Abstract
In Yersinia pestis, the causative agent of plague, two inorganic iron transport systems have been partially characterized. The yersiniabactin (Ybt) system is a siderophore-dependent transport system required for full virulence. Yfe is an ABC transport system that accumulates both iron and manganese. We have identified and cloned a Y. pestis yfuABC operon. The YfuABC system is a member of the cluster of bacterial ABC iron transporters that include Sfu of Serratia, Hit of Haemophilus, and Yfu of Yersinia enterocolitica. The Y. pestis KIM6+ system is most homologous to that in Y. enterocolitica, showing identities of 84% for YfuA (periplasmic binding protein), 87% for YfuB (inner membrane permease), and 75% for YfuC (ATP hydrolase). We constructed a yfuABC promoter-lacZ fusion to examine regulation of transcription. This promoter contains a potential Fur binding sequence and is iron and Fur regulated. Significant expression from the yfuABC promoter occurred during iron-deficient growth conditions. In vitro transcription and translation of a recombinant plasmid encoding yfuABC indicates that YfuABC proteins are expressed. Escherichia coli 1017 (an enterobactin-deficient mutant) carrying this plasmid was able to grow in an iron-restrictive complex medium. We constructed a deletion encompassing the yfuABC promoter and most of yfuA. This mutation was introduced into strains with mutations in Ybt, Yfe, or both systems to examine the role of Yfu in iron acquisition in Y. pestis. Growth of the yfu mutants in a deferrated, defined medium (PMH2) at 26 and 37°C failed to identify a growth or iron transport defect due to the yfu mutation. Fifty percent lethal dose studies in mice did not demonstrate a role for the Yfu system in mammalian virulence.
Nearly all living organisms require trace amounts of iron. For pathogens, the iron- and heme-chelating proteins of mammalian hosts are barriers to iron acquisition that must be overcome. A number of iron and hemoprotein transport systems from a variety of pathogens have been characterized and have demonstrated roles in the infectious process (9, 11, 22, 23, 51, 53).
Yersinia pestis, the etiologic agent of bubonic and pneumonic plague, has three partially characterized iron transport systems. The hemoprotein uptake (Hmu) system of Y. pestis allows the bacterium to use hemin, hemoglobin, haptoglobin-hemoglobin, myoglobin, heme-hemopexin, and heme-albumin as iron sources (25, 50). A siderophore-dependent system (Ybt) synthesizes yersiniabactin, ABC transport components, and a regulatory protein that are all encoded within a high-pathogenicity island that is present in highly pathogenic strains of Y. pestis, Yersinia pseudotuberculosis, Yersinia enterocolitica, and several types of Escherichia coli pathogens. In Y. pestis, the high-pathogenicity island lies within the pgm locus, a 102-kb chromosomal region subject to high-frequency deletion (10, 18, 20, 24, 28). The Ybt system is essential for iron acquisition during the early stages of plague (4, 5, 16). The YfeABCD system of Y. pestis belongs to an ABC family of bacterial cation transporters and transports both iron and manganese. It plays a role in iron acquisition during the later stages of plague (5, 6). Studies with iron chelators suggest that Y. pestis possesses an iron transport system that functions at 26 to 30°C but not at 37°C. This putative 26°C iron transport system is independent of the Ybt and Yfe transport systems (5, 29).
In this study, we describe the identification, cloning, and initial characterization of a Y. pestis ABC transporter called YfuABC. It has high homology to iron transporters in Y. enterocolitica (YfuABC) (40) and Serratia marcescens (SfuABC) (2). The Y. pestis system is iron and Fur regulated and enhanced growth of a siderophore-deficient strain of E. coli under iron-chelated conditions.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains and plasmids used in this study are listed in Table 1. Y. pestis strains missing the low-calcium-response (Lcr) virulence plasmid pCD1 are completely avirulent (34) and were used in all physiological and genetic experiments. All strains were stored in phosphate-buffered glycerol at −20°C. Y. pestis strains were grown in heart infusion broth (HIB), in Luria broth (LB), or on tryptose blood agar base (TBA). The pigmentation (Pgm) phenotype of strains was confirmed on Congo red plates (48). For growth under iron-restricted conditions, a colony from a Congo red plate was inoculated onto TBA slants and incubated for 24 to 48 h at 26 or 37°C. Cells on the TBA slant were suspended in PMH2 deferrated by Chelex 100 extraction (46), diluted to an optical density at 620 nm (OD620) of 0.1 in deferrated PMH2 broth, and grown aerobically for ∼8 h before inoculation of a second transfer containing fresh deferrated PMH2. PMH2 is a modified, defined medium derived from PMH (46); PMH2 contains 50 μM PIPES (piperazine-N,N′-bis[2-ethansulfonic acid]) in place of 50 μM HEPES. This change in buffers reduces acidification of the medium due to bacterial growth (data not shown). For iron transport assays only, bacterial cells were grown and assayed in PMH. Growth of the cultures was monitored by determining the OD620 with a Genesys5 spectrophotometer (Spectronic Instruments, Inc.) at regular intervals. For some studies, PMH2 was supplemented with either 2,2′-dipyridyl (DIP) or ethylenediamine-di(o-hydroxyphenyl acetic acid) (EDDA) to chelate residual iron in the medium. Contaminating iron in EDDA was extracted as previously described (38). E. coli strains were grown in either LB, nutrient broth with 85.6 mM NaCl (NB), or Tris-glucose-thymidine medium without FeCl3 (TG-Fe) (43). All glassware used in these studies was cleaned in a chromic-sulfuric acid solution (46.3 g of potassium dichromate per liter of ∼11.5 M sulfuric acid) or Scotclean (Owl Scientific, Inc.) to remove contaminating iron and then rinsed copiously with deionized water. All reagents and media were made with deionized water after passage through a Nanopure cartridge system (Barnstead). When appropriate, media included antibiotics at the following concentrations: ampicillin, 100 μg/ml; tetracycline, 12.5 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 30 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5α | Cloning host | 3 |
| DH5α(λpir) | Strain for propagating plasmid with R6K origins; derived from DH5α | S. C. Straley |
| 1017 | ent::Tn5 Kmr; derived from HB101 | 14 |
| Y. pestis strainsa | ||
| KIM6+ | Lcr− Pgm+ (Ybt+) Hmu+ Yfe+ Yfu+ | 18; this study |
| KIM6 | Lcr− Pgm− (Δpgm Ybt−) Hmu+ Yfe+ Yfu+ | 18; this study |
| KIM6-2030 | Lcr− Pgm− (Δpgm Ybt−) Hmu+ Yfe+ Yfu+ Fur− (fur::kan-9) | 44 |
| KIM6-2031.1+ | Lcr− Pgm+ (Ybt+) Hmu+ Yfe− (ΔyfeAB2031.1) Yfu+ | 5 |
| KIM6-2031.1 | Lcr− Pgm− (Δpgm Ybt−) Hmu+ Yfe− (ΔyfeAB2031.1) Yfu+ | 5 |
| KIM6-2082+ | Lcr− Pgm+ (Ybt+) Hmu+ Yfe+ Yfu− (ΔyfuA2082); derived from KIM6+ | This study |
| KIM6-2082 | Lcr− Pgm− (Δpgm Ybt−) Hmu+ Yfe+ Yfu− (ΔyfuA2082); derived from KIM6 | This study |
| KIM6-2082.1+ | Lcr− Pgm+ (Ybt+) Hmu+ Yfe− (ΔyfeAB2031.1) Yfu− (ΔyfuA2082); derived from KIM6-2031.1+ | This study |
| KIM6-2082.1 | Lcr− Pgm− (Δpgm Ybt−) Hmu+ Yfe− (ΔyfeAB2031.1) Yfu− (ΔyfuA2082); derived from KIM6-2031.1 | This study |
| KIM5-2031.12+ | Lcr+ (pCD1Ap, 'yadA::bla) Pgm+ (Ybt+) Hmu+ Yfe− (ΔyfeAB2031.1) Yfu+ Apr; derived from KIM6-2031.1+ | This study |
| KIM5-2082.3+ | Lcr+ (pCD1Ap, 'yadA::bla) Pgm+ (Ybt+) Hmu+ Yfe+ Yfu− (ΔyfuA2082) Apr; derived from KIM6-2082+ | This study |
| KIM5(pCD1Ap)+ | Lcr+ (pCD1Ap, 'yadA::bla) Pgm+ (Ybt+) Hmu+ Yfe+ Yfu+ Apr; derived from KIM6+ | This study |
| Plasmids | ||
| pACYC184 | 4.2-kb cloning vector; Cmr Tcr | 12 |
| pBluescript II KS+ | 3.0-kb cloning vector; Apr | 3 |
| pBGL2 | 4.8-kb cloning vector; Apr Tcr | 35 |
| pBR322 | 4.4-kb cloning vector; Apr Tcr | 3 |
| pEU730 | 15.2-kb single copy reporter vector; promoterless lacZ; Spr | 19 |
| pKNG101 | 6.8-kb suicide vector; SacB+ R6K origin; Smr | 27 |
| pRL494e | 3.7-kb vector with ampicillin resistance cassette (bla); Apr Kmr | 15 |
| pWSK129 | 6.7-kb low-copy-number cloning vector; Kmr | 52 |
| pCD1 | 70.5-kb Lcr plasmid of KIM5 | 36 |
| pCD1Ap | 70.5-kb pCD1 with bla cassette inserted into 'yadA; 71.7-kb Lcr+ Apr | This study |
| pBGCD3 | 9.98-kb BglII fragment from pCD1 ligated into pBGL2; 14.8 kb 'yadA'/'yadA, Apr Tcr | This study |
| pWSKYadA | 3.7-kb BglII-SmaI fragment from pBGCD3 ligated into the BamHI-EcoRV sites of pWSK129; 10.4 kb, yadA'/'yadA, Kmr | This study |
| pACYCYadA | 1.9-kb ScaI-HindIII fragment from pWSKYadA ligated into the EcoRV-HindIII sites of pACYC184; 6.0 kb, 'yadA, Cmr | This study |
| pACYCYadAp | 1.2-kb EcoRV fragment containing bla cassette from pRL494e ligated into pACYCYadA; 7.2 kb, 'yadA::bla, Cmr Apr | This study |
| pKNGYadAp | 3.6-kb XbaI-SalI fragment from pACYCYadAp ligated into pKNG101; 10.1 kb, 'yadA::bla, R6K origin, Smr | This study |
| pYFU238 | 8.9-kb Sau3AI Y. pestis chromosomal fragment ligated into BamHI site of pLG338; 16.2 kb, yfuAB+yfuC', Kmr | 35; this study |
| pYFU1 | 9.8-kb XhoI-PstI Y. pestis chromosomal fragment ligated into pBluescript II KS+; 12.8 kb, Yfu+ Apr | This study |
| pYFU2 | 6.7-kb BglII-SalI fragment from pYFU238 ligated into BamHI-SalI sites of pWSK129; 13.4 kb, Yfu− (yfuAB+yfuC') Kmr | This study |
| pYFU3 | Deletion of 1.8- and 0.2-kb BamHI fragments in yfuA from pYFU2; 11.4 kb, Yfu− (ΔyfuA2082 [YfuABC−]) Kmr | This study |
| pYFU3.1 | 4.7-kb XbaI-SalI fragment from pYFU3 ligated into pKNG101; 11.2 kb, ΔyfuA2082 (YfuABC−), Smr | This study |
| pYFU4 | 310-bp yfuABC promoter ligated into pEU730; 15.5-kb yfu::lacZ fusion; Spr | This study |
| pYFU5 | 10.1-kb PvuII-XbaI fragment from pYFU1 cloned into pBR322; 14.5 kb, Yfu+ Apr Kmr | This study |
| pYFU6 | 6.7-kb XbaI-XhoI fragment from pYFU2 cloned into pBR322; 10.7 kb, Yfu− (yfuAB+yfuC) Apr Kmr | This study |
Strains designated by a “+” suffix possess an intact pgm locus (i.e., Ybt+ Hms+); other strains have either deletion of the 102-kb pgm chromosomal locus or a mutation within the locus (Ybt− or Hms−). KIM6 strains lack the pCD1 plasmid (Lcr−), while KIM5 strains possess pCD1 or pCNAp (Lcr+); all strains possess the hemin utilization locus (Hmu+).
Recombinant DNA techniques and plasmids.
Plasmids were isolated by alkaline lysis (7, 26) and transformed into various E. coli strains by standard calcium chloride transformation (3) or electroporated into Y. pestis (17). Bacterial genomic DNA was isolated by a method utilizing lysozyme-sodium dodecyl sulfate (SDS)-proteinase K (3) and further purified by phenol and chloroform extractions (3). DNA restriction endonucleases, T4 DNA ligase, and calf intestinal alkaline phosphatase were used according to the manufacturer's specifications.
To isolate Y. pestis yfuABC, a PCR probe was generated using a digoxigenin-labeling kit (Roche Biochemical) and primers Ypyfu5.2 (5′-TGTTGCTTTACTGGCGTCTG-3′) and Ypyfu3.1 (5′-TAGGATTGGAAGCGGCATTC-3′). Reactions were performed in a GeneAmp PCR System 2400 (Perkin-Elmer) and run for 3 min at 94°C, 15 s at 94°C, 30 s at 50°C, and 2 min at 72°C for 30 cycles followed by a single cycle at 72°C for 7 min. The resulting 890-bp amplicon is within the yfuA coding region. The labeled probe was used to screen dot blot filters of our Y. pestis KIM6+ Sau3AI genomic library (35).
A 310-bp fragment from the yfu promoter region was amplified by PCR using primers P1 (5′-AGCTTTGTTTAAACACAAATAAGTGATAGCTA-3′) and P2 (5′-GGGGTACCATAGCGATCCTTTTAAAAG-3′). Reactions containing 250 μM deoxynucleoside triphosphates, 1.5 mM MgCl2, and 1 μM primers were performed for 5 min at 94°C, 30 s at 94°C, 45 s at 55°C, and 1 min at 72°C for 25 cycles followed by a single cycle at 72°C for 5 min. The products were purified on low-melting-point agarose gels and cloned into the reporter plasmid pEU730, a low-copy-number cloning vector that contains a multicloning site preceding a promoterless lacZ gene (19). A clone containing the unaltered yfu promoter sequence in the correct orientation to drive lacZ expression (pYFU4) was identified by sequencing and used in expression studies. Sequencing reactions were performed via the dideoxynucleotide chain termination method (42) using [35S]dATP (Amersham/USB), Sequenase version 2.0 (Amersham/USB), and 7-deaza-dGTP (Boehringer Mannheim Biochemicals). Samples were electrophoresed through a 6% polyacrylamide gel containing 8.3 M urea (Sigma) cast in Tris-borate-EDTA buffer (41). Dried gels were exposed at room temperature to Kodak Biomax MR film.
β-Galactosidase assays.
Y. pestis KIM6+, KIM6, and KIM6-2030 cells containing pYFU4 (yfu::lacZ) were harvested during exponential growth from second-transfer cultures in PMH2 broth containing either no added iron source or 10 μM FeCl3. β-Galactosidase activities from whole-cell lysates of these cultures were measured as previously described (30). Since Y. pestis is naturally β-galactosidase negative in this assay, the activity obtained from strains carrying reporter plasmids correlates directly with promoter activity of the lacZ fusion reporter (21, 46).
Construction of Y. pestis mutants.
A deletion encompassing an upstream open reading frame (ORF), the yfuABC promoter, and most of the yfuA gene was made by deleting 1,808- and 198-bp BamHI fragments (Fig. 1) from pYFU2 to generate pYFU3. The mutated region was then cloned into the suicide vector pKNG101 (27). The resulting recombinant plasmid, pYFU3.1 (Table 1), was introduced separately into Y. pestis KIM6+ (Ybt+ Yfe+), KIM6 (Ybt− Yfe+), KIM6-2031.1+ (Ybt+ Yfe−), and KIM6-2031.1 (Ybt− Yfe−) by allelic exchange. Y. pestis merodiploid strains were selected on TBA plates containing 50 μg of streptomycin/ml. Subsequent screening of these strains for exchange of the mutant alleles for wild-type alleles was accomplished by selection for sucrose resistance as described previously (4). To confirm that the deletion mutation had been exchanged, the yfu region of each strain was amplified by PCR using primer YFUP1 (5′-ACTGCCATACTGCCATCG-3′) and YFUP2 (5′-ACTCAGTGCAGCCTGTGC-3′). Reactions containing 250 μM deoxynucleoside triphosphates, 1.5 mM MgCl2, and 1 μM primers were performed for 10 min at 94°C, 45 s at 94°C, 30 s at 50°C, and 30 s at 72°C for 25 cycles followed by a single cycle at 72°C for 5 min. These primers used should amplify a 2,486-bp product in the yfu+ strains and a 480-bp product in the Δyfu strains. Products of the expected sizes were observed in all yfu+ and Δyfu strains; both products were observed in all merodiploid strains (data not shown).
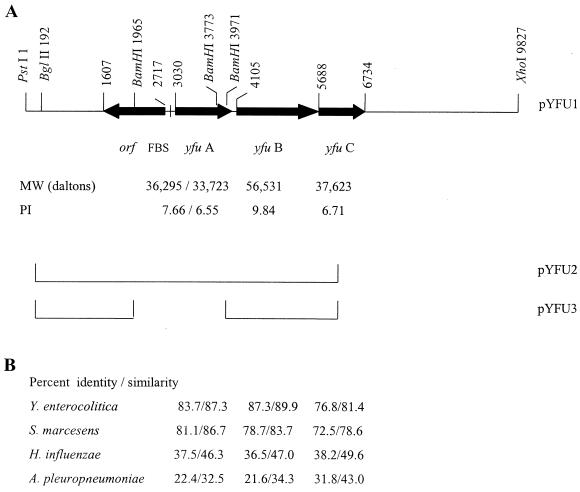
FIG. 1.
Genetic organization of the Y. pestis yfuABC operon and similarities to selected iron ABC transport systems. (A) Relevant genes and restriction sites of Y. pestis DNA in pYFU1. Base pair numbering is shown on the top line. A putative Fur binding sequence (FBS) is 95 bp from the start of yfuA. For YfuA, the unprocessed and processed molecular masses (MW) and pIs are given. Y. pestis DNA present in pYFU2 and pYFU3 are indicated by the lines shown. (B) Percent identity/similarity to each of four iron ABC transport systems is shown below the corresponding Y. pestis gene.
Iron transport assays.
Y. pestis KIM6 (Ybt− Yfe+ Yfu+), KIM6-2031.1 (Ybt− Yfe− Yfu+) and KIM6-2082.1 (Ybt− Yfe− Yfu−) cells were acclimated to iron-deficient growth at 37°C or 26°C in PMH medium for five to six generations, and 0.1 μCi of 55FeCl3/ml was added to exponentially growing Y. pestis cells. Samples of 0.5 ml were withdrawn at regular intervals for 40 min, collected by vacuum filtration through 0.45-μm-pore-size GN-6 nitrocellulose membranes (Gelman Sciences), and washed twice with PMH medium. Unfiltered samples determined the total radioisotope content of cultures in each experiment. Sample filters were suspended in Bio-Safe II counting cocktail (Research Products International), and the counts per minute of each sample was measured in a Beckman LS3801 liquid scintillation spectrometer with a counting window of 0 to 1,000 keV. To demonstrate energy-dependent uptake and correct for nonspecific binding, cells were poisoned metabolically with 100 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) 10 min before the addition of isotope.
Protein analysis.
In vitro transcription-translation of plasmid-encoded proteins was performed with an E. coli S30 cell extract system (Promega Corp.). Proteins were radiolabeled with 35S-labeled amino acids (DuPont NEN Research Products) according to the manufacturer's recommendations, and equal amounts of trichloroacetic acid-precipitable counts were resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Dried gels were exposed to Kodak BioMax MR film at room temperature. Homology searches of protein databases were performed with BLAST version 2.1 (1). Alignments were performed using CLUSTAL W (49). Molecular masses and pIs were determined using DNAMAN 4.16 (Lynnon Biosoft). Signal sequence cleavage sites were determined using Signal P (32).
Virulence testing.
Previously, we have used plasmid pCDl::MudI1734–73 (yopJ::MudII1734; Kmr) to transform Lcr− strains for virulence testing (4, 5, 16). Although a previous study with a YopJ− mutant did not show a significant effect on virulence in mice injected intravenously (47), in Y. pseudotuberculosis YopJ is required for inducing apoptosis in macrophages (31). To construct a marked pCD1 with no mutations in expressed genes, a 9,978-bp BglII fragment from pCD1, containing a portion of the pseudogene yadA'/'yadA, was cloned into pBGL2 to generate pBGCD3. A 3,674-bp BglII-SmalI piece was isolated from pBGCD3 and inserted into BamHI and EcoRV-digested pWSK129 to yield pWSKYadA. Using a HindIII site within the polylinker of pWSK129, a 1,858-bp Scal-HindIII fragment was excised from pWSKYadA and cloned into the EcoRV-HindIII sites of pACYC184 to create pACYCYadA. An approximately 1,200-bp EcoRV fragment containing an ampicillin gene cassette (bla) was excised from pRL494e and inserted into the unique EcoRV site within the 'yadA gene of pACYCYadA. This plasmid was named pACYCYadAp. To create the suicide vector, pKNGYadAp, an approximately 3.6-kb XbaI-SalI piece from pACYCYadAp was inserted into the corresponding sites in pKNG101.
pKNGYadAp was electroporated into KIM5 (Pgm− Lcr+) and incubated for 1 h at 37°C in HIB. Cointegrants were selected by incubation on TBA plates containing streptomycin and ampicillin for 2 days at 30°C. An Smr and Apr isolate was grown overnight at 30°C in HIB containing ampicillin and diluted to an OD620 of 0.01, and aliquots were spread onto TBA plates supplemented with ampicillin and 5% sucrose. Sucrose-resistant colonies were grown overnight at 30°C in HIB in the presence of ampicillin and screened by PCR for the presence of the mutant 'yadA::bla allele. PCRs utilized primers yadA1 (5′-TCGATATTAAATGATGCT-3′) and yadA2 (5′-CAAACGAGTTGACAAAGG-3′) and consisted of a 4-min incubation at 94°C followed by 30-s incubations at 94, 42, and 72°C for 25 cycles. The marked plasmid, designated pCD1Ap, has the bla cassette inserted downstream of the 1-bp deletion that generated the pseudogene yadA'/'yadA (Table 1) (36).
To generate strains suitable for virulence testing in mice, pCD1Ap was electroporated into KIM6+ (Ybt+ Yfe+ Yfu+), KIM6-2082+ (Ybt+ Yfe+ Yfu−), KIM6-2031.1+ (Ybt+ Yfe− Yfu+), and KIM6-2082.1+ (Ybt+ Yfe− Yfu−) to yield KIM5 (pCD1Ap)+, KIM5-2082.3+, KIM5-2031.12+, and KIM5-2082.11+, respectively. These virulent or potentially virulent strains were constructed and used in a BL3 facility. Pgm and Lcr phenotypes were confirmed on Congo red plates (48) and TBA plates supplemented with 20 mM sodium oxalate and 20 mM MgCl2 (33). Strains were grown at 26°C in PMH2 supplemented with 50 μM hemin and ampicillin (100 μg/ml) to approximate conditions that the bacteria might encounter in the flea gut and to force retention of pCD1Ap. Bacteria were grown under these conditions through two transfers for a total of six to seven generations. Cells were harvested at an OD620 of ∼0.4, pelleted, and resuspended in mouse isotonic phosphate-buffered saline (149 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4 [pH 7.0]). Five- to seven-week-old female NIH/Swiss Webster mice were injected subcutaneously with 0.1 ml of 10-fold serial dilutions of the bacterial suspensions. Four mice were used for each bacterial dose. The number of cells injected was determined by plating serial dilutions on TBA-ampicillin plates. Mice were monitored daily for a period of 3 weeks. Fifty percent lethal doses (LD50s) were calculated by the method of Reed and Muench (37).
RESULTS
Sequence analysis.
BLAST searches and analyses of the Y. pestis CO92 genome sequence database at the Sanger Centre (Yersinia pestis CO92 genomic sequence database [ftp://ftp.sanger.ac.uk/pub/pathogenic/yp/YP.dbs]) and the Y. pestis KIM10+ genome sequence database at the University of Wisconsin (UW) Genome Project (http://magpie.genome.wise.edu/ cgi-bin/Authenticate.cgi/uwgp_blast.html) with the deduced amino acid sequence of YfuA from Y. enterocolitica identified a potentially functional yfuABC operon in both plague biotypes. Y. pestis yfuABC appear to be in a single operon with a Fur binding sequence in the promoter region (Fig. 1). The Y. pestis Yfu system is a member of the cluster of bacterial ABC iron transport systems (39; http://www-biology.ucsd.edu/∼msaier/transport/titlepage.html) that include Sfu of S. marcescens, Hit of Haemophilus influenzae, Yfu of Y. enterocolitica, and Afu of Actinobacillus pleuropneumoniae (2, 8, 13, 40). Thus, YfuA likely acts as the periplasmic binding protein (PBP) which passes on the substrate to a dimer of YfuB, the inner membrane (IM) permease. Translocation across the IM is probably energized via ATP hydrolysis by YfuC, the ATP-binding protein or hydrolase. The Y. pestis KIM10+ system is most homologous to that in Y. enterocolitica, showing identities of 83.7% for YfuA, 87.3% for YfuB, and 76.8% for YfuC. Percents similarities/identities to Sfu of S. marcescens are nearly as high as those to Yfu of Y. enterocolitica, while those to Hit of H. influenzae and Afu of A. pleuropneumoniae (8, 13) are lower (Fig. 1). The higher degree of similarity among the enteric organisms is shown in Fig. 2, an alignment of Y. pestis YfuA with the PBPs from Y. enterocolitica, S. marcescens, Neisseria meningitidis, and H. influenzae.
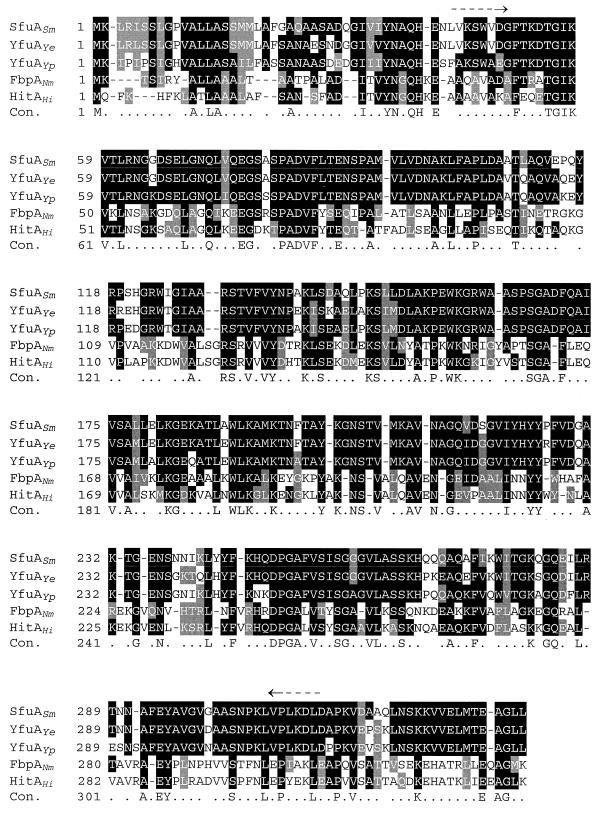
FIG. 2.
CLUSTAL W amino acid sequence alignments of the PBPs from S. marcescens (SfuASm), Y. enterocolitica (YfuAYe), Y. pestis (YfuAYp), N. meningitidis (FbpANm), and H. influenzae (HitAHi). Identical amino acids are shown in black boxes, while similar amino acids are shown in gray boxes. The consensus line (Con.) shows identical (capital letters) and similar (dots) amino acids. Locations of the yfuA primers from the Y. enterocolitica sequence used in reference 6 are shown by arrows.
Cloning and expression of Y. pestis yfuABC genes.
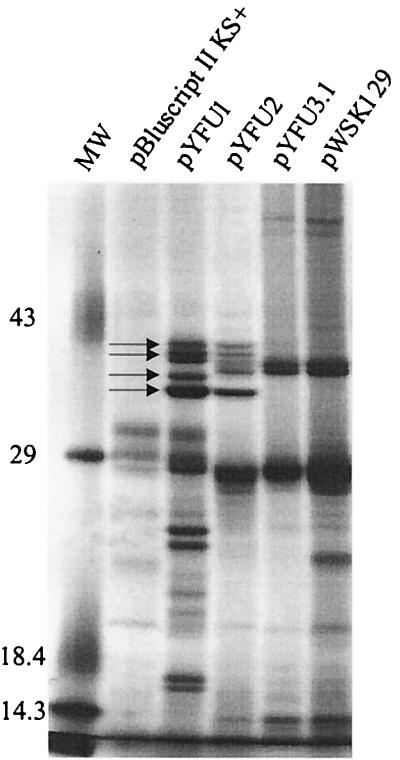
A PCR product generated from primers within the yfuA gene (see Materials and Methods) was used as a probe to screen our Sau3AI library of Y. pestis KIM6+ genomic DNA (35). Subcloning of pYFU238 to yield pYFU2 (Table 1) and restriction site mapping indicated that the library clone was missing ∼500 bp of the 3′ end of yfuC. To recover a full-length operon, a 9.8-kb XhoI-PstI Y. pestis chromosomal DNA fragment encoding yfuABC was cloned into pBluescript II KS+, generating pYFU1. In vitro transcription-translation of pYFU1 and pYFU2 followed by SDS-PAGE analysis of the products identified four polypeptides that could correspond to YfuA, YfuC, and the upstream ORF (Fig. 1 and 3). The larger YfuB was not detected (Fig. 3); IM permeases are often not detected by SDS-PAGE due to their hydrophobicity. Similar analysis of pYFU3.1, which contains the deletion encompassing the upstream ORF, the yfu promoter, and yfuA (Table 1), suggests that this deletion eliminates expression of all yfu genes as expected (Fig. 3).
FIG. 3.
Autoradiogram of plasmid-encoded proteins labeled with 35S-amino acids by in vitro transcription-translation and separated by SDS-PAGE. Molecular weight markers (MW) and their corresponding masses in kilodaltons are shown. Four relevant proteins are identified by arrows.
To analyze iron and Fur regulation of this operon, the yfuABC promoter region was fused to lacZ in a single-copy-number reporter plasmid, pYFU4 (Table 1). Table 2 shows that expression of β-galactosidase was repressed by iron four to fivefold in Pgm+ (Ybt+) and Pgm− (Ybt−) strains of Y. pestis. To demonstrate that this iron repression was controlled by Fur, we used the Pgm− (Ybt−) Fur− strain KIM6-2030 to avoid the more severe iron toxicity observed in Pgm+ strains (45). In KIM6-2030(pYFU4), iron-regulated repression of β-galactosidase expression was abolished (Table 2). Thus, the yfuABC promoter is iron repressible via Fur.
TABLE 2.
β-Galactosidase activities of Y. pestis strains containing pYFU4
| Strain | β-Galactosidase activity (mean ± SE)a
|
Ratio, −Fe/+Fe | |
|---|---|---|---|
| −Fe | +Fe | ||
| KIM6(pYFU4)+ (ybt+yfu::lacZ) | 9,221 ± 160 | 1,680 ± 107 | 5.5 ± 0.5 |
| KIM6(pYFU4)(Δybt yfu::lacZ) | 7,499 ± 79 | 1,713 ± 77 | 4.4 ± 0.2 |
| KIM6-2030(pYFU4)(Δybt fur::kan-9 yfu::lacZ) | 7,849 ± 783 | 7,393 ± 899 | 1.1 ± 0.03 |
Cells were grown without (−Fe) or with (+Fe) 10 μM iron.
Iron-deficient growth of E. coli 1017.
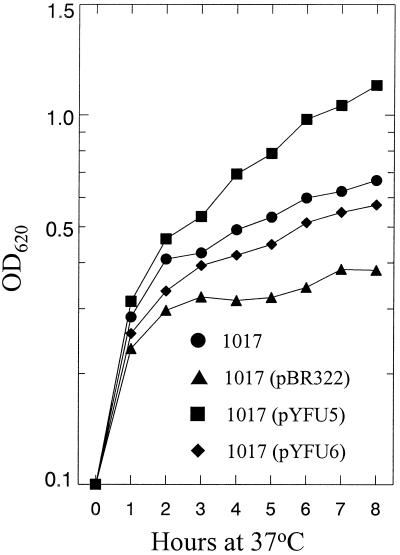
To determine if Y. pestis yfuABC genes enhanced growth of E. coli 1017 (an enterobactin-deficient mutant) under iron-chelated conditions, we transformed this strain with pYFU5 (Yfu+), pYFU6 (yfuAB+ yfuC'), or pBR322 (the moderate-copy-number vector for both recombinant plasmids) (Table 1). Transformed 1017 cells were grown overnight in NB at 37°C and then diluted into NB containing 50 μM DIP for growth analysis (Fig. 4). Growth of 1017 and growth of 1017(pYFU6) cells in this iron-chelated medium were nearly identical to each other and significantly inhibited compared to 1017(pYFU5). For unknown reasons, growth of 1017(pBR322) was inhibited compared to 1017 without any recombinant plasmid. These results suggest that an intact yfuABC operon enhanced growth of 1017 by acquiring iron from the chelated medium and that YfuC is essential for this function. The truncated YfuC' product is either nonfunctional or unstable. However, in defined TG-Fe with and without EDDA supplementation to 10, 25, or 50 μM, growth enhancement of 1017(pYFU5) compared to 1017 and 1017(pBR322) was not observed (data not shown).
FIG. 4.
Growth of E. coli 1017 and derivatives in NB medium supplemented with 50 μM DIP at 37°C. pBR322 is the vector for plasmids pYFU5 and pYFU6. pYFU5 encodes an intact yfuABC operon, while pYFU6 is yfuAB+ yfuC'.
Iron-deficient growth of Y. pestis yfu mutants.
Previously, iron acquisition defects of Yfe− mutants were masked by the more efficient Ybt-siderophore-dependent system (5). Consequently, we constructed single, double, and triple iron transport mutants to analyze the role of Yfu in iron uptake. The triple mutant (KIM6-2082.1; Ybt− Yfe− Yfu−) grew as well at 26°C on solidified PMH2 containing 60 or 80 μM DIP as its parental strain (KIM6-2031.1; Ybt− Yfe− Yfu+) (data not shown). Thus, the Yfu system is not the undefined 26°C iron transport system described by Lucier et al. (29).
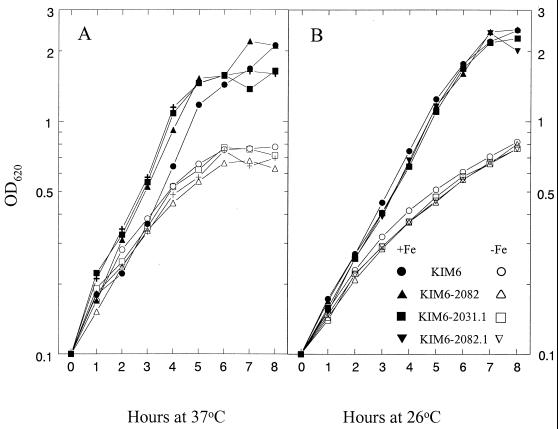
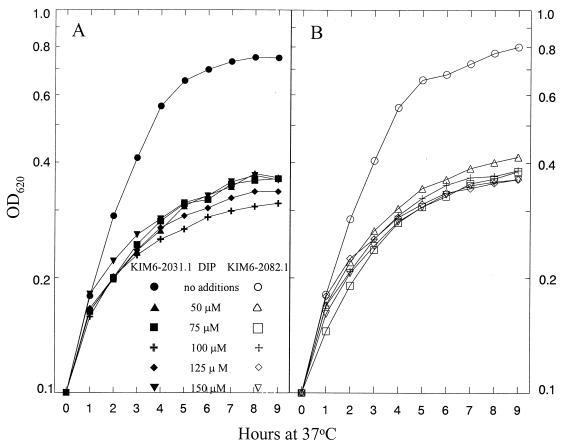
Cells of KIM6 (Ybt− Yfe+ Yfu+), KIM6-2082 (Ybt− Yfe+ Yfu−), KIM6-2031.1 (Ybt− Yfe− Yfu+), and KIM6-2082.1 (Ybt− Yfe− Yfu−) were grown in PMH2 at 26 and 37°C with and without iron supplementation to identify growth defects due to the Yfu system. In all backgrounds, Yfu− mutants grew as well as their Yfu+ parental strains (Fig. 5). We also tested the ability of Yfu− mutants to respond to supplementation of PMH2 with low concentrations of iron (0.1 to 2 μM). Again both KIM6-2031.1 (Ybt− Yfe− Yfu+) and KIM6-2082 (Ybt− Yfe− Yfu−) had similar growth responses to all concentrations of added iron at 26°C and at 37°C (data not shown). Growth defects due to mutation of the Yfe system were more clearly observed in iron-chelated media (5); consequently, we assayed growth of KIM6-2031.1 and KIM6-2082.1 in PMH2 at 37°C with increasing concentrations of DIP. At all concentrations, no growth defects due to the ΔyfuA2082 mutation were observed (Fig. 6). Similar growth studies performed at 26°C yielded similar results (data not shown).
FIG. 5.
Growth of Y. pestis strains KIM6 (Ybt− Yfe+ Yfu+), KIM6-2082 (Ybt− Yfe+ Yfu−), KIM6-2031.1 (Ybt−Yfe− Yfu+), and KIM6-2082.1 (Ybt− Yfe− Yfu−) in deferrated PMH2 with (+Fe) and without (−Fe) FeCl3 supplementation to 10 μM. Cultures were incubated at 37°C (A) or 26°C (B).
FIG. 6.
Growth of Y. pestis strains at 37°C in deferrated PMH2 with increasing concentrations of the iron chelator DIP. (A) KIM6-2031.1 (Ybt− Yfe− Yfu+); (B) KIM6-2082.1 (Ybt− Yfe− Yfu−).
In E. coli 1017, we observed a growth effect for the Yfu system only in a complex medium that might contain a bound iron source absent in defined media. Consequently, we tested the growth of Yfu+ and Yfu− strains of Y. pestis in LB and HIB supplemented with different concentrations of DIP. The Yfu system played no significant role in growth under these conditions (data not shown).
Iron uptake of Y. pestis yfu mutants.
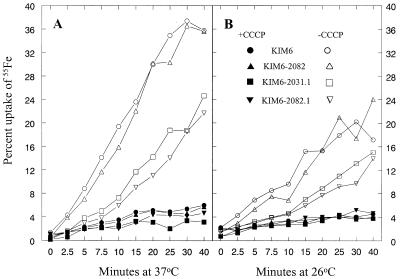
We next compared the abilities of Yfu+ and Yfu− strains to accumulate 55FeCl3. For these studies, KIM6 (Ybt− Yfe+ Yfu+), KIM6-2082 (Ybt− Yfe+ Yfu−), KIM6-2031.1 (Ybt− Yfe− Yfu+), and KIM6-2082.1 (Ybt− Yfe− Yfu−) were grown in PMH at 26 and 37°C; 55FeCl3 and, where appropriate, the energy poison CCCP were added to growing cell cultures. Energy-dependent iron uptake was observed in all four strains (Fig. 7). Levels of iron accumulation by KIM6 and KIM6-2082 were nearly identical at both temperatures. The Yfe− strain KIM6-2031.1 accumulated less iron than its KIM6 parent, as previously demonstrated (5). However, the triple iron transport mutant KIM6-2082.1 (Ybt− Yfe− Yfu−) was as effective as its KIM6-2031.1 (Ybt− Yfe− Yfu+) parent in iron accumulation at both temperatures.
FIG. 7.
Uptake of 55FeCl3 by Y. pestis strains KIM6 (Ybt− Yfe+ Yfu+), KIM6-2082 (Ybt− Yfe+ Yfu−), KIM6-2031.1 (Ybt− Yfe− Yfu+), and KIM6-2082.1 (Ybt− Yfe− Yfu−). Where indicated (closed symbols), cells were metabolically poisoned by addition of 100 μM CCCP 10 min prior to addition of isotope. These data are from a single assay but are representative of three independent experiments.
LD50 studies in mice.
To determine the contribution of the Y. pestis Yfu transport system to virulence in mice, we compared KIM5(pCD1Ap)+ (wild type) to KIM5-2082.3+ (Yfu−). By subcutaneous injection (to mimic a flea bite), both strains were fully virulent. In two separate trials, KIM5(pCD1Ap)+ had LD50s of 15 and <4.6, while KIM5-2082.3+ had an LD50 of 10.8. We also tested the effect of Yfu in a Yfe− background. KIM5-2031.12+ (Ybt+ Yfe− Yfu+) was no more virulent than KIM5-2082.11+ (Ybt+ Yfe− Yfu−); respective LD50s were 74.3 and <82 (lowest concentration tested).
DISCUSSION
Using Y. pestis genomic DNA, we previously failed to generate a PCR product using primers from the DNA sequence of Y. enterocolitica which showed high amino acid conservation to the PBPs from S. marcescens and N. gonorrhoeae (6). One region of high amino acid conservation, upon which one primer (shown in Fig. 2) was based, differs in Y. pestis YfuA compared to Y. enterocolitica YfuA and SfuA (Fig. 2) and accounts for our previous negative results. In this study, we used BLAST searches with the Y. enterocolitica YfuA sequence to identify the yfuABC operon in Y. pestis KIM10+ (UW Genome Project) and CO92 (Sanger Centre). Unlike the Ybt and Hmu/Hem iron and hemoprotein transport systems of Y. pestis and Y. enterocolitica, which are nearly identical (20, 50), YfuABC in these two organisms shows more divergence despite the high degree of similarity (81.4 to 89.9%) (this study and reference 40). Although the three enteric PBPs are more closely related to each other than to those of N. meningitidis and H. influenzae, YfuA of Y. enterocolitica and SfuA of S. marcescens are more similar to each other than to YfuA of Y. pestis (Fig. 2).
Based on the deduced amino acid sequence, the Y. pestis Yfu ABC transport system belongs to the TC 3.A.1.10 cluster of ABC iron transporters that includes Sfu of S. marcescens, Hit of H. influenzae, Yfu of Y. enterocolitica, and others (39; http://www-biology.ucsd.edu/∼msaier/transport/titlepage.html). YfuA serves as the PBP, while YfuB is the IM permease and YfuC is the ATP hydrolase. Saken et al. (40) described a fourth gene, yfuD, that is downstream of yfuC in Y. enterocolitica. YfuD of Y. enterocolitica showed homology to several hypothetical proteins and modest similarity to several bacterial transporters (40). A similar gene is not present within DNA 1 kb downstream of yfuABC in Y. pestis. The best match from a BLAST search of the Y. pestis KIM10+ genome (UW Genome Project) was an ORF on a separate contig than that containing yfuABC. Using this ORF, the best match from a BLAST search of the database was a threonine efflux protein from Salmonella enterica serover Typhimurium (probability score of 5 × 10−85). The probability score for the aligned regions of this ORF and YfuD of Y. enterocolitica was 2 × 10−22. We conclude that Y. pestis does not have a yfuD homolog. Although we believe that YfuD is not part of the Yfu system, it is possible that the Y. pestis Yfu system may not function efficiently due to the absence of YfuD. No outer membrane porin or receptor is associated with the Y. pestis yfuABC locus. In Y. enterocolitica and S. marcescens the Yfu and Sfu systems are not TonB dependent (40, 54), suggesting a TonB-independent receptor or porin. Our experimental results show that the Y. pestis yfuABC promoter is repressed by excess iron via the Fur regulator (Table 2). These results and in vitro transcription-translation (Fig. 3) indicate that the genes are expressed and protein products are made. The recombinant operon enhances growth of E. coli 1017 (an enterobactin-deficient mutant) in NB chelated with DIP. However, Yfu is not the putative 26°C iron transport system hypothesized from iron chelators studies (29).
It is possible that the Y. pestis Yfu system transports a cation other than iron as its primary substrate. However, complementation of an iron transport defect in E. coli by Y. pestis yfuABC, iron-repressible expression of Y. pestis yfuABC, and the degree of similarity of the Y. pestis Yfu system to ABC iron transport systems of other organisms all suggest that iron is the primary substrate. Nonetheless, the Yfu system does not appear to be a major system for iron acquisition by Y. pestis in mice or under the in vitro conditions tested in this study. No differential effect on growth of Yfu− mutants in PMH2 supplemented with the iron chelator DIP or EDDA was observed. Short-term iron uptake assays did not detect any uptake due to the Yfu system in Y. pestis (Fig. 7). As measured by LD50 studies, Y. pestis Yfu− and Yfe− Yfu− mutants were no less virulent than their Yfu+ parental strains. Saken et al. (40) also failed to demonstrate a role for Yfu in the virulence of Y. enterocolitica in mice.
The residual growth and iron uptake of a Ybt− Yfe− Yfu− strain of Y. pestis at 37°C suggests that an unidentified system that acquires iron even under iron-chelating conditions is functioning. Whether this system is specific for iron or iron is accumulated by a system designed for uptake of another cation or substrate remains to be determined.
The role and importance of Yfu in the survival and disease properties of plague are uncertain. Yfu might be an ancestral system that is no longer essential to the lifestyle of Y. pestis. Alternatively, Yfu could be important for survival under environmental conditions that we have not tested. Perhaps the appropriate bound iron source for Yfu uptake was not present in the media that we used. Yfu might have a role in pneumonic plague but not bubonic plague. We have previously hypothesized that different iron/hemoprotein transport systems are effective in different organ systems in mammals (5); this may extend to different rodent species. Yfu appears to be irrelevant in the mouse but may be important for iron acquisition in one or more of the many other rodent hosts sensitive to plague. Finally, the Yfu system could be important in acquiring iron during growth in the flea gut. Determination of the validity of any of these speculations awaits future testing.
ACKNOWLEDGMENT
This study was supported by Public Health Service grant AI-33481 from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angerer A, Gaisser S, Braun V. Nucleotide sequences of the sfuA, sufB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990;172:572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 4.Bearden S W, Fetherston J D, Perry R D. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearden S W, Perry R D. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol. 1999;32:403–414. doi: 10.1046/j.1365-2958.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 6.Bearden S W, Staggs T M, Perry R D. An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J Bacteriol. 1998;180:1135–1147. doi: 10.1128/jb.180.5.1135-1147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun V. Avoidance of iron toxicity through regulation of bacterial iron transport. Biol Chem. 1997;378:779–786. [PubMed] [Google Scholar]
- 9.Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Vol. 35. New York, N.Y: Marcel Dekker, Inc; 1998. pp. 67–145. [PubMed] [Google Scholar]
- 10.Buchrieser C, Prentice M, Carniel E. The 102-kilobase unstable region of Yersinia pestis comprises a high-pathogenicity island linked to a pigmentation segment which undergoes internal rearrangement. J Bacteriol. 1998;180:2321–2329. doi: 10.1128/jb.180.9.2321-2329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byers B R, Arceneaux E L. Microbial iron transport: iron acquisition by pathogenic microorganisms. In: Sigel A, Sigel H, editors. Metal ions in biological systems: iron transport and storage in microorganisms plants, and animals. Vol. 35. New York, N.Y: Marcel Deker, Inc.; 1998. pp. 37–66. [PubMed] [Google Scholar]
- 12.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin N, Frey J, Chang C F, Chang Y F. Identification of a locus involved in the utilization of iron by Actinobacillus pleuropneumoniae. FEMS Microbiol Lett. 1996;143:1–6. doi: 10.1111/j.1574-6968.1996.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 14.Daskaleros P A, Stoebner J A, Payne S M. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect Immun. 1991;59:2706–2711. doi: 10.1128/iai.59.8.2706-2711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 16.Fetherston J D, Bertolino V J, Perry R D. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol Microbiol. 1999;32:289–299. doi: 10.1046/j.1365-2958.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- 17.Fetherston J D, Lillard J W, Jr, Perry R D. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fetherston J D, Schuetze P, Perry R D. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol Microbiol. 1992;6:2693–2704. doi: 10.1111/j.1365-2958.1992.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 19.Froehlich B, Husmann L, Caron J, Scott J R. Regulation of rns, a positive regulatory factor for pili of enterotoxigenic Escherichia coli. J Bacteriol. 1994;176:5385–5392. doi: 10.1128/jb.176.17.5385-5392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehring A M, DeMoll E, Fetherston J D, Mori I, Mayhew G F, Blattner F R, Walsh C T, Perry R D. Iron acquisition in plague: modular logic in enzymatic biogenesis of yersiniabactin by Yersinia pestis. Chem Biol. 1998;5:573–586. doi: 10.1016/s1074-5521(98)90115-6. [DOI] [PubMed] [Google Scholar]
- 21.Geoffroy G A, Fetherston J D, Perry R D. Yersinia pestis YbtU and YbtT are involved in synthesis of the siderophore yersiniabactin but have different effects on regulation. Infect Immun. 2000;68:4452–4461. doi: 10.1128/iai.68.8.4452-4461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths E. The iron-uptake systems of pathogenic bacteria. In: Bullen J J, Griffiths E, editors. Iron and infection: molecular, physiological, and clinical aspects. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 69–137. [Google Scholar]
- 23.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 24.Hare J M, Wagner A K, McDonough K A. Independent acquisition and insertion into different chromosomal locations of the same pathogenicity island in Yersinia pestis and Yersinia pseudotuberculosis. Mol Microbiol. 1999;31:291–303. doi: 10.1046/j.1365-2958.1999.01172.x. [DOI] [PubMed] [Google Scholar]
- 25.Hornung J M, Jones H A, Perry R D. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem-protein complexes as iron sources. Mol Microbiol. 1996;20:725–739. doi: 10.1111/j.1365-2958.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys G O, Willshaw G A, Anderson E S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975;383:457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- 27.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 28.Lucier T S, Brubaker R R. Determination of genome size, macrorestriction pattern polymorphism, and nonpigmentation-specific deletion in Yersinia pestis by pulsed-field gel electrophoresis. J Bacteriol. 1992;174:2078–2086. doi: 10.1128/jb.174.7.2078-2086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucier T S, Fetherston J D, Brubaker R R, Perry R D. Iron uptake and iron-repressible polypeptides in Yersinia pestis. Infect Immun. 1996;64:3023–3031. doi: 10.1128/iai.64.8.3023-3031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 31.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Perry R D, Brubaker R R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983;40:166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F R. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed L J, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 38.Rogers H J. Iron-binding catechols and virulence in Escherichia coli. Infect Immun. 1973;7:445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saier M H., Jr A functional-phylogenetic system for the classification of transport proteins. J Cell Biochem Suppl. 1999;32/33:84–94. doi: 10.1002/(sici)1097-4644(1999)75:32+<84::aid-jcb11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Saken E, Rakin A, Heesemann J. Molecular characterization of a novel siderophore-independent iron transport system in Yersinia. Int J Med Microbiol. 2000;290:51–60. doi: 10.1016/S1438-4221(00)80106-X. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon E H, Tessman I. Thymidine-requiring mutants of phage T4. Proc Natl Acad Sci USA. 1963;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staggs T M, Fetherston J D, Perry R D. Pleiotropic effects of a Yersinia pestis fur mutation. J Bacteriol. 1994;176:7614–7624. doi: 10.1128/jb.176.24.7614-7624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staggs T M, Greer M K, Baseman J B, Holt S C, Tryon V V. Identification of lactoferrin-binding proteins from Treponema pallidum subspecies pallidum and Treponema denticola. Mol Microbiol. 1994;12:613–619. doi: 10.1111/j.1365-2958.1994.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 46.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surgalla M J, Beesley E D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969;18:834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson J M, Jones H A, Perry R D. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect Immun. 1999;67:3879–3892. doi: 10.1128/iai.67.8.3879-3892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wandersman C, Stojiljkovic I. Bacterial heme sources: the role of heme, hemoprotein receptors, and hemophores. Curr Opin Microbiol. 2000;3:215–220. doi: 10.1016/s1369-5274(00)00078-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 53.Weinberg E D, Weinberg G A. The role of iron in infection. Curr Opin Infect Dis. 1995;8:164–169. [Google Scholar]
- 54.Zimmermann L, Angerer A, Braun V. Mechanistically novel iron(III) transport system of Serratia marcescens. J Bacteriol. 1989;171:238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]