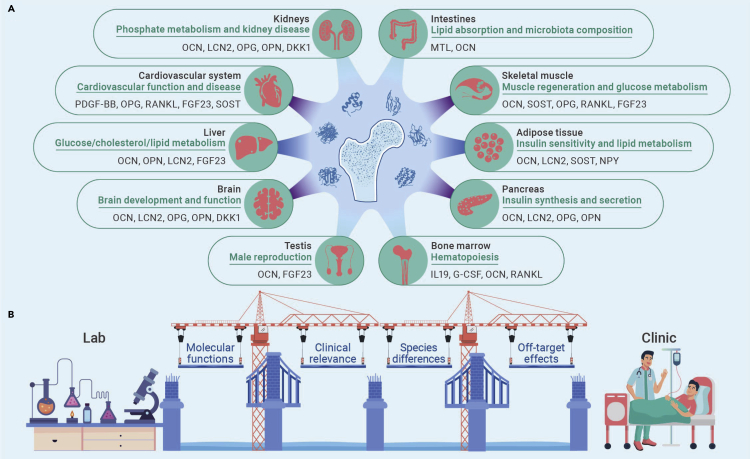
Osteokines in inter-organ communications
The concept of “organic wholeness” permeates all the fields of traditional Chinese medicine, which is also widely accepted by modern medicine. The wealth of knowledge regarding inter-organ communications generated in the latest decades provided solid evidence that human physiology and pathophysiology involve systematic interactions between multiple organs or tissues. Therefore, advancing our understanding of the inter-organ communication process is believed to provide a new perspective for treating various human diseases.
Bone is a vertebrate-specific tissue that supports the body, stores minerals, and protects internal organs in classical views. Interestingly, the diverse bone-derived cytokines identified in the recent decade have shown an “endocrine” role of bone that communicates closely with various system organs or tissues (Figure 1). Bone-derived cytokines, or “osteokines,” are bioactive peptides or proteins secreted by bone cells that exert autocrine or paracrine effects locally and through the endocrine system to regulate the functions of distal organs or tissues. These osteokine-mediated inter-organ communications are vital for maintaining proper physiologic homeostasis as well as being key regulators of disease pathologies (Figure 1). Moreover, clinical research indicates that targeting osteokines is a promising therapeutic strategy for disease treatment. For example, the FDA has approved burosumab (Crysvita), a neutralizing antibody against fibroblast growth factor 23, to treat X-linked hypophosphatemia. Furthermore, some recent exciting studies have shown that more osteokines are on their way to clinical applications.
Figure 1.
Opportunities and challenges in translating osteokines from lab to clinic
(A) Osteokines mediated inter-organ communications in health and disease. The green words above the line denote the biological effects of osteokines on their target organs or tissues. The black words below the line indicate the osteokines that acted on specific organs or tissues.
(B) Major challenges faced by osteokines from lab to clinic. DKK1, Dickkopf 1; FGF23, fibroblast growth factor 23; G-CSF, granulocyte colony-stimulating factor; IL-19, interleukin-19; LCN2, lipocalin 2; MTL, metabolitin; NPY, neuropeptide Y; OCN, osteocalcin; OPN, osteopontin; OPG, osteoprotegerin; OSTN, osteocrin; PDGF–BB, platelet-derived growth factor–BB; RANKL, receptor activator of nuclear factor κB ligand; SOST, sclerostin.
Osteocalcin (OCN), secreted by osteoblasts, is suggested to regulate the biological processes of multiple organs. OCN can cross the blood–brain barrier and bind to GPR158 receptors located in pyramidal neurons in the hippocampus, regulating individuals’ spatial learning and memory. OCN can also act on GPR37 receptors in glial cells, regulating oligodendrocytes’ differentiation and myelin homeostasis. Moreover, maternal OCN can cross the placenta during pregnancy and regulate brain development and the acquisition of cognitive function in the offspring. In a recent study published in Microbiome, Liu and colleagues established an interesting regulation circuit of bone–gut microbiota–brain.1 In this work, they find that the administration of recombined OCN prevented dopaminergic neuronal loss and ameliorated motor deficits in Parkinson disease mice models. More interestingly, they revealed that OCN-induced gut microbiota alterations and the increased levels of gut microbiota-derived metabolite propionate were essential mechanisms responsible for the neuroprotective effects of OCN on Parkinson disease. These exciting studies strongly suggest that OCN and its receptors may be potential targets for treating central nervous system diseases.
Platelet-derived growth factor–BB (PDGF–BB), an osteokines secreted by preosteoclasts, is a crucial mediator of bone type H vessels and bone formation. In a recent study published in The Journal of Clinical Investigation, Wan and colleagues investigated the role of the bone-derived cytokine PDGF–BB in vascular stiffening.2 They demonstrate that aging or a high-fat diet results in significantly elevated serum levels of PDGF-BB. Mice models with conditional PDGF–BB transgenic or knockout indicate that preosteoclasts-derived PDGF–BB is an essential mediator of vascular stiffening in response to aging and metabolic stress. Notably, although PDGF–BB is vital for maintaining bone homeostasis in young mice, aberrantly elevated PDGF–BB harms osteoblastic bone formation and leads to bone loss in the elderly. Therefore, these findings suggest that neutralizing PDGF–BB may benefit patients with cardiovascular diseases and osteoporosis in the elderly.
In addition to these well-characterized osteokines, the newly identified osteokines are also worth looking forward to. In a recent study published in Blood, Bai and colleagues identified that interleukin 19 (IL-19) was a new osteokine secreted by osteocytes. They demonstrate that osteocyte-derived IL-19 was a potent regulator of granulopoiesis and neutrophil formation by stimulating IL-20Rβ/Stat3 signaling in neutrophil progenitors.3 More importantly, their work showed that IL-19 is a promising cytokine for chemotherapy or irradiation-induced neutropenia, with therapeutic efficiency better than the clinically used drug granulocyte colony-stimulating factor administrated in a significantly lower dose.
Undoubtedly, more osteokines with interesting inter-organ communications stories and bright clinical transformation prospects can be discussed here. Notwithstanding, we should accept that the successful translational application of osteokines is still scarce. Therefore, we want to discuss the challenges faced by osteokines from bench to bedside in the following part.
Challenges of osteokine clinical transformation
Firstly, the molecular functions and regulatory mechanisms of osteokines need in-depth exploration. Currently, only a few osteokines have well-defined biological functions and regulatory mechanisms. OCN is the most-studied molecule among these osteokines, but its physical role is still debated. For example, two recent studies found that deleting OCN did not result in abnormal glucose metabolism or testosterone synthesis in mice. In addition, an OCN-deficient rat model even displays improved insulin sensitivity and glucose tolerance. The phenotype of these newly generated animal models was controversial compared with the original reports, which showed significant abnormalities in glucose metabolism and male fertility in OCN-deficient mice. Considering the different methodologies applied in these studies (gene-editing strategy, strain, age, sex, back-crossing times, detection indicators, and animal number) may affect the phenotype, a more thorough analysis of these models is required to make a more precise conclusion in the future. On the other hand, our understanding of the upstream regulatory mechanism controlling osteokine production, secretion and modification is still limited. Revealing the upstream regulation mechanism may provide a new perspective and intervention strategy to manipulate the expression levels of osteokines. Remarkably, bone is a mechanosensor organ, and exercise has significantly influenced the expression of many osteokines. As a result, a deep investigation of the molecular mechanisms of this aspect will provide us with a roadmap to modulate osteokines by exercise therapy to prevent diseases or delay aging.
Secondly, the species differences and the clinical relevance of osteokines need deep investigation. The existing studies about the biological functions of osteokines are primarily from animal models. We still have a limited understanding of the differences in the osteokines between humans and animals. For instance, the serum concentrations of mouse OCN is five to ten times higher in mice than in humans. Does this mean that the dosage of OCN used in humans does not need to be as high as that of mice or that the biological activity of human OCN is much higher than mice? Recently, an interesting study published in eLife uncovered a novel O-glycosylation modification on a single serine (S8) of mouse OCN, which is essential to increase the stability of OCN in circulation, while the human OCN (hOCN) cannot be O-glycosylated as the corresponding amino acid residue is a tyrosine (Y12).4 Moreover, they demonstrated that a single-point mutation (Y12S) is sufficient to mediate the O-glycosylate and then increase the half-life of hOCN ex vivo. These findings provide an explanation of the differences in circulation levels of OCN between humans and mice. Despite the Y12S mutation on the activity of hOCN needing further exploration, this work provides a strategy to improve the stability of hOCN, which could benefit the clinical transformation of hOCN. Prospective and cross-sectional clinical analysis is an important means to determine the clinical relevance of a factor. Although a large number of studies have analyzed the disease correlation of most osteokines, only a few osteokines have established clear causal associations between human diseases. The role of OCN in human glucose metabolism has been confirmed in most cross-sectional studies. However, due to technical limitations, the existing studies did not accurately measure all forms of hOCN in circulation, which may weaken the findings related to the clinical relevance of hOCN. Genome-wide association study (GWAS) is also a robust approach to establishing disease correlation, but limited GWASs have thus far established a link between osteokines and human disorders. Therefore, extensive prospective studies, more GWASs, and sensitive detection methods are necessary to infer more clinical relevance relationships of osteokines.
Lastly, most osteokines mediate communications with multiple organs and have numerous biological activities, which is not a good property of a drug candidate. Therefore, minimizing the potential off-site effects of osteokines is a crucial question to be resolved. Recently, Ren and colleagues identified a new OCN-derived peptide hormone named metabolitin (MTL), which could bind to the OCN receptor GPRC6A expressed in the intestines and inhibit triglyceride gut absorption.5 In a high-fat-diet-induced non-alcoholic fatty liver disease model, they demonstrated that oral or intraperitoneal MTL treatment significantly inhibits hepatic steatosis and improves systemic insulin resistance of the mice. Although it is unclear whether this MTL can act on the other two OCN receptors expressed in the brain, the bioactivity and biosafety highlight that MTL has therapeutic potential for treating patients with non-alcoholic fatty liver disease and obesity. Artificial intelligence has shown great potential in protein structure prediction and oligopeptide drug development. It may be an excellent way to optimize osteokine sequences with an artificial-intelligence-assisted algorithm to achieve better targeting and activity.
Concluding remarks
The knowledge of osteokines has revolutionized the fields of endocrinology and bone biology and provided promising therapeutic targets to treat various disorders. However, some unresolved issues, as outlined above, are still challenging and should be addressed before osteokine-based therapeutics can move to clinical. The emergence of new technologies, such as organoids, multi-organ chips, big data, and artificial intelligence, will provide powerful research tools to overcome these challenges and significantly accelerate the clinical transformation process of osteokines. Undoubtedly, studies on osteokines are an evolving story with a bright prospect in diagnosing and treating clinical diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 92049201).
Declaration of interests
The authors declare no competing interests.
Published Online: December 20, 2022
Contributor Information
Dake Zhang, Email: dakezhang@buaa.edu.cn.
Xiaogang Wang, Email: xiaogangwang@buaa.edu.cn.
References
- 1.Hou Y.F., Shan C., Zhuang S.Y., et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease. Microbiome. 2021;9:34. doi: 10.1186/s40168-020-00988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santhanam L., Liu G., Jandu S., et al. Skeleton-secreted PDGF-BB mediates arterial stiffening. J. Clin. Invest. 2021;131:e147116. doi: 10.1172/JCI147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao M., Zhang W., Liu W., et al. Osteocytes regulate neutrophil development through IL-19: a potent cytokine for neutropenia treatment. Blood. 2021;137:3533–3547. doi: 10.1182/blood.2020007731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Rifai O., Julien C., Lacombe J., et al. The half-life of the bone-derived hormone osteocalcin is regulated through O-glycoslation in mice, but not in humans. Elife. 2020;9:e61174. doi: 10.7554/eLife.61174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng B., Huang C., Cheng C.L., et al. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor GPRC6A. J. Hepatol. 2020;73:383–393. doi: 10.1016/j.jhep.2020.02.026. [DOI] [PubMed] [Google Scholar]