Abstract
Central nervous system (CNS) tumors are the most common solid malignancy in the pediatric population. Based on adoptive cellular therapy's clinical success against childhood leukemia and the preclinical efficacy against pediatric CNS tumors, chimeric antigen receptor (CAR) T cells offer hope of improving outcomes for recurrent tumors and universally fatal diseases such as diffuse intrinsic pontine glioma (DIPG). However, a major obstacle for tumors of the brain and spine is ineffective T cell chemotaxis to disease sites. Locoregional CAR T cell delivery via infusion through an intracranial catheter is currently under study in multiple early phase clinical trials. Here, we describe the Seattle Children's single-institution experience including the multidisciplinary process for the preparation of successful, repetitive intracranial T cell infusion for children and the catheter-related safety of our 307 intracranial CAR T cell doses.
Keywords: Chimeric antigen receptor (CAR) T cells, Central nervous system (CNS) tumor, Ommaya, CNS catheter, Ventriculoperitoneal (VP) shunt, HER2, EGFR, B7-H3
Introduction
Chimeric antigen receptor (CAR) T cells have induced dramatic responses in pediatric patients with hematologic malignancies [1], yet there are numerous obstacles to translating this success to children with central nervous system (CNS) tumors [2]. A fundamental concern is that systemic delivery limits on-tumor on-target efficiency due to the blood-brain barrier and may require higher dosing to achieve adequate T cell migration into the CNS [3]. However, with higher systemic doses, there is increased risk of systemic and CNS toxicities such as cytokine release syndrome (CRS) [4] or immune effector cell-associated neurotoxicity syndrome (ICANS) [5]. Ultimately, systemically delivered adoptive cellular therapy is a challenging way to target CNS tumors in children, particularly as the baseline deficits in pediatric neuro-oncology patients may predispose them to neurologic risks.
Locoregional delivery of chemotherapeutic agents has long been an attempted solution for bypassing the blood-brain barrier and effectively treating CNS tumors [6,7]. Surgically implanted Ommaya or Rickham reservoir catheters that can be accessed with a small needle for repeated drug delivery have proven an effective and safe approach, even for long-term use [8,9]. Complications of repeated drug delivery utilizing intraventricular shunt systems include infectious and non-infectious complications. The risk of infection from long-term Ommaya use has been shown to be comparable to or less than that of indwelling peripherally-inserted central catheters [10]. The risk of non-infectious complications, such as catheter obstruction/malposition, cerebrospinal fluid (CSF) leaks, and hemorrhage, were shown to be less than 12% [7]. Ventriculoperitoneal (VP) shunts with “on-off” valves also have been shown to be an efficient system to deliver effective intraventricular therapies against many cancers, including leptomeningeal carcinomatosis [11,12] and recurrent CNS lymphoma [13]. Most recently, locoregional delivery of CAR T cells was also demonstrated to be tolerable in adults with CNS tumors [14].
Based on this preliminary adult experience, the historical success of delivering therapeutics intracranially, and the desire to limit systemic toxicity, Seattle Children's Hospital opened three CNS CAR T cell trials (BrainChild-01 targeting HER2 [15] (NCT03500991), BrainChild-02 targeting EGFR [16] (NCT03638167), and BrainChild-03 targeting B7-H3 [17] (NCT04185038)) that exclusively deploy repeated, locoregional delivery. Across our three phase 1 trials, we have now delivered 307 intracranial CAR T cell doses to 41 children and young adults. Here, we describe the clinical preparation for enrolling patients, the procedure for delivering locoregional CAR T cells to children in the clinic, and the preliminary feasibility and safety of this approach.
Results
Clinical preparation prior to intracranial CAR T cell delivery
Prior to a patient's enrollment onto Seattle Children's BrainChild-01, -02, or -03 (NCT03500991, NCT03638167, and NCT04185038, respectively), the clinical coordinating nurse identifies the patient's existing intracranial hardware (e.g. Ommaya reservoir or ventriculoperitoneal (VP) shunt). Patients with a VP shunt are evaluated in neurosurgery clinic prior to the clinical trial consent conference in order to establish shunt care and to confirm appropriate shunt function. In preparation for this neurosurgical visit, patients undergo a shunt series that allows the neurosurgical provider to assess the current hardware system. The preferred shunt system features a programmable Certas valve, as it allows for a “virtual off” setting. Patients with VP shunts also undergo a computerized tomography (CT) of the brain obtained to assess the size of the lateral ventricles to ensure that safe cerebrospinal fluid (CSF) aspiration from their hardware is possible. Specifically, imaging is obtained to eliminate any concern that CSF aspiration could collapse the ventricle or compromise the mechanical operation of the shunt. Patients with other infusible hardware devices, such as an Ommaya catheter, do not require a pre-enrollment visit with the neurosurgical team and catheter location is confirmed by the immunotherapy clinical coordination nurse prior to enrollment. Following the enrollment visit (which includes a consent conference, apheresis catheter placement under sedation by general surgery, apheresis by a dedicated apheresis team in oncology clinic, and apheresis catheter removal without sedation – all performed outpatient over 3 days), patients return home to await CAR T cell production and release testing (which takes ∼24 days), and then return to Seattle Children's to begin treatment.
After confirming initial infusion eligibility, including adequate protocol-directed laboratory and clinical evaluations, the research nurse notifies the investigational drug service (IDS) pharmacy to thaw the cryopreserved vial containing the scheduled CAR T cell dose. The CAR T dose is verified and signed by two oncology providers for concordance between treatment roadmap and electronic medical record. For patients with a programmable shunt, the infusion nurse simultaneously notifies a neurosurgery team member. In situations where it is deemed clinically safe, which is the norm, a neurosurgical provider will increase the shunt setting to the highest resistance just prior to CAR T cell infusion. This setting change limits CAR T cell egression into the peritoneal (or other end-catheter) space and promotes CAR T cell flow into the CSF space. Specifically, for the programmable Certas valve, the “virtual off” setting of 8 is utilized. Patients’ shunts are kept at the higher resistance setting for 3 h while being observed in the clinic following infusion. If clinical signs of increased intracranial pressure develop, the duration of increased resistance may be shortened and personalized for each patient based on neurosurgical guidance.
Protocol for outpatient locoregional CAR T cell delivery to children
Prior to aspirating CSF, the size of the patient's ventricles is reviewed on prior imaging. This allows the provider to determine whether aspiration or CSF free flow to gravity is more appropriate. Approximately 45–60 min prior to the procedure, topical anesthetic is applied over the intracranial catheter site. Approximately 30 min prior to the procedure, the patient is given pre-medications (i.e., acetaminophen, diphenhydramine, and ondansetron) as per protocol.
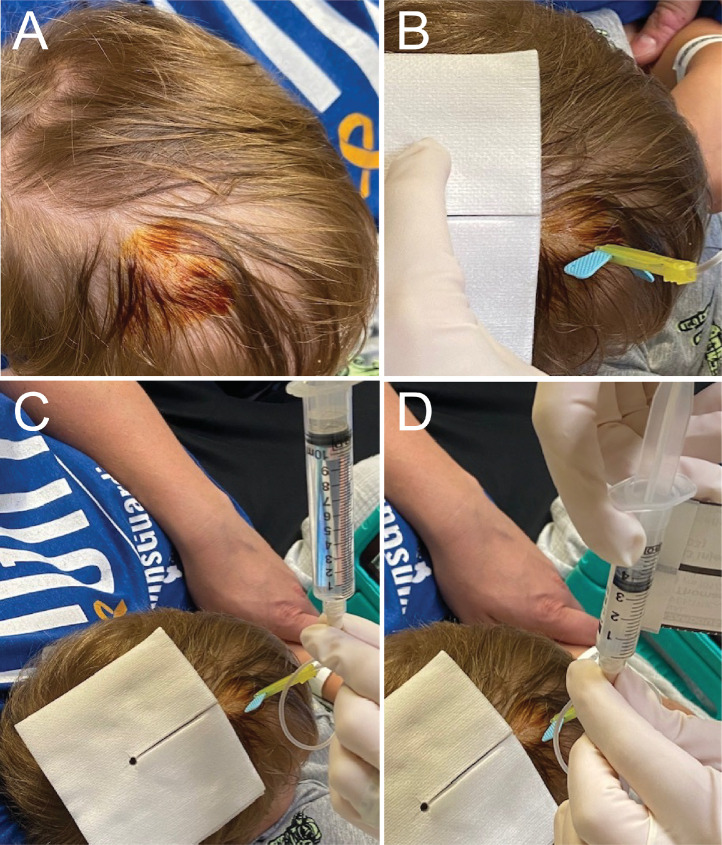
At the time of procedure, a time-out is called to review standard patient, procedure, and procedure location details. Distraction and anxiety relief techniques are provided by parents or Child Life Specialists, and the patient is placed in a position that allows access to the external reservoir of the intracranial catheter. The topical anesthetic is removed, and, if needed, the hair overlying the catheter site is trimmed using scissors. The site is cleaned with povidone-iodine solution (Fig. 1A), allowed to dry, and then draped in typical sterile technique. A 25-gauge butterfly needle, that has been primed with preservative-free normal saline (PFNS), is used to access the reservoir (Fig. 1B). Correct placement is verified by injection of 0.5-1 mL PFNS and aspiration of CSF. A sterile syringe for collection of CSF (∼10 mL) is attached; CSF is gently aspirated for clinical and correlative research studies (Fig. 1C) and then removed. The syringe of prepared CAR T cells (<5 mL) is then attached, and the CAR T cells are manually infused slowly over 1 min (Fig. 1D). Following completion of the infusion, the CAR T cell syringe is disconnected and a syringe with PFNS is connected. The volume required to flush the catheter tubing is predetermined in conjunction with neurosurgical guidance, though it is most often 2.5 mL. The PFNS is manually infused slowly over 1 min. The needle is then removed, and gentle pressure is applied by hand with sterile gauze over the procedure site. The area is gently wiped with an alcohol swab to remove the remaining povidone-iodine solution.
Fig. 1.
Intracranial dosing procedure. ∼45 min after a topical anesthetic agent is applied, (A) the Ommaya or shunt reservoir site is sterilized with betadine, (B) a 25-guage butterfly needle is inserted, and 0.5 mL preservative-free normal saline (PFNS) is infused. The sterile syringe of PFNS is removed and (C) a new 10 mL syringe is connected to withdraw CSF for clinical and correlative studies. That syringe is removed, and (D) a syringe with CAR T cells is connected. Finally, that syringe is removed and a syringe with 2.5 mL PFNS is connected and infused, after which the needle, with the syringe connected, is removed.
Following the administration, the child is observed in the outpatient oncology clinic for a minimum of 3 h with heart rate, oxygen saturations, blood pressure, and clinical assessments. Children under the age of 5 years receiving their first infusion are often admitted for observation to the inpatient oncology unit for 18–24 h; however, most children and young adults are discharged home from clinic. CSF obtained prior to CAR T cell infusion and CSF obtained at other study timepoints is evaluated for glucose, protein, and cytology – along with correlative research studies – to evaluate for disease dissemination and early evidence of potential infection.
Preliminary safety of intracranial CAR T cell delivery to children
As of October 2022, 62 children and young adults have been enrolled on BrainChild-01 (9 patients), BrainChild-02 (11 patients), and BrainChild-03 (42 patients). In total, 41 patients have been treated and received 307 intra-Ommaya (266; 87%) or intra-VP shunt (41; 13%) CAR T doses delivered at Seattle Children's Hospital (Table 1). 276 infusions (89.9%) have been given in the outpatient oncology clinic and 31 infusions (10.1%) have been delivered on the inpatient oncology unit. For patients who received their infusion on the inpatient oncology unit, this was because the prescribed dose was due while admitted for other care. Here, we refer exclusively to patients who received at least one CAR T cell infusion. Demographics for all patients are shown in Table 1. These patients’ (N = 41) tumors included diffuse intrinsic pontine glioma (DIPG)/diffuse midline glioma, H3 K27M-altered (DMG) (N = 15, 37%), embryonal tumors (N = 7, 17%), ependymoma (N = 16, 39%), or high-grade glioma (HGG) (N = 3, 7%).
Table 1.
Demographic and clinical characteristics of the patients.
| Characteristic | Value |
|---|---|
| Median age in years (range) | 12 (2-25) |
| Male | 25 (61%) |
| Diagnosis | |
| DIPG/DMG | 15 (37%) |
| Embryonal tumor | 7 (17%) |
| Ependymoma | 16 (39%) |
| HGG | 3 (7%) |
| Device | |
| Ommaya catheter | 35 (85%) |
| Shunt reservoir | 6 (15%) |
| CAR T cell doses | 307 |
| Shunt removals | 0 |
Abbreviations: diffuse intrinsic pontine glioma (DIPG), diffuse midline glioma H3K27M-altered (DMG)
Overall, there have been no serious adverse events (SAE) related to the intracranial catheter. One patient (2%) who had their shunt placed at an outside institution experienced skin breakdown along the shunt tubing of the distal neck. The shunt was replaced at Seattle Children's without complications, and the skin breakdown was considered unrelated to CAR T cell therapy. No CAR T cell doses were canceled due to high pressure resistance or had other mechanical complications during infusion. No patients reported site pain at or near the CNS catheter site following the infusion procedure. No patients developed an allergic reaction at the CNS catheter site or systemically. No patients developed meningitis at any point on protocol therapy. No patients developed hematomas, bleeding, or cellulitis at the site of the CNS catheter site. No patients required removal of their Ommaya or VP shunt reservoirs during the study period.
Discussion
We present our clinical experience of delivering outpatient repeated intracranial CAR T cell doses to children on Seattle Children's phase 1 BrainChild clinical trials with a focus on catheter-related preparation and safety. Specifically, we show the preliminary feasibility of delivering 307 locoregional intracranial infusions to 41 patients across 3 active CNS CAR T cell trials targeting HER2, EGFR, and B7-H3. Delivery through the CNS catheter, whether an Ommaya or shunt reservoir, has been tolerable regardless of the CAR T cell target, which is likely in part to the therapeutic window allotted by our selected targets that are expressed more highly on CNS tumors than normal CNS tissue [15], [16], [17], our manufacturing strategy, and also the streamlined delivery system that we have utilized. Currently, only a select number of pediatric centers have open CAR T cell clinical trials for children with brain and spinal cord tumors. Despite feasibility of intracranial delivery in adult CNS CAR T cell trials [14,18] and preclinical reports of effective and tolerable locoregional delivery to tumor-bearing mice [3,[15], [16], [17],19], there remains a debate in the field regarding the role of systemic versus locoregionally dosed adoptive cellular therapies. Seattle Children's has utilized locoregional CAR T cell delivery on three CAR T cell trials since their activation, and as we continue this therapeutic approach and other programs adopt these systems, it is critical to share early experiences.
While prior reports showed a risk of infectious complications with intra-Ommaya therapy [10,20,21], no intra-Ommaya or CSF infections were identified in the patients treated on our BrainChild CAR T cell trials. There were no patients who required removal of their Ommaya or shunt reservoir and very limited complications related to the use of these devices. In fact, when compared to prior reports, the rate of any complication related to the Ommaya or shunt, was significantly less [7]. Our methods were well tolerated, even in very young children, and easily replicated by dedicated, well-prepared providers, highlighting the feasibility of this therapeutic approach. Furthermore, our program has been able to deliver CAR T cells as an outpatient in 90% of infusions, with inpatient infusions only undertaken when patients are admitted to the inpatient unit for other clinical reasons. Beyond safety, these considerations are critical as we aim to maximize quality of life for affected children and young adults.
In addition to optimization of clinical procedures and cellular products for these patients, the technology through which these are delivered is also evolving. For most patients, an Ommaya was placed prior to enrollment to provide repetitive access to the intrathecal space for administration of CAR T cell infusions in the same way a patient requiring repetitive systemic therapy may require a central line placed prior to therapy. Less frequently, VP shunts - that were already in place for treatment of hydrocephalus - were also used to administer CAR T cells. The ability to infuse through a VP shunt for administration is dependent on multiple features, such as location, device type, and programmability. For unprogrammable VP shunts, there is a theoretical risk of CAR T cells being shunted away from the CNS and to the end-catheter location (e.g. peritoneum, lung, etc.). For these patients, the placement of Ommaya or revision of their shunt value to be programmable should be considered. Two key technical and procedural areas for improvement exist with regards to CNS infusion. First, harnessing technology such as the Synchromed II intrathecal baclofen pump (Medtronic, Minneapolis MN USA) could allow for a slower, more continuous infusion of intrathecal agent without the need of repeated percutaneous access [22]. However, currently there are several barriers including the stability of a stored cellular product within an intrathecal continuous delivery system. Another area of exploration could include laser interstitial thermal therapy (LITT) in conjunction with administration of intrathecal immunological agents [23,24]. Early experience suggests that LITT may play a role in modifying the blood brain barrier and/or enhancing target effects [25,26]. We hope that the delivery of locoregional immunotherapies, such as CAR T therapy, will improve by considering the incorporation of other technological advances in delivery and in clinical neurosurgical expertise.
Clinical experience with intracranial CAR T cell dosing [14,18] and preclinical success with locoregional delivery [3,[15], [16], [17],19,[27], [28], [29]], led our initial clinical efforts on BrainChild-01 [15], BrainChild-02, and BrainChild-03 [17]. However, optimal location of intracranial delivery is not yet known and may differ based on age, pathology, prior therapy, and general physiology. Endogenous T cells traffic within perivascular compartments both under physiological and pathological conditions [30] may reflect the key anatomical route providing access to CAR T cells injected into the CSF to parenchymal tumors. The glymphatic pathway is a brain-wide network of perivascular pathway along which solutes and particles within the CSF and brain interstitium exchange [31], [32], [33]. Through ongoing preclinical work, we are investigating the optimal anatomical delivery point for intracranial adoptive cellular therapy and the preferred physiologic timing of doses that could also be informed by serial proteogenomic profiling of these tumors [17,34]. These discoveries could inform future clinical trials or lead to amendments of ongoing clinical work.
As adoptive cellular therapies against pediatric CNS tumors continue to advance preclinically, there will be an ongoing need to optimize delivery to administer potent and safe immunotherapies to our patients. Through collaborative efforts with international consortiums and partnerships with other leading institutions, we aim to perfect these targeted therapeutics for those children and young adults in need.
Methods
Design of DNA constructs and lentivirus; Preclinical analysis
The DNA constructs, lentivirus, and preclinical studies have been previously published [15], [16], [17].
Study design and participants
Clinical data through October 2022 is included in this manuscript. BrainChild-01 [15] and BrainChild-03 [17] clinical methods have been described, while BrainChild-02 mirrors BrainChild-01 only delivering an EGFR-specific CAR rather than a HER2-specific CAR. BrainChild-01, -02, and -03 are phase 1 studies of CNS locoregional adoptive therapy with autologous CD4+ and CD8+ T cells lentivirally transduced to express either HER2, EGFR, or B7-H3-specific chimeric antigen receptor (CAR) and EGFRt, delivered intracranially to children and young adults with recurrent or refractory CNS tumors or diffuse intrinsic pontine glioma (DIPG)/diffuse midline glioma, H3 K27M-altered (DMG) (NCT03500991, NCT03638167, and NCT04185038). These studies are conducted in accordance with US Food and Drug Administration and International Conference on Harmonisation Guidelines for Good Clinical Practice, the Declaration of Helsinki, and applicable institutional review board requirements (study protocol approved by the Seattle Children's Institutional Review Board). All patients or their guardians provided written informed consent in accordance with local regulatory review. Enrollment criteria included: age ≥ 1 and ≤ 26 years (except for the first 3 patients on each study who were ≥ 15 and ≤ 26); evidence of refractory/recurrent CNS disease or DMG (with DIPG allowed only on BrainChild-03 Arm C) at any timepoint following completion of standard radiation; ability to tolerate apheresis; presence of a CNS catheter; life expectancy ≥ 8 weeks; Lansky/Karnofsky performance of ≥ 60; defined washout periods from prior therapies; adequate organ function including absolute lymphocyte count (ALC) ≥ 100 cells/µL, absolute neutrophil count ≥ 500 cells/µL, hemoglobin ≥ 9 g/dL, platelets ≥ 100,000/µL, creatinine ≤ upper limit of normal (ULN) for age, total bilirubin < 3x ULN for age or conjugated bilirubin < 2 mg/dL, an oxygen saturation ≥ 90% on room air without dyspnea at rest, adequate neurologic function defined as stable deficits for ≥ 1 week, ≤ 2 anti-epileptic agents required to control seizures, and no encephalopathy; negative virology for HIV, Hepatitis B, and Hepatitis C; and use of highly effective contraception in patients of child-bearing age. Exclusion criteria included: severe cardiac dysfunction; primary immunodeficiency or bone marrow failure syndrome; evidence of impending CNS herniation; presence of > Grade 3 dysphagia (for Arm C patients); another active malignancy; severe, active infection; active receipt of any anti-cancer therapy; or pregnancy or breastfeeding.
Enrolled patients underwent leuko-pheresis. CD4+ and CD8+ T cells from apheresis products were bioengineered to express the second-generation CAR. Requirements to receive CAR T cell infusions included: a CNS catheter in place; ≥ 5 days from surgery; evidence of persistent, evaluable disease; not breastfeeding nor pregnant; meeting defined washout periods from any bridging therapy; adequate organ function defined by specified laboratory values used for eligibility; no encephalopathy or uncontrolled seizure activity; compliance with prescribed anti-epileptic drug administration; and no evidence of active severe infection. To receive subsequent infusions, patients additionally were also required to have had no dose limiting toxicity (DLT). Beyond Course 2, patients were eligible to receive additional infusions at the previous maximum tolerated dose level, if above criteria were met and sufficient CAR T cells were available. Response was assessed following Course 2, and subsequent even numbered courses, using MRI brain and spine and CSF cytology. Correlative studies collections varied by biospecimen and by assigned Arm.
Clinical evaluations
The primary objectives of these studies are to assess the feasibility, safety, and tolerability. Feasibility is defined as generating sufficient therapeutic product to receive all scheduled doses in Courses 1 and 2 at the intended Dose Level (DL) per assigned Dose Regimen (DR) after two attempts using a single apheresis product for starting material. Safety and tolerability are determined by data that include history/physical exams, laboratory/radiographic evaluations, and Common Terminology Criteria for Adverse Events (CTCAE v5.0). A DLT is defined as an event which, in the opinion of the investigator, is possibly, probably, or definitely attributable to the CAR T product and which occurs from the time of initial CAR T cell infusion through 28 days following the final CAR T cell infusion. A DLT includes all ≥ grade 3 CTCAE v5.0 toxicities except ≥ grade 3 toxicities that are known to be related to CAR T cells, including grade 3 CRS that decreases to ≤ grade 2 within 72 h; ≥ grade 3 hypotension, fever, and/or chills not controlled with medical intervention that decrease to ≤ grade 2 within 72 h; ≥ grade 3 activated PTT, fibrinogen, and/or INR that are asymptomatic and resolve within 72 h; ≥ grade 3 hypoglycemia and/or electrolyte imbalance that are asymptomatic and resolve within 72 h; ≥ grade 3 nausea and/or vomiting that decrease to ≤ grade 2 with 7 days; grade 3 neurologic symptoms that decrease to ≤ grade 2 with 7 days (for all Arms A and B); and grade 3 neurologic symptoms that decreases to ≤ grade 2 within 21 days (treatment with dexamethasone and/or bevacizumab is allowed; for BrainChild-03 Arm C). The definition of a DLT also includes any toxicity lasting > 14 days that prevents the patient from meeting criteria for subsequent CAR T cell infusion in Courses 1 or 2. Patients are considered DR escalation-evaluable if they were evaluable for toxicity and were counted as part of a 3-patient dose escalation cohort. Our radiologic response criteria uses the standard sum of the two longest 2D perpendicular diameters to distinguish: stable disease, progressive disease (>25% increase), partial response (>50% decrease), and complete response (no evaluable or measurable disease).
Cell product manufacture
Most recent cell product manufacturing procedures have been published [15,17].
Authorship contributions statement
N.A.V. is the CNS CAR T cell lead and the principal investigator of BrainChild-01 and -03. J.G. is the principal investigator of BrainChild-02. N.A.V., C.H., M.B., J.B.F, A.L.W., J.H.S., and J.R.P. conceived of the project. N.A.V., R.A.G., M.C.J., and J.R.P. oversaw regulatory affairs. N.A.V., J.G., M.C.J., and J.R.P. planned, designed, and wrote the clinical trials. C.B., A.B., J.G., and M.C.J. oversaw development for cellular manufacturing. N.A.V., R.R., M.C., C.H., M.B., A.B-A., A.W., D.K., B.M.L., S.E.S.L., E.E.C., C.H., S.H., H.E.G., S.R.B., A.L, J.G.O., N.P., J.G., J.S.H., and J.R.P. participated in patient care. A.K. oversaw the investigational drug preparation. N.A.V., R.R., M.C., C.H., M.B., A.B-A., A.W., J.J.I., and J.S.H. wrote the manuscript. All authors reviewed and edited the manuscript. J.S.H. and J.R.P. oversaw all aspects of the work.
Funding and support
We are grateful for generous support from Amazon, The Andrew McDonough B+ Foundation, the Aven Foundation, The Avery Huffman DIPG Foundation, Kristie and Joe Berg, Erin Cordry and Eric Hanson, Freckles from Heaven, the Julianna Sayler Foundation, The Kellen Joyce Heart of a Warrior Research Fund, Kick Childhood Cancer, Liv Like a Unicorn, Hope for Harlee Foundation, Live Gray's Way, Love for Lucy, the McKenna Claire Foundation, the Pediatric Brain Tumor Research Fund Guild of Seattle Children's, Kate and Tom Peters, the Run of Hope Seattle, Sam Day Foundation, Starbucks, the Seattle Sounders, Team Beans Infant Brain Tumor Fund, Team Cozzi Foundation, Tommy Strong Foundation, Top Pot Doughnuts, Unravel Pediatric Cancer, and Jessica and Jared Wray. Funding is also provided by Cookies for Kid's Cancer Young Investigator Grant (N.A.V.), DIPG All-In (N.A.V.), Matthew Larson Research Grant (N.A.V.), and the We Love You Connie Foundation (N.A.V.). St. Baldrick's Stand Up to Cancer Dream Team Translational Cancer Research Grants (SU2C-AACR-DT-27-17; N.A.V., R.A.G., M.C.J., J.R.P.; Stand Up to Cancer is a division of the Entertainment Industry Foundation and research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C), Alex's Lemonade Stand Foundation for Childhood Cancer (R.A.G.), and by the National Center for Advancing Translational Sciences of the National Institutes of Health (U01TR002487; A.L.W., R.A.G., J.R.P.; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health).
Data Availability
All requests for raw and analyzed data and materials will be promptly reviewed by the intellectual property office of Seattle Children's Research Institute to verify if the request is subject to any intellectual property or confidentiality obligations. Raw preclinical and clinical data is stored at Seattle Children's with indefinite appropriate backup. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a Material Transfer Agreement.
Declaration of Competing Interest
M.C.J. has interests in Umoja Biopharma, City of Hope and Juno Therapeutics, a Bristol-Myers Squibb (BMS) company. M.C.J. is a seed investor and holds ownership equity in Umoja, serves as a member of the Umoja Joint Steering Committee, and is a Board Observer of the Umoja Board of Directors. M.C.J. is an inventor of patents licensed to Umoja Biopharma, City of Hope, and Juno Therapeutics. The remaining authors declare no competing financial interests. R.A.G. serves on a study steering committee for and is an inventor on a patent licensed to Juno Therapeutics, a Bristol Myers Squibb company, has served on advisory boards for Novartis and Sobi, and is a consultant for Crispr Therapeutics. A.J.J. and J.B.F. are inventors on issued and pending patents related to CAR T cell therapies. All other authors declare no competing interests.
Acknowledgments
We thank the children and families who bravely shoulder the burden of their disease and place their trust in Seattle Children's for their care. We are indebted to A. Thomsen for her expert immunotherapy clinical coordination. We thank our clinical research team, including H. Ullom, S. Bakotich, S. Bagchi, J. Chau, D. Chen, M. Guthrie, K. Hoon, C. Krein, R. Persona, Z. Maino, M. MacQuivey, and A. Westby. We thank our neuro-oncology team, including R. Geyer, J. Olson, N. Millard, A. Sato, A. Laurine, C. Verda, W. Iwata, V. Klein, and Z. Reinke. We thank our neurosurgical team including L. Augenthaler, E. Bruchmiller, M. Hedger, K. Hoban, C. Lance, C. Reilly-Shapiro, and L. Zapata. We thank J. Stevens, the Seattle Children's Hospital's Department of Anatomic Pathology, and the TTS Brain Tumor Committee. We thank the Investigational Drug Service team. We thank the Seattle Children's Therapeutics team, especially the Therapeutic Cell Production Core for their tireless efforts to manufacture cellular products and the Correlative Studies Lab for assistance in correlative sample processing and analysis.
References
- 1.Gardner R.A., Finney O., Annesley C., Brakke H., Summers C., Leger K., Bleakley M., Brown C., Mgebroff S., Kelly-Spratt K.S., et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty E., Downey K., Ferrerosa L., Flores C., Hegde B., Raskin S., Hwang E., Vitanza N., Okada H. Considerations when treating high-grade pediatric glioma patients with immunotherapy. Expert. Rev. Neurother. 2021;21:205–219. doi: 10.1080/14737175.2020.1855144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theruvath J., Sotillo E., Mount C.W., Graef C.M., Delaidelli A., Heitzeneder S., Labanieh L., Dhingra S., Leruste A., Majzner R.G., et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 2020;26:712–719. doi: 10.1038/s41591-020-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gust J., Finney O.C., Li D., Brakke H.M., Hicks R.M., Futrell R.B., Gamble D.N., Rawlings-Rhea S.D., Khalatbari H.K., Ishak G.E., et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann. Neurol. 2019;86:42–54. doi: 10.1002/ana.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaichana K.L., Pinheiro L., Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther. Deliv. 2015;6:353–369. doi: 10.4155/tde.14.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Pfeffer J.L., Gururangan S., Lester T., Lim D.A., Shaywitz A.J., Westphal M., Slavc I. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr. Neurol. 2017;67:23–35. doi: 10.1016/j.pediatrneurol.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Peyrl A., Chocholous M., Azizi A.A., Czech T., Dorfer C., Mitteregger D., Gojo J., Minichmayr E., Slavc I. Safety of Ommaya reservoirs in children with brain tumors: a 20-year experience with 5472 intraventricular drug administrations in 98 patients. J. Neurooncol. 2014;120:139–145. doi: 10.1007/s11060-014-1531-1. [DOI] [PubMed] [Google Scholar]
- 9.Sagnella S.M., White A.L., Yeo D., Saxena P., van Zandwijk N., Rasko J.E.J. Locoregional delivery of CAR-T cells in the clinic. Pharmacol. Res. 2022;182 doi: 10.1016/j.phrs.2022.106329. [DOI] [PubMed] [Google Scholar]
- 10.Mead P.A., Safdieh J.E., Nizza P., Tuma S., Sepkowitz K.A. Ommaya reservoir infections: a 16-year retrospective analysis. J. Infect. 2014;68:225–230. doi: 10.1016/j.jinf.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Burger M.C., Wagner M., Franz K., Harter P.N., Bahr O., Steinbach J.P., Senft C. Ventriculoperitoneal shunts equipped with on-o... valves for intraventricular therapies in patients with communicating hydrocephalus due to leptomeningeal metastases. J. Clin. Med. 2018;7(8):216. doi: 10.3390/jcm7080216. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://pubmed.ncbi.nlm.nih.gov/30110924/.
- 12.Nayar G., Ejikeme T., Chongsathidkiet P., Elsamadicy A.A., Blackwell K.L., Clarke J.M., Lad S.P., Fecci P.E. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget. 2017;8:73312–73328. doi: 10.18632/oncotarget.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubenstein J.L., Li J., Chen L., Advani R., Drappatz J., Gerstner E., Batchelor T., Krouwer H., Hwang J., Auerback G., et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121:745–751. doi: 10.1182/blood-2012-07-440974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.C., Naranjo A., Starr R., Wagner J., Wright C., et al. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitanza N.A., Johnson A.J., Wilson A.L., Brown C., Yokoyama J.K., Kunkele A., Chang C.A., Rawlings-Rhea S., Huang W., Seidel K., et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat. Med. 2021;27:1544–1552. doi: 10.1038/s41591-021-01404-8. [DOI] [PubMed] [Google Scholar]
- 16.Ravanpay A.C., Gust J., Johnson A.J., Rolczynski L.S., Cecchini M., Chang C.A., Hoglund V.J., Mukherjee R., Vitanza N.A., Orentas R.J., et al. EGFR806-CAR T cells selectively target a tumor-restricted EGFR epitope in glioblastoma. Oncotarget. 2019;10:7080–7095. doi: 10.18632/oncotarget.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitanza N.A., Wilson A.L., Huang W., Seidel K., Brown C., Gustafson J.A., Yokoyama J.K., Johnson A.J., Baxter B.A., Koning R.W., et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2022 doi: 10.1158/2159-8290.CD-22-0750. CD-22-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://pubmed.ncbi.nlm.nih.gov/36259971/.
- 18.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donovan L.K., Delaidelli A., Joseph S.K., Bielamowicz K., Fousek K., Holgado B.L., Manno A., Srikanthan D., Gad A.Z., Van Ommeren R., et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020;26:720–731. doi: 10.1038/s41591-020-0827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mechleb B., Khater F., Eid A., David G., Moorman J.P. Late onset Ommaya reservoir infection due to Staphylococcus aureus: case report and review of Ommaya Infections. J. Infect. 2003;46:196–198. doi: 10.1053/jinf.2002.1111. [DOI] [PubMed] [Google Scholar]
- 21.Lishner M., Perrin R.G., Feld R., Messner H.A., Tuffnell P.G., Elhakim T., Matlow A., Curtis J.E. Complications associated with Ommaya reservoirs in patients with cancer. The princess margaret hospital experience and a review of the literature. Arch. Intern. Med. 1990;150:173–176. [PubMed] [Google Scholar]
- 22.Farid R., Binz K., Emerson J.A., Murdock F. Accuracy and precision of the synchroMed II pump. Neuromodulation. 2019;22:805–810. doi: 10.1111/ner.12934. [DOI] [PubMed] [Google Scholar]
- 23.Kuo C.H., Feroze A.H., Poliachik S.L., Hauptman J.S., Novotny E.J., Jr., Ojemann J.G. Laser ablation therapy for pediatric patients with intracranial lesions in eloquent areas. World Neurosurg. 2019;121:e191–e199. doi: 10.1016/j.wneu.2018.09.074. [DOI] [PubMed] [Google Scholar]
- 24.Feroze A.H., McGrath M., Williams J.R., Young C.C., Ene C.I., Buckley R.T., Cole B.L., Ojemann J.G., Hauptman J.S. Laser interstitial thermal therapy for pediatric atypical teratoid/rhabdoid tumor: case report. Neurosurg. Focus. 2020;48:E11. doi: 10.3171/2019.10.FOCUS19746. [DOI] [PubMed] [Google Scholar]
- 25.Butt O.H., Zhou A.Y., Huang J., Leidig W.A., Silberstein A.E., Chheda M.G., Johanns T.M., Ansstas G., Liu J., Talcott G., et al. A phase II study of laser interstitial thermal therapy combined with doxorubicin in patients with recurrent glioblastoma. Neurooncol. Adv. 2021;3:vdab164. doi: 10.1093/noajnl/vdab164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salehi A., Paturu M.R., Patel B., Cain M.D., Mahlokozera T., Yang A.B., Lin T.H., Leuthardt E.C., Yano H., Song S.K., et al. Therapeutic enhancement of blood-brain and blood-tumor barriers permeability by laser interstitial thermal therapy. Neurooncol. Adv. 2020;2:vdaa071. doi: 10.1093/noajnl/vdaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priceman S.J., Tilakawardane D., Jeang B., Aguilar B., Murad J.P., Park A.K., Chang W.C., Ostberg J.R., Neman J., Jandial R., et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin. Cancer Res. 2018;24:95–105. doi: 10.1158/1078-0432.CCR-17-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown C.E., Aguilar B., Starr R., Yang X., Chang W.C., Weng L., Chang B., Sarkissian A., Brito A., Sanchez J.F., et al. Optimization of IL13Ralpha2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol. Ther. 2018;26:31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster J.B., Griffin C., Rokita J.L., Stern A., Brimley C., Rathi K., Lane M.V., Buongervino S.N., Smith T., Madsen P.J., et al. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. J. Immunother. Cancer. 2022;10(9):e004450. doi: 10.1136/jitc-2021-004450. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://pubmed.ncbi.nlm.nih.gov/36167467/.
- 30.Smolders J., van Luijn M.M., Hsiao C.C., Hamann J. T-cell surveillance of the human brain in health and multiple sclerosis. Semin. Immunopathol. 2022;44(6):855–867. doi: 10.1007/s00281-022-00926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliff J.J., Lee H., Yu M., Feng T., Logan J., Nedergaard M., Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iliff J.J., Wang M., Zeppenfeld D.M., Venkataraman A., Plog B.A., Liao Y., Deane R., Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Findlay I.J., De Iuliis G.N., Duchatel R.J., Jackson E.R., Vitanza N.A., Cain J.E., Waszak S.M., Dun M.D. Pharmaco-proteogenomic profiling of pediatric diffuse midline glioma to inform future treatment strategies. Oncogene. 2022;41:461–475. doi: 10.1038/s41388-021-02102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All requests for raw and analyzed data and materials will be promptly reviewed by the intellectual property office of Seattle Children's Research Institute to verify if the request is subject to any intellectual property or confidentiality obligations. Raw preclinical and clinical data is stored at Seattle Children's with indefinite appropriate backup. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a Material Transfer Agreement.