33.786.176-33.968.102 | 181,927 bp | Duplication | AP2B1, LINC02001, LOC107985033, PEX12, SLFN12L, SLFN14, SNORD7 |
| CNV of unknown inheritance | |||||
| Individual 11 | del9p24.1 | Chr9:4.973.080-5.000.904 | 27,825 bp | Deletion | JAK2 |
Individual 10 presented with ARM in form of a perineal fistula. He carried a 181.925 bp maternally inherited duplication of chromosomal region dup17q1.
Individual 5 presented with an isolated ARM in form of a recto-vestibular fistula. Besides her ARM, she had no co-occurring anomalies. She carried a maternally inherited 316.074 bp deletion of chromosomal region 2p13.2. The first deleted SNP was located on chromosomal position Chr2:72.623.204, the last at chromosomal position Chr2:72.939.279. Within this deletion resides the developmental gene EXOC6B. EXOC6B has been associated with autosomal-recessive inherited spondyloepimetaphyseal dysplasia with joint laxity, type 3 (MIM #618395).
Individuals 6 and 7 are brothers who both presented with ARM in form of a perineal fistula. In addition, Individual 6 presented with a testicular appendage and right-sided deafness. His brother presented with additional epispadias. Both carried a maternally inherited 132,652 bp duplication of chromosomal region 2p13.2. The first duplicated SNP was located at chromosomal position Chr2:72.882.934, the last at chromosomal position Chr2:73.015.587. Within this duplication resides the above-mentioned developmental gene EXOC6B. While the above EXOC6B-associated spondyloepimetaphyseal dysplasia with joint laxity, type 3 does not present with any visceral organ affection, the findings in individual 5 and in the two brothers 6 and 7 suggests a possible involvement of EXOC6B or the respective genomic region in ARM formation.
Individual 8 presented with ARM in form of a rectoprostatic fistula, aganglionosis of the Meissner’s plexus (submucosa, M. Hirschsprung) and craniofacial Goldenhar syndrome. He carried a paternally inherited 265.097 bp deletion of chromosomal region 4p16.2. The first deleted SNP was located at chromosomal position Chr4:5.162.593, the last SNP at chromosomal position Chr4:5.427.691. Within this deletion resides the developmental gene STK32B, which has been associated with Ellis-van Creveld syndrome, an autosomal-recessive skeletal dysplasia with co-occurring genital anomalies, e.g., hypospadias.
Individual 9 presented with an unclassified ARM as part of her VATER/VACTERL association, with further phenotypic data missing. She carried a paternally inherited 47,083 bp deletion of chromosomal region 7q31.33. The first deleted SNP was located at chromosomal position Chr7:126.392.369, the last at chromosomal position Chr7:126.439.453. Within this deletion resides the developmental gene GRM8, so far associated with any human disease.
Individual 10 presented with ARM in form of a perineal fistula. He carried a 181.925 bp maternally inherited duplication of chromosomal region 17q1. The first duplicated SNP was located at chromosomal position Chr17:33.786.176, the last duplication at chromosomal position Chr17:33.968.102. Within this duplication reside five coding genes comprising AP2B, PEX12, SLFN12L, SLFN14, and SNORD7 and two long non-coding RNAs LINC02001 and LOC107985033. Of the coding genes, PEX12 has been associated with the autosomal-recessive Peroxisome biogenesis disorder 3A (Zellweger, MIM #614859). SLFN14 has been associated with the autosomal-dominant “Bleeding disorder, platelet-type, 20 (MIM #616913)”.
Individual 11 presented with ARM in form of a recto-vesical fistula as part of his VATER/VACTERL association. He presented with additional CHD, right-sided kidney agenesis, and cryptorchidism. His chromosomal anomaly comprised a 27.823 bp deletion of chromosomal region 9q24.1 (Table 2). The first deleted SNP was located on chromosomal position Chr9:49.73.080, the last at chromosomal position Chr9:5.000.904. Here, we had no parental samples available for testing and can therefore not conclude, if the CNV has occurred de novo or has been transmitted from a healthy parent. Within the deletion resides the developmental gene JAK2, a gene that has been associated with broad spectrum of human hematologic malignancies (MIM *147796) but not with congenital visceral anomalies.
Rare submicroscopic de novo CNVs
In total we detected and confirmed three submicroscopic de novo CNVs (Table 3).
Table 3.
Patients with de novo CNVs.
| Individual 12 | Individual 13 | Individual 14 | |
|---|---|---|---|
| CNV | del16q12.1 | del18q32 | del22q11.21 |
| Type of ARM | Perineal fistula | Rectoprostatic fistula | Perineal fistula |
| Phenotypes | |||
| Heart | PDA, VSD, pulmonary valve stenosis | ||
| CNS | – | – | – |
| Esophagus | – | – | – |
| Extremities | – | – | – |
| Vertebral bodies | – | – | – |
| Hips | – | – | – |
| Kidney | – | Duplex kidney with megaureter | – |
| Urinary tract | – | – | – |
| Genital organs | – | Maldescensus testis (right) | – |
| Other | Auricular hypoplasia (right) | – | Luxation of the arytenoid cartilages, Abdominal wall hernia |
ARM anorectal malformation, CNS central nervous system, PDA patent ductus arteriosus, VSD ventricular septal defect, NA not available.
Individual 12 presented with an ARM in form of a perineal fistula and right-sided auricular hypoplasia. She carried a 1.38 Mb de novo deletion of chromosomal region 16q12.1. The first deleted SNP was located at chromosomal position chr16:50.135.837, the last at chromosomal position chr16:51.522.044. Within the deleted region reside nine coding genes including ADCY7, HEATR3, PAPD5, BRD7, CYLD, NKD1, NOD2, SNX20, and SALL1. Furthermore, the deletion comprised two microRNAs (MIR6771 and MIR3181) and four long non-coding RNAs (LINC02127, LINC02168, LINC02178, LOC101927272).
Individual 13 presented with ARM in form of a rectoprostatic fistula, duplicated kidney with megaureter, and right-sided maldescensus testis. He carried a 0.81 Mb de novo deletion of chromosomal region 18q32 (Fig. 1c). The first deleted SNP was located at chromosomal position chr18:76.501.085, the last at chromosomal position 18:77.373.296. Within the deletion reside three coding genes comprising SALL3, ATP9B, and NFATC1.
Individual 14 presented with an ARM in form of a perineal fistula. In addition, she presented with patent ductus arteriosus, ventricular septal defect and pulmonary valve stenosis. Furthermore, she had luxation of the arytenoid cartilages and an abdominal wall hernia. Her chromosomal anomaly comprised an isolated 2.58 Mb deletion of 22q11.21 (see above Individual 2). The first deleted SNP was located on chromosomal position Chr22:18.875.445, the last at chromosomal position Chr22:21.461.607. DECIPHER database (DatabasE of genomiC varIation and Phenotype in Humans using Ensembl Resources) [17] lists 1267 CNVs of chromosomal region 22q11.2. These comprise 667 heterozygous deletions. Within these 667 deletions are 14 deletions that have been described in association with ARM. The smallest region of overlap of all these deletions comprises 1.39 Mb. Here, further genetic studies are warranted to prioritize a possible ARM associated human disease-gene.
Discussion
Congenital ARM represent a rare but severe spectrum of birth defects of the hindgut, with life-long impairments. The current knowledge of the underlying molecular mechanisms is still limited.
The primary aim of our present study was to identify novel highly penetrant structural genomic variants at the size of CNVs, and to describe in-depth the overall contribution of highly penetrant CNVs to the expression of the ARM spectrum. Among 450 ARM individuals, we identified three individuals with trisomy 21, two unbalanced chromosomal translocations, and two microscopic large de novo duplications (Table 1). Furthermore, we detected and confirmed five very rare, putatively novel submicroscopic CNVs in six affected individuals (four independent individuals and one sib-pair) (Table 2). None of these CNVs were found in any of our 4,066 healthy inhouse controls (CNV frequency <0.0003) nor in DGV. To the best of our knowledge, none of these inherited CNVs have been previously associated with human ARM phenotypes or other human diseases (PubMed search February 2022). Furthermore, we detected and confirmed three submicroscopic de novo CNVs in three independent ARM individuals. Our results confirm our own previous observations in which about 5% of affected individuals were found to carry putative disease-causing CNVs.
All large microscopic chromosomal rearrangements detected here comprise previously syndromic ARM associated regions. Within the rare inherited CNVs, we were unable to identify any candidate gene that would have stood out due to its function or association with known human ARM related disease. However, our findings in Individual 5 and the brothers Individual 6 and 7 suggest a possible dosage effect of EXOC6B to be involved in human ARM formation. Biallelic recessive variants in EXOC6B have been associated with spondyloepimetaphyseal dysplasia with joint laxity, type 3, which does not present with visceral organ affection. However, according to Mouse Genome Database (MGD) Exoc6b shows strong expression in the mouse visceral organ system during embryonic development from embryonic day (ED) 10.5–18, including the critical embryonic timeframe for ARM organ formation [18]. Furthermore, we detected a heterozygous deletion in Individual 8 comprising STK32B, which has been associated with Ellis-van Creveld syndrome, an autosomal-recessive skeletal dysplasia with co-occurring genital anomalies, e.g., hypospadias. Hence, STK32B dosage effects might as well be implicated in ARM formation as assumed for EXOC6B, especially since we did not explore the respective trans alleles for disease-causing variants on the level of single base-pairs.
In five individuals we detected microscopic and submicroscopic chromosomal de novo rearrangements in which we prioritized three putative candidate genes. In Individual 12 we detected a 1.38 Mb de novo deletion of chromosomal region 16q12.1 (Table 3). Within the deleted region reside nine coding genes including ADCY7, HEATR3, PAPD5, BRD7, CYLD, NKD1, NOD2, SNX20, and SALL1. Previously, heterozygous deletions spanning SALL1 have been associated with Townes–Brocks syndrome (MIM # 107480) [19]. Key phenotypic features of Townes–Brocks syndrome include microcephaly, auricular anomalies such as microtia, CHD, ARM, hypospadias, CAKUT, skeletal anomalies of the hands and feet. Hence, the present genetic finding and the individual’s phenotype suggest the diagnosis of Townes–Brocks syndrome in Individual 12.
In Individual 13 we detected and confirmed a 0.81 Mb de novo deletion of chromosomal region 18q32 (Table 3 and Fig. 1c). Within the deletion reside three coding genes comprising SALL3, ATP9B, and NFATC1. Previously, the two developmental genes SALL1 and SALL4 have been associated with syndromic ARM [19, 20]. Furthermore, DECIPHER database [17] lists 47 individuals with heterozygous de novo losses of the SALL3 comprising genomic region. Two individuals presented with vaginal atresia, five with hypospadias, three with renal agenesis, and two with vesicoureteral reflux. Moreover, according to MAMEP database (http://mamep.molgen.mpg.de/index.php), Sall3 is expressed in the early mouse embryo (Theiler stage, TS15) in distinct structures. The highest expression is detected in the neural tube along the complete body axis and in the caudal end mesenchyme. Additional expression domains are detected in the developing limb buds and the brain area. In younger TS12 embryos, weaker staining is also detected in the endoderm areas, from which the hindgut will develop [21]. Together with these previous findings and the here observed findings in Individual 13, we suggest a dosage effect of SALL3 to be involved in the formation of human ARM or uro-rectal malformations in a broader spectrum. In Individual 3 we detected a 25.1 Mb duplication of chromosome 3q26.31-q29 (Fig. 1a). Within this large duplication resides among other genes the developmental gene LPP. Genetic variants of different size in LPP have been previously associated with the VATER/VACTERL association [22–25]. The acronym VATER/VACTERL association (OMIM #192350) refers to the non-random co-occurrence of the following component features (CFs): vertebral defects (V), anorectal malformations (ARM) (A), cardiac defects (C), tracheoesophageal fistula with or without esophageal atresia (TE), renal malformations (R), and limb defects (L) [24]. Individuals may present with additional anomalies; however, the clinical diagnosis requires the presence of at least three CFs [22]. These previously associated dosage effects of LPP include an approximate 451,000 bp heterozygous loss spanning nucleotides 189,395,885–189,951,376 on chromosome 3q (nucleotide position based on hg18) comprising only LPP gene [22]. According to MAMEP database Lpp is expressed widespread in TS15 embryos with distinct expression domains in the developing somites and the mesenchyme [21]. Additional expression domains are detected in the gut and in older TS17 embryos expression can be also detected in the hindgut area [21]. Together, we suggest LPP as a putative ARM candidate gene. In Individual 4 we detected a 0.48 Mb duplication of 17q25.3-qter (Fig. 1b). Within this duplicated region resides the developmental gene FOXK2. Very recently, the FOX-gene-cluster on human chromosome 16q24 has been associated with multifactorial esophageal atresia, the most common congenital intestinal atresia of the upper intestinal tract [26]. Furthermore, Foxk2 has a widespread expression pattern in the TS15 stage mouse embryo with distinct expression domains in the branchial arches, developing limbs and the epithelial structure of the forebrain and midbrain [27]. Moreover, Foxk2 is strongly expressed in almost all developing urinary tract structures at mouse embryonic day 15.5 with distinct expression patterns in mouse embryonic kidney [28]. Taken together, our findings and previous observations suggest dosage effects of FOXK2 to be implicated in human ARM formation. Our focus on CNVs comprising coding regions limited our current and previous analysis. Thereby we might have missed deep intronic small CNVs affecting only regulatory regions such as TATA boxes or TAD boundaries, possibly implicated in the formation of human ARM [29]. An additional limitation of our study was the method we carried out for CNV calling. For molecular karyotyping we employed an SNP-based array containing >600,000 SNPs distributed across the genome, followed by the estimation of the CNV-positions through PennCNV based on gains or losses of the SNPs. As a result, the precise CNV breakpoints remain unknown. We therefore cannot determine exactly, if patients with the apparent same CNV such as patients 5, 6 and 7 share the exact same breakpoints.
Further studies on our generated SNP-based array datasets, which were clearly beyond the scope of our present study might elucidate the complete contribution of CNVs to the formation of human ARM and explain larger proportions of its unknown genetic background.
Conclusions
This large scale CNV analysis with a focus on high penetrant chromosomal rearrangements in 450 individuals with ARM identified putative disease-causing microscopic and submicroscopic chromosomal rearrangements in 3% of cases. Within these CNVs reside putative candidate genes FOXK2, LPP, and SALL3. The confirmation of the putative candidate genes as ARM disease-genes will lead to new diagnostic possibilities, provide families and clinicians with greater knowledge of the causes of the disorder, and allow a precise estimation of a recurrence risk.
Supplementary information
Acknowledgements
We thank all patients and their families for their participation, as well as the German self-help organizations for individuals with anorectal malformations (SoMA e.V.) for their assistance with recruitment.
Author contributions
Conceptualization: HR and ACH. Methodology: JF, SHH, SS, AB, and GCD. Software: SS and AB. Validation: JF, CB, LS, and LW. Formal analysis: JF, SS, and ACH. Investigation: JF, GCD, HR, and ACH. Resources: HR, NS, TMB, ES, JN, JL, FK, SW, HMG, SH, CK, MP, SM, GG, ML, MS, MS, CK, BU, KK, UR, and MZ. Data curation: SS. Writing—original draft preparation: JF, ACH, PG, and HR. Writing—review and editing: all authors. Visualization: all authors. Supervision: HR and ACH. Funding acquisition: HR. All authors have read and agreed to the published version of the manuscript.
Funding
ACH was supported by BONFOR grant O-149.0123 and by the Else Kröner-Fresenius-Stiftung and the Eva Luise und Horst Köhler Stiftung – Project No: 2019_KollegSE.04. HR was supported by the grants RE 1723/1-1 and RE 1723/1-3 from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG). GCD has been supported by BONFOR grant O-120.0001 and by the Herbert-Reeck foundation (2019). We acknowledge financial support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding program “Open Access Publication Funding”. Open Access funding enabled and organized by Projekt DEAL.
Data availability
CNV data that support the findings of this study have been deposited in “ClinVar-Database” [“https://www.ncbi.nlm.nih.gov/clinvar/”] with the accession codes SCV002576529–SCV002576543.
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments involving human materials adhered to the recommendations of the World Medical Association, as stated in the revised Declaration of Helsinki from Seoul 2008. The proposed project has been approved by the Ethics Commission of the University of Bonn (Lfd. Nr. 031/19). In addition, the applicants abided to all regulations specified in German Data Protection Law. Informed consent was obtained from all subjects involved in the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heiko Reutter, Email: heiko.reutter@uk-erlangen.de.
Alina C. Hilger, Email: alina.hilger@uk-erlangen.de
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01216-5.
References
- 1.Jenetzky E. Prevalence estimation of anorectal malformations using German diagnosis related groups system. Pediatr Surg Int. 2007;23:1161–5. doi: 10.1007/s00383-007-2023-6. [DOI] [PubMed] [Google Scholar]
- 2.Marcelis C, Dworschak G, de Blaauw I, van Rooij IALM. Genetic counseling and diagnostics in anorectal malformation. Eur J Pediatr Surg. 2021;31:482–91. doi: 10.1055/s-0041-1740338. [DOI] [PubMed] [Google Scholar]
- 3.Schramm C, Draaken M, Bartels E, Boemers TM, Aretz S, Brockschmidt FF, et al. De novo microduplication at 22q11.21 in a patient with VACTERL association. Eur J Med Genet. 2011;54:9–13. doi: 10.1016/j.ejmg.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Schramm C, Draaken M, Bartels E, Boemers TM, Schmiedeke E, Grasshoff-Derr S, et al. De novo duplication of 18p11.21-18q12.1 in a female with anorectal malformation. Am J Med Genet A. 2011;155A:445–9. doi: 10.1002/ajmg.a.33820. [DOI] [PubMed] [Google Scholar]
- 5.Baudisch F, Draaken M, Bartels E, Schmiedeke E, Bagci S, Bartmann P, et al. CNV analysis in monozygotic twin pairs discordant for urorectal malformations. Twin Res Hum Genet. 2013;16:802–7. doi: 10.1017/thg.2013.29. [DOI] [PubMed] [Google Scholar]
- 6.Dworschak GC, Draaken M, Marcelis C, de Blaauw I, Pfundt R, van Rooij IALM, et al. De novo 13q deletions in two patients with mild anorectal malformations as part of VATER/VACTERL and VATER/VACTERL-like association and analysis of EFNB2 in patients with anorectal malformations. Am J Med Genet A. 2013;161A:3035–41. doi: 10.1002/ajmg.a.36153. [DOI] [PubMed] [Google Scholar]
- 7.Hilger A, Schramm C, Pennimpede T, Wittler L, Dworschak GC, Bartels E, et al. De novo microduplications at 1q41, 2q37.3, and 8q24.3 in patients with VATER/VACTERL association. Eur J Hum Genet. 2013;21:1377–82. doi: 10.1038/ejhg.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworschak GC, Draaken M, Hilger AC, Schramm C, Bartels E, Schmiedeke E, et al. Genome-wide mapping of copy number variations in patients with both anorectal malformations and central nervous system abnormalities. Birth Defects Res A Clin Mol Teratol. 2015;103:235–42. doi: 10.1002/bdra.23321. [DOI] [PubMed] [Google Scholar]
- 9.Dworschak GC, Crétolle C, Hilger A, Engels H, Korsch E, Reutter H, et al. Comprehensive review of the duplication 3q syndrome and report of a patient with Currarino syndrome and de novo duplication 3q26.32-q27.2. Clin Genet. 2017;91:661–71. doi: 10.1111/cge.12848. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Marsch F, Kause F, Degenhardt F, Schmiedeke E, Märzheuser S, et al. Array-based molecular karyotyping in 115 VATER/VACTERL and VATER/VACTERL-like patients identifies disease-causing copy number variations. Birth Defects Res. 2017;109:1063–9. doi: 10.1002/bdr2.1042. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 12.Wong EH, Cui L, Ng CL, Tang CS, Liu XL, So MT, et al. Genome-wide copy number variation study in anorectal malformations. Hum Mol Genet. 2013;22:621–31. doi: 10.1093/hmg/dds451. [DOI] [PubMed] [Google Scholar]
- 13.Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J. 2002;144:212–8. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 14.Geoffroy V, Guignard T, Kress A, Gaillard JB, Solli-Nowlan T, Schalk A, et al. AnnotSV and knotAnnotSV: a web server for human structural variations annotations, ranking and analysis. Nucleic Acids Res. 2021;49:W21–W28. doi: 10.1093/nar/gkab402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattina T, Perrotta CS, Grossfeld P. Jacobsen syndrome. Orphanet J Rare Dis. 2009;7:9. doi: 10.1186/1750-1172-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcelis C, de Blaauw I, Brunner H. Chromosomal anomalies in the etiology of anorectal malformations: a review. Am J Med Genet A. 2011;155A:2692–704. doi: 10.1002/ajmg.a.34253. [DOI] [PubMed] [Google Scholar]
- 17.Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D, et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet. 2009;84:524–33. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE, et al. Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 2019;47:D801–D806. doi: 10.1093/nar/gky1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borozdin W, Steinmann K, Albrecht B, Bottani A, Devriendt K, Leipoldt M, et al. Detection of heterozygous SALL1 deletions by quantitative real time PCR proves the contribution of a SALL1 dosage effect in the pathogenesis of Townes-Brocks syndrome. Hum Mutat. 2006;27:211–2. doi: 10.1002/humu.9396. [DOI] [PubMed] [Google Scholar]
- 20.Al-Baradie R, Yamada K, St Hilaire C, Chan WM, Andrews C, McIntosh N, et al. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am J Hum Genet. 2002;71:1195–9. doi: 10.1086/343821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geffers L, Herrmann B, Eichele G. Web-based digital gene expression atlases for the mouse. Mamm Genome. 2012;23:525–38. doi: 10.1007/s00335-012-9413-3. [DOI] [PubMed] [Google Scholar]
- 22.Arrington CB, Patel A, Bacino CA, Bowles NE. Haploinsufficiency of the LIM domain containing preferred translocation partner in lipoma (LPP) gene in patients with tetralogy of Fallot and VACTERL association. Am J Med Genet A. 2010;152A:2919–23. doi: 10.1002/ajmg.a.33718. [DOI] [PubMed] [Google Scholar]
- 23.Hernández-García A, Brosens E, Zaveri HP, de Jong EM, Yu Z, Namwanje M, et al. Contribution of LPP copy number and sequence changes to esophageal atresia, tra-cheoesophageal fistula, and VACTERL association. Am J Med Genet A. 2012;158A:1785–7. doi: 10.1002/ajmg.a.35391. [DOI] [PubMed] [Google Scholar]
- 24.Kolvenbach CM, van der Ven AT, Kause F, Shril S, Scala M, Connaughton DM, et al. Exome survey of indi-viduals affected by VATER/VACTERL with renal phenotypes identifies phenocopies and novel candidate genes. Am J Med Genet A. 2021;185:3784–92. doi: 10.1002/ajmg.a.62447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon BD. VACTERL/VATER association. Orphanet J Rare Dis. 2011;16:56. doi: 10.1186/1750-1172-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gehlen J, Giel AS, Köllges R, Haas SL, Zhang R, Trcka J, et al. First genome-wide association study of esophageal atresia with or without tracheoesophageal fistula (EA/TEF) identifies three genetic risk loci at CTNNA3, FOXF1/FOXC2/FOXL1 and HNF1B. HHG Adv. 2022;3:100093. doi: 10.1016/j.xhgg.2022.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, et al. The GUDMAP database – an online resource for genitourinary research. Development. 2011;138:2845–53. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–7. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- 29.Lifton RP, Goldberg ML, Karp RW, Hogness DS. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symposia Quant Biol. 1978;42:1047–51. doi: 10.1101/SQB.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CNV data that support the findings of this study have been deposited in “ClinVar-Database” [“https://www.ncbi.nlm.nih.gov/clinvar/”] with the accession codes SCV002576529–SCV002576543.