Abstract
This study aimed to evaluate the effect of isoleucine (Ile) on growth performance, meat quality and lipid metabolism of broilers fed a low-protein diet (LPD). The 396 one-day-old male Cobb broilers were allocated to 4 treatment groups as follows: control diet (CON), LPD, LPD + 0.13% Ile (LPD-LI) and LPD + 0.26% Ile (LPD-HI), with nine replicates of 11 broilers each for 42 d. The Ile increased average daily gain, average daily feed intake, fiber density and the mRNA level of myosin heavy chain (MyHC)-I in breast muscle, and decreased feed to gain ratio, shear force, fiber diameter and the mRNA level of MyHC-IIb in breast muscle, which were impaired by the LPD. Compared to the LPD group, broilers in LPD-LI and LPD-HI groups had lower serum lipid levels, liver fat content, abdominal adipose percentage and mRNA levels of peroxisome proliferator-activated receptor-γ, CCAAT/enhancer binding protein-α, ki-67, topoisomerase II alpha (TOP2A) and thioredoxin-dependent peroxidase 2 in abdominal adipose and liver X receptors-α, sterol regulatory element binding protein 1 (SREBP1), acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) in liver, and higher mRNA levels of peroxisome proliferator activated receptor-α, carnitine palmitoyl-transferase 1 (CPT-1), and acyl-CoA oxidase 1 (ACOX1) in liver, which were equal to the CON levels. A LPD supplemented with Ile decreased enzyme activities of ACC and FAS in liver and glycerol-3-phosphate dehydrogenase and TOP2A in abdominal adipose, and increased enzyme activities of CPT-1 and ACOX1 in liver. Furthermore, Ile supplementation enhanced the mRNA level of leptin receptor and protein levels of phospho-5′ adenosine monophosphate-activated protein kinase (AMPK), mechanistic target of rapamycin, ribosomal protein 70 S6 kinase, janus kinase 2 (JAK2), and signal transducer and activator of transcription 3 (STAT3), and decreased the protein level of SREBP1 in the liver of broilers in LPD group. In conclusion, dietary supplementation with Ile to 0.83% could improve growth performance and meat quality and alleviate lipid deposition of broilers fed a LPD through activating AMPK and JAK2/STAT3 signaling pathways.
Key words: broiler, isoleucine, lipid metabolism, liver, low-protein diet
INTRODUCTION
In order to save protein resources and reduce nitrogen excretion, a low-protein diet (LPD) is proposed, with the dietary crude protein content reduced by 2% to 3% (Kidd et al., 2021). However, accumulating evidence demonstrated that a LPD depresses growth and deteriorates meat quality of broilers characterized by incremental drip loss and increased muscle fiber area (Chodová et al., 2021; Wang et al., 2022). More importantly, a LPD lacks adequate amounts of amino acids required for lipoprotein synthesis, thereby impeding lipid transport from the liver and disrupting the dynamic balance of lipid metabolism in the liver, blood, and peripheral tissues, eventually leading to excessive lipid deposition (Sigolo et al., 2017). Branched-chain amino acids (BCAA), including leucine, valine, and isoleucine (Ile), are important nutrient regulators of protein synthesis and strongly associated with glucose and lipid metabolism (Nie et al., 2018). Numerous studies have reported that BCAA supplemented in LPDs could alleviate the lipid deposition in adipose tissue and liver of pigs (Li et al., 2017; Duan et al., 2018; Heng et al., 2020; Zhang et al., 2021). However, the effect and mechanism of BCAA supplemented in LPDs on regulating lipid metabolism in broilers remain largely unknown. Moreover, most studies of LPDs supplemented with BCAA have focused on either leucine or valine (Erwan et al., 2011; Nascimento et al., 2016), Ile has not attracted wide attention.
Ile, as the fourth co-limiting amino acid with valine for broilers fed corn-soybean meal-based diets, is critical to maintain growth performance, meat quality, and lipid metabolism (Dozier Iii et al., 2011). Broilers fed with Ile-deficient diet were reported to have lower body weight gain and feed intake and a higher feed to gain ratio (F:G) than with control diet (Kidd et al., 2004). In a previous study, increased Ile intake was found to promote accumulation of intramuscular fat (IMF) in finishing pigs by inhibiting phosphorylation of 5′ adenosine monophosphate-activated protein kinase (AMPK)-acetyl CoA carboxylase (ACC) and stimulating the expression of lipogenesis-related genes in skeletal muscle (Luo et al., 2018). In addition, dietary Ile supplementation was reported to reduce the mass of white adipose tissue and ameliorate hepatic steatosis in obese mice by promoting the browning of white adipose tissue and regulating expression of genes associated with lipid metabolism (Ma et al., 2020). However, it remains unclear whether supplementation of a LPD with Ile can improve meat quality and alleviate lipid deposition in broilers.
The liver is the primary site of lipid metabolism in broilers (Cai et al., 2011). AMPK, the main sensor of cellular energy status, plays a pivotal role in the regulation of lipid, glucose, and protein metabolism in liver (Hu et al., 2019). Leptin is a hormone secreted by white adipose tissue and binds to the leptin receptor (LEPR) via the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway to regulate lipid metabolism, energy homeostasis, and immune responses (Park and Ahima, 2014). The effects of Ile on the AMPK/mechanistic target of rapamycin (mTOR) and Leptin/JAK2/STAT3 pathways in broilers are unknown. Therefore, the aim of the present study was to investigate the effects of Ile on the growth performance, meat quality, and lipid metabolism of broilers fed a LPD, and to determine whether the effect of Ile on lipid metabolism is linked to activation of the AMPK/mTOR and Leptin/JAK2/STAT3 pathways.
MATERIALS AND METHODS
Materials
The Ile (with a purity of 98%) was purchased from CJ (Shanghai) Trading Co., Ltd. (Shanghai, China).
Animals and Experimental Design
The study protocol was approved by the Animal Care and Use Committee of Qingdao Agricultural University (Qingdao, China). A total of 396 one-day-old male Cobb 500 broilers were purchased from Yantai Dadi Animal Husbandry Co., Ltd. (Yantai, China). Broilers with similar initial body weights (46.24 ± 0.44 g) were randomly divided into 4 treatment groups composed of 9 replicated cages of 11 broilers each for a 42-d feeding period. The treatments consisted of a control (CON) diet, LPD, LPD + 0.13% Ile (LPD-LI), and LPD + 0.26% Ile (LPD-HI). The CON diet was formulated to meet all requirements recommended by the National Research Council (NRC, 1994; Table 1). The digestible Ile: lysine ratio was formulated at 0.74, meeting the amino acid requirement of broilers. Compared to the CON diet, the crude protein content of the LPD was reduced by 3%, while the levels of four limiting amino acids (lysine, methionine, threonine, and tryptophan) were the same. The Ile levels of the LPD-LI and LPD-HI groups were adjusted to that of the CON diet and 0.13% higher than that of the CON diet, respectively. All the diets were fed in mash form. During the experiment, feed and fresh water were provided ad libitum. Temperature was maintained at 33 to 35°C for the first week and then gradually reduced by 2°C every week to a final temperature of 24°C. Feed consumption of each group was measured daily on a replicate basis and the average daily feed intake (ADFI) was calculated. The body weight per replicate of each group was measured on days 0, 21, and 42 and the average daily gain (ADG) and F:G were calculated.
Table 1.
Composition and nutrient levels of experimental diets (air-dry basis) %.
| Item | Days 0 to 21 |
Days 21 to 42 |
||
|---|---|---|---|---|
| CON1 | LPD1 | CON | LPD | |
| Ingredients | ||||
| Corn | 51.26 | 60.29 | 58.05 | 67.50 |
| Soybean meal | 38.20 | 29.53 | 32.95 | 24.22 |
| Soybean oil | 6.72 | 5.84 | 5.64 | 4.44 |
| Limestone | 1.26 | 1.28 | 1.43 | 1.41 |
| CaHPO4 | 1.68 | 1.77 | 1.13 | 1.22 |
| NaCl | 0.35 | 0.35 | 0.35 | 0.35 |
| Vitamin premix2 | 0.10 | 0.10 | 0.10 | 0.10 |
| Mineral premix3 | 0.15 | 0.15 | 0.15 | 0.15 |
| Choline chloride | 0.07 | 0.07 | 0.07 | 0.07 |
| L-lysine hydrochloride | 0.04 | 0.23 | 0.05 | 0.24 |
| DL-methionine | 0.17 | 0.21 | 0.08 | 0.12 |
| L-threonine | 0.00 | 0.13 | 0.00 | 0.13 |
| L-tryptophan | 0.00 | 0.05 | 0.00 | 0.05 |
| Nutrient levels | ||||
| Metabolizable energy (MJ/kg) | 13.14 | 13.12 | 13.09 | 13.07 |
| Calcium (%) | 0.98 | 0.98 | 0.91 | 0.90 |
| Available phosphorus (%) | 0.45 | 0.45 | 0.35 | 0.35 |
| Crude protein (%)4 | 21.06 | 18.07 | 19.06 | 16.01 |
| Lysine (%)4 | 1.12 | 1.11 | 1.00 | 1.02 |
| Digestible lysine (%) | 0.96 | 0.98 | 0.87 | 0.89 |
| Methionine (%)4 | 0.52 | 0.51 | 0.38 | 0.40 |
| Threonine (%)4 | 0.87 | 0.86 | 0.80 | 0.79 |
| Tryptophan (%)4 | 0.31 | 0.32 | 0.26 | 0.27 |
| Isoleucine (%)4 | 0.83 | 0.70 | 0.75 | 0.62 |
| Digestible Isoleucine (%) | 0.71 | 0.59 | 0.64 | 0.53 |
CON, control diet; LPD, low-protein diet.
Provided per kilogram of diet: vitamin A (trans-retinyl acetate), 8000 IU; vitamin D3 (cholecalciferol), 3000 IU; vitamin E (all-rac-α-tocopherol acetate), 20 IU; vitamin K3 (menadione), 2.0 mg; vitamin B1 (thiamin), 4.2 mg; vitamin B2 (riboflavin), 4.0 mg; vitamin B6 (pyridoxine HCl), 4.5 mg; vitamin B12 (cobalamin), 0.02 mg; nicotinic acid, 10 mg; calcium pantothenate, 11 mg; folic acid, 1.0 mg; biotin, 0.15 mg.
Provided per kilogram of diet: Fe (as ferrous sulfate), 80 mg; Cu (as copper sulfate), 10 mg; Zn (as zinc sulfate), 75 mg; Mn (as manganese sulfate), 80 mg; Se (as sodium selenite), 0.30 mg; I (as potassium iodide), 0.40 mg.
Analyzed value.
Chemical Analysis
The crude protein and amino acid contents of the diets were determined in accordance with the methods described by the Association of Official Analytical Chemists (976.06 and 994.12, respectively). Amino acids, except tryptophan, methionine and cystine, were detected using the Hitachi L-8900 amino acid analyzer (Hitachi, Tokyo, Japan) after hydrolyzing with 6 mol/L HCl at 110°C for 24 h. Tryptophan was detected using the Agilent 1200 Series high performance liquid chromatography (Aligent, Santa Clara, CA) after hydrolyzing with 4 mol/L LiOH at 110°C for 24 h. Methionine and cysteine were detected using the Hitachi L-8900 amino acid analyzer after cold performic acid oxidation and hydrolyzing with 6.8 mol/ L HCl at 110°C for 24 h.
Sample Collections
On day 42, after fasting for 12 h, one broiler per replicate was randomly selected and blood samples were collected from the jugular vein, centrifuged at 3,000 × g and 4°C for 10 min, and the separated serum was stored at −80°C. After blood collection, the broilers were sacrificed by cervical dislocation and the abdominal and thoracic cavities were opened to harvest the left and right breast muscles, liver, and abdominal adipose tissue. The left breast muscle was stored at 4°C for meat quality assessment. Parts of the right breast muscle, liver, and abdominal adipose tissue were fixed in 4% neutral paraformaldehyde for histochemical analysis, while the remaining tissues were immediately frozen in liquid nitrogen and stored at −80°C for further analysis.
Measurement of Meat Quality
After a 30-min blooming period, the meat color parameters, including lightness (L*), redness (a*) and yellowness (b*) values, were measured at room temperature using a colorimeter (CR-410; standard observer, 2°; illuminant, D65; Konica Minota, Inc., Tokyo, Japan), as described by Zhang et al. (2022). The meat color parameters were immediately measured 3 times at different locations of the medial portion of each sample. Ultimate pH values were measured at 45 min (pH45min) and 24 h (pH24h) postmortem using a portable pH meter (Seven2Go; Mettler Toledo GmbH, Giessen, Germany) inserted 1 cm into the superior portion of the breast muscle, as described by Liu et al. (2021). Each measurement for each sample was performed with 3 replicates. Drip loss was calculated according to the equation: drip loss (%) = (initial weight − final weight)/(initial weight) × 100%, as described by Honikel (1998). Cooking loss was determined as described in a previous study (Liu et al., 2016). Briefly, the breast muscle samples were placed into Ziploc plastic bags and heated to an internal temperature of 77 to 80°C, then cooled to room temperature, wiped with absorbent paper, and reweighed. Shear force was calculated as described by Castellini et al. (2002). The mid-portion of each heated sample was cut into sections measuring 1.25 × 2 cm along the direction of the fibers. The shear force of every section was measured 3 times using a digital meat tenderness meter (model C-LM3; Tenovo International Co., Ltd., Beijing, China).
Fat Content Measurement and Histological Analysis
The liver and breast muscle tissues were cut into small sections, which were freeze-dried and ground into powder. The liver fat and IMF contents were measured using the Soxhlet extraction method with petroleum and ether. The IMF content was converted to the percentage of wet meat weight in accordance with the following equation: IMF (%) = (fat weight/wet weight of breast muscle) × 100% (Luo et al., 2018).
The breast muscle and abdominal adipose tissues were stained with hematoxylin and eosin, as described by Bai et al. (2015). Briefly, the breast muscle and abdominal adipose were cut into sections, which were fixed with Carnoy's solution at 4 ± 1°C for 24 h, then dehydrated with ethanol/xylene, and embedded in paraffin. The paraffin-embedded sections were cut into 4 μm-thick slices that were mounted on glass slides and stained with hematoxylin and eosin. For each sample, 3 sections were randomly photographed to calculate the muscle fiber diameter, muscle fiber density, and area of adipocytes.
Oil red O staining was conducted as described by Li et al. (2019). Briefly, frozen liver samples (5 µm) were fixed with 1% calcium chloride and 4% formaldehyde for 1 h, then treated with 60% isopropanol, and stained with oil red O solution and Mayer's hematoxylin solution for 15 min. The integrated optical density was measured under a microscope (Olympus Corporation, Tokyo, Japan) using the IAS––2000D image analysis system (Quality Engineering Associates, Inc., Acton, MA). Three images of each section were randomly captured for each sample.
Measurement of Biochemical Parameters and Enzyme Activities
Commercial enzyme-linked immunosorbent kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) were used to measure serum levels of triglyceride (TG; cat. no. YJ90993), total cholesterol (TC; cat. no. ML036921), high-density lipoprotein cholesterol (HDL-C; cat. no. ML036973), low-density lipoprotein cholesterol (LDL-C; cat. no. ML036983), free fat acid (FFA; cat. no. YJ68293), leptin (cat. YJ45283) and insulin (cat. no. YJ09873), enzyme activities of ACC (cat. no. ML09632), fatty acid synthase (FAS; cat. no. ML02302), carnitine palmitoyl-transferase 1 (CPT-1; cat. no. ML25412) and acyl-CoA oxidase 1 (ACOX1; cat. no. ML36214) in liver and enzyme activities of glycerol-3-phosphate dehydrogenase (G3PDH; cat. no. ML41236) and topoisomerase II alpha (TOP2A; cat. no. ML85214) in abdominal adipose according to the manufacturer's instructions.
Real-Time Quantitative PCR Analysis
Total RNA was extracted from the liver, abdominal adipose, and breast muscle samples using TRIzol reagent (Tiangen Biotech (Beijing) Co., Ltd., Beijing, China) in accordance with the manufacturer's instructions. The concentration and quality of RNA were measured using SpectraMax QuickDrop Micro-Volume Spectrophotometer (Molecular Devices, LLC, San Jose, CA). Samples with A260 nm/A280 nm values of 1.8 to 2.0 were considered acceptable. Then, the isolated RNA was reverse-transcribed into cDNA using the Evo M-MLV RT Mix Kit with gDNA Clean for qPCR (Hunan Aikerui Biological Engineering Co., Ltd., Hunan, China) in accordance with the manufacturer's instructions. All cDNA samples were stored at −20°C until further use. Relative gene expression was quantified by real-time PCR using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) with TB Green Premix Ex Taq polymerase (Takara Bio, Inc., Shiga, Japan) and calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The primers for PCR analysis are listed in Table 2.
Table 2.
Primers used for real time-PCR.
| Gene1 | Primer sequence (5’ to 3’) | Product size (bp) | GenBank no. |
|---|---|---|---|
| MyHC-I | F: AACGCCGCAACAACCT R: TTCTTCTTCATCCGCTCC |
330 | U85023.1 |
| MyHC-IIa | F: CCACCAATCCATACGACT R: CTGGCTCTGCTTGCTCT |
216 | NM 204228.1 |
| MyHC-IIb | F: TGTGAGTCAAGGCGAGAT R: CCAGAGCACCTACAGCAT |
347 | NM_001013396.1 |
| LXRα | F: GTGCAGAGAGTGACGAGCTT R: AGAGGTTTACGTGCGTGGAG |
175 | NM_204542 |
| SREBP-1 | F: TCACCGCTTCTTCGTGGAC R: CTGAAGGTACTCCAACGCATC |
220 | AY029224 |
| ACC | F: AATGGCAGCTTTGGAGGTGT R: TTCTGTTTGGGTGGGAGGTG |
137 | NM_205505.2 |
| FAS | F: AACTCTCTGCCATCTCCCGA R: TAGCCTGGTAGCCAGTTCGT |
165 | NM_205155.4 |
| PPARα | F: AATCATAAAGGAGTTTAAGTGACCG R: GCTGGTGAAAGGGTGTCTGT |
264 | NM_001001464.1 |
| CPT-1 | F: GGCTCTGGCAGGAGCTACA R: AGTAGGTCAGGACACGCTCA |
108 | XM_040700878.1 |
| ACOX1 | F: ATGTCACGTTCACCCCATCC R: AGGTAGGAGACCATGCCAGT |
133 | NM_001006205 |
| PPARγ | F: CAGTGGATCTGTCTGCGATG R: CTTTGGCAATCCTGGAGCTTG |
172 | NM_001001460 |
| C/EBPα | F: ATGGAGCAAGCCAACTTCTAC R: GCCAGGAACTCGTCGTTGAA |
230 | NM_001031459 |
| KI67 | F: AGGATGGAAGCAAGTCACCTGGAT R: CTTCTGAACGGGGACTGGAATCTT |
129 | XM_004020367 |
| TOP2A | F: TAAGCCCACTAGCACGGTTG R: CCAATCCCTTCTGCCCCATT |
142 | NM_204791.3 |
| TPX2 | F: TGGAGGGTGGGCCAATC R: TTGGCTGTGTGAGTTCCTTCAC |
57 | NM_204437.1 |
| LEPR | F: TCCCCCAGTGACCCGACCTCT R: CGCCTCATTTCCCAGCTCCCA |
201 | NM_204323 |
| GAPDH | F: GGGCACGCCATCACTATCTT R: TAACACGCTTAGCACCACCC |
148 | NM_204305.1 |
MyHC, myosin heavy chain; LXRα, liver X receptors-α; SREBP1, sterol regulatory element binding protein 1; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; PPARα, peroxisome proliferator activated receptor-α; CPT-1, carnitine palmitoyl-transferase 1; ACOX1, acyl-CoA oxidase 1; KI67, ki-67; TOP2A, topoisomerase II alpha; TPX2, thioredoxin-dependent peroxidase 2; PPARγ, peroxisome proliferator-activated receptor-γ; C/EBPα, CCAAT/enhancer binding protein-α; LEPR, leptin receptor; GAPGH, glyceraldehyde-3-phosphate dehydrogenase.
Western Blot Analysis
Liver tissue proteins were extracted using radio immunoprecipitation assay lysis buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and quantified using the bicinchoninic acid assay (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts of proteins from each group were separated by electrophoresis and then transferred to polyvinylidene difluoride membranes (Merck Millipore, Darmstadt, Germany), which were blocked with 5% skimmed milk and then incubated overnight at 4°C with primary antibodies against β-actin (Beyotime Institute of Biotechnology), AMPK (Cell Signaling Technology, Inc., Danvers, MA, USA), p-AMPK (Thr172; Cell Signaling Technology, Inc.), mTOR (Cell Signaling Technology, Inc.), p-mTOR (Ser2448; Cell Signaling Technology, Inc.), ribosomal protein 70 S6 kinase (p70S6K; Cell Signaling Technology, Inc.), p-p70S6K (Thr389; Cell Signaling Technology, Inc.), sterol regulatory element binding protein 1 (SREBP1; Wanlei Biotechnology Co., Ltd., Shenyang, China), JAK2 (Cell Signaling Technology, Inc.), p-JAK2 (Tyr1007/1008; Cell Signaling Technology, Inc.), STAT3 (Santa Cruz Biotechnology, Inc., Dallas, TX), and p-STAT3 (Tyr705; Santa Cruz Biotechnology, Inc.). After washing 3 times with 1 × Tris-buffered saline with 0.1% Tween 20 detergent (TBST), the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Beyotime Institute of Biotechnology) for 1 h at room temperature, then washed 5 times with 1 × TBST. The signals were visualized with an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology) and imaged with a CanoScan LiDE 100 scanner (Canon, Inc., Tokyo, Japan). Relative protein levels were determined using ImageJ software.
Statistical Data Analysis
All data were analyzed by one-way analysis of variance using IBM SPSS Statistics for Windows, version 20.0. (IBM Corporation, Armonk, NY). The results are presented as the mean ± SEM and compared using Turkey's multiple range test. Statistical significance was defined as P < 0.05.
RESULTS
Growth Performance
As shown in Table 3, there were no significant differences in the ADG, ADFI, and F:G among the 4 groups from d 0 to 21. From d 21 to 42 and d 0 to 42, ADG and ADFI were significantly greater (P < 0.001) in the CON, LPD-LI, and LPD-HI groups than the LPD group, while there were no significant differences among the CON, LPD-LI, and LPD-HI groups. The F:G was significantly greater (P < 0.001) in the LPD group than the other groups, with no significant differences among the CON, LPD-LI, and LPD-HI groups.
Table 3.
Effect of a LPD supplemented with isoleucine (Ile) on growth performance of broilers.
| Item1 | CON2 | LPD | LPD-LI | LPD-HI | SEM | P-value |
|---|---|---|---|---|---|---|
| Days 0–21 | ||||||
| ADG (g) | 35.86 | 35.02 | 35.69 | 35.23 | 0.420 | 0.175 |
| ADFI (g) | 50.32 | 49.48 | 50.27 | 49.97 | 0.584 | 0.464 |
| F:G | 1.40 | 1.41 | 1.41 | 1.42 | 0.023 | 0.948 |
| Days 21–42 | ||||||
| ADG (g) | 77.51a | 66.59b | 77.87a | 77.74a | 0.962 | <0.001 |
| ADFI (g) | 134.88a | 124.73b | 137.28a | 137.88a | 1.637 | <0.001 |
| F:G | 1.74b | 1.87a | 1.77b | 1.78b | 0.020 | <0.001 |
| Days 0–42 | ||||||
| ADG (g) | 56.63a | 50.61b | 56.61a | 56.30a | 0.521 | <0.001 |
| ADFI (g) | 92.48a | 86.48b | 93.44a | 93.18a | 0.801 | <0.001 |
| F:G | 1.63b | 1.71a | 1.65b | 1.66b | 0.014 | <0.001 |
ADFI, average daily feed intake; ADG, average daily gain; F:G, feed to gain ratio.
CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile.
Mean values within a row with no common superscript differ significantly (P < 0.05).
Meat Quality
Compared with the CON and LPD groups, the L* value (P = 0.017) and drip loss (P = 0.026) were significantly lower in the LPD-HI group, with no significant differences between the LPD-LI and LPD-HI groups (Table 4). Meanwhile, the a* value was significantly lower (P = 0.001) and the pH24h value (P = 0.014) and the IMF content (P = 0.012) were significantly higher in the LPD-HI group than the other groups, but there were no significant differences among the CON, LPD, and LPD-LI groups. Shear force was significantly increased (P = 0.048) in the LPD group compared to the other groups, with no significant differences among the CON, LPD-LI, and LPD-HI groups.
Table 4.
Effect of a LPD supplemented with isoleucine (Ile) on meat quality of broilers.
| Item1 | CON2 | LPD | LPD-LI | LPD-HI | SEM | P-value |
|---|---|---|---|---|---|---|
| L* | 53.23a | 53.91a | 52.92ab | 51.90b | 0.596 | 0.017 |
| a* | 5.23a | 5.26a | 5.24a | 5.14b | 0.028 | 0.001 |
| b* | 2.24 | 2.22 | 2.23 | 2.27 | 0.034 | 0.488 |
| pH45min | 6.80 | 6.78 | 6.82 | 6.81 | 0.023 | 0.345 |
| pH24h | 6.56b | 6.52b | 6.57b | 6.65a | 0.039 | 0.014 |
| Drip loss (%) | 2.55a | 2.58a | 2.51ab | 2.39b | 0.064 | 0.026 |
| Cooking loss (%) | 14.53 | 14.74 | 13.68 | 14.44 | 0.970 | 0.711 |
| Shear force (N) | 8.64b | 9.06a | 8.62b | 8.66b | 0.175 | 0.048 |
| IMF (%) | 2.35b | 2.33b | 2.36b | 2.48a | 0.052 | 0.012 |
| Fiber diameter (µm) | 69.44b | 86.87a | 68.40b | 64.37b | 3.094 | <0.001 |
| Fiber density (number/mm2) | 455.04a | 418.34b | 456.67a | 457.84a | 8.265 | <0.001 |
L*, lightness; a*, redness; b*, yellowness; pH45min, postmortem pH at 45 min; pH24h, post-mortem pH at 24 h; IMF, intramuscular fat.
CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile.
Mean values within a row with no common superscript differ significantly (P < 0.05).
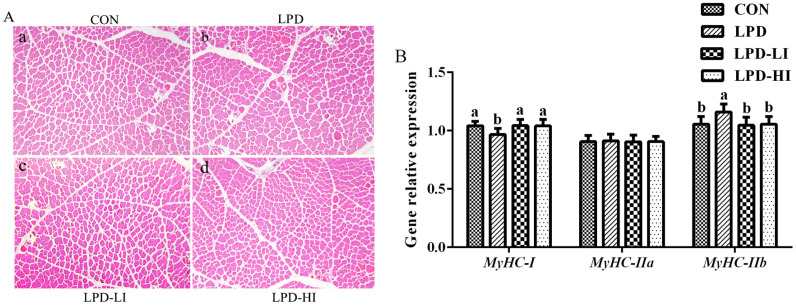
Hematoxylin and eosin staining of the breast muscle showed that the diameter of the muscle fibers was significantly increased (P < 0.001), while the density was significantly decreased (P < 0.001) in the LPD group compared to the other groups, with no significant differences among the CON, LPD-LI, and LPD-HI groups (Table 4 and Figure 1A). Compared to the other groups, relative expression of myosin heavy chain (MyHC)-I was significantly decreased (P = 0.038) and that of MyHC-IIb was significantly increased (P = 0.027) in the LPD group, but there were no significant differences among the CON, LPD-LI, and LPD-HI groups (Figure 1B).
Figure 1.
Effect of a LPD supplemented with isoleucine (Ile) on muscle fiber of broilers. (A) Histological analysis of breast muscle by staining with hematoxylin and eosin. (B) Relative MyHC expressions. MyHC, myosin heavy chain; CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile. a,bBars with no common superscript differ significantly (P < 0.05).
Lipid-Related Metabolite and Hormone Levels in Serum
As shown in Table 5, the TG (P = 0.041) and insulin (P = 0.006) levels were higher in the LPD group than the CON and LPD-HI groups, with no significant differences among the CON, LPD-LI and LPD-HI groups. The TC level was higher (P = 0.009) in the LPD group than the CON, LPD-LI and LPD-HI groups, while there were no significant differences among the CON, LPD-LI, and LPD-HI groups. Meanwhile, the HDL-C (P = 0.006), leptin (P = 0.003), and FFA (P = 0.026) levels were lower in the LPD group than the other groups, but there were no significant differences among the CON, LPD-LI, and LPD-HI groups.
Table 5.
Effect of a LPD supplemented with isoleucine (Ile) on serum lipid-related metabolite and hormone levels in broilers.
| Item1 | CON2 | LPD | LPD-LI | LPD-HI | SEM | P-value |
|---|---|---|---|---|---|---|
| TG (mmol/L) | 1.17b | 1.22a | 1.20ab | 1.16b | 0.022 | 0.041 |
| TC (mmol/L) | 3.08b | 3.22a | 3.11b | 3.11b | 0.039 | 0.009 |
| LDL-C (mmol/L) | 0.54 | 0.56 | 0.56 | 0.56 | 0.021 | 0.640 |
| HDL-C (mmol/L) | 1.87a | 1.77b | 1.85a | 1.89a | 0.032 | 0.006 |
| Leptin (µg/L) | 1.77a | 1.69b | 1.76a | 1.76a | 0.020 | 0.003 |
| FFA (µmol/L) | 368.30a | 356.30b | 367.58a | 365.07a | 4.008 | 0.026 |
| Insulin (mU/L) | 17.63b | 19.04a | 18.44ab | 17.66b | 0.403 | 0.006 |
TG, triglyceride; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; FFA, free fat acid.
CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile.
Mean values within a row with no common superscript differ significantly (P < 0.05).
Lipid Metabolism in Liver and Abdominal Adipose Tissues
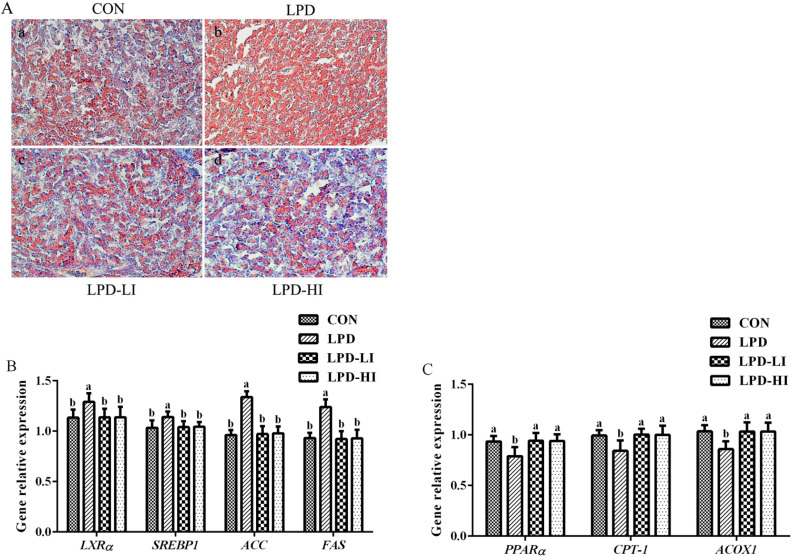
The liver fat content and integrated optical density were significantly higher (P < 0.001) in the LPD group compared to the other groups, while there were no significant differences among the CON, LPD-LI, and LPD-HI groups (Table 6 and Figure 2A). Compared to the LPD group, Ile supplementation significantly downregulated gene expressions of liver X receptors-α (LXRα; P = 0.015), SREBP1 (P = 0.016), ACC (P < 0.001) and FAS (P < 0.001), and enzyme activities of ACC (P = 0.014) and FAS (P = 0.001), while the gene expression levels of PPARα (P = 0.003), CPT-1 (P = 0.005) and ACOX1 (P = 0.002) and the enzyme activities of CPT-1 (P = 0.011) and ACOX1 (P = 0.010) were significantly upregulated, with no significant differences among the CON, LPD-LI, and LPD-HI groups (Table 6 and Figure 2B, C).
Table 6.
Effect of a LPD supplemented with isoleucine (Ile) on hepatic lipid metabolism in broilers.
| Item1 | CON2 | LPD | LPD-LI | LPD-HI | SEM | P-value |
|---|---|---|---|---|---|---|
| Liver fat content (%) | 12.90b | 16.61a | 12.97b | 12.80b | 0.343 | < 0.001 |
| Integrated optical density | 2.43b | 5.46a | 2.63b | 2.48b | 0.224 | < 0.001 |
| Enzyme activity (IU/g) | ||||||
| ACC | 6.93b | 7.94a | 6.83b | 6.79b | 0.364 | 0.014 |
| FAS | 35.52b | 47.26a | 39.57b | 38.74b | 2.360 | 0.001 |
| CPT-1 | 4.38a | 3.36b | 4.39a | 4.42a | 0.335 | 0.011 |
| ACOX1 | 3.93a | 3.02b | 3.94a | 4.14a | 0.319 | 0.010 |
ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; CPT-1, carnitine palmitoyl-transferase 1; ACOX1, acyl-CoA oxidase 1.
CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile.
Mean values within a row with no common superscript differ significantly (P < 0.05).
Figure 2.
Effect of a LPD supplemented with isoleucine (Ile) on hepatic lipid metabolism in broilers. (A) Histological analysis of liver sections by staining with oil red O. (B) Key genes related to lipogenesis. (C) Key genes related to lipolysis. LXRα, liver X receptors-α; SREBP1, sterol regulatory element-binding protein 1; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; PPARα, peroxisome proliferator activated receptor-α; CPT-1, carnitine palmitoyl-transferase 1; ACOX1, acyl-CoA oxidase 1; CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile. a,bBars with no common superscript differ significantly (P < 0.05).
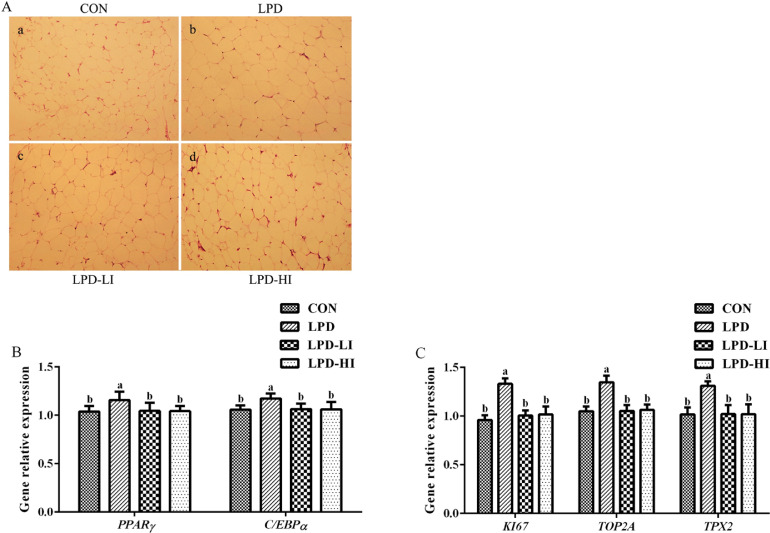
The abdominal adipose percentage (P < 0.001) and adipocyte area (P = 0.003) in the abdominal adipose tissue were higher in the LPD group than the other groups, but there were no significant differences among the CON, LPD-LI, and LPD-HI groups (Table 7 and Figure 3A). Relative expression levels of peroxisome proliferator-activated receptor-γ (PPARγ; P = 0.023), CCAAT/enhancer binding protein-α (C/EBPα; P = 0.006), ki67 (KI67; P < 0.001), TOP2A (P < 0.001) and thioredoxin-dependent peroxidase 2 (TPX2; P < 0.001) and enzyme activities of G3PDH (P = 0.002) and TOP2A (P = 0.005) were significantly increased in the LPD group compared to the CON group, while Ile supplementation inhibited (P < 0.05) this trend and the expression levels of these genes and the activities of these enzymes were equal to those of the CON group (Table 7 and Figure 3B, C).
Table 7.
Effect of a LPD supplemented with isoleucine (Ile) on lipid metabolism in abdominal adipose tissue of broilers.
| Item1 | CON2 | LPD | LPD-LI | LPD-HI | SEM | P-value |
|---|---|---|---|---|---|---|
| Abdominal adipose percentage (%) | 1.41b | 2.49a | 1.53b | 1.44b | 0.105 | < 0.001 |
| Adipocyte area (µm2) | 226.70b | 325.90a | 263.48b | 244.99b | 23.066 | 0.003 |
| Enzyme activity (IU/g) | ||||||
| G3PDH | 1.86b | 2.55a | 2.08b | 1.96b | 0.159 | 0.002 |
| TOP2A | 2.81b | 3.44a | 2.78b | 2.85b | 0.186 | 0.005 |
G3PDH, glycerol-3-phosphate dehydrogenase; TOP2A, topoisomerase II alpha.
CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile.
Mean values within a row with no common superscript differ significantly (P < 0.05).
Figure 3.
Effect of a LPD supplemented with isoleucine (Ile) on lipid metabolism in abdominal adipose tissue of broilers. (A) Histological analysis of abdominal adipose tissue sections by staining with hematoxylin and eosin. (B) Key genes related to preadipocyte differentiation. (C) Key genes related to adipocyte proliferation. PPARγ, peroxisome proliferator-activated receptor-γ; C/EBPα, CCAAT/enhancer binding protein-α; KI67, ki67; TOP2A, topoisomerase II alpha; TPX2, thioredoxin-dependent peroxidase 2; CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile. a,bBars with no common superscript differ significantly (P < 0.05).
AMPK/mTOR and Leptin/JAK2/STAT3 Signaling Pathways in Liver
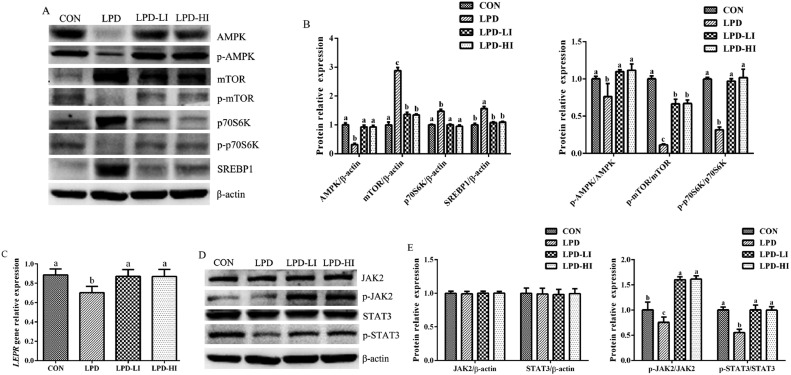
As shown in Figure 4, dietary supplementation with Ile significantly increased the downregulated relative gene expression of LEPR (P < 0.001) and relative protein expression levels of AMPK/β-actin (P < 0.001), p70S6K/β-actin (P < 0.001), p-AMPK/AMPK (P = 0.009), p-p70S6K/p70S6K (P < 0.001), and p-STAT3/STAT3 (P < 0.001) in the LPD group, and decreased (P < 0.001) the upregulated relative protein expression level of SREBP1/β-actin, whereas there were no significant differences among the CON, LPD-LI, and LPD-HI groups. Meanwhile, dietary Ile supplementation significantly decreased (P < 0.001) the upregulated relative protein expression of mTOR/β-actin and increased (P < 0.001) the downregulated relative protein expression of p-JAK2/JAK2 in the LPD group, but were higher (P < 0.001) than in the CON group. Relative protein expressions of p-mTOR/mTOR were higher (P < 0.001) in the LPD-LI and LPD-HI groups compared to the LPD group, but lower (P < 0.001) than in the CON group.
Figure 4.
Effect of a LPD supplemented with isoleucine (Ile) on the AMPK/mTOR and Leptin/JAK2/STAT3 signaling pathways in the liver of broilers. (A) Western blot analysis of proteins associated with the AMPK/mTOR signaling pathway. (B) Relative expression level of proteins associated with the AMPK/mTOR signaling pathway. (C) LEPR gene expression level. (D) Western blot analysis of proteins associated with the JAK2/STAT3 signaling pathway. (E) Relative expression level of proteins associated with the JAK2/STAT3 signaling pathway. AMPK, 5′ adenosine monophosphate-activated protein kinase; mTOR, mechanistic target of rapamycin; p70S6K, ribosomal protein 70 S6 kinase; SREBP1, sterol regulatory element-binding protein 1; LEPR, leptin receptor; JAK2, janus kinase 2; STAT3, signal transducer and activator of transcription 3; CON, control diet; LPD, low-protein diet; LPD-LI, low-protein diet + 0.13% Ile; LPD-HI, low-protein diet + 0.26% Ile. a,b,cBars with no common superscript differ significantly (P < 0.05).
DISCUSSION
Considering the economic and environmental advantages of a LPD, to meet the critical amino acid requirements for optimum performance of broilers fed a LPD is crucial (Attia et al., 2020). Mixed BCAA are reportedly vital to the regulation of growth performance and meat quality in pigs fed a LPD (Zhang et al., 2022). However, it is unknown whether a LPD supplemented with Ile promotes growth performance and meat quality. In the present study, growth performance was depressed in broilers fed a LPD, similar to a report by Liu et al. (2022). However, Ile supplementation significantly improved growth performance of broilers fed a LPD, partially in agreement with a previous study that reported significantly increased ADG and decreased F:G of broilers fed a diet supplemented with Ile (Corrent and Bartelt, 2011). Chicken meat is an excellent source of animal protein for human nutrition, thus meat quality is especially important to the consumer (Tang et al., 2021). The pH is an important parameter of meat quality. Within limits, pH is negatively correlated with the L* value, a* value, drip loss, and shear force (Le Bihan-Duval et al., 2008). The results of the present study showed that the pH24h value was higher, while the L* value, a* value, drip loss, and shear force were lower in the LPD-HI group, similar to report by Xu et al. (2020) that high Ile intake significantly increased the pH24h value and tended to decrease the a* value and drip loss of pork. To elucidate the possible mechanism affected by Ile supplementation on improving meat quality, the fiber characteristics and expression profiles of MyHC isoforms in breast muscle were examined in the present study. It is reported that there is a close relationship between fiber characteristics and shear force (An et al., 2010). In the present study, Ile supplementation decreased the fiber diameter and increased fiber density in broilers fed a LPD, which is a possible reason for the decreased shear force caused by Ile. Muscle fibers of broilers are classified into 3 types based on enzyme activity (Huo et al., 2022). Muscles with a higher proportion of MyHC-I fibers have higher pH and IMF content (Hou et al., 2020). In contrast, muscles with a higher proportion of MyHC-IIb fibers exhibit a quite opposite trend because of the increased content of glycogen and activities of glycolytic enzymes (Ismail and Joo, 2017). In the present study, Ile supplementation increased expression of MyHC-I and decreased expression of MyHC-IIb, which could explain the effect of Ile on the pH24h value and IMF content.
The abdomen is the main site of lipid deposition in broilers (Li et al., 2020). In the present study, the abdominal adipose percentage and adipocyte area were increased in broilers fed a LPD, in agreement with the findings of a previous study (Sharma et al., 2022). Dietary Ile supplementation markedly decreased lipid deposition in the abdomen as reported in a previous study of broilers (Maynard et al., 2022). The formation of adipose tissue includes the differentiation of preadipocytes to adipocytes and the proliferation of adipocytes (Bai et al., 2017). The PPARγ and C/EBPα are the main regulators of preadipocyte differentiation, while KI67, TOP2A, and TPX2 are markers of adipocyte proliferation. G3PDH, an indirect marker of triacylglycerol synthesis, is closely associated with preadipocyte differentiation (Sledzinski et al., 2013). In this study, Ile decreased the gene expressions of PPARγ, C/EBPα, KI67, TOP2A, and TPX2, and the enzyme activities of G3PDH and TOP2A, suggesting that Ile reduced lipid deposition in the abdomen by inhibiting preadipocyte differentiation and adipocyte proliferation.
In avian species, the liver accounts for about 95% of de novo synthesis of fatty acids, indicating that liver is the most important organ for intermediary metabolism of lipids and energy (Wan et al., 2021). Compared to the more vigorous lipid deposition in the liver of broilers fed a LPD, the fat content and integrated optical density in the liver of broilers fed a LPD supplemented with Ile were closer to those of the CON group. Consistently, dietary supplementation with Ile significantly reduced serum lipids levels, further suggesting that Ile alleviates lipid deposition. Lipid deposition in liver is reportedly dependent on the dynamic balance between fatty acid synthesis and β-oxidation (Li et al., 2019). LXRα, as the primary regulator of hepatic lipid metabolism, regulates fatty acid synthesis via activation of SREBP1, which binds to the promoter regions of major genes associated with lipogenesis, including ACC and FAS, to enhance expression (Schultz et al., 2000). The results of the present study showed that Ile downregulated the increased gene expressions of LXRα, SREBP1, ACC, and FAS, and decreased the elevated enzyme activities of ACC and FAS in broilers fed a LPD, resulting in decreased fatty acid synthesis. PPARα, as a member of the nuclear receptor superfamily, serves an important role in fatty acid β-oxidation (Huang et al., 2013). Activation of PPARα is reported to upregulate the expression levels of genes related to lipolysis (CPT-1 and ACOX1) and enhance fatty acid β-oxidation (Li et al., 2021). In this study, decreased gene expressions of PPARα, CPT-I, and ACOX1 and downregulated enzyme activities of CPT-1 and ACOX1 in broilers fed a LPD were drastically increased by Ile. Genes and enzymes related to both lipogenesis and lipolysis are regulated by the AMPK/mTOR and Leptin/JAK2/STAT3 signaling pathways. Activation of hepatic AMPK suppresses fatty acid synthesis and stimulates fatty acid β-oxidation via inhibition of the activities of mTOR and p70S6K by phosphorylation at Ser2448 and Thr389 and reducing protein expression of SREBP1 (Wang et al., 2017). Leptin suppresses food intake, reduces blood TG content, and increases energy expenditure (Wang et al., 2020). The binding of leptin and LEPR phosphorylates JAK2 and activates protein kinase sites that recruit STAT3 and tyrosine phosphorylation by receptor-associated JAK2. Phosphorylated STAT3 dimerizes and translocates into the nucleus, where it binds to the promoters of target genes to regulate lipid metabolism (Chen et al., 2019). In the present study, Ile supplementation upregulated the decreased serum leptin level and expressions of LEPR at the mRNA level and p-AMPK/AMPK, p-mTOR/mTOR, p-p70S6K/p70S6K, p-JAK2/JAK2, and p-STAT3/STAT3 at the protein level, and downregulated the increased protein relative expression of SREBP1. These modulation patterns were in line with the regulatory roles of the AMPK/mTOR and Leptin/JAK2/STAT3 signaling pathways, suggesting that Ile could alleviate the LPD-induced lipid deposition through activation of these two pathways.
In summary, the results of the present study demonstrated that dietary supplementation with Ile improved the growth performance and meat quality of broilers fed a LPD. Furthermore, Ile supplementation activated the AMPK/mTOR and Leptin/JAK2/STAT3 signaling pathways, decreased lipogenesis and promoted lipolysis in the liver, and inhibited differentiation and proliferation of abdominal adipocytes, thereby alleviating the lipid deposition induced by a LPD.
AUTHOR CONTRIBUTIONS
Shengnan Ma: Data collection, Data analyses, Writing-Original Draft. Kai Zhang: Data collection & Data analyses. Shuyan Shi: Investigation. Xuemin Li: Formal analysis. Chuanyan Che: Investigation. Peng Chen: Visualization; Huawei Liu: Conceptualization, Writing-Review & Editing, Funding acquisition. All authors have read and approved the published version of the manuscript.
ACKNOWLEDGMENTS
This study was financially supported by the Shandong Provincial Natural Science Foundation (grant no. ZR2021MC118), the Qingdao Science and Technology Program (grant no. 22-3-7-xdny-11-nsh) and the Postgraduate Innovation Program of Qingdao Agricultural University (grant no. QNYCX21055). The technical assistance of Jianlin Wang and Kun Xu was gratefully appreciated. We would like to thank CJ (Shanghai) Trading Co., Ltd. for supplying isoleucine and the many graduate students from the Department of Animal Nutrition and Feed Science for excellent assistance in conducting the animal trial.
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
REFERENCES
- An J.Y., Zheng J.X., Li J.Y., Zeng D., Qu L.J., Xu G.Y., Yang N. Effect of myofiber characteristics and thickness of perimysium and endomysium on meat tenderness of chickens. Poult. Sci. 2010;89:1750–1754. doi: 10.3382/ps.2009-00583. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Bovera F., Wang J., Al-Harthi M.A., Kim W.K. Multiple amino acid supplementations to low-protein diets: effect on performance, carcass yield, meat quality and nitrogen excretion of finishing broilers under hot climate conditions. Animals (Basel) 2020;10:973. doi: 10.3390/ani10060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S.P., Wang G.Q., Zhang W., Zhang S., Rice B.B., Cline M.A., Gilbert E.R. Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015;189:115–123. doi: 10.1016/j.cbpa.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Bai S.P., Pan S.Q., Zhang K.Y., Ding X.M., Wang J.P., Zeng Q.F., Xuan Y., Su Z.W. Dietary overload lithium decreases the adipogenesis in abdominal adipose tissue of broiler chickens. Environ. Toxicol. Pharmacol. 2017;49:163–171. doi: 10.1016/j.etap.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Cai Y.L., Song Z.G., Wang X.J., Jiao H.C., Lin H. Dexamethasone-induced hepatic lipogenesis is insulin dependent in chickens (Gallus gallus domesticus) Stress. 2011;14:273–281. doi: 10.3109/10253890.2010.543444. [DOI] [PubMed] [Google Scholar]
- Castellini C., Mugnai C., Dal Bosco A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002;60:219–225. doi: 10.1016/s0309-1740(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Chen Y.N., Lu W., Jin Z.Y., Yu J., Shi B.M. Carbenoxolone ameliorates hepatic lipid metabolism and inflammation in obese mice induced by high fat diet via regulating the JAK2/STAT3 signaling pathway. Int. Immunopharmacol. 2019;74 doi: 10.1016/j.intimp.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Chodová D., Tůmová E., Ketta M., Skřivanová V. Breast meat quality in males and females of fast-, medium- and slow-growing chickens fed diets of 2 protein levels. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrent E., Bartelt J. Valine and isoleucine: the next limiting amino acids in broiler diets. Lohmann. Inform. 2011;46:59. [Google Scholar]
- Dozier Iii W.A., Corzo A., Kidd M.T., Tillman P.B., Branton S.L. Determination of the fourth and fifth limiting amino acids in broilers fed on diets containing maize, soybean meal and poultry by-product meal from 28 to 42 d of age. Br. Poult. Sci. 2011;52:238–244. doi: 10.1080/00071668.2011.561282. [DOI] [PubMed] [Google Scholar]
- Duan Y.H., Li F.N., Wen C.Y., Wang W.L., Guo Q.P., Li Y.H., Yin Y.L. Branched-chain amino acid ratios in low-protein diets regulate the free amino acid profile and the expression of hepatic fatty acid metabolism-related genes in growing pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 2018;102:43–51. doi: 10.1111/jpn.12698. [DOI] [PubMed] [Google Scholar]
- Erwan E., Alimon A.R., Sazili A.Q., Yaakub H., Karim R. Effects of levels of L-leucine supplementation with sub-optimal protein in the diet of grower-finisher broiler chickens on carcass composition and sensory characteristics. Asian Austral. J. Anim. 2011;24:650–654. [Google Scholar]
- Heng J.H., Wu Z.H., Tian M., Chen J.M., Song H.Q., Chen F., Guan W.T., Zhang S.H. Excessive BCAA regulates fat metabolism partially through the modification of m6A RNA methylation in weanling piglets. Nutr. Metab. (Lond) 2020;17:10. doi: 10.1186/s12986-019-0424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Hou Y.R., Su L., Su R.N., Luo Y.L., Wang B.H., Yao D., Zhao L.H., Jin Y. Effect of feeding regimen on meat quality, MyHC isoforms, AMPK, and PGC-1α genes expression in the biceps femoris muscle of Mongolia sheep. Food Sci. Nutr. 2020;8:2262–2270. doi: 10.1002/fsn3.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Wang Y.F., Sheikhahmadi A., Li X.L., Buyse J., Lin H., Song Z.G. Effects of glucocorticoids on lipid metabolism and AMPK in broiler chickens' liver. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019;232:23–30. doi: 10.1016/j.cbpb.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Huang J.B., Zhang Y., Zhou Y.B., Zhang Z.Z., Xie Z.W., Zhang J.S., Wan X.C. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agr. Food Chem. 2013;61:8565–8572. doi: 10.1021/jf402004x. [DOI] [PubMed] [Google Scholar]
- Huo W.R., Weng K.Q., Li Y., Zhang Y., Zhang Y., Xu Q., Chen G.H. Comparison of muscle fiber characteristics and glycolytic potential between slow- and fast-growing broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I., Joo S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M.T., Burnham D.J., Kerr B.J. Dietary isoleucine responses in male broiler chickens. Br. Poult. Sci. 2004;45:67–75. doi: 10.1080/00071660410001668888. [DOI] [PubMed] [Google Scholar]
- Kidd M.T., Maynard C.W., Mullenix G.J. Progress of amino acid nutrition for diet protein reduction in poultry. J. Anim. Sci. Biotechnol. 2021;12:45. doi: 10.1186/s40104-021-00568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Debut M., Berri C.M., Sellier N., Sante-Lhoutellier V., Jego Y., Beaumont C. Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genet. 2008;9:53. doi: 10.1186/1471-2156-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.F., Zhang K., Pan Z.X., Yu M.L., Lu Y.L., Wang G.Y., Wu J.F., Zhang J., Zhang K.N., Du W.X. Antibiotics promote abdominal fat accumulation in broilers. Anim. Sci. J. 2020;91:e13326. doi: 10.1111/asj.13326. [DOI] [PubMed] [Google Scholar]
- Li L.L., Zhang H.H, Yao Y., Yang Z.M., Ma H.T. (-)-Hydroxycitric acid suppresses lipid droplet accumulation and accelerates energy metabolism via activation of the adiponectin-AMPK signaling pathway in broiler chickens. J. Agr. Food Chem. 2019;67:3188–3197. doi: 10.1021/acs.jafc.8b07287. [DOI] [PubMed] [Google Scholar]
- Li X.W., Liu S.P., Wang J., Yi J.N., Yuan Z.H., Wu J., Wen L.X., Li R.F. Effects of ND vaccination combined LPS on growth performance, antioxidant performance and lipid metabolism of broiler. Res. Vet. Sci. 2021;135:317–323. doi: 10.1016/j.rvsc.2020.10.007. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Wei H.K., Li F.N., Duan Y.H., Guo Q.P., Yin Y.L. Effects of low-protein diets supplemented with branched-chain amino acid on lipid metabolism in white adipose tissue of piglets. J. Agr. Food Chem. 2017;65:2839–2848. doi: 10.1021/acs.jafc.7b00488. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Li K., Lv M.B., Zhao J.S., Xiong B.H. Effects of chestnut tannins on the meat quality, welfare, and antioxidant status of heat-stressed lambs. Meat Sci. 2016;116:236–242. doi: 10.1016/j.meatsci.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Zhao F., Zhang K., Zhao J.S., Wang Y. Investigating the growth performance, meat quality, immune function and proteomic profiles of plasmal exosomes in Lactobacillus plantarum-treated broilers with immunological stress. Food Funct. 2021;12:11790–11807. doi: 10.1039/d1fo01936h. [DOI] [PubMed] [Google Scholar]
- Liu Q.W., Feng J.H., Wei L.M., Hu C.J., Zheng X.L., Sun R.P., Zhang M.H. Interactive effects of high temperature and crude protein levels on growth performance, nitrogen excretion, and fecal characteristics of broilers. Trop. Anim. Health Prod. 2022;54:392. doi: 10.1007/s11250-022-03380-8. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y.H., Zhang X., Zhu Z.P., Jiao N., Qiu K., Yin J.D. Surplus dietary isoleucine intake enhanced monounsaturated fatty acid synthesis and fat accumulation in skeletal muscle of finishing pigs. J. Anim. Sci. Biotechnol. 2018;9:88. doi: 10.1186/s40104-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q.Q., Zhou X.B., Hu L.L., Chen J.Y., Zhu J.L., Shan A.S. Leucine and isoleucine have similar effects on reducing lipid accumulation, improving insulin sensitivity and increasing the browning of WAT in high-fat diet-induced obese mice. Food Funct. 2020;11:2279–2290. doi: 10.1039/c9fo03084k. [DOI] [PubMed] [Google Scholar]
- Maynard C.W., Mullenix G.J., Maynard C.J., Wells-Crafton S.C., Lee J.T., Rao S.K., Butler L.D., Orlowskl S.K., Kidd M.T. Titration of dietary isoleucine and evaluation of branched-chain amino acid levels in female Cobb 500 broilers during a 22-to 42-day finisher period. J. Appl. Poultry Res. 2022;31 [Google Scholar]
- Nascimento G.R., Murakami A.E., Ospina-Rojas I.C., Diaz-Vargas M., Picoli K.P., Garcia R.G. Digestible valine requirements in low-protein diets for broilers chicks. Braz. J. Poult. Sci. 2016;18:381–386. [Google Scholar]
- Nie C.X., He T., Zhang W.J., Zhang G.L., Ma X. Branched chain amino acids: beyond nutrition metabolism. Int. J. Mol. Sci. 2018;19:954. doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nutrient Requirements of Poultry. 9th ed. National Academy Press; Washington, DC: 1994. [Google Scholar]
- Park H.K., Ahima R.S. Leptin signaling. F1000prime Rep. 2014;6:73. doi: 10.12703/P6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J.R., Tu H., Luk A., Repa J.J., Medina J.C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D.J., Lustig K.D., Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.K., Cadogan D.J., Chrystal P.V., McGilchrist P., Wilkinson S.J., Inhuber V., Moss A.F. Guanidinoacetic acid as a partial replacement to arginine with or without betaine in broilers offered moderately low crude protein diets. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigolo S., Zohrabi Z., Gallo A., Seidavi A., Prandini A. Effect of a low crude protein diet supplemented with different levels of threonine on growth performance, carcass traits, blood parameters, and immune responses of growing broilers. Poult. Sci. 2017;96:2751–2760. doi: 10.3382/ps/pex086. [DOI] [PubMed] [Google Scholar]
- Sledzinski T., Korczynska J., Goyke E., Stefaniak T., Proczko-Markuszewska M., Kaska L., Swierczynski J. Association between cytosolic glycerol 3-phosphate dehydrogenase gene expression in human subcutaneous adipose tissue and BMI. Cell Physiol. Biochem. 2013;32:300–309. doi: 10.1159/000354438. [DOI] [PubMed] [Google Scholar]
- Tang J.Y., He Z., Liu Y.G., Jia G., Liu G.M., Chen X.L., Tian G., Cai J.Y., Kang B., Zhao H. Effect of supplementing hydroxy selenomethionine on meat quality of yellow feather broiler. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.L., Yang Z.F., Ji H.R., Li N., Yang Z., Xu L., Yang H.M., Wang Z.Y. Effects of lycopene on abdominal fat deposition, serum lipids levels and hepatic lipid metabolism-related enzymes in broiler chickens. Anim. Biosci. 2021;34:385–392. doi: 10.5713/ajas.20.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Mao Y.J., Wang B., Wang S.S., Lu H., Ying L.L., Li Y. Quercetin improving lipid metabolism by regulating lipid metabolism pathway of ileum mucosa in broilers. Oxid. Med. Cell Longev. 2020;2020 doi: 10.1155/2020/8686248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.J., Li X.J., Guo H.L., Yuan Z.H., Wang T., Zhang L.Y., Jiang Z.Z. Emodin alleviates hepatic steatosis by inhibiting sterol regulatory element binding protein 1 activity by way of the calcium/calmodulin-dependent kinase kinase-AMP-activated protein kinase-mechanistic target of rapamycin-p70 ribosomal S6 kinase signaling pathway. Hepatol. Res. 2017;47:683–701. doi: 10.1111/hepr.12788. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Yuan T., Yang J., Zheng W.X., Wu Q.L., Zhu K.X., Mou X.Y., Wang L.Z., Nie K.K., Li X.Y., Zhu Y.W. Responses of combined non-starch polysaccharide enzymes and protease on growth performance, meat quality, and nutrient digestibility of yellow-feathered broilers fed with diets with different crude protein levels. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.946204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.D., Wang Y.B., Jiao N., Qiu K., Zhang X., Wang L.Q., Wang L., Yin J.D. The coordination of dietary valine and isoleucine on water holding capacity, pH value and protein solubility of fresh meat in finishing pigs. Meat Sci. 2020;163:74. doi: 10.1016/j.meatsci.2020.108074. [DOI] [PubMed] [Google Scholar]
- Zhang K., Li X.M., Zhao J.S., Wang Y., Hao X.J., Liu K.D., Liu H.W. Protective effects of chlorogenic acid on the meat quality of oxidatively stressed broilers revealed by integrated metabolomics and antioxidant analysis. Food Funct. 2022;13:2238–2252. doi: 10.1039/d1fo03622j. [DOI] [PubMed] [Google Scholar]
- Zhang L.Y., Li F.N., Guo Q.P., Duan Y.H., Wang W.L., Yang Y.H., Yin Y.J., Gong S.M., Han M.M., Yin Y.L. Different proportions of branched-chain amino acids modulate lipid metabolism in a finishing pig model. J. Agr. Food Chem. 2021;69:7037–7048. doi: 10.1021/acs.jafc.1c02001. [DOI] [PubMed] [Google Scholar]
- Zhang L.Y., Li F.N., Guo Q.P., Duan Y.H., Wang W.L., Yang Y.H., Yin Y.J., Gong S.M., Han M.M., Yin Y.L. Balanced branched-chain amino acids modulate meat quality by adjusting muscle fiber type conversion and intramuscular fat deposition in finishing pigs. J. Sci. Food Agr. 2022;102:3796–3807. doi: 10.1002/jsfa.11728. [DOI] [PubMed] [Google Scholar]