Abstract
Staphylococcus aureus is a major pathogen of bone that has been shown to be internalized by osteoblasts via a receptor-mediated pathway. Here we report that there are strain-dependent differences in the uptake of S. aureus by osteoblasts. An S. aureus septic arthritis isolate, LS-1, was internalized some 10-fold more than the laboratory strain 8325-4. Disruption of the genes for the fibronectin binding proteins in these two strains of S. aureus blocked their ability to be internalized by osteoblasts, thereby demonstrating the essentiality of these genes in this process. However, there were no differences in the capacity of these two strains to bind to fibronectin or osteoblasts. Analysis of the kinetics of internalization of the two strains by osteoblasts revealed that strain 8325-4 was internalized only over a short period of time (2 h) and to low numbers, while LS-1 was taken up by osteoblasts in large numbers for over 3 h. These differences in the kinetics of uptake explain the fact that the two strains of S. aureus are internalized by osteoblasts to different extents and suggest that in addition to the fibronectin binding proteins there are other, as yet undetermined virulence factors that play a role in the internalization process.
Staphylococcus aureus is a major human pathogen that has a propensity for causing bone infections. Bone infections with this organism are responsible for causing orthopedic implant failure (27), osteomyelitis (17), and septic arthritis (15) (reviewed in reference 22). These infections are extremely difficult to treat and usually require prolonged antibiotic treatment and surgical intervention.
How (and why) S. aureus targets bone has not been determined but may relate to its ability to adhere to bone by expressing receptors (adhesins) for components of bone matrix such as fibronectin (14), collagen (24), and bone sialoprotein (28). The fibronectin binding proteins FnBPA and FnBPB have been found to be expressed by most clinical isolates of S. aureus, and it has been suggested that these proteins may be important in the adherence of this bacterium to implants that have been coated in plasma proteins (22). Ninety percent of the organic matrix of bone is composed of collagen; thus, it was supposed that expression of the collagen binding adhesin, Cna, by S. aureus would be essential for its tropism to bone. However, only about 38 to 56% of S. aureus isolates associated with bone infection express Cna (30, 32). On the other hand, the bone sialoprotein binding protein of S. aureus has been shown to be preferentially expressed by isolates from patients with osteomyelitis and/or septic arthritis (28, 29, 33). Thus, it is not clear which, if any, of these staphylococcal proteins are responsible for targeting S. aureus to bone.
There is increasing evidence that S. aureus is internalized by a variety of cell types including epithelial (3) and endothelial (20) cells. We and others have shown that S. aureus is internalized by osteoblasts (12, 16, 18, 26). It has been proposed that this internalization may provide a means by which the bacteria can escape from the host immune system and/or antibiotic therapy. This may help to explain the recurrent nature of bone diseases such as osteomyelitis.
We have previously shown that internalization of S. aureus by osteoblasts is via a receptor-mediated pathway that requires cytoskeletal elements (18). Neither the osteoblast receptor nor the S. aureus ligand involved in this process has been identified. However, it is well established that a number of bacteria utilize proteins that bind to fibronectin to gain entry to host cells (10, 21, 31). Indeed, S. aureus fibronectin binding proteins have been shown to be required for adhesion and invasion of epithelial cells (19) and endothelial cells (25).
The aims of this study were to determine whether internalization of S. aureus by osteoblasts was mediated by the fibronectin binding proteins of this organism and whether these proteins were also responsible for any strain variations in the capacity to become internalized. We examined four S. aureus strains for the ability to be internalized by osteoblasts. Strain LS-1 was internalized to the highest degree and was used in comparison to strain 8325-4 and isogenic mutants of both of these strains, disrupted in the genes for the fibronectin binding proteins, to determine the role of the fibronectin binding proteins in this process.
MATERIALS AND METHODS
Osteoblast cell culture.
The osteoblast cell line MG63 was routinely cultured in growth medium consisting of Dulbecco's modified Eagle's medium (DMEM), containing 25 mM HEPES and supplemented with 10% fetal calf serum (FCS) (Pierce & Warriner, Chester, United Kingdom), 2 mM glutamine, 100 U of penicillin/ml and 100 μg of streptomycin/ml (Sigma-Aldrich Ltd., Poole, United Kingdom). The assay medium was the same as the growth medium but without antibiotics.
Bacterial strains and growth media.
The laboratory strains of S. aureus used in this study were NCTC6571 and 8325-4 (NCTC8325 cured of prophages [23]). The other strains used were FRI326, a food isolate (6), and LS-1, a strain isolated from a swollen joint of a spontaneously arthritic NZB/W mouse (7, 8). Also used in this study was strain DU5883 (a kind gift from Timothy Foster, Microbiology Department, Moyne Institute of Preventive Medicine, Trinity College, Dublin, Ireland), an isogenic mutant of strain 8325-4 disrupted in the fnbA (fnbA::Tc) and fnbB (fnbB::Em) genes. The fnbA::Tc and fnbB::Em mutations were cotransduced from DU5883 into strain LS-1 by phage 85-mediated transduction (4). Transductants resistant to 2 μg of tetracycline and 10 μg of erythromycin ml−1 were selected, and the disruptions were confirmed by Southern blotting. The isogenic mutants were also assessed in the fibronectin binding assay to confirm loss of this phenotype.
Prior to assays, bacteria were grown overnight in 10 ml of brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) aerobically in a 37°C shaking incubator. Bacterial cell numbers were estimated spectrophotometrically at 650 nm. Bacteria were harvested by centrifugation and resuspended in assay medium to give 1.5 × 107 CFU per 100 μl.
S. aureus associated with osteoblasts.
The assay determined the total number of bacteria associated with osteoblasts, i.e., adherent and internalized S. aureus. MG63 cells were seeded at 50,000 cells per well into 24-well tissue cultures plates (Sarstedt Ltd., Leicester, United Kingdom) in 1 ml of growth medium. MG63 cells were cultured for 2 days; at the end of the first day, the cells were washed twice with 1 ml of DMEM and then incubated with 1 ml of assay medium. Bacteria (100 μl) were added to the MG63 cells and incubated for 2 h at 37°C in a 5% CO2 incubator. The cultures were then washed three times with 1 ml of DMEM, and the bacteria were harvested by adding 1 ml of 0.1% Triton X-100 to each well. Enumeration was performed by serial dilution and plate counting on 5% blood agar plates containing Wilkins-Chalgren agar (Oxoid).
S. aureus internalization by osteoblasts.
The assay was a modification of the above procedure for determining the numbers of bacteria associated with osteoblasts. After 2 h of coculture of bacteria (multiplicity of infection [MOI] of 300:1) and MG63 cells, cultures were washed twice with 1 ml of DMEM. To each well, 1 ml of fresh assay medium containing gentamicin (100 μg ml−1; Gibco, Paisley, United Kingdom) was added, and the cultures were incubated for a further 2 h. The bacteria were harvested and enumerated by the same procedure as used for the association assay. To determine the kinetics of S. aureus uptake by osteoblasts, the above procedure was modified so that cocultures were incubated for between 30 min and 3 h before the cultures were washed and then incubated with gentamicin for a further 2 h. To examine survival of S. aureus inside osteoblasts, MG63 cells were incubated in the presence of S. aureus for 2 h, washed, and then incubated with assay medium containing gentamicin for between 2 and 6 h.
Fibronectin binding assay.
This assay was essentially a modification of that used by Hartford et al. (13) to determine fibrinogen binding. Nunc MaxiSorp 96-well plates (Gibco) were coated with 100 μl of 0.02% sodium carbonate (pH 9.6) containing fibronectin (10 μg ml−1; Sigma) overnight at 4°C. The plates were washed three times with phosphate-buffered saline (PBS; Sigma) and then blocked with 100 μl of a 2% bovine serum albumin solution for 1 h at 37°C. The wells were washed four times with 100 μl of PBS; 100 μl of bacteria (corresponding to 106, 107, or 108 cells) was added, in quadruplicate, to the appropriate wells and incubated for 2 h at 37°C. The wells were washed four times with 100 μl of PBS. Bacteria were fixed with 100 μl of 25% formaldehyde (Sigma) for 10 min. Then 100 μl of 0.5% crystal violet (Sigma) was added to each well for 1 min, cells were washed four times with PBS, and the absorbance was measured at 570 nm using a Multiskan plate reader.
Statistics.
All data are shown as means ± the standard deviations. Data were compared using Student's t test.
RESULTS
Internalization of S. aureus strains by osteoblasts.
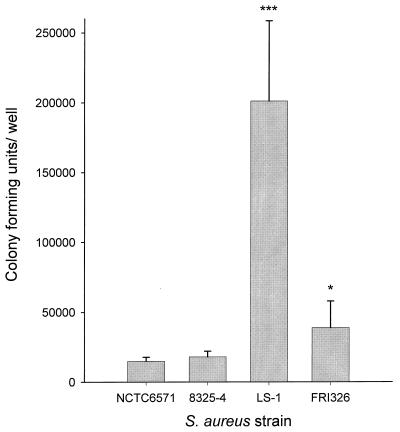
We examined the abilities of four strains of S. aureus to become internalized by osteoblasts. Strains NCTC6571 and 8325-4 are commonly used laboratory strains, while FRI326 was a food isolate and LS-1 was a septic arthritis isolate. Figure 1 shows that NCTC6571 and 8325-4 were internalized by osteoblasts to similar levels, while FRI326 was internalized to a slightly higher degree. LS-1 was internalized by osteoblasts to a far higher level than any of the other strains tested (about 10-fold higher than 8325-4). S. aureus 8325-4 and LS-1 were chosen for further study as being representative of weakly invasive and highly invasive strains, respectively.
FIG. 1.
Abilities of four strains of S. aureus to become internalized by osteoblasts. Strains NCTC6571, 8325-4, LS-1, and FRI326 were cocultured with osteoblasts at an MOI of 300:1. The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. The abilities of S. aureus strains to become internalized by osteoblasts were compared to that of strain 8325-4 using Student's t test. ∗, P < 0.05; ∗∗∗, P < 0.001.
S. aureus associated with osteoblasts.
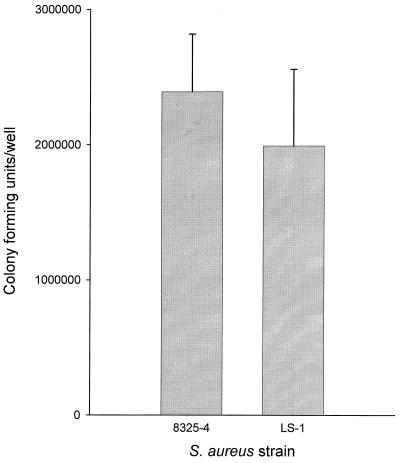
The total numbers of bacteria associated (adherent and internalized) with osteoblasts for strains 8325-4 and LS-1 are shown in Fig. 2. There were slightly more 8325-4 than LS-1 bacteria associated with osteoblasts, though the difference was not statistically significant. There were approximately 100-fold more S. aureus strain 8325-4 bacteria associated with the osteoblasts than internalized (Fig. 1 and 2). In the case of LS-1, there were approximately 10-fold more bacteria associated with the osteoblasts than internalized (Fig. 1 and 2). These data demonstrated that binding of S. aureus to osteoblasts did not correlate with the levels of internalization.
FIG. 2.
Numbers of bacteria associated (adherent and internalized) with osteoblasts for strains 8325-4 and LS-1 at an MOI of 300:1. The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. Comparison of the data using Student's t test revealed no significant difference between the two strains.
S. aureus binding to fibronectin.
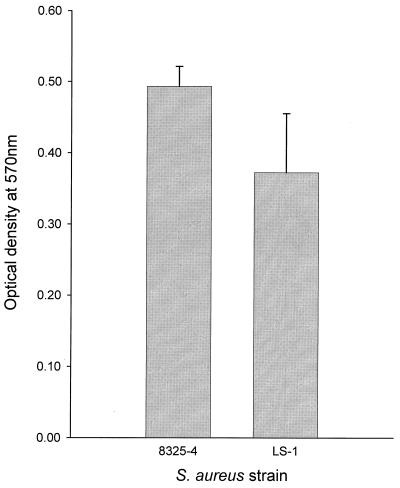
The ability of S. aureus strains 8325-4 and LS-1 to bind to fibronectin was determined using a microtiter plate adherence assay. Figure 3 shows the binding of S. aureus strains (107 bacteria) to fibronectin. Both strains bound to fibronectin, with strain 8325-4 binding slightly more than LS-1. When 106 bacteria were used, similar results were obtained (data not shown). Once again the capacity to bind to fibronectin (Fig. 3) did not correlate with the ability of the strains to become internalized by osteoblasts (Fig. 1). However, the capacity of the two strains to bind fibronectin did mirror their ability to bind to osteoblasts (Fig. 2 and 3).
FIG. 3.
Abilities of S. aureus strains 8325-4 and LS-1 to bind to fibronectin. The results shown are for 107 bacteria and are representative of one of at least three experiments; data are the means and standard deviations of four replicate wells. Comparison of the data using Student's t test revealed no significant difference between the two strains.
Role of S. aureus fibronectin binding proteins in adhesion and internalization.
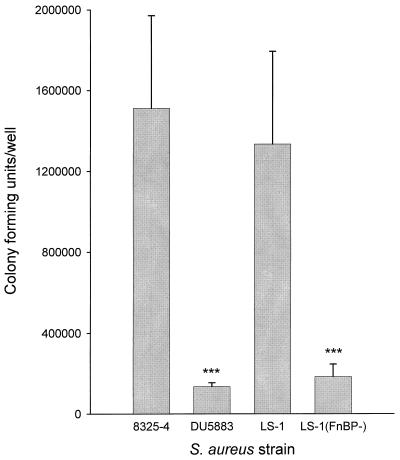
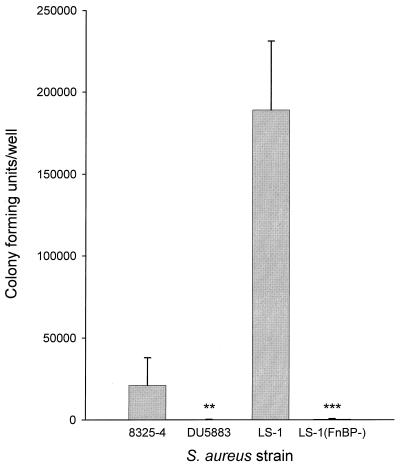
To determine the role of FnBPA and FnBPB in the ability of strains 8325-4 and LS-1 to bind to, and become internalized by, osteoblasts, isogenic mutants disrupted in the genes for these proteins were compared to the parental strains. Disruption of the genes for FnBPA and FnBPB in either strain 8325-4 or strain LS-1 resulted in a 10-fold reduction in the numbers bacteria associated with osteoblasts (Fig. 4). The isogenic mutants of 8325-4 and LS-1, disrupted in the fnbA and fnbB genes, were not internalized by osteoblasts, as shown in Fig. 5. These data taken together demonstrate that the fibronectin binding proteins of S. aureus are the major adhesins for osteoblasts and are essential for bacterial internalization. These results are somewhat contradictory when one considers that the weakly invasive strain of S. aureus 8325-4 was marginally better at binding to osteoblasts and fibronectin than the highly invasive strain and suggest that other factors may be involved in the process of internalization.
FIG. 4.
Numbers of bacteria associated with osteoblasts for strains 8325-4 and LS-1 and their respective isogenic mutants DU5883 and LS-1(FnBP-). Cocultures were performed at an MOI of 300:1. The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. Student's t test was used to compare differences between the isogenic mutant and its wild-type strain. ∗∗∗, P < 0.001.
FIG. 5.
Abilities of S. aureus strains 8325-4, and LS-1 and their respective isogenic mutants DU5883 and LS-1(FnBP-) to become internalized by osteoblasts. Cocultures were performed at an MOI of 300:1. The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. Student's t test was used to compare differences between the isogenic mutant and its wild-type strain. ∗∗, P < 0.01, ∗∗∗, P < 0.001.
Kinetics of S. aureus internalization by osteoblasts.
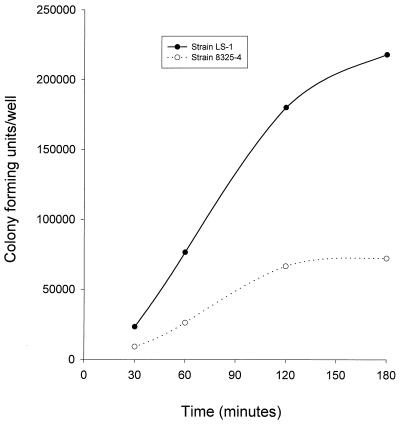
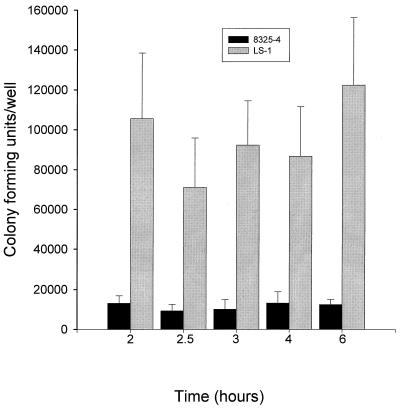
The kinetics of S. aureus internalization were examined by incubating bacteria with osteoblasts for time intervals of between 30 min and 3 h. Figure 6 shows that internalization of S. aureus strain 8325-4 reached a maximum at 2 h, while internalization of strain LS-1 had not reached a maximum by 3 h. The numbers of strain 8325-4 internalized were significantly lower than those of LS-1 at all time points examined. Since the internalization assay is based on the recovery of viable intracellular bacteria, the differences seen for the two strains in this kinetic assay could have been due to variations in the ability of these bacteria to grow and/or survive in the intracellular environment of the osteoblast. To examine this possibility, recovery of viable bacteria from inside osteoblasts was monitored over time. Figure 7 shows that there were no significant differences in the levels of either 8325-4 or LS-1 recovered from within osteoblasts over a time period of 6 h, thus demonstrating that survival and/or growth within the intracellular environment was not affecting the results obtained from internalization assays.
FIG. 6.
Kinetics of S. aureus internalization by osteoblasts. S. aureus strains 8325-4 and LS-1, at an MOI of 300:1, were cocultured in the presence of osteoblasts for between 30 min and 3 h. The results are from one representative experiment of at least three.
FIG. 7.
Abilities of S. aureus strains 8325-4 and LS-1 to grow and/or survive in the intracellular environment of the osteoblast, determined by the numbers of bacteria recovered over a 6-h time period. The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. Comparison of the data for each bacterial strain using Student's t test revealed no significant difference between the numbers of bacteria recovered at the different time points.
DISCUSSION
S. aureus was once considered to exist exclusively as an extracellular pathogen. However, there is now a growing body of evidence indicating that this organism can be taken up by a range of nonphagocytic cells such as epithelial cells (3), endothelial cells (5, 25), and osteoblasts (16, 18, 26).
In this study, we have found that there are strain differences in the ability of S. aureus to become internalized by osteoblasts. Thus, two laboratory strains (8325-4 and NCTC6571) were internalized to a lower degree than a food isolate (FRI326), and all of these strains were internalized about 10-fold less than a septic arthritis isolate (LS-1). S. aureus strain LS-1 is commonly used in animal models of septic arthritis (1, 2, 7, 9) because of its highly virulent character, and thus it is tempting to speculate that this may be related to its ability to become internalized by osteoblasts. However, while the results presented herein do show that there are strain differences in the capacity to become internalized by osteoblasts, no conclusion can be drawn as to whether the source from which the S. aureus strain was isolated had any influence on this ability because of the limited number of strains examined. Having established differences in the ability of S. aureus strains to become internalized by osteoblasts, we selected a strain that was internalized to a low degree (8325-4) and one that was internalized to a high degree (LS-1) for further study. When we examined the numbers of bacteria from each of these strains associated with osteoblasts, we discovered that there was no significant difference between 8325-4 and LS-1, although the numbers of 8325-4 were consistently higher. This finding demonstrated that adherence of S. aureus to osteoblasts was not alone sufficient to cause internalization.
Recently S. aureus FnBPA and FnBPB have been shown to be required for internalization of this organism by epithelial (11) and endothelial (25) cells. We examined the capacity of S. aureus 8325-4 and LS-1 to bind to fibronectin in order to determine if differences in binding could account for the variation in internalization of the two strains. Although 8325-4 bound slightly more to fibronectin than LS-1 the difference was not significant, suggesting that the ability to bind to fibronectin was not related to the number of recovered organisms.
To determine the role of FnBPA and FnBPB in adhesion to, and internalization by, osteoblasts, we used isogenic mutants of S. aureus strain 8325-4 and LS-1 with disruptions of the genes for these two proteins. The isogenic mutants were 10-fold less adherent to osteoblasts than the parental strains, demonstrating that the fibronectin binding proteins of S. aureus were the predominant adhesins for osteoblasts. However, this does not rule out a role for other adhesins. Similar reductions in the levels of adherence of isogenic mutants, defective in the fibronectin binding proteins, to endothelial cells have been reported (25). However, Dziewanowska et al. (11) recently reported that similar isogenic mutants of S. aureus were reduced in the ability to adhere to epithelial cells by only 40% compared to the parental strain. The differences in adhesion to different mammalian cell types are likely to be due to differences in matrix molecules expressed by these cells. Isogenic mutants of 8325-4 and LS-1 were not internalized in significant numbers by osteoblasts, demonstrating the essential role of the fibronectin binding proteins in this process. This finding was in agreement with those reported for epithelial (11) and endothelial (25) cells.
These findings raised the question of why S. aureus strain LS-1 was apparently more capable of being internalized by osteoblasts than 8325-4, when it bound to neither osteoblasts nor fibronectin to a greater degree than 8325-4. One possible answer was that since the internalization assay relies on the recovery of viable bacteria from within the osteoblasts, the results obtained may have been influenced by the ability of bacteria to grow and/or survive within this intracellular environment. However, we found no differences in the abilities of 8325-4 and LS-1 to grow and/or survive within osteoblasts over a 6-h time period, demonstrating that this was not responsible for the variation in the capacity of these strains to become internalized by osteoblasts. The kinetics of uptake by osteoblasts of the two strains showed dramatic differences. The levels of internalization of S. aureus 8325-4 by osteoblasts reached a plateau by 2 h, while internalization of LS-1 had not leveled out at 3 h. Thus, the kinetics of internalization, at least in part, account for the higher levels of S. aureus LS-1 found in osteoblasts. These findings establish that while the fibronectin binding proteins of S. aureus are essential in the process of internalization by osteoblasts, there are other strain-dependent factors that influence the kinetics of internalization. Similar findings were reported by Dziewanowska et al. (11) for internalization of S. aureus by epithelial cells. Two possible ways in which the kinetics of internalization by osteoblasts could be controlled by the bacterium are (i) if S. aureus strain LS-1 was producing virulence factors that caused an up-regulation in the number of mammalian cell surface receptors (or the rate of receptor cycling) responsible for internalizing this bacterium and (ii) if S. aureus strain 8325-4 was producing a virulence factor that inhibited its internalization by osteoblasts. Some evidence in favor of this second theory was recently provided by Yao et al. (34), who reported that S. aureus produces a lipoprotein that apparently inhibits internalization by endothelial cells. Whether the increased uptake of certain strains of S. aureus by osteoblasts involves the production of stimulatory or inhibitory factors by this organism remains to be defined; however, these factors are potential additional therapeutic targets for the prevention and/or treatment of persistent bone infections.
ACKNOWLEDGMENT
We are grateful to the Arthritis Research Campaign for Program Grant funding (grant H0600).
REFERENCES
- 1.Abdelnour A, Arvidson S, Bremell T, Ryden C, Tarkowski A. The accessory gene regulator (Agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelnour A, Tarkowski A. Polyclonal B-cell activation by an arthritogenic Staphylococcus aureus strain: contribution of T-cells and monokines. Cell Immunol. 1993;147:279–293. doi: 10.1006/cimm.1993.1069. [DOI] [PubMed] [Google Scholar]
- 3.Almeida R A, Matthews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 4.Asheshov E H. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperatures. J Gen Microbiol. 1966;42:403–410. doi: 10.1099/00221287-42-3-403. [DOI] [PubMed] [Google Scholar]
- 5.Beekhuizen H, van de Gevel J S, Olsson B, van Benten I J, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 6.Brehm R D, Tranter H S. Purification of staphylococcal enterotoxin E. Nat Toxins. 1993;1:250–254. doi: 10.1002/nt.2620010409. [DOI] [PubMed] [Google Scholar]
- 7.Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremell T, Lange S, Svensson L, Jennische E, Grondahl K, Carlsten H, Tarkowski A. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–1744. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- 9.Bremell T, Lange S, Yacoub A, Ryden C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehio C, Gray-Owen S D, Meyer T F. Host cell invasion by pathogenic Neisseriae. Subcell Biochem. 2000;33:61–96. doi: 10.1007/978-1-4757-4580-1_4. [DOI] [PubMed] [Google Scholar]
- 11.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellington J K, Reilly S S, Ramp W K, Smeltzer M S, Kellam J F, Hudson M C. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 13.Hartford O, Francois P, Vaudaux P, Foster T J. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol Microbiol. 1997;25:1065–1076. doi: 10.1046/j.1365-2958.1997.5291896.x. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R, Lew P D, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 15.Ho G. Bacterial arthritis. In: McCarty D J, Koopman W P, editors. Arthritis and allied conditions. Philadelphia, Pa: Lea & Febiger; 1993. pp. 2003–2024. [Google Scholar]
- 16.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe H L. Metabolic, degenerative, and inflammatory diseases of bones and joints. Philadelphia, Pa: Lea & Febiger; 1972. pp. 1015–1031. [Google Scholar]
- 18.Jevon M, Guo C, Ma B, Mordan N, Nair S P, Harris M, Henderson B, Bentley G, Meghji S. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun. 1999;67:2677–2681. doi: 10.1128/iai.67.5.2677-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammers A, Nuijten P J, Smith H E. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol Lett. 1999;180:103–109. doi: 10.1111/j.1574-6968.1999.tb08783.x. [DOI] [PubMed] [Google Scholar]
- 20.Menzies B E, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66:5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinari G, Chhatwal G S. Role played by the fibronectin-binding protein SfbI (protein F1) of Streptococcus pyogenes in bacterial internalization by epithelial cells. J Infect Dis. 1999;179:1049–1050. doi: 10.1086/314681. [DOI] [PubMed] [Google Scholar]
- 22.Nair S P, Williams R J, Henderson B. Advances in our understanding of the bone and joint pathology caused by Staphylococcus aureus infection. Rheumatology (Oxford) 2000;39:821–834. doi: 10.1093/rheumatology/39.8.821. [DOI] [PubMed] [Google Scholar]
- 23.Novick R P. Properties of a cryptic high frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:156–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 24.Patti J M, Jonsson H, Guss B, Switalski L M, Wiberg K, Lindberg M, Hook M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 25.Peacock S J, Foster T J, Cameron B J, Berendt A R. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology. 1999;145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 26.Reilly S S, Hudson M C, Kellam J F, Ramp W K. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone. 2000;26:63–70. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 27.Ross A C. Infections complicating joint replacement and other orthopedic conditions. Curr Opin Rheumatol. 1993;5:461–467. doi: 10.1097/00002281-199305040-00010. [DOI] [PubMed] [Google Scholar]
- 28.Ryden C, Maxe I, Franzen A, Ljungh A, Heinegard D, Rubin K. Selective binding of bone matrix sialoprotein to Staphylococcus aureus in osteomyelitis. Lancet. 1987;2:515. doi: 10.1016/s0140-6736(87)91830-7. [DOI] [PubMed] [Google Scholar]
- 29.Ryden C, Yacoub A I, Maxe I, Heinegard D, Oldberg A, Franzen A, Ljungh A, Rubin K. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem. 1989;184:331–336. doi: 10.1111/j.1432-1033.1989.tb15023.x. [DOI] [PubMed] [Google Scholar]
- 30.Ryding U, Flock J I, Flock M, Soderquist B, Christensson B. Expression of collagen-binding protein and types 5 and 8 capsular polysaccharide in clinical isolates of Staphylococcus aureus. J Infect Dis. 1997;176:1096–1099. doi: 10.1086/516520. [DOI] [PubMed] [Google Scholar]
- 31.Schorey J S, Li Q, McCourt D W, Bong-Mastek M, Clark-Curtiss J E, Ratliff T L, Brown E J. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect Immun. 1995;63:2652–2657. doi: 10.1128/iai.63.7.2652-2657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Switalski L M, Patti J M, Butcher W, Gristina A G, Speziale P, Hook M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol. 1993;7:99–107. doi: 10.1111/j.1365-2958.1993.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 33.Yacoub A, Lindahl P, Rubin K, Wendel M, Heinegard D, Ryden C. Purification of a bone sialoprotein-binding protein from Staphylococcus aureus. Eur J Biochem. 1994;222:919–925. doi: 10.1111/j.1432-1033.1994.tb18940.x. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Bengualid V, Berman J W, Lowy F D. Prevention of endothelial cell cytokine induction by a Staphylococcus aureus lipoprotein. FEMS Immunol Med Microbiol. 2000;28:301–305. doi: 10.1111/j.1574-695X.2000.tb01490.x. [DOI] [PubMed] [Google Scholar]