Abstract
Escherichia coli is the primary cause of uncomplicated infections of the urinary tract including cystitis. More serious infections, characterized as acute pyelonephritis, can also develop. Type 1 fimbriae of E. coli contribute to virulence in the urinary tract; however, only recently has the expression of the type 1 fimbriae been investigated in vivo using molecular techniques. Transcription of type 1 fimbrial genes is controlled by a promoter that resides on a 314-bp invertible element capable of two orientations. One places the promoter in the ON orientation, allowing for transcription; the other places the promoter in the OFF orientation, preventing transcription. A PCR-based assay was developed to measure the orientation of the invertible element during an experimental urinary tract infection in mice. Using this assay, it was found that the percentage of the population ON in urine samples correlated with the respective CFU per gram of bladder (P = 0.0006) but not with CFU per gram of kidney (P > 0.069). Cystitis isolates present in the urine of mice during the course of infection had a higher percentage of their invertible elements in the ON orientation than did pyelonephritis isolates (85 and 34%, respectively, at 24 h; P < 0.0001). In general, cystitis isolates, unlike pyelonephritis isolates, were more likely to maintain their invertible elements in the ON orientation for the entire period of infection. E. coli cells expressing type 1 fimbriae, expelled in urine, were shown by scanning electron microscopy to be densely packed on the surface of uroepithelial cells. These results suggest that expression of type 1 fimbriae is more critical for cystitis strains than for pyelonephritis strains in the early stages of an infection during bladder colonization.
Escherichia coli is the most common cause of uncomplicated cystitis (38). Between 40 and 50% of adult women will have at least one urinary tract infection (UTI) during their lifetime (24). Approximately 90% of community-acquired UTIs and 30% of hospital-acquired UTIs are caused by E. coli (13). Clearly, UTI caused by this bacterium represents a major human health concern in the United States and worldwide.
UTIs can be classified as asymptomatic bacteriuria, cystitis, or acute pyelonephritis (38). Cystitis predominantly involves colonization of the bladder. The more severe upper urinary tract disease, acute pyelonephritis, involves colonization of the kidneys and represents an infection capable of progressing to bacteremia (15). It is unclear whether there are subpopulations of uropathogenic E. coli that specifically cause cystitis or specifically cause pyelonephritis or whether all uropathogenic E. coli strains are capable of causing either disease, with the clinical outcome depending more on the host response. There are studies to support either hypothesis. For example, the same organism that previously caused acute pyelonephritis can, on recurrence, cause cystitis (2, 32). There is also ample evidence suggesting that there are differences between cystitis isolates and pyelonephritis isolates from UTIs. For example, Sandberg et al. demonstrated that strains isolated from patients with cystitis may differ in their O, K, and H serotypes from strains isolated from those with pyelonephritis (32). Cystitis isolates are also less likely to be P fimbriated, adhere less well to uroepithelial cells, and are less capable of mannose-resistant hemagglutination than are pyelonephritis isolates (2, 32, 36). In more recent studies using the experimental mouse model of UTI, cystitis isolates were found in greater numbers in the bladder and caused greater histological changes within the bladder than did pyelonephritis isolates (17).
Type 1 fimbriae of E. coli may be in part responsible for the above observations and may also serve as a possible marker to subdivide uropathogenic strains of E. coli into two distinct populations. Type 1 fimbriae are hair-like projections that extend from the surface of E. coli and other genera of the Enterobacteriaceae (35). These fimbriae bind mannose-containing oligosaccharides via the FimH adhesive tip protein (23, 34). Type 1 fimbriae are required for colonization of the urinary tract by uropathogenic E. coli (5, 7, 14, 20, 25, 29, 33). Indeed, it is the only virulence factor in uropathogens (besides Dr fimbriae in chronic pyelonephritis infections [10]) for which the molecular Koch's postulates have been satisfied (5, 20). The presence of type 1 fimbriae increases the numbers of E. coli successfully infecting the urinary tract, as well as enhancing the persistence of the bacteria (5). More recent work has suggested a specific role for type 1 fimbriae in the colonization of the bladder through binding to uroepithial cells (29).
Production of type 1 fimbriae is under the control of a 314-bp phase-variable invertible element that contains the promoter controlling the transcription of the fimbrial genes fimACDFGH (1). When the invertible element is in the ON position, the promoter is in the correct orientation to transcribe the fimbrial genes; however, when the element is in the OFF position, the orientation of the promoter does not allow transcription and no type 1 fimbriae are expressed by the bacteria. Through flipping of the orientation of this invertible element, E. coli strains are capable of rapidly changing between the fimbriated and nonfimbriated states. In a previous study, our laboratory investigated the ability of E. coli strains specifically isolated from patients with cystitis or acute pyelonephritis to switch from the nonfimbriated to the fimbriated state under in vitro growth conditions. In those studies, we observed that cystitis isolates were more likely to switch their invertible elements from the OFF to the ON orientation than were pyelonephritis isolates (27).
In the present study, we present data that further define the role of type 1 fimbriae in the colonization of the bladder and exfoliation of host uroepithial cells. We have observed that during the course of a UTI, cystitis isolates maintain a larger percentage of their invertible elements in the ON orientation, thus allowing type 1 fimbriae expression. In contrast, pyelonephritis isolates have a significantly lower average percentage of their invertible elements in the ON orientation. This indicates that there may be at least two distinct groups of uropathogenic E. coli, one adapted to colonize the lower urinary tract (cystitis) and one group more capable of infecting the upper urinary tract (acute pyelonephritis). The regulation of type 1 fimbria expression during the course of the infection may account, in part, for these differences.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in these investigations (Table 1) were selected from the strain library described previously (27). Growth conditions for strains on agar plates and in liquid culture were also described previously (27). For growth on solid medium, individual strains were streaked onto Luria agar plates and incubated at 37°C for 18 h. A single colony was selected for use in DNA extraction. Alternatively, strains were inoculated into Luria broth (100 ml) and incubated at 37°C for 18 h with aeration (200 rpm/min). In this case, bacteria were harvested by centrifugation (10,000 × g for 5 min at 4°C), and the pellet was used for DNA extraction.
TABLE 1.
E. coli strains used in this studya
| Strain | Original date of isolation (mo/day/yr) | Source of sample | Clinical syndrome | fim+ | MSHAb | pap+ | MRHAc | Binding to Gal-Gal latex | hly+ | sfa+ | cnf+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFT 073 | 8/12/82 | Blood | Pyelonephritis | +c | + | + | + | + | + | + | − |
| CFT 204 | 12/15/82 | Urine | Pyelonephritis | + | + | + | + | + | + | − | − |
| CFT 325 | 4/24/83 | Blood | Pyelonephritis | + | + | − | + | +, −d | − | − | − |
| CPZ 005 | 3/19/80 | Blood | Pyelonephritis | + | + | + | + | +, −d | NDe | − | − |
| CYS 38 | ND | Urine | Cystitis | + | + | ND | ND | ND | ND | − | ND |
| F 11 | 3/31/94 | Urine | Cystitis | + | + | + | + | ND | + | ND | ND |
| F 3 | 3/31/94 | Urine | Cystitis | + | + | + | + | ND | + | ND | ND |
| F 38 | 3/31/94 | Urine | Cystitis | + | + | + | + | ND | + | ND | ND |
| F 63 | 3/31/94 | Urine | Cystitis | + | + | + | + | ND | + | ND | ND |
Values in the table are positive (+) or negative (−) for genotype (determined by hybridization) or phenotype (determined by agglutination). Some data are derived from references 17 and 39.
Phenotype for type 1 fimbriae determined by the mannose-sensitive hemagglutination assay (MSHA).
Phenotype for P fimbriae determined by the mannose-resistant hemagglutination assay (MRHA) (of human red blood cells).
Values for multiple assays.
ND, not determined.
Murine model of ascending UTI.
CBA mice (20 to 22 g, 6 to 8 weeks old [Harlan Sprague-Dawley, Indianapolis, Ind.]) were inoculated transurethrally with a suspension containing approximately 107 CFU of the E. coli strain, using a 0.28-mm sterile polyethylene catheter connected to an infusion pump (Harvard Apparatus, Millis, Mass.) as described previously (27). Inocula were prepared from bacteria grown on Luria agar plates incubated at 37°C for 18 h; the resulting bacterial lawns were suspended in 2 ml of phosphate-buffered saline (PBS). Urine samples were collected at 4, 24, 48, 72, and 96 h and frozen immediately at −70°C. At 96 h, mice were sacrificed and the bladders and kidneys were removed, homogenized, and used to determine the CFU per gram of tissue (17). Mice were also sacrificed at 24, 48, and 72 h to establish a correlation between the type 1 fimbria invertible-element orientation and colony counts in the urine, bladder, and kidneys.
Preparation of DNA template for PCR, PCR amplification, restriction enzyme digestion, and densitometric analysis.
Bacteria present in urine samples or other suspensions were harvested by centrifugation (10,000 × g for 5 min at 4°C) and resuspended in 25 μl of 50 mM Tris (pH 8.5)–1 mM EDTA–0.5% Tween 20 containing proteinase K (200 μg/ml). The samples were incubated for 30 min at 55°C followed by 10 min at 96°C to inactivate the proteinase K. Samples were stored at −20°C until used as templates in the PCR assay. The conditions for amplification of the invertible element region were described previously (27). The resulting PCR products were digested with SnaBI (New England Biolabs) and visualized on 2% agarose gels. The DNA bands resulting from this process were quantitated as described previously (27). The densitometric measurements were made using the Eagle Eye gel documentation system and the Eagle Sight software version 3.0 (Stratagene). More information on this assay can be found on our laboratory's web site (http://134.192.128.199/res.htm).
Filter assay of mouse urine samples.
Urine samples were collected 4, 24, 48, 72, and 96 h postinoculation from mice infected with E. coli pyelonephritis isolate CFT073. Immediately after collection, the urine samples were diluted to a volume of 200 μl in PBS and passed through a syringe tip filter containing a polycarbonate membrane with pores 12 μm in diameter (Millipore Isopore 12.0-μm TKTP membrane filters and Swinnex 13-mm syringe tip filter holder). The filters were washed by passage of an additional 100 μl of PBS. Material retained on the filter was resuspended in 100 μl of PBS by gentle agitation. The samples resuspended from the filter and the filtrate (suspension that passed through the filter) were concentrated separately by centrifugation (10,000 × g for 5 min at 4°C) and then processed to prepare the DNA template for use in PCR amplification and densitometric analysis as described previously (27).
Electron microscopy.
Material that was retained on the surface of 12-μm-pore-diameter filters after filtration of mouse urine was visualized by scanning electron microscopy. Immediately after filtration, the filters were removed from their support and fixed in 4% formaldehyde–1% glutaraldehyde for 18 h. Samples were washed in a 0.2 M sucrose–0.1 M sodium cacodylate buffer, placed in 2% OsO4 for 30 min, and washed with distilled water. They were then placed in a saturated solution of thiocarbohydrazide (Polyscience, Warrington, Pa.) for 20 min, washed with distilled water, placed in OsO4 again for 20 min, and given final wash in distilled water. They were then dehydrated by washing twice for 10 min each in 70, 95, and 100% ethanol successively. The samples were critical-point dried using a Sandri 790B drying system (Tousimis Research Corp.) and gold coated with a sputter-type machine (Humer model; Technics, Alexandria, Va.). An AMR model 1000 scanning electron microscope was used to examine the filter surfaces at magnifications of ×500 to ×2,000
RESULTS
The percentage of invertible elements in the ON orientation correlates with the CFU per gram of bladder tissue of experimentally infected mice.
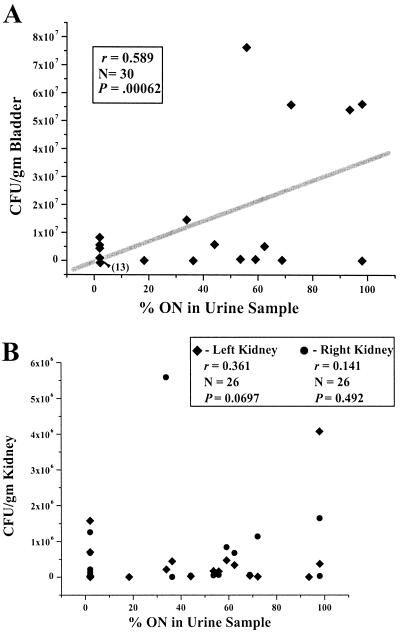
To use the orientation of the invertible elements of the E. coli present in the urine of mice as a marker for in vivo events occurring in the bladders and kidneys of mice, we correlated the percent ON of the invertible elements in urine samples with colony counts of E. coli present in corresponding bladder and kidney tissue. The percentage of E. coli type 1 fimbria invertible elements in the ON orientation for a series of urine samples collected from experimentally infected mice was determined. Immediately after urine samples were collected, the mice were sacrificed and the bladders and kidneys were removed, weighed, homogenized, and spread onto nutrient agar plates; after overnight incubation, the CFU per gram of bladder or kidney was determined. The percent ON in the urine samples correlated significantly with the CFU per gram in bladder tissue (P < 0.001) (Fig. 1A). In contrast, there was no significant correlation between the percent ON of the invertible elements in the urine samples and the CFU per gram of kidney (Fig. 1B). Thus, the percentage of E. coli cells with the invertible element ON in urine samples represents a proxy for colonization of bladder but not kidney tissue.
FIG. 1.
Correlation of CFU in bladder or kidney tissue with percent ON in urine samples. Mice were transurethrally inoculated with E. coli CFT073 (107 CFU/g). At various time points, urine was collected and the mice were sacrificed. The percent ON from the urine and the CFU per gram of bladder and kidney were determined. Please note the different ranges of the y axes in panels A and B. (A) The percentage of the bacteria in urine with the type 1 fimbria invertible element in the ON orientation correlates with the number of CFU per gram of bladder in the corresponding specimens (r = 0.59, P = 0.0006). Note that 13 bladder samples with CFU per gram values ranging from 0 to 9.4 × 105 are represented by a single diamond. (B) The percentage of the bacteria in urine with the type 1 fimbria invertible element in the ON orientation does not correlate with the number of CFU per gram of right kidney (r = 0.141, P = 0.492) or left kidney (r = 0.361, P = 0.0697) in the corresponding specimens.
Adherent and nonadherent bacteria in mouse urine separated on a 12-μm-pore-size filter.
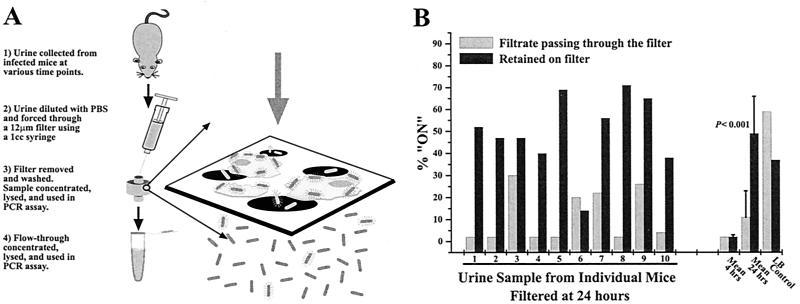
To determine the percent ON for E. coli adherent to epithelial cells versus nonadherent planktonic E. coli, urine samples from infected mice were passed through filters with pores 12 μm in diameter. Samples that passed through the filter and samples retained by the filter were collected and analyzed using the PCR assay (Fig. 2A). Urine samples were collected from each of 10 mice at 24 h, processed, and assayed (Fig. 2B). The percent ON was significantly higher in bacteria retained on the filter (49%) than in bacteria that passed throught the filter (11%) (P < 0.001). The mean values for the 4-h samples did not differ significantly between the samples retained on the filter (2%) and the samples from the flowthrough (2%) (P > 0.1).
FIG. 2.
Percent ON of invertible elements adherent and nonadherent bacteria in urine. (A) Urine samples collected from mice experimentally infected with E. coli CFT073 were passed through 12-μm-pore-size filters. Bacteria that passed through the filters and bacteria that were retained on the filters were individually assayed to determine the type 1 fimbria invertible-element orientation for the bacterial populations. (B) The percent ON values of the invertible elements of the bacteria that were retained on the filter and the bacteria that passed through the filter for individual mouse urine samples are shown. The mean percent ON values for all samples are also shown. A control experiment with CFT073 grown in static Luria broth showed that bacteria on the membrane surface are not simply retained there by virtue of expressing type 1 fimbriae.
Scanning electron microscopy of E. coli retained on 12-μm-pore-size filters.
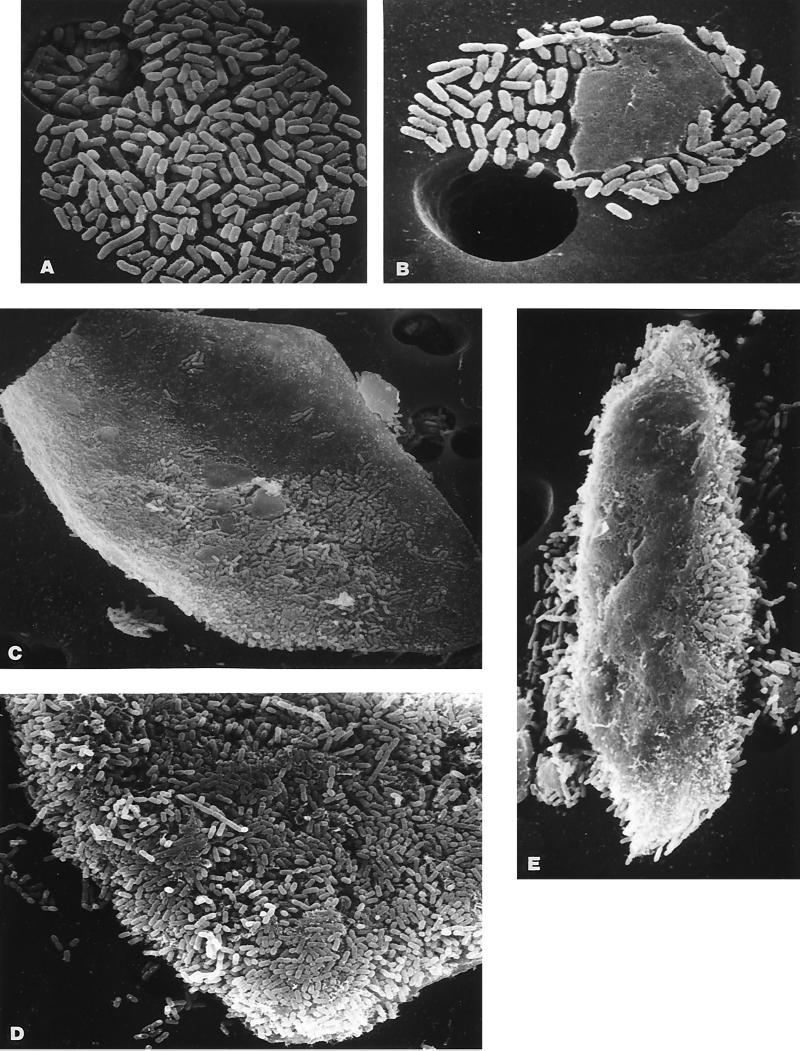
It has been previously shown in other laboratories that E. coli strains released in the urine of experimentally infected mice are associated with host uroepithelial cells (14, 21). To determine whether these E. coli strains, capable of expressing type 1 fimbriae and captured on the surface of the 12-μm filters, were also associated with uroepithelial cells, we examined the filters by scanning electron microscopy. The resulting images (Fig. 3) are representative of the different associations for the E. coli cells observed on the filter surface. Most uncommonly, E. coli cells were found aggregated in tight clusters of bacteria alone (Fig. 3A). A slightly larger number of the E. coli cells were found attached to what appeared to be cell fragments which were too large to pass through the filter pores (Fig. 3B). Most frequently, bacteria were found in extraordinarily large numbers in close association with what appear to be host uroepithelial cells (Fig. 3C and D). Instances were observed where what may have been the basolateral side of a recently exfoliated cell was devoid of bacteria whereas the corresponding apical side was obscured by attaching bacteria (Fig. 3E).
FIG. 3.
Scanning electron microscopy of bacteria retained by filters following the filtration of urine from infected mice. The filters used to retain material of >12 μm present in infected mouse urine were subjected to scanning electron microscopy. Representative images are shown. (A) An aggregate of E. coli CFT073. Magnification, ×1,760. (B) E. coli binding to a eukaryotic cell fragment. Magnification, ×880. (C) A large number of E. coli cells binding to a eukaryotic cell with the morphological characteristics of a uroepithelial cell. Magnification, ×440. (D) E. coli binding to a uroepithelial cell. Note the large number of bacteria bound to the cell exfoliated into the urine of the infected mouse. Magnification, ×880. (E) Another host cell showing the top surface of the cell (presumed to be the basolateral surface) devoid of bacteria while the face-down surface (presumed to be the apical surface) appears coated with E. coli. Magnification, ×440.
Invertible-element orientation in a cystitis isolate during experimental UTI.
Previous work from our laboratory suggested that a difference may exist in the propensity for turning ON the fim genes between cystitis and pyelonephritis isolates and that examination of the controlling switch position of these two strain types during an experimental infection may be instructive (21).
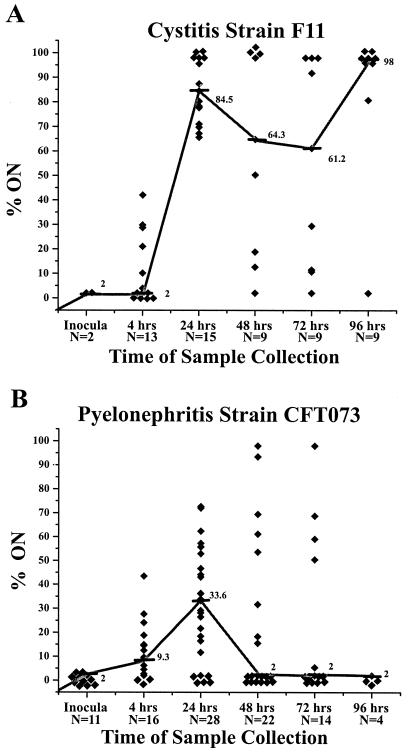
The mouse model of ascending UTI was used to determine the orientation of the type 1 fimbria invertible element under in vivo conditions. Cystitis strain F11 was heavily inoculated onto Luria agar plates and incubated for 18 h, resulting in a lawn of E. coli cells that had their invertible elements exclusively in the OFF orientation (≤2% ON) (data not shown). Bacteria from three plates were resuspended in ∼4 ml of PBS for use as an inoculum. Female CBA mice were transurethrally inoculated with 107 CFU of the F11 strain as described above, and urine was collected from the mice at 4, 24, 48, 72, and 96 h. Samples were processed, and the percentage of the invertible elements in the ON orientation in each urine sample at each time point was determined. The percent ON of invertible elements of the E. coli cells in each urine sample was plotted against sample collection time (Fig. 4A). A clear progression for the switching of the invertible elements from the OFF orientation (≤2% ON) to an ON orientation (median peak value of 84.5% at 24 h) was observed for F11. After 24 h, the median percentage of the bacterial population's invertible elements in the ON orientation declined slightly at the 48- and 72-h time points but never dropped below 61%. At 96 h, the percent ON for the F11 samples increased to a median value of 98%.
FIG. 4.
Percentage of invertible elements in the ON orientation of F11 or CFT073 isolates present in the urine of infected mice. Bacteria present in the collected urine of infected mice were assayed to determine the orientation of the type 1 fimbria invertible elements. (A) Urine samples taken from mice infected with cystitis isolate F11. Significant differences were observed between the median values at the 4- and 24-h time points (P < 0.0001). No significant differences were observed between the median values at 24, 48, 72, and 96 h. Symbols: ⧫, one unique mouse urine sample; —, median values; N, number of samples collected at each time point. (B) Urine samples taken from mice infected with pyelonephritis isolate CFT073. Significant differences were observed between the median values at the 4- and 24-h time points (P < 0.007). The median values for CFT073 samples differ significantly from the median values for F11 samples at the 24-, 48-, 72-, and 96-h time points.
Invertible-element orientation in pyelonephritis isolate CFT073 during experimental UTI.
The previous set of experiments was repeated using the pyelonephritis isolate CFT073. The CFT073 inoculum was prepared in the identical manner and contained bacteria with the invertible elements exclusively in the OFF orientation (data not shown). Mice were transurethrally inoculated, and urine samples were collected at the same time points and processed and assayed in an identical manner. A strikingly different pattern was observed for the percent ON orientation of the invertible elements for pyelonephritis isolate CFT073 with respect to F11 (Fig. 4B). Initially (at 4 h), the pyelonephritis isolate CFT073 behaved in a manner similar to the cystitis isolate F11. The median percentages of invertible elements in the ON orientation for F11 and CFT073 at the 4-h time point were 2 and 9.3%, respectively (not significantly different [P = 0.44]). However, after the early period of the infection, the median values for the percent ON orientation of invertible elements of the cystitis strain and pyelonephritis strain diverged significantly. At 24 h, the median value for strain CFT073 increased to only 33.6%, which differed significantly from the median value for strain F11 of 84.5% at the same time point (P < 0.0001). At 48 and 72 h, pyelonephritis isolate CFT073 also began a downward trend in percent ON invertible-element orientation, decreasing to median values of just 2% ON in both cases. At 96 h, the pyelonephritis isolate again differed significantly from the cystitis isolate in that it did not reverse the downward trend, since the median percent ON values for CFT073 remained at ≤2% (i.e., the invertible element was almost exclusively in the OFF orientation) at this time point.
To confirm that the differences observed between strains F11 and CFT073 were accurate representations of the invertible-element orientation at the time of sampling and not the product of preferential switching during sample processing, the following experiment was performed. Identical samples of CFT073 and F11 were processed immediately or allowed to sit on ice for either 5 or 30 min. After all of the samples were processed, the percentages of the invertible elements in the ON orientation for each strain at the various time points were compared. Samples of strain F11 held on ice for 0, 5, and 30 min were found to have 20, 22, and 18% of their invertible elements in the ON orientation, respectively. For strain CFT073 the same time intervals resulted in 43, 54, and 53% of the invertible elements in the ON orientation. The lack of significant variation in the orientation of the invertible element of samples held for different intervals on ice demonstrated that there was no appreciable switching of the invertible element after collection but before completion of the assay.
Invertible-element orientations of three cystitis and pyelonephritis isolates under in vivo conditions.
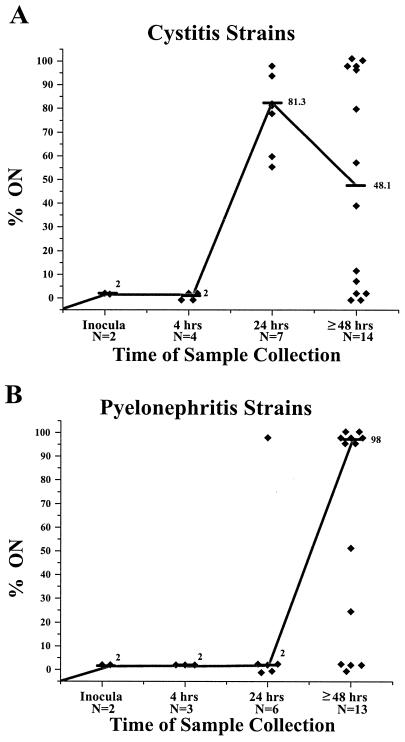
The significant variation between the representative cystitis and pyelonephritis isolates prompted a comparison of additional cystitis and pyelonephritis isolates. Three additional cystitis isolates (F3, F38, and F63) and three additional pyelonephritis isolates (CFT204, CFT325, and CPZ005) were selected based on their ability to reliably infect CBA mice (17). The isolates were tested in the same manner as strains F11 and CFT073 (Fig. 5).
FIG. 5.
Percentage of invertible elements in the ON orientation of three cystitis or three pyelonephritis isolates present in the urine of infected mice. Bacteria present in the collected urine of infected mice were assayed to determine the orientation of the type 1 fimbria invertible elements. (A) Urine samples from three additional cystitis isolates (F3, F38, and F63). Significant differences were observed between the median values at the 4- and 24-h time points for these strains (P < 0.05). However, the median values for the three isolates do not differ significantly from the median values determined for strain F11 at the 4- and 24-h time points (Fig. 4A). Symbols: ⧫, one unique mouse urine sample; —, median values; N, number of samples collected at each time point. (B) Urine samples from three additional pyelonephritis isolates (CFT204, CFT325, and CPZ005). The mean values for the three pyelonephritis isolates do not differ significantly from the mean values determined for strain CFT073 at the 4- and 24-h time points (Fig. 4B). The median value for the three cystitis isolates differ significantly from the median value for the three pyelonephritis isolates at the 24-h time point (P = 0.035) but not at the ≥48-h time point (P = 0.5).
The invertible elements of the cystitis strains used to infect the mice started almost exclusively in the OFF orientation (≤2%) (Fig. 5A). However by 24 h, the cystitis isolate in each urine sample switched the majority of the invertible elements into the ON orientation. A peak median value of 81.3% ON was reached at 24 h. This was similar to cystitis strain F11 (Fig. 4A). After a peak mean value at the 24 h, the median values for the three cystitis isolates began to decrease, reaching a median value of only 48.1% ON for all samples taken after the 48-h time point.
The pyelonephritis isolates also began with their invertible elements almost exclusively in the OFF orientation (≤2%) (Fig. 5B). At 24 h, the E. coli invertible elements in the samples assayed remained predominantly in the OFF orientation, with a median of only 2% ON. In samples taken at 48 h or later, the pyelonephritis isolates switched a sizable proportion of their invertible elements into the ON orientation, with a median value of 98%. The median percent ON values for the three new pyelonephritis isolates differed from the pattern observed for the original pyelonephritis strain CFT073 at this time point (Fig. 4B).
DISCUSSION
Type 1 fimbriae have been proven to be a virulence factor of uropathogenic E. coli. It may not only be the adhesive properties of the surface organelle alone, however, that dictate virulence. Rather, the control mechanism of type 1 fimbrial expression, the invertible element, may function as a virulence factor in its own right, a virulence factor that, depending on its orientation during the course of an infection, may preferentially localize the organisms to the bladder or kidneys, causing cystitis or pyelonephritis, respectively. This would suggest that it possible to divide uropathogenic E. coli strains into cystitis strains and pyelonephritis strains, based not only on established genotypic differences but also on their ability to switch the expression of type 1 fimbriae ON or OFF at specific times during the establishment of infection. Thus, interactions between cystitis-specific uropathogens and the host would lead more readily to cystitis, while similar interactions between pyelonephritis-specific uropathogens and the host would lead more readily to acute pyelonephritis.
Cystitis accounts for the vast majority of UTIs. Pyelonephritis occurs less frequently and is considered a more serious type of infection, involving the kidneys; 30% of such cases lead to bacteremia (16). E. coli strains are capable of causing either of these infections; however, it is not known why cystitis develops in one individual and acute pyelonephritis develops in another. Are there specific genotypic or phenotypic features of the infecting bacterium, or do host factors dictate the outcome of cystitis or pyelonephritis? What traits of an E. coli strain makes it a cystitis-causing strain as opposed to a pyelonephritis-causing strain?
Johnson et al. (17) investigated the first of these two questions. In their study, three cystitis isolates and three pyelonephritis isolates were compared in the mouse model of ascending UTI (12). They observed that on average, the cystitis isolates infected the mouse bladders in 100- to 10,000-fold-larger numbers than did the pyelonephritis isolates (17). However, no consistently significant differences were observed in the kidneys of the mice between the two types of strains (i.e., cystitis and pyelonephritis isolates). Upon histological examination of infected bladders, greater morphological changes were observed in bladder tissue infected with cystitis isolates than in bladder tissue infected with pyelonephritis isolates.
In previous studies from our laboratory, possible genotypic differences between pyelonephritis and cystitis isolates were sought by determining the presence or absence of pathogenicity island (PAI)-associated genes in these strains (11, 18). Using 11 different DNA probes, specific to a PAI that had been sequenced in pyelonephritis strain CFT073, we probed a collection of E. coli isolates from patients with pyelonephritis or cystitis or from fecal samples of healthy individuals. The PAI-specific probes hybridized significantly more often to the genomic DNA of the cystitis and pyelonephritis isolates than of the fecal isolates. However, no significant differences were observed between the binding of the 11 separate probes to cystitis isolates and to pyelonephritis isolates. Although the probes represent a small percentage of the total PAI sequences, the results suggested that simple genotypic differences alone may not be sufficient to differentiate cystitis and pyelonephritis isolates.
We have also previously described differences in the ability of cystitis and pyelonephritis isolates to switch their invertible elements into the ON orientation during growth in vitro (27). These observations, in conjunction with those made by Johnson et al. (17), led us to hypothesize that the expression of type 1 fimbriae, controlled by the invertible element, may be responsible for the differences observed between the cystitis and pyelonephritis strains during the experimental mouse infections. To test this hypothesis, we compared, in vivo, the orientations of the invertible elements of E. coli cystitis strain F11 and pyelonephritis strain CFT073 during the course of an infection of the mouse urinary tract. Significant differences between the bacterial strains in their invertible-element orientation, and therefore their type 1 fimbria expression, were observed during the course of infection (Fig. 4). These differences were pronounced 24 hs after infection. At this point, bacteria in urine samples from the F11 (cystitis strain)-infected animals had the majority of their invertible elements in the ON orientation (median value, 85%). At this same time, urine samples from the CFT073 (pyelonephritis strain)-infected animals had significantly fewer of their invertible elements in the ON orientation (median value, 34%). Furthermore, at 48 h and beyond, samples from CFT073-infected mice had less and less of their invertible elements in the ON orientation, until by 96 h the strain had its invertible elements almost exclusively in the OFF orientation. In contrast, when urine samples from F11-infected mice were examined at 48 hs and beyond, a majority of their invertible elements were found in the ON orientation, as far out as 96 h (median value, 98%). These differences suggested that different strategies were employed by the two bacterial strain types for type 1 fimbria usage during UTIs. For cystitis strain F11, the large majority of bacteria were capable of producing type 1 fimbriae 24 h after inoculation with a bacterial population in which the invertible elements are >98% in the OFF position. At this time, shedding of bladder uroepithelial cells into the urine was evident (Fig. 2). The cystitis strain F11 also maintained the ON orientation for type 1 fimbria expression during the entire course of infection, which may serve to localize a greater number of the infecting bacteria to the bladder. In contrast, at 24 h, a smaller percentage of E. coli CFT073 cells are capable of expressing type 1 fimbriae, which may account for the lower bacterial counts observed in the bladder. Furthermore, E. coli CFT073 turns OFF expression of the type 1 fimbriae later in the course of the infection, perhaps allowing the bacteria to both ascend to the kidneys and avoid a host immune response mounted against the surface adhesion.
When three additional cystitis isolates were assayed in the same manner as F11 and CFT073, it became apparent that, in general, during an infection cystitis isolates have a large majority of the invertible elements in the ON orientation at 24 h. In contrast when three additional pyelonephritis isolates were tested in the mouse model, these isolates maintained their invertible elements primarily in the OFF orientation at 24 h (median ON value, 2%). Therefore, it appears as though the invertible-element orientation of type 1 fimbriae at 24 h postinfection is a point of differentiation between cystitis and pyelonephritis isolates.
It is important to note that one of the three additional pyelonephritis isolates assayed is not a confirmed invasive strain. Strain CFT204 was isolated from the urine of a patient diagnosed with pyelonephritis, unlike strains CPZ005, CFT325, and CFT073, which were isolated from the blood of patients diagnosed with pyelonephritis that had progressed to bacteremia (Table 1). The difference in the site of collection for CFT204 calls into question whether this was in fact an invasive strain. Indeed, if we look only at data for strains of proven invasiveness (isolated from the blood), only 2 of the 19 samples from mice infected with these strains had ON values above 2% (one at 48 h and one at 96 h), and both of these came from the same mouse. Indeed, it is strain CFT204 that accounts for a majority of the pyelonephritis samples with a large percentage of invertible elements in the ON orientation at the later time points.
We have presented data that suggest differences in the magnitude and timing of the control of type 1 fimbria expression by cystitis and pyelonephritis isolates during UTIs. However, the bacterial genotype and mechanism responsible for these observed differences remain the subjects of investigation. Besides the FimB and FimE recombinases, other accessory proteins are essential for normal invertible-element inversion, namely, integration host factor (IHF) (4, 6), leucine-responsive regulatory protein (LRP) (3, 9), and the histone-like protein (H-NS) (19, 30). FimE appears responsible for switching of the invertible element exclusively from the ON to the OFF orientation, while FimB mediates bidirectional switching (ON to OFF and OFF to ON) (8, 22, 28). The action of FimB is detrimentally affected by the excision of a PAI (Pai II) in a uropathogenic strain of E. coli (31). The excision of Pai II results in the truncation of the leuX tRNA gene that is necessary for expression of the FimB recombinase. It should also be noted, however, that in certain E. coli strains, switching to the ON orientation as well as expression of type 1 fimbriae can occur in the absence of a functional FimB recombinase (37).
gene that is necessary for expression of the FimB recombinase. It should also be noted, however, that in certain E. coli strains, switching to the ON orientation as well as expression of type 1 fimbriae can occur in the absence of a functional FimB recombinase (37).
Another possible mechanism for the differences observed between the cystitis and pyelonephritis strains in invertible-element switching can be found in the differences observed in the sequence in and around the invertible element of several E. coli strains (26). The sequence changes were observed in areas where the FimB, FimE, and other accessory proteins (IHF, LRP, and H-NS) are believed to bind. Certain sequence changes in and around the switch region that lead to increased switching to the ON or OFF orientation could explain the differences in the switching we observed in the cystitis and pyelonephritis isolates during infections. Leathart and Gally suggested that certain sequence changes may select uropathogens to become pyelonephritis-causing strains and that other changes would favor a cystitis-specific strains (26).
A recent study has demonstrated regulatory cross talk between the control of type 1 fimbriae expression and the expression of P fimbriae (40). The investigation showed that expression of a gene in the pap operon, PapB, could affect the recombinases FimB and FimE in ways that led to the type 1 fimbria invertible-element switching to the OFF orientation, blocking fimbria expression. This regulation within a pyelonephritis strain such as CFT073 could allow initial colonization of the bladder by type 1 fimbriae at 24 h. After colonization of the bladder, P fimbria expression could increase and allow bacteria to ascend the ureters and colonize the kidneys. This could lead to down regulation of type 1 fimbria expression, allowing bacteria to avoid being segregated to the bladder, as may be the case with cystitis isolates. The current research presents many interesting avenues of investigation that may eventually explain the novel phenotypic differences that we have reported between cystitis and pyelonephritis strains in their usage of type 1 fimbriae during infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI43363 from the National Institutes of Health.
We also thank Maritza Patton, Perry Comegys, and Seung H. Chang, all members of University of Maryland, Baltimore, Pathology Department's Electron Microscopy laboratory, for their help in our EM work, and we thank Richard Hebel for assistance with statistical analysis. Finally, we thank the members of the Mobley laboratory for critical evaluation of the manuscript.
REFERENCES
- 1.Abraham J M, Freitag C S, Clements J R, Eisenstein B I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur M, Johnson C E, Rubin R H, Arbeit R D, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989;57:303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomfield I C, Calie P J, Eberhardt K J, McClain M S, Eisenstein B I. Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J Bacteriol. 1993;175:27–36. doi: 10.1128/jb.175.1.27-36.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomfield I C, Kulasekara D H, Eisenstein B I. Integration host factor stimulates both. Mol Microbiol. 1997;23:705–717. doi: 10.1046/j.1365-2958.1997.2241615.x. [DOI] [PubMed] [Google Scholar]
- 5.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenstein B I, Sweet D S, Vaughn V, Friedman D I. Integration host factor is required for the DNA inversion that controls phase variation in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:6506–6510. doi: 10.1073/pnas.84.18.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gally D L, Bogan J A, Eisenstein B I, Blomfield I C. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol. 1993;175:6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gally D L, Leathart J, Blomfield I C. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol Microbiol. 1996;21:725–738. doi: 10.1046/j.1365-2958.1996.311388.x. [DOI] [PubMed] [Google Scholar]
- 9.Gally D L, Rucker T J, Blomfield I C. The leucine-responsive regulatory protein binds to the fim switch to control phase variation of type 1 fimbrial expression in Escherichia coli K-12. J Bacteriol. 1994;176:5665–5672. doi: 10.1128/jb.176.18.5665-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goluszko P, Moseley S L, Truong L D, Kaul A, Williford J R, Selvarangan R, Nowicki S, Nowicki B. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Investig. 1997;99:1662–1672. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyer D M, Kao J S, Mobley H L. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect Immun. 1998;66:4411–4417. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg E C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley R W, Culver D H, White J W, Morgan W M, Emori T G. The nationwide nosocomial infection rate. A new need for vital statistics. Am J Epidemiol. 1985;121:159–167. doi: 10.1093/oxfordjournals.aje.a113988. [DOI] [PubMed] [Google Scholar]
- 14.Hultgren S J, Porter T N, Schaeffer A J, Duncan J L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985;50:370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikaheimo R, Siitonen A, Karkkainen U, Kuosmanen P, Makela P H. Characteristics of Escherichia coli in acute community-acquired cystitis of adult women. Scand J Infect Dis. 1993;25:705–712. doi: 10.3109/00365549309008567. [DOI] [PubMed] [Google Scholar]
- 16.Ikaheimo R, Siitonen A, Karkkainen U, Mustonen J, Heiskanen T, Makela P H. Community-acquired pyelonephritis in adults: characteristics of E. coli isolates in bacteremic and non-bacteremic patients. Scand J Infect Dis. 1994;26:289–296. doi: 10.3109/00365549409011797. [DOI] [PubMed] [Google Scholar]
- 17.Johnson D E, Lockatell C V, Russell R G, Hebel J R, Island M D, Stapleton A, Stamm W E, Warren J W. Comparison of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect Immun. 1998;66:3059–3065. doi: 10.1128/iai.66.7.3059-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao J S, Stucker D M, Warren J W, Mobley H L. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawula T H, Orndorff P E. Rapid site-specific DNA inversion in Escherichia coli mutants lacking the histonelike protein H-NS. J Bacteriol. 1991;173:4116–4123. doi: 10.1128/jb.173.13.4116-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keith B R, Maurer L, Spears P A, Orndorff P E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986;53:693–696. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisielius P V, Schwan W R, Amundsen S K, Duncan J L, Schaeffer A J. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect Immun. 1989;57:1656–1662. doi: 10.1128/iai.57.6.1656-1662.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986;5:1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krogfelt K A, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990;58:1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunin C M. Urinary tract infections in females. Clin Infect Dis. 1994;18:1–10. doi: 10.1093/clinids/18.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 26.Leathart J B, Gally D L. Regulation of type 1 fimbrial expression in uropathogenic Escherichia coli: heterogeneity of expression through sequence changes in the fim switch region. Mol Microbiol. 1998;28:371–381. doi: 10.1046/j.1365-2958.1998.00802.x. [DOI] [PubMed] [Google Scholar]
- 27.Lim J K, Gunther N W, Zhao H, Johnson D E, Keay S K, Mobley H L. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun. 1998;66:3303–3310. doi: 10.1128/iai.66.7.3303-3310.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain M S, Blomfield I C, Eisenstein B I. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991;173:5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 30.Olsen P B, Klemm P. Localization of promoters in the fim gene cluster and the effect of H- NS on the transcription of fimB and fimE. FEMS Microbiol Lett. 1994;116:95–100. doi: 10.1111/j.1574-6968.1994.tb06681.x. [DOI] [PubMed] [Google Scholar]
- 31.Ritter A, Gally D L, Olsen P B, Dobrindt U, Friedrich A, Klemm P, Hacker J. The Pai-associated leuX specific tRNA5(Leu) affects type 1 fimbriation in pathogenic Escherichia coli by control of FimB recombinase expression. Mol Microbiol. 1997;25:871–882. doi: 10.1111/j.1365-2958.1997.mmi517.x. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg T, Kaijser B, Lidin-Janson G, Lincoln K, Orskov F, Orskov I, Stokland E, Svanborg-Eden C. Virulence of Escherichia coli in relation to host factors in women with symptomatic urinary tract infection. J Clin Microbiol. 1988;26:1471–1476. doi: 10.1128/jcm.26.8.1471-1476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaeffer A J, Schwan W R, Hultgren S J, Duncan J L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokurenko E V, Chesnokova V, Dykhuizen D E, Ofek I, Wu X R, Krogfelt K A, Struve C, Schembri M A, Hasty D L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci USA. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soto G E, Hultgren S J. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999;181:1059–1071. doi: 10.1128/jb.181.4.1059-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stapleton A, Moseley S, Stamm W E. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163:773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- 37.Stentebjerg-Olesen B, Chakraborty T, Klemm P. Type 1 fimbriation and phase switching in a natural Escherichia coli fimB null strain, Nissle 1917. J Bacteriol. 1999;181:7470–7478. doi: 10.1128/jb.181.24.7470-7478.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren J W. Clinical presentation and epidemiology of urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections—molecular pathogenesis and clinical management. Washington, D.C.: ASM Press; 1996. pp. 3–28. [Google Scholar]
- 39.Warren J W, Miller E H, Jr, Fitzpatrick B, DiFranco D E, Caplan E S, Tenney J H, Anthony W C. A randomized, controlled trial of cefoperazone vs. cefamandole-tobramycin in the treatment of putative, severe infections with gram-negative bacilli. Rev Infect Dis. 1983;5(Suppl. 1):S173–S180. doi: 10.1093/clinids/5.supplement_1.s173. [DOI] [PubMed] [Google Scholar]
- 40.Xia Y, Gally D, Forsman-Semb K, Uhlin B E. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 2000;19:1450–1457. doi: 10.1093/emboj/19.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]