Abstract
This study was conducted to investigate the effects of fermented mixed feed (FMF) on growth performance, carcass traits, meat quality, muscle amino acid and fatty acid composition and mRNA expression levels of genes related to lipid metabolism in finishing pigs. In the present study, 144 finishing pigs (Duroc × Berkshire × Jiaxing Black) were randomly allocated to 3 dietary treatments with 4 replicate pens per group and 12 pigs per pen. The dietary treatments included a basal diet (CON), a basal diet + 5% FMF and a basal diet + 10% FMF. The experiment lasted 38 d after 4 d of acclimation. The results showed that 5% and 10% FMF significantly increased the average daily gain (ADG) of the females but not the males (P < 0.05), but FMF supplementation showed no impact on carcass traits. Moreover, 10% FMF supplementation increased the meat color45 min and meat color24 h values, while it decreased the shear force relative to CON (P < 0.05). In addition, 10% FMF significantly increased the contents of flavor amino acids (FAA), total essential AA (EAA), total non-EAA (NEAA) and total AA relative to CON (P < 0.05). Furthermore, the diet supplemented with 10% FMF significantly increased the concentration of n-3 polyunsaturated fatty acids (PUFA), n-6 PUFA and total PUFA, and the PUFA to saturated fatty acids ratio (P < 0.05), suggesting that FMF supplementation increased meat quality. Moreover, compared with the CON, 10% FMF supplementation increased the mRNA expression of lipogenic genes, including CEBPα, PPARγ, SREBP1 and FABP4, and upregulated the expression of unsaturated fatty acid synthesis (ACAA1 and FADS2). Together, our results suggest that 10% FMF dietary supplementation improved the female pigs’ growth performance, improved the meat quality and altered the profiles of muscle fatty acids and amino acids in finishing pigs. This study provides a reference for the production of high-quality pork.
Keywords: Fermented mixed feed, Growth performance, Meat quality, Amino acid profile, Fatty acid composition, Finishing pig
1. Introduction
Pork is one of the most widely consumed meats and among the most important sources of protein for humans. In recent years, consumer demand for high-quality and healthy pork has been increasing, hence improving the meat quality of pork is an effective practice in the development of animal husbandry. The evaluation indices of meat quality include shear force, drip loss, meat color, pH value and intramuscular fat (IMF) content (Frank et al., 2016; Lomiwes et al., 2014), among which the IMF content is closely related to the sensory characteristics of meat, including flavor, juiciness and tenderness (Hao et al., 2020; Van Elswyk and Mcneill, 2014). Additionally, the composition and content of amino acids, especially the FAA, greatly affect the flavor and taste of meat (Pereira and Vicente, 2013). Furthermore, the content and composition of fatty acids in muscle play a key role in meat quality, which determines the nutritional value and flavor of the meat. Previous studies have demonstrated that polyunsaturated fatty acids (PUFA) are tied to anti-obesity and anti-inflammatory properties, particularly n-3 PUFA (Buckley and Howe, 2009; Tortosa-Caparrós et al., 2017). Nutritional regulation is an effective means to improve pork quality. Indeed, some feed additives, such as lycopene, β-glucan and betaine, have been shown to increase the IMF content and improve the pork amino acid and fatty acid profiles in finishing pigs (Luo et al., 2019; Wen et al., 2022; Zhong et al., 2021).
Fermented mixed feed (FMF) is a product of microbial fermentation, which effectively degrades antinutritional factors in feed while producing probiotics and their metabolites (Cao et al., 2012; Shi et al., 2015). Our previous study showed that microbial fermentation improved feed nutritional value and bioavailability (Hao et al., 2020; Shi et al., 2017; Wang et al., 2018). Furthermore, dietary FMF supplementation contributes to improving growth performance and meat quality, enhancing gut health and reducing the diarrhea rate in animal production (Ding et al., 2020; Kiers et al., 2003; Missotten et al., 2013; Tang et al., 2021). Our group also found that growth performance and meat quality were improved in finishing pigs (Duroc × Landrace × Large White) fed FMF (Hao et al., 2020). Taken together, FMF shows great application prospects to improve the growth performance of pigs and the quality of meat produced.
However, the effects of dietary FMF supplementation on growth performance and meat quality in Duroc × Berkshire × Jiaxing Black pigs have not been reported. Here, we used corn, soybean meal, and wheat bran as substrates and fermented them with a complex microbial combination (Bacillus subtilis and Enterococcus faecalis) to obtain FMF. Notably, the basal substrate and microbial combination were different from our previous studies (Hao et al., 2020). Therefore, this study aimed to explore the effects of FMF supplementation on the growth performance, carcass traits, meat quality, muscle fatty acid and amino acid profiles and mRNA expression levels of genes related to lipid metabolism in the longissimus dorsi muscle (LDM) in finishing Duroc × Berkshire × Jiaxing Black pigs.
2. Materials and methods
2.1. Animal ethics statement
All the procedures were approved by the Institutional Animal Care and Use Committee at Zhejiang University.
2.2. Fermented mixed feed preparation and chemical analysis
The FMF preparation method was based on previous research (Wang et al., 2018). Enterococcus faecium (E. faecium) was obtained from Baolai-leelai Biotech Co., Ltd (Tai'an, China). The B. subtilis (B. subtilis) ZJU12 used in this study was isolated from traditional fermented food (pickled vegetables). Pilot production of FMF was carried out at the Kesheng Feed Co., Ltd, Zhejiang, China. The basal substrate contained 40% corn, 40% soybean meal and 20% wheat bran. Whilst stirring evenly, sterile water was added to make the total moisture in system 40%. One kilogram of E. faecium powder (108 cfu/g) and 1.25 kg of B. subtilis powder (3 × 108 cfu/g) was inoculated into 1,000 kg of wet mixed substrate. Then, the wet mixed substrate was transferred to a plastic bag with a one-way valve (Rou Duoduo Biotechnology Co., Beijing, China), sealed, and fermented at room temperature for 72 h.
The contents of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF) and acid detergent fiber (ADF) in mixed feed (MF) and fermented mixed feed (FMF) were analyzed according to the AOAC International guidelines (2005). The trichloroacetic acid-soluble protein (TCA-SP) was measured according to reported methods (Ovissipour et al., 2009). The lactic acid content was evaluated using a commercial assay kit (Nanjing Jiancheng Bio Co., Nanjing, China) according to the manufacturer's instructions. Chemical analysis of the MF and FMF is presented in Table 1.
Table 1.
Nutrient composition of mixed feed and fermented mixed feed (as-fed basis).
| Item | MF | FMF |
|---|---|---|
| DM, % | 92.77 | 93.01 |
| CP, % | 26.39 | 28.03 |
| TCA-SP, % | 2.58 | 5.05 |
| TCA-SP:CP ratio, % | 9.77 | 18.01 |
| NDF, % | 34.11 | 32.45 |
| ADF, % | 20.02 | 19.42 |
| Hemicellulose, % | 14.09 | 13.03 |
| Lactic acid, mmol/kg | 96.67 | 117.78 |
| pH | 6.62 | 3.84 |
| Live BS cells, cfu/g | – | 1.3 × 108 |
| Live EF cells, cfu/g | – | 8.0 × 108 |
MF = mixed feed; FMF = fermented mixed feed (40% corn, 40% soybean meal, 20% wheat bran); CP = crude protein; TCA-SP = trichloroacetic acid–soluble protein (small peptides); TCA-SP:CP ratio = TCA-SP to CP ratio; NDF = neutral detergent fiber; ADF = acid detergent fiber; Hemicellulose = NDF-ADF; BS = Bacillus subtilis; EF = Enterococcus faecium.
2.3. Experimental design and diets
A total of 144 finishing pigs (body weight = 77.21 ± 8.31 kg) were randomly divided into 3 dietary treatments with 4 replications (12 pigs per replication). Each treatment contained 2 male replicates and 2 female replicates. In addition, each group consisted of half males and half females. The dietary treatments included a basal diet (CON), a basal diet + 5% FMF and a basal diet + 10% FMF. All experimental diets were formulated to meet the nutritional requirements of finishing pigs according to the Chinese National Feeding Standard for swine and contained similar levels of crude protein (CP) and metabolizable energy (ME) (Table 2). All pigs were weighed after 4 d of prefeeding. The experiment lasted 38 days. All pigs had free access to water and food and were fed 3 times (at 07:30, 13:30 and 17:30) per day.
Table 2.
Ingredients and nutrient levels of experimental diets (%, as-fed basis).1
| Item | Diet |
||
|---|---|---|---|
| CON | 5% FMF | 10% FMF | |
| Ingredients | |||
| Corn | 40.50 | 36.20 | 32.10 |
| Soybean meal | 14.00 | 13.30 | 12.60 |
| Barley | 15.00 | 15.00 | 15.00 |
| Rice bran meal | 4.00 | 3.00 | 1.80 |
| Flour | 8.00 | 8.00 | 8.00 |
| Defatted rice bran | 8.00 | 8.00 | 8.00 |
| Wheat middlings | 6.00 | 6.00 | 6.00 |
| Soyabean oil | 1.00 | 2.00 | 3.00 |
| FMF | 0.00 | 5.00 | 10.00 |
| Premix2 | 3.50 | 3.50 | 3.50 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient level | |||
| Digestible energy, MJ/kg | 13.44 | 13.43 | 13.43 |
| Crude protein | 15.37 | 15.37 | 15.36 |
| Crude fat | 4.43 | 5.33 | 6.23 |
| Crude fiber | 3.38 | 3.34 | 3.28 |
| Calcium | 0.57 | 0.57 | 0.58 |
| Total phosphorus | 0.58 | 0.56 | 0.54 |
| Available phosphorus | 0.21 | 0.20 | 0.20 |
| Lysine | 0.95 | 0.95 | 0.96 |
| Methionine | 0.30 | 0.30 | 0.30 |
| Methionine + Cystine | 0.57 | 0.57 | 0.57 |
| Threonine | 0.65 | 0.65 | 0.65 |
| Tryptophan | 0.17 | 0.17 | 0.17 |
| Valine | 0.71 | 0.71 | 0.71 |
CON = basal diet; FMF = fermented mixed feed.
Basal diet formulated according to the Chinese National Feeding Standard for swine.
Provided the following per kilogram of complete diet: vitamin A, 7,500 IU; vitamin D3, 1,800 IU; vitamin E, 54 IU; vitamin K3, 4.8 mg; vitamin B12, 0.024 mg; vitamin B1, 2.7 mg; vitamin B2, 7.2 mg; vitamin B6, 5.4 mg; D-biotin: 0.18 mg; folic acid, 0.9 mg; nicotinamide, 36 mg; D-pantothenic acid, 24 mg; Cu, 15 mg; Fe, 50 mg; Zn, 45 mg; Mn, 20 mg; I, 0.5 mg; Se, 0.35 mg.
2.4. Sample collection and carcass characteristics measurements
All pigs were weighed on d 38 and fasted for 12 h before slaughter. Then, 18 pigs (6 pigs/treatment group) were selected and slaughtered humanely after electrical stunning in a commercial slaughterhouse (Qinglian Food Co., Ltd., Zhejiang, China). After slaughter, the hot carcass weight of each pig was measured and used to calculate carcass yield. The oblique length and straight length were measured using band tape. The backfat thickness at the thickest part of the shoulder, thoracolumbar junction and lumbar-sacral junction were recorded and used to calculate the average backfat value. Additionally, the LDM was removed for on-site testing and the rest of the muscle samples were quickly frozen in liquid nitrogen and stored at - 80 °C to determine LDM amino acids, fatty acids and gene expression.
2.5. Meat quality measurements
The LDM samples stored at 4 °C were used for pH, meat color, drip loss, shear force and marbling score analyses. Meat quality evaluation was carried out according to a previous report (Nong et al., 2020). After slaughter, the LDM pH value was measured at 45 min and 24 h with a portable pH meter (pH-Star Matthäus GmbH, Pöttmes, Germany). The meat color values, including lightness (L∗), redness (a∗) and yellowness (b∗), were also evaluated at 45 min and 24 h after slaughter using a Minolta CM-2002 spectrophotometer (Osaka, Japan). For drip loss, approximately 10 g of each LDM sample was hung in a special sealed plastic tube at 4 °C and weighed after 24 h. The shear force was measured with a C-LM tenderness tester (Tenovo International Co., Limited, Beijing, China) according to the manufacturer's instructions. The marbling score at 45 min after slaughter was evaluated according to an NPPC meat color chart (Nanjing Mingao Instrument Equipment Co., Ltd., Nanjing, China).
2.6. Amino acid composition
First, approximately 0.1 g LDM of each sample was weighed and digested with 5 mL of 6 mol/L HCl solution in 105 °C oven for 24 h. Then, the volume was bought up to 50 mL in a volumetric flask and the sample was filtered through a 0.22-μm water phase filter into a centrifuge tube. Next, 2 mL of the filtrate was placed in an evaporating dish in a 60 °C water bath for evaporation and 4 mL of 0.02 mol/L HCl solution was added. After dissolution, the sample was stored at 4 °C for detection with an ion-exchange AA analyzer (L8900, Hitachi, Tokyo, Japan).
2.7. Fatty acid composition
The fatty acid composition was determined by gas chromatography (GC) as described previously (Hao et al., 2020). First, the LDM samples were extracted with a mixture of chloroform and methanol (2:1; vol/vol). Approximately 20 g of each LDM sample was weighed and dried in an oven at 105 °C for 1 h, and then 1 g of each dried sample was weighed and leached with petroleum ether for 3 h. A total of 60 mg of the extracted fat was placed in a test tube, 4 mL of isooctane was added to fully dissolve the sample, and then 200 μL potassium hydroxide-methanol and 1 g of sodium bisulfate were added. After salt precipitation, the solution containing the methyl esters was drawn into the upper layer and stored in a refrigerator at 4 °C. Each sample was filtered through a 0.22-nm filter membrane before GC detection (Model 7890 A, Agilent Technologies, Palo Alto, CA, USA). Finally, the fatty acid concentration was analyzed by GC ChemStation software (Agilent Technologies, Palo Alto, CA, USA).
2.8. Hematoxylin-eosin staining
After slaughter, the LDM was isolated and fixed in fixative purchased from Servicebio (CAT: G1111-100ML) followed by paraffin sectioning to obtain tissue sections. Then, the LDM paraffin sections were stained using a hematoxylin-eosin staining kit (Servicebio, Wuhan, China). In brief, after deparaffinization with xylene, the sections were stained with hematoxylin solution for 5 min. After that, they were immersed in 1% acid ethanol (1% HCl in 70% ethanol) for 10 s and rinsed with running water. Finally, the sections were stained with eosin for 2 to 3 min, dehydrated with pure alcohol and rendered transparent with xylene. The morphological structure and size of the cells were observed with an Olympus BX61 fluorescence microscope (Japan).
2.9. Intramuscular fat and triglyceride measurement
The IMF content was evaluated by following the Chinese Agriculture Industry Standard NY/T 2793-2015. For triglyceride measurement, the samples of LDM were homogenized with normal saline solution (tissue weight:normal saline solution = 1:9) and centrifuged for 10 min. The concentrations of triglycerides in the LDM were measured with the corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions. In addition, the concentration of protein in the homogenate was detected using a BCA Protein Assay Reagent Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.10. Real-time quantitative PCR
RNA extraction, library construction and quantitative real-time PCR (qPCR) of the LDM samples were performed as described in our previous study (Shan et al., 2013; Xu et al., 2021). Briefly, total RNA was isolated from the LDM muscle using TRIzol reagent (Thermo Fisher, Waltham, MA, USA) according to the instructions of the manufacturer as previously described (Shan et al., 2013; Xu et al., 2021). RNA quality and concentration were measured with a NanoDrop 2000 instrument (Gene Company Limited, Hong Kong, China). A ReverAid First Strand cDNA Synthesis Kit (Thermo Fisher) and random primers were used to generate cDNA by reverse transcription. The primer sequences are listed in Table 3. Quantitative real-time PCR was performed with an Applied Biosystems StepOnePlus Real-Time PCR System with SYBR Green Master Mix (Roche, Indianapolis, IN, United States). Relative gene expression was analyzed by the 2−ΔΔCT method.
Table 3.
Primer sequences used in this study.
| Gene | Primer | Sequence (5′ to 3′) | GenBank ID | Size, bp |
|---|---|---|---|---|
| GAPDH | Forward Reverse |
AAGGAGTAAGAGCCCCTGGA TCTGGGATGGAAACTGGAA |
NM_001206359.1 | 140 |
| CEBPα | Forward Reverse |
AACAACTGAGCCGCGAACTGGA CTTGAGATCTGGAGACCCGAAACC |
XM_003127015.4 | 170 |
| PPARγ | Forward Reverse |
AAGACGGGGTCCTCATCTCC CGCCAGGTCGCTGTCATCT |
NM_214379.1 | 149 |
| SREBP1 | Forward Reverse |
AAGCGGACGGCTCACAATG CGCAAGACGGCGGATTTAT |
NM_214157.1 | 122 |
| ACCα | Forward Reverse |
TTCCAGGCACAGTCCTTAGG TCATCCAACACGAGCTCAGT |
NM_001114269.1 | 161 |
| FABP4 | Forward Reverse |
TGGAAACTTGTCTCCAGTG GGTACTTTCTGATCTAATGGTG |
NM_001002817.1 | 147 |
| ACAA1 | Forward Reverse |
CGAGCTTCTCTCTGCAGTCAT TGGGATGTCACTCAGAAACTGG |
XM_003132103.4 | 148 |
| FADS2 | Forward Reverse |
GCTGGATTCCAACCCTCATG AGCCTGGGCCTGAGAGGTA |
NM_001171750.1 | 56 |
| SCD | Forward Reverse |
GCCACCTTTCTTCGTTACG CCTCACCCACAGCTCCCAAT |
NM_213781.1 | 142 |
GAPDH = glyceraldehyde-3-phosphate dehydrogenase; CEBPα = CCAAT enhancer-binding protein α; PPARγ = peroxisome proliferator-activated receptor γ; SREBP1 = sterol regulatory element binding protein 1; ACCα = acetyl CoA carboxylase α; FABP4 = fatty acid-binding protein 4; ACAA1 = acetyl-CoA acyltransferase 1; FADS2 = fatty acid desaturase 2; SCD = stearoyl Coenzyme A desaturase.
2.11. Statistical analyses
All data in the current study were analyzed by one-way analysis of variance (ANOVA) with the statistical software SPSS 20.0 (Chicago, IL, USA) followed by Duncan's multiple range analysis. P < 0.05 was considered to be statistically significant. All results are shown as the means and standard error of the mean (SEM).
3. Results
3.1. Nutrient composition of the fermented mixed feed
The nutrient compositions of MF and FMF are shown in Table 1. The contents of DM, CP and lactic acid in FMF were higher than those in unfermented MF, while FMF contained less NDF, ADF and hemicellulose. Furthermore, as presented in the Supplementary Data (Tables S1 and S2), we also analyzed the amino acid and fatty acid compositions of MF and FMF. After fermentation, the contents of EAA, NEAA and total AA were higher in FMF than those in unfermented MF. Regarding fatty acid composition, microbial fermentation improved the contents of monounsaturated fatty acid (MUFA) and PUFA, while reducing the levels of saturated fatty acids (SFA).
3.2. Growth performance
The growth performance of the finishing pigs is presented in Table 4. The ADG values of the females in the 5% and 10% FMF groups were significantly higher than those in the CON group (P = 0.026), however, the ADG of the males showed no significant difference. In addition, there was no difference in the other indices of growth performance.
Table 4.
Effects of fermented mixed feeds on the growth performance of finishing pigs.
| Item | Treatment |
SEM | P-value | ||
|---|---|---|---|---|---|
| CON | 5% FMF | 10% FMF | |||
| Males | |||||
| Initial weight, kg | 76.88 | 77.40 | 75.75 | 0.95 | 0.776 |
| Final weight, kg | 108.73 | 109.42 | 109.13 | 1.22 | 0.974 |
| ADG, kg/d | 0.84 | 0.84 | 0.88 | 0.01 | 0.459 |
| ADFI, kg/d | 3.32 | 3.28 | 3.31 | ||
| F:G | 4.06 | 3.99 | 3.88 | ||
| Females | |||||
| Initial weight, kg | 79.67 | 76.35 | 77.52 | 1.03 | 0.412 |
| Final weight, kg | 107.07 | 106.00 | 107.74 | 1.19 | 0.837 |
| ADG, kg/d | 0.72b | 0.78a | 0.79a | 0.01 | 0.026 |
| ADFI, kg/d | 3.19 | 2.90 | 3.22 | ||
| F:G | 4.41 | 3.75 | 4.14 | ||
CON = basal diet; FMF = fermented mixed feed; ADG = average daily gain; ADFI = average daily feed intake; F:G = the ratio of feed intake to body weight gain.
a,b Within a row, values with different superscripts differ significantly at P < 0.05. Data are expressed as means and SEM, n = 24.
3.3. Carcass characteristics
As shown in Table 5, the live weights and carcass traits were higher in the 10% FMF group than those in the CON group (P < 0.05). No difference in carcass characteristics was found between the 5% FMF group and the CON group. In addition, compared with the 5% FMF group, 10% FMF supplementation significantly increased backfat thickness at the lumbar-sacral junction (P = 0.018).
Table 5.
Effects of fermented mixed feeds on the carcass traits of finishing pigs.
| Item | Treatment |
SEM | P-value | ||
|---|---|---|---|---|---|
| CON | 5% FMF | 10% FMF | |||
| Live weight, kg | 104.58b | 111.33ab | 116.08a | 1.88 | 0.032 |
| Carcass weight, kg | 77.35b | 81.33ab | 85.23a | 1.32 | 0.039 |
| Carcass yield, % | 72.34 | 73.04 | 74.56 | 0.46 | 0.129 |
| Carcass oblique length, cm | 84.17 | 88.08 | 88.17 | 0.98 | 0.167 |
| Carcass straight length, cm | 97.08 | 101.33 | 100.92 | 1.29 | 0.354 |
| Skin thick, mm | 3.35 | 3.30 | 2.93 | 0.13 | 0.377 |
| Backfat thickness at the thickest part of the shoulder, mm | 46.38 | 46.07 | 49.37 | 1.46 | 0.622 |
| Backfat thickness at thoracolumbar junction, mm | 29.48 | 33.15 | 34.18 | 1.14 | 0.218 |
| Backfat thickness at lumbar-sacral junction, mm | 22.83ab | 19.13b | 26.22a | 1.09 | 0.018 |
| Backfat, mm | 31.75 | 31.77 | 37.37 | 1.50 | 0.222 |
| Loin-eye area, cm | 45.40 | 48.74 | 51.07 | 1.27 | 0.191 |
CON = basal diet; FMF = fermented mixed feed.
a,b Within a row, values with different superscripts differ significantly at P < 0.05. Data are expressed as means and SEM, n = 6.
3.4. Meat quality
The effects of FMF on the meat quality of finishing pigs are presented in Table 6. On the one hand, compared with CON, dietary 5% FMF supplementation significantly increased the meat color45 min value while significantly reducing the shear force (P < 0.05). On the other hand, 10% FMF supplementation significantly increased meat color45 min and meat color24 h values, and significantly decreased shear force relative to CON (P < 0.05). In addition, the pH24 h of 10% FMF supplementation was higher than that in the CON group (P = 0.051).
Table 6.
Effects of fermented mixed feeds on the meat quality of finishing pigs.
| Item | Treatment |
SEM | P-value | ||
|---|---|---|---|---|---|
| CON | 5% FMF | 10% FMF | |||
| pH45 min | 6.41 | 5.88 | 6.21 | 0.09 | 0.054 |
| pH24 h | 5.59 | 5.52 | 5.92 | 0.07 | 0.051 |
| pH48 h | 5.67 | 5.61 | 5.79 | 0.07 | 0.659 |
| Meat color45 min | 77.09b | 82.60a | 82.72a | 1.06 | 0.034 |
| L∗45 min | 45.03 | 42.99 | 41.69 | 0.70 | 0.141 |
| a∗45 min | 9.56 | 9.91 | 10.08 | 0.19 | 0.557 |
| b∗45 min | 9.28 | 8.47 | 8.44 | 0.19 | 0.112 |
| Meat color24 h | 69.95b | 72.97b | 79.36a | 1.40 | 0.010 |
| L∗24 h | 50.00 | 49.06 | 45.70 | 0.86 | 0.091 |
| a∗24 h | 11.18 | 12.03 | 11.28 | 0.34 | 0.578 |
| b∗24 h | 11.58 | 12.51 | 10.49 | 0.45 | 0.188 |
| Drip loss, % | 1.96 | 1.65 | 1.55 | 0.08 | 0.082 |
| Shear force, N | 149.33a | 93.62b | 96.50b | 9.31 | 0.012 |
| Marbling scores | 1.83 | 1.67 | 2.17 | 0.18 | 0.537 |
CON = basal diet; FMF = fermented mixed feed.
a,b Within a row, values with different superscripts differ significantly at P < 0.05. Data are expressed as means and SEM, n = 6.
3.5. Free amino acid profiles in the longissimus dorsi muscle
The free amino acid profiles in the LDM are shown in Table 7. Both 5% and 10% FMF supplementation significantly increased the contents of flavor AA (FAA), total EAA, total NEAA and total AA relative to CON (P < 0.05). In detail, the concentrations of EAA (lysine, methionine, and threonine) and NEAA (alanine, aspartate, glutamate, arginine, serine, and tyrosine) were significantly increased with FMF supplementation (P < 0.05). In addition, only 10% FMF supplementation improved the concentration of isoleucine compared with CON (P = 0.047). The content of leucine in the 5% FMF group was higher than that in the CON group (P = 0.044). Moreover, the concentration of phenylalanine showed a linear increase in response to the FMF supplementation ratio, with a maximum observed in the 10% FMF group (P < 0.001). Notably, 5% FMF supplementation markedly increased the total protein content relative to CON (P = 0.023).
Table 7.
Effects of fermented mixed feeds on the free amino acid profile of the longissimus dorsi muscle in finishing pigs (g/kg, as-fresh basis).
| Item | Treatment |
SEM | P-value | ||
|---|---|---|---|---|---|
| CON | 5% FMF | 10% FMF | |||
| EAA | |||||
| Lysine | 18.45b | 19.58a | 19.51a | 0.17 | 0.003 |
| Methionine | 4.42b | 4.75a | 4.80a | 0.07 | 0.048 |
| Valine | 10.47 | 10.87 | 10.88 | 0.08 | 0.055 |
| Isoleucine | 9.88b | 10.23ab | 10.33a | 0.08 | 0.047 |
| Leucine | 16.12b | 16.80a | 16.65ab | 0.12 | 0.044 |
| Phenylalanine | 6.68c | 7.10b | 7.47a | 0.09 | <0.001 |
| Histidine | 9.35 | 9.65 | 9.50 | 0.09 | 0.395 |
| Threonine | 8.60b | 9.20a | 9.18a | 0.10 | 0.008 |
| NEAA | |||||
| Alanine | 11.12b | 11.73a | 11.63a | 0.10 | 0.009 |
| Aspartate | 17.80b | 19.08a | 18.97a | 0.20 | 0.005 |
| Glutamate | 28.45b | 30.48a | 30.25a | 0.35 | 0.024 |
| Arginine | 12.48b | 13.27a | 13.07a | 0.13 | 0.026 |
| Glycine | 9.15 | 9.17 | 9.23 | 0.08 | 0.921 |
| Serine | 7.10b | 7.60a | 7.60a | 0.09 | 0.016 |
| Tyrosine | 6.78b | 7.23a | 7.17a | 0.07 | 0.005 |
| Proline | 7.52 | 7.60 | 7.43 | 0.08 | 0.743 |
| FAA1 | 79.00b | 83.73a | 83.15a | 0.80 | 0.018 |
| Total EAA | 83.97b | 88.18a | 88.32a | 0.74 | 0.012 |
| Total NEAA | 100.40b | 106.17a | 105.35a | 0.99 | 0.024 |
| Total AA | 184.50b | 194.67a | 193.67a | 1.71 | 0.016 |
| Total protein | 205.33b | 219.67a | 214.50ab | 2.29 | 0.023 |
CON = basal diet; FMF = fermented mixed feed; EAA = essential amino acids; NEAA = non-essential amino acids; FAA = flavor amino acids.
a,b Within a row, values with different superscripts differ significantly at P < 0.05. Data are expressed as means and SEM, n = 6.
1FAA = glutamate + aspartate + alanine + arginine + glycine.
3.6. Fatty acid profiles in the longissimus dorsi muscle
Diets supplemented with FMF greatly altered the fatty acid profiles in the LDM (Table 8). Compared with CON, 10% FMF supplementation significantly increased the concentration of n-3 PUFA, n-6 PUFA, total PUFA and the PUFA:SFA ratio (P < 0.05). The concentrations of C18:2n6c, C18:3n3, and C20:3n3 were higher in the 10% FMF group than in the CON group (P < 0.05). Additionally, 5% FMF supplementation significantly decreased the concentrations of C14:0 and C20:0 (P < 0.05), whereas the concentration of C24:1 markedly increased relative to CON (P = 0.022). However, the concentration of C20:1 in the 10% FMF group was higher than that in the 5% FMF supplementation group (P = 0.029). There were no pronounced differences in the concentrations of MUFA or the n-6 to n-3 ratio (P > 0.05).
Table 8.
Effects of fermented mixed feeds on the fatty acid profile of the longissimus dorsi muscle in finishing pigs (g/kg, as-fresh basis).
| Item | Treatment |
SEM | P-value | ||
|---|---|---|---|---|---|
| CON | 5% FMF | 10% FMF | |||
| C10:0 | 0.11 | 0.09 | 0.10 | 0.00 | 0.228 |
| C14:0 | 1.45a | 0.99b | 1.36a | 0.08 | 0.034 |
| C16:0 | 26.27 | 23.45 | 24.67 | 1.06 | 0.580 |
| C16:1 | 2.10 | 1.75 | 1.77 | 0.11 | 0.331 |
| C17:0 | 0.26 | 0.23 | 0.26 | 0.02 | 0.617 |
| C18:0 | 11.71 | 5.61 | 9.86 | 1.15 | 0.077 |
| C18:1n9c | 36.58 | 31.03 | 38.20 | 1.57 | 0.149 |
| C18:2n6c | 13.47b | 12.87b | 17.82a | 0.69 | 0.001 |
| C18:3n3 | 0.83b | 0.90b | 1.39a | 0.09 | 0.010 |
| C20:0 | 0.27a | 0.17b | 0.24a | 0.00 | 0.001 |
| C20:1 | 0.25ab | 0.20b | 0.27a | 0.00 | 0.029 |
| C20:2 | 0.75 | 0.65 | 0.83 | 0.03 | 0.118 |
| C20:3n3 | 0.13b | 0.13b | 0.18a | 0.01 | 0.003 |
| C20:3n6 | 0.13 | 0.14 | 0.15 | 0.00 | 0.242 |
| C20:4n6 | 0.27 | 0.37 | 0.38 | 0.02 | 0.088 |
| C22:1n9 | 0.09 | 0.08 | 0.09 | 0.01 | 0.796 |
| C24:1 | 0.08b | 0.18a | 0.12ab | 0.02 | 0.022 |
| SFA1 | 40.07 | 30.54 | 36.48 | 1.84 | 0.095 |
| MUFA2 | 39.11 | 33.25 | 40.46 | 1.66 | 0.170 |
| PUFA3 | 15.58b | 15.05b | 20.74a | 0.80 | 0.001 |
| PUFA:SFA ratio | 0.40b | 0.50ab | 0.59a | 0.29 | 0.022 |
| n-3 PUFA4 | 0.96b | 1.02b | 1.57a | 0.10 | 0.008 |
| n-6 PUFA5 | 13.86b | 13.37b | 18.34a | 0.70 | 0.001 |
| n-6:n-3 PUFA ratio | 14.50 | 13.13 | 12.21 | 0.94 | 0.131 |
CON = basal diet; FMF = fermented mixed feed; SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid.
a,b Within a row, values with different superscripts differ significantly at P < 0.05. Data are expressed as means and SEM, n = 6.
SFA = C10:0 + C14:0 + C16:0 + C17:0 + C18:0 + C20:0.
MUFA = C16:1 + C18:1n9c + C20:1 + C22:1n9 + C24:1.
PUFA = C18:2n6c + C18:3n3 + C20:2 + C20:3n3 + C20:3n6 + C20:4n6.
n-3 PUFA = C18:3n3 + C20:3n3.
n-6 PUFA = C18:2n6c + C20:3n6 + C20:4n6.
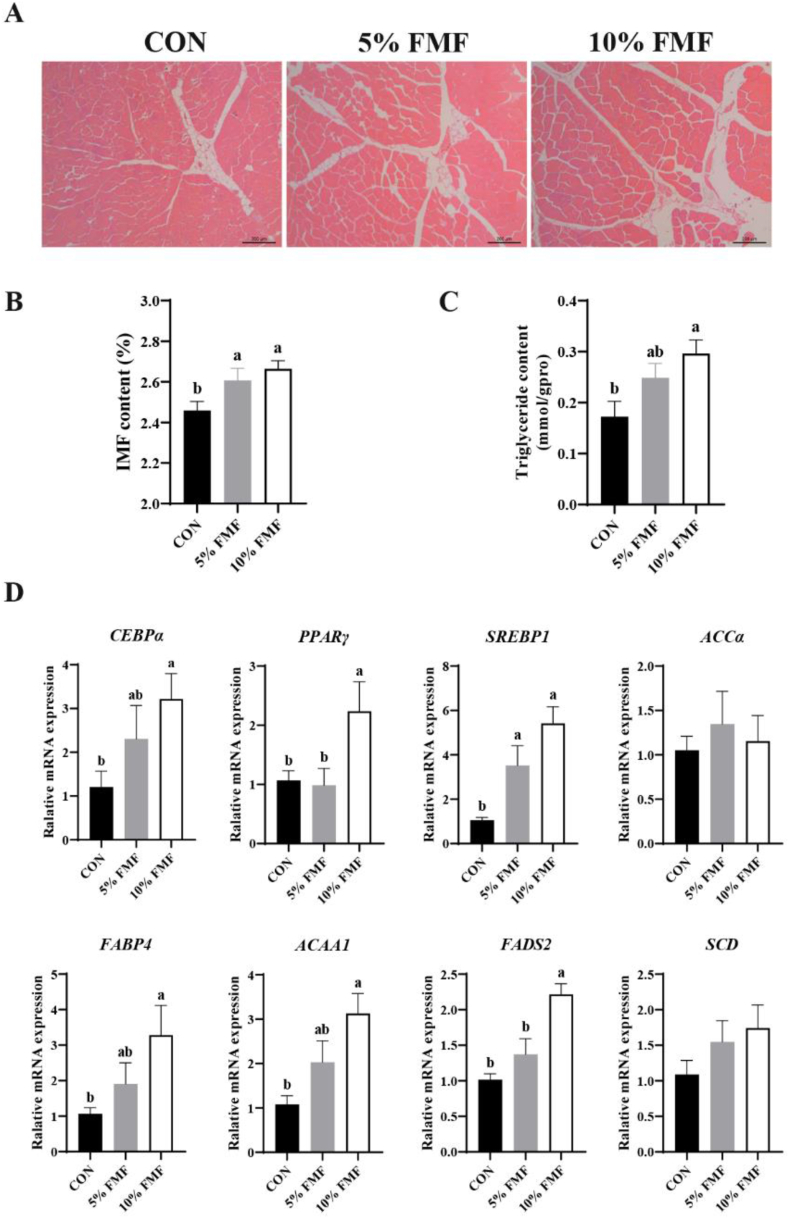
3.7. The IMF contents and mRNA expression levels of genes related to lipid metabolism
As shown in Fig. 1A, FMF supplementation increased the number of adipocytes by H&E staining. Furthermore, 5% and 10% FMF supplementation significantly increased the IMF content in the LDM (P < 0.05) (Fig. 1B). Among the three groups, the TG content in the 10% FMF group was significantly higher than that in CON (P < 0.05) (Fig. 1C). To further explore how FMF improves the IMF content and fatty acid profile, we detected the mRNA expression levels of genes related to lipid metabolism, such as fatty acid synthesis and transport, in the LDM. As shown in Fig. 1D, the expression of CEBPα PPARγ and SREBP1, which are key transcription factors for lipid metabolism, in the 10% FMF supplementation group was significantly higher than that in the CON group (P < 0.05). Moreover, compared with CON, 10% FMF supplementation significantly upregulated the expression levels of genes related to fatty acid uptake and transport, such as FABP4 (P < 0.05). Furthermore, 10% FMF supplementation significantly increased the expression levels of the unsaturated fatty acid synthesis genes ACAA1 and FADS2 (P < 0.05). However, there was no dramatic difference in the mRNA expression levels of ACCα and SCD.
Fig. 1.
Effects of fermented mixed feeds on the intramuscular fat content and relative mRNA expression of genes related to lipid metabolism in longissimus dorsi muscle (LDM) of finishing pigs. (A) Hematoxylin and eosin staining analyzed the percentage of adipocytes and the morphological structure of LDM. (B) Intramuscular fat (IMF) content in LDM. (C) Triglyceride (TG) content in LDM. (D) Real-time quantitative PCR analyzed the mRNA expressions of CCAAT enhancer-binding protein α (CEBPα), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element binding protein 1 (SREBP1), acetyl CoA carboxylase α (ACCα), fatty acid-binding protein 4 (FABP4), acetyl-CoA acyltransferase 1 (ACAA1) fatty acid desaturase 2 (FADS2) and stearoyl Coenzyme A desaturase (SCD). Data are presented as the mean and SEM (n = 6). a, b Bars with different letters indicate significant difference (P < 0.05). CON = basal diet; FMF = fermented mixed feed.
4. Discussion
The present study was designed to examine the effects of FMF supplementation on growth performance, carcass characteristics, meat quality and muscle amino acid and fatty acid composition in finishing pigs (Duroc × Berkshire × Jiaxing Black). We found that FMF supplementation improved the growth performance, meat quality and muscle amino acid and fatty acid compositions. Furthermore, we also found that FMF supplementation upregulated the mRNA expression of genes related to adipogenesis.
Previous studies have shown that FMF provides many advantages to animal growth, such as increased feed intake, nutrient utilization and gut health maintenance (Canibe and Jensen, 2003; Ding et al., 2020; Mukherjee et al., 2016). These effects are attributed to the ability of microorganisms, such as B. subtilis, to degrade antinutritional factors and macromolecular nutrients in the feed (Chun-Hua et al., 2016). Our previous study found that B. subtilis and E. faecium co-fermentation decreased the contents of β-conglycinin and glycinin, two antinutritional factors, while increasing the contents of CP, TCA-SP, Ca and total P in the feed (Wang et al., 2018). Furthermore, FMF improved the performance of females and their progeny. Therefore, in this study, we focused on the effects of B. subtilis and E. faecium co-fermentation on feed nutrient levels, such as fatty acid and amino acid compositions. We observed that B. subtilis and E. faecium co-fermentation increased the concentrations of CP, total AA and FAA, and improved the contents of MUFA and PUFA. Intriguingly, the SFA content decreased after B. subtilis and E. faecium co-fermentation. In line with previous reports, microbial fermentation also increased the concentrations of TCA-SP, a class of easily absorbed small molecule nutrients, and lactic acid (Hao et al., 2020; Wang et al., 2018).
Previous studies have shown that diets supplemented with FMF improved growth performance and nutrient digestibility in finishing pigs (Tian et al., 2020; Xu et al., 2017; Yan et al., 2012). In the present study, we found that FMF supplementation increased the ADG of females, but did not affect the ADG of males. This may be due to gender differences in the effect of FMF on growth performance, but the specific mechanism is unclear. Notably, although the carcass weights were higher in the 10% FMF group than in the CON group, this could be attributed to the difference in live weight and did not account for the benefits of FMF. The slaughter weight was not consistent with the final weight, because we calculated final weights using 48 finishing pigs per group while the calculation of carcass weights only referred to 6 randomly selected pigs. Unlike previous reports (Hao et al., 2020), we did not observe an increase in ocular muscle area with dietary FMF addition. This difference may be attributed to the different breeds of pigs.
The evaluation indicators of meat quality included IMF contents, marbling score, meat color, shear force and drip loss. Previous studies have shown that FMF supplementation increased marbling scores and IMF contents in finishing pigs (Duroc × Landrace × Large White) (Hao et al., 2020). In the present study, the IMF contents were significantly increased in the FMF supplementation group compared to the CON group, but there was no significant difference in marbling scores between the three groups. In line with previous studies (Guo et al., 2020; Hao et al., 2020), we observed that FMF improved the meat color of the LDM in finishing pigs, as the meat color45 min and meat color24 h values were greater in the 10% group in comparison to CON, whereas 5% FMF administration also significantly increased the meat color45 min values. Muscle shear force represents the tenderness of meat. Others have shown that FMF supplementation had no effect on muscle shear force (Lu et al., 2020; Tian et al., 2020). Contrary to previous reports, we demonstrated that FMF markedly decreased the shear force of the LDM in finishing pigs. Together, the present findings confirm that dietary FMF supplementation improved meat quality, especially in the 10% FMF group. A popular explanation is that B. subtilis and E. faecium in FMF play a key role because the antibiotic metabolites from these microbes have been shown to improve meat quality (Meng et al., 2010). Specifically, metabolites produced by microorganisms may be beneficial for improving meat quality, such as through promoting the deposition of IMF and the conversion of fast-twitch fibers to slow-twitch fibers. However, these explanations need further verification.
The amino acid profiles of muscles are closely related to the flavor and nutritional value of meat (Moeller et al., 2010). Recently, nutritional intervention has been recognized as an effective means to improve the quality of pork, including amino acid composition (Xu et al., 2019; Yu et al., 2020). In this study, we observed that dietary FMF supplementation increased the concentrations of total EAA, NEAA and AA, whereas 5% FMF significantly improved the total protein content. Specifically, our results showed that pigs fed FMF had increased concentrations of lysine, methionine, phenylalanine, threonine, alanine, aspartate, glutamate, arginine, serine and tyrosine. However, previous research has not found such significant benefits from FMF (Lu et al., 2020). We speculate that this might be due to differences in the experimental animals, fermentation substrates and strains. In addition, the content of FAA in muscles is closely related to meat flavor (Pereira and Vicente, 2013). Furthermore, in the current study, the contents of flavor amino acids (FAA), such as glutamate, aspartate, alanine and arginine, were increased with FMF supplement. The findings are directly in line with previous studies (Li et al., 2022; Tian et al., 2020). To date, the exact mechanism that regulates amino acid composition remains unclear. Notably, the contents of CP, total AA and FAA increased after microbial fermentation in this study, which may be the main reason for the change in the amino acid profiles (Table S1).
The fatty acid profiles in the IMF determine the nutritional value and oxidative stability of muscle and are particularly important for meat quality and meat product acceptability (Duan et al., 2016). A high intake of SFAs has been reported to increase the risk of type-2 diabetes (T2D) and heart disease (Lenighan et al., 2019), while PUFA, in particular EPA and DHA, have been found to possess numerous benefits, such as anti-inflammatory, glycolipid metabolism regulation and muscle development properties (Tachtsis et al., 2018; Tortosa-Caparrós et al., 2017; Vaidya and Cheema, 2014; Vissers et al., 2019). In the current study, only 10% FMF supplementation increased the content of PUFA and the PUFA:SFA ratio. These results tie in well with previous studies (Hao et al., 2020). Specifically, our results indicated that 10% FMF supplementation increased the concentrations of n-3 PUFA and n-6 PUFA, such as C18:2n6c, C18:3n3, and C20:3n3. Previous studies have shown that high n-6 and low n-3 PUFA intake (average ratio 15:1) contributes to the development of nonalcoholic fatty liver disease (NAFLD) (Toshimitsu et al., 2007). Our results suggested that the n-6 to n-3 PUFA ratio in the LDM was lower than 15:1 in all groups. Although FMF supplementation reduced the n-6 to n-3 ratio, no significant difference was found. However, there have been few published studies on how FMF affects the fatty acid profiles of pork, and it is difficult to explain the exact mechanism by which the fatty acid profiles of pigs fed FMF were altered. A plausible explanation may be that microbial fermentation optimized the fatty acid composition of the feed, as microbial fermentation increased the contents of MUFA and PUFA and decreased the content of SFAs in this study. As for why the effect of the 10% FMF group was more pronounced than that of the 5% FMF group, we speculated that the main reason was that the addition of FMF was positively correlated with the nutritional levels of amino acids and fatty acids in the feed. As shown in Table S1 and Table S2, microbial fermentation increased the content of amino acids and fatty acids in the feed, resulting in a more significant effect on the composition of muscle amino acids and fatty acids in the 10% FMF group.
To date, few studies have been conducted on the role of FMF on lipid metabolism-related genes. As stated above, the IMF content increased with 5% and 10% FMF supplementation. However, it is unclear whether the expression of genes involved in the control of fat deposition was altered. ACCα and FABP4 are two key genes involved in fatty acid synthesis and transport (Furuhashi and Hotamisligil, 2008; Munday, 2002). In the present study, 10% FMF supplementation upregulated the expression level of FABP4. These results are not entirely consistent with previous reports (Yu et al., 2020), as there was no difference in ACCα expression. The addition of FMF significantly increased the contents of unsaturated fatty acids and changed the fatty acid composition. We further examined the expression of unsaturated fatty acid synthesis-related genes, including ACAA1, FADS2 and SCD (Xu et al., 2021). We observed that 10% FMF supplementation increased the expression levels of ACAA1 and FADS2. CEBPα, PPARγ and SREBP are key nuclear transcription factors related to lipid synthesis and metabolism that can regulate the expression of downstream target genes, including ACCα and FABP4 (Oishi et al., 2017; Stoeckman and Towle, 2002; Wang et al., 2020). In the present study, the mRNA expression levels of CEBPα, PPARγ and SREBP1 were all markedly upregulated in the 10% FMF group. In summary, this study provides evidence that 10% FMF supplementation promoted fatty acid synthesis and transport, which was attributed to fatty acid profile alterations and IMF deposition.
5. Conclusion
In conclusion, the current study demonstrated that dietary 5% and 10% FMF supplementation improved the ADG in female finishing pigs. In addition, dietary FMF supplementation improved the meat quality and altered the amino acid and fatty acid compositions in the LDM. Furthermore, the upregulated expression of genes related to lipid metabolism might mediate these benefits. However, further research is needed to explore the mechanism by which FMF improves meat quality in finishing pigs. Taken together, this study suggests that FMF has great prospects for improving growth performance and meat quality in finishing pigs.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
We thank all the members of the Shan Laboratory for their support and constructive comments. This work was partially supported by the National Key R&D Program of China (2021YFC2103005) and the Zhejiang Provincial Key R&D Program of China (2021C02008).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2022.09.003.
Contributor Information
Tenghao Wang, Email: wangth85@126.com.
Tizhong Shan, Email: tzshan@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Buckley J.D., Howe P.R. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes Rev. 2009;10:648–659. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- Canibe N., Jensen B.B. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J Anim Sci. 2003;81:2019–2031. doi: 10.2527/2003.8182019x. [DOI] [PubMed] [Google Scholar]
- Cao F.L., Zhang X.H., Yu W.W., Zhao L.G., Wang T. Effect of feeding fermented ginkgo biloba leaves on growth performance, meat quality, and lipid metabolism in broilers. Poultry Sci. 2012;91:1210–1221. doi: 10.3382/ps.2011-01886. [DOI] [PubMed] [Google Scholar]
- Chun-Hua Chi, Cho Seong-Jun. Improvement of bioactivity of soybean meal by solid-state fermentation with bacillus amyloliquefaciens versus lactobacillus spp. and saccharomyces cerevisiae. LWT - Food Sci Technol (Lebensmittel-Wissenschaft -Technol) 2016 [Google Scholar]
- Ding X., Li H., Wen Z., Hou Y., Wang G., Fan J., Qian L. Effects of fermented tea residue on fattening performance, meat quality, digestive performance, serum antioxidant capacity, and intestinal morphology in fatteners. Animals (Basel) 2020;10 doi: 10.3390/ani10020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Li F., Li Y., Guo Q., Ji Y., Tan B., Li T., Yin Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids. 2016;48:2131–2144. doi: 10.1007/s00726-016-2223-2. [DOI] [PubMed] [Google Scholar]
- Frank D., Joo S.T., Warner R. Consumer acceptability of intramuscular fat. Korean J Food Sci Anim Resour. 2016;36:699–708. doi: 10.5851/kosfa.2016.36.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Zhang Y., Xv J., Hou Y., Ding B. Partial substitution of fermented soybean meal for soybean meal influences the carcass traits and meat quality of broiler chickens. Animals. 2020;10:225. doi: 10.3390/ani10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Su W., Zhang Y., Wang C., Lu Z. Effects of supplementing with fermented mixed feed on the performance and meat quality in finishing pigs. Anim Feed Sci Technol. 2020;266 [Google Scholar]
- Kiers J.L., Meijer J.C., Nout M.J., Rombouts F.M., Nabuurs M.J., Van Der Meulen J. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J Appl Microbiol. 2003;95:545–552. doi: 10.1046/j.1365-2672.2003.02011.x. [DOI] [PubMed] [Google Scholar]
- Lenighan Y.M., Mcnulty B.A., Roche H.M. Dietary fat composition: replacement of saturated fatty acids with pufa as a public health strategy, with an emphasis on α-linolenic acid. Proc Nutr Soc. 2019;78:234–245. doi: 10.1017/S0029665118002793. [DOI] [PubMed] [Google Scholar]
- Li H., Duan Y., Yin F., Zhu Q., Hu C., Wu L., Xie P., Li F., Cheng R., Kong X. Dietary addition of fermented sorghum distiller's dried grains with soluble improves carcass traits and meat quality in growing-finishing pigs. Trop Anim Health Prod. 2022;54:97. doi: 10.1007/s11250-022-03089-8. [DOI] [PubMed] [Google Scholar]
- Lomiwes D., Farouk M.M., Wiklund E., Young O.A. Small heat shock proteins and their role in meat tenderness: a review. Meat Sci. 2014;96:26–40. doi: 10.1016/j.meatsci.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Lu J., Han Q., Wang S., Wang Z., Shi X. Effect of fermented corn–soybean meal on carcass and meat quality of grower-finisher pigs. J Anim Physiol Anim Nutr. 2020 doi: 10.1111/jpn.13444. [DOI] [PubMed] [Google Scholar]
- Luo J., Zeng D., Cheng L., Mao X., Yu J., Yu B., Chen D. Dietary β-glucan supplementation improves growth performance, carcass traits and meat quality of finishing pigs. Anim Nutr. 2019;5:380–385. doi: 10.1016/j.aninu.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.W., Yan L., Ao X., Zhou T.X., Kim I.H. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J Anim Sci. 2010;88:3320. doi: 10.2527/jas.2009-2308. [DOI] [PubMed] [Google Scholar]
- Missotten J.A., Michiels J., Dierick N., Ovyn A., Akbarian A., De Smet S. Effect of fermented moist feed on performance, gut bacteria and gut histo-morphology in broilers. Br Poultry Sci. 2013;54:627–634. doi: 10.1080/00071668.2013.811718. [DOI] [PubMed] [Google Scholar]
- Moeller S.J., Miller R.K., Edwards K.K., Zerby H.N., Logan K.E., Aldredge T.L., Stahl C.A., Boggess M., Box-Steffensmeier J.M. Consumer perceptions of pork eating quality as affected by pork quality attributes and end-point cooked temperature. Meat Sci. 2010;84:14–22. doi: 10.1016/j.meatsci.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Mukherjee R., Chakraborty R., Dutta A. Role of fermentation in improving nutritional quality of soybean meal - a review. Asian-Australas J Anim Sci. 2016;29:1523–1529. doi: 10.5713/ajas.15.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday M.R. Regulation of mammalian acetyl-coa carboxylase. Biochem Soc Trans. 2002;30:1059–1064. doi: 10.1042/bst0301059. [DOI] [PubMed] [Google Scholar]
- Nong Q., Wang L., Zhou Y., Sun Y., Chen W., Xie J., Zhu X., Shan T. Low dietary n-6/n-3 pufa ratio regulates meat quality, reduces triglyceride content, and improves fatty acid composition of meat in heigai pigs. Animals (Basel) 2020;10 doi: 10.3390/ani10091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y., Spann N.J., Link V.M., Muse E.D., Strid T., Edillor C., Kolar M.J., Matsuzaka T., Hayakawa S., Tao J., Kaikkonen M.U., Carlin A.F., Lam M.T., Manabe I., Shimano H., Saghatelian A., Glass C.K. Srebp1 contributes to resolution of pro-inflammatory tlr4 signaling by reprogramming fatty acid metabolism. Cell Metabol. 2017;25:412–427. doi: 10.1016/j.cmet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovissipour M., Abedian A., Motamedzadegan A., Rasco B., Safari R., Shahiri H. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (acipenser persicus) viscera. Food Chem. 2009;115:238–242. [Google Scholar]
- Pereira P.M., Vicente A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013;93:586–592. doi: 10.1016/j.meatsci.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Shan T., Liang X., Bi P., Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the ampk-pgc1α-fndc5 pathway in muscle. Faseb J. 2013;27:1981–1989. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., He J., Yu J., Yu B., Huang Z., Mao X., Zheng P., Chen D. Solid state fermentation of rapeseed cake with aspergillus Niger for degrading glucosinolates and upgrading nutritional value. J Anim Sci Biotechnol. 2015;6:13. doi: 10.1186/s40104-015-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Zhang Y., Lu Z., Wang Y. Solid-state fermentation of corn-soybean meal mixed feed with bacillus subtilis and enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J Anim Sci Biotechnol. 2017;8:50. doi: 10.1186/s40104-017-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckman A.K., Towle H.C. The role of srebp-1c in nutritional regulation of lipogenic enzyme gene expression. J Biol Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- Tachtsis B., Camera D., Lacham-Kaplan O. Potential roles of n-3 PUFA during skeletal muscle growth and regeneration. Nutrients. 2018;10 doi: 10.3390/nu10030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Liu X., Zhang K. Effects of microbial fermented feed on serum biochemical profile, carcass traits, meat amino acid and fatty acid profile, and gut microbiome composition of finishing pigs. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.744630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z., Deng D., Cui Y., Chen W., Yu M., Ma X. Diet supplemented with fermented okara improved growth performance, meat quality, and amino acid profiles in growing pigs. Food Sci Nutr. 2020;8:5650–5659. doi: 10.1002/fsn3.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa-Caparrós E., Navas-Carrillo D., Marín F., Orenes-Piñero E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit Rev Food Sci Nutr. 2017;57:3421–3429. doi: 10.1080/10408398.2015.1126549. [DOI] [PubMed] [Google Scholar]
- Toshimitsu K., Matsuura B., Ohkubo I., Niiya T., Furukawa S., Hiasa Y., Kawamura M., Ebihara K., Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46–52. doi: 10.1016/j.nut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Vaidya H., Cheema S.K. Sea cucumber and blue mussel: new sources of phospholipid enriched omega-3 fatty acids with a potential role in 3t3-l1 adipocyte metabolism. Food Funct. 2014;5:3287–3295. doi: 10.1039/c4fo00330f. [DOI] [PubMed] [Google Scholar]
- Van Elswyk M.E., Mcneill S.H. Impact of grass/forage feeding versus grain finishing on beef nutrients and sensory quality: the u.S. Experience. Meat Sci. 2014;96:535–540. doi: 10.1016/j.meatsci.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Vissers L.E.T., Rijksen J., Boer J.M.A., Verschuren W.M.M., Van Der Schouw Y.T., Sluijs I. Fatty acids from dairy and meat and their association with risk of coronary heart disease. Eur J Nutr. 2019;58:2639–2647. doi: 10.1007/s00394-018-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Lin C., Su W., Zhang Y., Wang F., Wang Y., Shi C., Lu Z. Effects of supplementing sow diets with fermented corn and soybean meal mixed feed during lactation on the performance of sows and progeny. J Anim Sci. 2018;96:206–214. doi: 10.1093/jas/skx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wu B., Zhang L., Cui Y., Zhang B., Wang H. Laquinimod prevents adipogenesis and obesity by down-regulating ppar-γ and c/ebpα through activating ampk. ACS Omega. 2020;5:22958–22965. doi: 10.1021/acsomega.0c02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Chen X., Huang Z., Chen D., Yu B., He J., Luo Y., Yan H., Chen H., Zheng P., Yu J. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim Nutr. 2022;8:256–264. doi: 10.1016/j.aninu.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen X., Chen D., Yu B., Yin J., Huang Z. Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs. Food Funct. 2019;10:7426–7434. doi: 10.1039/c9fo01304k. [DOI] [PubMed] [Google Scholar]
- Xu X., Li L.M., Li B., Guo W.J., Ding X.L., Xu F.Z. Effect of fermented biogas residue on growth performance, serum biochemical parameters, and meat quality in pigs. Asian-Australas J Anim Sci. 2017;30:1464–1470. doi: 10.5713/ajas.16.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Chen W., Wang L., Zhou Y., Nong Q., Valencak T.G., Wang Y., Xie J., Shan T. Cold exposure affects lipid metabolism, fatty acids composition and transcription in pig skeletal muscle. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.748801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Meng Q.W., Kim I.H. Effects of fermented garlic powder supplementation on growth performance, nutrient digestibility, blood characteristics and meat quality in growing-finishing pigs. Anim Sci J. 2012;83:411–417. doi: 10.1111/j.1740-0929.2011.00973.x. [DOI] [PubMed] [Google Scholar]
- Yu M., Li Z., Rong T., Wang G., Liu Z., Chen W., Li J., Li J., Ma X. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J Anim Sci Biotechnol. 2020;11:78. doi: 10.1186/s40104-020-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Yan Z., Song B., Zheng C., Duan Y., Kong X., Deng J., Li F. Dietary supplementation with betaine or glycine improves the carcass trait, meat quality and lipid metabolism of finishing mini-pigs. Anim Nutr. 2021;7:376–383. doi: 10.1016/j.aninu.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.