Abstract
Lipopolysaccharide (LPS) is a very potent inducer of tumor necrosis factor alpha (TNF-α) expression from monocytes and macrophages. Another inflammatory cytokine, gamma interferon (IFN-γ), can potentiate the effects of LPS, but the mechanism is not thoroughly understood. Previous reports emphasized the ability of IFN-γ to upregulate CD14 expression (the receptor for LPS), and nearly all studies have utilized sequential stimulation with IFN-γ followed by LPS to exploit this phenomenon. This study demonstrates that IFN-γ can upregulate the effect of LPS at the level of transcription. Human monoblastic Mono-Mac-6 cells produced up to threefold-greater levels of TNF-α when simultaneously stimulated with LPS and IFN-γ compared to treatment with LPS alone. RNase protection studies showed a similar increase in RNA beginning as early as within 30 min. The synthesis of TNF-α mRNA in IFN-γ- and LPS-treated Mono-Mac-6 cells was also temporally prolonged even though the message turnover rate was identical to that seen in LPS stimulated cells. The modulatory effect of IFN-γ may be mediated by Jak2.
Tumor necrosis factor alpha (TNF-α) is secreted in response to inflammatory stimuli by immunologically relevant cells including macrophages, lymphocytes, natural killer cells, and dendritic cells. Its biological actions include upregulating the production of proinflammatory cytokines, chemokines, and adhesion molecules (3). TNF-α produced inappropriately or excessively may lead to deleterious immunologic consequences, as is the case in overwhelming sepsis, rheumatoid arthritis, and systemic lupus erythematosus (1, 20, 36, 43).
Lipopolysaccharide (LPS) is an integral component of gram-negative bacterial cell walls which stimulates TNF-α production. LPS binds CD14 and activates the mitogen-activated protein kinase (MAPK) signaling pathway via p56Lyn (30, 44). LPS also induces NF-κB release from IκB including p50-p65 heterodimers, p50 homodimers, and Rel-p65 heterodimers (7, 37). The induction of TNF-α by LPS has been widely used to investigate TNF-α transcriptional regulation. NF-κB, as well as C/EBPβ and c-Jun, has been demonstrated to play a role in the regulation of TNF-α transcription in myelomonocytic cells (32, 48, 54). The ability of LPS to activate multiple inflammatory pathways, including the upregulation of TNF-α, is believed to be central to the development of septic shock (10, 35).
Gamma interferon (IFN-γ) has been shown to prime cells and potentiate the effects of LPS (23, 24, 33, 49). As IFN-γ engages with its receptor, cytoplasmic Janus kinases (Jak1 and Jak2) become activated which phosphorylate specific tyrosine residues on the signal transducers and activators of transcription (STAT)-1α molecules (9, 40, 53). Activated and phosphorylated STAT1α forms homodimers, translocates to the nucleus, and binds specific STAT-binding DNA sequences (15, 16). Monocytes and macrophages become more responsive and sensitive to LPS after pretreatment with IFN-γ (6). Primary human monocytes increased TNF-α transcription and formed more stable TNF-α mRNA when they were primed with IFN-γ for several hours prior to LPS stimulation (23, 24, 33, 49). The mechanism underlying the priming effect appears to be complex. In part, IFN-γ upregulates the receptor for LPS, CD14. It also appears to alter proteins which target mRNA turnover (42, 47).
In addition to this well-known priming effect, there have been some studies suggesting that IFN-γ may have a direct transcriptional effect. In macrophages isolated from LPS-resistant mice (C3H/HeJ), IFN-γ was able to overcome the unresponsiveness of LPS and to induce TNF-α gene and protein expression (4). Other early studies have supported the idea that IFN-γ may have a direct transcriptional effect (5, 13, 27). Recent studies have identified signaling pathways which are coactivated by both LPS and IFN-γ. In one, IFN-γ was shown to dramatically augment NF-κB induction by LPS. This effect required preincubation with IFN-γ and was dependent on protein synthesis (24). A second study demonstrated that both LPS and IFN-γ can lead to phosphorylation of the proto-oncogene vav (17). This effect was dependent on the kinase hck, which is known to modulate TNF-α production. Another signaling pathway in which LPS and IFN-γ effect converge is at STAT1. STAT1 is typically considered to be phosphorylated at Y701 by Jak1 and Jak2. Phosphorylation at S727 potentiates the transcriptional effects of STAT1. The pathway responsible for phosphorylation of S727 has not been clearly defined; however, LPS is capable of inducing phosphorylation at that site (28). Therefore, these studies suggest that LPS and IFN-γ may coactivate certain signaling pathways relevant to the transcription of TNF-α. Our studies attempt to define which of the pathways might underlie the increased TNF-α production from human cells when both LPS and IFN-γ are presented simultaneously as opposed to a priming strategy. The simultaneous administration of these two stimuli might mimic the effects of an early in vivo bacterial infection more closely than a priming strategy. Understanding the interplay between proinflammatory cytokines could have potential in developing new cytokine-directed therapeutic strategies for overwhelming infections or autoimmune diseases.
MATERIALS AND METHODS
Tissue culture.
Mono-Mac-6 cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin-6 phosphate per ml, 10 mM minimal essential medium nonessential amino acids, 10 μg of insulin-transferrin-sodium selenite per ml, and 100 mM sodium pyruvate. Tissue culture media and cell culture supernatants were periodically screened for endotoxin and mycoplasma. All plasticware used in these experiments was certified pyrogen-free.
Reagents.
LPS (Escherichia coli O11:B4) and actinomycin D were obtained from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human IFN-γ (rhIFN-γ) from Endogen, Inc. (Woburn, Mass.), had a specific activity of 3 × 107 U/mg. Tyrphostin AG-490 was purchased from Calbiochem (San Diego, Calif.) and suspended in dimethyl sulfoxide (DMSO).
TNF-α assays.
Mono-Mac-6 cells were plated in 96-well round-bottom tissue culture plates at 2 × 105 cells per 200 μl. Cells were stimulated in duplicates or triplicates with 2 μg of LPS per ml with or without varyious concentrations of rhIFN-γ. After various time points, supernatants were harvested and were stored at −70°C until enzyme-linked immunosorbent assays (ELISAs) were performed. Human matched antibody pairs for TNF-α were purchased from Endogen for a conventional sandwich ELISA. Nunc-Immune Maxisorp plates were coated overnight with capture antibodies [mouse anti-human TNF-α monoclonal immonoglobulin G1 κ]. Supernatants from cultures and standard curve TNF-α were allowed to bind capture antibodies before addition of the biotin-labeled detecting antibodies [mouse anti-human TNF-α monoclonal immunoglobulin G1(κ)]. Streptavidin-conjugated horseradish peroxidase catalyzing TMB (3,3′,5,5′-tetramethylbenzidine) substrate color conversion was used for detection. Enzyme reactions were terminated by the addition of 0.18 M H2SO4 before being measured at 450 nm on the Dynatech MR4000 spectrophotometer (Dynatech Laboratories, Inc., Chantilly, Va.). ELISA sensitivity was <5.0 pg/ml, and the backgrounds were negligible.
RNase protection assay.
Mono-Mac-6 cells were cultured and stimulated in T-25 tissue culture flasks at 2 × 106 cells/ml. Unless otherwise noted, cells were stimulated with 2 μg of LPS per ml with or without 50 ng of rhIFN-γ per ml. In the experiments to analyze mRNA turnover, actinomycin D at a final concentration of 3 μg/ml was added into the cultures after various periods of time (1, 2, or 4 h). In the experiments to examine the effects of Jak2 inhibition, AG-490 at a final concentration of 20 μM was added at the time of initial culture. As a control for the AG-490 diluent, equal volumes of DMSO were added to the second set of cultures. Culture flasks containing light-sensitive AG-490 were wrapped in aluminum foil to protect against its deactivation. For time course studies, approximately 2 × 106 to 4 × 106 cells were removed at designated time points. After pelleting of the cells, supernatants were removed and saved for ELISAs, while the cell pellets were resuspended in RNAzol B. RNA was isolated utilizing the RNAzol B protocol (Tel-Test, Inc., Friendswood, Tex.). The quality of RNA was first checked on 1% agarose-formaldehyde gel prior to further processing. RNase protection assays were performed using the RiboQuant multiprobe system (Pharmingen, San Diego, Calif.) by following the manufacturer's instructions. Then, 10 μg of total RNA was hybridized with radioactive probe. Hybridized RNA was separated on 5% acrylamide gel and quantified on a PhosphorImager using the ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). TNF-α mRNA quantity was normalized to the L32 concentrations in the same hybridization reaction. Densitometric units were calculated as follows: (TNF-α/L32) × the average of L32 within the entire set of one experiment.
Statistical analysis.
Student t tests were performed to determine the statistical significance of TNF-α protein secretion among sets of stimulated cells. The paired Student t test was utilized to compare the quantitative differences of mRNA in the RNase protection assays. A P value of <0.05 was used to indicate significance.
RESULTS
IFN-γ and LPS induced greater TNF-α production than LPS alone.
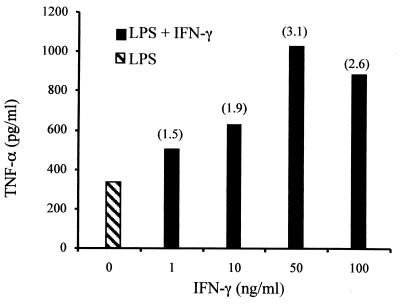
Mono-Mac-6 cells simultaneously treated with LPS and IFN-γ consistently secreted from 1.5- to 3-fold greater amounts of TNF-α compared to LPS stimulation alone (Fig. 1). This upregulation by IFN-γ was dose dependent. IFN-γ alone could not induce TNF-α secretion in repeated experiments. IFN-γ effects were observed with LPS in doses ranging from 20 ng/ml to 2 μg/ml (data not shown). From these experiments, LPS at 2 μg/ml and IFN-γ at 50 ng/ml were determined to be concentrations that optimally stimulated TNF-α production in Mono-Mac-6 cells.
FIG. 1.
Stimulation of Mono-Mac-6 cells with LPS and IFN-γ. Human Mono-Mac-6 cells (2 x 105 per well) were stimulated for 5 h with 2 μg of LPS (E. coli O111:B4) per ml, with increasing concentrations of rhIFN-γ. Duplicate ELISAs were performed as described in Materials and Methods for each sample or condition from triplicate stimulations. The numbers in parenthesis above the solid bars indicate the fold increase over LPS stimulation alone (striped bar). The figure is representative of five separate experiments.
IFN-γ may potentiate the ability of LPS to induce TNF-α by increasing the rate or efficiency of TNF-α mRNA synthesis.
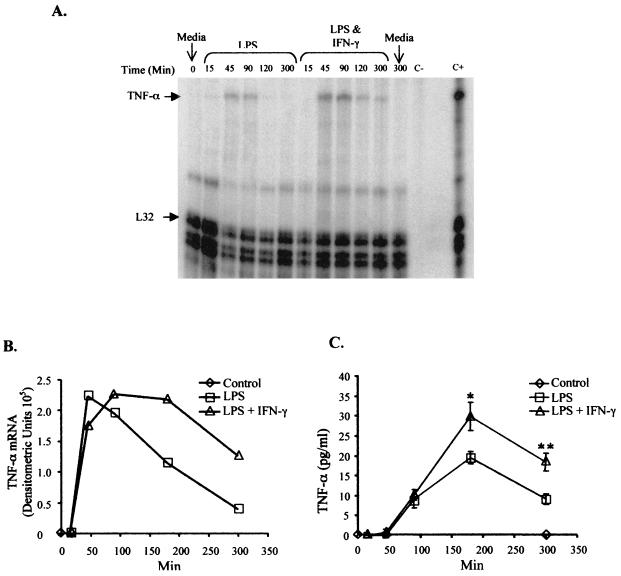
To assess whether the effect of IFN-γ was mediated at a pre- or a posttranscriptional level, we compared mRNA production over time in Mono-Mac-6 cells treated with LPS alone or with a combination of LPS and IFN-γ. Employing the RNase protection assay, TNF-α transcripts isolated from stimulated cells were hybridized to radiolabeled TNF-α RNA probes. The amount of TNF-α RNA protected from RNase was quantified and normalized to the L32 RNA (Fig. 2A and B). Induction of TNF-α mRNA by LPS was rapid and transient. At the time points analyzed, transcripts accumulated rapidly between 15 and 45 min post-LPS stimulation and began to fall progressively after 45 min (Fig. 2B). TNF-α transcripts harvested from cells stimulated with LPS and IFN-γ showed similar patterns initially. Interestingly, the levels of transcript harvested from dually stimulated cells did not decline as quickly as in LPS-stimulated cells (Fig. 2B). Therefore, stimulation with LPS plus IFN-γ seemed to prolong the presence of TNF-α mRNA or alter the kinetics of transcription. It also suggested that IFN-γ modulated LPS-induced TNF-α production at the transcriptional level.
FIG. 2.
TNF-α mRNA and protein persist longer in Mono-Mac-6 cells treated with LPS and IFN-γ compared to those treated with LPS alone. Mono-Mac-6 cells (2 × 106/ml) were stimulated with 2 μg of LPS per ml with or without 50 ng of rhIFN-γ per ml. Controls were untreated. At various time points approximately 4 × 106 cells were harvested for RNA isolation. RNase protection assays were performed. A representative autoradiograph is shown in panel A. C− indicates the use of yeast tRNA as negative control; C+ indicates RNA from previously assayed Mono-Mac-6 cells treated for 3 h with LPS and IFN-γ as a positive control. (B) TNF-α mRNA densitometric units shown graphically. (C) Supernatants from these cells were also analyzed for TNF-α protein secretion by ELISA. Error bars represent the standard deviations of triplicate analyses for each condition. ∗, P = 0.01 comparing the difference between LPS versus LPS and IFN-γ; ∗∗, P = 0.04. Figures are representative of at least four separate experiments.
The patterns of TNF-α protein secretion over time correlated with those of the TNF-α mRNA levels. We measured TNF-α secretion in supernatants of the same Mono-Mac-6 cell cultures where RNA was also isolated. As measured by sandwich ELISA, TNF-α production by LPS-stimulated cells began to increase by between 45 and 90 min and peaked at 180 min or 2 h (Fig. 2C). The peak of protein synthesis was delayed by approximately 2 hours compared to that of the mRNA (Fig. 2B). Similar kinetics of TNF-α secretion is initially observed for cells stimulated with both LPS and IFN-γ (Fig. 2C). However, LPS- and IFN-γ-treated cells secreted significantly greater amounts of TNF-α after 180 and 300 min of stimulation (P < 0.05). This correlated strongly with the prolonged presence of TNF-α transcripts in these cells.
Stability of TNF-α mRNA is comparable in Mono-Mac-6 cells stimulated with LPS alone or LPS with IFN-γ.
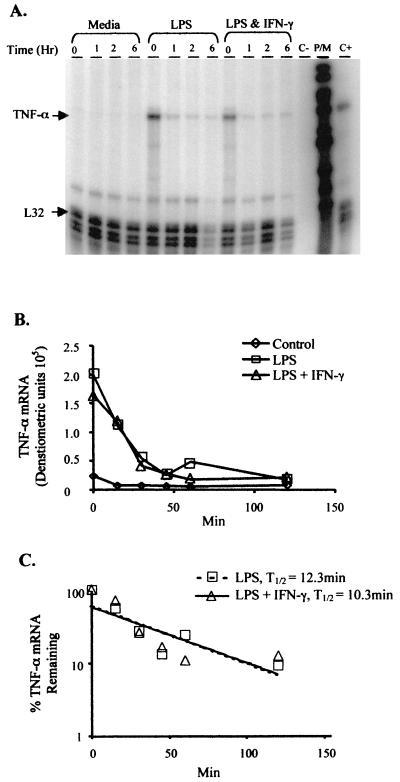
To determine whether the prolonged presence of TNF-α mRNA in LPS- and IFN-γ-treated cells is due to increased stability of the transcripts, we measured RNA levels accumulated after 1 h of stimulation with further transcription inhibited. Mono-Mac-6 cells were treated with or without IFN-γ in the presence of LPS. After 1 h, cells were treated with actinomycin D, a potent inhibitor of RNA polymerase-dependent transcription. Transcripts that were synthesized within 1 h were monitored over time and analyzed by the RNase protection assay (Fig. 3A and B). TNF-α transcripts decreased rapidly 15 to 30 min after synthesis, with the rate of degradation in LPS- stimulated cells comparable to that in LPS- and IFN-γ-stimulated cells (Fig. 3B). Half-lives of the transcripts were calculated by determining the time at which 50% of the mRNA remained (Fig. 3C). Among three separate experiments, the half-lives of TNF-α transcripts from cells stimulated by LPS were 28.1 ± 23.7 (mean ± the standard deviation) min without IFN-γ and 27.3 ± 21.1 min with IFN-γ. Similar rates of mRNA degradation were observed from transcripts accumulated after 2 h (LPS, 27.0 min; LPS plus IFN-γ, 24.4 min) and after 4 h (LPS, 22.4 min; LPS plus IFN-γ, 21.5 min) of stimulation. This demonstrated that the increase in TNF-α mRNA and protein in LPS- and IFN-γ-stimulated cells was not due to increased stability of the transcript. This suggests that IFN-γ acts directly to modulate the LPS-induced TNF-α at the level of gene transcription. This is unlike the results of previous reports showing priming with IFN-γ for several hours before LPS treatment both greatly increased TNF-α transcription and enhanced mRNA stability (23, 42).
FIG. 3.
Stability of TNF-α mRNA is comparable in Mono-Mac-6 cells treated with either LPS with IFN-γ or LPS alone. Mono-Mac-6 cells (2 × 106/ml) were stimulated with 2 μg of LPS per ml with or without 50 ng of rhIFN-γ per ml. Controls were untreated. After 1 h, the cells were treated with 3 μg of actinomycin D per ml. (A) RNase protection assay of RNA isolated at various time points after actinomycin D treatment. C− indicates the yeast tRNA negative control; P/M indicates undigested probe-marker; C+ indicates Mono-Mac-6 cell RNA treated for 3 h with LPS and IFN-γ used as a positive control. (B) TNF-α mRNA densitometric units shown graphically. (C) The t1/2 of TNF-α mRNA was calculated for each condition. The experiment with the shortest half-life is shown, but no experiment showed a difference in t1/2. The figures are representative of three separate experiments.
Inhibition of Jak2 by tyrphostin AG-490 compromised the induction of TNF-α by LPS with or without of IFN-γ.
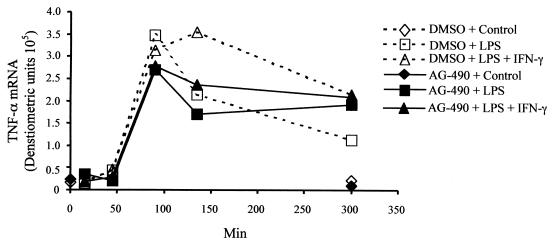
Our findings of very rapid induction of TNF-α transcripts by LPS with a prolonged peak induced by IFN-γ could not be explained by changes in message turnover. This, in turn, suggested that IFN-γ was directly activating transcription in the presence of LPS, although it had no activity on its own. This could be due to a direct transcriptional effect of STAT1α or due to an interaction of the IFN-γ signaling pathway with the LPS signaling pathway. To begin to explore the mechanisms of the modulatory effects of IFN-γ, we utilized tyrphostin AG-490, a competitive inhibitor of Jak2 and Jak3 (8, 26, 34, 52). AG-490 is a tyrosine kinase inhibitor first identified as an inhibitor of the expansion of acute lymphoblastic leukemia cells (34). The kinase activities of Jak1 and of other kinases, Lck, Lyn, Btk, Syk, and Src, were unaffected by AG-490 (19, 31). Mono-Mac-6 cells were stimulated with LPS with or without IFN-γ; one set of cells was treated with AG-490 and the other with DMSO carrier as a control. TNF-α gene transcripts from all the experimental conditions exhibited similar profiles through at least the first 90 min of stimulation (Fig. 4). Thereafter, RNA levels began to decline in cells treated with LPS (Fig. 4). TNF-α message from LPS–IFN-γ-stimulated cells exhibited the prolonged peak, as previously shown in Fig. 2B. In the presence of AG-490, this prolonged transcript synthesis was no longer observed. AG-490 had an unexpected inhibitory effect on LPS-induced TNF-α mRNA synthesis (Fig. 4). At 130 min, this inhibitory effect was significant in comparing the amount of transcripts in the LPS versus the LPS- and AG-490-stimulated cells (P = 0.005). The difference in the amount of TNF-α mRNA levels between the LPS–IFN-γ- versus the LPS–IFN-γ–AG-490-stimulated cells was also significant (P = 0.025). We focused on the 130-min time point since the prolonged presence of TNF-α mRNA was the most obvious in LPS–IFN-γ-treated cells, and the inhibitory effects of AG-490 appeared to be the most marked. At that time point it is clear that AG-490 inhibited TNF-α transcription in cells stimulated with LPS alone even though Jak2 and Jak3 are not known to play a role in LPS signaling. AG-490 also altered the kinetics of TNF-α transcript accumulation such that the prolonged peak typically seen with IFN-γ–LPS stimulation was no longer evident. The most likely explanation for these findings is that the LPS signaling pathway is altered by Jak2 and/or Jak3 inhibition and that the loss of the characteristic prolonged peak was due to the expected inhibition of Jak2 which is required for IFN-γ signaling. We cannot exclude the possibility that AG-490 is having effects on multiple signaling pathways, however.
FIG. 4.
IFN-γ effects are sensitive to a Jak2 inhibitor, as revealed by an RNase protection assay examining the effect of AG-490. Mono-Mac-6 cells (2 × 106/ml) were stimulated with 2 μg of LPS per ml with or without 50 ng of IFN-γ per ml. One set of cells was treated with 20 μM AG-490, and a second set was treated with equal volumes of the DMSO carrier. TNF-α mRNA densitometric units were calculated. Panel A is representative of three separate experiments. The differences between the LPS and LPS+AG-490 treatments and between the LPS+IFN-γ and LPS+IFN-γ–AG+490 treatments were not statistically different at 300 min (P = 0.699 and P = 0.465, respectively).
DISCUSSION
In this study, we demonstrated that IFN-γ could directly potentiate the ability of LPS to induce the expression of TNF-α at the level of gene transcription. Human Mono-Mac-6 cells secreted significantly more TNF-α when stimulated simultaneously with IFN-γ and LPS compared to stimulation with LPS alone. The production of TNF-α mRNA was also prolonged from cells treated with both IFN-γ and LPS. This effect was regulated at the level of transcription but not at the level of message stability since the rate of message degradation was similar whether LPS alone or LPS with IFN-γ induced the expression. Our studies suggest that IFN-γ has a direct transcriptional effect in addition to the well-characterized “priming” activity of IFN-γ (23).
The production of TNF-α is regulated at various stages from the agonist initiation of signaling pathways to the exportation and cleavage of the mature protein. Interactions of signaling pathways launched upon receptor engagement remain largely undefined. However, studies of the promoter region of TNF-α have provided much insight into the complex choreography of DNA-binding proteins that is coordinated to initiate transcription and derepress inhibitory transcription repressors (18, 21, 29, 45, 46, 50, 54). Interactions of NF-κB, C/EBPβ, Jun, and other proteins contribute to the transcriptional regulation of TNF-α. Other studies to understand the regulation of TNF-α expression have included analyses of transcription rates, message stability, and targets for mRNA degradation (11, 14, 39). In our studies, the rapid kinetics and the absence of an effect on message stability strongly suggest that IFN-γ modulation of LPS-induced TNF-α expression in human Mono-Mac-6 cells is at the level of gene transcription.
No definitive conclusions can be drawn as to whether Jak2 took part in the prolonged presence of TNF-α mRNA in LPS–IFN-γ-stimulated cells because there was a general inhibition of TNF-α expression. However, it did appear that the characteristic kinetics seen with LPS–IFN-γ stimulation were abolished by AG-490 treatment. This suggests that the effect on the kinetics may be mediated by Jak2. Potential mechanisms include direct and indirect effects. The IFN-γ potentiation effect could be due to protein-protein interactions of STAT1α with the LPS-induced transcription factors on the TNF-α promoter. STAT molecules have been demonstrated to complex with other transcription factors such as NF-κB leading to synergistic effects (24).
In addition to altering the characteristic kinetics, AG-490 had the unexpected effect of inhibiting LPS-induced transcription of TNF-α. This suggests that it may be acting on shared signaling pathways. The glycosyl-phosphatidylinositol-anchored cell surface receptor, CD14, has been shown to directly associate with a member of the src family protein tyrosine kinase, p56lyn (41, 44), to activate the MAPK cascades (30, 38, 55). The MAPK cascade through Raf-1 targets the activation of c-Jun and c-Fos, components of the AP-1 transcription dimer, to induce TNF-α expression (22, 25). Both Jak2 and Jak3 can activate the MAPK cascade, suggesting one potential mechanism for IFN-γ potentiation of LPS induction of TNF-α (12, 51, 52, 55). Our AG-490 data are consistent with that hypothesis. AG-490 would abolish the IFN-γ effect on kinetics through its inhibition of Jak2 and could also affect LPS stimulation due to the intersection of Jak2 and the MAPK pathway. However, there are other possible explanations for our data. It is possible that AG-490 targets and inhibits the phosphorylation of kinases in the LPS signaling pathways. Another LPS-induced signaling pathway results in the activation of the NF-κB family of transcription factors which is critical for TNF-α transcription (2, 21, 48, 50).
In summary, we have shown that IFN-γ has the ability to upregulate the LPS-inducible TNF-α mRNA and protein production using a stimulation protocol which administers IFN-γ and LPS simultaneously. The data we present here suggest that the effect is at the level of gene transcription since mRNA turnover rates were similar in cells stimulated by LPS with or without IFN-γ. This phenomenon could be of relevance early in the course of bacterial infections when both LPS and IFN-γ would be present at high levels.
ACKNOWLEDGMENTS
We thank Laurie Kilpatrick for critical review of the manuscript.
J.Y.L. is a recipient of the National Eye Institute Pre-doctoral Training Grant and the Gina Finzi Memorial Fellowship from the Lupus Foundation of America, Inc. K.E.S. is supported by the Wallace Chair of Pediatrics and the RO1 AI/AR 44127.
REFERENCES
- 1.Ashkenazi A, Marsters S A, Capon D J, Chamow S M, Figari I S, Pennica D, Goeddel D V, Palladino M A, Smith D H. Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin. Proc Natl Acad Sci USA. 1991;88:10535–10539. doi: 10.1073/pnas.88.23.10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes P J, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 4.Beutler B, Tkacenko V, Milsark I, Krochin N, Cerami A. Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med. 1986;164:1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biragyn A, Nedospasov S A. Lipopolysaccharide-induced expression of TNF-alpha gene in the macrophage cell line ANA-1 is regulated at the level of transcription processivity. J Immunol. 1995;155:674–683. [PubMed] [Google Scholar]
- 6.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 7.Bohuslav J, Kravchenko V V, Parry G C, Erlich J H, Gerondakis S, Mackman N, Ulevitch R J. Regulation of an essential innate immune response by the p50 subunit of NF-κB. J Clin Investig. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bright J J, Du C, Sriram S. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol. 1999;162:6255–6262. [PubMed] [Google Scholar]
- 9.Briscoe J, Guschin D, Rogers N C, Watling D, Muller M, Horn F, Heinrich P, Stark G R, Kerr I M. JAKs, STATs and signal transduction in response to the interferons and other cytokines. Phil Trans R Soc London Ser B Biol Sci. 1996;351:167–171. doi: 10.1098/rstb.1996.0013. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Baumgartner J D, Grau G E, Wu M M, Lambert P H, Schellekens J, Verhoef J, Glauser M P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990;161:982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 11.Carballo E, Lai W S, Blackshear P J. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 12.Carter-Su C, Smit L S. Signaling via JAK tyrosine kinases: growth hormone receptor as a model system. Rec Prog Hormone Res. 1998;53:61–83. [PubMed] [Google Scholar]
- 13.Chung I Y, Kwon J, Benveniste E N. Role of protein kinase C activity in tumor necrosis factor-alpha gene expression. Involvement at the transcriptional level. J Immunol. 1992;149:3894–3902. [PubMed] [Google Scholar]
- 14.Crawford E K, Ensor J E, Kalvakolanu I, Hasday J D. The role of 3′ poly(A) tail metabolism in tumor necrosis factor-alpha regulation. J Biol Chem. 1997;272:21120–21127. doi: 10.1074/jbc.272.34.21120. [DOI] [PubMed] [Google Scholar]
- 15.Darnell J E, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 16.Decker T, Kovarik P, Meinke A. GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. J Interferon Cytokine Res. 1997;17:121–134. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- 17.English B K, Orlicek S L, Mei Z, Meals E A. Bacterial LPS and IFN-gamma trigger the tyrosine phosphorylation of vav in macrophages: vidence for involvement of the hck tyrosine kinase. J Leukoc Biol. 1997;62:859–864. doi: 10.1002/jlb.62.6.859. [DOI] [PubMed] [Google Scholar]
- 18.Fong C-L, Siddiqui A H, mark D F. Identification and characterization of a novel repressor site in the human tumor necrosis factor a gene. Nucleic Acids Res. 1994;22:1108–1114. doi: 10.1093/nar/22.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazit A, Yaish P, Gilon C, Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 20.Georgopoulos S, Plows D, Kollias G. Transmembrane TNF is sufficient to induce localized tissue toxicity and chronic inflammatory arthritis in transgenic mice. J Inflamm. 1996;46:86–97. [PubMed] [Google Scholar]
- 21.Goldfeld A E, Doyle C, Maniatis T. Human tumor necrosis factor a gene regulation by virus and lipopolysaccharide. Proc Natl Acad Sci USA. 1990;87:9769–9773. doi: 10.1073/pnas.87.24.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hambleton J, McMahon M, DeFranco A L. Activation of Raf-1 and mitogen-activated protein kinase in murine macrophages partially mimics lipopolysaccharide-induced signaling events. J Exp Med. 1995;182:147–154. doi: 10.1084/jem.182.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes M P, Freeman S L, Donnelly R P. IFN-gamma priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and MRNA stability. Cytokine. 1995;7:427–435. doi: 10.1006/cyto.1995.0058. [DOI] [PubMed] [Google Scholar]
- 24.Held T K, Weihua X, Yuan L, Kalvakolanu D V, Cross A S. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect Immun. 1999;67:206–212. doi: 10.1128/iai.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin M, Delhase M. JNK or IKK, AP-1 or NF-kappaB, which are the targets for MEK kinase 1 action? Proc Natl Acad Sci USA. 1998;95:9067–9069. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirken R A, Erwin R A, Taub D, Murphy W J, Behbod F, Wang L, Pericle F, Farrar W L. Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J Leukoc Biol. 1999;65:891–899. doi: 10.1002/jlb.65.6.891. [DOI] [PubMed] [Google Scholar]
- 27.Koerner T J, Adams D O, Hamilton T A. Regulation of tumor necrosis factor (TNF) expression: interferon-gamma enhances the accumulation of mRNA for TNF induced by lipopolysaccharide in murine peritoneal macrophages. Cell Immunol. 1987;109:437–443. doi: 10.1016/0008-8749(87)90326-1. [DOI] [PubMed] [Google Scholar]
- 28.Kovarik P, Stoiber D, Novy M, Decker T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 1998;17:3660–3668. doi: 10.1093/emboj/17.13.3660. . (Erratum, 17:4210.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuprash D V, Udalova I A, Turetskaya R L, Kwiatkowski D, Rice N R, Nedospasov S A. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–4052. [PubMed] [Google Scholar]
- 30.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 31.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Sidiropoulos P, Song G, Pagliari L J, Birrer M J, Stein B, Anrather J, Pope R M. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- 33.Medvedev A E, Flo T, Ingalls R R, Golenbock D T, Teti G, Vogel S N, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- 34.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder J S, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman C M. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 35.Michie H R, Manogue K R, Spriggs D R, Revhaug A, O'Dwyer S, Dinarello C A, Cerami A, Wolff S M, Wilmore D W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 36.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, Widmer M B, Blosch C M. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 37.Oeth P A, Parry G C, Kunsch C, Nantermet P, Rosen C A, Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol Cell Biol. 1994;14:3772–3781. doi: 10.1128/mcb.14.6.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 39.Raabe T, Bukrinsky M, Currie R A. Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem. 1998;273:974–980. doi: 10.1074/jbc.273.2.974. [DOI] [PubMed] [Google Scholar]
- 40.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 41.Stefanova I, Corcoran M L, Horak E M, Wahl L M, Bolen J B, Horak I D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 42.Suk K, Erickson K L. Differential regulation of tumour necrosis factor-alpha mRNA degradation in macrophages by interleukin-4 and interferon-gamma. Immunology. 1996;87:551–558. doi: 10.1046/j.1365-2567.1996.500561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan K E, Wooten C, Schmeckpeper B, Goldman D, Petri M. A promoter polymorphism of tumor necrosis alpha is associated with systemic lupus erythematosus in African Americans. Arthritis Rheum. 1997;40:2207–2211. doi: 10.1002/art.1780401215. [DOI] [PubMed] [Google Scholar]
- 44.Sweet M J, Hume D A. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 45.Takashiba S, Shapira L, Amar S, Van Dyke T E. Cloning and characterization of human TNF alpha promoter region. Gene. 1993;131:307–308. doi: 10.1016/0378-1119(93)90314-s. [DOI] [PubMed] [Google Scholar]
- 46.Takashiba S, Van Dyke T E, Shapira L, Amar S. Lipopolysaccharide-inducible and salicylate sensitive nuclear factor(s) on human tumor necrosis factor alpha promoter. Infect Immun. 1995;63:1529–1534. doi: 10.1128/iai.63.4.1529-1534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeshita S, Nakatani K, Takata Y, Kawase H, Sekine I, Yoshioka S. Interferon-gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha) enhance lipopolysaccharide binding to neutrophils via CD14. Inflamm Res. 1998;47:101–103. doi: 10.1007/s000110050290. [DOI] [PubMed] [Google Scholar]
- 48.Trede N S, Tsytsykova A V, Chatila T, Goldfeld A E, Geha R S. Transcriptional activation of the human TNF-alpha promoter by superantigen in human monocytic cells: role of NF-kappa B. J Immunol. 1995;155:902–908. [PubMed] [Google Scholar]
- 49.Ucla C, Roux-Lombard P, Fey S, Dayer J M, Mach B. Interferon gamma drastically modifies the regulation of interleukin-1 genes by endotoxin in U937 cells. J Clin Investig. 1990;85:185–191. doi: 10.1172/JCI114411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Udalova I A, Knight J C, Vidal V, Nedospasov S A, Kwiatkowski D. Complex NF-kappaB interactions at the distal tumor necrosis factor promoter region in human monocytes. J Biol Chem. 1998;273:21178–21186. doi: 10.1074/jbc.273.33.21178. [DOI] [PubMed] [Google Scholar]
- 51.Vanderkuur J A, Butch E R, Waters S B, Pessin J E, Guan K L, Carter-Su C. Signaling molecules involved in coupling growth hormone receptor to mitogen-activated protein kinase activation. Endocrinology. 1997;138:4301–4307. doi: 10.1210/endo.138.10.5453. [DOI] [PubMed] [Google Scholar]
- 52.Wang L H, Kirken R A, Erwin R A, Yu C R, Farrar W L. JAK3, STAT, and MAPK signaling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol. 1999;162:3897–3904. [PubMed] [Google Scholar]
- 53.Weber-Nordt R M, Mertelsmann R, Finke J. The JAK-STAT pathway: signal transduction involved in proliferation, differentiation and transformation. Leukemia Lymphoma. 1998;28:459–467. doi: 10.3109/10428199809058353. [DOI] [PubMed] [Google Scholar]
- 54.Yao J, Mackman N, Edgington T S, Fan S-T. Lipopolysaccharide induction of the tumor necrosis factor-a promoter in human monocytic cells. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 55.Zhu W, Downey J S, Gu J, Di Padova F, Gram H, Han J. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J Immunol. 2000;164:6349–6358. doi: 10.4049/jimmunol.164.12.6349. [DOI] [PubMed] [Google Scholar]