Abstract
Radiation exposure is an undeniable health threat encountered in various occupations and procedures. High energy waves in ionizing radiation cause DNA damage and induce reactive oxygen species (ROS) production, which further exacerbate DNA, protein, and lipid damage, increasing risk of mutations. Although endogenous antioxidants such as superoxide dismutase have evolved to upregulate and neutralize ROS, exogenous dietary antioxidants also have the potential to combat ionizing radiation (IR)-induced ROS production. We evaluated a cocktail of ingredients (AOX) purported to have antioxidant and mitochondrial protective properties on the acute effects of IR. We show that IR stimulates DNA damage through phosphorylation of DNA repair proteins in the heart, brain, and liver of mice. AOX showed partial protection in brain and liver, through a lack of significant activation in given repair proteins. In addition, AOX attenuated the IR-induced increase in NF-kβ mRNA and protein expression in brain and liver. Lastly, cytochrome c oxidase complex transcripts were significantly higher in heart and brain following radiation, which was also diminished by prior ingestion of AOX. Together, our findings suggest that a multi-ingredient AOX supplement may attenuate the IR-induced cellular damage response and represents a feasible and cost-effective preventative supplement for at-risk populations of radiation exposure.
Keywords: antioxidants, ionizing radiation, γ-H2AX, mtDNA
1. Introduction
Radiation exposure is a concern for many populations and professions, as high energy waves, particularly ionizing radiation (IR), alter chemical structure in cells [1], induce reactive oxygen species (ROS) [2], and are involved in tissue damage [3] and increased risk of cancer [4]. Specifically, due to their persistent exposure to IR, nuclear industry workers, aircrews, medical radiology technicians, and patients of frequent diagnostic imaging may be at increased risk [5,6]. Indeed, it has been estimated that in some developed countries, over 3% of cancers can be attributed to radiation exposure from diagnostic imaging [7]. In addition, with recent concerns about nuclear accidents rising due to miliary conflict, there is a risk of excessive radiation exposure to the local populations, potentially affecting long term health [8,9].
The mutagenic effects and cellular damage associated with IR are primarily caused by two cellular events [10,11,12]. First, radiation induces double stranded breaks in DNA, which cause mutations, loss of heterozygosity, and can lead to cancer or cell death. Imperative to repair the breaks, a damage repair response is triggered where proteins are phosphorylated and localized in the nucleus to initiate the repair. These proteins include γ-H2AX and ataxia telengiectasia mutated (ATM), and together with cell cycle arrest proteins, cyclin-dependent kinase inhibitor 1A (CDKN1A) and tumor protein 53 (TP53), have been proposed as the top four biomarker candidates of IR-induced DNA damage [12]. In addition to nuclear DNA damage, IR can also cause damage to mitochondrial DNA (mtDNA), which may be more detrimental to the health of the cell, as mtDNA lacks the excision and recombination repair mechanism [13], making it more susceptible to mutagenesis [14].
Secondly, IR-induced effects include the generation of ROS, which oxidize molecules and cause further damage to DNA, lipids, and proteins in the cell [10]. The high energy waves from IR interact with water molecules in the cell generating H2O+ and a free electron, which subsequently produces free radicals and ROS [15]. Molecular damage induced by ROS will then further exacerbate DNA damage and initiate an inflammatory response [16]. As protection against ROS, cells have evolved to endogenously produce free scavenger proteins such as superoxide dismutase (SOD), which break down and neutralize ROS, making SOD an endogenous antioxidant [17]. Exogenous free scavengers can also be supplemented with dietary antioxidants and have been shown to be protective against IR-induced DNA damage [18,19]. Previous studies in patients undergoing diagnostic imaging have shown that ingesting oral antioxidants prior to their procedure can prevent nuclear DNA damage in blood cells, as reflected in an attenuation of γ-H2AX [19,20]. However, whether similar protection can be conferred to mitochondrial DNA has yet to be investigated. In our current study, we created a formula containing 10 different ingredients with antioxidant properties, which have been shown to be beneficial against radiation-induced damage by reducing lethality [21] or attenuating markers of DNA and cellular damage [18,19,20], including quercetin [22,23], astaxanthin [24], zeaxanthin [25], vitamin C [26], vitamin B12 [27], selenium [28], folate [29], CoQ10 [30,31], α-lipoic acid [32], and vitamin E [33]. Importantly, we specifically chose to include CoQ10, vitamin E, and α-lipoic acid, as they were the main components of a multi-ingredient supplement that we have shown lowered lactate and oxidative stress markers in patients with primary mitochondrial myopathy [34]. Consequently, the novelty of our proposal is that we have proposed a multi-ingredient supplement that would target multiple final common pathways of IR-induced damage including mitochondrial DNA and membrane protection. We also chose to evaluate brain and heart given that these have been shown to be negatively affected by repeated doses [35,36] and even a single [37] dose of IR exposure.
Thus, the aim of our study was to test the hypothesis that our multi-ingredient antioxidant supplement (AOX) would provide cellular protection to rodents exposed to a single high dose of IR (2 Gy). We employed varying molecular biology techniques to assess nuclear and mitochondrial DNA damage, inflammatory response, and mitochondrial stress response, following acute high dose IR in mice. We provide evidence that partial cellular protection may be achieved when ingesting AOX prior to IR exposure. This AOX represents a feasible, cost-effective therapeutic intervention that has the potential to reduce the increased risk in mutagenesis when exposed to IR. We specifically designed the study to interrogate the potential for the supplement to prophylactically protect against IR exposure that would be of relevance to patients undergoing diagnostic imaging, radiation workers and those at risk of nuclear accidents.
2. Materials and Methods
Animals and study design. All animal experiments and procedures were performed with the approval of McMaster University Animal Research Ethics Board and conformed to the standards of the Canadian Council on Animal Care (AUP#20-04-17). Male C57BL/6J mice were ordered from Jackson Laboratories (Bar Harbor, ME, USA) and randomly assigned to one of two separate cohorts, acute (+30 min) or later (+24 h) post-IR collection times. Furthermore, each of these two groups were further divided into one of three study groups: CON (no RAD nor AOX), RAD, or RAD + AOX. Animals were maintained in standard mouse cages (3 mice per cage) on an ad libitum diet of standard chow throughout the experimental period. Mice were fasted for 12 h prior to gavage feeding and experimental procedures. An antioxidant cocktail (based around the provision of nuclear and mitochondrial DNA protection, Table 1), was administered to RAD + AOX mice via oral gavage two hours prior to irradiation. The RAD group received a vehicle control of corn oil and water. The control group (CON) was not gavage fed. Animals in the RAD and RAD + AOX groups were also subjected to 2 Gy of whole-body radiation (Gammacell® 3000, Best Theratronics, Kanata, ON, Canada). Animals in the acute and later cohorts were sacrificed at +30 min and +24 h post radiation exposure, respectively. The blood, liver, heart, and brain were rapidly removed, snap-frozen, and stored at −80 °C until analysis. Samples intended for flow cytometry were incubated in 1 mL of buffer containing 10 g/L collagenase B (Roche, Basel, Switzerland, 11088831001), 4 g/L Dispase II (Roche, 494207800) and Ham’s F-10 media, at 37 °C.
Table 1.
Multi-ingredient antioxidant supplement list.
| Ingredient | Dose (mg/kg) | Vendor | Cat. Number |
|---|---|---|---|
| Quercetin | 41 | Cayman Chemical Company | 10005169 |
| CoQ10 | 27.5 | MyBioSource LLC | MBS165643 |
| α-Lipoic Acid | 27.5 | Sigma-Aldrich | T1395 |
| Vitamin E | 27.5 | Sigma-Aldrich | T3126 |
| Vitamin C | 41 | Sigma-Aldrich | A5960 |
| Astaxanthin | 0.82 | Sigma-Aldrich | SML0982 |
| Zeaxanthin | 0.51 | Cayman Chemical Company | 10009992 |
| Folate | 0.082 | Sigma-Aldrich | F7876 |
| Selenium | 0.0205 | Sigma-Aldrich | 229865-5G |
| Vitamin B12 | 0.0103 | Sigma-Aldrich | V6629-250MG |
Flow cytometry. Flow cytometry technique was performed on liver, heart, and brain cells, as well as bone marrow (BMCs) and peripheral blood mononuclear cells (PBMCs). Prior to fixation, cells were stained with LIVE/DEAD® Fixable Dead Cell Stain Kit (ThermoFisher Scientific, Waltham, MA, USA) viability dye for 10 min at 4 °C in the dark. Samples were then washed and fixed with BD Cytofix/Cytoperm Buffer™ (BD Bioscience, Mississauga, ON, Canada, 554714) to permeabilize the cells, incubating at 4 °C for 20 min. After fixation, cells were washed, centrifuged at 500× g for 5 min and cell pellets were resuspended in 100 μL BD Perm/Wash™ buffer (BD Bioscience, 554714). Cells were incubated with Phospho-Histone H2A.X (Ser139) (20E3, Cell Signaling Technology, Danvers, MA, USA) Rabbit mAb (Alexa Fluor® 488 Conjugate) antibody (Cell Signaling Technology, 9719S) for 1 h at 4 °C in the dark. Following incubation, cells were washed and resuspended in 400 µL PBS (ThermoFisher Scientific, 14190250) and spun at 500× g for 5 min at 4 °C. Tissues with a prominent pellet were resuspended in up to 300 μL PBS while PBMCs and BMCs were resuspended in 150 μL PBS. The cell suspension was filtered through a 100 µm cell strainer prior to analysis. All samples were immediately analyzed using a flow cytometer (MoFlo™ XDP, Beckman Coulter, Brea, CA, USA).
Immunoblotting. Approximately 20 mg of snap-frozen tissue was homogenized in Axygen™ Snaplock Microcentrifuge tubes (14-222-156, Fisher Scientific, Waltham, MA, USA) via the FastPrep-24 Tissue and Cell Homogenizer in chilled RIPA lysis and extraction buffer (ThermoFisher Scientific, 89901) supplemented with protease and phosphatase inhibitor cocktail (Halt™, ThermoFisher Scientific, 78440). The homogenate was centrifuged for 20 min at 4 °C and 12,000× g, followed by removal of the supernatant. Total protein concentrations were determined using a BCA Protein Assay Kit (Pierce, Thermo Scientific, 23225) and protein samples were prepared in 1× Laemmli Buffer (Fisher Scientific, AAJ61337AC). Prior to gel electrophoresis, samples were heated at 95 °C for 5 min and micro-centrifuged. Immunoblots were resolved using a 4–20% Criterion TGX Precast Midi Protein Gel (5671094, Bio-Rad Laboratories, Hercules, CA, USA) with a PageRuler Plus Prestained Protein Ladder at 10 to 250 kDA (ThermoFisher Scientific, 26619). All blots were run in 1× Tris/Glycine/SDS buffer (Bio-Rad Laboratories, 1610772). Protein samples were transferred to a membrane using Trans-Blot Turbo Midi Nitrocellulose Transfer Packs (Bio-Rad Laboratories, 1704159EDU) and subsequently stained with Ponceau S solution (P7170-1L, Sigma, St. Louis, MO, USA) for 5 min. Membranes were imaged using the ChemiDoc MP Imaging System (Bio-Rad Laboratories) and placed in blocking solution (5% BSA in 1× TBS) for 1 h. Blots were left to incubate in primary antibody at 4 °C overnight with constant agitation. All primary antibodies were prepared in 5% BSA in TBS (Table 2). Membranes were serially washed (3 × 5 min) in 1× TBST and incubated with the appropriate secondary antibody at 1:20,000 (diluted in 5% BSA in 1× TBS) for 1 h. Membranes were serially washed again (3 × 5 min) in 1× TBST, then incubated with Clarity Max Western ECL substrate (Bio-Rad Laboratories, 1705062) for 5 min at room temperature (RT). All membranes were visualized using the ChemiDoc MP Imaging System (Bio-Rad Laboratories). Furthermore, band intensities were quantified using Image Lab 6.1 software (Bio-Rad Laboratories) and normalized to Ponceau S staining.
Table 2.
Antibody information.
| Primary Antibodies | ||||
| Antibody | Species | Vendor | Cat. Number | Dilution |
| pATM | M | Thermo Scientific | MA1-2020 | 1:3000 in 5% BSA in 1× TBS |
| γH2AX | R | CST | 9718S | 1:5000 in 5% BSA in 1× TBS |
| NF-kβ | R | CST | 4764T | 1:1000 in 5% BSA in 1× TBS |
| SODI/II | R | abcam | ab16831 | 1:1000 in 5% BSA in 1× TBS |
| Total OXPHOS | M | abcam | ab110411 | 1:1000 in 5% BSA in 1× TBS |
| Secondary Antibodies | ||||
| Antibody | Vendor | Cat. Number | Dilution | |
| Peroxidase AffiniPure Donkey Anti-Mouse IgG (H + L) | Jackson ImmunoResearch | 715-035-151 | 1:20,000 in 5% BSA in 1× TBS | |
| Peroxidase AffiniPure Donkey Anti-Rabbit IgG (H + L) | Jackson ImmunoResearch | 711-035-152 | 1:20,000 in 5% BSA in 1× TBS | |
RNA isolation. RNA was isolated from 20 mg of tissue using the Trizol/RNeasy method. All samples were homogenized with 500 μL Trizol Reagent with the FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals, Solon, OH, USA) for 40 s at a speed of 6 m/s. All samples were incubated at room temperature for 5 min. 10µL chloroform was added to each sample, mixed vigorously for 30 s, incubated at RT for 3 min, and spun at 12,000× g for 15 min at 4 °C. The aqueous phase containing RNA was transferred to a new tube, precipitated with 250 µL isopropanol, incubated for 10 min at 4 °C, and centrifuged at 12,000× g for 10 min at 4 °C. The pellet was immediately resuspended in 500 µL 75% ethanol, vortexed briefly, and centrifuged at 7500× g for 5 min at 4 °C. Prior to solubilization, the pellet was air dried for 10 min. The pellet was resuspended in 30 µL RNase-free water. RNA concentration (ng/mL) and purity (260/280) were determined with the Nano-Drop 1000 Spectrophotometer (ThermoFisher Scientific).
cDNA Synthesis. Reverse transcription was performed on 1 μg cell lysate of RNA using a SuperScript™ IV VILO™ Master Mix (ThermoFisher Scientific, 11756500), as per the manufacturer’s protocol. The reaction mixtures were incubated in a T100 Thermal Cycler (Bio-Rad Laboratories) with thermocycling conditions of 25 °C for 10 min (Annealing), 50 °C for 10 min (Elongation), and 85 °C for 5 min (Enzyme Inactivation). Synthesized cDNA was diluted with nuclease-free water to obtain a concentration of 5.5 ng/µL prior to storage at −80 °C.
Quantitative PCR. Gene expression was determined by running 4.5 µL of cDNA template (5.5 ng/µL) to a final volume of 10 uL on a CFX384 Real-Time System (Bio-Rad Laboratories) using the TaqMan Fast Advanced Master Mix (ThermoFisher Scientific, 4444557), using the following TaqMan cycling conditions: 20 s at 95 °C for initial denaturation, followed by 40 cycles with 3 s denaturation at 95 °C, and 30 s annealing and elongation at 60 °C. The fluorescence threshold was automatically set above the background level. Fold-increase in mRNA probes of interest (Table 3) was calculated by the 2−ΔΔCt method and expression levels were normalized to the reference gene B2M (Beta-2-microglobulin). The absence of genomic contamination was ensured using an NTC control for each probe. All RT-qPCR reactions were run in duplicates.
Table 3.
TaqMan probe gene assay ID.
| Gene Symbol | Gene Name | Vendor | Assay ID |
|---|---|---|---|
| B2M | Beta-2-microglobulin | Thermo Fisher | Mm00437762_m1 |
| NF-kβ2 | Nuclear factor of kappa light polypeptide gene enhancer in B cells 2 | Thermo Fisher | Mm00479807_m1 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (P21) | Thermo Fisher | Mm00432448_m1 |
| STAT3 | Signal transducer and activator of transcription 3 | Thermo Fisher | Mm01219775_m1 |
| COX1 | Cytochrome c oxidase subunit 1 | Thermo Fisher | Mm04225243_g1 |
| COX4I1 | Cytochrome c oxidase subunit 4I1 | Thermo Fisher | Mm01250094_m1 |
LORD-Q assay. Tissue fragments from heart, brain, and liver were homogenized in lysis buffer using the FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals). DNA was then isolated and purified, as per manufacturer instructions with DNeasy Blood and Tissue Kit (69506, Qiagen, Hilden, Germany). DNA concentration (ng/mL) and purity (260/280) was determined by spectrometric analysis using the Nano-Drop 1000 Spectrophotometer (Thermo Scientific). Samples were then diluted to 7.5 ng/uL where 1 µL was used per 10 µL reaction. In addition, the rt-PCR reaction contained forward and reverse primers for mtDNA, 1× KAPA2G Fast Hot Start ReadyMix, and LightCycler 480 ResoLight dye. The ResoLight dye is provided at 20× concentration, which was optimized and diluted to 0.2×. Primers used for amplification of the mitochondrial short (S) and long (L) DNA fragment are listed in Table 4. mtDNA lesions were derived and calculated from Ct (Cp) scores of short (CpS) and long fragments (CpL), using the formula below (1). Non-irradiated mice were used as the control (Ref). Amplification efficiencies (E) for both primer sets were determined through a standard curve with serial dilutions of 24, 16, 8, 4, 2, 1, 0.5 and 0.25 ng/uL. This protocol was adapted from Lehle et al. (2014), and Dannenmann et al. (2017) [38,39].
| (1) |
Table 4.
LORD-Q primers.
| Locus | Base Pairs | Primer Denotation | Primer Sequence |
|---|---|---|---|
| mtDNA (L) mouse | 3921 | MM.mtDNA.F | 5′-TCCTACTGGTCCGATTCCAC-3′ |
| MM.mtDNA.L.R | 5′-CGGTCTATGGAGGTTTGCAT-3′ | ||
| mtDNA (S) mouse | 74 | MM.mtDNA.F | 5′-TCCTACTGGTCCGATTCCAC-3′ |
| MM.mtDNA.S.R | 5′-GGCTCCGAGGCAAAGTATAG-3′ | ||
| Col1a1 (L) murine | 2637 | MM.col1a1.L1.F | 5′-CCGTTTGTCCCATTACTGCT-3′ |
| MM.col1a1.L1.R | 5′-AGCAAGGACGAGGACTTTGA-3′ | ||
| Col1a1 (S) murine | 60 | MM.col1a1.S.F | 5′-AAAGTGGGAATCTGGACACG-3′ |
| MM.col1a1.S.R | 5′-CAGAGGCCTTATTTCATTTTCG-3′ |
3. Results
3.1. Ionizing Radiation Causes Acute DNA Damage across Multiple Tissues 30 Min following Exposure
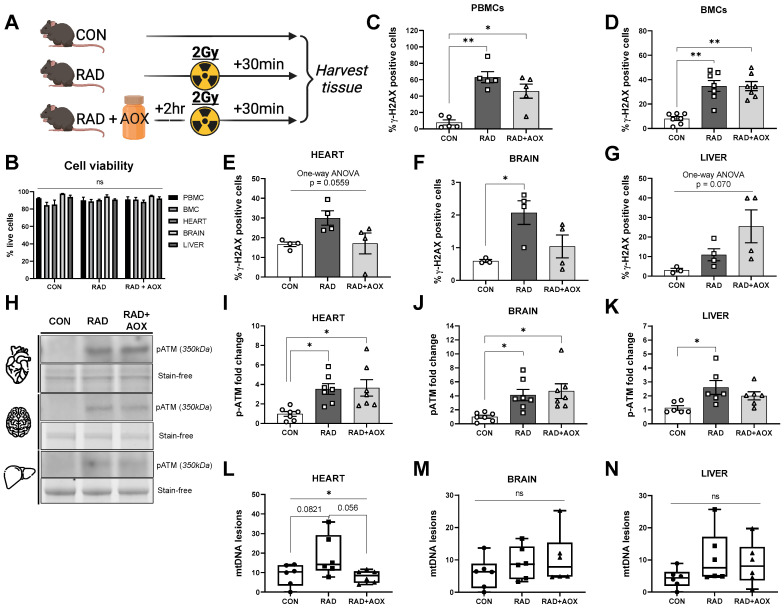
A higher percentage of γ-H2AX-positive cells was observed, as a result of IR, across multiple tissues (Figure 1C–G). In isolated PBMCs, the percentage of γ-H2AX cells significantly increased from 7.9% (CON) to 63.1% in RAD, while a significant increase to 46.2% was observed in RAD + AOX animals (Figure 1C). Similarly, BMCs isolated from mouse femurs saw a significant increase in γ-H2AX-positive cells following radiation, in both RAD (34.8%) and RAD + AOX (34.7%) groups, compared to CON (8.1%) (Figure 1D). In the heart, brain, and liver, the percentage of γ-H2AX positive cells was higher in RAD and RAD + AOX groups than in CON, however, only in brain tissue did this increase reach statistical significance (Figure 1E–G). Evidently, the employed dose of IR was sufficient to induce a cellular response detectable by the utilized outcome measures.
Figure 1.
Ionizing radiation causes DNA damage across multiple tissues in mice. (A) Design of experiment. Mice were harvested 30 min following 2 Gy radiation. 2 h prior, mice were gavage fed with vehicle placebo (RAD) or antioxidant (RAD + AOX). Control mice (CON) were not gavage fed or irradiated. Flow cytometry of (B) viable cells, stained for γ-H2AX were analyzed and represented as % of positive cells in (C) Peripheral blood mononuclear cells (PBMCs), (D) Bone marrow cells (BMCs) (E) heart, (F) brain, and (G) liver. (H) Representative Western blot images for p-ATM protein expression and densitometric quantification in the (I) heart, (J) brain and (K) liver. Protein expression data is expressed as fold change mean (±SEM) relative to CON. (L–N) mtDNA lesions were analyzed and graphed through LORD-Q assay. Individual data points shown. Statistical analysis was done with One-way ANOVA with Tukey’s post hoc. *, ** denote a significant difference between groups of p < 0.05, and p < 0.01, respectively.
Higher pATM expression was found in RAD and RAD + AOX groups compared to CON across all three interrogated tissues (Figure 1H–K). The fold-changes of pATM in RAD mice relative to CON for heart, brain, and liver were 3.6, 4.1, and 2.5-fold, respectively (p < 0.05). pATM expression was also higher in RAD + AOX animals compared to CON heart and brain tissue. Specifically, pATM expression was 3.6-fold higher in the heart (p < 0.05), and 4.6-fold in the brain (p < 0.05); whereas, in liver, there was no significant difference in pATM levels between RAD + AOX and CON.
Using a previously validated method [39] of long-run real-time PCR (LORD-Q) mtDNA damage quantification, we found a significant main effect between groups in heart tissue, where RAD was trending towards significantly higher mtDNA lesions compared to CON (p = 0.082) (Figure 1L). Importantly, heart tissue of RAD + AOX mice did not show a higher number of mtDNA lesions vs. CON (Figure 1L). No significant effect of IR was observed upon mtDNA lesions in the brain and liver of either RAD or RAD + AOX mice compared to CON (Figure 1M,N).
3.2. Multi-Ingredient Antioxidant Supplement Dampens the Inflammatory Stress Response Induced 24 h following Ionizing Radiation
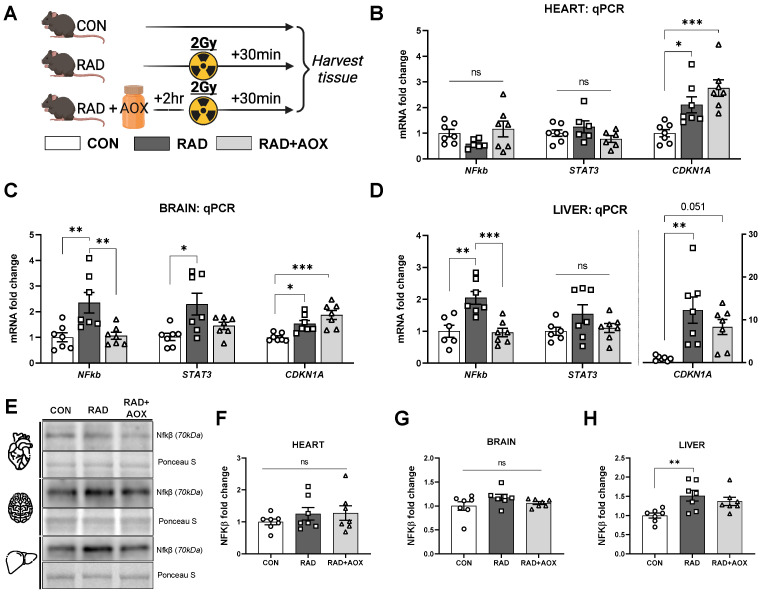
To investigate the protective effect of AOX upon IR-induced DNA damage, we measured the mRNA abundance of inflammatory transcription factors NF-κβ, STAT3, and CDKN1A in heart, brain, and liver harvested 24 h following IR.
Relative to CON, NF-κβ mRNA abundance was significantly higher in RAD animals in both the brain and liver by 2.3 and 2.0-fold, respectively. RAD + AOX animals showed no significant difference compared to CON (Figure 2B–D). Relative to CON, significantly higher (1.51-fold-change) NF-κβ protein content was observed in the RAD group, with RAD + AOX showing no significant difference (Figure 2H). In contrast, NF-κβ protein content was not significantly different across the three groups in heart or brain (Figure 2F,G).
Figure 2.
Antioxidant multi-ingredient supplement attenuates increase in NF-kβ induced by ionizing radiation. (A) Design of experiment. Mice were harvested 24 h following 2 Gy radiation. 2 h prior to IR exposure, mice were gavage with vehicle placebo (RAD) or antioxidant (RAD + AOX). Control mice (CON) were not gavage fed or irradiated. The mRNA abundance of; NF-kβ, STAT3, and CDKN1A in the (B) heart, (C) brain, and (D) liver were measured using qRT-PCR. Analysis was done using the 2−Δ method normalized to B2M housekeeper gene. (E) Representative Western blot images for NFkβ protein expression and densitometric quantification in the (F) heart, (G) brain and (H) liver. Data is expressed as fold change mean (±SEM) relative to CON. Individual data points shown, n = 7 per group. Statistical analysis was done with One-way ANOVA with Tukey’s post hoc. *, **, and *** denote a significant difference between groups of p < 0.05, p < 0.01, and p < 0.001, respectively.
The mRNA abundance of STAT3 transcripts were significantly higher in brain tissue of RAD mice (2.3-fold), with no significant elevation in the RAD + AOX group. Heart and liver tissues did not show a significant difference in STAT3 transcription between groups (Figure 2B–D).
The cell cycle arrest transcription factor CDKN1A was higher in RAD than CON in all tissues (2.1, 1.6, and 12.2-fold-change for heart, brain, and liver, respectively; Figure 2B–D). In contrast to results for other transcripts, the IR-induced increase in CDKN1A mRNA transcripts was not attenuated in RAD + AOX (Figure 2B–D).
3.3. Multi-Ingredient Antioxidant Supplement Attenuates Increase in Mitochondrial and Nuclear Encoded Mitochondrial Transcripts 24 h following Radiation Induced Damage
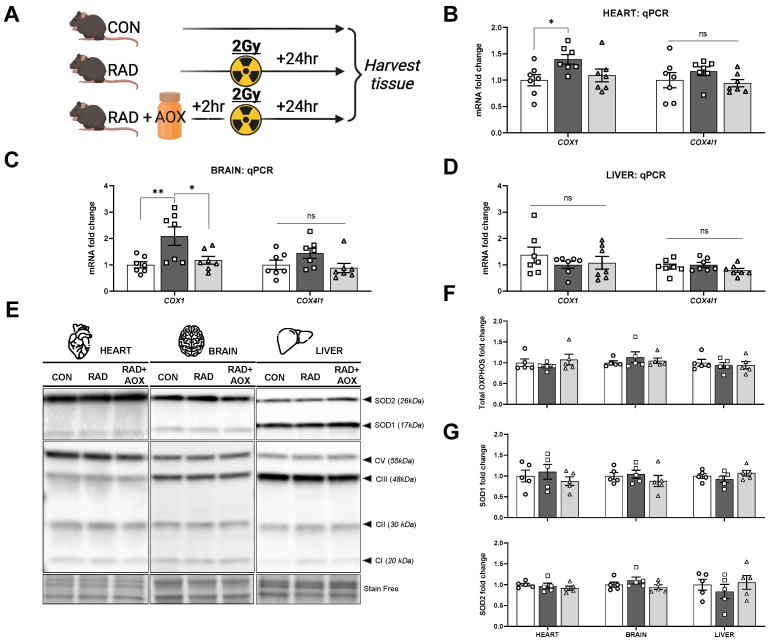
The mRNA abundance of the mtDNA encoded COX1 subunit was higher in RAD vs. CON for both heart (1.4-fold) and brain (2.1-fold), with no radiation-induced elevations observed in RAD + AOX animals vs. CON (Figure 3B,C). Expression of the nuclear DNA encoded transcript (COX4I1), showed a similar pattern for both heart and brain, but did not reach statistical significance. No effect of IR was found on mRNA abundance for either COX1 or COX4I1 in the liver (Figure 3D). Given the mRNA abundance changes, total protein content for representative mitochondrial electron transport proteins were evaluated, however no differences between groups were observed in any of the tissues tested (Figure 3F). Additionally, no IR-effect was observed on total protein content of endogenous antioxidant proteins SODI/II 24 h after IR exposure, in heart, brain, or liver (Figure 3G).
Figure 3.
Antioxidant multi-ingredient supplement attenuates increase in COX mRNA abundance. (A) Design of experiment. Mice were harvested 24 h following 2 Gy radiation. 2 h prior, mice were gavaged with vehicle placebo (RAD) or antioxidant (RAD + AOX). Control mice (CON) were not gavage fed or irradiated. mRNA abundance for genes of mitochondrial complex proteins I and IV in the (B) heart, (C) brain, and (D) liver were measured using qRT-PCR. Analysis was done using the 2−Δ method normalized to B2M housekeeper gene. (E) Representative Western blot images for superoxide dismutase I/II proteins (SODI/II) and total OXPHOS proteins, and (F,G) densitometric quantification in the heart, brain, and liver. Data is expressed as fold change mean (±SEM) relative to CON. Individual data points shown. Statistical analysis was done with One-way ANOVA with Tukey’s post hoc. *, and ** denote a significant difference between groups of p < 0.05, and p < 0.01, respectively.
4. Discussion
The current study provides evidence for the potential benefit of an AOX multi-ingredient supplement to attenuate some of the cellular consequences of IR exposure, mainly in more terminally differentiated tissues, but also in peripheral blood mononuclear cells.
Specifically, we showed that IR induced DNA damage in isolated PBMCs, BMCs, heart, brain, and liver cells via staining of γ-H2AX, which was partially attenuated with AOX. Furthermore, ingestion of AOX attenuated the IR-induced increase in NF-kβ transcript and protein, as well as mitochondrial protein complex transcripts. The dose of IR administered in the present study (2 Gy) is higher than the typical dose of exposure during nuclear medicine procedures (~0.02 Gy for PET/CT scan); however, it is known that prolonged and repeated IR exposure can nevertheless compound within the body and lead to adverse effects in the heart and brain [23,40]. Survivors of radiation therapy display elevated prevalence of cardiac fibrosis and increased risk of coronary artery disease, cardiomyopathy, arrhythmia, and other cardiovascular complications [40]. Cognitive and memory dysfunction are also prevalent in patients undergoing radiation therapy, which may accelerate age-related dementia [41]. Given these consequences of radiation, a protective antioxidant supplement could be of potential benefits to cosmic radiation (X-ray, particle-based radiation) and other frequent radiation exposures encountered during occupational or diagnostic events. In contrast, radiation doses at or above 2 Gy are possible following nuclear disasters such as nuclear reactor accidents or nuclear war exposure [9,42,43]. Consequently, prophylaxis with the current formulation could potentially attenuate some of the cellular consequences of such high exposures.
Indeed, earlier studies of patients undergoing diagnostic imaging have shown promising results. Velauthapillai and colleagues showed that when taking oral antioxidants prior to technetium-99m methylene diphosphonate (99mTc MDP) bone scans, an attenuation of the increase in percentage of γ-H2AX foci was observed in isolated PBMCs when compared to PBMCs isolated from individuals that did not preemptively supplement with antioxidants, suggesting that antioxidants taken prior to radiation exposure may prevent DNA injury [19]. In addition, when incubating human blood lymphocytes with a cocktail of antioxidants before administering a CT-scan-comparable dose of radiation (0.02 Gy), antioxidant-treated cells show a lower percentage of γ-H2AX positive foci, again, suggesting a protection of antioxidant supplementation [18]. Literature highlighting individual antioxidant ingredients within our AOX formula further supports the idea of partial nuclear DNA protection against IR. In particular, blood lymphocytes incubated in various antioxidants for 1 h prior to IR, namely selenium, vitamin E, vitamin C, and CoQ10, have all demonstrated significant 12–25% reductions in γ-H2A foci number compared to placebo treated cells [20]. However, perhaps the most convincing evidence supporting the assertion that antioxidants may provide protection against radiation-induced DNA damage comes from randomized controlled trials of antioxidant supplementation studies in cancer patients undergoing radiation therapy [44]. Studies supplementing with vitamin E (ingredient used in our study) have reported a significant 38% reduction in radiation-induced adverse effects [45]. However, counterproductively, they also report reductions in local tumor control rates, and even more concerning, patients receiving antioxidants had poorer overall survival, suggesting that antioxidants do not only provide protection for healthy cells, but also cancer cells, thereby reducing the effectiveness of radiation therapy [45]. Although meta-analyses have concluded that antioxidants may not be a feasible supplement during radiation therapy, their use may indeed provide protection to tissue when given prior to IR exposure [45], in situations such as diagnostic imaging, nuclear accidents, or in workers exposed to radiation (i.e., interventional radiologists, flight crews, etc.).
In addition to protecting against nuclear DNA damage, protection to mitochondrial DNA damage is crucial due to limited repair mechanisms of mtDNA and its increased rate of mutagenesis [13,14]. Indeed, a potent stress signal such as 2 Gy of IR may cause an increase in cytosolic Ca2+ [46,47], which can activate mitochondrial phosphatases. This in turn dephosphorylates the cytochrome c protein complex thereby removing the inhibition on ATP production, increasing respiration and mitochondrial membrane potential to elevate ROS production. Excess ROS may then cause further damage to mtDNA and nuclear DNA. We saw elevations in mtDNA lesions in the heart of RAD mice compared to CON and RAD + AOX mice. In addition, COX1 and COX4I1 mRNA abundance were robustly (COX1) and trending (COX4I1) higher in heart and brain tissue of RAD animals, but not in those treated with AOX and exposed to IR when compared to CON. Numerous studies have shown induction of mtDNA lesions by radiation, both ionizing and ultraviolet [39,48], likely causing a mitochondrial-stress response accompanied by an increase in transcription of COX complex protein transcripts. Given the mitochondrial-related consequences of radiation, our antioxidant formula contains ingredients (CoQ10, vitamin E, and α-lipoic acid) that have been shown to lower oxidative stress and improve mitochondrial function in mitochondrial myopathy patients [34]. Interestingly, within the current study, AOX attenuated the increase in mtDNA lesions in heart and COX mRNA transcripts in heart and brain. In liver tissue, however, no changes in gene transcripts were detected following radiation. The liver possesses greater regenerative properties, with increased rate of proliferation [49,50], possibly accounting for observed discrepancies in the 24 h timepoint following radiation. Indeed, we chose to assess our endpoints immediately post, and 24 h post radiation for two reasons; namely, to assess immediate protein activity (phosphorylation of H2AX and ATM), and to allow for response in gene transcription, 24 h post radiation. However, given the different rates of cellular turnover in different tissues [50], the 24 h timepoint could have been too late to see differences in liver COX mRNA transcripts, whereas an investigation 12 h post radiation may have allowed for the observation of significant changes. In addition, although not significant, our results suggest a trend towards an increase in mtDNA lesions in the liver immediately after radiation. Interrogating an intermediary timepoint, possibly 12 h post radiation, may have allowed more time for mtDNA lesions to develop and be observed. Together, we provide evidence that our formula may be beneficial and protective against mitochondrial-related radiation-induced damage, in heart, brain and possibly liver given a more inclusive timeline.
Furthermore, it stands to reason that combining several individual antioxidant supplements may have a synergistic effect, not only related to nuclear and mitochondrial DNA protection, but also other radiation-mediated consequences such as inflammation, and possibly even greater benefits with continuous administration of AOX. The inflammatory response is a protective mechanism by which the immune system responds to foreign pathogens and damaged cells [51]. Herein, gene expression of inflammatory transcription factor NF-kβ was significantly higher in liver and brain of RAD mice 24 h post-IR. This translated into an upregulation in protein expression in liver specifically. It is well known that NF-kβ is activated following radiation [52]. It has been shown that IR activates NF-kB, which in turn is involved in promoting invasion and migration of cancer cells, in vitro [53]. Inhibitors of the transcription factor have been proposed as targets to minimize and reduce the adverse effects of radiation mediated by NF-kβ [54]. Interestingly, in the current study, our AOX ameliorated the increase in mRNA abundance and protein expression following radiation in brain and liver tissue. Although it is difficult to discern the specific mechanism, ingredients within our AOX have been shown in the literature to inhibit NF-kβ. In particular, quercetin inhibits osteoclastic differentiation and potentially protects against bone loss via inhibition of an NF-kβ-dependent pathway [55]. In addition, CoQ10 has also been shown to successfully ameliorate increases in NF-kβ and IL-6 in intestinal tissue of irradiated mice [30].
Collectively, our study provides evidence that a single dose of a multi-ingredient antioxidant supplement can have some beneficial effects in vivo, by partially negating the consequences of radiation exposure. Although not tested in our current investigation, it is possible that multiple intermittent doses of antioxidants prior to exposure may result in greater protection. In addition, the timing of oral gavage feeding represents a challenge, as it is possible that different ingredients have different timing required to be bioavailable to tissue. Furthermore, studies of continuous administration of antioxidants following high exposure to radiation increased survival in mice [21,56], suggesting that supplementation following exposure may further increase the beneficial effects of AOX observed within the current investigation. Future studies may investigate the effects of continuous AOX administration, pre- and post-IR, in mice or humans to further elucidate the beneficial effects of these combined ingredients. Nevertheless, the collective results presented here suggest that oral antioxidants can offer a feasible option to attenuate the cellular damage incurred by radiation exposure when given prophylactically. Populations at high risk of accidental radiation exposure may benefit from a prophylactic supplementation strategy.
5. Patents
PCT/CA2020/051439 filed at the European patent and trademark office (PCT) covers the formulation used in this research work.
Acknowledgments
We would like acknowledge Alessandra Chiarot and Katherine Manta for assisting with animal procedures. We would like to thank Minomi Subapanditha for assisting with flow cytometry analysis. Lastly, we would also like to thank Amy Gillgrass for providing irradiator training and access.
Author Contributions
Conceptualization, D.X., I.A.R., K.M., J.P.N. and M.A.T.; methodology, D.X., I.A.R., M.M. and L.M.; validation, D.X., I.A.R., M.M. and L.M.; formal analysis, D.X. and I.A.R.; investigation, D.X., I.A.R., M.M., L.M. and J.P.N.; resources, M.A.T.; data curation, D.X. and I.A.R.; writing—original draft preparation, D.X.; writing—review and editing, I.A.R., K.M., J.P.N. and M.A.T.; visualization, D.X.; supervision, I.A.R., J.P.N. and M.A.T.; funding acquisition, M.A.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animal experiments and procedures were performed with the approval of McMaster University Animal Research Ethics Board and conformed to the standards of the Canadian Council on Animal Care (AUP#20-04-17).
Data Availability Statement
Data can be made available from corresponding author upon reasonable request.
Conflicts of Interest
Exerkine Corporation is a biotechnology company that develops and commercializes therapies based on nutritional supplements, exercise-derived factors (“exerkines”), and extracellular vesicles to treat and diagnose genetic disorders and chronic disease. MAT is the founder, CEO, and CSO of Exerkine Corporation. Exerkine supported this study by providing salary for DX, IAR, and MM. MAT and LM are shareholders in the company. Exerkine did not provide any other funding for this study. Exerkine holds shares in Cora Therapeutics. Cora Therapeutics is a biotechnology company delivering innovative research-based wellness solutions. KM is the founder and president of Cora Therapeutics and is a shareholder in the company.
Funding Statement
This work was partially supported by Exerkine Corporation (Hamilton, Canada) and partially supported by Cora Therapeutics (Toronto, Canada). Cora Therapeutics did not have roles in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Reisz J.A., Bansal N., Qian J., Zhao W., Furdui C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid Redox Signal. 2014;21:260–292. doi: 10.1089/ars.2013.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tominaga H., Kodama S., Matsuda N., Suzuki K., Watanabe M. Involvement of Reactive Oxygen Species (ROS) in the induction of genetic instability by radiation. J. Radiat. Res. 2004;45:181–188. doi: 10.1269/jrr.45.181. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.H., Jenrow K.A., Brown S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014;32:103–105. doi: 10.3857/roj.2014.32.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgio E., Piscitelli P., Migliore L. Ionizing Radiation and Human Health: Reviewing Models of Exposure and Mechanisms of Cellular Damage. An Epigenetic Perspective. Int. J. Environ. Res. Public Health. 2018;15:1971. doi: 10.3390/ijerph15091971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee W.J., Choi Y., Ko S., Cha E.S., Kim J., Kim Y.M., Kong K.A., Seo S., Bang Y.J., Ha Y.W. Projected lifetime cancer risks from occupational radiation exposure among diagnostic medical radiation workers in South Korea. BMC Cancer. 2018;18:1206. doi: 10.1186/s12885-018-5107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaiserman A., Koliada A., Zabuga O., Socol Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose-Response. 2018;16:1559325818796331. doi: 10.1177/1559325818796331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De González A.B., Darby S. Risk of cancer from diagnostic X-rays: Estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 8.Kamiya K., Ozasa K., Akiba S., Niwa O., Kodama K., Takamura N., Zaharieva E.K., Kimura Y., Wakeford R. Long-term effects of radiation exposure on health. Lancet. 2015;386:469–478. doi: 10.1016/S0140-6736(15)61167-9. [DOI] [PubMed] [Google Scholar]
- 9.Zablotska L.B. 30 years After the Chernobyl Nuclear Accident: Time for Reflection and Re-evaluation of Current Disaster Preparedness Plans. J. Urban Health. 2016;93:407–413. doi: 10.1007/s11524-016-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redon C.E., Dickey J.S., Bonner W.M., Sedelnikova O.A. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv. Space Res. 2009;43:1171–1178. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivieri F., Albertini M.C., Orciani M., Ceka A., Cricca M., Procopio A.D., Bonafè M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget. 2015;6:35509–35521. doi: 10.18632/oncotarget.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchetti F., Coleman M.A., Jones I.M., Wyrobek A.J. Candidate protein biodosimeters of human exposure to ionizing radiation. Int. J. Radiat. Biol. 2006;82:605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- 13.Miquel J. An update on the mitochondrial-DNA mutation hypothesis of cell aging. Mutat. Res./DNAging. 1992;275:209–216. doi: 10.1016/0921-8734(92)90024-J. [DOI] [PubMed] [Google Scholar]
- 14.Yakes F.M., Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia S., Ge S., Fan X., Leong K.W., Ruan J. Promoting reactive oxygen species generation: A key strategy in nanosensitizer-mediated radiotherapy. Nanomedicine. 2021;16:759–778. doi: 10.2217/nnm-2020-0448. [DOI] [PubMed] [Google Scholar]
- 16.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Branicky R., Noë A., Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuefner M.A., Brand M., Ehrlich J., Braga L., Uder M., Semelka R.C. Effect of antioxidants on X-ray-induced γ-H2AX foci in human blood lymphocytes: Preliminary observations. Radiology. 2012;264:59–67. doi: 10.1148/radiol.12111730. [DOI] [PubMed] [Google Scholar]
- 19.Velauthapillai N., Barfett J., Jaffer H., Mikulis D., Murphy K. Antioxidants Taken Orally prior to Diagnostic Radiation Exposure Can Prevent DNA Injury. J. Vasc. Interv. Radiol. 2017;28:406–411. doi: 10.1016/j.jvir.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Brand M., Sommer M., Ellmann S., Wuest W., May M.S., Eller A., Vogt S., Lell M.M., Kuefner M.A., Uder M. Influence of Different Antioxidants on X-Ray Induced DNA Double-Strand Breaks (DSBs) Using γ-H2AX Immunofluorescence Microscopy in a Preliminary Study. PLoS ONE. 2015;10:e0127142. doi: 10.1371/journal.pone.0127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S.L., Kolozsvary A., Liu J., Jenrow K.A., Ryu S., Kim J.H. Antioxidant Diet Supplementation Starting 24 Hours after Exposure Reduces Radiation Lethality. Radiat. Res. 2010;173:462–468. doi: 10.1667/RR1716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Özyurt H., Çevik O., Özgen Z., Özden A.S., Çadırcı S., Elmas M.A., Ercan F., Gören M.Z., Şener G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014;48:1247–1255. doi: 10.3109/10715762.2014.945925. [DOI] [PubMed] [Google Scholar]
- 23.Kale A., Piskin Ö., Bas Y., Aydin B.G., Can M., Elmas Ö., Büyükuysal Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018;59:404–410. doi: 10.1093/jrr/rry032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue X.-L., Han X.-D., Li Y., Chu X.-F., Miao W.-M., Zhang J.-L., Fan S.-J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. 2017;8:1–14. doi: 10.1186/s13287-016-0464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firdous A.P., Sindhu E.R., Ramnath V., Kuttan R. Amelioration of radiation-induced damages in mice by carotenoid meso-zeaxanthin. Int. J. Radiat. Biol. 2012;89:171–181. doi: 10.3109/09553002.2013.741283. [DOI] [PubMed] [Google Scholar]
- 26.Sato T., Kinoshita M., Yamamoto T., Ito M., Nishida T., Takeuchi M., Saitoh D., Seki S., Mukai Y. Treatment of Irradiated Mice with High-Dose Ascorbic Acid Reduced Lethality. PLoS ONE. 2015;10:e0117020. doi: 10.1371/journal.pone.0117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Meer R.S., El-Habit O.H.M., Al-Hazaa A.A. Adaptive response to ionizing radiation and the role of vitamin B12 in amelioration radiation protection standards. J. King Saud. Univ. Sci. 2011;23:197–204. doi: 10.1016/j.jksus.2010.07.009. [DOI] [Google Scholar]
- 28.Karami M., Asri-Rezaei S., Dormanesh B., Nazarizadeh A. Comparative study of radioprotective effects of selenium nanoparticles and sodium selenite in irradiation-induced nephropathy of mice model. Int. J. Radiat. Biol. 2017;94:17–27. doi: 10.1080/09553002.2018.1400709. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q., Wei Z., Weng H., Chen Y., Zhang J., Mei S., Wei J., Zhu X., Nong Y., Ruan J., et al. Folic Acid Preconditioning Alleviated Radiation-Induced Ovarian Dysfunction in Female Mice. Front. Nutr. 2022;9:1358. doi: 10.3389/fnut.2022.854655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed H.A., Said R.S. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-kB/TGF-β/MMP-9 pathways. Int. Immunopharmacol. 2021;92:107347. doi: 10.1016/j.intimp.2020.107347. [DOI] [PubMed] [Google Scholar]
- 31.Noh Y.H., Kim K.-Y., Shim M.S., Choi S.-H., Choi S., Ellisman M.H., Weinreb R.N., Perkins G.A., Ju W.-K. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013;4:e820. doi: 10.1038/cddis.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manda K., Ueno M., Moritake T., Anzai K. Alpha-Lipoic acid attenuates x-irradiation-induced oxidative stress in mice. Cell Biol. Toxicol. 2007;23:129–137. doi: 10.1007/s10565-006-0137-6. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan V., Weiss J.F. Radioprotection by vitamin E: Injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int. J. Radiat. Oncol. Biol. Phys. 1992;23:841–845. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez M.C., MacDonald J.R., Mahoney D.J., Parise G., Beal M.F., Tarnopolsky M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 35.Roguin A., Goldstein J., Bar O. Brain tumours among interventional cardiologists: A cause for alarm. Report of four new cases from two cities and a review of the literature. EuroIntervention. 2012;7:1081–1086. doi: 10.4244/EIJV7I9A172. [DOI] [PubMed] [Google Scholar]
- 36.Hatch M., Cardis E. Somatic health effects of Chernobyl: 30 years on. Eur. J. Epidemiol. 2017;32:1047–1054. doi: 10.1007/s10654-017-0303-6. [DOI] [PubMed] [Google Scholar]
- 37.Hauptmann M., Byrnes G., Cardis E., Bernier M.O., Blettner M., Dabin J., Engels H., Istad T.S., Johansen C., Kaijser M., et al. Brain cancer after radiation exposure from CT examinations of children and young adults: Results from the EPI-CT cohort study. Lancet Oncol. 2022. In Press . [DOI] [PubMed]
- 38.Lehle S., Hildebrand D.G., Merz B., Malak P.N., Becker M.S., Schmezer P., Essmann F., Schulze-Osthoff K., Rothfuss O. LORD-Q: A long-run real-time PCR-based DNA-damage quantification method for nuclear and mitochondrial genome analysis. Nucleic Acids Res. 2014;42:e41. doi: 10.1093/nar/gkt1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dannenmann B., Lehle S., Lorscheid S., Huber S.M., Essmann F., Schulze-Osthoff K. Simultaneous quantification of DNA damage and mitochondrial copy number by long-run DNA-damage quantification (LORD-Q) Oncotarget. 2017;8:112417–112425. doi: 10.18632/oncotarget.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belzile-Dugas E., Eisenberg M.J. Radiation-induced cardiovascular disease: Review of an underrecognized pathology. J. Am. Heart Assoc. 2021;10:e021686. doi: 10.1161/JAHA.121.021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turnquist C., Harris B.T., Harris C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol. Adv. 2020;2:1–10. doi: 10.1093/noajnl/vdaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mettler F.A., Gus’kova A.K., Gusev I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007;93:462–469. doi: 10.1097/01.HP.0000278843.27969.74. [DOI] [PubMed] [Google Scholar]
- 43.Bouville A., Chumak V., Inskip P.D., Kryuchkov V., Luckyanov N. The chornobyl accident: Estimation of radiation doses received by the Baltic and Ukrainian cleanup workers. Pt 2Radiat. Res. 2006;1:158–167. doi: 10.1667/RR3370.1. [DOI] [PubMed] [Google Scholar]
- 44.Lawenda B.D., Kelly K.M., Ladas E.J., Sagar S.M., Vickers A., Blumberg J.B. Should Supplemental Antioxidant Administration Be Avoided During Chemotherapy and Radiation Therapy? JNCI J. Natl. Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 45.Bairati I., Meyer F., Gélinas M., Fortin A., Nabid A., Brochet F., Mercier J.-P., Têtu B., Harel F., Masse B., et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J. Natl. Cancer Inst. 2005;97:481–488. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 46.Ramzan R., Vogt S., Kadenbach B. Stress-mediated generation of deleterious ROS in healthy individuals—Role of cytochrome c oxidase. J. Mol. Med. 2020;98:651–657. doi: 10.1007/s00109-020-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadenbach B., Hüttemann M., Arnold S., Lee I., Bender E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic. Biol. Med. 2000;29:211–221. doi: 10.1016/S0891-5849(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 48.Birch-Machin M.A., Russell E.V., Latimer J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013;169((Suppl. S2)):9–14. doi: 10.1111/bjd.12207. [DOI] [PubMed] [Google Scholar]
- 49.Abu Rmilah A., Zhou W., Nelson E., Lin L., Amiot B., Nyberg S.L. Understanding the marvels behind liver regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2019;8:e340. doi: 10.1002/wdev.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sender R., Milo R. The distribution of cellular turnover in the human body. Nat. Med. 2021;27:45–48. doi: 10.1038/s41591-020-01182-9. [DOI] [PubMed] [Google Scholar]
- 51.Najafi M., Motevaseli E., Shirazi A., Geraily G., Rezaeyan A., Norouzi F., Rezapoor S., Abdollahi H. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: Clinical implications. Int. J. Radiat. Biol. 2018;94:335–356. doi: 10.1080/09553002.2018.1440092. [DOI] [PubMed] [Google Scholar]
- 52.Singh V., Gupta D., Arora R. NF-kB as a key player in regulation of cellular radiation responses and identification of radiation countermeasures. Discoveries. 2015;3:e35. doi: 10.15190/d.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang A.R., Cho J.H., Lee N.G., Kwon J.H., Song J.Y., Hwang S.G., Jung I.S., Kim J.S., Um H.D., Oh S.C., et al. Radiation-induced IL-1β expression and secretion promote cancer cell migration/invasion via activation of the NF-κB-RIP1 pathway. Biochem. Biophys. Res. Commun. 2021;534:973–979. doi: 10.1016/j.bbrc.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 54.Molavi Pordanjani S., Jalal Hosseinimehr S. The Role of NF-kB Inhibitors in Cell Response to Radiation. Curr. Med. Chem. 2016;23:3951–3963. doi: 10.2174/0929867323666160824162718. [DOI] [PubMed] [Google Scholar]
- 55.Wattel A., Kamel S., Prouillet C., Petit J.P., Lorget F., Offord E., Brazier M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NFκB and AP-1. J. Cell Biochem. 2004;92:285–295. doi: 10.1002/jcb.20071. [DOI] [PubMed] [Google Scholar]
- 56.Wambi C., Sanzari J., Wan X.S., Nuth M., Davis J., Ko Y.-H., Sayers C.M., Baran M., Ware J.H., Kennedy A.R. Hematopoietic Cells and Improve Animal Survival after Total-Body Irradiation. Radiat. Res. 2008;169:384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available from corresponding author upon reasonable request.