Abstract
Pulmonary hypertension (PH) is caused by a range of conditions and is important to recognize as it is associated with increased mortality. Pulmonary arterial hypertension refers to a group of PH subtypes affecting the distal pulmonary arteries for which effective treatment is available. The hemodynamic definition of pulmonary arterial hypertension has recently changed which may lead to greater case recognition and earlier treatment. The prevalence of specific PH etiologies may differ depending on geographic region. PH caused by left heart disease is the most common cause of PH worldwide. In Asia, there is greater proportion of congenital heart disease– and connective tissue disease– (especially systemic lupus erythematosus) related PH relative to the West. This review summarizes the definition, classification, and epidemiology of PH as it pertains to Asia.
Key Words: pulmonary arterial hypertension, Asia
Abbreviations and Acronyms: BPA, balloon pulmonary angioplasty; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RHC, right heart catheterization; WSPH, World Symposium on Pulmonary Hypertension
Central Illustration
Highlights
-
•
There are significant epidemiological differences in pulmonary hypertension between Asian and Western regions.
-
•
Asian data are scarce and are based on national registries and small cohort studies.
-
•
Larger multinational Asian epidemiological studies are needed to inform regional pulmonary hypertension trends.
Pulmonary hypertension (PH) refers to elevated pressure within the pulmonary artery and is an important cause of morbidity and mortality worldwide. Varied underlying diseases may lead to PH, with left heart disease (LHD) and chronic lung disease being the most common.1 Of particular interest is pulmonary arterial hypertension (PAH), a specific subgroup of PH where the disease is characterized by a primary pulmonary vasculopathy of the distal pulmonary arteries for which pulmonary vasodilator drug treatment is effective.2 Identifying and classifying PH can be challenging, but has received increasing attention over the past 20 years in concert with the development of PAH therapies.2 The presence of PH, irrespective of etiology, is associated with increased mortality.3 PH results in an increase in right ventricular afterload and may result in progressive maladaptation of the right ventricle and ultimately right heart failure.4
Hemodynamic Definitions
PH has historically been defined as a mean pulmonary artery pressure (mPAP) ≥25 mm Hg measured by right heart catheterization (RHC).5 The Sixth World Symposium on Pulmonary Hypertension (6th WSPH) in 2018 proposed a new hemodynamic threshold of mPAP >20 mm Hg.6, 7, 8 A number of reasons led to the proposed change in the hemodynamic definition of PH. In a meta-analysis of RHC data from normal subjects, resting mPAP was 14 ± 3.3 mm Hg with a limit of 20 mm Hg using 2 SDs above the normal value.9 Normal resting mPAP was also found to be independent of age, ethnicity, and sex. In addition, large cohort studies have clearly established increased mortality and risk for hospitalization for mPAP 21 to 24 mm Hg or previously so-called “borderline PH.”10, 11, 12, 13 These studies are summarized in Table 1.
Table 1.
Cohort Studies Examining Mildly Elevated Mpap and Mortality
| Maron et al10 | Douschann et al11 | Kovacs et al12 | Valerio et al13 | Heresi et al66 | |
|---|---|---|---|---|---|
| Country | USA | Austria | Austria | England | USA |
| Years enrolled | 2007-2012 | 2005-2014 | 2006-2010 | <2010 | 1990-2012 |
| N | 21,727 | 547 | 141 | 228 | 1,491 |
| Population | U.S. Veterans cohort | Two large COPD cohorts | PH clinic cohort | Systemic sclerosis cohort | Cleveland clinic PH registry |
| No. of subjects with normal mPAP (Reference) | 4,207 | 193 | 109 | 142 | 85 |
| No. of subjects with mildly elevated mPAP | 5,030 | 64 | 32 | 86 | 63 |
| Definition of mildly elevated mPAP, mm Hg | 19-24 | 20.6-24.9 | 20-25 | 21-24 | 21-24 |
| Mortality HR (95% CI) | 1.23 (1.12-1.36), P < 0.000166 | 2.37 (1.14-4.97) | 4.75 | 3.7a (1.7-8.0), P < 0.001 | 3.08 (0.60-15.90), P = 0.18 |
COPD = chronic obstructive pulmonary disease; mPAP = mean pulmonary artery pressure; PH = pulmonary hypertension.
HR to develop PH, not mortality.
The change in hemodynamic definition of PH poses new challenges in the field. The updated definition will lead to increased case finding of patients with PH. It has the potential to identify patients with PH at an earlier stage of the disease, and optimistically, permit introduction of therapeutics at an earlier timeframe with potential to improve outcomes. However, clinical therapeutics in PH have not been proven to be of benefit in patients with mPAP 21 to 24 mm Hg because all clinical trials have universally used mPAP ≥25 mm Hg as an inclusion criteria before the definition change at the 6th WSPH.14
The finding of elevated pulmonary artery pressure in isolation is insufficient to determine the underlying mechanism(s) leading to PH. Elevated pulmonary artery pressure does not imply a primary pulmonary vasculopathy and may occur in the setting of elevated cardiac output in certain physiological states (eg, exercise and pregnancy) and noncardiopulmonary disease states (eg, liver disease and anemia). Furthermore, LHD can lead to elevated left atrial pressure and passive increased in mPAP via pulmonary venous hypertension. For these reasons, right heart catheter measurements of cardiac output and pulmonary artery wedge pressure (PAWP), and calculation of pulmonary vascular resistance (PVR = (mPAP − PAWP)/cardiac output) are integral to diagnosing PH and determining its hemodynamic classification. The 6th WSPH further proposed measurement of PVR ≥3 Wood units (WU) in conjunction with mPAP ≥20 mm Hg and PAWP ≤15 mm Hg as the hemodynamic definition of precapillary PH.8 A PAWP >15 mm Hg designates postcapillary PH (ie, PH caused by LHD [PH-LHD]), which can be further subdivided into isolated postcapillary forms (PVR <3 WU) and combined precapillary and postcapillary (CpcPH) forms (PVR ≥3 WU). The hemodynamic definitions are summarized in Table 2.
Table 2.
Hemodynamic Definitions of PH
| Characteristics | Clinical Group No | |
|---|---|---|
| Precapillary PH | mPAP >20 mm Hg | 1, 3, 4, and 5 |
| PAWP ≤15 mm Hg | ||
| PVR ≥3 WU | ||
| Isolated postcapillary PH | mPAP >20 mm Hg | 2 and 5 |
| PAWP >15 mm Hg | ||
| PVR <3 WU | ||
| Combined precapillary and postcapillary PH | mPAP >20 mm Hg | 2 and 5 |
| PAWP >15 mm Hg | ||
| PVR ≥3 WU |
Reproduced with permission from Simonneau et al.6
Clinical groups: 1 = pulmonary arterial hypertension; 2 = pulmonary hypertension (PH) caused by left heart disease; 3 = PH caused by lung disease and/or hypoxia; 4 = PH caused by pulmonary artery obstruction; 5 = PH with unclear and/or multifactorial mechanisms; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; other abbreviations as in Table 1.
Although the 6th WSPH has provided clarity in terms of the issue of mildly elevated mPAP, there remains debate on the most appropriate threshold of PVR for the diagnosis of precapillary PH. A recent study involving more than 4,000 subjects who had undergone RHC showed that the all-cause mortality hazard for PVR was increased at ∼2.2 WU compared with PVR of 1.0 WU. Among patients with an mPAP of >19 mm Hg and PAWP <15 mm Hg, the adjusted HR for mortality was 1.71 (95% CI: 1.59-1.84; P < 0.0001) and for heart failure hospitalization was 1.27 (95% CI: 1.13-1.43; P = 0.0001), using a cutoff PVR threshold of 2.2 WU. These data suggest that risk of adverse outcomes may begin at a level less than the current PVR >3 WU threshold for definition of precapillary PH.15,16 Furthermore, a small observation study showed that patients with mPAP >25 mm Hg, PAWP <15 mm Hg, and PVR between 2 and 3 WU may show response to pulmonary vasodilator therapy.15 It is important to recognize that lowering the PVR threshold will introduce increased risk of inappropriately treating patients with pulmonary vasodilators who do not have true pulmonary vascular disease. Furthermore, it is unclear whether mildly elevated PVR may simply represent a biomarker for increased mortality in patients with cardiopulmonary comorbidities, and patients may not necessarily die from pulmonary vascular disease and right heart failure. The clinical implications of PVR between 2 and 3 WU is an area which requires further study.
Clinical Classification of PH
PH is divided into 5 major groups for clinical classification purposes. These are: 1) PAH; 2) PH-LHD; (3) PH caused by lung disease and/or hypoxia (PH-LD); 4) chronic thromboembolic PH (CTEPH); and 5) PH with unclear and/or multifactorial mechanisms.5,8 In addition to hemodynamic differences (Table 2), the groups encompass a broad range of underlying conditions, with varied prevalence and therapeutic approach. The current PH clinical classification system is presented in Table 3.
Table 3.
Updated Clinical Classification of Pulmonary Hypertension. Description of Groups 1-5
| 1 PAH |
| 1.1 Idiopathic PAH |
| 1.2 Heritable PAH |
| 1.3 Drug- and toxin-induced PAH |
| 1.4 PAH associated with: |
| 1.4.1 Connective tissue disease |
| 1.4.2 HIV infection |
| 1.4.3 Portal hypertension |
| 1.4.4 Congenital heart disease |
| 1.4.5 Schistosomiasis |
| 1.5 PAH long-term responders to calcium channel blockers |
| 1.6 PAH with overt features of venous/capillaries (PVOD/PCH) involvement |
| 1.7 Persistent PH of the newborn syndrome |
| 2 PH caused by left heart disease |
| 2.1 PH caused by heart failure with preserved LVEF |
| 2.2 PH caused by heart failure with reduced LVEF |
| 2.3 Valvular heart disease |
| 2.4 Congenital/acquired cardiovascular conditions leading to postcapillary PH |
| 3 PH caused by lung diseases and/or hypoxia |
| 3.1 Obstructive lung disease |
| 3.2 Restrictive lung disease |
| 3.3 Other lung disease with mixed restrictive/obstructive pattern |
| 3.4 Hypoxia without lung disease |
| 3.5 Developmental lung disorders |
| 4 PH caused by pulmonary artery obstructions |
| 4.1 Chronic thromboembolic PH |
| 4.2 Other pulmonary artery obstructions |
| 5 PH with unclear and/or multifactorial mechanisms |
| 5.1 Hematological disorders |
| 5.2 Systemic and metabolic disorders |
| 5.3 Others |
| 5.4 Complex congenital heart disease |
Reproduced with permission from Simonneau et al.6
LVEF = left ventricular ejection fraction; PAH = pulmonary arterial hypertension; PCH = pulmonary capillary hemangiomatosis; PVOD = pulmonary veno-occlusive disease.
Group 1: PAH
PAH is uncommon relative to other forms of PH, with an estimated prevalence of 15 to 50 per million.17,18 Group 1 PAH conditions are characterized by pulmonary artery vascular cell proliferation, luminal narrowing, and loss of cross-sectional area.4 Common causes of PAH include idiopathic, heritable, drug-induced, and connective tissue disease– and congenital heart disease–associated. Rarer causes of PAH include HIV, schistosomiasis, and portal hypertension.
Randomized trials have shown effectiveness of vasodilator agents, including phosphodiesterase-5 inhibitors, endothelin receptor antagonists, soluble guanylate cyclase inhibitors, and prostacyclin analogues in group 1 PAH. Historically, idiopathic PAH was predominantly recognized in younger females; however, there is increasing prevalence of PAH affecting more elderly patients with a more balanced sex ratio, comorbidities, and potentially less favorable response to therapy.19 Hemodynamically, PAH is characterized by precapillary PH with mPAP >20 mm Hg, PAWP <15 mm Hg and PVR >3 WU.8
Group 2 PH
Group 2 PH refers to PH-LHD, including left ventricular systolic and diastolic dysfunction and valvular heart disease. PH-LHD is overwhelmingly the most common cause of PH, constituting 75% of all causes of PH in population studies.20,21 Hemodynamic distinction relies on accurate measurement of PAWP >15 mm Hg which designates “postcapillary PH.”8 Postcapillary PH can be further defined based on measurement of diastolic pressure gradient (DPG) as follows: isolated postcapillary PH with DPG <7 mm Hg and/or PVR ≤3 WU, or CpcPH with DPG >7 mm Hg and/or PVR >3 WU.22 Elevated PVR in PH-LHD (ie, CpcPH) has been associated with increased mortality.22,23 To date, vasodilator treatments have not shown any benefit, and in some trials have shown harm.24,25
Group 3 PH
Group 3 PH or chronic lung disease–related PH (CLD-PH) is the second most common cause of PH. Hemodynamically, most CLD-PH tends to be associated with mild to moderate elevation rather than very severe PH seen in group 1 PAH. However, a small proportion of CLD-PH may present with very severe precapillary PH. The presence of PH, regardless of severity, has been associated with increased mortality in the setting of CLDs such as chronic obstructive pulmonary disease and interstitial lung disease.26 Expert guidelines suggest complex lung function testing cutoffs of forced expiratory volume in the first second <60% (obstructive pathologies) and forced vital capacity <70% (restrictive pathologies) in conjunction with moderate to severe computed tomography parenchymal abnormality as designating CLD-PH.26 A recent randomized controlled trial of inhaled treprostinil in PH caused by interstitial lung disease showed improvements in 6-minute walk distance and time to clinical worsening, and it is the only drug with regulatory approval for treatment in CLD-PH.27 In CLD-PH associated with hypoxic respiratory failure, long-term oxygen therapy is indicated.
Group 4 PH
This group consists of conditions where obstructive lesions of the pulmonary artery results in PH, with CTEPH being the most common. Rarely, pulmonary artery obstruction can be the result of pulmonary artery sarcoma or large-vessel pulmonary vasculitis (eg, Takayasu arteritis). CTEPH is an uncommon but underdiagnosed condition caused by fibrotic narrowing of the more proximal elastic pulmonary arteries from nonresolved venous thromboembolism. CTEPH is estimated to complicate 3% to 4% of pulmonary embolism cases and is defined as PH in the setting of unresolved pulmonary emboli despite 3 months of anticoagulation.28 Pathologically, nonresolved thrombus leads to organization and fibrosis within the pulmonary arterial bed causing impairment to blood flow and PH.28 Pulmonary endarterectomy is the treatment of choice for CTEPH and can be curative.29 For nonoperable patients, balloon pulmonary angioplasty (BPA) and pharmacological therapy is available.30,31 BPA had been pioneered and refined over many years in Japan, with worldwide adoption of their modern approach beginning in 2012. This has led to significant differences between Western and Japanese approaches to CTEPH treatment.32
Group 5 PH
Group 5 PH includes so-called miscellaneous and rare causes of PH. These may include PH associated with myeloproliferative disorders, chronic renal failure, sarcoidosis, and thyroid disease. Epidemiological data and therapeutic trial data are limited to small case series and reports.
Epidemiology of PH in Asia
An estimated prevalence of PH at the population level is approximately 1% to 3%.33,34 PAH is rare, with an estimated prevalence of 15 to 30 per million.20,33 The incidence and prevalence of PH is increasing, likely for multifactorial reasons.35 Heightened awareness, access to echocardiogram, and right heart catheter studies and an ageing population are likely the chief contributors. Lesser factors which will continue to play a role include increased case finding related to definition changes (mPAP >20 mm Hg) and improved treatments leading to prolonged survival.
Contemporary Asian data regarding pulmonary hypertension is more limited relative to the West. Differences in PH epidemiology may be expected based on genetic, environmental, behavioral and socioeconomic factors, although such factors are heterogenous throughout Asia.1
Variable socioeconomic, dietary, and lifestyle factors have contributed to a rising epidemic of cardiovascular disease in Asia.36 As in the West, PH-LHD is likely to be the most common PH subtype, although the etiology is likely more varied in Asia. The incidence of congenital heart disease is as high as 25 per 1,000 in developing countries in Asia, relative to approximately 10 per 1,000 worldwide.37 Rheumatic heart disease occurs with significantly higher prevalence, approximately 1,000-fold, in South Asian countries compared to Western countries, and has not declined significantly in the past 30 years.38
Tobacco smoking remains a significant public health problem in most Asian countries, and in some countries rates of smoking continue to increase.39 Rates of PH caused by lung disease (ie, chronic obstructive pulmonary disease) may be higher than observed in Western registries where smoking rates have fallen. In addition, tuberculosis incidence in a number of Asian countries is the highest in the world, which could be expected to lead to higher rates of PH-LD.40 Hoeper et al33 summarized crude prevalence estimates of PH-LHD (15 million) and PH-LD (11 million) in Asia to be the highest of all world regions.
In socioeconomically disadvantaged Asian regions, mirroring middle- and low-income countries globally, there is likely increased prevalence of PH related to infections including HIV, schistosomiasis, and rheumatic fever compared to Western countries.41
Finally, high altitude pulmonary hypertension occurs in persons living above 2,500 m sea level, and the Asian Himalayas are home to more than 50 million people. Although right heart catheter diagnostic confirmation is rarely performed, epidemiological studies of Kyrgyzstan highlanders found that 14% had electrocardiographic features of cor pulmonale.42
Western PH Registries
Registries serve as useful cohort studies to inform epidemiological traits, natural history, survival, and treatment data in rare diseases such as PAH. The first primary PH registry was commissioned by the U.S.-based National Institutes of Health in 1981.43 Since then, numerous PH registries have been established and reported, with major focus on group 1 PAH. One of the largest is the U.S. REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management) registry, based on 55 U.S. centers between 2006 and 2009, which described the demographics, disease characteristics, hemodynamics, and survival data for patients with group 1 PAH.44 Other major registries include the French (FPHN [French Pulmonary Hypertension Network] COMPERA (Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension) (German), Aspire (United Kingdom) and PHSANZ (Pulmonary Hypertension Society of Australia and New Zealand). In Western countries, unlike many Asian registries, idiopathic PAH is the most common etiology, comprising 30% to 50% of all PAH cases, followed by connective tissue disorder (CTD)–PAH and congenital heart disease (CHD)–PAH. Survival rates at 1, 3, and 5 years in the REVEAL registry (85% prevalent cases) from diagnostic right heart catheter were 85%, 68%, and 57%, respectively. The characteristics of selected Western PAH registries are presented in Table 4.
Table 4.
Western PAH Registries
| REVEAL44 | FPHN17 | FPHN-R67 | COMPERA45 | Aspire68 | PHSANZ69 | |
|---|---|---|---|---|---|---|
| Country | USA | France | France | Germany | United Kingdom | Australia and New Zealand |
| No. of patients | 2,716 | 674 | 1,611 | 1,655 | 600 | 2,558 |
| No. of centers | 54 | 17 | 50 | 1 | 21 | |
| Years enrolled | 2006-2009 | 2002-2003 | 2006-2018 | 2009-2020 | 2001-2010 | 2011-2019 |
| Analysis type | Incident + Prevalent | Incident only | Incident only | Incident only | Incident only | Incident + Prevalent |
| Female, % | 79 | 65 | 56 | 64 | 62 | 74 |
| Mean age (SD/IQR as applicable), y | 50.4 (16.8) | 50 ± 15 | 60 ± 17 | 65.7 (15.5) | 59 ± 17 | 58.2 ± 16.3 |
| WHO subtype prevalence, n (%) | ||||||
| 1.1. IPAH | 1,262 (47) | 264 (39) | 1,201 (75) | 1,182 (72)a | 175 (29)a | 1,043 (41)a |
| 1.2. CTD-PAH | 648 (24) | 103 (15) | not applicable | 330 (20) | 188 (31) | 313 (31) |
| 1.3. CHD-PAH | 319 (12) | 76 (11) | not applicable | 46 (2.8) | 198 (33) | 260 (10) |
| 1.4. Portal HTN | 138 (5) | 70 (10) | not applicable | 83 (5) | 24 (4) | 80 (3) |
| 1.5. HIV | 51 (2) | 42 (6) | not applicable | 14 (1) | not applicable | 15 (0.6) |
| 1.6. FPAH | 79 (3) | 26 (4) | 133 (8) | not applicable | not applicable | 45 (2) |
| 1.7. DPAH | 134 (5) | 64 (10) | 277 (17) | not applicable | not applicable | not applicable |
CHD = congenital heart disease; COMPERA = Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; CTD = connective tissue disease; DPAH = drug associated pulmonary arterial hypertension; FPAH = familial pulmonary arterial hypertension; FPHN = French Pulmonary Hypertension Network; HTN = hypertension; IPAH = idiopathic pulmonary arterial hypertension; not applicable = not available; PHSANZ = Pulmonary Hypertension Society of Australia and New Zealand; REVEAL = Registry to Evaluate Early and Long-term PAH Disease Management; other abbreviation as in Table 3.
Includes DPAH + FPAH (with exception of PHSANZ).
One of the key epidemiological changes in the past 15 years has been the increasing age of PAH patients enrolled in Western registries. In the COMPERA registry, the average age of patients with PAH was 65.7 ± 15.5 years.45 In the same registry, a cluster analysis revealed 3 distinct phenotypes of PAH characterized by: cluster 1 — median age 45 years, 76% females, no comorbidities, and diffusing capacity of lung for carbon monoxide (DLCO) ≥45%; cluster 2 — median age 75 years, 98% females, frequent comorbidities, no smoking history, DLCO mostly ≥45%; and cluster 3 — median age 72 years, 72% males, frequent comorbidities, history of smoking, and low DLCO.46 Importantly treatment response and survival were best in cluster 1, suggesting a distinct phenotypic difference for more elderly patients with multiple comorbidities. Further studies are needed to help refine our approach to PAH therapy according to different phenotypes of PAH.
Asian PH Registries
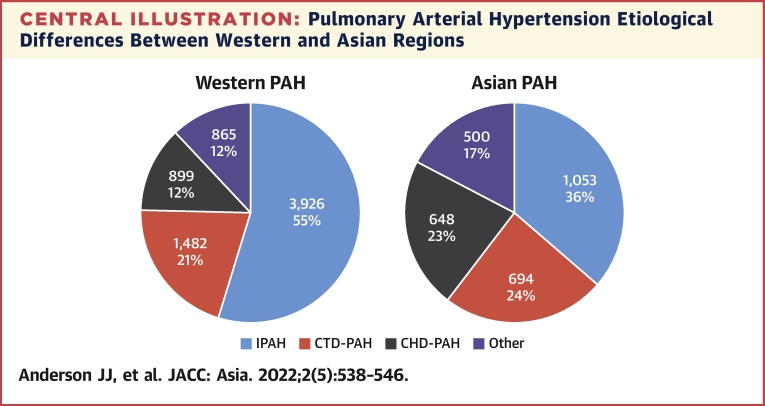
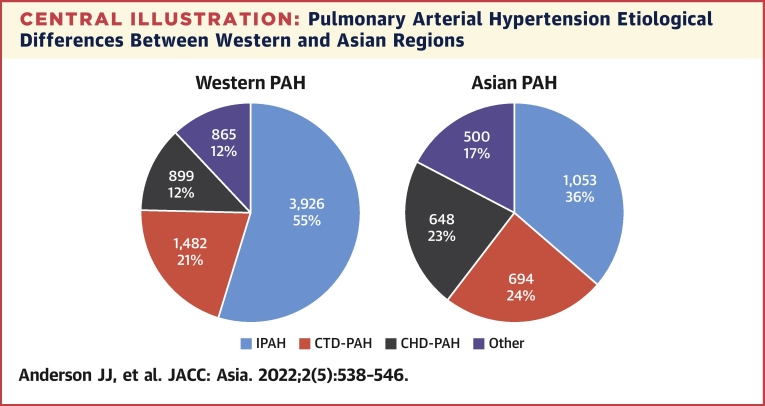
The largest Asian registry describing all PH subtypes is the PRO-KERALA registry based in the state of Kerala, India.47 Fifty hospitals across the state registered data for consecutive PH patients in 2003, finding mean age 56 ± 16.1 years, female predominance (52%), with etiologies PH-LHD (59%), group 1 PAH (42%), PH-LD (13.3%), CTEPH (3.8%), and group 5 miscellaneous PH (2.4%).47 Lim et al48 recently reported the characteristics of pulmonary arterial hypertension in a multiethnic Asian registry, with findings similar to other large registries. The mean age was 50.8 ± 14.9 years, cases were predominantly female (77%), with etiologies of CHD-PAH (35.8%), idiopathic PAH (29.7%), and CTD- PAH (24.3%).48 In addition, survival rates were similar to Western registries, with 1-year survival at 85.8%, 5-year survival at 66.9%, and 10-year survival at 55.4%. Sex distribution was also similar, at approximately ranging 52% to 81%; however, Asian registries record greater proportions of CTD-PAH and CHD-PAH, and 2 Chinese registries report a younger mean age at diagnosis in PAH.49,50 Results are summarized in Table 5, and a comparison of PAH etiology between Western and Asian regions is shown in the Central Illustration.
Table 5.
Epidemiology of Asian PAH Registries
| KORPAH70,71,a | Lim et al48 | PRO-KERALA47,a | JHPAR72 | Zhang et al49,b | Jing et al50 | |
|---|---|---|---|---|---|---|
| Country | South Korea | Indonesia | India | Japan | China | China |
| No. of patients | 625 | 148 | 2,003 | 189 | 276 | 956 |
| No. of centers | 35 | 1 | 50 | 8 | 5 | 9 |
| Years enrolled | 2008-2011 | 2003-2016 | 2015 | 2008-2013 | 2007-2009 | 2008-2011 |
| Female, % | 81 | 77 | 52 | 76 | 76 | 70 |
| Mean age, y | 47.6 ± 15.7 | 50.8 ± 15.9 | 56 ± 16.1 | 43.9 ± 16.9 | 36.4 | 36 ± 13 |
| WHO subtype prevalence, n (%) | ||||||
|
145 (23) 311 (50) 159 (25) |
44 (30) 36 (24) 53 (36) |
424 (21) 117 (28) 9 (4) 291 (69) |
105 (56) 48 (25) 16 (9) 20 9 (11) |
173 (63) 103 (37) not applicable not applicable |
335 (35) 182 (19) 411 (43) not applicable |
|
not applicable | not applicable | 1,189 (59) | not applicable | not applicable | not applicable |
|
not applicable | not applicable | 266 (13.3) | not applicable | not applicable | not applicable |
|
not applicable | not applicable | 77 (3.8) | not applicable | not applicable | not applicable |
|
not applicable | not applicable | 47 (2.4) | not applicable | not applicable | not applicable |
CLD = chronic lung; CTEPH = chronic thromboembolic pulmonary hypertension; JHPAR = Japan pulmonary hypertension registry; KORPAH = Korean registry of pulmonary arterial hypertension; LHD = left heart disease; PRO-KERALA = pulmonary hypertension registry of Kerala, India; other abbreviations as in Tables 1, 3, and 4.
Registry used echocargiographic criteria for inclusion rather than accepted right heart catheter parameters.
Includes only IPAH and CTD-PAH.
Central Illustration.
Pulmonary Arterial Hypertension Etiological Differences Between Western and Asian Regions
Group 1 pulmonary arterial hypertension (PAH) in Western and Asian regions based on registry data. Asian registries report greater proportions of connective tissue disease–PAH (CTD-PAH) and congenital heart disease–associated PAH (CHD-PHD). Other PAH includes PAH associated with portal hypertension, HIV infection, and schistosomiasis. IPAH = idiopathic pulmonary arterial hypertension.
In terms of heritability PAH, no significant differences have been shown between Asian cohorts to Western cohorts in small studies to date. Bone morphogenetic receptor 2 protein (BMPR2) is the most common genetic abnormality in PAH. Korean and Chinese investigators have identified rates from 14% to 22% for BMPR2 mutation in patients with sporadic PAH, similar to Western cohorts.51, 52, 53 Chinese investigators have consistently identified younger age at diagnosis in their cohort in BMPR2 carriers relative to Western series, but whether this precocity is simply related to overall background epidemiology is uncertain.52,53
Systemic lupus erythematosus (SLE) prevalence is higher in Asians compared to Caucasians, although both are low compared to Black ethnic groups.54 In Western registries, scleroderma-associated PAH (SSc-PAH) is the overwhelmingly the most frequent CTD-PAH subtype.55 Estimates of SSc-PAH prevalence in patients with scleroderma in Western centers ranges from 7% to 12%.56,57 The recognition of high rates of SSc-PAH led to development of systematic screening recommendations based on complex lung function testing and echocardiography, and was incorporated into latest guidelines.5,57 There are significantly higher rates of PH caused by SLE in Asia. Hao et al58 identified SLE in 49% of 129 consecutive adult patients referred to 3 Chinese PH centers in a 5-year period compared to just 6% with SSc-PAH. Prevalence of PH in a cross-sectional sample of 1,934 Chinese patients with SLE has been estimated at 3.8% (based on echocardiographic parameters), although other estimates range from 2.8% to 23.3% depending on reference criteria.59,60 Although evidence is limited, a similar approach to screening in Asian patients with high-risk SLE as SSc-PAH in Western patients may be warranted.
Takayasu arteritis (TA) is a rare large-vessel vasculitis with higher incidence in Asian populations relative to the West, predominantly affecting females in their second and third decades.61 A small proportion of TA patients may develop pulmonary arteritis, estimated at 6.3% in a large Korean cohort.62 TA-PH (classified as group 4) may occur in more than half of patients who develop pulmonary arteritis, and these patients may exhibit responses to pulmonary vasodilator therapy.62, 63, 64
Finally, a recent international prospective CTEPH registry highlighted differences in epidemiology and approach to treatment between Western and Asian centers. In Japan, 70% of patients are offered BPA as a first-choice treatment (compared to 72% offered pulmonary endarterectomy in Western centers), and there is an as-yet unexplained higher proportion (75%) of females with CTEPH in Japanese centers.65
Conclusions
Definitions and classification of PH have matured over time, with Western data underpinning the framework. Right heart catheter mPAP > 20 mm Hg is the new accepted definition of PH.8 Asian registry data suggests that PH-LHD is the most common PH subtype, as is the case in the West, and is in keeping with reported cardiovascular disease trends. Epidemiological differences between PH in Asia compared to that described in the literature should be suspected based on multiple geographic, socioeconomic, and behavioral factors. Although rare relative to PH-LHD and CLD-PH, conditions including SLE-PAH, TA-PH, and HIV-PH should be considered in Asian patients with PH. In terms of group 1 PAH, Asian registry data suggest a similar mean age of diagnosis of PAH and female preponderance, with a greater proportion of CTD-PAH and CHD-PAH relative to idiopathic PAH.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Mocumbi A.O., Thienemann F., Sliwa K. A global perspective on the epidemiology of pulmonary hypertension. Can J Cardiol. 2015;31(4):375–381. doi: 10.1016/j.cjca.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N., Channick R.N., Frantz R.P., et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strange G., Stewart S., Celermajer D.S., et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol. 2019;73(21):2660–2672. doi: 10.1016/j.jacc.2019.03.482. [DOI] [PubMed] [Google Scholar]
- 4.Schermuly R.T., Ghofrani H.A., Wilkins M.R., Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galiè N., Humbert M., Vachiery J.-L., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). 2015. Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Resp J. 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda K., Date H., Doi S., et al. Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017) Circ J. 2019;83(4):842–945. doi: 10.1253/circj.CJ-66-0158. [DOI] [PubMed] [Google Scholar]
- 8.Park J.H., Na J.O., Lee J.S., Kim Y.H., Chang H.-J. 2020 KSC/KATRD guideline for the diagnosis and treatment of pulmonary hypertension: executive summary. Tuberc Respir Dis (Seoul) 2022;85(1):1–10. doi: 10.4046/trd.2021.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs G., Bergholt A., Scheidl S., Olscheski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 10.Maron B.A., Hess E., Maddox T.M., et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douschan P., Kovacs G., Avian A., et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197(4):509–516. doi: 10.1164/rccm.201706-1215OC. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs G., Avian A., Tscherner M., et al. Characterization of patients with borderline pulmonary arterial pressure. Chest. 2014;146(6):1486–1493. doi: 10.1378/chest.14-0194. [DOI] [PubMed] [Google Scholar]
- 13.Valerio C.J., Schreiber B.E., Handler C.P., Denton C.P., Coghlan J.G. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheumatol. 2013;65(4):1074–1084. doi: 10.1002/art.37838. [DOI] [PubMed] [Google Scholar]
- 14.Barst R.J., Rubin L.J., Long W.A., et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 15.Maron B.A., Brittain E.L., Hess E., et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med. 2020;8(9):873–884. doi: 10.1016/S2213-2600(20)30317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratwatte S., Anderson J., Strange G., et al. Pulmonary arterial hypertension with below threshold pulmonary vascular resistance. Eur Repir J. 2020;56(1) doi: 10.1183/13993003.01654-2019. [DOI] [PubMed] [Google Scholar]
- 17.Humbert M., Sitbon O., Chaouart A., et al. Pulmonary arterial hypertension in France. Am J Resp Crit Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 18.Peacock A.J., Murphy M.F., McMurray J.J.V., Caballero L., Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30(1):104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 19.Hoeper M.M., Huschen D., Ghofrani H.A., et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168(2):871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Strange G., Playford D., Stewart S., et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98(24):1805–1811. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenkranz S., Gibbs J.S.R., Wachter R., DeMarco T., Vonk-Noordegraaf A., Vachiery J.-L. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37(12):942–954. doi: 10.1093/eurheartj/ehv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vachiéry J.-L., Delcroix M., Al-Hitti H., et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1) [Google Scholar]
- 23.Caravita S., DeWachter C., Sorarna D., et al. Haemodynamics to predict outcome in pulmonary hypertension due to left heart disease: a meta-analysis. Eur Respir J. 2018;51(4) doi: 10.1183/13993003.02427-2017. [DOI] [PubMed] [Google Scholar]
- 24.Vachiery J.L., Delcroix M., Al-Hiti H., et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51(2) doi: 10.1183/13993003.01886-2017. [DOI] [PubMed] [Google Scholar]
- 25.Bermejo J., Yotti R., García-Orta R., et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J. 2018;39(15):1255–1264. doi: 10.1093/eurheartj/ehx700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan S.D., Barbera J.A., Gaine S.P., et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waxman A., Restrepo-Jaramillo R., Thenappan T., et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 28.Simonneau G., Torbicki A., Dorfmuller P., Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143) doi: 10.1183/16000617.0112-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepke-Zaba J., Delcroix M., Lang I., et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 30.Phan K., Jo H.E., Xu J., Lau E.M. Medical therapy versus balloon angioplasty for CTEPH: a systematic review and meta-analysis. Heart Lung Circ. 2018;27(1):89–98. doi: 10.1016/j.hlc.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Ghofrani H.A., GalièN, Grimminger E., et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 32.Lang I.M., Matsubara H. Balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension: is Europe behind? Eur Respir J. 2019;53(5) doi: 10.1183/13993003.00843-2019. [DOI] [PubMed] [Google Scholar]
- 33.Hoeper M.M., Humbert M., Souza R., et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–322. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 34.Moreira E.M., Gall H., Leening M.J.G., et al. Prevalence of pulmonary hypertension in the general population: the Rotterdam study. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijeratne D.T., Lajkosz K., Brogly S.R., et al. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11(2) doi: 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC: Asia. 2021;1(1):1–13. doi: 10.1016/j.jacasi.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W., He J., Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine (Baltimore) 2020;99(23) doi: 10.1097/MD.0000000000020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins D.A., Jognson C.O., Colquhon S.A., et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377(8):713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 39.Yang J.J., Yu D., Wen W., et al. Tobacco smoking and mortality in Asia: a pooled meta-analysis. JAMA Netw Open. 2019;2(3) doi: 10.1001/jamanetworkopen.2019.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Global Tuberculosis Report 2020. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 41.Rich S., Haworth S.G., Hassoun P.M., Yacoub M.H. Pulmonary hypertension: the unaddressed global health burden. Lancet Respir Med. 2018;6(8):577–579. doi: 10.1016/S2213-2600(18)30268-6. [DOI] [PubMed] [Google Scholar]
- 42.Aldashev A.A., Sarybaev A.S., Sydkov A.S., et al. Characterization of high-altitude pulmonary hypertension in the Kyrgyz. Am J Respir Crit Care Med. 2002;166(10):1396–1402. doi: 10.1164/rccm.200204-345OC. [DOI] [PubMed] [Google Scholar]
- 43.D'Alonzo G.E., Barst R.J., Ayres S.M., et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 44.Benza R.L., Miller D.P., Gomberg-Maitland M., et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 45.Hoeper M., Pausch C., Olsson K.M., et al. COMPERA 2.0: a refined 4-strata risk assessment model for pulmonary arterial hypertension. Eur Respir J. 2021 doi: 10.1183/13993003.02311-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoeper M.M., Pausch C., Gruning E., et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant. 2020;39(12):1435–1444. doi: 10.1016/j.healun.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Harikrishnan S., Sanjay G., Ashishkumar M., et al. Pulmonary hypertension registry of Kerala, India (PRO-KERALA) — clinical characteristics and practice patterns. Int J Cardiol. 2018;265:212–217. doi: 10.1016/j.ijcard.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 48.Lim Y., Low T.-T., Chan S.-P., et al. Pulmonary arterial hypertension in a multi-ethnic Asian population: characteristics, survival and mortality predictors from a 14-year follow-up study. Respirology. 2019;24(2):162–170. doi: 10.1111/resp.13392. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R., Dai L.-Z., Xie W.-P., et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest. 2011;140(2):301–309. doi: 10.1378/chest.10-2327. [DOI] [PubMed] [Google Scholar]
- 50.Jing Z.-C., Xu X.-Q., Han Z.-Y., et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132(2):373–379. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 51.Jang A.Y., Kim B.-G., Kwon S., et al. Prevalence and clinical features of bone morphogenetic protein receptor type 2 mutation in Korean idiopathic pulmonary arterial hypertension patients: the PILGRIM explorative cohort. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng Q., Yang H., Liu B., et al. Clinical characteristics and survival of Chinese patients diagnosed with pulmonary arterial hypertension who carry BMPR2 or EIF2KAK4 variants. BMC Pulm Med. 2020;20(1):150. doi: 10.1186/s12890-020-01179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu D., Liu Q.-Q., Eyries M., et al. Molecular genetics and clinical features of Chinese idiopathic and heritable pulmonary arterial hypertension patients. Eur Respir J. 2012;39(3):597–603. doi: 10.1183/09031936.00072911. [DOI] [PubMed] [Google Scholar]
- 54.Rees F., Doherty M., Grainger M.J., Lanyon P., Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 55.McGoon M.D., Miller D.P. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Resp Rev. 2012;21(123):8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Pavec J., Hubert M., Mouthon C., Hassoun P.M. Systemic sclerosis-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181(12):1285–1293. doi: 10.1164/rccm.200909-1331PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coghlan J.G., Denton C.P., Grimminger E., et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–1349. doi: 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao Y.-J., Jiang X., Zhou W., et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44(4):963–972. doi: 10.1183/09031936.00182813. [DOI] [PubMed] [Google Scholar]
- 59.Li M., Wang Q., Lhao J., et al. Chinese SLE Treatment and Research group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosus. Lupus. 2014;23(10):1085–1091. doi: 10.1177/0961203314527366. [DOI] [PubMed] [Google Scholar]
- 60.Xia Y.K., Tu Sh, HU Y.A., et al. Pulmonary hypertension in systemic lupus erythematosus: a systematic review and analysis of 642 cases in Chinese population. Rheumatol Int. 2013;33(5):1211–1217. doi: 10.1007/s00296-012-2525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onen F.N., Akkoc N. Epidemiology of Takayasu arteritis. Presse Médicale. 2017;46(7-8 Part 2):e197–e203. doi: 10.1016/j.lpm.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 62.Yang J., Peng M., Shi J., Zhong W., Yu X. Pulmonary artery involvement in Takayasu’s arteritis: diagnosis before pulmonary hypertension. BMC Pulm Med. 2019;19(1):225. doi: 10.1186/s12890-019-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X., Dang A., Chen B., Lv N., Liu Q. Takayasu arteritis-associated pulmonary hypertension. J Rheumatol. 2015;42(3):495–503. doi: 10.3899/jrheum.140436. [DOI] [PubMed] [Google Scholar]
- 64.Jiang X., Zhu Y.-J., Zhou Y.-P. Clinical features and survival in Takayasu’s arteritis-associated pulmonary hypertension: a nationwide study. Eur Heart J. 2021;42(42):4298–4305. doi: 10.1093/eurheartj/ehab599. [DOI] [PubMed] [Google Scholar]
- 65.Guth S., D'Armini A.M., Delcroix M., et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH Registry. ERJ Open Res. 2021;7(3):00850–2020. doi: 10.1183/23120541.00850-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heresi G.A., Minai O.A., Tonelli A.R., et al. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3(4):916–925. doi: 10.1086/674756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boucly A., Savale L., Jaïs X., et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;204(7):842–854. doi: 10.1164/rccm.202009-3698OC. [DOI] [PubMed] [Google Scholar]
- 68.Hurdman J., Condliffe R., Elliot C.A., et al. ASPIRE registry: Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Centre. Eur Respir J. 2012;39(4):945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 69.Anderson J.J., Lau E.M., Lavender M., et al. Retrospective validation of the REVEAL 2.0 risk score with the Australian and New Zealand Pulmonary Hypertension Registry Cohort. Chest. 2020;157(1):162–172. doi: 10.1016/j.chest.2019.08.2203. [DOI] [PubMed] [Google Scholar]
- 70.Jang A.Y., W J., Chung W.-J. Current status of pulmonary arterial hypertension in Korea. Korean J Int Med. 2019;34(4):696–707. doi: 10.3904/kjim.2019.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung W.-J., Park Y.B., Jeon C.H., et al. Baseline characteristics of the Korean Registry of Pulmonary Arterial Hypertension. J Korean Med Sci. 2015;30(10):1429–1438. doi: 10.3346/jkms.2015.30.10.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tamura Y., Kumamaru H., Satoh T., et al. Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ J. 2017;82(1):275–282. doi: 10.1253/circj.CJ-17-0139. [DOI] [PubMed] [Google Scholar]