Abstract
Over the last three decades, the use of dermoscopy has been extended to inflammatory and infectious dermatoses. Regarding the latter, while the first applications concerned skin parasitoses, there has been a significant increase in the publication trend regarding nonparasitic dermatoses over recent years, yet data on this topic are sparse and often lack a standardized analytical approach. This systematic literature review summarizes published data on dermoscopy of bacterial, viral, and fungal dermatoses (dermoscopic findings, used setting, pathological correlation, and level of evidence of studies) and provides a homogeneous terminology of reported dermoscopic features according to a standardized methodology. A total of 152 papers addressing 43 different dermatoses and describing 184 different dermoscopic findings were included in the analysis. The majority of them displayed a level of evidence of V (107 single case reports and 40 case series), with only 5 studies showing a level of evidence of IV (case–control studies). Moreover, our analysis also underlined a high variability in the terminology used in published articles (even for the same dermatosis). Therefore, despite significant potential, future studies designed according to a systematic and standardized approach are required for a better characterization of dermoscopy of nonparasitic skin infections.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00855-2.
Keywords: Bacteria, Dermoscopy, Entodermoscopy, Entomodermoscopy, Fungus, Infectiouscopy, Mycoses, Viruses
Key Points
| Many articles on dermoscopy of bacterial, viral, and fungal dermatoses have been published over the last two decades, yet information is sparse and lacks an analytical approach. |
| This systematic literature review summarizes published data on dermoscopy of bacterial, viral, and fungal dermatoses and provides a homogeneous terminology of reported dermoscopic features according to a standardized methodology. |
Introduction
In addition to the classical applications in dermato-oncology, the use of dermoscopy has expanded over the last three decades to non-neoplastic dermatoses (“general dermatology”), including both inflammatory and infectious dermatoses [1]. In this regard, such a technique has been demonstrated to reduce the number of cases requiring further time-consuming and expensive investigations, thereby being an ideal supportive tool for a rapid diagnosis in every dermatologist's office [1–6]. This becomes more relevant for infections (“infectiouscopy”) as they are quite common in rural and resource-poor areas that are often limited by unavailability of second-level tests (e.g., microscopic and laboratory tests) [3–6].
Interestingly, whereas the first uses of infectiouscopy concerned parasitoses (scabies and larva migrans), there has been a significant increase in the publication trend regarding nonparasitic infections over recent years, especially in the last 5 years (36 versus 9 addressing parasitoses in the time span between 2016 and 2020) [1]. However, knowledge on dermoscopy of nonparasitic infectious dermatoses is sparse and often comes from studies lacking a systematic analytical approach [1–6]. Even though a few narrative reviews on the topic have been published [3–6], there is no systematic review analysis.
The objective of this review is to provide a systematic summary of available data on dermoscopy of nonparasitic skin infections (i.e., bacterial, viral, and fungal dermatoses), as well as to try to align dermoscopic terminology of described findings according to a standardized methodology. It is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Methods
This systematic review was performed according to Preferred Reporting Items for Systematic Reviews and MetaAnalyses (PRISMA) guidelines. A search of papers published up to 30 December 2021 was done via PubMed electronic database with the following search terms: “dermoscopy,” “dermatoscopy,” “videodermatoscopy,” or “epiluminescence microscopy.” Titles and abstracts were screened by two independent reviewers to identify papers reporting dermoscopic findings of bacterial, viral, and fungal skin infections; articles dealing with hair, nail, and mucosal conditions were not considered and duplicates were excluded. A manual search was also performed by assessing the reference sections of all significant studies or reviews on this topic. English-language original articles, case series, and case reports were included in the analysis, while non-English articles, reviews, personal opinions, and nonrelevant articles were ruled out after full-text reading.
All of the retrieved studies were classified based on standard definitions for diagnostic accuracy studies [7, 8], and their level of evidence was assigned according to The Oxford 2011 Levels of Evidence [9]. Dermoscopic findings, corresponding histopathological features (if specified), dermoscopic setting (polarized versus nonpolarized/magnification degree), and number of cases were assessed and summarized. Additionally, standardized terminology based on the International Dermoscopy Society (IDS) consensus document on dermoscopy in general dermatology was specified for each dermoscopic finding retrieved from the review [10]. Notably, in the body of the manuscript, we reported the original terminology adopted in the articles, while standardized nomenclature is shown as supplementary material (see “Results” section).
Results
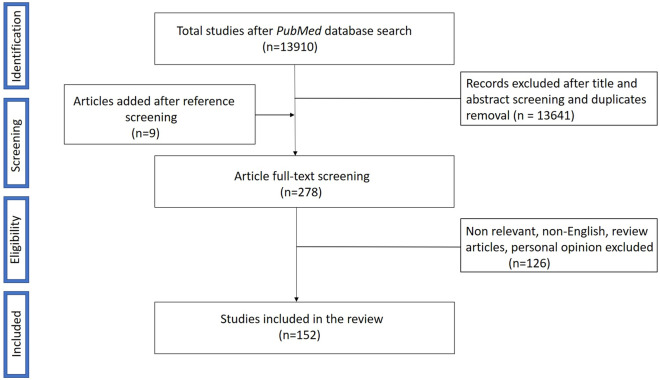
The initial PubMed search yielded 13,910 publications, with 269 articles included for full-text reading following title and abstract screening and excluding duplicates. Of these, 126 papers were ruled out according to exclusion criteria, whereas 9 articles were added after additional reference screening, with a total of 152 papers being eventually admitted to the review procedure. A flow chart describing the study selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow chart displaying the selection process for study inclusion in the systematic review
The full-text review included 107 single case reports, 40 case series, and 5 case–control studies, whereas no cross-sectional study was found. Therefore, most of the studies had a level of evidence of V, with only 5 studies displaying a level of evidence of IV. In total, 43 different dermatoses (also taking into account relevant disease variants typified by dermoscopic peculiarities) were assessed, including 21 bacterial infections, 7 viral infections, and 15 fungal infections. Table 1 shows all the conditions analyzed along with the number of studies and total number of included patients for each disease.
Table 1.
Total number of studies, patients, and dermoscopic findings of bacterial, viral, and fungal skin infections
| Dermatoses | Total number of studies | Total number of patients | Dermoscopic findings |
|---|---|---|---|
| Leprosy | 11 | 178 | |
| Borderline tuberculoid (BT) | 4 | 63 | White areas, decreased density of hairs/paucity of appendageal structures, yellow dots and globules, decreased/absent white dots, branching vessels, structureless yellowish-orange areas, diminished/loss of pigment network, lack of blood vessels, and violaceous to erythematous background |
| Tuberculoid (TL) | 2 | 6 | Central yellowish-white structureless areas, surrounding zone of erythema, linear branching vessels, broken pigmentary network, lesional loss of hair follicles and eccrine gland openings, and orange yellow areas |
| Borderline lepromatous (BL) | 2 | 27 | Broken and loss of pigmentary network, linear chrysalis-like structures, relative paucity of appendages/hair follicles, focal areas of hyperpigmentation, and relative sparing of appendages |
| Lepromatous (LL) | 5 | 35 | Yellowish-orange structureless areas, dilated branching/arborizing vessels, sparse appendages/diminished hair follicles with preserved eccrine ducts, diffuse yellow coloration/reddish-brown background, discrete brownish halo in the center, scarring nacreous center/whitish streaks, heteromorphic telangiectasias more concentrated peripherally, yellowish/orange-brown globules, patchy xerosis, shiny skin, white scaling, hypotrichosis, and accentuation of normal reticular pigment |
| Type 1 reaction | 4 | 34 (10 with BT spectrum) |
Scales (grayish-white, semi-adherent, triangular, and/or star shaped silvery white) prominent keratinous plugs, yellowish-orange/reddish-orange areas, vessels, diffuse erythematous background, sparse hair follicles, white globules, accentuation of normal skin markings, and violaceous to brown perifollicular and periappendageal pigmentation |
| Type 2 reaction | 3 | 22 (12 with LL spectrum) | Blanching/increased erythema, vascular dilation/vessels, hyperpigmentation, scales, xerosis, and red dots + BL and LL dermoscopic features |
| Histoid leprosy | 6 | 13 | Structureless white areas/shiny white area/crystalline lines/rosettes, rim of brownish hyperpigmentation, linear branching/crown/hairpin vessels, well circumscribed dome shaped yellowish brown nodules, yellowish-brown to pink background, orange yellow areas, scaling, and follicular plugs |
| Syphilis | 6 | 6 | |
| Condyloma lata | 1 | 1 | Red to milky red globules at periphery with glomerular vessels in the center, whitish-pink network on the raised border, and yellowish structureless areas at the periphery with multiple, white, small, round structures in the center |
| Palmoplantar syphiloderm | 2 | 2 | Yellowish-red/orangish background, circular scaling edge progressing outward with erythematous halo, and vessels |
| Penile annular syphiloderm | 2 | 2 | White scales, vessels (dotted, short linear, linear irregular, and/or hairpin), central clear area, and whitish/yellowish background |
| Secondary syphilis | 1 | 1 | Scaling within furrows and peripheral scaling, relatively clear central area, central ill-defined paler area fading toward periphery, and orange color |
| Lupus vulgaris | 4 | 40 | Orange-yellowish areas, vessels (linear branching, dotted, and/or linear), white reticular streaks, milia-like cysts/follicular plugs, white/yellow scales, pigmentation structures, yellowish-white/reddish globules, pinkish-red background, ulceration, white structureless areas, patulous follicles, and bluish hue |
| Tuberculosis verrucosa cutis | 1 | 1 | Yellowish to reddish background, papillated surface, dirty white thick scales, yellow orange globular areas, and irregularly dilated vessels |
| Lichen scrofulosorum | 2 | 2 | Follicular and perifollicular lesions, pale round monomorphic grouped perifollicular dots, central brown follicular plug/clod, marginal rim of white scales, halo of pallor/hyperpigmentation, and telangiectasias |
| Mycobacterium marinum infection | 1 | 1 | Whitish areas with surrounding erythematous background, purplish background, multiple structured orangish rounded areas, fine scaling, and vessels (crown-shaped looped vessels, and/or dotted vessels) |
| Peruvian wart | 1 | 1 | Vascular component, circular scale, and vascular component with central depression |
| Staphylococcal scalded skin syndrome | 1 | 1 | Skin exfoliation still having an epidermis with less leachate |
| Pitted keratolysis | 2 | 43 | Multiple pits having heterogeneous architecture/size/configuration, interrupted dermatoglyphic lines, “bead sign,” and white black or brown opaques |
| Folliculitis | 2 | 56 | |
| Common folliculitis | 1 | 55 | Central round pustule and peripheral sparse dotted vessels |
| Pseudomonal folliculitis | 1 | 1 | Pinkish background, paler center, and central vellus hair |
| Trichobacteriosis | 7 | 8 | Waxy and yellowish/yellowish-white/golden yellow adherent nodules and concretions along entire length of hair |
| Warts | 30 | 819 | |
| Genital warts | 12 | 221 | Exophytic papillary structures/irregular projection (finger-like, knob-like, mosaic, cerebriform, unspecific or mixed), peripheral white halo/white band, vessels (red dots, comma shaped, hairpin, dendritic, curved, annular, polymorphic, glomerular, looped, and/or mixed), pigmentation, keratosis, grayish dots, and blue–white structureless background |
| Flat warts | 5 | 113 | Light brown/yellow/red/red–gray/whitish/pale background, vessels (dotted/globular/linear), comedo-like openings, presence of papillae/surrounding halo, and interrupted skin markings |
| Palmoplantar warts | 12 | 346 | Waved hyperkeratosis, papilliform surface/frogspawn appearance, vessels (homogeneous black to red dots and globules, dotted vessel, linear, and/or coiled), brown/yellow/pink background, pigmented parallel ridge pattern, surrounding white halo interrupting skin dermatoglyphics (falooda seed appearance), yellow/yellow–grey structureless pattern, and hemorrhages/crusting |
| Common warts | 6 | 139 | Exophytic keratotic projection (mosaic, finger-like, filiform, cone, knob, daisy flower pattern, or nonspecific), vessels (dotted, looped, coiled, linear, hairpin, and/or glomerular), shiny white/yellow/brown/pink background, hemorrhagic crust, keratosis, presence of papillae/surrounding halo, interrupted skin markings, and white halo surrounding vessels |
| Molluscum contagiosum | 22 | 93 (278 lesions) | White–yellow/pearly-white/pinkish-white polyglobular/rounded/four-leaf clover-like shiny clods/amorphous structures/whitish discoid area (white target pattern) in the center, vessels (crown, red corona, hairpin, linear, fine, blurred, branching, punctiform, radial, serpentine, and/or mixed), orifice/central umbilicated core/depression, white shiny/white–yellow clods, smooth reddish and pink surface, and smooth shiny clods (swimming google appearance) |
| Orf nodule | 6 | 6 (26 lesions) | Central crust/erosion/ulceration, white/yellow structureless area, white shiny/orange–yellow/grayish-white streaks, vessels (dotted, hairpin, comma, polymorphic, and/or glomerular), scaling (fine peripheral), blue–gray area, yellow–white globules, yellow–white/erythematous ring, erythema, black/brown/brown-grayish dot, red-brownish branched lines, milky red globules/red–pink clods in a linear arrangement on the white background, lacunas at the periphery and milky red globules in nonlinear arrangement surrounded by white network |
| Milker’s nodule | 1 | 20 lesions | Blue–gray area, orange–yellow/grayish-white streaks, central yellow–white areas, crust, erosion–ulceration, yellow–white globules, yellow–white/erythematous ring, erythema, black dot, and vessels (dotted, comma, polymorphic, hairpin, glomerular, and/or milky-red globules) |
| Sporotrichosis | 2 | 5 | Yellowish-orange areas, generalized erythema, ulceration/crusting, yellow tears, white scar-like areas/radiating white streams, clustered pustules at periphery, and unfocused linear/arborizing vessels |
| Cutaneous blastomycosis | 2 | 2 | White to pink overlapping papillomatous structures, white and red structureless areas, blood spots, hemorrhagic crusting, scattered thin plates of scale, and polymorphous vessels (irregular, dotted, coiled, and/or serpentine) |
| Tinea nigra | 16 | 33 | Homogeneous, superficial, nonmelanocytic fine, wispy, dark brown/gray–brown pigment with spicule forming filamentous/reticular pattern not following dermatoglyphic lines, brown to gray colored, fine dotted and granule like structure over amorphous dark brown pigmentation, and parallel ridge pattern |
| White piedra | 5 | 18 | Creamy yellow/white nodules/concretion/ovular masses distributed along the hair shaft |
| Tinea manuum | 3 | 12 | White scales mainly in palmar furrows, brownish scales showing dried vesicles, dotted vessels localized in the furrows, and intense erythema |
| Tinea corporis | 6 | 74 | Diffuse erythema/erythematous background, scales (white/yellow/both, diffuse/patchy/central/peripheral/perifollicular, and moth-eaten), micropustules, multiple brown spots some with loss of vellus hair and surrounding white-yellowish halo, scaly broken, wavy hair, peeling with outward direction, vessels (globular/linear), and Morse code of vellus hair |
| Tinea incognito | 5 | 55 | Erythematous background, vessels (nonspecific, linear, tortuous, and/or dotted), scaly, broken/bent/deformable weakened transparent hair showing unusual bends, comma, corkscrew, translucent hair/Morse code hair, micropustules, black dots surrounded by white-yellowish halo, scales (white, peripheral, and/or perifollicular) and crusts, tinea of vellus hair, reddish-brown hemorrhagic spots, and concentric areas of erythema separated by scales |
| Tinea of vellus hair | 3 | 8 | Erythematous background, follicular pustules, empty follicles, yellow scales, black dots, broken, dystrophic, translucent, corkscrew, Morse code hair, white sheath around hair shaft, and scattered linear vessels |
| Majocchi’s granuloma | 2 | 2 | Yellowish/orange/blue structureless areas, central yellowish crust, polymorphic vessels (serpentine, coiled, looped with bulbous endings, or clod vessels of different sizes), and hemorrhages |
| Talaromyces marneffei infection | 3 | 3 (63 lesions) | Round or homogeneous whitish background with central keratin plug or hemorrhagic ulcer, molluscum like lesions, folliculitis/acne like lesions, xanthoma-like lesions, and vessels (radial, tufted, hairpin, and/or punctiform) |
| Cutaneous cryptococcosis | 1 | 1 | White structureless area, vessels (linear irregular, branching, and/or serpentine), surrounding structureless yellowish halo, pinkish background, and white lines on the periphery |
| Chromoblastomycosis | 6 | 6 | Yellow–orange ovoid structures, reddish black–brown dots, white net-like pattern, white and pink areas, scales, crust, and polymorphic vessels |
| Mycetoma | 4 | 4 | Yellow/white/blue areas, yellow/brown–black, red/white globules, small black grains and surrounding white halo, white scales, vessels (polymorphic and dotted), and blood spots |
| Pityriasis versicolor | 4 | 157 | Background of hypopigmented or hyperpigmented skin, scaling (mostly along furrows, or diffuse, patchy, and/or perifollicular), contrast halo sign, folliculocentricity, and hypopigmentation of hair |
| Pityrosporum folliculitis | 2 | 67 | Folliculocentric papules and pustules with surrounding erythema, perilesional dirty white scaling, keratosis pilaris like features, hypopigmentation of hair shaft, and perilesional brownish discoloration in resolving lesions |
A total of 184 different dermoscopic findings were evaluated (Table 1); dermoscopic setting (polarized versus nonpolarized) was reported in 55 records (46 polarized; 4 nonpolarized; 5 both), magnification in 74 records (47 × 10 magnification; 2 × 16 magnification; 5 × 20 magnification; 1 × 40 magnification; 6 × 50 magnification; 1 × 100 magnification; 2 × 200 magnification; 10 variable magnification), and dermoscopic–pathological correlation in 66 records. The Supplementary Table provides a summary of all such data, along with analytical description of each study assessed in the review (number of patients, type of study, and level of evidence), dermoscopic features, and corresponding terminology based on the International Dermoscopy Society consensus document on dermoscopy in general dermatology.
Bacterial Infections
Leprosy
Leprosy (Hansen’s disease) is a chronic granulomatous disorder caused by Mycobacterium leprae and typified by a wide clinical spectrum ranging from paucibacillary tuberculoid pole to multibacillary lepromatous pole [5, 11, 12].
Dermoscopy of various subtypes of leprosy has been described (Fig. 2a–f), with borderline tuberculoid (BT) form being the first variant studied from a dermoscopic point of view in a study by Ankad et al. [13] In detail, the main findings observed in this analysis included white areas, yellow globules, decreased density of hairs/white dots, and branching vessels histologically related to decreased number of melanocytes, dermal granulomas, destruction of appendageal structures by granulomatous inflammation, and dilated dermal vessels, respectively [13]. Similar findings were reported by other subsequent studies [11, 14, 15]. Additionally, the analysis by Ankad et al. also highlighted that facial lesions tend to feature more prominent vascular structures and yellow areas along with coiled hairs due to histological changes typical of this area, i.e., richer vascularity, thinner epidermis, and involvement of hair shaft of facial vellus [13].
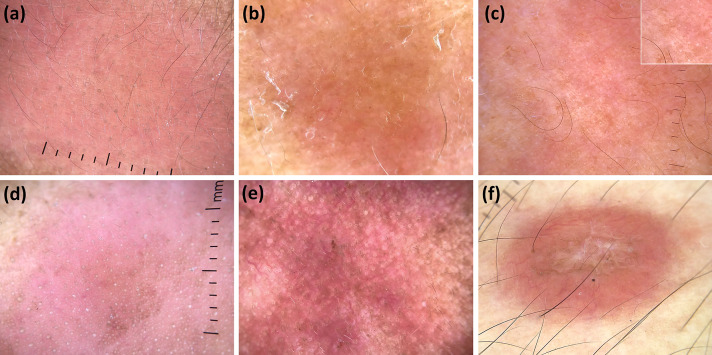
Fig. 2.
Dermoscopic images (×10 magnification): borderline tuberculoid leprosy (subtle white and decreased density of hairs and eccrine glands openings—white dots) (a); lepromatous leprosy (orange background along with subtle branching vessels and reduction of hairs) (b); lepromatous leprosy (diminished hair density with relative sparing of eccrine glands openings—white dots—and accentuation of normal reticular pigment; better seen in the box) (c); type 1 lepromatous reaction (follicular plugs with perifollicular pigmentation and sparse hairs over a diffuse erythematous background) (d); type 2 lepromatous reaction (increased erythema, vascular dilation, and brown scales) (e); and histoid leprosy (central structureless white areas along with peripheral linear branching vessels over an orange background) (f)
Vinay et al. and Mohta et al. described dermoscopic features across the entire spectrum of leprosy, with yellow/yellowish-orange areas, appendageal structures (eccrine dots and hairs) reduction, and vascular structures (mainly branching vessels) being the most common findings in all subtypes [11, 14]. Moreover, diminished pigment network was observed in all forms except lepromatous leprosy, in which the authors found an increased pigment network resulting from basal layer pigmentation [11, 14].
Of note, Mohta et al. also described a relative sparing of vellus hair and the presence of focal areas of hyperpigmentation along with loss of pigment network in tuberculoid and borderline lepromatous leprosy, respectively [14]. Further dermoscopic features reported by another three case series/report in lepromatous leprosy/leproma included white streaks, white scaling, and cicatricial nacreous center [15–17].
When it comes to lepromatous reactions, four studies are available for type 1 reaction (T1R) and three for type 2 reaction (T2R) [11, 12, 14, 15, 18]. The most common dermoscopic features of T1R included intense erythema, vessels, and violaceous to brown periappendageal pigmentation, white globules resulting from dermal edema, and scaling/follicular plugging owing to hyperkeratosis/follicular hyperkeratosis [11, 14, 15, 18]. Additionally, Mohta et al. also reported accentuation of normal skin markings with triangular and star-shaped silvery-white scaling [14]. On the other hand, T2R has been described as typified by vascular structures related to increased vascularity/vasculitis on histology, including erythema, vascular dilatation, red dots, and milky-red structureless areas; hyperpigmentation may also be seen [11, 12, 14, 15]. Notably, dermoscopic findings typical of underlying leprosy subtype have also been reported in lepromatous reactions [11, 12, 14, 15, 18].
Finally, histoid leprosy has also been extensively investigated, with six studies reporting its findings [11, 14, 19–22]. Main characterizing dermoscopic features included white/white–yellow areas and yellowish-orange areas histologically related to whorled arrangement of spindle-shaped histiocytes in the expansile granuloma; shiny white structures (shiny white area, crystalline lines, or rosettes), yellowish-brown to pink background, peripheral hyperpigmentation, scaling, follicular plugs and vessels (linear branching, hairpin, and crown-like) are possible additional findings [11, 14, 19–22].
Syphilis
Syphilis is an infectious disorder caused by Treponema pallidum, usually transmitted through sexual contact that is characterized by a variable clinical presentation [5]. Dermoscopy has been found to be helpful in facilitating the recognition of several types of syphilitic lesions (Fig. 3a–c).
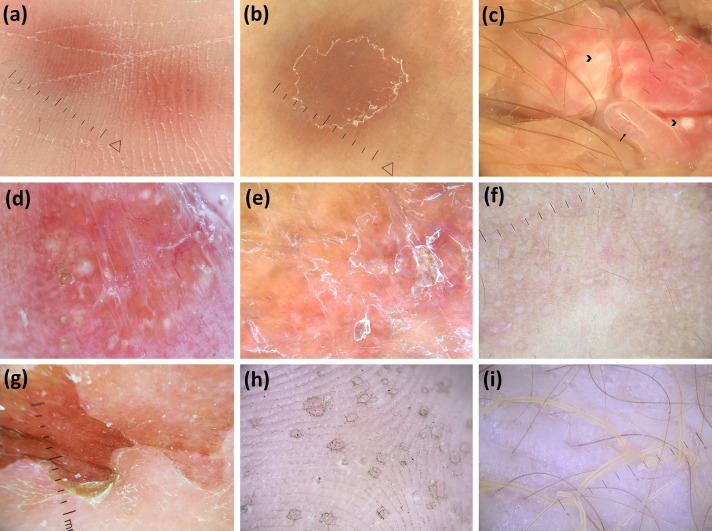
Fig. 3.
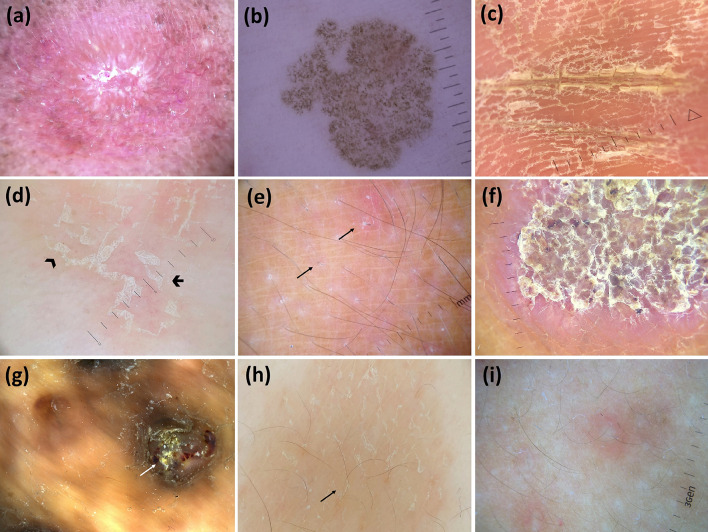
Dermoscopic images (×10 magnification): palmar syphiloderm (orange background and white scales) (a); secondary syphilis (extra-palmoplantar lesions) (orange background and peripheral scaling with an inner free edge) (b); condyloma lata (white round structures—arrowheads—and dotted/glomerular vessels—arrow—over a red–white background) (c); lupus vulgaris (orange areas, focused linear branching vessels and milia-like cysts) (adapted from Dermoscopy in General Dermatology, Lallas A, Errichetti E, Ioannides D, eds. CRC Press 2018) (d); tuberculosis verrucosa cutis (orange areas along with dirty white thick scales) (e); lichen scrofulosorum (pale/white, round, monomorphic, grouped perifollicular dots/globules) (adapted from Dermoscopy in General Dermatology for Skin of Color, Errichetti E, Lallas A, eds. CRC Press 2021) (f); staphylococcal scalded skin syndrome (skin exfoliation having an epidermis with scarce leachate) (g); pitted keratolysis (multiple pits having heterogeneous architecture/size/configuration with interruption of dermatoglyphic lines) (h); and trichomycosis axillaris (waxy and yellow adherent concretions along entire length of hair) (i)
Peripheral scaling with outward progression, often surrounded by an erythematous halo, was reported to be the most common finding in palmar syphiloderm according to two case reports [23, 24]. Diffuse dotted vessels and orangish background (related to dermal hemosiderin deposits) were other described findings [23, 24], with the latter one being a helpful clue to differentiate syphilis from papular palmar psoriasis, which lacks such a feature but may display peripheral scaling similarly to syphilis [24]. The presence of orange color on palmar lesions was also described in another case report investigating dermoscopic features of skin lesions at different stages of secondary syphilis [25]. The same report observed a change in scaling pattern on nonpalmar lesions over the time, with white scales in skin furrows evolving into a peripheral rim of thin scales [25].
A single case report on dermoscopy of condyloma lata is available, with four different main findings being reported, i.e., milky-red globules and glomerular vessels (due to papillary dermal capillary dilatation), peripheral yellowish structureless area (due to macerated horny layer), whitish-pink network on the raised border (related to prominent acanthosis), and central multiple, white, small, round structures (corresponding to follicular plugs) [26].
Finally, nonspecific vascular findings (dotted, short-linear, linear-irregular, and hairpin vessels) along with white scales were observed in a single instance of penile annular syphiloderm [27, 28].
Lupus Vulgaris
Lupus vulgaris is the most common subtype of cutaneous tuberculosis seen in previously sensitized patients [1, 29]. Dermoscopy of lupus vulgaris was first reported in four patients by Brasiello et al., who identified two main findings, i.e., orange–yellow areas and focused linear-branching vessels, histologically corresponding to granulomas and dilated vessels in the dermis (Fig. 3d) [29]; such findings were then confirmed by several studies [30, 31].
Further additional dermoscopic features described in the literature include milia-like cysts, whitish scales, pigmentation structures, follicular plugs, and whitish reticular streak [29–31]. Notably, in a recent study on 19 patients by Ankad et al. [32], yellowish-white globules, pinkish-red background, white structureless areas due to fibrosis, and white/yellow scales were found to be present in all cases.
Tuberculosis Verrucosa Cutis
Tuberculosis verrucosa cutis (TBVC) is a primary form of tuberculosis occurring in a previously sensitized host due to direct bacterial inoculation [33]. A single report on dermoscopy of TBVC has been published, with the following findings being described [34]: yellow–orange globular areas and yellowish–reddish background resulting from epithelioid cell granuloma, as well as papillated surface, dirty white thick scales, and irregularly dilated vessels due to papillomatosis, hyperkeratosis, and angiogenesis, respectively (Fig. 3e) [34].
Lichen Scrofulosorum
Lichen scrofulosorum (LS) is the most common tuberculid, usually seen in children and adolescents [35, 36]. Reported dermoscopic findings of LS include follicular and perifollicular dots, central brown follicular plug, marginal rim of white scales, halo of pallor, marginal hyperpigmentation, and telagiectasias (Fig. 3f) [35, 36]. The perifollicular dots are believed to be secondary to granulomatous infiltration in the perifollicular lesion [35].
Mycobacterium marinum Infection
Mycobacterium marinum is a slow-growing atypical mycobacterium which grows in saltwater conditions; infection is commonly seen upon exposure to nonchlorinated aqueous environment [37]. There is a single report on dermoscopy of Mycobacterium marinum infection that described two lesions, one displaying orange-whitish central areas with looped vessels surrounded by an erythematous background along with fine scaling and dotted vessels, and the other one showing multiple structured, orangish, rounded areas with surrounding crown-shaped looped vessels [37]. Orange color was correlated with dermal tuberculoid granulomas composed of inflammatory nodular infiltrate [37].
Peruvian Wart
Peruvian wart is the eruptive phase of Carrion’s disease, an infection caused by Bartonella bacilliformis; on the basis of the size of the lesions, there are three clinical types, i.e., miliary (1–4 mm), mular (> 5 mm), and nodular or subdermic [38]. A single dermoscopy report on Peruvian wart is available, with miliary lesions showing vascular component with circular scale, mular lesions featuring a vascular component with a central depression, and nodular lesions exhibiting a nonspecific pattern [38].
Staphylococcal Scalded Skin Syndrome
Staphylococcal scalded skin syndrome (SSSS) is caused by exfoliative toxin produced by some strains of Staphylococcus aureus. It is imperative to differentiate SSSS from toxic epidermal necrolysis (TEN) to initiate an appropriate treatment [39]. Dermoscopy has been found to be helpful in this regard by showing a partially intact epidermis and less leachate at fresh exfoliation site, suggesting subcorneal detachment (Fig. 3g); this is different from TEN, in which epidermis is destroyed and there is more leachate [39].
Pitted Keratolysis
Pitted keratolysis (PK) is a plantar infection caused by Corynebacterium species manifesting as multiple small pits over the soles [40]. Lockwood et al. first reported dermoscopic findings of PK, with pit walls of heterogeneous architecture being the main feature (Fig. 3h) [40]. In a recent study on 40 naval cadets, white opaque or, less commonly, black/brown opaque pits of various sizes and configurations were found to be the most common finding; other reported features included interrupted dermatoglyphic lines and “bead” sign [41].
Folliculitis
Bacterial folliculitis typically presents as papulopustular eruption and is most commonly due to Staphylococcus aureus, yet pseudomonas folliculitis is also frequently seen in daily practice as edematous papular/papulopustular lesions resulting from direct contact with contaminated water [5, 42, 43]. Dermoscopic features of staphylococcal folliculitis was investigated in 55 patients in a prospective study, and central round pustules with peripheral sparse dotted vessels were the main finding [42]. Conversely, Errichetti et al. observed a pinkish-pale background due to dermal edema along with a central vellus hair in an instance of pseudomonas folliculitis [43].
Trichobacteriosis
Trichobacteriosis is a superficial bacterial infection caused by Corynebacterium species (mainly C. flavescens) presenting as yellow, black, or red concretions attached to axillary or pubic hair shafts [5]. Several studies investigated dermoscopic features of this condition and found waxy white-yellowish concretions adherent along the entire length of hair shafts to be the typical feature; various configuration of such concretions have been reported, including flame, skewer, brush-like or plume appearance (Fig. 3i) [44–50].
Viral Infections
Warts
Warts are frequent skin infections caused by various strains of human papillomavirus (HPV); the main clinical subtypes include anogenital, flat, palmoplantar, and common warts [2–4]. Dermoscopy can aid in diagnosis and treatment follow-up of such lesions [6].
Anogenital warts are the most studied variant from a dermoscopic point of view (Fig. 4a, b) [2, 51–61]. Three main patterns have been reported, i.e., “mosaic” (relatively flattened and rounded structures, which are similar in diameter and resemble a jigsaw puzzle among each other), “knob-like” (short and closely aggregated knob-like projections, which are similar in both diameter and length among each other), and “finger-like” (relatively long, usually markedly separated finger-like projections, which are similar in diameter but different in length among each other) [2, 6, 51–61]. A combination of such patterns and unspecific presentations (not classifiable according to the previous three patterns) may also be observed [2, 51–58]. Several vascular structures can be observed in anogenital warts, including dotted and glomerular, looped, coiled, comma, and hairpin vessels [2, 6, 51–61]. Interestingly, Dong et al. correlated the clinical and dermoscopic features of genital warts of 61 lesions from 48 patients and found mosaic pattern to be more common in relatively flat lesions, whereas more raised lesions predominantly showed knob-like and finger-like pattern [2]. Additionally, the same study found hairpin, glomerular/dotted, and no vessels being associated to finger-like, knob-like/mosaic, and unspecified patterns, respectively [2]. Other features reported in the literature include “brain-like” pattern (exophytic papillary structures, some with jet black pigmentation, along with red dots surrounded by whitish halo) in a case of pigmented, hemorrhagic genital wart [59], and grayish dots surrounded by white halo over a blue–white structureless background in a case of plaque-like wart over the glans penis [60].
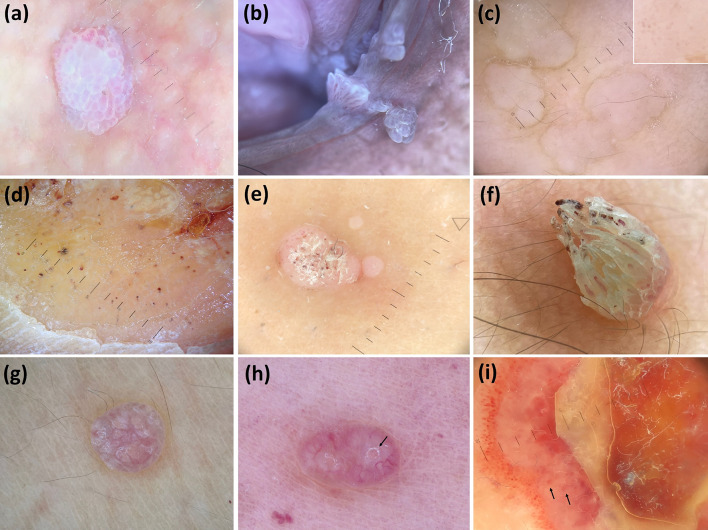
Fig. 4.
Dermoscopic images (×10 magnification): genital wart (globular white structures centered by vessels, mainly dotted—“mosaic-like” pattern) (a); genital warts (white papillary projections centered by vessels, mainly linear—“finger-like” pattern) (b); plane warts (dotted vessels—better seen in the box—over a white background with interruption of skin markings) (c); plantar wart (interruption of dermatoglyphics along with dotted vessels and hemorrhagic dots/spots, hyperkeratosis, yellow areas, and papilliform surface) (d); common warts (dotted vessels, hemorrhagic dots, and white hyperkeratosis over a white background) (e); filiform common wart (exophytic keratotic projection with elongated/linear vessels and hemorrhagic spots) (f); molluscum contagiosum (multiple white/yellow globular structures) (g); molluscum contagiosum (multiple confluent white globular structures along with crown-like vessels at the periphery and central orifice—arrow) (h); and orf (central necrotic crust surrounded with a white structureless rim containing red globular structures—arrows—surrounded by numerous dotted/globular vessels) (i)
Moving to flat warts, according to literature data, the typical dermoscopic pattern consists of regularly distributed dotted or globular vessels histologically corresponding to dilated vessels in dermal papillae over a light red, yellow–brown, red-gray or pale background; perivascular white halos are often seen (Fig. 4c) [4, 62–68].
When it comes to palmoplantar warts, dermoscopy has been reported to show a quite repetitive appearance typified by homogeneous black to red dots and globules over a white or light brownish background with interrupted skin lines (Fig. 4d) [66, 67, 69–76]. Additional described features include linear and coiled vessels surrounded by white halos, hemorrhages/crusting, papillomatous surface, yellow to yellow–gray structureless areas, “frogspawn-like” appearance, “falooda seed-like” appearance (regular black dots interrupting the dermatoglyphics), and “parallel ridge” pattern (band-like pigmentation on the ridges) [66, 67, 69–76]. Dermoscopic examination has also been found to be helpful in assessing post-treatment response/recurrence of palmoplantar warts [66, 67, 74, 77].
Finally, dermoscopy of common warts was first described by Zalaudek et al., who reported multiple densely packed papillae, with central red dot or loop surrounded by whitish halo giving rise to a “frogspawn” appearance [4]. Subsequent studies have described other features, including “mosaic” pattern, “keratotic projection” pattern, “finger-like” pattern, “knob-like” pattern, “frogspawn” pattern, “daisy flower” pattern, vascular structures (dotted, linear, looped, coiled, and hairpin vessels), and hemorrhagic crusting (Fig. 4e, f) [66, 79–81]. Usefulness of dermoscopy in post-treatment follow-up of common warts has also been demonstrated to assess complete clearance of lesions [67, 78].
Molluscum Contagiosum
Molluscum contagiosum (MC) is a frequent skin viral infection due to a poxvirus (molluscum contagiosum virus), commonly seen in children and sexually active adults [3, 6]. It clinically presents as dome-shaped, umbilicated, skin-colored to pinkish-white papules with translucent, glossy appearance [3, 6]. Dermoscopy can be an useful supportive diagnostic tool, especially in atypical presentations, such as central umbilication absence and single/giant lesions [3, 6].
The most commonly reported dermoscopic features of MC include white/yellow-white globules that often coalesce to form polylobular structures and peripheral vascular structures usually not crossing the center of the lesions; central pore or umbilication may also be seen (Fig. 4g, h) [82–92]. Several shapes of vessels have been reported, i.e., crown-like, linear, branching, hairpin, punctiform, radial, dotted, and serpentine [82–92]; combinations of vascular structures are possible and may give rise to particular morphologies, such as “flower” pattern (radial vessels along with crown-like vessels) [84]. From a dermoscopic–pathological correlation point of view, white/yellow-white globules correspond to lobules containing hyalinized molluscum bodies (Henderson–Paterson bodies), while peripheral vessels represent dilated vessels in the dermis [82, 83].
Further patterns reported in the literature include the “white target pattern,” consisting of a small, central whitish discoid area and surrounding hairpin vessels [93], and “swimming goggle” appearance, typified by smooth, shiny clods, with reddish surface and surrounding vessels [94]. Additionally, extraction dermoscopy of MC has been reported to show white bodies with visible vessels running across the surface which corresponds to the in vivo finding of crown vessels [95].
Contagious Ecthyma (Orf)
Ecthyma contagiosum (orf) is a zoonotic disease caused by a DNA parapoxvirus usually transmitted to humans from infected goat and sheep, and presenting on the hands, fingers, feet, legs, and face as single or multiple papules/nodules that ulcerate and then crust [102, 103].
Several articles on dermoscopy of orf have been published (Fig. 4i) [102–106], with the study by Ayhan et al. on 26 lesions reporting the following main dermoscopic features: central erosion–ulceration, peripheral yellow–white or erythematous ring, and black dots [102]. Additional findings described by the same study included blue–gray areas, orange–yellow streaks, grayish–whitish streaks, central yellow–white areas, crusting, yellow–white globules, and vessels (dotted, comma-like, polymorphic, and glomerular) [102].
Of note, the authors identified four different general patterns: central yellow–white area and surrounding erythematous ring in type 1; central orange–yellow streaks with violaceous erythematous base, surrounding grayish-white streaks and erythematous ring outside in type 2; central ulceration, yellow–white ring around and ring of erythema outside in type 3; and central erythema or ulcer-crusted area with surrounding yellow–white ring in type 4 [102].
Other reports showed similar features, though further findings were also described in single instances, i.e., hairpin and arborizing vessels, milky-red globules and lacunae, and linearly arranged multiple red–pink clods with surrounding white network [103–106].
Milker’s Nodule
Milker’s nodule (MN) is a cutaneous zoonotic infection caused by paravaccinia virus and transmitted to humans from infected cattle’s udder, body, and oral cavity [102]. A single study assessing 20 lesions is available from the literature [102]. According to this analysis, the most common dermoscopic features of MN included erosion–ulceration with an erythematous ring, black dots, and dotted vessels [102]. Other possible findings were yellow–white globule, yellow–white ring, blue–grey areas, orange–yellow streaks, grayish–whitish streaks, central yellow–white area, crusting, comma vessels, polymorphic vessels, glomerular vessels, hairpin vessels, and milky-red globules [102] The study demonstrated no significant dermoscopic differences between the lesions of Milker’s nodule and orf, with the four general patterns described for the latter being visible even in the former [102].
Fungal Infections
Sporotrichosis
Sporotrichosis is a subcutaneous mycosis caused by Sporothrix schenckii, which is endemic in tropical/subtropical areas [108]. Clinical presentations include cutaneous, lymphocutaneous, and disseminated subtype; dermoscopy has recently been employed in all these three variants [108, 109].
In detail, yellowish structureless areas, white-scar like areas, arborizing telangiectasia over generalized erythema, and clustered pustules at the periphery were observed in a case of disseminated cutaneous sporotrichosis [109]. When it comes to cutaneous and lymphocutaneous sporotrichosis, Vinay et al. found yellow–orange areas (histologically related to granulomas) on an erythematous background, linear unfocused telangiectatic vessels, superficial ulcerations, and hemorrhagic crusting to be the most salient findings [108]. Of note, the authors also observed a dermoscopic variability based on disease stage, with early and evolving lesions being typified by diffuse background erythema (representing ongoing inflammation), yellowish-orange areas and variable telangiectatic vessels and healing lesions by white fibrotic strands and linear telangiectatic vessels [108]. Additionally, they also found follicular plugs (named “yellow tears”) on lesions located on the face (Fig. 5a) [108].
Fig. 5.
Dermoscopic images (×10 magnification): sporotrichosis [follicular plugs (yellow tears) and unfocused linear vessels over an orange-erythematous background] (adapted from Dermoscopy in General Dermatology for Skin of Color, Errichetti E, Lallas A, eds. CRC Press 2021) (a); tinea nigra (wispy brown pigment with spicule forming filamentous/reticular pattern not following dermatoglyphic lines) (adapted from “Dermoscopy in General Dermatology, Lallas A, Errichetti E, Ioannides D, eds. CRC Press 2018) (b); tinea manuum (white scales in palmar furrows) (c); tinea corporis (peripheral white scaling with both inner—arrowhead—and outer free edge—arrow) (d); tinea incognito (perifollicular white scaling and broken hairs—arrows) (e); chromoblastomycosis (reddish black–brown dots and white scaling with a peripheral yellow erythematous rim) (adapted from Dermoscopy in General Dermatology for Skin of Color, Errichetti E, Lallas A, eds. CRC Press 2021) (f); mycetoma (yellow globules, white scales, and blood spots) (adapted from Dermoscopy in General Dermatology for Skin of Color, Errichetti E, Lallas A, eds. CRC Press 2021) (g); pityriasis versicolor (white scaling in the skin furrows and brownish background) (h); and pityrosporum folliculitis (white round follicular globule surrounded by an erythematous halo) (i)
Blastomycosis
Blastomycosis is a deep mycosis endemic in North America that is caused by inhalation of Blastomyces dermatitidis, clinically manifesting as verrucous, ulcerated, or subcutaneous nodules [110, 111]. In the report by Wang et al., overlapping papillomatous structures showing a pink hue along with scales, irregular vessels, and hemorrhagic crusting were observed on dermoscopy [110]. A similar pattern was also seen in another instance, yet vessels were described as polymorphic, and white/red structureless areas as well as blood spots were also described [111].
Tinea Nigra
Tinea nigra is a superficial fungal infection caused by dematiaceous fungus Hortaea werneckii that manifests as a gradually progressive, irregular brown to black macule over glabrous skin [4, 6]. Dermoscopy of tinea nigra has been extensively investigated by several studies [112–127], with the typical pattern consisting of homogeneous, fine, wispy pigmented spicules distributed either irregularly or in a reticular arrangement without following dermatoglyphics (Fig. 5b) [112–121]. Such spicules have been reported to represent pigmented hyphae in the stratum corneum [123]. More uncommon but possible dermoscopic presentations include irregularly distributed brownish dots displaying filamentous arrangement not adhering to dermatoglyphic lines [117, 122, 123] and a parallel ridge pattern [124, 125].
White Piedra
White piedra is a superficial mycosis caused by several Trichosporon species, which presents as multiple white to tan irregular nodules along the hair shaft [62]. Dermoscopy has been shown to support its diagnosis by revealing multiple, irregularly, scattered, yellow to white concretions or ovular masses along the hair shaft [50, 128, 129]. Notably, in a report by Zhuang et al. dermoscopic appearance of white piedra turned out to be similar to that of trichobacteriosis, with yellow nodules being distributed along the entire length of hair shafts to form a sheath [130].
Tinea Manuum
Tinea manuum (TM) is superficial dermatophyte infection occurring on palms and interdigital spaces appearing as more or less diffuse white scaling or keratoderma [131, 132]. According to two case reports, dermoscopy may be of aid in assisting clinical diagnosis by showing white scaling predominantly located in the skin furrows (Fig. 5c). This particular distribution pattern is typical of TM compared with other forms of keratoderma and is due to the tropism of the fungus form more humid areas [131, 132]. Additional dermoscopic findings described in the literature include whiting scaling in adjoining dermatoglyphics, brown scales resulting from dried vesicles, intense erythema, and dotted vessels in the skin furrows [132, 133].
Tinea Corporis
Tinea corporis (TC) is a dermatophyte infection presenting as annular erythematous plaques with peripheral scaling often involving trunk and limbs. Initial descriptions came from single case reports or small case series [63, 132, 134], with several findings being identified, including erythematous background, scales, follicular micropustules, brown spots surrounded by white-yellowish halo with or without vellus hair loss, and mixed vessels (dotted and linear) [63, 132, 134]. Subsequently, in a study on 30 patients, Bhat et al. found “follicular” findings to be relevant clues to recognize TC, especially scaly broken hairs but also “wavy” hair and “Morse code” hair [132]. Additionally, Lekkas et al. studied dermoscopic findings in 36 microscopically positive cases of TC and reported peripherally distributed white scales, outward peeling direction of scales, and randomly distributed dotted vessels to be strong indicators of TC (Fig. 5d) [136].
Besides diagnostic purposes, dermoscopy has also been found helpful in choosing treatment, with vellus hair involvement on dermoscopic assessment (seen as either brown spots surrounded by white halo or loss of vellus hair) being an indicator for starting systemic antifungal therapy owing to lower efficacy of topical treatments in this scenario [134]. Recently, dermoscopy of treated TC healed as hypertrophic scars and multiple comedones has been described, with evidence of double opening comedones with a “dominant” orifice connected with a smaller orifice by a thin layer of epidermis [137].
Tinea Incognito
Tinea incognito typically results from corticosteroid misuse in TC; dermoscopy of this condition has been described by three single case reports and two case series [132, 138–141]. Besides erythematous background, peripheral whitish scales and micropustules at the borders, the main dermoscopic features of tinea incognito turned out to be follicular findings, mainly including Morse code hairs of vellus hairs, follicular micropustules, and easily deformable, weakened, and transparent hairs with unusual bends (Fig. 5e) [132, 138–141].
Tinea of Vellus Hair
Tinea of vellus hair is an uncommon infection affecting mostly children and caused by zoophilic or geophilic species presenting as follicular micropustules and inflammatory or excoriated papules [142]. Dermoscopic features described in this condition include yellowish scaling, follicular micropustules, broken hairs, corkscrew hairs, black dots, dystrophic hairs, “Morse code” hairs, coiled hairs, white sheath around vellus hair shafts, empty follicles, and erythematous background and vessels [142–144].
Majocchi’s Granuloma
Majocchi’s granuloma is a deep and persistent suppurative and granulomatous folliculitis due to dermatophytes [145]. Dermoscopic features have been described in two case reports and included yellowish-orange structureless areas, central crust surrounded by a blue structureless area, polymorphous vessels (serpentine, coiled, and looped vessels showing bulbous endings), hemorrhages and clod vessels of different sizes [145, 146].
Talaromyces marneffei Infection
Talaromyces marneffei infection is a systemic mycosis often involving the skin with nonspecific lesions that mainly affects people with severe immunodeficiency (e.g., AIDS) [147]. Dermoscopy has been reported to be helpful in providing clues for early diagnosis of skin lesions, including homogeneous round whitish background (granulomas comprised of yeast ingested histiocytes) along with central keratin plug or hemorrhagic ulcer surrounded by punctiform, tufted, and hairpin vessels [147, 148]. Moreover, Xu et al. reported four dermoscopic patterns; i.e., molluscum type, folliculitis/acne type, xanthoma type, and ulcers [149].
Cutaneous Cryptococcosis
Cryptococcosis is a systemic fungal infection caused by C. neoformans seen predominantly in patients with reduced cell-mediated immunity [150]. Dermoscopy of skin lesions has been reported in a single instance, which described a central white structureless area and a peripheral yellowish halo histologically corresponding to fibrosis and granulomatous inflammation, respectively; vessels may also be seen as the result of angiogenesis [150].
Chromoblastomycosis
Chromoblastomycosis is a subcutaneous mycosis caused by Fonsacea and Cladophialophora species [151]. Dermoscopy of this condition has been investigated in six single case reports, with yellow–orange ovoid structures and pink–white areas along with interspersed brown, reddish-brown, or black dots being found to be the most common finding (Fig. 5f) [151–156]. Pigmented dots are believed to represent trans-epithelial elimination of inflammatory cells and fungal elements, while yellow–orange areas would correspond to mycotic granulomas [151–156]. Additional features included scales, crust, white net-like pattern, and polymorphic vessels [151–156].
Mycetoma
Mycetoma is a subcutaneous mycosis presenting with the triad of soft tissue swelling, sinuses, and grains [12]. Dermoscopy has been reported to facilitate its diagnosis by showing yellow, brown–black, or red globules/grains, especially when grains are missing clinically (Fig. 5g) [157–160]. Other possible dermoscopic findings include yellow and white areas, blue–white areas surrounded by white halo, scales, erosions, blood spots, and polymorphic vessels [157–160]. Some authors also found dermoscopy to be helpful in post-treatment monitoring by observing a reduction in yellow globules, scaling, blood spots, and vessels [158].
Pityriasis Versicolor
Pityriasis versicolor (PV) is a superficial cutaneous mycosis caused by Malassezia yeasts presenting as either hypo- or hyperpigmented patches with fine scaling mainly affecting the trunk [161, 162]. The main study on dermoscopy of PV was performed on 125 patients by Mathur et al., who reported three common features for both hypo- and hyperpigmented lesions, i.e., nonuniform pigmentation, inconspicuous ridges and furrows, and scaling (mainly patchy or in the furrows) (Fig. 5h) [163]. The presence of white scaling in skin creases has been confirmed as a dermoscopic clue for PV by other reports [161, 162].
Other relevant findings described for both hypo- and hyperpigmented PV by an observational study of 30 patients included altered pigmentary network, “contrast halo” sign (increased or decreased pigmentation around primary decreased or increased pigmentary network, respectively), and folliculocentric pattern of pigmentary changes [164].
Pityrosporum Folliculitis
Pityrosporum folliculitis is a hair follicle infection due to yeasts of the Malassezia family [165]. Jakhar et al. assessed dermoscopic pattern in 15 patients and found several features, with the most common ones being folliculocentric papules and pustules with surrounding erythema and perilesional dirty white scaling (Fig. 5i) [165]. Additionally, they also observed keratosis pilaris-like appearance (coiled/looped hair follicle with surrounding erythema and scaling) as well as perilesional hypopigmentation or brownish discoloration occurring in regressing lesions [165].
Subsequently, Durdu et al. studied dermoscopic findings of 52 cases of pityrosporum folliculitis, comparing them with other causes of both infectious and noninfectious folliculitis and concluded that peripheral, regularly distributed dotted vessels in the absence of other diagnostic findings were the main dermoscopic clue of such a condition, with a sensitivity and specificity of 67.3 and 93.1, respectively [42].
Limitations
The search was restricted to the PubMed database. Since truncations were not considered, some records reporting the terms “dermatoscopic” and “dermoscopic” might have been missed.
Conclusions
The present systematic review highlights that there is a large body of literature when it comes to dermoscopy of nonparasitic skin infections, with a higher number of bacterial and fungal dermatoses having at least one description compared with viral diseases. Of note, while articles published on dermoscopy of skin parasitoses, bites, and stings are mainly single case reports or small case series [166], several larger studies are available in the field of nonparasitic infectious dermatoses, likely because they are more common than the former group of conditions. However, level of evidence analysis showed that most of the published articles are of poor quality with a lack of comparative cohort, gold-standard diagnostic reference, blinding, and/or consecutive recruitment. Additionally, as previously reported for skin parasitoses, bites, and stings [166], dermoscopic terminology used in published articles on bacterial, viral, and fungal dermatoses is highly variable (even for the same condition), thus generating confusion for use in clinical practice.
This review paper emphasizes that dermoscopy of nonparasitic skin infections has significant potential, as it may allow for the appreciation of subclinical findings strictly related to specific histological and/or microbiological features, yet future studies designed according to a systematic and standardized approach are required for better characterization.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding has been received for this paper.
Author Contributions
All authors contributed to concept and design of the article and drafting of the manuscript.
Disclosures
Payal Chauhan, Dilip Meena, and Enzo Errichetti have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The patients in this manuscript have given written informed consent for the publication of their case details.
Data Availability
Data sharing not applicable to this article as no datasets were generated during the current study.
References
- 1.Errichetti E. Dermoscopy in general dermatology (non-neoplastic dermatoses): the journey so far. Dermatol Ther (Heidelb) 2021;11:1871–1877. doi: 10.1007/s13555-021-00633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong H, Shu D, Campbell TM, et al. Dermatoscopy of genital warts. J Am Acad Dermatol. 2011;64:859–864. doi: 10.1016/j.jaad.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Sonthalia S, Agrawal M, Bhatia J, et al. Entodermoscopy update: a contemporary review on dermoscopy of cutaneous infections and infestations. Indian Dermatol Online J. 2021;12:220–236. doi: 10.4103/idoj.IDOJ_559_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14–23. doi: 10.1159/000109353. [DOI] [PubMed] [Google Scholar]
- 5.Bakos RM, Reinehr C, Escobar GF, Leite LL. Dermoscopy of skin infestations and infections (entomodermoscopy)—Part I: dermatozoonoses and bacterial infections. An Bras Dermatol. 2021;96:735–745. doi: 10.1016/j.abd.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakos RM, Leite LL, Reinehr C, Escobar GF. Dermoscopy of skin infestations and infections (entomodermoscopy)—Part II: viral, fungal and other infections. An Bras Dermatol. 2021;96:746–758. doi: 10.1016/j.abd.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JM, Kulgar M, Ding S, et al. Chapter 9: diagnostic test accuracy systematic reviews. In: Joanna Briggs Institute reviewer’s manual (Aromataris E, Munn Z, eds). The Joanna Briggs Institute, 2017. https://reviewersmanual.joannabriggs.org. Accessed 4 Dec 2021.
- 8.Rutjes AW, Reitsma JB, Vandenbroucke JP, et al. Case–control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005;51:1335–1341. doi: 10.1373/clinchem.2005.048595. [DOI] [PubMed] [Google Scholar]
- 9.Oxford Centre for Evidence-Based Medicine. The Oxford 2011 levels of evidence. https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf. Accessed 4 Dec 2021.
- 10.Errichetti E, Zalaudek I, Kittler H, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182:454–467. doi: 10.1111/bjd.18125. [DOI] [PubMed] [Google Scholar]
- 11.Vinay K, Kamat D, Chatterjee D, Narang T, Dogra S. Dermatoscopy in leprosy and its correlation with clinical spectrum and histopathology: a prospective observational study. J Eur Acad Dermatol Venereol. 2019;33:1947–1951. doi: 10.1111/jdv.15635. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34–44. doi: 10.4103/idoj.IDOJ_543_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ankad BS, Sakhare PS. Dermoscopy of borderline tuberculoid leprosy. Int J Dermatol. 2018;57:74–76. doi: 10.1111/ijd.13731. [DOI] [PubMed] [Google Scholar]
- 14.Mohta A, Jain SK, Agrawal A, et al. Dermoscopy in leprosy: a clinical and histopathological correlation study. Dermatol Pract Concept. 2021;11:e2021032. doi: 10.5826/dpc.1102a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopra A, Mitra D, Agarwal R, Saraswat N, Talukdar K, Solanki A. Correlation of dermoscopic and histopathologic patterns in leprosy—a pilot study. Indian Dermatol Online J. 2019;10:663–668. doi: 10.4103/idoj.IDOJ_297_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kansal NK, Joshi PP, Dhanta A, Hazarika N, Divyalakshmi C. A masquerading solitary skin lesion: unusual presentation of multibacillary leprosy with dermoscopic assistance in diagnosis. Indian Dermatol Online J. 2020;11:632–634. doi: 10.4103/idoj.IDOJ_495_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miola AC, Bicudo NP, Tsutsui GM, Miot HA. Leproma's dermoscopy. An Bras Dermatol. 2020;95:383–385. doi: 10.1016/j.abd.2019.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha AK, Zeeshan MD, Tiwary P, Singh A, Roy PK, Chaudhary RKP. Dermoscopy of type 1 lepra reaction in skin of color. Dermatol Pract Concept. 2020;10:e2020083. doi: 10.5826/dpc.1003a83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ankad BS, Sakhare PS. Dermoscopy of histoid leprosy: a case report. Dermatol Pract Concept. 2017;7:63–65. doi: 10.5826/dpc.0702a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharya P, Mathur MC. Clinicodermoscopic study of histoid leprosy: a case series. Int J Dermatol. 2020;59:365–368. doi: 10.1111/ijd.14731. [DOI] [PubMed] [Google Scholar]
- 21.Mathur M, Acharya P, Karki A. Visual dermatology: crown vessels in dermoscopy of histoid leprosy. J Cutan Med Surg. 2019;23:333. doi: 10.1177/1203475419825759. [DOI] [PubMed] [Google Scholar]
- 22.Abadías-Granado I, Navarro-Bielsa A, Gómez-Mateo MC, Bermúdez-Cameo R, Gilaberte Y. Crown vessels and shiny white structures in dermoscopy of histoid leprosy. JAAD Case Rep. 2020;6:1147–1149. doi: 10.1016/j.jdcr.2020.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tognetti L, Sbano P, Fimiani M, Rubegni P. Dermoscopy of Biett's sign and differential diagnosis with annular maculo-papular rashes with scaling. Indian J Dermatol Venereol Leprol. 2017;83:270–273. doi: 10.4103/0378-6323.196318. [DOI] [PubMed] [Google Scholar]
- 24.Errichetti E, Stinco G. Dermoscopy in differentiating palmar syphiloderm from palmar papular psoriasis. Int J STD AIDS. 2017;28:1461–1463. doi: 10.1177/0956462417714178. [DOI] [PubMed] [Google Scholar]
- 25.Mathur M, Acharya P, Karki A, Shah J, Kc N. Dermoscopic clues in the skin lesions of secondary syphilis. Clin Case Rep. 2019;7:431–434. doi: 10.1002/ccr3.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda E, Goto A, Suzaki R, Sawada M, Dekio I, Ishizaki S, Fujibayashi M, Takahashi H, Tanaka M. Condylomata lata on the ankle: an unusual location. Dermatol Pract Concept. 2016;6:49–51. doi: 10.5826/dpc.0602a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li FG, Huang WB, Chen HS, Wang T, Fan YM. Clinicopathological, dermoscopic, and ultrastructural observation of annular secondary syphilis on the penis. Int J STD AIDS. 2020;31:699–701. doi: 10.1177/0956462419900092. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Wu H, Huang Z, Fan YM. Penile annular and scrotal eczematoid syphilids with penile chancre redux. Sex Transm Infect. 2021;97:119. doi: 10.1136/sextrans-2020-054935. [DOI] [PubMed] [Google Scholar]
- 29.Brasiello M, Zalaudek I, Ferrara G, et al. Lupus vulgaris: a new look at an old symptom—the lupoma observed with dermoscopy. Dermatology. 2009;218:172–174. doi: 10.1159/000182255. [DOI] [PubMed] [Google Scholar]
- 30.Lallas A, Argenziano G, Apalla Z, et al. Dermoscopic patterns of common facial inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2014;28:609–614. doi: 10.1111/jdv.12146. [DOI] [PubMed] [Google Scholar]
- 31.Bombonato C, Argenziano G, Lallas A, Moscarella E, Ragazzi M, Longo C. Orange color: a dermoscopic clue for the diagnosis of granulomatous skin diseases. J Am Acad Dermatol. 2015;72:S60–S63. doi: 10.1016/j.jaad.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 32.Ankad BS, Adya KA, Gaikwad SS, Inamadar AC, Manjula R. Lupus vulgaris in darker skin: dermoscopic and histopathologic incongruity. Indian Dermatol Online J. 2020;11(6):948–952. doi: 10.4103/idoj.IDOJ_100_20.PMID:33344345;PMCID:PMC7734991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco-Paredes C, Marcos LA, Henao-Martínez AF, Rodríguez-Morales AJ, Villamil-Gómez WE, Gotuzzo E, Bonifaz A. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32:e00069–e118. doi: 10.1128/CMR.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakhar D, Gupta RK, Sarin N. Dermoscopy of tuberculosis verrucosa cutis. Indian Dermatol Online J. 2020;12:206–207. doi: 10.4103/idoj.IDOJ_292_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jassi R, Yadav A, Chander R. Dermoscopy of Lichen Scrofulosorum. Indian Dermatol Online J. 2020;11:876–877. doi: 10.4103/idoj.IDOJ_191_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg T, Kumar P, Das S, Singh S, Madan S. Lichen scrofulosorum coexisting with phlyctenular keratoconjuctivitis: dermoscopy and ocular findings. Indian Dermatol Online J. 2021;12:941–943. doi: 10.4103/idoj.IDOJ_689_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conforti C, Zalaudek I, Vichi S, Di Meo N. Dermoscopy of Mycobacterium marinum skin infection: a challenging diagnosis. Acta Dermatovenerol Croat. 2019;27:278–279. [PubMed] [Google Scholar]
- 38.Durand D, Quijano E, Sanz ME. Clinical and dermoscopic characteristics of a patient with cutaneous and mucosal manifestations of Peruvian wart. Int J Dermatol. 2017;56:1442–1444. doi: 10.1111/ijd.13796. [DOI] [PubMed] [Google Scholar]
- 39.Miyashita K, Ogawa K, Iioka H, et al. Adult case of staphylococcal scalded skin syndrome differentiated from toxic epidermal necrolysis with the aid of dermoscopy. J Dermatol. 2016;43:842–843. doi: 10.1111/1346-8138.13281. [DOI] [PubMed] [Google Scholar]
- 40.Lockwood LL, Gehrke S, Navarini AA. Dermoscopy of pitted keratolysis. Case Rep Dermatol. 2010;2:146–148. doi: 10.1159/000319792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pattanaprichakul P, Kulthanan K, Bunyaratavej S, et al. The correlations between clinical features, dermoscopic and histopathological findings, and treatment outcomes of patients with pitted keratolysis. Biomed Res Int. 2021;2021:3416643. doi: 10.1155/2021/3416643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durdu M, Errichetti E, Eskiocak AH, Ilkit M. High accuracy of recognition of common forms of folliculitis by dermoscopy: an observational study. J Am Acad Dermatol. 2019;81:463–471. doi: 10.1016/j.jaad.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 43.Errichetti E, Stinco G. Dermoscopy: a useful tool for assisting the diagnosis of Pseudomonas folliculitis. An Bras Dermatol. 2016;91:835–836. doi: 10.1590/abd1806-4841.20165382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guiotoku MM, Ramos PM, Miot HA, Marques SA. Trichobacteriosis: case report and dermoscopic study. An Bras Dermatol. 2012;87:315–316. doi: 10.1590/s0365-05962012000200023. [DOI] [PubMed] [Google Scholar]
- 45.Salim G, Zahra MF. Trichobacteriosis: contribution of dermoscopy. Dermatol Online J. 2014;20:9. [PubMed] [Google Scholar]
- 46.Rojas Mora E, Freites Martínez A, Hernández-Núñez A, Borbujo MJ. Trichomycosis axillaris: clinical, wood lamp, and dermoscopic diagnostic images. Actas Dermosifiliogr. 2017;108:264–266. doi: 10.1016/j.ad.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Almazán-Fernández FM, Fernández-Crehuet SP. Trichomycosis axillaris dermoscopy. Dermatol Online J. 2017;23:6. [PubMed] [Google Scholar]
- 48.Larrondo J, Porte L, Gosch M, Cabrera R, Weitzel T. Trichobacteriosis axillaris caused by Dermabacter hominis. J Eur Acad Dermatol Venereol. 2017;31:e267–e268. doi: 10.1111/jdv.14082. [DOI] [PubMed] [Google Scholar]
- 49.Gupta V, Sharma VK. Four views of trichomycosis axillaris: clinical, Wood's lamp, dermoscopy and microscopy. Indian J Dermatol Venereol Leprol. 2018;84:748–749. doi: 10.4103/ijdvl.IJDVL_567_17. [DOI] [PubMed] [Google Scholar]
- 50.Lacarrubba F, Verzì AE, Micali G. Trichoscopy in the differential diagnosis of pseudonits. Skin Appendage Disord. 2019;5:142–145. doi: 10.1159/000493741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SH, Seo SH, Ko HC, Kwon KS, Kim MB. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353–355. doi: 10.1016/j.jaad.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Yoshida Y, Yamamoto O. Differential diagnosis of pearly penile papules and penile condyloma acuminatum by dermoscopy. Eur J Dermatol. 2010;20:414–415. doi: 10.1684/ejd.2010.0944. [DOI] [PubMed] [Google Scholar]
- 53.Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501–1505. doi: 10.1111/j.1365-4632.2011.05087.x. [DOI] [PubMed] [Google Scholar]
- 54.Lacarrubba F, Dinotta F, Nasca MR, Micali G. Enhanced diagnosis of genital warts with videodermatoscopy: histopatologic correlation. G Ital Dermatol Venereol. 2012;147(2):215–216. [PubMed] [Google Scholar]
- 55.Veasey JV, Framil VM, Nadal SR, Marta AC, Lellis RF. Genital warts: comparing clinical findings to dermatoscopic aspects, in vivo reflectance confocal features and histopathologic exam. An Bras Dermatol. 2014;89:137–140. doi: 10.1590/abd1806-4841.20141917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang S, Zhang Y, Zou X. Condyloma acuminata at urethral orifice complicated with hemophilia A. An Bras Dermatol. 2017;92:289–290. doi: 10.1590/abd1806-4841.20176092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seong SH, Jung JH, Kwon DI, et al. Dermoscopic findings of genital keratotic lesions: Bowenoid papulosis, seborrheic keratosis, and condyloma acuminatum. Photodiagn Photodyn Ther. 2021;36:102448. doi: 10.1016/j.pdpdt.2021.102448. [DOI] [PubMed] [Google Scholar]
- 58.García-Lozano JA, Cárdenas-de la Garza JA, Cuellar-Barboza A, Ocampo-Candiani J, Garza-Cortés R, González-Ramírez RA. Vascular dermoscopic features of intrameatal warts. Australas J Dermatol. 2020;61:e70–e72. doi: 10.1111/ajd.13113. [DOI] [PubMed] [Google Scholar]
- 59.Ozdemir F, Kilinc-Karaarslan I, Akalin T. A pigmented, hemorrhagic genital wart: clinical, dermoscopic, and histopathologic features. Arch Dermatol. 2008;144:1072–1073. doi: 10.1001/archderm.144.8.1072. [DOI] [PubMed] [Google Scholar]
- 60.Campos MA, Sousa A, Lage G, et al. Blue-gray plaque of the penis. JAAD Case Rep. 2018;4:531–533. doi: 10.1016/j.jdcr.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Jiang S, Lin H, Guo X, Zou X. Application of dermoscopy image analysis technique in diagnosing urethral condylomata acuminata. An Bras Dermatol. 2018;93:67–71. doi: 10.1590/abd1806-4841.20186527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee M, Neema S. Dermatoscopy of infections and infestations. Indian Dermatol Online J. 2021;12:14–23. doi: 10.4103/idoj.IDOJ_589_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vázquez-López F, Kreusch J, Marghoob AA. Dermoscopic semiology: further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150:226–231. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 64.Kim WJ, Lee WK, Song M, et al. Clinical clues for differential diagnosis between verruca plana and verruca plana-like seborrheic keratosis. J Dermatol. 2015;42:373–377. doi: 10.1111/1346-8138.12760. [DOI] [PubMed] [Google Scholar]
- 65.Hui D, Hong-Yan J, Ai-E X. Evaluation of verruca plana by in vivo reflectance confocal microscopy and dermoscopy. Skin Res Technol. 2017;23:437–440. doi: 10.1111/srt.12336. [DOI] [PubMed] [Google Scholar]
- 66.Al Rudaisat M, Cheng H. Dermoscopy features of cutaneous warts. Int J Gen Med. 2021;14:9903–9912. doi: 10.2147/IJGM.S335276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal M, Khunger N, Sharma S. A dermoscopic study of cutaneous warts and its utility in monitoring real-time wart destruction by radiofrequency ablation. J Cutan Aesthet Surg. 2021;14:166–171. doi: 10.4103/JCAS.JCAS_47_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piccolo V. Update on dermoscopy and infectious skin diseases. Dermatol Pract Concept. 2019;10:e2020003. doi: 10.5826/dpc.1001a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee DY, Park JH, Lee JH, Yang JM, Lee ES. The use of dermoscopy for the diagnosis of plantar wart. J Eur Acad Dermatol Venereol. 2009;23:726–727. doi: 10.1111/j.1468-3083.2009.03184.x. [DOI] [PubMed] [Google Scholar]
- 70.Tanioka M, Nakagawa Y, Maruta N, Nakanishi G. Pigmented wart due to human papilloma virus type 60 showing parallel ridge pattern in dermoscopy. Eur J Dermatol. 2009;19:643–644. doi: 10.1684/ejd.2009.0781. [DOI] [PubMed] [Google Scholar]
- 71.Bae JM, Kang H, Kim HO, Park YM. Differential diagnosis of plantar wart from corn, callus and healed wart with the aid of dermoscopy. Br J Dermatol. 2009;160:220–222. doi: 10.1111/j.1365-2133.2008.08937.x. [DOI] [PubMed] [Google Scholar]
- 72.Quast DR, Nauck MA, Bechara FG, Meier JJ. A case series of verrucae vulgares mimicking hyperkeratosis in individuals with diabetic foot ulcers. Diabet Med. 2017;34:1165–1168. doi: 10.1111/dme.13387. [DOI] [PubMed] [Google Scholar]
- 73.Nirmal B, George R, Kodiatte TA. Dermatoscopy of palmar wart with falooda seed appearance. Australas J Dermatol. 2018;59:155–156. doi: 10.1111/ajd.12676. [DOI] [PubMed] [Google Scholar]
- 74.Barkat MT, Abdel-Aziz RTA, Mohamed MS. Evaluation of intralesional injection of bleomycin in the treatment of plantar warts: clinical and dermoscopic evaluation. Int J Dermatol. 2018;57:1533–1537. doi: 10.1111/ijd.14092. [DOI] [PubMed] [Google Scholar]
- 75.Arpaia N, Filotico R, Mastrandrea V, Cassano N, Vena GA. Acral viral wart showing a parallel ridge pattern on dermatoscopy. Eur J Dermatol. 2009;19:381–382. doi: 10.1684/ejd.2009.0670. [DOI] [PubMed] [Google Scholar]
- 76.Kaçar N, Demirkan N. Plantar wart with parallel ridge pattern in a patient with a previous history of melanoma: a diagnostic challenge. Australas J Dermatol. 2013;54:e78–79. doi: 10.1111/ajd.12083. [DOI] [PubMed] [Google Scholar]
- 77.Albalat W, Attwa E, Ebrahim HM. Intralesional cryotherapy versus cryotherapy spray for the treatment of recalcitrant plantar warts: a prospective, randomized study. J Dermatol Treat. 2020;26:1–7. doi: 10.1080/09546634.2020.1782821. [DOI] [PubMed] [Google Scholar]
- 78.Hassan SNE, Hussein TM, Eldeeb ME. Photodynamic therapy using methylene blue and intense pulsed light versus intense pulsed light alone in treatment of verruca: A randomized controlled study. Photodiagn Photodyn Ther. 2021;36:102541. doi: 10.1016/j.pdpdt.2021.102541. [DOI] [PubMed] [Google Scholar]
- 79.Yoong C, Di Stefani A, Hofmann-Wellenhof R, Campbell T, Soyer HP. Unusual clinical and dermoscopic presentation of a wart. Australas J Dermatol. 2009;50:228–229. doi: 10.1111/j.1440-0960.2009.00548.x. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Yu J, Thomas S, Lee K, Soyer HP. Clinical and dermoscopic features of common warts. J Eur Acad Dermatol Venereol. 2017;31:e308–e310. doi: 10.1111/jdv.14093. [DOI] [PubMed] [Google Scholar]
- 81.Veasey JV, Erthal ALN, Lellis RF. In vivo and ex vivo dermoscopy of lesions from implantation of human papillomavirus in tattoos: report of two cases. An Bras Dermatol. 2020;95:78–81. doi: 10.1016/j.abd.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morales A, Puig S, Malvehy J, Zaballos P. Dermoscopy of molluscum contagiosum. Arch Dermatol. 2005;141(12):1644. doi: 10.1001/archderm.141.12.1644. [DOI] [PubMed] [Google Scholar]
- 83.Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of molluscum contagiosum: a useful tool for clinical diagnosis in adulthood. J Eur Acad Dermatol Venereol. 2006;20(4):482–483. doi: 10.1111/j.1468-3083.2006.01480.x. [DOI] [PubMed] [Google Scholar]
- 84.Ianhez M, CestariSda C, Enokihara MY, Seize MB. Dermoscopic patterns of molluscum contagiosum: a study of 211 lesions confirmed by histopathology. An Bras Dermatol. 2011;86(1):74–79. doi: 10.1590/s0365-05962011000100009. [DOI] [PubMed] [Google Scholar]
- 85.Ku SH, Cho EB, Park EJ, Kim KH, Kim KJ. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40(2):208–210. doi: 10.1111/ced.12444. [DOI] [PubMed] [Google Scholar]
- 86.Zhuang K, Ran Y, Xu F, Lama J. Atypical infantile genital Molluscum contagiosum. An Bras Dermatol. 2015;90:403–405. doi: 10.1590/abd1806-4841.20153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uzuncakmak TK, Kuru BC, Zemheri EI, Zindanci I, Turkoglu Z, Kavala M. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71–73. doi: 10.5826/dpc.0603a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oya K, Ishitsuka Y, Fujimoto M. Coccygeal nodule in an infant: a quiz. Acta Derm Venereol. 2018;98:824–825. doi: 10.2340/00015555-2955. [DOI] [PubMed] [Google Scholar]
- 89.Atzori L, Corbeddu M, Mou M, Pilloni L, Rongioletti F. Molluscum contagiosum arising in a melanocytic congenital nevus. Pediatr Dermatol. 2018;35(5):e310–e311. doi: 10.1111/pde.13591. [DOI] [PubMed] [Google Scholar]
- 90.Elmas ÖF, Kilitçi A. Plantar molluscum contagiosum with dermoscopic features. Dermatol Pract Concept. 2020;10:e2020037. doi: 10.5826/dpc.1002a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sil A, Bhanja DB, Chandra A, Biswas SK. BOTE sign in molluscum contagiosum. BMJ Case Rep. 2020;13:e239142. doi: 10.1136/bcr-2020-239142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monteagudo Sánchez B, León-Muiños E, Piñeyro-Molina F, Vázquez-Bueno JÁ. Dermoscopy of plantar molluscum contagiosum. Actas Dermosifiliogr (Engl Ed) 2021;S0001–7310(21):00195–202. doi: 10.1016/j.ad.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Panasiti V, Devirgiliis V, Roberti V, Curzio M, Calvieri S. Molluscum contagiosum on a tattoo: usefulness of dermoscopy. Int J Dermatol. 2008;47:1318–1319. doi: 10.1111/j.1365-4632.2008.03747.x. [DOI] [PubMed] [Google Scholar]
- 94.Pradhan S, Ran X, Xu X, Yang Y, Lei S, Ran Y. Image gallery: dermoscopy of perianal molluscum contagiosum in a child caused by molluscum contagiosum virus subtype I. Br J Dermatol. 2019;180:e68. doi: 10.1111/bjd.17401. [DOI] [PubMed] [Google Scholar]
- 95.Daruwalla SB, Dhurat RS, Agrawal S, Mahobia S, Naidu KS. "Extraction dermoscopy": expanding the utility of epiluminescence microscopy. Skin Appendage Disord. 2020;6:220–223. doi: 10.1159/000508518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alfaro-Castellón P, Mejía-Rodríguez SA, Valencia-Herrera A, Ramírez S, Mena-Cedillos C. Dermoscopy distinction of eruptive vellus hair cysts with molluscum contagiosum and acne lesions. Pediatr Dermatol. 2012;29:772–773. doi: 10.1111/j.1525-1470.2012.01771.x. [DOI] [PubMed] [Google Scholar]
- 97.Mun JH, Ko HC, Kim BS, Kim MB. Dermoscopy of giant molluscum contagiosum. J Am Acad Dermatol. 2013;69:e287–288. doi: 10.1016/j.jaad.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 98.Demirdag HG, Ayanoglu BT, Durmus B, Bulut M, Akay BN. Molluscum contagiosum with dermoscopic features in an unusual areola and nipple location. Dermatol Online J. 2019;25:4. [PubMed] [Google Scholar]
- 99.Elmas ÖF, Kilitçi A. Subungual molluscum contagiosum: a rare presentation. Skin Appendage Disord. 2020;6:55–57. doi: 10.1159/000503978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar S, Aggarwal D, Chatterjee D, Vinay K. Atypical presentation of genital molluscum contagiosum mimicking genital warts. Int J STD AIDS. 2020;31:1420–1422. doi: 10.1177/0956462420920410. [DOI] [PubMed] [Google Scholar]
- 101.Sławińska M, Hlebowicz M, Iżycka-Świeszewska E, et al. Dermoscopic features of giant molluscum contagiosum in a patient with acquired immunodeficiency syndrome. Acta Dermatovenerol Croat. 2020;28:233–235. [PubMed] [Google Scholar]
- 102.Ayhan E, Aktaş H. Dermoscopic features and types of orf and milker's nodule. Postepy Dermatol Alergol. 2017;34:357–362. doi: 10.5114/ada.2017.69317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rezende ALRA, Bernardes Filho F, de Paula NA, Towersey L, Hay R, Frade MAC. Clinical manifestation, dermoscopy, and scanning electron microscopy in two cases of contagious ecthyma (orf nodule) Case Rep Dermatol Med. 2018;2018:2094086. doi: 10.1155/2018/2094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chavez-Alvarez S, Barbosa-Moreno L, Villarreal-Martinez A, Vazquez-Martinez OT, Ocampo-Candiani J. Dermoscopy of contagious ecthyma (orf nodule) J Am Acad Dermatol. 2016;74(5):e95–96. doi: 10.1016/j.jaad.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 105.Tognetti L, Cinotti E, Habougit C, et al. Ecthyma contagiosum (Orf): reflectance confocal microscopy and histopathological correlates. Skin Res Technol. 2019;25:234–237. doi: 10.1111/srt.12618. [DOI] [PubMed] [Google Scholar]
- 106.Aslan Kayıran M, Uzunçakmak TK, Cebeci F, Akdeniz N, Çobanoğlu ŞB. Dermoscopic features of Orf disease. Int J Dermatol. 2018;57:115–116. doi: 10.1111/ijd.13805. [DOI] [PubMed] [Google Scholar]
- 107.Alnami A, Shokor N, Alajlan A, AlJasser MI. Clinical and dermoscopic features of orf transmitted from camels. Int J Dermatol. 2021;60:e276–e277. doi: 10.1111/ijd.15479. [DOI] [PubMed] [Google Scholar]
- 108.Vinay K, Bhattacharjee R, Bishnoi A, Chatterjee D, Rudramurthy S, Dogra S. Dermatoscopic features of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2020;34:e718–e720. doi: 10.1111/jdv.16539. [DOI] [PubMed] [Google Scholar]
- 109.Dabas G, Kaur H, Vinay K, Kumaran MS, Shivaprakash MR, Saikia UN, Dogra S. Dermoscopy in disseminated sporotrichosis. J Eur Acad Dermatol Venereol. 2019;33:e33–e35. doi: 10.1111/jdv.15152. [DOI] [PubMed] [Google Scholar]
- 110.Wang S, Martini MC, Groth JV, Hernandez C. Dermatoscopic and clinicopathologic findings of cutaneous blastomycosis. J Am Acad Dermatol. 2015;73:e169–170. doi: 10.1016/j.jaad.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 111.Elmas ÖF, Uyar B, Kilitçi A. A 25-year history of leg ulceration: cutaneous blastomycosis. Dermatol Pract Concept. 2020;10:e2020054. doi: 10.5826/dpc.1003a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gupta G, Burden AD, Shankland GS, Fallowfield ME, Richardson MD. Tinea nigra secondary to Exophiala werneckii responding to itraconazole. Br J Dermatol. 1997;137:483–484. doi: 10.1111/j.1365-2133.1997.tb03775.x. [DOI] [PubMed] [Google Scholar]
- 113.Smith SB, Beals SL, Elston DM, Meffert JJ. Dermoscopy in the diagnosis of tinea nigra plantaris. Cutis. 2001;68:377–380. [PubMed] [Google Scholar]
- 114.Xavier MH, Ribeiro LH, Duarte H, Saraça G, Souza AC. Dermatoscopy in the diagnosis of tinea nigra. Dermatol Online J. 2008;14:15. [PubMed] [Google Scholar]
- 115.Criado PR, Delgado L, Pereira GA. Dermoscopy revealing a case of Tinea Nigra. An Bras Dermatol. 2013;88:128–129. doi: 10.1590/S0365-05962013000100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rossetto AL, Corrêa PR, Cruz RC, Pereira EF, Haddad Filho Jr V. A case of Tinea nigra associated to a bite from a European rabbit (Oryctolagus cuniculus, Leporidae): the role of dermoscopy in diagnosis. An Bras Dermatol. 2014;89:165–166. doi: 10.1590/abd1806-4841.20142539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guarenti IM, Almeida HL, Jr, Leitão AH, Rocha NM, Silva RM. Scanning electron microscopy of tinea nigra. An Bras Dermatol. 2014;89:334–336. doi: 10.1590/abd1806-4841.20142780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas CL, Samarasinghe V, Natkunarajah J, Fogo A. Entodermoscopy: a spotlight on tinea nigra. Int J Dermatol. 2016;55:e117–e118. doi: 10.1111/ijd.13057. [DOI] [PubMed] [Google Scholar]
- 119.Veasey JV, Avila RB, Ferreira MAMO, Lazzarini R. Reflectance confocal microscopy of tinea nigra: comparing images with dermoscopy and mycological examination results. An Bras Dermatol. 2017;92:568–569. doi: 10.1590/abd1806-4841.20176808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veasey JV, Avila RB, Miguel BAF, Muramatu LH. White piedra, black piedra, tinea versicolor, and tinea nigra: contribution to the diagnosis of superficial mycosis. An Bras Dermatol. 2017;92:413–416. doi: 10.1590/abd1806-4841.20176018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Valentim FO, Lacerda PN, Haddad GR. Tinea nigra: a series of three cases observed in Botucatu, São Paulo. Rev Soc Bras Med Trop. 2021;54:e0563–2020. doi: 10.1590/0037-8682-0563-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paschoal FM, de Barros JA, de Barros DP, de Barros JC, Filho CD. Study of the dermatoscopic pattern of tinea nigra: report of 6 cases. Skinmed. 2010;8:319–321. [PubMed] [Google Scholar]
- 123.Maia Abinader MV, Carvalho Maron SM, Araújo LO, Silva AA. Tinea nigra dermoscopy: a useful assessment. J Am Acad Dermatol. 2016;74:e121–122. doi: 10.1016/j.jaad.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 124.Noguchi H, Hiruma M, Inoue Y, Miyata K, Tanaka M, Ihn H. Tinea nigra showing a parallel ridge pattern on dermoscopy. J Dermatol. 2015;42:518–520. doi: 10.1111/1346-8138.12830. [DOI] [PubMed] [Google Scholar]
- 125.Nazzaro G, Ponziani A, Cavicchini S. Tinea nigra: a diagnostic pitfall. J Am Acad Dermatol. 2016;75:e219–e220. doi: 10.1016/j.jaad.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 126.Piliouras P, Allison S, Rosendahl C, Buettner PG, Weedon D. Dermoscopy improves diagnosis of tinea nigra: a study of 50 cases. Australas J Dermatol. 2011;52:191–194. doi: 10.1111/j.1440-0960.2011.00790.x. [DOI] [PubMed] [Google Scholar]
- 127.Nogita A, Hiruma J, Maeda T, Harada K, Tsuboi R, Umebayashi Y. Case of tinea nigra on the sole: correspondences between dermoscopic and histological findings. J Dermatol. 2019;46:e187–e188. doi: 10.1111/1346-8138.14720. [DOI] [PubMed] [Google Scholar]
- 128.Landero J. Long-term pubic dermatitis diagnosed as white piedra. Cutis. 2017;100:448–450. [PubMed] [Google Scholar]
- 129.Bonifaz A, Tirado-Sánchez A, Araiza J, et al. White Piedra: clinical, mycological, and therapeutic experience of fourteen cases. Skin Appendage Disord. 2019;5:135–141. doi: 10.1159/000493374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhuang K, Ran X, Dai Y, et al. An unusual case of white Piedra due to Trichosporon inkin mimicking trichobacteriosis. Mycopathologia. 2016;181:909–914. doi: 10.1007/s11046-016-0049-9. [DOI] [PubMed] [Google Scholar]
- 131.Errichetti E, Stinco G. Dermoscopy in tinea manuum. An Bras Dermatol. 2018;93:447–448. doi: 10.1590/abd1806-4841.20186366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhat YJ, Keen A, Hassan I, Latif I, Bashir S. Can dermoscopy serve as a diagnostic tool in dermatophytosis? A pilot study. Indian Dermatol Online J. 2019;10:530–535. doi: 10.4103/idoj.IDOJ_423_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jakhar D, Kaur I, Sonthalia S. Dermoscopy of tinea manuum. Indian Dermatol Online J. 2019;10:210–211. doi: 10.4103/idoj.IDOJ_95_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Knöpfel N, del Pozo LJ, Escudero Mdel M, Martín-Santiago A. Dermoscopic visualization of vellus hair involvement in tinea corporis: a criterion for systemic antifungal therapy? Pediatr Dermatol. 2015;32:e226–227. doi: 10.1111/pde.12648. [DOI] [PubMed] [Google Scholar]
- 135.Kobayashi M, Kitahara H, Yaguchi T, Sato T. A case of tinea corporis on the arm caused by Nannizzia gypsea with dermatoscopic images. J Dtsch Dermatol Ges. 2018;16:784–786. doi: 10.1111/ddg.13549. [DOI] [PubMed] [Google Scholar]
- 136.Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy of tinea corporis. J Eur Acad Dermatol Venereol. 2020;34:e278–e280. doi: 10.1111/jdv.16277. [DOI] [PubMed] [Google Scholar]
- 137.Llamas Carmona JA, Vera Casaño Á, Martin González MT, Martinez Pilar L, Gómez ME. Flexural comedones and scar formation caused by inflammatory Tinea corporis. Pediatr Dermatol. 2021;38:960–961. doi: 10.1111/pde.14617. [DOI] [PubMed] [Google Scholar]
- 138.Gómez Moyano E, Crespo Erchiga V, Martínez Pilar L, Martinez GS. Correlation between dermoscopy and direct microscopy of morse code hairs in tinea incognito. J Am Acad Dermatol. 2016;74:e7–8. doi: 10.1016/j.jaad.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 139.Piccolo V, Corneli P, Russo T, Zalaudek I, Alfano R, Argenziano G. Dermoscopy as a useful tool in diagnosis of tinea incognito. Int J Dermatol. 2019;58:e32–e34. doi: 10.1111/ijd.14278. [DOI] [PubMed] [Google Scholar]
- 140.Sonthalia S, Ankad BS, Goldust M, Jha AK. Dermoscopy—a simple and rapid in vivo diagnostic technique for tinea incognito. An Bras Dermatol. 2019;94:612–614. doi: 10.1016/j.abd.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singal A, Jakhar D, Kaur I, Pandhi D, Das S. Tinea pseudoimbricata as a unique manifestation of steroid abuse: a clinico-mycological and dermoscopic study from a tertiary care hospital. Indian Dermatol Online J. 2019;10:422–425. doi: 10.4103/idoj.IDOJ_385_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tang J, Ran Y. Polarized and ultraviolet dermoscopy for the diagnosis of dermatophytosis of vellus hair. Indian J Dermatol Venereol Leprol. 2020;86:607. doi: 10.4103/ijdvl.IJDVL_65_19. [DOI] [PubMed] [Google Scholar]
- 143.Gómez-Moyano E, Crespo Erchiga V, Martínez Pilar L, Martínez García S, Martín González T, Godoy Diaz DJ, Vera CA. Using dermoscopy to detect tinea of vellus hair. Br J Dermatol. 2016;174:636–638. doi: 10.1111/bjd.14085. [DOI] [PubMed] [Google Scholar]
- 144.Liu ZH, Zhi HL, Xia XJ. Combination dermoscopy highlights fungal invasion of vellus hair in tinea vellus. Clin Exp Dermatol. 2022;47:138–139. doi: 10.1111/ced.14839. [DOI] [PubMed] [Google Scholar]
- 145.Piccolo V, Di Brizzi EV, Russo T, et al. Majocchi's granuloma on the face: dermoscopy and reflectance confocal microscopy. Int J Dermatol. 2019;58:e180–e182. doi: 10.1111/ijd.14496. [DOI] [PubMed] [Google Scholar]
- 146.Akay BN, Demirdag HG, Evren E, Karahan ZC, OkcuHeper A, Kundakci N. Deep fungal infection caused by Trichophyton rubrum after heart transplantation: a case report with dermoscopy. Australas J Dermatol. 2019;60:e252–e254. doi: 10.1111/ajd.12993. [DOI] [PubMed] [Google Scholar]
- 147.Xian J, Huang X, Li Q, Peng X, Peng X. Dermatoscopy for the rapid diagnosis of Talaromyces marneffei infection: a case report. BMC Infect Dis. 2019;19:707. doi: 10.1186/s12879-019-4351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Q, Wang C, Zeng K, Peng X, Wang F. AIDS-associated disseminated talaromycosis (penicilliosis) marneffei. J Dtsch Dermatol Ges. 2018;16:1256–1259. doi: 10.1111/ddg.13663. [DOI] [PubMed] [Google Scholar]
- 149.Xu X, Ran X, Pradhan S, Lei S, Ran Y. Dermoscopic manifestations of Talaromyces (Penicillium) marneffei infection in an AIDS patient. Indian J Dermatol Venereol Leprol. 2019;85:348. doi: 10.4103/ijdvl.IJDVL_118_17. [DOI] [PubMed] [Google Scholar]
- 150.Sławińska M, Hlebowicz M, Iżycka-Świeszewska E, et al. Dermoscopic observations in disseminated cryptococcosis with cutaneous involvement. J Eur Acad Dermatol Venereol. 2018;32:e223–e224. doi: 10.1111/jdv.14744. [DOI] [PubMed] [Google Scholar]
- 151.Chauhan P, Jindal R, Shirazi N. Dermoscopy of Chromoblastomycosis. Indian Dermatol Online J. 2019;10:759–760. doi: 10.4103/idoj.IDOJ_213_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arguello-Guerra L, Gatica-Torres M, Dominguez-Cherit J. Chromomycosis. BMJ Case Rep. 2016;2016:bcr2016215391. doi: 10.1136/bcr-2016-215391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tang J, Zhuang K, Ran X, Dai Y, Ran Y. Chromoblastomycosis caused by Cladophialophora carrionii. Indian J Dermatol Venereol Leprol. 2017;83:482–485. doi: 10.4103/ijdvl.IJDVL_707_16. [DOI] [PubMed] [Google Scholar]
- 154.Subhadarshani S, Yadav D. Dermoscopy of chromoblastomycosis. Dermatol Pract Concept. 2017;7:23–24. doi: 10.5826/dpc.0704a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jayasree P, Malakar S, Raja H, Gopinathan NN. Dermoscopic features in nodular chromoblastomycosis. Int J Dermatol. 2019;58:e107–e109. doi: 10.1111/ijd.14344. [DOI] [PubMed] [Google Scholar]
- 156.Katoch S, Barua TN, Barua KN. The curious case of an elusive solitary plaque. Indian Dermatol Online J. 2020;11:288–290. doi: 10.4103/idoj.IDOJ_457_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reis LM, Lima BZ, ZilloFda C, Rezende CM, Fabricio LH, Pinto CA. Dermoscopy assisting the diagnosis of mycetoma: case report and literature review. An Bras Dermatol. 2014;89:832–833. doi: 10.1590/abd1806-4841.20143008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ankad BS, Manjula R, Tejasvi T, Nikam BP. Dermoscopy of eumycotic mycetoma: a case report. Dermatol Pract Concept. 2019;9:297–299. doi: 10.5826/dpc.0904a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ankad BS, Beergoudar SL, Nikam BP. Dermatoscopy in actinomycetoma: an observation. Indian Dermatol Online J. 2019;10:330–331. doi: 10.4103/idoj.IDOJ_268_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Litaiem N, Midassi O, Zeglaoui F. Detecting subclinical mycetoma’s black grains using dermoscopy. Int J Dermatol. 2019;58:231–232. doi: 10.1111/ijd.14187. [DOI] [PubMed] [Google Scholar]
- 161.Zhou H, Tang XH, De Han J, Chen MK. Dermoscopy as an ancillary tool for the diagnosis of pityriasis versicolor. J Am Acad Dermatol. 2015;73:e205–206. doi: 10.1016/j.jaad.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 162.Thomas N, Malakar S. Dermoscopy: an easy way to solve the diagnostic puzzle in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2019;85:664–665. doi: 10.4103/ijdvl.IJDVL_816_16. [DOI] [PubMed] [Google Scholar]
- 163.Mathur M, Acharya P, Karki A, Kc N, Shah J. Dermoscopic pattern of pityriasis versicolor. Clin Cosmet Investig Dermatol. 2019;12:303–309. doi: 10.2147/CCID.S195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaur I, Jakhar D, Singal A. Dermoscopy in the evaluation of pityriasis versicolor: a cross sectional study. Indian Dermatol Online J. 2019;10:682–685. doi: 10.4103/idoj.IDOJ_502_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jakhar D, Kaur I, Chaudhary R. Dermoscopy of pityrosporum folliculitis. J Am Acad Dermatol. 2019;80:e43–e44. doi: 10.1016/j.jaad.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 166.Chauhan P, Jindal R, Errichetti E. Dermoscopy of skin parasitoses, bites and stings: a systematic review of the literature. J Eur Acad Dermatol Venereol. 2022 doi: 10.1111/jdv.18352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated during the current study.