Abstract
Purpose
Surface-guided radiation therapy (SGRT) has been investigated intensively to ensure correct patient positioning during a radiation therapy course. Although the implementation is well defined for photon-beam facilities, only a few analyses have been published for ion-beam therapy centers. To investigate the accuracy, reliability, and efficiency of SGRT used in ion-beam treatments against the conventional skin marks, a retrospective study of a unique SGRT installation in an ion gantry treatment room was conducted, where the environment is quite different to conventional radiation therapy.
Methods and Materials
There were 32 patients, divided into 3 cohorts—pelvis, limb, and chest/spine tumors—and treated with ion-beams. Two patient positioning workflows based on 300 fractions were compared: workflow with skin marks and workflow with SGRT. Position verification was followed by planar kilo voltage imaging. After image matching, 6 degrees of freedom corrections were recorded to assess interfraction positioning errors. In addition, the time required for patient positioning, image matching, and the number of repeated kilo voltage imaging also were gathered.
Results
SGRT decreased the translational magnitude shifts significantly (P < .05) by 0.5 ± 1.4 mm for pelvis and 1.9 ± 0.5 mm for limb, whereas for chest/spine, it increased by 0.7 ± 0.3 mm. Rotational corrections were predominantly lowered with SGRT for all cohorts with significant differences in pitch for pelvis (P = .002) and chest/spine (P = .009). The patient positioning time decreased by 18%, 9%, and 15% for pelvis, limb, and chest/spine, respectively, compared with skin marks. By using SGRT, 53% of all studied patients had faster positioning time, and 87.5% had faster matching time. Repositioning and consequent reimaging decreased from about 7% to 2% with a statistically significant difference of .042.
Conclusions
The quality of patient positioning before ion-beam treatments has been optimized by using SGRT without additional imaging dose. SGRT clearly reduced inefficiencies in the patient positioning workflow.
Introduction
The primary objective of radiation therapy is to deliver a prescribed dose to a tumor-bearing target volume as precisely as possible while sparing the adjacent healthy tissues to the furthest extent.1, 2, 3 This goal becomes more essential when implementing tumor treatment approaches that involve using high doses with extremely steep dose gradients. Compared with photon beams, ion-beams exhibit the advantage of delivering the same therapeutic dose to a deep located target with a much less integral dose to the organs at risk (OAR) due to Bragg peak and with increased biological effectiveness.1,4,5 One of the most difficult challenges for such a treatment technique is having a precise method for patient positioning during the entire course of therapy. Hence, it is ensured that deviations from the planned computed tomography (CT) are minimized.6
Conventionally, the patient positioning process for ion-beam treatments is based on planar kilo voltage (kV) imaging and/or CT,7 whereas cone beam CT (CBCT) is limited to few institutions. At our institution, HIT, we use in-room lasers (IRLs) and permanent tattoos as skin marks for initial patient positioning and then verify the patient position with planar kV imaging per fraction. However, skin marks may have a negative effect, especially on pediatric patients and patients with breast cancer, and potentially lead to psychological effects because the skin marks can serve as a reminder for the patients of their tumor treatments long after treatments have ceased.8, 9, 10 Moser et al11 found that 70% of women who received skin marks during the radiation therapy of breast cancer have negative feelings about the tattoos.
Furthermore, a disadvantage of using skin marks for patient prepositioning is that the skin marks on the elastic patient skin may be hard to find and align, for example, in case of darker-colored skin and changes on the skin surface over the course of therapy.12 Therefore, the skin marks may deviate from the desired position, the accuracy may not be ensured, and the patient must be repositioned.12 In addition, the main drawback of planar kV imaging (and to some extent also CBCT) is that position variations only can be assessed and verified using bony landmarks, irrespective of changes in water-equivalent thickness along the beam path (eg, tissue swelling, effusion, changes in tumor size). Such an approach is unable to precisely match and consider anatomic changes because of soft-tissue variations. This may result in inaccuracies, leading to an altered dose distribution within the target volume and OAR. As a supplementary method, a control CT image may be used but will not be evaluated directly or performed frequently because only 1 CT scanner is available in one of our treatment rooms for position verification at HIT, and also because of concerns about the additional imaging dose, particularly in case of pediatric patients.
The demand for nonionizing skin-based image guidance that relies on the patient skin surface but not only on 3 skin marks is needed.13 For this purpose, a surface-guided radiation therapy (SGRT) system (VisionRT Ltd, London, United Kingdom) combined with a unique mounting structure frame has been installed in the isocentric ion-beam gantry of our institution. SGRT may support the initial patient positioning without any additional dose and can track the position of the patient during the entire irradiation time.14 Furthermore, SGRT may supply faster and a more precise patient positioning compared with skin marks.15 For this aim, it compares the current patient skin surface and the reference patient skin surface from the planning CT data within a user-defined region of interest (ROI).16
The aim of this retrospective study was first to quantify the reliability, accuracy, and efficiency of SGRT in positioning of patients before planar kV imaging in comparison with skin mark-based patient positioning. Second, we investigated whether SGRT could improve workflow (WF) efficiency by reducing the patient setup time (positioning and image matching) and the frequency of incorrect positioning (immobilization setup errors). Finally, we studied whether repeated planar kV imaging during patient positioning may be reduced.
Methods and Materials
Ethics
This study was approved and confirmed by the local ethical review committee of the Faculty of Medicine at the University of Heidelberg in Heidelberg, Germany (S-053/2022).
Gantry treatment room at HIT
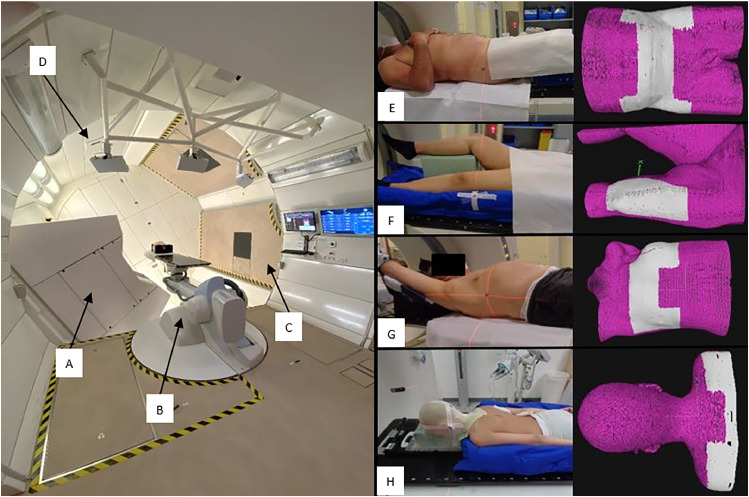
HIT operates 3 treatment rooms. Two rooms deliver the beam in fixed horizontal direction, and the third one is an isocentric gantry with a length of 25 m, a diameter of 13 m, and the ability to rotate around 360° (Fig. 1). Despite the considerable mass of the gantry (660 tons), an accuracy of less than 1 mm in beam position is achievable. Proton, carbon, and helium ions can be delivered at the gantry.5 Indications such as craniospinal irradiation, focal irradiation of head and neck or brain tumors, lymphoma, prostate cancer, skull base, and spinal sarcoma are treated. Planar kV imaging is used for patient position verification and final alignment at the gantry.
Figure 1.
Gantry treatment room at HIT including (A) beam nozzle at 240°, (B) robotic treatment table, (C) foldable floor, and (D) SGRT system installation including 3 AlignRT pods on the left image. On the right side, different immobilization setups at the planning CT with different ROIs dependent on the indication and anatomy for (E) pelvis patients were positioned using ProSTEP, (F) limb patients positioned using BlueBAG vacuum cushion, (G) chest patients positioned using WingSTEP, and (H) spine patients positioned using BlueBAG vacuum cushion and thermoplastic mask. Source: HIT. ProSTEP, HeadSTEP, WingSTEP, and BlueBAG vacuum cushions are developed by Elekta (Stockholm, Sweden). Abbreviations: CT = computed tomograpy; ROI = region of interest; SGRT = surface-guided radiation therapy.
SGRT system
In this study, the AlignRT software, version 5.1.2, developed by VisionRT Ltd, was used. As a result of the special construction of our ion gantry treatment room, a standard installation of a SGRT system was not possible because of the moveable ceiling of the gantry while the gantry is rotating, the size of the beam nozzle, and the foldable floor in the rotation area of the gantry (Fig. 1). Thus, an individual mounting was created by a third-party company (S. Bleyer GmbH, Schorndorf, Germany). This spiderweb-like structure frame was screwed directly to the gantry bearing enabling optimal 3-dimensional (3D) data acquisition by avoiding (1) transfer of gantry rotation movements into the system and (2) shading of the optical system by the beam nozzle. The system hardware consists of 3 camera pods mounted to the gantry bearing, each with 2 image sensors and a projector that displays an optical random speckle pattern on body surface of patient. The AlignRT software is able to calculate position deviations, the so-called real-time deltas for 3 translations (vertical, longitudinal, and lateral), and 3 rotations (iso, roll, and pitch). For the calculation, a user-defined ROI (Fig. 1) and a rigid-body transformation between the reference surface and the current surface of the patient is used. The calculation is based on active stereo photogrammetry and triangulation.16, 17, 18, 19
The system was inspected through conducting, on a yearly basis, a specialized quality assurance (QA) program composed of monthly calibration, WF test, and gantry angle dependency tests to ensure that all components in the room, such as robotic treatment table, robotic imager, IRL, gantry, and SGRT system interacted properly. In addition, relative to the monthly reference calibration, the position of the pods was monitored based on daily QA before therapy start.
SGRT system accuracy
The positioning accuracy of the AlignRT system at HIT has been, over the technical commissioning and evaluation phase, thoroughly investigated, and is presented briefly here. By using the planning CT, the absolute positioning accuracy of the SGRT system compared with planar kV imaging system for the Quasar phantom developed by Modus QA (London, Canada) and the virtual human male CIRS pelvic phantom developed by CIRS (Norfolk, VA) was examined. For the Quasar phantom, the average translational differences between AlignRT and planar kV imaging were –0.1 ± 0.2 mm in lateral, –0.2 ± 0.3 mm in longitudinal, and –0.3 ± 0.2 mm in the vertical direction, respectively. The average rotational discrepancy was 0.0° ± 0.2° in iso, –0.0° ± 0.1° in pitch, and 0.0° ± 0.1° in roll. For the CIRS-Pelvis phantom, deviations of 0.2 ± 0.1 mm in lateral, –0.3 ± 0.4 mm in longitudinal, and 0.0 ± 0.3 mm in vertical direction as well as –0.1° ± 0.1° in iso, 0.2° ± 0.2° in pitch, and 0.0° ± 0.3° in roll were recorded. Furthermore, the tracking accuracy of the AlignRT system in the isocenter was tested relative to a 3D FARO laser tracker developed by Faro Technologies Inc. (Lake Mary, FL) by moving the treatment table to predefined translational and rotational coordinates. The CIRS-Pelvis phantom was used for this test. The maximum differences between AlignRT computations and calculated values of the laser tracker were 0.01 ± 0.01 mm in translation (±100 mm for each translation) and 0.0° ± 0.2° in rotation (±5° for each rotation).
ROI design
The aim of drawing an ROI is to focus the 6 degrees of freedom match on a surface region, which can provide more relevant and precise surface tracking.18 This depends on the SGRT system application. To draw an appropriate ROI on the reference patient skin surface, it was required to obtain so-called reference captures using AlignRT at the first treatment fraction to observe which portions of the patient surface are processed as the result of limitations, for example, shadowing of camera by the beam nozzle. The reference capture in AlignRT is defined as an option to take a new reference of the current patient position. This capture differs from the reference surface reconstructed from the planning CT data and was merely used for an optimized ROI definition on the planning CT (Fig. 1). The planning CT used as reference is called the DICOM reference surface, or “Digital Imaging and Communication in Medicine.” Figure 1 shows the optimized ROIs using the reference captures.
Treatment Planning System (TPS)
The patient plan data produced by the TPS using the planning CT are required for SGRT. The RayStation TPS (RS10A; RaySearch Laboratories, Stockholm, Sweden) was used for contouring at HIT. The lower Hounsfield unit (HU) thresholds used for the contouring of the reference skin surface in planning CT ranged from –250 to –350 HU, and the upper thresholds ranged from 1500 to 3071 HU depending on patient anatomy.
Study design
This study was conducted in 2 WFs for initial patient positioning before planar kV imaging: WF with skin marks and WF with SGRT. In total, 32 patients were included and divided into 3 cohorts: pelvis, limb, and chest/spine (Table 1). The first 8 to 10 treatment fractions of each patient were evaluated, and accordingly the statistical analysis was performed based on 300 fractions. The 2 WFs were compared for each patient. To keep the overall treatment time and study-related patient burden as low as possible, the SGRT WF was performed not on a daily basis but 2 to 3 times a week. The following parameters were analyzed: the translational and rotational residual shifts of patient position based on kV image matching, the total setup time required for patient positioning including matching time, and the so-called reimaging frequency including number of repeated planar kV imaging and repositioning for final confirmation.
Table 1.
Patient and setup specifications
| Site and patient number | Age (y), median (range) | Diagnosis | Immobilization | Matching bony landmarks | Fractions analyzed* (total treated fractions) |
|---|---|---|---|---|---|
| Pelvis P1-11 |
50 (18-86) | 7 sarcomas 4 chordomas |
10 ProSTEP 1 BlueBAG |
Hips, coccyx, femur, spine | 100 (248) |
| Limb L1-11 |
41 (14-73) | 11 sarcomas | 11 BlueBAG | Knee, tibia, femur, hips | 100 (230) |
| Chest/spine C1-10 |
24.5 (9-80) | 7 sarcomas 3 lymphomas |
3 WingSTEP 4 BlueBAG 3 HeadSTEP Thermoplastic masks |
Sternum, spine, vertebral body | 100 (235) |
Analyzed fractions distributed equally for each workflow.
Patient and setup specifications including tumor region, matching bony landmarks for planar kV imaging, immobilization devices, and patient posture in the planning CT are introduced in Table 1. All patients obtained a free-breathing planning CT with 3-mm slice thickness.
Clinical WF: Skin marks versus SGRT
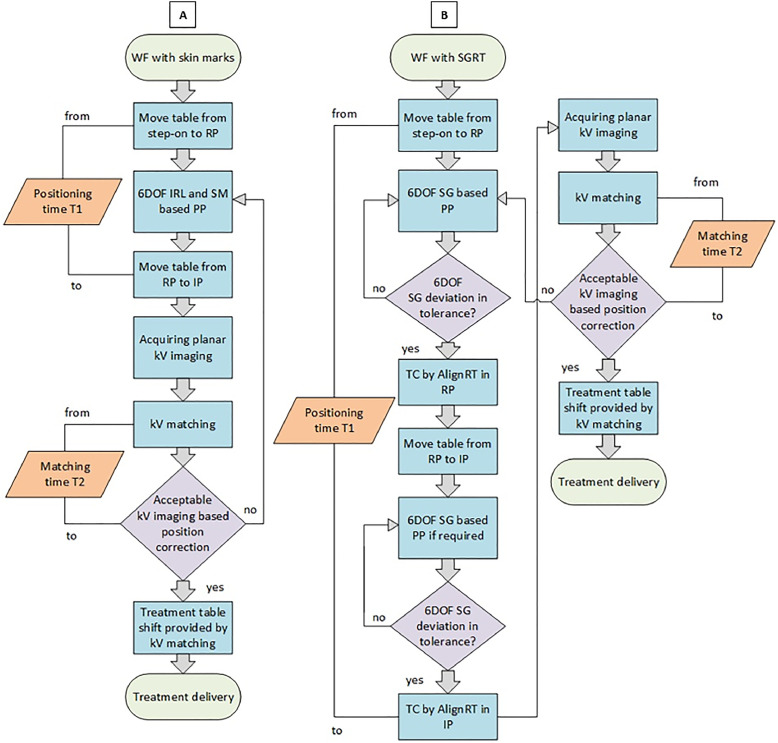
For both WFs (Fig. 2), the patient was initially moved with the 6 degrees of freedom robotic treatment table20 from a step-on position to so-called reference point in our WF (isocenter) at a gantry angle of 0° using the hand-held control. The skin marks and the IRL are used for patient prepositioning in the WF with skin marks.
Figure 2.
WF explanation. (A) WF with skin marks. (B) WF with SGRT. Abbreviations: IP = imaging point; IRL = in-room-laser; kV = kilo voltage; PP = patient positioning; RP = reference point; SG = surface guidance; SGRT = surface-guided radiation therapy; SM = skin marks; TC = treatment capture; WF = workflow; 6DOF = 6 degrees of freedom.
The AlignRT was used for aligning the patient in the WF with SGRT instead of the skin marks and IRL. Subsequently, the patient moved to the imaging point that was previously defined by the radiotherapist, relative to the reference point. The reference point was determined by the skin marks and deemed to be the reference for all later points (imaging point and beam isocenter points). The imaging point was qualified as the position verification of the patient using bony landmarks and it could differ from reference and beam isocenter points.
In the WF with SGRT, patients initially were positioned relative to the DICOM reference surface using AlignRT real-time imaging and predefined thresholds of 3 mm for translations and 2° for rotations for all regions in both reference and then imaging point if the imaging point was not equal to the reference point. The so-called treatment captures in AlignRT were captured twice, after both positioning in the reference point and in the following imaging point. Treatment captures are snapshots of the patient for documentation purposes and can be performed either static or gated depending on the region. The corresponding real-time deltas in the imaging point were collected only but not applied for patient prepositioning because the treatment machine was not connected to the AlignRT system. The final patient position was verified through planar kV imaging including anteroposterior and left–right images in both WFs. The bony landmarks in the imaging point region were used to match the planar kV images acquired in anteroposterior and left–right with the digitally reconstructed radiographs. The digitally reconstructed radiographs were generated from the projections of the planning CT series projected to a 2-dimensional plane (Table 1). After the kV matching process, rotational and translational correction vectors were applied to precisely position the patient at the beam isocenter point(s). The later correction vectors were compared with the remaining offsets indicated by the AlignRT.
The matching process of the first fraction was always evaluated through the physician in charge and during the remaining fractions by the therapists. If the post-kV imaging adjustments in both WFs, in translations and/or rotations, exceeded the position correction constraints, which are dependent on the treated indication and employed setup devices, the entire WF was repeated from the beginning, including repositioning and reimaging. In our WF protocol, the criteria for repositioning are defined by the physician regarding the ALARA principle, “as low as reasonably achievable,” and by the medical physicist regarding collision constraints in the treatment room resulting in dose effects (ie, moving material edges into the beam path), and movement limits of the treatment couch robot.
Data collection and statistical analysis
Patient data were collected between May 2020 and December 2021 as part of the clinical routine and reviewed for this retrospective study. To assess the reliability, accuracy, and efficiency of SGRT in detection and quantification, all position deviations calculated by AlignRT after treatment capture were reviewed for each patient treatment fraction. All kV image matching correction vectors applied in the imaging points after both WFs were also recorded by the treatment machine. The average (µ) and the standard deviation (SD) were calculated for all studied treatment fractions. The resulting residual setup imaging correction vectors were divided into 2 kinds of setup error, which were defined by Bijhold et al21 and De Boer et al22: cohort residual systematic setup errors (Ʃ) and cohort residual random setup errors (σ). The Ʃ corresponds to the SD of all patient averages, and σ corresponds to the average of all SDs. The time spent on patient positioning and kV image matching was recorded separately. The additional time for repeating the patient setup WF including repositioning and reimaging is not included in this work, but it usually takes several minutes, which should be taken to account. The number of repeated planar kV images when repositioning the patient also was documented. The statistical analysis was performed using SPSS software (IBM Corp, Armonk, NY). Statistical differences of the residual corrections of 2 WFs were evaluated using the paired Wilcoxon signed-rank test, and correlation tests were evaluated using the Spearman test. Results were considered significant when P < .05.
Results
Patient characteristics
We assessed 32 patients with tumors of pelvis and limb and tumor locations in the chest and spine. Patient characteristics are depicted in Table 1.
Reliability and accuracy of patient positioning: Skin marks versus SGRT
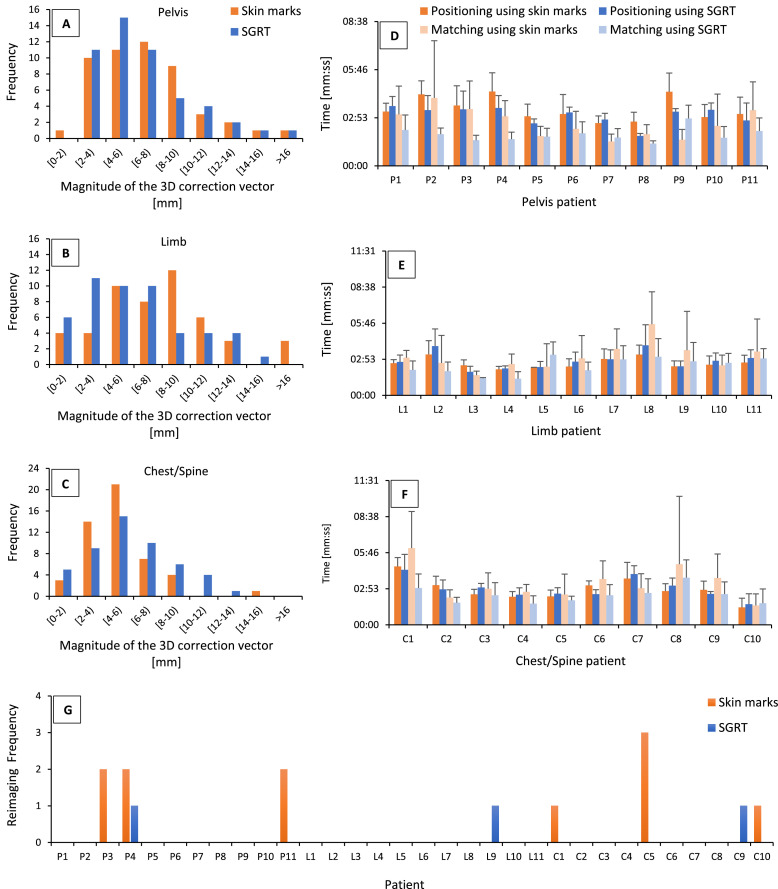
Comparison of SGRT versus skin marks in patient positioning before treatment for 32 patients, a total of 300 fractions, was analyzed. The translational and rotational residual µ, Ʃ, and σ setup errors for each cohort after kV image matching are shown in Table 2. The P values for all residual corrections of translations and rotations also are demonstrated in Table 2. In addition, cumulative histograms showing the residual magnitude of postimaging 3D correction vectors for both WFs, skin marks and SGRT, for 3 cohorts are introduced in Fig. 3.
Table 2.
Residual setup postimaging correction vectors after patient prepositioning using both WFs for all patient cohorts (at α = .05 significance level)
| Site | Skin marks |
SGRT |
|||||
|---|---|---|---|---|---|---|---|
| µ | Ʃ | σ | µ | Ʃ | σ | P value | |
| Pelvis | |||||||
| LAT, mm | 1.4 | 2.6 | 3.7 | 0.2 | 3.1 | 3.2 | .167 |
| LNG, mm | 0.1 | 4.0 | 3.4 | –0.7 | 2.4 | 2.7 | .054 |
| VRT, mm | 1.9 | 3.4 | 2.2 | 2.4 | 3.2 | 2.3 | .457 |
| Mag, mm | 7.1 | 2.3 | 3.8 | 6.6 | 2.3 | 2.4 | .011 |
| Iso, ° | 0.2 | 0.5 | 0.4 | 0.0 | 0.5 | 0.5 | .077 |
| Pitch, ° | –0.6 | 1.2 | 1.1 | 0.1 | 1.2 | 0.8 | .002 |
| Roll, ° | –0.3 | 0.4 | 0.6 | –0.3 | 0.7 | 0.7 | .391 |
| Limb | |||||||
| LAT, mm | 0.7 | 2.3 | 2.4 | 0.9 | 2.0 | 1.9 | .349 |
| LNG, mm | 0.0 | 4.4 | 4.0 | –0.4 | 2.1 | 2.8 | .452 |
| VRT, mm | 2.6 | 4.8 | 2.3 | 2.1 | 4.6 | 2.2 | .309 |
| Mag, mm | 7.9 | 3.8 | 3.0 | 6.0 | 2.8 | 2.5 | .006 |
| Iso, ° | –0.1 | 0.6 | 0.5 | 0.1 | 0.6 | 0.4 | .150 |
| Pitch, ° | 0.2 | 0.6 | 0.6 | 0.1 | 0.6 | 0.4 | .152 |
| Roll,° | –0.2 | 0.7 | 1.3 | –0.7 | 1.4 | 1.4 | .083 |
| Chest/spine | |||||||
| LAT, mm | 0.4 | 2.2 | 2.2 | 0.5 | 1.4 | 2.2 | .401 |
| LNG, mm | 0.1 | 3.0 | 2.2 | –1.1 | 2.9 | 2.9 | .028 |
| VRT, mm | 0.2 | 2.3 | 2.0 | 1.9 | 2.8 | 2.5 | .001 |
| Mag, mm | 5.0 | 1.6 | 1.9 | 5.7 | 2.2 | 2.2 | .001 |
| Iso, ° | –0.2 | 0.8 | 0.6 | –0.5 | 0.5 | 0.8 | .022 |
| Pitch, ° | 0.5 | 0.8 | 0.9 | 0.0 | 0.5 | 0.6 | .009 |
| Roll,° | 0.3 | 0.9 | 1.1 | 0.2 | 0.5 | 0.7 | .382 |
Abbreviations: µ = cohort average; Ʃ = cohort residual systematic setup error; σ = cohort residual random setup error; LAT = lateral correction; LNG = longitudinal correction; Mag = magnitude; SGRT = surface-guided radiation therapy; VRT = vertical correction; WF = workflow.
Figure 3.
Cumulative histograms showing (A-C) the residual postimaging 3-dimensional correction vectors, and (D-F) summary of positioning average time, kV image matching average time, and standard deviation for the skin marks as well as SGRT method, and reimaging frequency (G) for all investigated patients: pelvis (P1-11), limb (L1-11), and chest/spine (C1-10), respectively. Abbreviations: C = chest/spine; L = limb; mm:ss = minutes and seconds; P = pelvis; SGRT = surface-guided radiation therapy.
Positioning and matching time: Skin marks versus SGRT
The average time and the SD recorded for the positioning and kV image matching process for each patient in both WFs are introduced in Fig. 3. The irradiation time is not included.
In addition, the total setup time, including the positioning on the treatment table and kV image matching, was as follows, with statistically significant differences of time by .018 and .023 for pelvis and chest/spine, respectively, whereas for the cohort of limb patients, a nonsignificant shortening of total setup time was observed (P = .05): (1) pelvis cohort: 05:57 ± 02:11 minutes and seconds (mm:ss) for skin marks versus 04:54 ± 01:06 (mm:ss) for SGRT; (2) limb cohort: 05:39 ± 02:12 (mm:ss) for skin marks versus 05:09 ± 01:34 (mm:ss) for SGRT; and (3) chest/spine cohort: 06:09 ± 02:26 (mm:ss) for skin marks versus 05:15 ± 01:30 (mm:ss) for SGRT.
Frequency of reimaging required before treatment: Skin marks versus SGRT
Another component of the analysis included the assessment of reimaging frequency. Reimaging means, as mentioned previously (Fig. 2), not only repeating the positioning WF but also more ionizing doses for the patient. Thus, the entire treatment WF efficiency is heavily dependent on the positioning process. Figure 3 depicts reimaging performed for each patient during the assessed number of the 8 to 10 fractions. By using SGRT for patient positioning, the reimaging frequency decreased from about 7% to 2%, with a statistically significant difference of .042.
Discussion
The initial patient positioning before treatment, merely based on 3 skin marks (ie, tattoos), which must be found by the therapists, is not adequate enough to avoid patient setup inefficiencies, especially in torsions. In addition, existing tattoos from previous radiation therapies may be confused with the marks of the actual treatment.23 In case of darker-colored or heavily freckled skin, hair follicles and moles may be mistaken with tattoos because of their similar appearance, resulting in potential patient setup errors.12 Furthermore, it is common for therapists to shift the skin surface to align the tattoos with IRL without changing the position of the internal anatomy.13 These pitfalls highlight the need for improvements in initial patient setup, because repeating the whole patient positioning process, including planar kV imaging, exposes the patient to more radiation. This can delay therapy, generates stress for both patient and treatment team, and extends the patient's treatment period. These inefficiencies could be minimized by implementing SGRT as a complementary for planar kV imaging for more accurate positioning.
Previous studies in conventional radiation therapy using a linear accelerator have investigated the reliability, stability, and reproducibility of different SGRT systems for setup and intrafraction motion monitoring during radiation therapy treatments of abdomen, pelvis, extremity, chest, and head and neck.13,24, 25, 26, 27, 28, 29, 30 These studies focus on techniques like stereotactic radiosurgery, stereotactic body radiation therapy, and deep inspiration breath hold in the photon beam treatments, and the final patient position mostly verified by CBCT, which is not available in our ion gantry treatment room. They showed that the use of nonionizing SGRT may replace the skin marks, provide more accurate initial patient positioning than skin marks before CBCT or planar kV imaging, and enable more secure intrafractional monitoring than the WF with skin marks. Furthermore, the total setup time when using the SGRT can be shorter than WF with skin marks.27,28,31,32
Our study design focuses on using the AlignRT system to optimize patient positioning WF in a unique treatment room, where the environment is quite different from conventional RT because of the considerably large size of the treatment machine and treatment room. Although the implementation is well defined for photon-beam facilities, only a few analyses have been published for ion-beam therapy centers. Batin et al31 investigated the setup accuracy of SGRT and a traditional planar radiographic technique for postmastectomy chest wall patients treated with proton therapy. The authors found that SGRT provided both more accurate and faster patient setups compared with conventional radiographic setup technique. The results of this study confirm these findings and extend them for further body regions. However, this study provides no points over the correlation between both skin marks and SGRT. Finally, the reliability, accuracy, and efficiency of optical surface imaging will be discussed in this study by comparing both WF schemes, skin marks, and SGRT, based on investigations of 3 patient cohorts (pelvis, limb, and chest/spine).
Reliability and accuracy of patient positioning: Skin marks versus SGRT
Our investigation indicates that the use of SGRT significantly decreases the cohort translational couch magnitude shifts during patient setup by 0.5 ± 1.4 mm for pelvis patients and by 1.9 ± 0.5 mm for limb patients (P < .05), whereas for chest/spine patients, SGRT significantly increases the magnitude shifts by 0.7 ± 0.3 mm (P = .001). The reason for the latter finding is probably the free-breathing CT scans used for chest/spine treatment planning, which are affected by the interplay between scanning in the CT and free breathing. Thus, the current position constantly deviates from the reference position, particularly in vertical, longitudinal, and pitch errors. Table 2 shows that rotational corrections were predominantly lowered with SGRT for all cohorts, with significant differences in pitch for pelvis (P = .002) and chest/spine (P = .009).
The reliability of SGRT to position the patient more accurately may be attributed to several factors. Skin marks use the positions of only 3 points on the skin surface, whereas SGRT uses a 3D ROI that covers nearly the entire treated region for surface matching. This study also shows that SGRT reduced most translational and rotational systematic and random errors, because positioning using the skin marks is more affected by circulation of personnel and individual experiences for each patient. The WF steps using SGRT are more consistent for every patient and less dependent on the personnel if the therapists’ team is well trained. In our center, 85% of our therapists’ team have more than 1 year of experience, and 61% have more than 3 years’ experience with the SGRT system. Furthermore, the SGRT WF can potentially replace the WF with skin marks and IRL for the 3 cohorts investigated in this study.
Positioning and matching time: Skin marks versus SGRT
Using SGRT resulted in a considerable reduction in total patient setup time (positioning and matching time) in our investigation. SGRT-based positioning before treatment required 18%, 9%, and 15% less time for the entire setup procedure for pelvis, limb, and chest/spine patients, respectively, than with the skin marks positioning approach. Furthermore, the SD by employing the SGRT system eased off, on average, by 49%, 29%, and 38% for pelvis, limb, and chest/spine patients, respectively. This latter finding implies that the initial patient positioning became more resilient against setup inefficiencies. Our data also have shown a strong correlation, .001 < P < .045 for SGRT, and .001 < P < .011 for skin marks between positioning time and matching time. Finally, 53% of all studied patients had faster positioning time, and 87.5% had faster matching time by using SGRT compared with skin marks (Fig. 3). Moreover, this can even be improved if the initial position is automatically corrected using SGRT.
Frequency of reimaging required before treatment: Skin marks versus SGRT
The data show a clear reduction of reimaging frequency when SGRT was used for the initial patient positioning of pelvis and chest/spine patients (Fig. 3). This indicates potentially a more consistent WF. The greater reimaging frequency at pelvis and chest/spine compared with limb is related to the different immobilization methods. Although most patients of the pelvis and chest/spine are immobilized using ProSTEP, HeadSTEP, and WingSTEP, enabling more mobility, using the skin marks in this case only is not sufficient to detect rotational setup errors, especially pitch and roll. In contrast, all patients of the limb are immobilized using BlueBAG vacuum cushions, with less mobility compared with pelvis and chest/spine immobilization approaches. However, SGRT is particularly helpful to position limbs.
The reason for the reimaging in the WF with SGRT is related to nonsufficient experiences by drawing an appropriate ROI, which may distort the true position of the patient. Thus, it is recommended to use more than 1 ROI during the positioning process.
Potential role of SGRT for detecting anatomic changes
Large discrepancies between skin marks and SGRT, or planar kV imaging and SGRT, may potentially serve as an indicator for anatomic changes (eg, weight fluctuations or tissue swelling). Even so, the capability of SGRT to possibly detect anatomic changes on the skin surface in the area of the anterior beam entry should be investigated and confirmed for ion-beam therapy with future studies, because ion-beam treatments are more sensitive to changes in water-equivalent thickness in beam entry path compared with photons due to Bragg peak, which may lead to an altered dose distribution within the target volume and OAR.
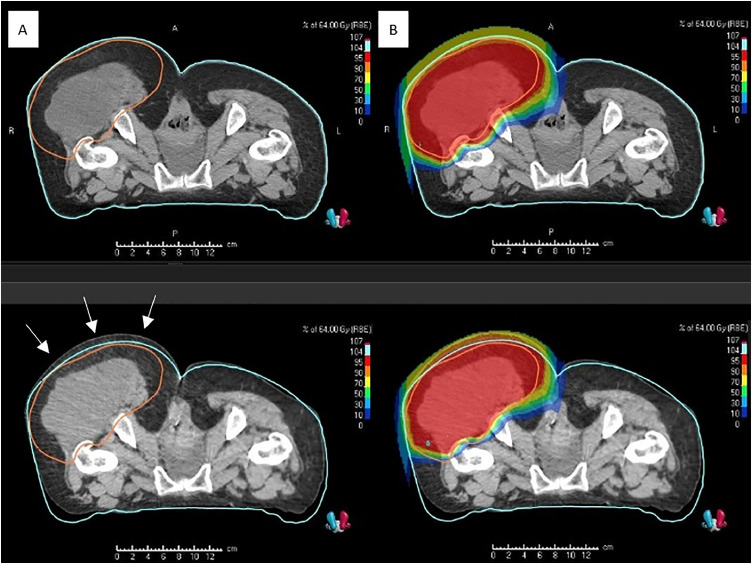
A chondrosarcoma was an example of anatomic changes in our investigation. The tumor showed a growth by approximately 1 cm in the beam entrance region when initially using SGRT. There were significant differences between planar kV imaging and SGRT in position. This was confirmed through a comparison between the regular control CT and the original planning CT. The irradiation plan had to be adapted using a new planning CT (Fig. 4). Another example of postoperative edema in the lower extremity at the time of planning CT was regredient in the interval of 11 days between planning and initiation of treatment. A comparison between the original planned CT and a regular control CT determined a decrease in the soft tissue by roughly 6 to 8 mm posterior of the target volume. These anatomic modifications had no effect on the irradiation. In this scenario, no replanning was necessary.
Figure 4.
Dose distribution degradation caused by tumor growth, approximately 1 cm during the treatment course of a patient with chondrosarcoma and confirmed by the control CT. The left upper panel (A) illustrates the planning CT including the contouring of the skin surface and clinical target volume (CTV), and the left lower panel shows the tumor growth on the control CT after 4 irradiated fractions. The right lower panel (B) illustrates the shifted dose distribution on the control CT transferred from the original plan (right upper). Source: HIT. Abbreviations: CT = computed tomograpy.
Finally, the main disadvantage of using skin marks for patient positioning before treatment is that the skin marks on the elastic patient skin might undergo significant deviations over the course of therapy due to anatomic changes, which may be detected by comparing both reference and current patient skin surface in AlignRT system on a daily basis. That would be a very important benefit for particle therapy centers where is no CBCT available.
Limitations and considerations of SGRT
The gantry construction, the large beam nozzle, patient geometry, and immobilization devices obscured and reduced the field of view of the 3 AlignRT pods in numerous instances. This resulted in unprocessed surface regions where the system was unable to acquire surface data. The position of the 3 pods was optimized before settling on a compromise position that resulted in reduced obstruction by the beam nozzle and sufficient field of view for patient positioning.
The inability to scan very dark skin tones, which results in surface degradations that look like holes, is another unavoidable technical constraint of using SGRT. This is crucial for institutions that serve a greater number of patients with darker skin tones.33
In addition, the AlignRT system in HIT is not connected to the treatment table, which is a significant difference from a typical linear accelerator setup. As a result, the computed shifts of AlignRT require manual adjustment by moving the treatment table using the hand control. Thus, we assume that direct feedback of the SGRT system to the treatment table may result in further decrease in residual setup errors, improved setup accuracy, and faster positioning process before treatment.
A further limitation of using SGRT to analyze patient position deviation is the ability to exclusively evaluate visible patient skin surface to the pods. In this case, brain and head and neck patients with a thermoplastic mask can benefit from SGRT by using open-face or minimal mask immobilization, which were investigated in previous studies.24,27,28
Conclusions
We found that employing SGRT as a complementary for planar kV imaging can optimize the patient positioning WF and can increase the positioning accuracy before treatments of patients with tumors of the pelvis, limb, and chest/spine without extra imaging dose. On the basis of our data, using SGRT for patient positioning considerably reduced the total setup time and reimaging frequency and enhanced the WF efficiency. Thus, the reliability of our unique SGRT installation is confirmed for patient positioning. We also found that a good ROI design can lead to a more robust patient positioning using SGRT. In addition, this SGRT system will be used for other purposes, such as intrafractional irradiation monitoring and gating methods.
Furthermore, SGRT also may provide the ability to detect anatomic changes in anterior beam regions that potentially influence dose distribution in CTV and OARs, particularly in superficial target volumes where the internal target potentially correlates with the patient skin surface. The correlation between the internal target and the external skin surface, which is influenced by several factors, such as tumor location, age, sex, and body mass index, should be investigated using SGRT to use the full benefits of SGRT.
Acknowledgments
We gratefully acknowledge the professional contributions of our therapists in the gantry treatment room. Without their support, the work would not have succeeded. We also thank Beata Koczur and Thomas Mielke for sharing their proficiency in treatment planning, as well as Dr Jakob Liermann and Dr Katharina Seidensaal for their helpful consultations regarding patient treatments. For a part of the publication fee, we acknowledge the financial support by Deutsche Forschungsgemeinschaft within the funding programme “Open Access Publikationskosten” as well as by the Heidelberg University.
Footnotes
Sources of support: The German Cancer Research Center (DKFZ) and Heidelberg Institute of Radiation Oncology (HIRO) funded the surface-guided radiation therapy project at the Heidelberg Ion Beam Therapy Center (HIT).
Disclosures: Dr Debus reports grants from the Clinical Research Institute, ViewRay Inc, Accuray International, Accuray Incorporated, RaySearch Laboratories AB, Vision RT, Merck Serono GmbH, Astellas Pharma GmbH, AstraZeneca GmbH, Siemens Healthcare GmbH, Solution Akademie GmbH, Eromed PLC Surrey Research Park, Quintiles GmbH, Pharmaceutical Research Associates GmbH, Boehringer Ingelheim Pharma GmbH Co, Physikalisch-Technische Werkstätten (PTW) Freiburg Dr Pychlau GmbH, and Nanobiotix, outside the submitted work. No other disclosures were reported.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Jäkel O. Medical physics aspects of particle therapy. Radiat Prot Dosimetry. 2009;137:156–166. doi: 10.1093/rpd/ncp192. [DOI] [PubMed] [Google Scholar]
- 2.Jäkel O, Schulz-Ertner D, Karger CP, Nikoghosyan A, Debus J. Heavy ion therapy: status and perspectives. Technol Cancer Res Treat. 2003;2:377–387. doi: 10.1177/153303460300200503. [DOI] [PubMed] [Google Scholar]
- 3.Willoughby T, Lehmann J, Bencomo JA, et al. Quality assurance for nonradiographic radiotherapy localization and positioning systems: Report of Task Group 147. Med Phys. 2012;39:1728–1747. doi: 10.1118/1.3681967. [DOI] [PubMed] [Google Scholar]
- 4.Karger CP, Jäkel O, Debus J, Kuhn S, Hartmann GH. Three-dimensional accuracy and interfractional reproducibility of patient fixation and positioning using a stereotactic head mask system. Int J Radiat Oncol Biol Phys. 2001;49:1493–1504. doi: 10.1016/s0360-3016(00)01562-5. [DOI] [PubMed] [Google Scholar]
- 5.Eichkorn T, König L, Held T, et al. Carbon ion radiation therapy: One decade of research and clinical experience at Heidelberg Ion Beam Therapy Center. Int J Radiat Oncol Biol Phys. 2021;111:597–609. doi: 10.1016/j.ijrobp.2021.05.131. [DOI] [PubMed] [Google Scholar]
- 6.Jiang SB. Radiotherapy of mobile tumors. Semin Radiat Oncol. 2006;16:239–248. doi: 10.1016/j.semradonc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Landry G, Hua CH. Current state and future applications of radiological image guidance for particle therapy. Med Phys. 2018;45:e1086–e1095. doi: 10.1002/mp.12744. [DOI] [PubMed] [Google Scholar]
- 8.Clow B, Allen J. Psychosocial impacts of radiation tattooing for breast cancer patients: A critical review. Can Woman Stud. 2010;28:46–52. [Google Scholar]
- 9.Landeg SJ, Kirby AM, Lee SF, et al. A randomized control trial evaluating fluorescent ink versus dark ink tattoos for breast radiotherapy. Br J Radiol. 2016;89:6–8. doi: 10.1259/bjr.20160288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bregnhøj A, Hædersdal M. Q-switched YAG laser vs. punch biopsy excision for iatrogenic radiation tattoo markers—A randomized controlled trial. J Eur Acad Dermatol Venereol. 2010;24:1183–1186. doi: 10.1111/j.1468-3083.2010.03617.x. [DOI] [PubMed] [Google Scholar]
- 11.Moser T, Creed M, Walker R, Meier G. Radiotherapy tattoos: Women's skin as a carrier of personal memory—What do we cause by tattooing our patients? Breast J. 2020;26:316–318. doi: 10.1111/tbj.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathod S, Munshi A, Agarwal J. Skin markings methods and guidelines: A reality in image guidance radiotherapy era. South Asian J Cancer. 2012;1:27–29. doi: 10.4103/2278-330X.96502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley DN, Mcconnell KA, Kirby N, Gutiérrez AN, Papanikolaou N, Rasmussen K. Comparison of initial patient setup accuracy between surface imaging and three point localization: A retrospective analysis. J Appl Clin Med Phys. 2017;18:58–61. doi: 10.1002/acm2.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiant DB, Wentworth S, Maurer JM, Vanderstraeten CL, Terrell JA, Sintay BJ. Surface imaging-based analysis of intrafraction motion for breast radiotherapy patients. J Appl Clin Med Phys. 2014;15:147–159. doi: 10.1120/jacmp.v15i6.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mast M, Perryck S. Introduction to: Surface guided radiotherapy (SGRT) Tech Innov Patient Support Radiat Oncol. 2022;22:37–38. doi: 10.1016/j.tipsro.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bert C, Metheany KG, Doppke K, Chen GTY. A phantom evaluation of a stereo-vision surface imaging system for radiotherapy patient setup. Med Phys. 2005;32:2753–2762. doi: 10.1118/1.1984263. [DOI] [PubMed] [Google Scholar]
- 17.Freislederer P, Kügele M, Öllers M, et al. Recent advanced in surface guided radiation therapy. Radiat Oncol. 2020;15(1) doi: 10.1186/s13014-020-01629-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoisak JDP, Paxton AB, Waghorn B, Pawlicki T. CRC Press; Boca Raton, FL: 2020. Surface Guided Radiation Therapy. [Google Scholar]
- 19.Batista V, Meyer J, Kügele M, Al-Hallaq H. Clinical paradigms and challenges in surface guided radiation therapy: Where do we go from here? Radiother Oncol. 2020;153:34–42. doi: 10.1016/j.radonc.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Nairz O, Winter M, Heeg P, Jäkel O. Accuracy of robotic patient positioners used in ion beam therapy. Radiat Oncol. 2013;8:1–7. doi: 10.1186/1748-717X-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijhold J, Lebesque JV, Hart AAM, Vijlbrief RE. Maximizing setup accuracy using portal images as applied to a conformal boost technique for prostatic cancer. Radiother Oncol. 1992;24:261–271. doi: 10.1016/0167-8140(92)90233-k. [DOI] [PubMed] [Google Scholar]
- 22.De Boer HCJ, Van Sörnsen De Koste JR, Creutzberg CL, Visser AG, Levendag PC, Heijmen BJM. Electronic portal image assisted reduction of systematic set-up errors in head and neck irradiation. Radiother Oncol. 2001;61:299–308. doi: 10.1016/s0167-8140(01)00437-6. [DOI] [PubMed] [Google Scholar]
- 23.Rigley J, Robertson P, Scattergood L. Radiotherapy without tattoos: Could this work? Radiography. 2020;26:288–293. doi: 10.1016/j.radi.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhao B, Maquilan G, Jiang S, Schwartz DL. Minimal mask immobilization with optical surface guidance for head and neck radiotherapy. J Appl Clin Med Phys. 2018;19:17–24. doi: 10.1002/acm2.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzerling JH, Hampton CJ, Robinson M, et al. Use of surface-guided radiation therapy in combination with IGRT for setup and intrafraction motion monitoring during stereotactic body radiation therapy treatments of the lung and abdomen. J Appl Clin Med Phys. 2020;21:48–55. doi: 10.1002/acm2.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bert C, Metheany KG, Doppke KP, Taghian AG, Powell SN, Chen GTY. Clinical experience with a 3D surface patient setup system for alignment of partial-breast irradiation patients. Int J Radiat Oncol Biol Phys. 2006;64:1265–1274. doi: 10.1016/j.ijrobp.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Huang S, Zhang L, et al. Accuracy of surface-guided patient setup for conventional radiotherapy of brain and nasopharynx cancer. J Appl Clin Med Phys. 2021;22:48–57. doi: 10.1002/acm2.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W, Ioannides PJ, Sehgal V, Daroui P. Quantifying the impact of optical surface guidance in the treatment of cancers of the head and neck. J Appl Clin Med Phys. 2020;21:73–82. doi: 10.1002/acm2.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitz D, Walter F, Schönecker S, et al. Stability and reproducibility of 6013 deep inspiration breath-holds in left-sided breast cancer. Radiat Oncol. 2020;15:1–9. doi: 10.1186/s13014-020-01572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naumann P, Batista V, Farnia B, et al. Feasibility of optical surface-guidance for position verification and monitoring of stereotactic body radiotherapy in deep-inspiration breath-hold. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.573279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batin E, Depauw N, MacDonald S, Lu HM. Can surface imaging improve the patient setup for proton postmastectomy chest wall irradiation? Pract Radiat Oncol. 2016;6:e235–e241. doi: 10.1016/j.prro.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen D, Farah J, Josserand-Pietri F, Barbet N, Khodri M. Benefits and challenges of standard ceiling-mounted surface guided radiotherapy systems for breast treatments on Halcyon. Radioprotection. 2021;56:295–301. [Google Scholar]
- 33.Al-Hallaq HA, Cerviño L, Gutierrez AN, et al. AAPM task group report 302: Surface-guided radiotherapy. Med Phys. 2022;49:e82–e112. doi: 10.1002/mp.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]