Abstract
Introduction
Upadacitinib, an oral, selective Janus kinase inhibitor, is approved in Japan for the treatment of moderate-to-severe atopic dermatitis (AD), a chronic inflammatory skin disease characterized by eczematous morphology and intense itch.
Methods
Rising Up is an ongoing phase 3, randomized, multicenter study evaluating the long-term safety and efficacy of upadacitinib in Japan. Patients with moderate-to-severe AD were randomized 1:1:1 to topical corticosteroids plus upadacitinib 15 mg (UPA15), upadacitinib 30 mg (UPA30), or placebo at baseline; at week 16, placebo patients were rerandomized 1:1 to UPA15 or UPA30 (plus topical corticosteroids per investigator discretion). This 2-year interim analysis evaluated safety and efficacy through 112 weeks (data cutoff date: 11 August 2021). Adverse events (AEs), AEs of special interest (AESIs), and laboratory data were assessed. Efficacy assessments included ≥ 75% and ≥ 90% improvement from baseline in Eczema Area and Severity Index (EASI 75/90), achievement of clear or almost clear on the validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD 0/1), and ≥ 4-point improvement in the Worst Pruritus Numerical Rating Scale (WP-NRS).
Results
A total of 272 patients were enrolled and 242 were ongoing at data cutoff (UPA15, n = 120; UPA30, n = 122). After 112 weeks of treatment, serious AEs, AEs leading to discontinuation, and most AESIs were generally infrequent, and rates were similar between the two upadacitinib groups. One event each of rectal cancer and cerebellar hemorrhage was reported in the UPA15 group; no thrombosis events were observed. The most common AEs included acne, nasopharyngitis, and herpes zoster. EASI 75, EASI 90, vIGA-AD 0/1, and WP-NRS response rates were maintained through week 112.
Conclusion
UPA15 and UPA30 were well tolerated through 112 weeks of treatment with similar safety profiles to short-term studies and demonstrated durable long-term efficacy for the treatment of moderate-to-severe AD in adults and adolescents.
Trial Registration
ClinicalTrials.gov identifier, NCT03661138.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00842-7.
Keywords: Atopic dermatitis, Clinical trial, Eczema, Janus kinase inhibitors, Safety, Topical corticosteroids, Upadacitinib
Key Summary Points
| Why carry out this study? |
| Medicinal products with long-term sustained efficacy and acceptable safety are needed for the treatment of atopic dermatitis, a chronic inflammatory skin disease characterized by eczematous morphology and intense itch. |
| This study evaluated the long-term safety and efficacy of upadacitinib for the treatment of adolescents and adults with moderate-to-severe atopic dermatitis. |
| What was learned from the study? |
| Upadacitinib 15 mg and 30 mg were well tolerated through 112 weeks of continuous treatment with a safety profile consistent with that identified in short-term studies; no new safety risks were identified. |
| Response rates for skin clearance and itch improvement were numerically greater for upadacitinib than for placebo at week 16 and remained durable through week 112. |
| These 2-year interim results support the favorable benefit–risk profile of upadacitinib for the treatment of adult and adolescent patients with moderate-to-severe atopic dermatitis. |
Introduction
Atopic dermatitis (AD) is a chronic, itchy, inflammatory skin disease that typically begins during childhood and can persist into adulthood [1–3]. Atopic dermatitis is characterized by eczematous morphology, sensitive and dry skin, and intense itch, which can significantly impair quality of life for patients and their families [1–3]. Atopic dermatitis is a heterogeneous disease, with a worldwide prevalence ranging from 0.2% to 24.6% that varies across countries [3–5]. While some studies evaluating racial differences in atopic dermatitis have suggested a higher prevalence in Asian populations than in white/European populations [5, 6], data on prevalence across countries are limited and further investigation is warranted. Unique clinical manifestations have been reported across different racial and ethnic groups: Asian patients generally have more well-demarcated lesions and stronger Th17/Th22 polarization than white/European patients [5–7].
First-line treatments for AD include local anti-inflammatory therapies such as topical corticosteroids and calcineurin inhibitors [3, 8]. In patients with more severe forms of AD, current guidelines (including those from the Japanese Dermatological Association) recommend systemic therapies if topical treatments do not adequately control AD symptoms or if patients’ quality of life is severely affected by AD [3, 8]. Multiple systemic therapies have recently been developed for the treatment of AD; however, long-term safety and efficacy data for these therapies are needed.
Upadacitinib is an oral, selective Janus kinase (JAK) inhibitor with greater potency for JAK1 than JAK2, JAK3, or tyrosine kinase 2 [9, 10]. In phase 3 studies, upadacitinib demonstrated superior efficacy to placebo both with and without concomitant topical corticosteroid therapy for the treatment of moderate-to-severe AD in adolescents and adults [1, 2]. Upadacitinib is approved in many countries and regions, including Japan, Europe, and the USA for the treatment of moderate-to-severe AD in adolescents and adults [11, 12].
Rising Up (NCT03661138) is an ongoing phase 3 study evaluating the safety and efficacy of upadacitinib in adolescents and adults in Japan with moderate-to-severe AD [13]. A planned 24-week interim safety analysis reported an acceptable safety profile for upadacitinib 15 mg and 30 mg in combination with topical corticosteroids for treating patients with moderate-to-severe AD [13]. Patients also experienced substantial improvements in skin clearance and itch reduction with either dose of upadacitinib after 24 weeks of treatment [13]. This 2-year interim analysis builds on findings from the 24-week analysis to report the long-term safety and efficacy of upadacitinib treatment for Japanese patients with AD through 112 weeks of continuous therapy.
Methods
Patients, Study Design, and Treatment
Detailed methods have been described previously [13]. Briefly, this ongoing phase 3 study (Rising Up, NCT03661138) was designed to evaluate the safety and efficacy of upadacitinib for the treatment of moderate-to-severe AD in adolescents (aged 12 to < 18 years, weight ≥ 40 kg) and adults (aged 18–75 years) in Japan. There were 42 study sites, all of which were in Japan. Patients were randomized 1:1:1 to receive either upadacitinib 15 mg, upadacitinib 30 mg, or placebo with concomitant topical corticosteroids for all arms from baseline to week 16 (double-blind period). Randomization was stratified according to baseline disease severity [validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 3 (moderate) versus 4 (severe)] and age (< 18 versus 18–40 versus > 40 years of age). Patients who received placebo during the double-blind period were rerandomized 1:1 at week 16 to receive either upadacitinib 15 mg or 30 mg; treatment was blinded from weeks 16–52 (blinded extension period) and open-label from week 52 up to week 160 (open-label phase). Starting at the week 16 visit, the use of any concomitant topical medication for AD was no longer required and was administered per investigator discretion. The study was conducted in accordance with the protocol, International Council for Harmonisation guidelines, and applicable regulations, guidelines, and ethical principles originating from the Declaration of Helsinki. The study protocol was reviewed and approved by an institutional review board and patients provided written informed consent before screening or undergoing study-specific procedures [13].
Assessments and Statistical Analysis
Safety assessments, exploratory efficacy endpoints, and statistical analyses through week 24 were previously reported [13]. In this 2-year interim analysis, safety and efficacy were evaluated through the 112-week cutoff date (11 August 2021). Short-term safety was reported as number (percent) of patients, while long-term safety was calculated as exposure-adjusted incidence rates [the number of patients with at least one event per 100 patient-years (n/100 PY)] to account for differences in upadacitinib exposure across treatment arms. Long-term safety data were censored with exposure only counted until the event. Exposure-adjusted event rates [calculated as the number of events per 100 patient-years (E/100 PY)] were also calculated through 52 weeks and 112 weeks of continuous upadacitinib treatment. Efficacy assessments included at least 75% and at least 90% improvement from baseline in Eczema Area and Severity Index (EASI 75/90), achievement of clear or almost clear on the vIGA-AD (vIGA-AD 0/1) with at least two grades of reduction from baseline, and ≥ 4-point improvement (reduction) on the Worst Pruritus Numerical Rating Scale (WP-NRS) in patients with WP-NRS scores ≥ 4 at baseline. Long-term efficacy data are reported using the observed cases approach (i.e., all observed data up to drug discontinuation were included and no imputation was applied for missing data). Data were descriptively reported, and hypothesis testing was not performed.
Results
Patients
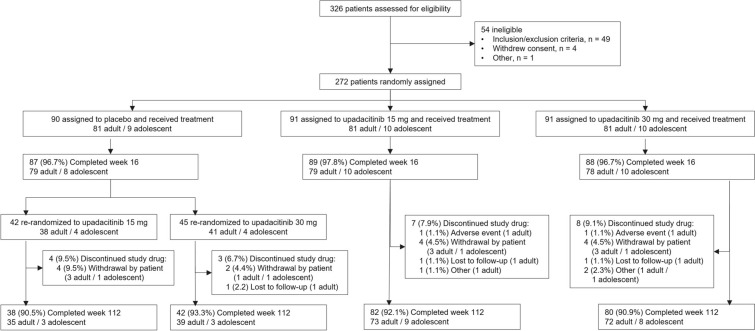
Among the 272 patients enrolled in the study, 242 were still ongoing at the time of data cutoff (120 in the upadacitinib 15 mg group and 122 in the upadacitinib 30 mg group; Fig. 1). A total of 29 adolescents were randomized, and 23 adolescents were still ongoing at the time of data cutoff (12 in the upadacitinib 15 mg group and 11 in the upadacitinib 30 mg group). Baseline demographics and characteristics were generally balanced across treatment groups in both the overall population [13] (Table 1) and in adolescents (Table S1).
Fig. 1.
Patient disposition. All patients received concomitant topical corticosteroids from baseline to week 16. After week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Upadacitinib 15 mga (N = 91) |
Upadacitinib 30 mga (N = 91) |
Placeboa (N = 90) |
|---|---|---|---|
| Age, years, mean (SD) | 35.9 (13.2) | 34.7 (12.7) | 36.3 (12.6) |
| Age group, n (%) | |||
| Adolescents (< 18 years) | 10 (11.0) | 10 (11.0) | 9 (10.0) |
| Adults (≥ 18 years) | 81 (89.0) | 81 (89.0) | 81 (90.0) |
| Sex, n (%) | |||
| Female | 23 (25.3) | 22 (24.2) | 16 (17.8) |
| Male | 68 (74.7) | 69 (75.8) | 74 (82.2) |
| Weight, kg, mean (SD) | 65.1 (14.2) | 66.2 (14.4) | 67.6 (12.8) |
| Affected BSA, %, mean (SD) | 61.7 (23.7) | 66.7 (21.2) | 62.0 (20.5) |
| Disease duration since diagnosis, years, mean (SD) | 23.0 (14.3) | 20.7 (14.1) | 24.7 (14.4) |
| Medical history, n (%) | |||
| Asthma | 29 (31.9) | 28 (30.8) | 34 (37.8) |
| Rhinitis allergic | 45 (49.5) | 45 (19.5) | 58 (64.4) |
| Conjunctivitis allergic | 8 (8.8) | 12 (13.2) | 11 (12.2) |
| vIGA-AD, n (%) | |||
| Moderate (< 4) | 47 (51.6) | 48 (52.7) | 47 (52.2) |
| Severe (4) | 44 (48.4) | 43 (47.3) | 43 (47.8) |
| EASI, mean (SD) | 34.2 (14.1) | 36.1 (14.4) | 34.4 (13.0) |
| WP-NRS, mean (SD) | 6.7 (1.4) | 7.0 (1.4) | 6.8 (1.3) |
| hsCRP, mg/L, mean (SD) | 2.3 (3.8) | 3.9 (8.8) | 3.1 (6.4) |
BSA body surface area, EASI Eczema Area and Severity Index, hsCRP high-sensitivity C-reactive protein, vIGA-AD validated Investigator Global Assessment for Atopic Dermatitis, WP-NRS Worst Pruritus Numerical Rating Scale
aAll patients received concomitant topical corticosteroids from baseline to week 16. After week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion
Safety
Overall, similar safety profiles were observed between the upadacitinib 15 mg group and upadacitinib 30 mg group through the week 112 data cutoff date (Table 2). The most common adverse events (AEs) with long-term upadacitinib treatment continued to be acne, nasopharyngitis, and herpes zoster. All cases of acne were mild or moderate in severity; one case of acne in the upadacitinib 30 mg group led to study drug discontinuation. Rates of acne, herpes zoster, increased blood creatine phosphokinase levels, and tinea pedis were slightly higher in the upadacitinib 30 mg group than in the 15 mg group. Rates for other common AEs were generally similar between the two upadacitinib treatment groups with some variability likely owing to the small sample size. Long-term incidence rates (n/100 PY) for severe AEs and AEs leading to discontinuation were similar between the two upadacitinib treatment groups. The rate of serious AEs was numerically higher with upadacitinib 15 mg than with upadacitinib 30 mg, likely owing to the small number of reports or simply a chance finding; no patterns in the type or timing of events were observed. There were no deaths during the interim analysis period. Similar patterns were observed for exposure-adjusted event rates (E/100 PY) through week 52 and week 112 (Table S2).
Table 2.
Short- and long-term safety overview of upadacitinib in the Rising Up study
| Parameter | Week 16, n (%) | Week 112; n/PY (n/100 PY) | |||
|---|---|---|---|---|---|
| Upadacitinib 15 mg + TCS (N = 91) |
Upadacitinib 30 mg + TCS (N = 91) |
Placebo + TCS (N = 90) |
Upadacitinib 15 mga (N = 133) |
Upadacitinib 30 mga (N = 136) |
|
| Overview | |||||
| Any AE | 51 (56.0) | 58 (63.7) | 38 (42.2) | 116/91.6 (126.6) | 117/66.6 (175.7) |
| AE with reasonable possibility of being drug related | 12 (13.2) | 24 (26.4) | 11 (12.2) | 61/207.1 (29.5) | 66/183.1 (36.0) |
| Severe AE | 2 (2.2) | 4 (4.4) | 0 | 16/274.8 (5.8) | 16/265.1 (6.0) |
| Serious AE | 1 (1.1) | 1 (1.1) | 1 (1.1) | 14/277.0 (5.1) | 8/276.8 (2.9) |
| AE leading to discontinuation of study drug | 2 (2.2) | 1 (1.1) | 1 (1.1) | 7/288.9 (2.4) | 5/285.4 (1.8) |
| Deaths | 0 | 0 | 0 | 0/289.4 | 0/287.7 |
| Most common AEsb | |||||
| Acne | 12 (13.2) | 18 (19.8) | 5 (5.6) | 30/237.7 (12.6) | 51/195.5 (26.1) |
| Nasopharyngitis | 12 (13.2) | 14 (15.4) | 14 (15.6) | 36/229.6 (15.7) | 43/213.8 (20.1) |
| Herpes zoster | 0 | 4 (4.4) | 0 | 18/271.1 (6.6) | 31/248.2 (12.5) |
| Pyrexia | 4 (4.4) | 3 (3.3) | 1 (1.1) | 13/275.6 (4.7) | 16/269.6 (5.9) |
| Blood CPK increased | 1 (1.1) | 2 (2.2) | 0 | 6/281.8 (2.1) | 12/274.2 (4.4) |
| Tinea pedis | 0 | 1 (1.1) | 2 (2.2) | 2/286.8 (0.7) | 11/279.7 (3.9) |
| Influenza | 1 (1.1) | 2 (2.2) | 1 (1.1) | 7/277.2 (2.5) | 11/268.3 (4.1) |
| Dermatitis atopic | 3 (3.3) | 0 | 3 (3.3) | 17/270.7 (6.3) | 9/278.0 (3.2) |
| Herpes simplex | 2 (2.2) | 2 (2.2) | 2 (2.2) | 11/272.5 (4.0) | 7/276.9 (2.5) |
| Oral herpes | 1 (1.1) | 2 (2.2) | 0 | 11/278.6 (3.9) | 8/275.5 (2.9) |
| Skin papilloma | 1 (1.1) | 0 | 0 | 13/273.9 (4.7) | 7/282.9 (2.5) |
| Folliculitis | 3 (3.3) | 2 (2.2) | 2 (2.2) | 13/270.5 (4.8) | 6/281.6 (2.1) |
| Eczema herpeticum | 0 | 0 | 0 | 11/281.5 (3.9) | 5/280.3 (1.8) |
Among patients who received at least one dose of study drug from baseline to week 16 and patients who received at least one dose of upadacitinib from week 16 through the week 112 data cutoff date
AE adverse event, CPK creatine phosphokinase, n/100 PY number of patients with at least one event per 100 patient-years, n/PY number of patients with at least one event divided by the total patient years for patients at risk of an event, TCS topical corticosteroids
aAfter week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion
bMost common AEs are defined as those occurring in > 10 patients in either group
Long-term incidence rates of AEs were lower in adolescents than in the overall study population and were consistent with the previously reported safety profile for adolescents; no new safety findings were reported (Table S3).
Similar to the 16-week placebo-controlled period, incidence rates of AEs of special interest were generally low at week 112 for both doses of upadacitinib (Table 3). All opportunistic infections (excluding tuberculosis and herpes zoster) were eczema herpeticum (Kaposi’s varicelliform eruption), aside from one onset of Pneumocystis jirovecii pneumonia. In the upadacitinib 15 mg group, six serious infections were reported and included appendicitis, Pneumocystis jirovecii pneumonia, herpes zoster, cellulitis, septic shock, and enteritis infectious; all cases were severe. All serious infections in the upadacitinib 15 mg group except cellulitis and septic shock were considered by the investigator as having a reasonable possibility of being related to study drug, and all had resolved as of the data cutoff date. In the upadacitinib 30 mg group, four serious infections were reported (one event of appendicitis, one event of herpes simplex, and two events of herpes zoster); all cases were severe, all were considered by the investigator as having a reasonable possibility of being related to study drug, and all had resolved as of the data cutoff date. Most cases of herpes zoster were non-serious, did not lead to treatment discontinuation, and involved one or two dermatomes. Most hepatic disorders were elevations in transaminase levels. Incidence rates of anemia, neutropenia, and elevations in creatine phosphokinase levels remained higher with upadacitinib 30 mg than with upadacitinib 15 mg. All laboratory-related AEs were generally mild or moderate in severity and were non-serious; few led to treatment discontinuation. One case of grade 1 rectal cancer was reported in the upadacitinib 15 mg group on study day 793 (patient age at onset, 38 years); the case resolved after the excision of the tumor, and was considered unrelated to study drug by the investigator. A 22-year-old male patient experienced a major adverse cardiovascular event (cerebellar hemorrhage) on study day 65, and assessed by the investigator as possibly related to study drug. There continued to be no cases of gastrointestinal perforation, active tuberculosis, or venous thromboembolic events. Clinical laboratory parameters were generally similar through 112 weeks of treatment (Table 4) Exposure-adjusted event rates for AEs of special interest at week 52 and week 112 are presented in Table S4.
Table 3.
Short-term incidence and long-term event rates for AESIs in the Rising Up study
| Parameter | Week 16, n (%) | Week 112, n/PY (n/100 PY) | |||
|---|---|---|---|---|---|
| Upadacitinib 15 mg + TCS (N = 91) |
Upadacitinib 30 mg + TCS (N = 91) |
Placebo + TCS (N = 90) |
Upadacitinib 15 mga (N = 133) |
Upadacitinib 30 mga (N = 136) |
|
| Serious infection | 0 | 1 (1.1) | 0 | 6/284.5 (2.1) | 4/279.8 (1.4) |
| Opportunistic infection excluding TB and herpes zosterb | 3 (3.3) | 1 (1.1) | 0 | 12/281.5 (4.3) | 6/279.9 (2.1) |
| Malignancy | 0 | 0 | 0 | 1/289.2 (0.3) | 0/287.7 |
| NMSC | 0 | 0 | 0 | 0/289.4 | 0/287.7 |
| Malignancy excluding NMSC | 0 | 0 | 0 | 1/289.2 (0.3) | 0/287.7 |
| Lymphomac | 0 | 0 | 0 | 0/289.4 | 1/287.6 (0.3) |
| Hepatic disorder | 1 (1.1) | 1 (1.1) | 0 | 10/278.8 (3.6) | 11/272.9 (4.0) |
| Gastrointestinal perforation | 0 | 0 | 0 | 0/289.4 | 0/287.7 |
| Anemia | 0 | 1 (1.1) | 0 | 4/285.8 (1.4) | 7/276.1 (2.5) |
| Neutropenia | 1 (1.1) | 4 (4.4) | 0 | 2/287.0 (0.7) | 7/273.9 (2.6) |
| Lymphopenia | 0 | 0 | 0 | 0/289.4 | 1/286.1 (0.3) |
| Herpes zosterd | 0 | 4 (4.4) | 0 | 20/268.6 (7.4) | 31/248.2 (12.5) |
| CPK elevation | 1 (1.1) | 2 (2.2) | 0 | 6/281.8 (2.1) | 12/274.2 (4.4) |
| Renal dysfunction | 0 | 0 | 0 | 0/289.4 | 0/287.7 |
| Active TB | 0 | 0 | 0 | 0/289.4 | 0/287.7 |
| Adjudicated MACEe,f | 1 (1.1) | 0 | 0 | 1/289.4 (0.3) | 0/287.7 |
| Adjudicated VTEg | 0 | 0 | 0 | 0/289.4 | 0/287.7 |
AESI adverse event of special interest, MACE major adverse cardiovascular event, MedDRA Medical Dictionary for Regulatory Activities Terminology, n/100 PY number of patients with at least one event per 100 patient-years, n/PY number of patients with at least one event divided by the total patient years for patients at risk of an event, NMSC non-melanoma skin cancer, TB tuberculosis, TCS topical corticosteroids, UPA upadacitinib, VTE venous thromboembolic events
aAfter week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion
bAll events were eczema herpeticum except for one case of Pneumocystis jirovecii pneumonia in the UPA 15 mg group and one case of Oesophageal candidiasis in the UPA 30 mg group
cAtypical lymphocytes seen in peripheral blood smear that resolved; event was not considered a malignancy
dOn the basis of group term search with the Company MedDRA Query
eMACE is defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke
f22-year-old male with no risk factors who experienced cerebellar hemorrhage on study day 65; assessed by investigator to be possibly related to study drug
gVTE is defined as deep vein thrombosis and pulmonary embolism (fatal and non-fatal)
Table 4.
Potentially clinically important laboratory values through week 112
| Parameter | Threshold | Patients, n (%) | |
|---|---|---|---|
| Upadacitinib 15 mg (N = 133)a |
Upadacitinib 30 mg (N = 136)a |
||
| Hemoglobin, g/L | < 80 g/L | 0 | 0 |
| Lymphocytes, 109/L | < 0.5 × 109/L | 1 (0.8) | 3 (2.2) |
| Neutrophils, 109/L | < 1.0 × 109/L | 2 (1.5) | 3 (2.2) |
| Platelets, 109/L | < 50 × 109/L | 0 | 0 |
| ALT, U/L | > 5.0 × ULN | 2 (1.5) | 1 (0.7) |
| Alkaline phosphatase, U/L | > 5.0 × ULN | 0 | 0 |
| AST, U/L | > 5.0 × ULN | 1 (0.8) | 0 |
| Creatinine, µmol/L | > 3.0 × ULN | 0 | 0 |
| CPK, U/L | > 5.0 × ULN | 4 (3.0) | 9 (6.6) |
ALT alanine aminotransferase, AST aspartate aminotransferase, CPK creatine phosphokinase, ULN upper limit of normal
aAll patients received concomitant topical corticosteroids from baseline to week 16. After week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion
Among adolescents, AEs of special interest were reported infrequently (≤ three cases) with no new safety findings relative to the overall study population (Table S5). A single event of serious infection (appendicitis, possibly related to study drug) was reported in an adolescent receiving upadacitinib 15 mg. In addition to the appendicitis, two serious AEs were reported in adolescents receiving upadacitinib 15 mg: one event of concussion due to an accident and one event of irritable bowel syndrome, both considered to be unrelated to the study drug by the investigator. Two events of herpes zoster were reported in adolescents receiving upadacitinib 30 mg. No malignancies, adjudicated major adverse cardiovascular events, or venous thromboembolic events were reported.
Efficacy
In patients randomized to receive upadacitinib at baseline, improvements in EASI scores observed by week 16 were sustained through 112 weeks of treatment (Fig. 2) At week 112, 69.5% and 74.4% of patients achieved EASI 75 with upadacitinib 15 mg and 30 mg, respectively (Fig. 2A), while 37.8% and 56.4% of patients achieved EASI 90 (Fig. 2B). Patients who switched from placebo to upadacitinib at week 16 also showed substantial improvements in EASI scores within 8 weeks that remained high and durable through week 112, regardless of upadacitinib dose; at week 112, 66.7% and 85.4% of patients achieved EASI 75 with upadacitinib 15 mg and 30 mg, respectively, and 36.1% and 56.1% of patients achieved EASI 90.
Fig. 2.
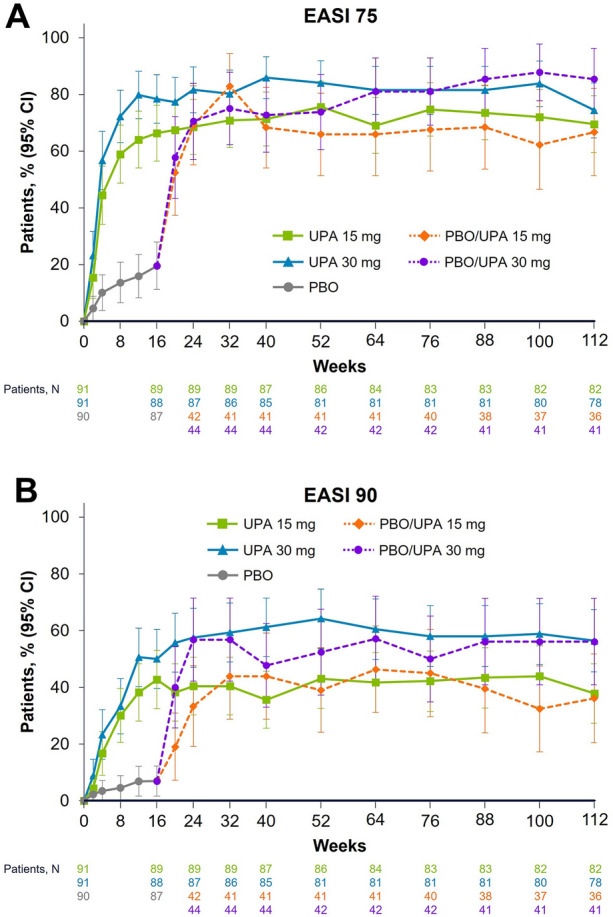
Patients achieving EASI 75 and EASI 90 from baseline to week 112. Data are presented using the observed cases approach. All patients received concomitant topical corticosteroids from baseline to week 16. After week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion. EASI 75/90 ≥ 75%/ ≥ 90% improvement in Eczema Area Severity Index, PBO placebo, UPA upadacitinib
Response rates for vIGA-AD 0/1 were numerically greater for patients treated with either dose of upadacitinib than for patients receiving placebo, and vIGA-AD 0/1 response rates were maintained through week 112 in all upadacitinib treatment groups (Fig. 3). At week 112, 32.9% and 46.2% of patients achieved vIGA-AD 0/1 with continuous upadacitinib 15 mg and 30 mg, respectively; among patients who switched from placebo to upadacitinib at week 16, 27.8% and 36.6% of patients achieved vIGA-AD 0/1 at week 112 with upadacitinib 15 mg and 30 mg, respectively.
Fig. 3.
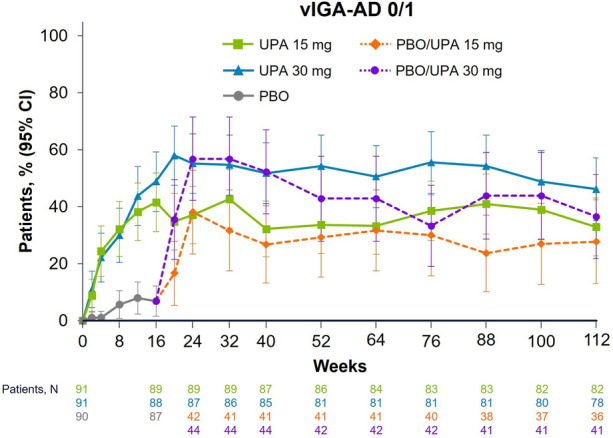
Patients achieving vIGA-AD 0/1a from baseline to week 112. Data are presented using the observed cases approach. All patients received concomitant topical corticosteroids from baseline to week 16. After week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion. aPatients achieving vIGA-AD 0/1 with at least two grades of reduction from baseline. PBO placebo, UPA upadacitinib, vIGA-AD 0/1 validated Investigator Global Assessment for AD score of clear or almost clear
Patients receiving upadacitinib also showed substantial reductions in WP-NRS scores within as early as 1 week of treatment that were maintained long term (Fig. 4). At week 112, 44.4% and 56.4% of patients with WP-NRS scores ≥ 4 at baseline achieved a ≥ 4-point improvement in WP-NRS scores with continuous upadacitinib 15 mg and 30 mg, respectively. Among patients who switched from placebo to upadacitinib at week 16, 44.4% and 63.4% of patients achieved a ≥ 4-point improvement in WP-NRS scores at week 112 with upadacitinib 15 mg and 30 mg, respectively.
Fig. 4.
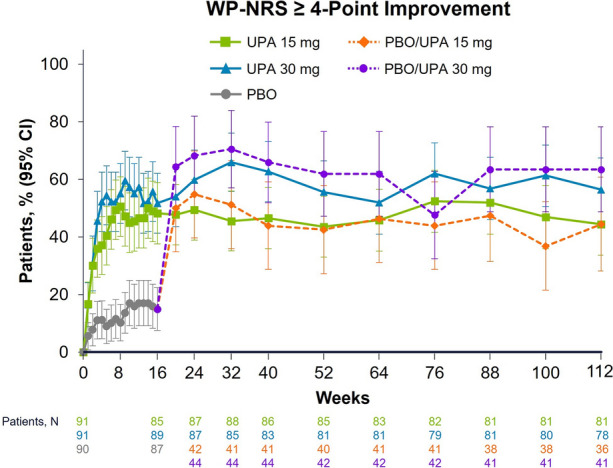
Patients achieving WP-NRS ≥ 4-point improvementa from baseline to week 112. Data are presented using the observed cases approach. All patients received concomitant topical corticosteroids from baseline to week 16. After week 16, the use of concomitant topical corticosteroids was no longer required and was administered per investigator discretion. aAmong patients with WP-NRS scores ≥ 4 at baseline. PBO placebo, UPA upadacitinib, WP-NRS Worst Pruritus Numerical Rating Scale
Adolescents also experienced substantial skin clearance and itch reduction that generally followed similar trends as the overall population; however, higher variability was observed owing to the small sample size, and the data should be interpreted with caution (Figs. S1–S3).
Discussion
Atopic dermatitis is a chronic, relapsing condition with substantial disease burden that requires long-term management for both adolescents and adults [3, 14–16]. However, most AD therapies are focused on short-term management of flares, with no consensus on standardized approaches for long-term control [15]. Many of these therapies, such as oral corticosteroids, are not recommended for long-term use owing to safety concerns [8, 17]. Despite recent advances in therapeutic options for AD, studies evaluating the long-term safety of AD treatments (including oral JAK inhibitors) are lacking, and treatments with acceptable long-term benefit–risk profiles are needed but still limited [3, 15, 17].
The 112-week interim results of the Rising Up study presented herein support the favorable benefit–risk profile for upadacitinib for the long-term treatment of moderate-to-severe AD. Upadacitinib was generally safe and well tolerated through 112 weeks of continuous treatment with a similar safety profile to that noted in short-term studies for both adults and adolescents [13]. No new safety risks were identified beyond those previously reported [13]. Slightly higher rates of acne, herpes zoster, increased blood creatine phosphokinase levels, and tinea pedis were observed in the upadacitinib 30 mg group than in the 15 mg group, which is consistent with the known upadacitinib safety profile from other global studies [1, 2, 13, 18]. In the double-blind, placebo-controlled period, response rates for skin clearance and itch improvement were numerically greater for either upadacitinib dose than for placebo at week 16 in both the overall study population and adolescents. Upadacitinib response rates were maintained during the blinded extension and open-label extension periods through over 2 years of continuous treatment with either upadacitinib dose.
Further investigation on the safety and efficacy of AD treatments within specific global populations may also be warranted, given the potential higher prevalence of AD and different clinical manifestations in Asian populations compared with white/European populations [5–7]. Although results from some studies have demonstrated no differences in treatment response across white, Black or African American, and Asian patients, most patients in clinical trials are white and there are little data on the efficacy of AD treatments in non-white patients [5]. More data describing the efficacy of systemic therapies across different ethnic groups are needed given the potential differences in skin phenotypes and pharmacokinetics between these groups [5]. Our findings in the current study contribute to the totality of safety data for upadacitinib, especially for long-term use in adults and adolescents in Japan.
Limitations of this study include small sample sizes in each treatment group, especially for the adolescent population, as well as the absence of a control group beyond week 16. Additionally, the open-label treatment period from weeks 52–112 could potentially introduce reporting bias for patients and attribution bias for investigators. No comparisons with other AD treatments can be made because of differences in study designs and the lack of comparator group in this study. Since the study was limited to Japanese patients, these findings may not be generalizable to patients with AD in other global populations. However, the results reported in this 2-year analysis are generally consistent with those reported in other phase 3 studies of upadacitinib that were performed in broader study populations [1, 2].
Conclusions
These 2-year interim results demonstrate an acceptable long-term safety profile of upadacitinib to treat adults and adolescents with moderate-to-severe AD through 112 weeks of treatment. A high proportion of patients improved and maintained clinical responses for over 2 years of upadacitinib therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AbbVie and the authors thank all study investigators for their contributions and the patients who participated in these studies
Funding
This study was sponsored and funded by AbbVie, Inc. AbbVie Inc. participated in the study design; study research; collection, analysis, and interpretation of data; and writing, reviewing, and approving this manuscript for publication. AbbVie also provided funding for the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
Medical writing support, funded by AbbVie, was provided by Callie A. S. Corsa, PhD, and Lamara D. Shrode, PhD, CMPP™, of JB Ashtin, who developed the first draft based on an author-approved outline and assisted in implementing author revisions. JB Ashtin adheres to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. No honoraria or payments were made by AbbVie for authorship.
Author Contributions
All authors critically reviewed this manuscript, contributed to data interpretation, and provided final approval for publication. John Liu and Henrique D. Teixeira contributed to study design. Norito Katoh, Yukihiro Ohya, Hiroyuki Murota, Masanori Ikeda, Takuya Sasaki, and Hidehisa Saeki contributed to data acquisition. Xiaofei Hu and Kimitoshi Ikeda contributed to statistical analysis.
Prior Presentation
Portions of these data were previously presented at the 31st Congress of the European Academy of Dermatology and Venerology, 7–10 September 2022 in Milan, Italy.
Disclosures
N. Katoh has received honoraria as a speaker/consultant for AbbVie, Celgene Japan, Jansen Pharma, Kyowa Kirin, LEO Pharma, Lilly Japan, Maruho, Mitsubishi Tanabe Pharma, Sanofi, Taiho Pharmaceutical, and Torii Pharmaceutical, and has received grants as an investigator from Abbvie, A2 Healthcare, Boehringer Ingelheim Japan, Eisai, Jansen Pharma, Kyowa Kirin, LEO Pharma, Lilly Japan, Maruho, Sun Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical. Y. Ohya has received honoraria as a speaker, consultant, and/or for data safety monitoring for AbbVie, Lilly Japan, Japan Tobacco, Maruho, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Pola Pharma, Sanofi/Regeneron, Shino-Test, Sysmex, Taiho Pharmaceutical, Thermo Fisher Scientific, and Torii Pharmaceutical. He has received grants as an investigator from Fam’s. H. Murota has received funding or grant support from Maruho, Mitsubishi Tanabe Pharma, SUN Pharma, and Taiho Pharmaceutical, and honorarium as a speaker/consultant from Japan Tobacco, Kaken Pharmaceutical, Maruho, Mitsubishi Tanabe Pharma, Sanofi, Shiseido Japan, Lily, Abbie, and Taiho Pharmaceutical. M Ikeda has received a scholarship donation from the Central Research Institute of Pias. X. Hu, K. Ikeda, J. Liu, T. Sasaki, E.M. Raymundo, and H.D. Teixeira are full-time employees of AbbVie Inc., and may hold AbbVie stock or stock options. H. Saeki has received honoraria for consultancy from AbbVie, LEO Pharma, and Sanofi, and funding/grant support from Eisai, Maruho, Mitsubishi Tanabe Pharma, and Torii Pharmaceutical.
Compliance with Ethics Guidelines
All study protocols were reviewed and approved by an institutional review board (Supplementary Table 6). The study was conducted in accordance with the protocol; International Council for Harmonisation guidelines; and applicable regulations, guidelines, and ethical principles originating from the Declaration of Helsinki. All patients provided written informed consent before screening or undergoing study-specific procedures.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials and studies we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This study data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 2.Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169–2181. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 3.Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21:21–40. doi: 10.1038/s41573-021-00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449–455. doi: 10.1016/j.anai.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340–357. doi: 10.1111/exd.13514. [DOI] [PubMed] [Google Scholar]
- 6.Nomura T, Wu J, Kabashima K, Guttman-Yassky E. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840–1852. doi: 10.1016/j.jaip.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol. 2021;32:23–33. doi: 10.1111/pai.13335. [DOI] [PubMed] [Google Scholar]
- 8.Katoh N, Ohya Y, Ikeda M, et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int. 2020;69:356–369. doi: 10.1016/j.alit.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voss J, Graff C, Schwartz A, et al. Pharmacodynamics of a novel Jak1 selective inhibitor in rat arthritis and anemia models and in healthy human subjects. Arthritis Rheum. 2013;65:S1015. [Google Scholar]
- 11.Rinvoq® Tablets [package insert]. AbbVie GK, August 2021. Accessed 7 Nov 2022. https://www.drugfuture.com/pmda/label/rx/112130_3999048G2024_1_10.
- 12.Rinvoq® [package insert]. AbbVie, Inc., January 2022. Accessed 1 Feb 2022. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf.
- 13.Katoh N, Ohya Y, Murota H, et al. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (Rising Up): an interim 24-week analysis. JAAD Int. 2022;6:27–36. doi: 10.1016/j.jdin.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsawi R, Dainty K, Smith Begolka W, et al. The multidimensional burden of atopic dermatitis among adults: results from a large national survey. JAMA Dermatol. 2022;158:887–892. doi: 10.1001/jamadermatol.2022.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abuabara K, Margolis DJ, Langan SM. The long-term course of atopic dermatitis. Dermatol Clin. 2017;35:291–297. doi: 10.1016/j.det.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boguniewicz M, Alexis AF, Beck LA, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5:1519–1531. doi: 10.1016/j.jaip.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Mendes-Bastos P, Ladizinski B, Guttman-Yassky E, et al. Characterization of acne associated with upadacitinib treatment in patients with moderate-to-severe atopic dermatitis: a post hoc integrated analysis of three phase 3 randomized, double-blind, placebo-controlled trials. J Am Acad Dermatol. 2022:S0190-9622(0122)00996-00993 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials and studies we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This study data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.