Abstract
Seeds are the most commonly used source of storage material to preserve the genetic diversity of plants. However, prior to the deposition of seeds in gene banks, several questions need to be addressed. Here, we illustrate the scheme that can be used to ensure that the most optimal conditions are identified to enable the long-term storage of seeds. The main questions that need to be answered pertain to the production of viable seeds by plants, the availability of proper protocols for dormancy alleviation and germination, seed tolerance to desiccation and cold storage at −20 °C. Finally, it is very important to fully understand the capability or lack thereof for seeds or their explants to tolerate cryogenic conditions. The proper storage regimes for orthodox, intermediate and recalcitrant seeds are discussed.
Keywords: seed storage, desiccation, orthodox, recalcitrant, intermediate, exceptional species, cryopreservation
1. Introduction
The global biodiversity crisis has become a problem over recent years, mainly due to human activity, and it is expected to continue. The causes can be direct including land use, pollution or climate change, and indirect, as demographic factors, as well as economic and government issues [1,2]. Currently, the increase in average year temperature and related changes in climate are undebatable. Indeed, we have been experiencing global climate warming since 1981—the rate of temperature increase has been 0.18 °C per decade, and the 9 years from 2013 through 2021 rank among the 10 warmest years on record (www.climate.gov/news-features/understanding-climate/climate-change-global-temperature, accessed on 28 July 2022). It has become clear that climate change entails the loss of biodiversity and impacts its organization at all levels, affecting genes, populations, species, ecosystems, and thus human livelihood [3,4,5,6,7]. Two general approaches exist for dealing with the consequences of climate change, which are an adaptation to adverse conditions by adjustment to high temperatures or mitigation of the consequences of a warming climate. In both approaches, however, conservation of the world’s biological resources and their diversity is crucial to enable future generations to thrive and adjust to altered climate conditions as well as counteract further degradation of ecosystems. Only by preserving diversified varieties of plants will we be able to secure all multiple and vastly diverse products and services provided by plants including food and medicine, timber and animal forage, as well as a regulation of water supply and carbon sequestration [4,8,9,10]. Although there are still few (0.2%) well-documented plant extinction examples, it has been estimated that 30–44% of land plants are threatened [9]. The understanding of the importance of biodiversity protection for future human prosperity has increased the number of efforts aiming at in situ actions such as expanding the protected areas and restoring degraded ecosystems [9]. Over the course of the last several decades, conservation activities away from the natural location of the plants (ex situ) have resulted in an increased number of gene banks worldwide and a rapid rise in the number of accessions that are preserved [11,12,13,14,15,16,17,18]. Gene banks provide a broad range of diversity for usage in breeding programs and research efforts, and they play a key role in disseminating seeds to farmers [19]. Lastly, they serve as an invaluable backup source of seeds in the event of a disaster [20]. Currently, there are approximately 1750 gene banks in existence which maintain more than 7.4 million accessions of plant genetic resources. Among these gene banks, we can distinguish approximately 130 large facilities that hold more than 10,000 accessions at each location. There are also substantial ex situ collections in botanical gardens, containing approximately 4 million accessions from over 80,000 species [20,21,22]. Importantly, not only are the crop varieties and their wild relatives currently preserved, but also wild species as well. Among the main largest-scale activities planned to enlarge the deposition of seeds was the operation of the Millennium Seed Bank: “Collect and conserve 10% of the world’s wild seed-bearing flora, principally from the drylands, by the year 2010”; currently, the aim has been expanded to 20% [23]. Another important initiative is the Consultative Group for International Agricultural Research (CGIAR) that operates gene banks preserving the collection of >700,000 crop seed accessions [11,19].
The hope and expectation that seed storage and gene banks can effectively contribute to the conservation of plant biodiversity in a time of complex global challenges relate to studies showing that seeds can remain viable for hundreds and even thousands of years [20,24,25]. Silene stenophylla Ledeb. buried in permanently frozen loess-ice deposits on the right bank of the lower Kolyma River for 32,000 years are the oldest known viable seeds that have been found [26]. However, these data are often burdened with error as the age of seeds was assessed by radiocarbon dating [27]. Nevertheless, the longest ongoing experiment and, in general, among the oldest trials in the world regarding seed longevity, was initiated in 1879 at Michigan State University by Dr. William James Beal. In this study, 20 bottles were buried underground on the MSU campus, with each containing 50 seeds from 21 different plant species. The intention was that a bottle would be dug up every 5 years but intervals were subsequently extended. The last published data of a 120-year storage period showed that 23 seeds of Verbascum blattaria L. and 2 seeds of Verbascum sp. germinated, which corresponded to 50% of total germination [28]. The last sample was taken in 2021 and the international community of seed researchers is still looking forward to a publication which will reveal seed germinability, as this experiment is a unique mix of seed storage in semi-natural conditions with carefully recorded data. Consequently, based on the natural features of seeds, the long-term security of seeds in cold storage facilities was established and projected.
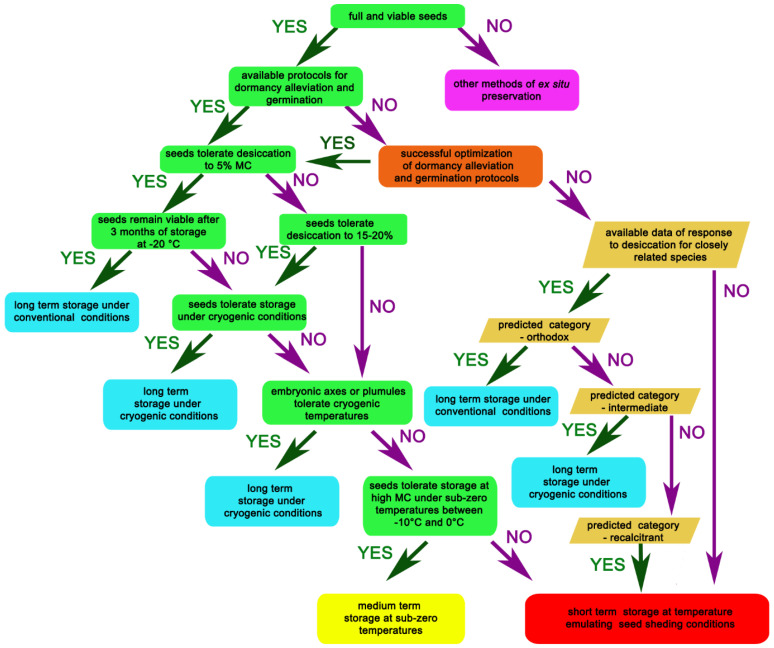
The aim of this review is to provide a comprehensive view on current possibilities for the long-term preservation of seeds. In previous publications, information pertaining to how seeds should be stored based on their post-harvest physiology [29,30,31] or how to improve conditions for more successful cryostorage of extracted plant tissues has been described [21,32]. However, in preparation of a decision-making path for the successful storage of plant germplasm, we focused on seeds, from their collection until conventional and cryogenic storage (Figure 1). Importantly, our decision-making path is enriched with information on how to establish and choose the optimal conditions of storage when protocols of dormancy alleviation and seed germination are not available. Unfortunately, this is a frequent and very problematic situation, especially in the case of endangered and rare species originated from biodiversity hot spots. This important information and guidance is regularly omitted in other reviews, as the most commonly presented decision-making process is typically based primarily on the desiccation tolerance of seeds [29,30,33]. Since several recent studies showed that there is high probability to predict seed desiccation tolerance [34,35], it is plausible that efforts to preserve valuable seeds could be initiated and proceeded until the dormancy breaking and germination protocols are provided. Therefore, there is a need for an indication regarding how to proceed with such seeds. We have described the respective stages of a decision-making pathway in more detail, and also provide the most important information regarding the influence of condition of storage on seeds’ viability. Finally, we provide some information on future possibilities of improving both conventional and cryogenic storage of seeds. Collectively, all of this information provides indications for how to search for the solution if problems in seed storage are encountered.
Figure 1.
A protocol to determine the optimal conditions of plant germplasm storage. Two paths are presented: experimental in rectangles and literature based in parallelograms.
2. Important Questions That Need to Be Addressed Prior to Seed Storage
The first question that needs to be answered is whether the species of interest produces viable seeds? This is an important question because even though there is the possibility to store other tissues, i.e., shoot tips, dormant buds, pollen, or vegetative clonal tissues, seeds are the first choice to protect biodiversity as each seed is an individual specimen containing a diverse genetic material that enables the protection of a wide gene pool [21,36,37,38,39,40]. Moreover, under appropriate conditions, seeds produce seedlings without the requirement of additional, costly and labor-intensive procedures, such as in vitro culture. Therefore, the higher plant accessions are usually stored in gene banks as seeds [40,41]. If the species of choice does not produce viable seeds that could be stored, other approaches to preserve the plant in gene banks are chosen, including storage of other organs and tissues mentioned above. The present article is focused on the preservation of seeds. We propose the decision-making path for the successful storage of plant germplasm, with a focus on seeds (Figure 1).
Numerous factors can influence the ability to successfully procure and use seeds to support plant reintroductions, including challenges with seed collection, storage, and germination [31,42]. Therefore, when seeds are available, and confirmed to be viable by, e.g., biochemical 2,3,5-tryphenyl tetrazolium chloride (TTC) test [43] and proved to be at good quality by, e.g., X-ray imaging [44,45], the next valid issue that requires an answer is the accessibility of the germination and dormancy breaking protocols if seeds become dormant upon maturation. Dormancy regulates germination through various physical and/or physiological means imposed by the seed coat, or within the embryo. Freshly collected seeds are considered to be dormant when they do not germinate within 4–6 weeks under conditions of sufficient moisture and suitable temperature [46,47]. Unfortunately, for many wild species, there are no such protocols. However, 50% to 90% of wild plant species produce dormant seeds, with specific dormancy traits driven by the geography of species’ occurrence, a form of growth, and genetic factors [47]. For example, more than 70% of the arctic-alpine plants produce dormant seeds, exhibiting mainly physiological dormancy, which evolved to prevent seed germination before or during cold autumn/winter seasons [46]. Unfortunately, the protocols for dormancy breaking require laborious, long-term procedures as seeds may require several months or years to lose their dormancy [47]. Therefore, specialized knowledge regarding the ecology and physiology of the species is a prerequisite. As a consequence, the accessibility to proper dormancy-breaking and germination protocols can be the bottleneck limiting conservation efforts, because this challenging question needs to be faced: How to proceed with the storage of seeds without proper protocols essential for testing seed viability before and after storage? Consequently, understanding seed dormancy and germination traits is critical to effective seed management and achieving planned restoration success [47]. In general, it is assumed that closely related species growing in similar conditions and climatic zones produce seeds of similar characteristics [48]. However, it should be remembered that this is not always a true assumption. For instance, the dormancy phenotype of Avena fatua L. seeds is determined 50% genetically, by 50% in the maternal environment, and therefore results in variation [49]. Additionally, the response of mature seeds of Amaranthus retroflexus L. to temperature and the light regime was affected by harvesting time as they varied in dormancy levels and requirement for after-ripening [50]. There was a difference in optimal germination temperature for seeds produced by Vicia sativa L. cultivars collected at a different elevations above sea level [51]. Species-specific dormancy alleviation protocols were reported for Violaceae, as some species required cold stratification while others responded to other procedures such as pretreatment with gibberellic acid and priming [42]. Thus, it is well known that dormancy alleviation and germination stimulation may vary between individual plants from one population, from year to year, and between sites of production which is attributed to differences in environmental factors before and during seed maturation [47,49,50,51]. Lack of knowledge about seed dormancy and germination requirements leads to failure in the regeneration of plantlets and seed wastage [47]. Therefore, if there is no species-specific information in the literature and on Royal Botanic Garden Kew’s Seed Information Database, the “move-along” experiment testing dormancy breakage and germination on a small batch of freshly collected seeds in conditions simulating a series of temperature regimes mirroring natural conditions in any bioregion is proposed [47,52,53]. In addition, the exact seed bank procedure for testing dormancy breakage and germination conditions is also established [29].
3. How to Successfully Store Seeds for a Long Time? The Role of Seed Moisture Content (MC) and Low Temperature in an Extension of Seed Longevity
Another important issue that must be tackled prior to the deposition of seeds in storage is the determination of their desiccation tolerance. Since the 1970s, the most common and popular classification of seeds based on their post-harvest physiology has been used [54]. Seeds were divided into two categories according to their resistance to desiccation which is defined as a reduction in their MC that is not detrimental to viability. Orthodox seeds are capable of tolerating desiccation below 5% of their MC and resume metabolism when rehydrated. This unusual feature of life objects is limited to microbes, invertebrates, resurrection plants, fungi, and seeds [55,56,57,58]. Mature orthodox seeds are developmentally arrested, metabolically quiescent, and dry. Thus, seeds shed or harvested at a MC of 20% or below are very likely to be orthodox [30,58,59]. In contrast, recalcitrant seeds are frequently shed from the mother plant at a MC of above 60% that may trigger germination [60]. However, these seeds are sensitive to desiccation as they do not tolerate drying below 20–40% of MC [30,54]. The distinction has been used as a management criterion for storage in gene banks [61,62]. Further exploration of seed behavior raised the awareness that this orthodox-recalcitrant paradigm is too restrictive, and in consequence, this binary division was extended by adding an intermediate category [63]. As the name suggests, this category of seeds exhibits to various extents some features of both orthodox and recalcitrant seeds. As a result, this seed category is less uniform in relative comparison to other categories. Indeed, these seeds (1) tolerate desiccation to a MC of 10%, although it is not so low as in the case of orthodox seeds. They are (2) sensitive to storage at sub-zero temperatures and (3) are characterized by a short lifespan regardless of the effort aiming to improve its extension by lowering the MC and temperature [62]. Among the explanations of short-lived behavior is that seeds are dispersed without a complete metabolic shut-down, as in the case of desiccation tolerant Poincianella pluviosa (DC.) L. P. Queiroz [59]. Another reason may be related to a lack of endosperm and an energy reservoir, as in the case of Populus sp. seeds [64]. Nevertheless, this categorization is useful for the decision-making process in seed management, however, it should be kept in mind that seeds exhibit a wide spectrum of responses to desiccation rather than the rigid ones defined by the categories [57,62]. Therefore, because the thresholds among orthodox, intermediate, and recalcitrant seed behavior are not always well defined, efforts to allocate seeds in a particular category may lead to varied conclusions. For example, in the case of Corylus avellana L., its seeds were already classified as recalcitrant, intermediate and orthodox [65,66,67].
Once the tolerance to desiccation is tested, the next step in the process is the determination of the proper temperature of storage. For decades, it has been assumed that lowering both the seed MC and temperature of storage would increase their lifespan. The first rules aiding in the establishment of the seed storage regime were “the rules of thumb” [68,69]. The following rules allow for an assessment of the effect of MC and temperature on seed storage, stating that (1) for every 1% decrease in seed MC, the lifespan of seeds doubles. This rule is applicable when seed MC ranges between 5% and 14%. (2) For every 5 °C (10 °F) decrease in the storage temperature, the life of the seed doubles when the temperature ranges from 0 to 50 °C. (3) Optimum seed storage is achieved when the sum of percent relative humidity (RH) in the storage environment and the storage temperature in F sum up to a hundred, but the contribution from temperature should not exceed 50 °F. In the 1980s and 1990s, the scientific debate regarding the most proper MC for the long-term storage of seeds continued. According to R.H. Ellis and collaborators, seed longevity increases as seeds are progressively dried and temperature and MC have an independent impact [70,71,72,73]. Therefore, seeds were suggested to be stored at an extremely low level of MC close to 10–12% of RH achieved during drying at 20 °C, regardless of the storage temperature. Conversely, Vertucci and collaborators [74,75,76] claimed that over-drying of seeds could be deleterious to their viability. The optimal seed MC for storage depends on the temperature of storage, and it decreases as temperature increases. Therefore, the optimal RH for seed storage at ambient temperature should be approximately 20–25% RH [76]. On the other hand, for cryogenically stored seeds (temperature approximately −150 °C), the RH should be higher (i.e., for pea seed, this is approximately 0.17 g H2O g−1 dry mass, which is equivalent to 70% RH) [75]. Nevertheless, desiccation tolerance and optimal temperature of storage, if not available in databases (i.e., Seed Information Database (SID; http://data.kew.org/sid/, accessed on 4 October 2022), need to be established experimentally. When there is a limited number of available seeds, desiccation tolerance can be assessed by a so called “100-seeds test”. This test was initially developed for palm [77], and was further successfully adapted to 91 native Caribbean woody species [78]. Additionally, if there is no reference to information pertaining to the storage of seeds for a particular species, the desiccation tolerance and category of seeds could be predicted by models that are based on seeds’ trait such as seed coat ratio and seed mass [35,79]. Additional research on 17,539 species showed that desiccation tolerance can be anticipated based on taxonomy, species traits, location and climate data. Importantly, the most important predictor variables were the response of relatives to desiccation, seed mass and annual precipitation. Three models with varying degrees of success rates for identifying the desiccation-sensitive species were constructed based upon these variables. Specifically, there was an 89% success rate for the genus-level model, 79% for the family-level model and 60% for the order-level model [48].
If information exists on how to store seeds of particular or closely related species, such seeds should be collected when ripe, preferably shed, and only slight subsequent surface desiccation is recommended prior to storage at low temperature. The temperature level is dependent on the climate conditions where maternal plants grow and the chosen storage temperature should be close to the average temperature for the winter season. However, such conditions of storage should be applied only for short-term storage until a proven method is developed. For example, a method can be determined based upon the step-by-step approach [29,30], in which the desiccation tolerance to 10–12% MC is first evaluated and then further to 5%, which subsequently enables the possibility to store seeds for 3 months at −20 °C. If seeds survive desiccation to 5% of MC and storage at −20 °C, it is very likely that they belong to the orthodox category. For other seeds, experimental data need to be collected as they require non-conventional storage methods [80]. The experimental path to determine intermediate or recalcitrant seeds has been proposed [30,81]. It includes storage at +5 and −20 °C and regular viability testing for a 5-year period. By using this approach, it can be determined whether seeds belong to the intermediate short-lived or intermediate freeze-sensitive categories. Examples of orthodox, intermediate, and recalcitrant seeds storage conditions, and related issues are presented in the sections below.
4. Conventional Storage of Seeds
The term “conventional storage” means that seeds are treated in a defined way. Specifically, they are desiccated to 10–25% of RH in a recommended drying environment of 5–20 °C, which corresponds to approximately 5% of MC [61]. According to the established protocol [30], for the short-term storage of dry seeds (<18 months), a temperature between 0 and 5 °C is sufficient to maintain their viability. For longer periods of storage, seeds should be stored at −18 to −20 °C. However, in Mediterranean gene banks, seeds of native species are stored at −25 °C [82,83].
4.1. Conventional Storage of Orthodox Seeds
Multiple reports have described the successful conventional storage of orthodox seeds [80,84,85,86,87,88,89,90,91]. In particular, results obtained in long-term (years and decades) studies are of high importance as they are proof of concept and provide actual information on the longevity of orthodox seeds. For instance, 16 Nordic agricultural and horticultural crops, each represented by two or three cultivars, were stored under ultra-dried conditions at a MC of 3–5% a temperature of −3.5 ± 0.2 °C for a 30-year period. The aforementioned seeds remained highly viable for 20–25 years. However, after 30 years of storage, the median value of germinability for all tested seed lots decreased to 80%, with the highest germinability decline (up to 49%) observed for Secale cereale L. found in seed lots with the highest initial MC [88]. Another study showed that the longevity of desiccation-tolerant seeds of 28 plants from 7 families conventionally stored at −18 °C and MC of 5 ± 2% varied from 20.41 years (Arachis hypogea L.) to 500 years (for Avena sativa L. and Triticum aestivum L.) [92]. During one of the most complex studies, 42,000 seed accessions representing 276 species were quantified after storage at 5 °C, followed by the storage of a select 178 species at −18 °C [93]. The MC of the investigated seeds ranged between 4% and 8%. This experiment showed that the time to reduce germination to 50% (P50) ranged among species from <13 years to an extrapolated estimate of >450 years, and the median P50 was 54 years. Some plant families possessed characteristically short-lived (i.e., Apiaceae and Brassicaceae) or long-lived (e.g., Malvaceae and Chenopodiaceae) seeds. Although data from that study support the statement that some species tend to survive longer than others in the seed bank, the information on the attributes of seeds affecting their storage performance is still incomplete. Importantly, a meta-analysis of germination data indicated that predicted germinability was often higher than actual values that were experimentally obtained [87]. Therefore, further research which focuses on obtaining more accurate predictions of orthodox seed longevity based upon the testing of new markers of seed viability and quality in real-time appear warranted and necessary [91,94,95,96,97,98,99].
4.2. Conventional Storage of Intermediate Seeds
The first definition of the intermediate category states that among the main features of these seeds is that they can be injured by low temperatures in a dry state [63]. Indeed, the viability of coffee seeds (Coffea arabica L.) stored at cool and sub-zero temperatures and at a low MC was shown to be lower in comparison to seeds stored at a higher MC and temperatures. In accordance with these observations, seeds of Citrus species behaved similarly. Moreover, in the case of Fagus sylvatica L. and F. crenata L., seeds of both species do not withstand desiccation below 7.6%, thus they are not orthodox, but they can be stored in and optimal temperature range of +10 to −20 °C [100]. These aforementioned seeds were classified as intermediate, as well as other oil-rich seeds such as Coffea arabica, Citrus sp., and Corylus sp. [101]. However, there are also short-lived seeds in the intermediate category, i.e., Salix sp. [102] or Populus sp. [103,104], that do not follow typical orthodox or recalcitrant seed storage behavior. Lastly, there are also seeds which do not fit the classification as recalcitrant or orthodox, that have been placed in the intermediate category. As a consequence, this group is highly heterogenous [62]. Nevertheless, the longevity of intermediate seeds increases with drying and cooling (as with orthodox seeds), but seeds still age rapidly during conventional storage and will die within approximately 5 years ([62], saveplants.org/best-practices/difference-between-orthodox-intermediate-and-recalcitrant-seed/ accessed on 28 July 2022). Relevant information regarding the storage behavior of intermediate seeds for their conservation appears to have gained importance since recent research showed that more seeds, particularly from the world’s biodiversity hotspots, should be classified as intermediate [80,81,105]. It has also been claimed that desiccation and temperature behavior during storage lasting longer than 2 years should be studied comparatively to properly determine the intermediate category [81].
4.3. Conventional Storage of Recalcitrant Species
Recalcitrant seeds do not withstand conditions of conventional storage due to mechanical damage, metabolism-induced damage and macromolecular denaturation. Importantly, recalcitrant storage behaviors are more prevalent in woody plant species [31]. Therefore, the most practical way to extend the storage life of intact recalcitrant seeds is by storing them at the lowest possible temperature that does not cause damages as mentioned above [106]. However, the lowest applicable temperature must be experimentally tested. For instance, recalcitrant seeds from the temperate zone can be stored for a short time at the temperature range between 0 and −10 °C, i.e., Quercus robur L. [107,108,109] and Acer pseudoplatanus L. [110], while short-term storage of tropical recalcitrant seeds, i.e., Hopea hainanensis Merr. at a high MC of ~33% and a much higher temperature of 15 and 20 °C was reported [111].
4.4. Conventional Storage of Woody Plant Species in Gene Banks
In recent decades, the importance of preservation of tree species in gene banks has become indisputable as approximately 30% of tree species are threatened with extinction and at least 142 tree species are recorded as extinct in the wild (https://www.bgci.org/resources/bgci-tools-and-resources/state-of-the-worlds-trees/, accessed on 20 October 2022). As a result, new gene banks that are focused mostly on preservation of forest tree species were established, e.g., Kostrzyca Forest Gene Bank in Poland [17,112]. Another example of conservation efforts was the UK National Tree Seed Project (UKNTSP), that was initiated in 2013 by Royal Botanic Garden Kew and completed in April 2018. This project included a collection and conventional storage of 10 million seeds from 60 woody species native for the UK (https://www.kew.org/read-and-watch/10-million-seeds-national-seed-project, accessed on 20 October 2022). Additionally, seeds of 131 threatened woody plant species collected worldwide are preserved at −20 °C in Millennium Seed Bank (Table S1 based on [90]. Moreover, in The World Agroforestry Center (ICRAF) in Nairobi, 5800 seed accessions, or unique seed samples, representing 189 tree species, are conserved (https://forestsnews.cifor.org/65471/world-agroforestry-gene-bank-germinates-a-future-for-healthy-ecosystems?fnl=en, accessed on 20 October 2022).
5. Cryogenic Storage of Seeds
Cryogenic storage offers a long-term method of plant germplasm preservation that, in principle, overcomes the problematic issues related to the storage of intermediate and recalcitrant seeds. In theory, it offers indefinite preservation of tissues in liquid nitrogen (LN) vapor (−130 °C) or directly immersed in LN (−196 °C), with cellular metabolism effectively halted and the cessation of cell aging [113,114,115]. Cryostorage is based on the premise that under specific conditions, water can be cooled to cryogenic temperatures in a manner that avoids the process of ice nucleation [39,113,116]. Therefore, a major effort is put into the prevention of ice crystal formation and the promotion of vitrification which can be described as a solidification of a solution by achieving an extremely high viscosity without ice crystallization, resulting in a so-called “glassy” state [117]. In nature, the progressive loss of water inducing such a solid-like state is observed during the maturation of orthodox seeds. As a result, storage at low temperatures for these seeds does not induce any damage, leading to a decline in viability. In contrast, water from seeds with a high cell water content needs to be removed. One way to avoid ice formation is complete drying out of the plant tissue leaving no water, but for obvious reasons, it is not applicable as it would lead to cell death. In the case of desiccation-sensitive seeds, the problem is not trivial, and the solution is usually excision of explants followed by a variety of combined techniques which are: air drying, freeze dehydration, application of penetrating and osmotic active cryoprotectants, and the induction of accumulation of metabolites in cells. Therefore, all cryogenic strategies aim to achieve the glassy state by dealing with two factors: concentration of soluble compounds in the cytoplasm and freezing rate [58,115]. Unfortunately, there are limited studies which aimed to test the viability of seeds and explants after long-term (years and decades) cryogenic storage. However, studies on seeds stored for more than 10 years in cryogenic conditions showed that even though seeds deteriorate faster than anticipated, the shelf life of, i.e., lettuce seeds stored in the vapor and liquid phases of LN was projected for ~500 and ~3400 years, respectively [118]. Another study showed that the initial viability of seeds is an important factor, that needs to be monitored, as short-lived seeds derived from 11 investigated species deteriorated faster during the time of cryostorage (12–20 years) when their initial viability was low [119]. Therefore, there is a continuous need for further research showing how seed viability changes during long-term cryogenic storage, as well as during conventional storage. Special attention should be placed on testing and characterizing the proper storage and recovery procedures, determination of the time of safe storage that does not lower seed viability, and identifying the consequences of any instabilities in stored plant material to ensure the conservation of viable and true-to-type plant germplasm [91,120].
5.1. Cryogenic Storage of Orthodox Seeds
Cryogenic storage of orthodox seeds is relatively easy and commonly used in gene banks for ensuring the secure long-term backup of especially valuable seeds; however, it has become a routine practice only in the past 40 years [118]. Prior to cryostorage, seeds are desiccated to the tolerable range of 8–10% MC. Afterwards, they are placed into LN-tolerant containers, i.e., cryovials, or CryoFlex (polyethylene bags, sealed at both ends), and subsequently immersed directly into LN or put into a vapor of LN. The thawing process is also relatively straightforward with seeds being required to be transferred from cryogenic conditions directly to water baths at 40–45 °C. Alternatively, they can be thawed on a laboratory bench at room temperature (15–25 °C). However, a warm water bath is preferred due to the assurance of a more rapid and even thawing, which limits the formation of lethal ice crystals that tend form during a slow thawing procedure. So far, successful cryopreservation protocols have been established for many economically important species or their wild relatives such as Secale cereale L. [121], Solanum lycopersicum Mill. [122], Medicago sativa L., Beta vulgaris L., Allium cepa L., Oryza sativa L., Zea mays L. [118]. Orthodox seeds of many tree species can be successfully cryostored as well, such as seeds of Fraxinus excelsior L. [123], Prunus armeniaca L. [124], Malus sylvestris (L.) Mill. [125], Pyrus communis (L.) [91,126], Prunus avium L. [127,128], Sorbus aucuparia L., P. padus L., and Cornus sanguinea L. [89]. However, even in the case of cryopreservation of orthodox seeds, which tolerate desiccation below 5% of MC, some studies showed that seeds at a MC lower than 5% may produce fewer seedlings after cryostorage [129].
5.2. Cryogenic Storage of Intermediate Seeds
For intermediate seeds that do not tolerate conventional storage, cryopreservation can be a potential alternative. However, in this scenario, a single “universal” protocol does not exist, as in the case of orthodox seeds, that enables cryopreservation. As a result, it is important to separately evaluate seeds from every species of interest in order to identify its optimal MC and tolerance to cryogenic storage. However, the number of experimental records demonstrating the successful cryopreservation of intermediate seeds has significantly increased during the past several decades. So far, cryogenic storage for oil-rich seeds as Coffea liberica Bull. ex Hiern has been successful at an experimentally indicated optimal MC (16.7%) [130]. Successful cryopreservation was also reported for four Citrus species (C. aurantifolia, C. grandis, C. madurensis, C. reticulata) [131]. The second group of intermediate seeds, which is referred to as ‘short-lived seeds’, are also competent for cryopreservation. Successful cryostorage protocols have been published for two Salix hybrids: S. rehderiana × S. caprea and S. × sericans × S. viminalis [132], S. xerophila, S. maximowiczii, and S. koreensis [133], S. caprea L. [102], Populus deltoides Bartr. [134] and P. nigra L. [104]. However, short-lived seeds require permanent monitoring during cryogenic storage because aging processes do not stop completely and have deleterious effects on their longevity [119].
5.3. Cryogenic Storage of Recalcitrant Seeds
Entire recalcitrant seeds are not capable of withstanding cryogenic storage, as their tissues do not tolerate desiccation below 35–40% of MC for temperate species and 50–60% of MC for tropical species. Such high hydration of cells promotes the formation of ice crystals during the freezing and thawing of seeds, leading to lethal injuries, in particular to cellular membranes [58]. Even though entire recalcitrant seeds cannot be cryostored, their germplasm can be successfully preserved in LN in the form of tissues isolated from seeds, i.e., embryonic axes (EA) containing both shoot and root meristems [60,135,136,137,138,139,140,141,142] or plumules (containing only shoot meristem). To date, however, only plumules isolated from seeds of Quercus robur L., Q. petraea (Mattuschka) Liebl. and Cocos nucifera L. have been successfully cryostored [112,143,144,145]. The regeneration of plantlets from EA or plumules can be obtained via in vitro culturing. However, even though the EA is often characterized by higher desiccation tolerance than whole seeds [146,147], when isolated from seeds, they do not tolerate cryogenic storage. As a result, additional pre-treatment before plugging in LN is necessary. These processes are mostly vitrification, encapsulation vitrification, and droplet vitrification procedures based upon the utilization of cryoprotectants that are (1) penetrable through the cell wall and into the protoplast, (2) penetrable through the cell wall only, or (3) unable to penetrate through the cell wall [148,149]. For many decades, the most common vitrification solution was plant vitrification solution 2 (PVS2), which contains 30% (w/v) glycerol, 15% (w/v) ethylene glycol and 15% (w/v) dimethyl sulfoxide (DMSO) and 0.4 M sucrose [150]. However, this solution is often modified by additional compounds such as Supercool X1000 [151]. In recent years, cryoprotectants, which lack DMSO, i.e., 50% glycerol, 50% sucrose, have been popular [152], due to the recognition and discussion of the genotoxicity of DMSO [153,154,155].
Cryostorage can be a successful method of securing recalcitrant germplasm derived from species of tropical and temperate zones. However, in many cases, the entire cryopreservation procedure is challenging as it may require several steps including pre-drying, pre-cooling, and the usage of varied vitrification solutions. In addition, it is also required to test the duration of exposure time to cryoprotectants, modification of the pace of drying and cooling in LN, selection of the conditions for optimal sterilization and regeneration during in vitro culture. Collectively, these aforementioned steps and procedures make the cryopreservation technique expensive and labor-consuming in comparison to maintaining deposited seeds in gene banks. Therefore, the cryopreservation methodologies can only be performed in laboratories with specialized equipment.
5.4. Cryogenic Storage of Woody Plant Species at Gene Banks
Due to a requirement of a special equipment and training, cryopreservation is not routinely applied in many seed banks. Therefore, cryostorage of seeds of woody plant species is not commonly used, although there are several exceptions. For instance, in Forest Gene Bank in Kostrzyca, there are seed accessions of 20 species of forest trees and shrubs cryopreserved (Table S2 based on [17]). Moreover, to achieve Global Strategy for Plant Conservation Target 8 (CBD 2012), it is necessary to conserve species native to tropical moist forests [156]. Significantly, for many of them, cryopreservation may be the only method to ensure the effective ex situ conservation [157,158]. So far, protocols for cryopreservation of zygotic embryos of tropical recalcitrant seeds of tree species were characterized with various outcome. Some reports showed some success, e.g., cryopreservation of embryonic axes of Aquilaria malaccensis Lam., Sterculia cordata or Acrocomia aculeata (JACQ.) Lodd. ex Mart. [159,160,161]. However, many of reports have informed about failures or unsuccessful attempts of cryostorage of tropical tree species embryos [162,163,164]. So far, only the successful protocol for cryopreservation of Quercus robur plumules is routinely applied in gene banks [17,112,144,145]. However, the availability of information about cryopreserved accession in seed banks is very scarce and needs to be updated.
6. Future Possibility of Improving Storability of Seeds with Conventional Methods
Improving seed storage procedures via reductions in seed MC and storage temperature has limitations. Consequently, other approaches have been developed in an effort to optimize storage conditions. For example, modulation of the gaseous environment has been evaluated since the aging processes of dry stored seeds is accelerated by the presence of oxygen in the storage environment [165]. Thus, storage of seeds at a low oxygen concentration has been investigated as a method to improve seed viability. Studies which aimed to increase seed longevity of celery and celeriac seeds showed the importance of storage in cool and anoxic or limited oxygen conditions as soon as possible after harvesting and drying [165]. However, it is important to note that storage of seeds under hypoxic conditions also has deleterious effects on viability when moisture levels are relatively high in stored seeds. At high water activity levels, seeds are metabolically active and oxygen deprivation will result in suffocation and anaerobic respiration, accompanied by the production of toxic acetaldehyde and ethanol. Conversely, studies on dry seeds have reported either neutral or positive effects of anoxic storage conditions on seed longevity [165,166]. Recently published studies on the storage of maize seeds showed that seeds stored at 75% and 85% RH and 25 °C benefited from anoxic conditions in terms of their vigor and germination. However, anoxia did not affect seeds when higher temperatures (30 and 35 °C) and 65% RH were investigated [167]. This clearly shows that anoxic conditions benefit seed quality only under particular combinations of MC and temperature, therefore the optimal conditions of storage should be separately investigated for every species. Another recent study showed that modulation of the gaseous environment using oxygen absorbers and/or silica gel have potential for enhancing seed longevity by trapping toxic volatiles emitted by seeds during artificial aging. Moreover, this same study showed that for two investigated species: Lolium perenne L. and Agrostemma githago L., seed longevity was greater when aged in the presence of silica gel due to its action as a volatile trap. No effects on seed MC or oxygen concentration were observed in the storage containers [168]. It was also shown that 98% of nitrogen in the atmosphere effectively protected the reactive oxygen species (ROS) scavenging systems in wheat seeds and alleviated the internal deterioration during storage of wheat seed at accelerated aging conditions (at 35°C and 86% of RH) [169]. Beneficial effects of nitrogen in the controlled storage atmosphere have been reported for two old Italian wheat cultivars (Verna and Cappelli). Under nitrogen-saturated conditions, the loss of functional molecules, especially vitamin E, was reduced [170]. Moreover, new approaches aiming to improve seed storage include the invigoration of seeds with, i.e., exogenous antioxidants [171,172,173] or usage of nanoparticles for seed protection against microbial infections and alteration of deteriorative physiological processes through ROS scavenging. As a result, strategies incorporating the usage of nanoparticles have the potential for beneficial impact on plant growth, development and stress resistance [7,174,175]. Moreover, recent research focused the attention on the important role of nitric oxide (NO) as a seed antiaging molecule that diminishes the negative effects of seed deterioration processes. Significantly, its role in seed vigor improvement has appeared to be very promising [176,177].
7. Future Prospects of Improving the Cryostorage of Seeds or Explants
Cryogenic storage is much more complex than conventional methods, especially when applied for the preservation of recalcitrant embryonic axes or plumules. In both of these latter examples, extraction of these tissues and further treatment requires scientific expertise and specialized equipment. Several parallel technical paths have been developed for cryopreservation of seed materials which depend on the methods of cryo-pretreatment including vitrification, encapsulation vitrification, and droplet vitrification. Since cryopreservation has been more commonly used to preserve shoot tips or embryogenic callus, methods that have been successfully applied for somatic tissues are therefore also utilized for embryonic axes. However, the appropriate design and selection of a suitable cryopreservation protocol is not easy task, especially when information is lacking regarding the cryopreservation of seed explants of closely related species. Therefore, cryopreservation protocols are being constantly modified to achieve the best outcomes, for instance with the application of activated charcoal (AC) to alginate beads. This modification allowed to enhance the post-cryopreservation shoot and root regrowth of AC-encapsulated embryonic axes to Castanea sativa Mill. [141]. Another option is the application of exogenous antioxidants at different procedural stages of cryopreservation. The most promising results have been observed with glutathione, ascorbic acid, alpha-tocopherol (vitamin E) and cathodic water. However, most of these components were primarily applied to improve the cryopreservation of shoot tips [178,179] and only cathodic water was successfully applied for the cryopreservation embryonic axes of Ekebergia capensis [60]. Recent studies have demonstrated that incorporation of an application of exogenous dehydrin NnRab18 protein improves the cryopreservation of Arabidopsis thaliana seedlings [180]. Moreover, it seems that the rapid development of protocols including the application of nanoparticles (i.e., gold, iron oxide, nano-zinc oxide, and selenium), especially in cryopreservation of human and animal cells [181,182,183], shows great promises for future optimizations of plant cryopreservation protocols. Indeed, the first reports of successful application of nanoparticles to improve plant callus or shoot-tip cryopreservation have been reported [184,185].
8. Conclusions
The deleterious effects of seed aging occur mostly due to oxidative damage, leading to deterioration of biomolecules. Therefore, the main concept in seed storage is anchored in the prerequisite that effective seed storage relies on slowing down the seed’s normal metabolism, as much as possible without causing damage [31,186]. Consequently, the key to successful post-harvest management and storage of collections of seeds and explants is to understand and control temperature and humidity of storage as well as air accessibility. Obviously, this demands well-equipped storage facilities [31].
Moreover, in addition to the economic importance and conservation efforts, another classification complying with the feasibility and limitations of seed banking was proposed [41]. In this classification, more factors affecting the suitability of seeds to be preserved have been taken into consideration, including their tolerance to desiccation and low temperatures of storage, their accessibility and initial viability, access to dormancy release protocols and germination procedures. This classification has been started from the discrimination of the group of “exceptional” species, which includes species that produce seeds which are ‘un-bankable’ under conventional conditions, species breeding few or no seeds, or for which seed collection is not practical [41]. The concept of exceptional species has been further developed [40,187], and exceptional species were divided into groups based on critical limitations in seed banking. Those limitations are described as exceptionality factors (EF). EF1 distinguishes species that are characterized by seeds that are not viable or infrequent or even not available, EF2 groups recalcitrant seeds, while EF3 corresponds to species producing seeds that partially tolerate desiccation and are short lived at −20 °C, therefore resembling the intermediate category. Finally, EF4 indicates species which produce deeply dormant seeds. Since there are fewer exceptional species than species producing bankable seeds, and their storage requires skills and technologies that are far more complex and expensive than conventional seed banking, they are therefore far more likely to be overlooked. However, this classification may be commonly used in the future, particularly by practicians in gene banks as the identification of a species as exceptional is a first step in developing targeted, effective, and scientifically informed species conservation strategies [40].
Even though we have significantly improved our knowledge about seed long-term storage over the last 50 years, there are many issues that still need to addressed. Firstly, for successful seed conservation, more attention should be placed into the development of germination and dormancy breaking protocols, as well as the determination of seed post-harvest physiology, and finally into the testing of long-term storage of seeds (years/decades). Because improvement in this area requires labor-consuming research and time that usually goes beyond the 3–4-year cycle of research funding, it is very difficult to reach solid experimental conclusions within the time frames required by financing agencies. However, an experiment conducted at Michigan State University [28] shows that seed science is a marathon, not a sprint. Therefore, new funding opportunities, taking into account long-term research pursuits, should be provided.
Cryobiotechnology, including cryopreservation and in vitro technologies, provides potential technical paths to preserve plant species for which seed banking is not an option, especially sub-tropical and tropical recalcitrant-seeded species. For example, a recent study demonstrated that the viability of plant tissues can be maintained over 20–30 years in cryogenic conditions [34,37]. Finally, it is crucial to ensure proper storage conditions that do not exert any deleterious impacts on seedling development. Therefore, in addition to the assessment of germination, other seed and seedling properties should also be more commonly tested such as morphological properties [188], proteome and metabolome status [98], and genetic and epigenetic stability [96,120,145,189,190]. Consequently, there is a huge need for new biochemical or molecular markers of seed viability that improve the process of monitoring plant material during storage in gene banks and to help with the understanding of the concomitant aging process occurring in seeds. These studies have developed rapidly over the last decade and substantiate the importance of volatile organic compounds [94,95,98], proteomic studies [191], RNA integrity [192,193,194,195,196] and DNA damage markers or epigenetic marks [96,97,99,190,197,198,199,200] that are highly promising and should be more widely explored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12010072/s1. Table S1: List of globally threatened woody plant species whose seeds are conserved in −20 °C for long-term storage at the MSB, RBG Kew; Table S2: The seed accessions of forest trees and shrubs cryopreserved at the Kostrzyca Forest Gene Bank.
Author Contributions
Conceptualization, M.M. and B.P.P.-M.; writing—original draft preparation, M.M., M.T. and B.P.P.-M.; visualization, M.M., M.T. and B.P.P.-M.; supervision, M.M. and B.P.P.-M.; project administration, M.M. and B.P.P.-M.; funding acquisition, M.M. and B.P.P.-M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Science Center, Poland, grant number UMO2016/21/D/NZ9/02489 awarded to B.P.P-M and by National Science Center, Poland, grant number UMO2017/26/E/NZ9/00909 awarded to M.M.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Roe D., Seddon N., Elliott J. Biodiversity Loss—More than an Environmental Emergency. Lancet Planet. Health. 2019;3:e287–e289. doi: 10.1016/S2542-5196(19)30113-5. [DOI] [PubMed] [Google Scholar]
- 2.Soler Z., Kostecka J. Biodiversity Loss, the Causes, the State and Basic Form of Nature Protection in Spain and Poland. Pol. J. Sustain. Dev. 2019;22:75–84. doi: 10.15584/pjsd.2018.22.2.9. [DOI] [Google Scholar]
- 3.Thuiller W., Lavorel S., Araújo M.B., Sykes M.T., Prentice I.C. Climate Change Threats to Plant Diversity in Europe. Proc. Natl. Acad. Sci. USA. 2005;102:8245–8250. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Applequist W., Brinckmann J., Cunningham A., Hart R., Heinrich M., Katerere D., Andel T. Scientists’ Warning on Climate Change and Medicinal Plants. Planta Med. 2019;86:10–18. doi: 10.1055/a-1041-3406. [DOI] [PubMed] [Google Scholar]
- 5.Martin M.A., Sendra O.A., Bastos A., Bauer N., Bertram C., Blenckner T., Bowen K., Brando P.M., Rudolph T.B., Büchs M., et al. Ten New Insights in Climate Science 2021: A Horizon Scan. Glob. Sustain. 2021;4:e25. doi: 10.1017/sus.2021.25. [DOI] [Google Scholar]
- 6.Aurelle D., Thomas S., Albert C., Bally M., Bondeau A., Boudouresque C.-F., Cahill A.E., Carlotti F., Chenuil A., Cramer W., et al. Biodiversity, Climate Change, and Adaptation in the Mediterranean. Ecosphere. 2022;13:e3915. doi: 10.1002/ecs2.3915. [DOI] [Google Scholar]
- 7.de Martins R.S., dos Santos Junior N.A., Barbedo C.J. Seed Pathology of Non-Domesticated Species of Tropical Ecosystems. J. Seed Sci. 2022;44:e202244029. doi: 10.1590/2317-1545v44262592. [DOI] [Google Scholar]
- 8.Kahane R., Hodgkin T., Jaenicke H., Hoogendoorn C., Hermann M., (Dyno) Keatinge J.D.H., d’Arros Hughes J., Padulosi S., Looney N. Agrobiodiversity for Food Security, Health and Income. Agron. Sustain. Dev. 2013;33:671–693. doi: 10.1007/s13593-013-0147-8. [DOI] [Google Scholar]
- 9.Corlett R.T. Safeguarding Our Future by Protecting Biodiversity. Plant Divers. 2020;42:221. doi: 10.1016/j.pld.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muluneh M.G. Impact of Climate Change on Biodiversity and Food Security: A Global Perspective—A Review Article. Agric. Food Secur. 2021;10:36. doi: 10.1186/s40066-021-00318-5. [DOI] [Google Scholar]
- 11.Hay F.R., Whitehouse K.J., Ellis R.H., Sackville Hamilton N.R., Lusty C., Ndjiondjop M.N., Tia D., Wenzl P., Santos L.G., Yazbek M., et al. CGIAR Genebank Viability Data Reveal Inconsistencies in Seed Collection Management. Glob. Food Secur. 2021;30:100557. doi: 10.1016/j.gfs.2021.100557. [DOI] [Google Scholar]
- 12.Singh N., Wu S., Raupp W.J., Sehgal S., Arora S., Tiwari V., Vikram P., Singh S., Chhuneja P., Gill B.S., et al. Efficient Curation of Genebanks Using next Generation Sequencing Reveals Substantial Duplication of Germplasm Accessions. Sci. Rep. 2019;9:650. doi: 10.1038/s41598-018-37269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A.K., Varaprasad K.S., Venkateswaran K. Conservation Costs of Plant Genetic Resources for Food and Agriculture: Seed Genebanks. Agric. Res. 2012;1:223–239. doi: 10.1007/s40003-012-0029-3. [DOI] [Google Scholar]
- 14.Panis B., Nagel M., Van den Houwe I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants. 2020;9:1634. doi: 10.3390/plants9121634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jose Diez M., De la Rosa L., Martin I., Guasch L., Elena Cartea M., Mallor C., Casals J., Simo J., Rivera A., Anastasio G., et al. Plant Genebanks: Present Situation and Proposals for Their Improvement. the Case of the Spanish Network. Front. Plant Sci. 2018;9:1794. doi: 10.3389/fpls.2018.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Börner A., Khlestkina E.K. Ex-Situ Genebanks—Seed Treasure Chambers for the Future. Russ. J. Genet. 2019;55:1299–1305. doi: 10.1134/S1022795419110036. [DOI] [Google Scholar]
- 17.Zimnoch-Guzowska E., Chmielarz P., Wawrzyniak M.K., Plitta-Michalak B.P., Michalak M., Pałucka M., Wasileńczyk U., Kosek P., Kulus D., Rucińska A., et al. Polish Cryobanks: Research and Conservation of Plant Genetic Resources. Acta Soc. Bot. Pol. 2022;91:9121. doi: 10.5586/asbp.9121. [DOI] [Google Scholar]
- 18.Engels J.M.M., Ebert A.W. A Critical Review of the Current Global Ex Situ Conservation System for Plant Agrobiodiversity. II. Strengths and Weaknesses of the Current System and Recommendations for Its Improvement. Plants. 2021;10:1904. doi: 10.3390/plants10091904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusty C., Sackville Hamilton R., Guarino L., Richards C., Jamora N., Hawtin G. Envisaging an Effective Global Long-Term Agrobiodiversity Conservation System That Promotes and Facilitates Use. Plants. 2021;10:2764. doi: 10.3390/plants10122764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusty C., Guarino L., Toll J., Lainoff B. Encyclopedia of Agriculture and Food Systems. Elsevier; Amsterdam, The Netherlands: 2014. Genebanks: Past, Present, and Optimistic Future; pp. 417–432. [Google Scholar]
- 21.Gonzalez-Arnao M.T., Martinez-Montero M.E., Cruz-Cruz C.A., Engelmann F. Sustainable Development and Biodiversity. Springer; Cham, Switzerland: 2014. Advances in Cryogenic Techniques for the Long-Term Preservation of Plant Biodiversity; pp. 129–170. [Google Scholar]
- 22.Hay F., Sershen N. Plant Genetic Resources: A Review of Current Research and Future Needs. Burleigh Dodds Science Publishing; London, United Kingdom: 2020. New Technologies to Improve the Ex Situ Conservation of Plant Genetic Resources. [Google Scholar]
- 23.Smith P.P., Trivedi C., Cochrane A., Crawford A., Way M. The Millennium Seed Bank Project Delivering Target 8 of The. BG J. 2007;4:9–12. [Google Scholar]
- 24.Ohga I. The Germination of Century-Old and Recently Harvested Indian Lotus Fruits, with Special Reference to the Effect of Oxygen Supply. Am. J. Bot. 1926;13:754–759. doi: 10.1002/j.1537-2197.1926.tb05908.x. [DOI] [Google Scholar]
- 25.Porsild A.E., Harington C.R., Mulligan G.A. Lupinus Arcticus Wats. Grown from Seeds of Pleistocene Age. Science. 1967;158:113–114. doi: 10.1126/science.158.3797.113. [DOI] [PubMed] [Google Scholar]
- 26.Yashina S., Gubin S., Maksimovich S., Yashina A., Gakhova E., Gilichinsky D. Regeneration of Whole Fertile Plants from 30,000-y-Old Fruit Tissue Buried in Siberian Permafrost. Proc. Natl. Acad. Sci. USA. 2012;109:4008–4013. doi: 10.1073/pnas.1118386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning S.W., Griggs C., Lorentzen B., Ramsey C.B., Chivall D., Jull A.J.T., Lange T.E. Fluctuating Radiocarbon Offsets Observed in the Southern Levant and Implications for Archaeological Chronology Debates. Proc. Natl. Acad. Sci. USA. 2018;115:6141–6146. doi: 10.1073/pnas.1719420115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telewski F.W., Zeevaart J.A.D. The 120-Yr Period for Dr. Beal’s Seed Viability Experiment. Am. J. Bot. 2002;89:1285–1288. doi: 10.3732/ajb.89.8.1285. [DOI] [PubMed] [Google Scholar]
- 29.Kameswara Rao N. Manual of Seed Handling in Genebanks. Bioversity International; Rome, Italy: 2006. [Google Scholar]
- 30.Hong T.D., Ellis R.H. A Protocol to Determine Seed Storage Behavior. International Plant Genetic Resources Institute; Rome, Italy: 1996. IPGRI Technical Bulletin. [Google Scholar]
- 31.De Vitis M., Hay F.R., Dickie J.B., Trivedi C., Choi J., Fiegener R. Seed Storage: Maintaining Seed Viability and Vigor for Restoration Use. Restor. Ecol. 2020;28:S249–S255. doi: 10.1111/rec.13174. [DOI] [Google Scholar]
- 32.Pence V.C., Bruns E.B. The Tip of the Iceberg: Cryopreservation Needs for Meeting the Challenge of Exceptional Plant Conservation. Plants. 2022;11:1528. doi: 10.3390/plants11121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breman E., Ballesteros D., Castillo-Lorenzo E., Cockel C., Dickie J., Faruk A., O’Donnell K., Offord C.A., Pironon S., Sharrock S., et al. Plant Diversity Conservation Challenges and Prospects—The Perspective of Botanic Gardens and the Millennium Seed Bank. Plants. 2021;10:2371. doi: 10.3390/plants10112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyse S.V., Dickie J.B., Willis K.J. Seed Banking Not an Option for Many Threatened Plants. Nat. Plants. 2018;4:848–850. doi: 10.1038/s41477-018-0298-3. [DOI] [PubMed] [Google Scholar]
- 35.Lan Q., Xia K. Seed Storage Behaviour of 101 Woody Species from the Tropical Rainforest of Southern China: A Test of the Seed-Coat Ratio–Seed Mass (SCR–SM) Model for Determination of Desiccation Sensitivity. Aust. J. Bot. 2014;62:305–311. doi: 10.1071/BT14037. [DOI] [Google Scholar]
- 36.Martinez-Montero M.E., Harding K. PlantOmics: The Omics of Plant Science. Springer; New Delhi, India: 2015. Cryobionomics: Evaluating the Concept in Plant Cryopreservation; pp. 655–682. [Google Scholar]
- 37.Pence V.C., Ballesteros D., Walters C., Reed B.M., Philpott M., Dixon K.W., Pritchard H.W., Culley T.M., Vanhove A.-C. Cryobiotechnologies: Tools for Expanding Long-Term Ex Situ Conservation to All Plant Species. Biol. Conserv. 2020;250:108736. doi: 10.1016/j.biocon.2020.108736. [DOI] [Google Scholar]
- 38.Walters C., Pence V.C. The Unique Role of Seed Banking and Cryobiotechnologies in Plant Conservation. Plants People Planet. 2021;3:83–91. doi: 10.1002/ppp3.10121. [DOI] [Google Scholar]
- 39.Benelli C. Plant Cryopreservation: A Look at the Present and the Future. Plants. 2021;10:2744. doi: 10.3390/plants10122744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pence V.C., Meyer A., Linsky J., Gratzfeld J., Pritchard H.W., Westwood M., Bruns E.B. Defining Exceptional Species—A Conceptual Framework to Expand and Advance Ex Situ Conservation of Plant Diversity beyond Conventional Seed Banking. Biol. Conserv. 2022;266:109440. doi: 10.1016/j.biocon.2021.109440. [DOI] [Google Scholar]
- 41.Pence V.C. In Vitro Methods and the Challenge of Exceptional Species for Target 8 of the Global Strategy for Plant Conservation1. Ann. Mo. Bot. Gard. 2013;99:214–220. doi: 10.3417/2011112. [DOI] [Google Scholar]
- 42.Kilgore S., Havens K., Kramer A., Lythgoe A., MacKechnie L., Vitis M.D. Seed Collection, Storage, and Germination Practices May Affect Viola Reintroduction Outcomes. Nativ. Plants J. 2022;23:40–55. doi: 10.3368/npj.23.1.40. [DOI] [Google Scholar]
- 43.Lopez Del Egido L., Navarro-Miró D., Martinez-Heredia V., Toorop P.E., Iannetta P.P.M. A Spectrophotometric Assay for Robust Viability Testing of Seed Batches Using 2,3,5-Triphenyl Tetrazolium Chloride: Using Hordeum vulgare L. as a Model. Front. Plant Sci. 2017;8:747. doi: 10.3389/fpls.2017.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musaev F., Priyatkin N., Potrakhov N., Beletskiy S., Chesnokov Y. Assessment of Brassicaceae Seeds Quality by X-Ray Analysis. Horticulturae. 2022;8:29. doi: 10.3390/horticulturae8010029. [DOI] [Google Scholar]
- 45.Kavelenova L., Roguleva N., Yankov N., Ruzaeva I., Pavlova E., Nakrainikova D., Potrachov N. Assessment of the Quality of Seeds Formed in Situ and Ex Situ as a Mandatory Element of Maintaining Seed Banks of Rare Plants. E3S Web Conf. 2021;265:05012. doi: 10.1051/e3sconf/202126505012. [DOI] [Google Scholar]
- 46.Baskin C., Baskin J.M. Seed Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press; Cambridge, MA, USA: 2014. p. 1586. [Google Scholar]
- 47.Kildisheva O.A., Dixon K.W., Silveira F.A.O., Chapman T., Di Sacco A., Mondoni A., Turner S.R., Cross A.T. Dormancy and Germination: Making Every Seed Count in Restoration. Restor. Ecol. 2020;28:S256–S265. doi: 10.1111/rec.13140. [DOI] [Google Scholar]
- 48.Wyse S., Dickie J. Taxonomic Affinity, Habitat and Seed Mass Strongly Predict Seed Desiccation Response: A Boosted Regression Trees Analysis Based on 17 539 Species. Ann. Bot. 2017;121:71–83. doi: 10.1093/aob/mcx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ņečajeva J., Bleidere M., Jansone Z., Gailīte A., Ruņģis D. Variability of Seed Germination and Dormancy Characteristics and Genetic Analysis of Latvian Avena Fatua Populations. Plants. 2021;10:235. doi: 10.3390/plants10020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cristaudo A., Gresta F., Restuccia A., Catara S., Onofri A. Germinative Response of Redroot Pigweed (Amaranthus retroflexus L.) to Environmental Conditions: Is There a Seasonal Pattern? Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016;150:583–591. doi: 10.1080/11263504.2014.987845. [DOI] [Google Scholar]
- 51.Li R., Chen L., Wu Y., Zhang R., Baskin C.C., Baskin J.M., Hu X. Effects of Cultivar and Maternal Environment on Seed Quality in Vicia Sativa. Front. Plant Sci. 2017;8:1411. doi: 10.3389/fpls.2017.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baskin C., Baskin J. When Breaking Seed Dormancy Is a Problem Try a Move-along Experiment. Nativ. Plants J. 2003;4:17–21. doi: 10.3368/npj.4.1.17. [DOI] [Google Scholar]
- 53.Baskin C., Thompson K., Baskin J. Mistakes in Germination Ecology and How to Avoid Them. Seed Sci. Res. 2006;16:165–168. doi: 10.1079/SSR2006247. [DOI] [Google Scholar]
- 54.Roberts E.H. Predicting the Storage Life of Seeds. Seed Sci. Technol. 1973;1:499–514. [Google Scholar]
- 55.Barbedo C.J., da Centeno D.C., de Ribeiro R.C.L.F. Do Recalcitrant Seeds Really Exist? Hoehnea. 2013;40:583–593. doi: 10.1590/S2236-89062013000400001. [DOI] [Google Scholar]
- 56.Sershen , Varghese B., Naidoo C., Pammenter N.W. The Use of Plant Stress Biomarkers in Assessing the Effects of Desiccation in Zygotic Embryos from Recalcitrant Seeds: Challenges and Considerations. Plant Biol. 2016;18:433–444. doi: 10.1111/plb.12428. [DOI] [PubMed] [Google Scholar]
- 57.Plitta-Michalak B.P., Naskret-Barciszewska M.Z., Kotlarski S., Tomaszewski D., Tylkowski T., Barciszewski J., Chmielarz P., Michalak M. Changes in Genomic 5-Methylcytosine Level Mirror the Response of Orthodox (Acer platanoides L.) and Recalcitrant (Acer pseudoplatanus L.) Seeds to Severe Desiccation. Tree Physiol. 2018;38:617–629. doi: 10.1093/treephys/tpx134. [DOI] [PubMed] [Google Scholar]
- 58.Matilla A. The Orthodox Dry Seeds Are Alive: A Clear Example of Desiccation Tolerance. Plants. 2021;11:20. doi: 10.3390/plants11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva J.P.N., Salatino M.L.F., Barbedo C.J., de Figueiredo-Ribeiro R.C.L., da Centeno D.C. Active Metabolism during Desiccation of the Desiccation Tolerant Short-Lived Seeds of Poincianella pluviosa (DC.) L. P. Queiroz. J. Seed Sci. 2022;44:e202244028. doi: 10.1590/2317-1545v44261911. [DOI] [Google Scholar]
- 60.Bharuth V., Naidoo C. Responses to Cryopreservation of Recalcitrant Seeds of Ekebergia Capensis from Different Provenances. S. Afr. J. Bot. 2020;132:1–14. doi: 10.1016/j.sajb.2020.04.007. [DOI] [Google Scholar]
- 61.FAO, editor. Genebank Standards for Plant Genetic Resources for Food and Agriculture. Food and Agriculture Organization of the United Nations; Rome, Italy: 2014. [Google Scholar]
- 62.Walters C. Orthodoxy, Recalcitrance and in-between: Describing Variation in Seed Storage Characteristics Using Threshold Responses to Water Loss. Planta. 2015;242:397–406. doi: 10.1007/s00425-015-2312-6. [DOI] [PubMed] [Google Scholar]
- 63.Ellis R.H., Hong T.D., Roberts E.H. An Intermediate Category of Seed Storage Behaviour? I. COFFEE. J. Exp. Bot. 1990;41:1167–1174. doi: 10.1093/jxb/41.9.1167. [DOI] [Google Scholar]
- 64.Jacobo-Pereira C., Muñiz-Castro M.Á., Vázquez-García J.A., Flores J., Muñoz-Urias A., Huerta-Martínez F.M., Jacobo-Pereira C., Muñiz-Castro M.Á., Vázquez-García J.A., Flores J., et al. Seed Integrity, Effect of Temperature and Storage Time on Germination of Populus Luziarum and P. Primaveralepensis, Endangered Subtropical Species from Mexico. Bot. Sci. 2022;100:192–203. doi: 10.17129/botsci.2810. [DOI] [Google Scholar]
- 65.Noor N., Reed B.M., Yu X. Seed Storage and Cryoexposure Behavior in Hazelnut (Corylus avellana L. Cv. Barcelona) CryoLetters. 1994;15:315–322. [Google Scholar]
- 66.Michalak M., Plitta B.P., Chmielarz P. Desiccation Sensitivity and Successful Cryopreservation of Oil Seeds of European Hazelnut (Corylus Avellana) Ann. Appl. Biol. 2013;163:351–358. doi: 10.1111/aab.12059. [DOI] [Google Scholar]
- 67.Pipinis E., Stampoulidis A., Milios E., Kitikidou K., Akritidou S., Theodoridou S., Radoglou K. Effects of Seed Moisture Content, Stratification and Sowing Date on the Germination of Corylus Avellana Seeds. J. For. Res. 2020;31:743–749. doi: 10.1007/s11676-018-0852-x. [DOI] [Google Scholar]
- 68.Harrington J.F. Thumb Rules of Drying Seeds. Crops Soils. 1960;13:16–17. [Google Scholar]
- 69.Harrington J.F. Insects, and Seed Collection, Storage, Testing, and Certification. Elsevier; Amsterdam, The Netherlands: 1972. Seed Storage and Longevity; pp. 145–245. [Google Scholar]
- 70.Ellis R.H., Hong T.D., Roberts E.H. A Comparison of the Low-Moisture-Content Limit to the Logarithmic Relation Between Seed Moisture and Longevity in Twelve Species. Ann. Bot. 1989;63:601–611. doi: 10.1093/oxfordjournals.aob.a087788. [DOI] [Google Scholar]
- 71.Ellis R.H., Hong T.D., Roberts E.H. The Low-Moisture-Content Limit to the Negative Logarithmic Relation Between Seed Longevity and Moisture Content in Three Subspecies of Rice. Ann. Bot. 1992;69:53–58. doi: 10.1093/oxfordjournals.aob.a088306. [DOI] [Google Scholar]
- 72.Ellis R.H., Hong T.D., Roberts E.H. Moisture Content and the Longevity of Seeds of Phaseolus Vulgaris. Ann. Bot. 1990;66:341–348. doi: 10.1093/oxfordjournals.aob.a088033. [DOI] [Google Scholar]
- 73.Ellis R.H., Hong T.D., Roberts E.H., Tao K.-L. Low Moisture Content Limits to Relations Between Seed Longevity and Moisture. Ann. Bot. 1990;65:493–504. doi: 10.1093/oxfordjournals.aob.a087961. [DOI] [Google Scholar]
- 74.Vertucci C.W., Roos E.E. Theoretical Basis of Protocols for Seed Storage. Plant Physiol. 1990;94:1019–1023. doi: 10.1104/pp.94.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vertucci C.W., Roos E.E. Theoretical Basis of Protocols for Seed Storage II. The Influence of Temperature on Optimal Moisture Levels. Seed Sci. Res. 1993;3:201–213. doi: 10.1017/S0960258500001793. [DOI] [Google Scholar]
- 76.Vertucci C.W., Roos E.E., Crane J. Theoretical Basis of Protocols for Seed Storage III. Optimum Moisture Contents for Pea Seeds Stored at Different Temperatures. Ann. Bot. 1994;74:531–540. doi: 10.1006/anbo.1994.1151. [DOI] [Google Scholar]
- 77.Pritchard H., Wood C.B., Hodges S.S., Vautier H.J. 100-Seed Test for Desiccation Tolerance and Germination: A Case Study on Eight Tropical Palm Species. Seed Sci. Technol. 2004;32:393–403. doi: 10.15258/sst.2004.32.2.11. [DOI] [Google Scholar]
- 78.Mattana E., Peguero B., Di Sacco A., Agramonte W., Encarnación Castillo W.R., Jiménez F., Clase T., Pritchard H.W., Gómez-Barreiro P., Castillo-Lorenzo E., et al. Assessing Seed Desiccation Responses of Native Trees in the Caribbean. New For. 2020;51:705–721. doi: 10.1007/s11056-019-09753-6. [DOI] [Google Scholar]
- 79.Daws M.I., Garwood N.C., Pritchard H.W. Prediction of Desiccation Sensitivity in Seeds of Woody Species: A Probabilistic Model Based on Two Seed Traits and 104 Species. Ann. Bot. 2006;97:667–674. doi: 10.1093/aob/mcl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayrinck R.C., Vilela L.C., Pereira T.M., Rodrigues-Junior A.G., Davide A.C., Vaz T.A.A. Seed Desiccation Tolerance/Sensitivity of Tree Species from Brazilian Biodiversity Hotspots: Considerations for Conservation. Trees. 2019;33:777–785. doi: 10.1007/s00468-019-01815-8. [DOI] [Google Scholar]
- 81.Chau M.M., Chambers T., Weisenberger L., Keir M., Kroessig T.I., Wolkis D., Kam R., Yoshinaga A.Y. Seed Freeze Sensitivity and Ex Situ Longevity of 295 Species in the Native Hawaiian Flora. Am. J. Bot. 2019;106:1248–1270. doi: 10.1002/ajb2.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Porceddu M., Santo A., Orrù M., Meloni F., Ucchesu M., Picciau R., Sarigu M., Cuena Lombraña A., Podda L., Sau S., et al. Seed Conservation Actions for the Preservation of Plant Diversity: The Case of the Sardinian Germplasm Bank (BG-SAR) Plant Sociol. 2017;54:111–117. doi: 10.7338/pls2017542S1/11. [DOI] [Google Scholar]
- 83.Bacchetta G., Belletti P., Brullo S., Cagelli L., Carasso V., Casas J.L., Cervelli C., Escribà M.C., Fenu G., Gorian F. Manual for the Collection, Study, Ex Situ Processing and Conservation of Germplasm. Agenzia per la Protezione Dell’ambiente e per i Servizi tecnici; Rome, Italy: 2006. [Google Scholar]
- 84.Kumar S., Jalli (J) R., Srinivasan K. Physiological and Biochemical Changes in the Seeds of Karanj (Pongamia Pinnata L) under Different Storage Conditions. Indian J. Agric. Sci. 2011;81:423–428. [Google Scholar]
- 85.Magrini S., De Vitis M., Torelli D., Santi L., Zucconi L. Seed Banking of Terrestrial Orchids: Evaluation of Seed Quality in Anacamptis Following 4-Year Dry Storage. Plant Biol. 2019;21:544–550. doi: 10.1111/plb.12936. [DOI] [PubMed] [Google Scholar]
- 86.Dhyani A., Baskin C., Nautiyal B., Nautiyal M. Overcoming Root Dormancy and Identifying the Storage Behaviour of Lilium Polyphyllum Seeds. Botany. 2019;97:161–166. doi: 10.1139/cjb-2018-0161. [DOI] [Google Scholar]
- 87.Solberg S.Ø., Yndgaard F., Andreasen C., von Bothmer R., Loskutov I.G., Asdal Å. Long-Term Storage and Longevity of Orthodox Seeds: A Systematic Review. Front. Plant Sci. 2020;11:1007. doi: 10.3389/fpls.2020.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Solberg S.Ø., Brodal G., von Bothmer R., Meen E., Yndgaard F., Andreasen C., Asdal Å. Seed Germination after 30 Years Storage in Permafrost. Plants. 2020;9:579. doi: 10.3390/plants9050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wawrzyniak M.K., Michalak M., Chmielarz P. Effect of Different Conditions of Storage on Seed Viability and Seedling Growth of Six European Wild Fruit Woody Plants. Ann. For. Sci. 2020;77:58. doi: 10.1007/s13595-020-00963-z. [DOI] [Google Scholar]
- 90.Liu U., Cossu T.A., Davies R.M., Forest F., Dickie J.B., Breman E. Conserving Orthodox Seeds of Globally Threatened Plants Ex Situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: The Status of Seed Collections. Biodivers. Conserv. 2020;29:2901–2949. doi: 10.1007/s10531-020-02005-6. [DOI] [Google Scholar]
- 91.Plitta-Michalak B.P., Naskręt-Barciszewska M.Z., Barciszewski J., Chmielarz P., Michalak M. Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions. Forests. 2021;12:288. doi: 10.3390/f12030288. [DOI] [Google Scholar]
- 92.Desheva G. The Longevity of Crop Seeds Stored Under Long-Term Condition in the National Gene Bank of Bulgaria. Agric. Polnohospodárstvo. 2016;62:90–100. doi: 10.1515/agri-2016-0010. [DOI] [Google Scholar]
- 93.Walters C., Wheeler L.M., Grotenhuis J.M. Longevity of Seeds Stored in a Genebank: Species Characteristics. Seed Sci. Res. 2005;15:1–20. doi: 10.1079/SSR2004195. [DOI] [Google Scholar]
- 94.Colville L., Bradley E.L., Lloyd A.S., Pritchard H.W., Castle L., Kranner I. Volatile Fingerprints of Seeds of Four Species Indicate the Involvement of Alcoholic Fermentation, Lipid Peroxidation, and Maillard Reactions in Seed Deterioration during Ageing and Desiccation Stress. J. Exp. Bot. 2012;63:6519–6530. doi: 10.1093/jxb/ers307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mira S., Hill L.M., González-Benito M.E., Ibáñez M.A., Walters C. Volatile Emission in Dry Seeds as a Way to Probe Chemical Reactions during Initial Asymptomatic Deterioration. J. Exp. Bot. 2016;67:1783–1793. doi: 10.1093/jxb/erv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mira S., Pirredda M., Martín-Sánchez M., Marchessi J.E., Martín C. DNA Methylation and Integrity in Aged Seeds and Regenerated Plants. Seed Sci. Res. 2020;30:92–100. doi: 10.1017/S0960258520000021. [DOI] [Google Scholar]
- 97.González-Benito M.E., Ibáñez M.Á., Pirredda M., Mira S., Martín C. Application of the MSAP Technique to Evaluate Epigenetic Changes in Plant Conservation. Int. J. Mol. Sci. 2020;21:7459. doi: 10.3390/ijms21207459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michalak M., Plitta-Michalak B.P., Nadarajan J., Colville L. Volatile Signature Indicates Viability of Dormant Orthodox Seeds. Physiol. Plant. 2021;173:788–804. doi: 10.1111/ppl.13465. [DOI] [PubMed] [Google Scholar]
- 99.Michalak M., Plitta-Michalak B.P., Naskręt-Barciszewska M.Z., Barciszewski J., Chmielarz P. DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L. Cells. 2022;11:2080. doi: 10.3390/cells11132080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.León-Lobos P., Ellis R.H. Seed Storage Behaviour of Fagus Sylvatica and Fagus Crenata. Seed Sci. Res. 2002;12:31–37. doi: 10.1079/SSR200195. [DOI] [Google Scholar]
- 101.Stavrinides A.K., Dussert S., Combes M.-C., Fock-Bastide I., Severac D., Minier J., Bastos-Siqueira A., Demolombe V., Hem S., Lashermes P., et al. Seed Comparative Genomics in Three Coffee Species Identify Desiccation Tolerance Mechanisms in Intermediate Seeds. J. Exp. Bot. 2020;71:1418–1433. doi: 10.1093/jxb/erz508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Popova E.V., Kim D.H., Han S.H., Pritchard H.W., Lee J.C. Narrowing of the Critical Hydration Window for Cryopreservation of Salix Caprea Seeds Following Ageing and a Reduction in Vigour. CryoLetters. 2012;33:220–231. [PubMed] [Google Scholar]
- 103.Suszka J., Plitta B.P., Michalak M., Bujarska-Borkowska B., Tylkowski T., Chmielarz P. Optimal Seed Water Content and Storage Temperature for Preservation of Populus nigra L. Germplasm. Ann. For. Sci. 2014;71:543–549. doi: 10.1007/s13595-014-0368-2. [DOI] [Google Scholar]
- 104.Michalak M., Plitta B.P., Tylkowski T., Chmielarz P., Suszka J. Desiccation Tolerance and Cryopreservation of Seeds of Black Poplar (Populus nigra L.), a Disappearing Tree Species in Europe. Eur. J. For. Res. 2015;134:53–60. doi: 10.1007/s10342-014-0832-4. [DOI] [Google Scholar]
- 105.Bareke T., Addi A., Jilo K., Kumsa T. Effect of Storage Temperature and Packing Materials on Seed Germination and Seed Storage Behavior of Schefflera Abyssinica. Nusant. Biosci. 2022;14:141–147. doi: 10.13057/nusbiosci/n140202. [DOI] [Google Scholar]
- 106.Umarani R., Aadhavan E.K., Faisal M.M. Understanding Poor Storage Potential of Recalcitrant Seeds. Curr. Sci. 2015;108:2023–2034. [Google Scholar]
- 107.Suszka B., Tylkowski T. Storage of acorns of the English oak (Quercus robur L.) over 1-5 winters. Arbor. Kórn. 1980;25:199–229. [Google Scholar]
- 108.Michalak M., Plitta-Michalak B., Naskręt-Barciszewska M.Z., Barciszewski J., Bujarska-Borkowska B., Chmielarz P. Global 5-Methylcytosine Alterations in DNA during Ageing of Quercus Robur Seeds. Ann. Bot. 2015;116:369–376. doi: 10.1093/aob/mcv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chmielarz P., Suszka J., Wawrzyniak M.K. Desiccation Does Not Increase Frost Resistance of Pedunculate Oak (Quercus robur L.) Seeds. Ann. For. Sci. 2022;79:3. doi: 10.1186/s13595-022-01121-3. [DOI] [Google Scholar]
- 110.Tylkowski T. Short-Term Storage of after Ripened Seeds of Acer platanoides L. and A. pseudoplatanus L. Arbor. Kórn. 1989;34:135–141. [Google Scholar]
- 111.Lan Q., Luo Y.L., Ma S., Lu X., Yang M.-Z., Tan Y., Jiang X., Tan Y., Wang X., Li Z. Development and Storage of Recalcitrant Seeds of Hopea Hainanensis. Seed Sci. Technol. 2012;40:200–208. doi: 10.15258/sst.2012.40.2.05. [DOI] [Google Scholar]
- 112.Pałucka M., Hrydziuszko P. Cryogenic Storage of Plant Matrial in the Kostrzyca Forest Gene Bank. In Current Technologies of Forest Seed Treatment; Muller, J., Kozioł, C., Pałucka, M., Eds.; The Kostrzyca Forest Gene Bank, Milkow, Poland: 2014; ISBN 978-83-88245-12-1
- 113.Benson E.E. Cryopreservation Theory. In: Reed B.M., editor. Plant Cryopreservation: A Practical Guide. Springer; New York, NY, USA: 2008. pp. 15–32. [Google Scholar]
- 114.Streczynski R., Clark H., Whelehan L.M., Ang S.-T., Hardstaff L.K., Funnekotter B., Bunn E., Offord C.A., Sommerville K.D., Mancera R.L. Current Issues in Plant Cryopreservation and Importance for Ex Situ Conservation of Threatened Australian Native Species. Aust. J. Bot. 2019;67:1–15. doi: 10.1071/BT18147. [DOI] [Google Scholar]
- 115.Panis B. Sixty Years of Plant Cryopreservation: From Freezing Hardy Mulberry Twigs to Establishing Reference Crop Collections for Future Generations. Acta Hortic. 2019;1234:1–8. doi: 10.17660/ActaHortic.2019.1234.1. [DOI] [Google Scholar]
- 116.Reed B.M. Plant Cryopreservation: A Continuing Requirement for Food and Ecosystem Security. In Vitro Cell. Dev. Biol. Plant. 2017;53:285–288. doi: 10.1007/s11627-017-9851-4. [DOI] [Google Scholar]
- 117.Wowk B. Thermodynamic Aspects of Vitrification. Cryobiology. 2010;60:11–22. doi: 10.1016/j.cryobiol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 118.Walters C., Wheeler L., Stanwood P.C. Longevity of Cryogenically Stored Seeds. Cryobiology. 2004;48:229–244. doi: 10.1016/j.cryobiol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 119.Ballesteros D., Pence V.C. Survival and Death of Seeds during Liquid Nitrogen Storage: A Case Study on Seeds with Short Lifespans. CryoLetters. 2017;38:278–289. [PubMed] [Google Scholar]
- 120.Choudhary P., Pramitha L., Aggarwal P.R., Rana S., Vetriventhan M., Muthamilarasan M. Biotechnological Interventions for Improving the Seed Longevity in Cereal Crops: Progress and Prospects. Crit. Rev. Biotechnol. 2022:1–17. doi: 10.1080/07388551.2022.2027863. [DOI] [PubMed] [Google Scholar]
- 121.Lu J., Greene S., Reid S., Cruz V.M.V., Dierig D.A., Byrne P. Phenotypic Changes and DNA Methylation Status in Cryopreserved Seeds of Rye (Secale cereale L.) Cryobiology. 2018;82:8–14. doi: 10.1016/j.cryobiol.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 122.Zevallos B., Cejas I., Escriba R.C., Yabor L., Aragón Abreu C.E., Gonzalez Olmedo J., Engelmann F., Martinez-Montero M., Lorenzo Feijoo J. Biochemical Characterization of Ecuadorian Wild Solanum Lycopersicum Mill. Plants Produced from Non-Cryopreserved and Cryopreserved Seeds. CryoLetters. 2013;34:413–421. [PubMed] [Google Scholar]
- 123.Chmielarz P. Cryopreservation of Dormant European Ash (Fraxinus Excelsior) Orthodox Seeds. Tree Physiol. 2009;29:1279–1285. doi: 10.1093/treephys/tpp064. [DOI] [PubMed] [Google Scholar]
- 124.Jaganathan G.K., Wu G.R., Song X.Y., Liu B.L. Cryopreservation of Dormant Prunus armeniaca L. Seeds. Seed Sci. Technol. 2015;43:456–466. doi: 10.15258/sst.2015.43.3.11. [DOI] [Google Scholar]
- 125.Michalak M., Plitta-Michalak B., Chmielarz P. Desiccation Tolerance and Cryopreservation of Wild Apple (Malus Sylvestris) Seeds. Seed Sci. Technol. 2015;43:480–491. doi: 10.15258/sst.2015.43.3.20. [DOI] [Google Scholar]
- 126.Reed B.M., Schwanke S., Shala R. Pear Seeds Retain Viability after Liquid Nitrogen Immersion. HortScience. 2001;36:1121–1122. doi: 10.21273/HORTSCI.36.6.1121. [DOI] [Google Scholar]
- 127.Chmielarz P. Cryopreservation of Dormant Orthodox Seeds of Forest Trees: Mazzard Cherry (Prunus avium L.) Ann. For. Sci. 2009;66:405. doi: 10.1051/forest/2009020. [DOI] [Google Scholar]
- 128.Michalak M., Plitta-Michalak B., Chmielarz P. A New Insight in Desiccation Tolerance and Cryopreservation of Mazzard Cherry (Prunus avium L.) Seeds. Cent. Eur. J. Biol. 2015;10:354–364. doi: 10.1515/biol-2015-0036. [DOI] [Google Scholar]
- 129.Chmielarz P. Cryopreservation of Dormant Orthodox Seeds of European Hornbeam (Carpinus Betulus) Seed Sci. Technol. 2010;38:146–157. doi: 10.15258/sst.2010.38.1.15. [DOI] [Google Scholar]
- 130.Normah M., Vengadasalam M. Effects of Moisture-Content on Cryopreservation of Coffea and Vigna Seeds and Embryos. CryoLetters. 1992;13:199–208. [Google Scholar]
- 131.Hor Y.L., Kim Y.J., Ugap A., Chabrillange N., Sinniah U.R., Engelmann F., Dussert S. Optimal Hydration Status for Cryopreservation of Intermediate Oily Seeds: Citrus as a Case Study. Ann. Bot. 2005;95:1153–1161. doi: 10.1093/aob/mci126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wood C., Pritchard H., Lindegaard K. Seed Cryopreservation and Longevity of Two Salix Hybrids. CryoLetters. 2003;24:17–26. [PubMed] [Google Scholar]
- 133.Ku J.J., Han S.H., Kim D.H. Extended Low Temperature and Cryostorage Longevity of Salix Seeds with Desiccation Control. Open Life Sci. 2019;14:1–11. doi: 10.1515/biol-2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pence V.C. Germination, Desiccation and Cryopreservation of Seeds of Populus Deltoides Bartr; Proceedings of the International Seed Testing Association; Opocno, Czech Republic. 9–11 October 1996. [Google Scholar]
- 135.Walters C., Touchell D.H., Power P., Wesley-Smith J., Antolin M.F. A Cryopreservation Protocol for Embryos of the Endangered Species Zizania Texana. CryoLetters. 2002;23:291–298. [PubMed] [Google Scholar]
- 136.Hazubska-Przybył T., Chmielarz P., Michalak M., Dering M., Bojarczuk K. Survival and Genetic Stability of Picea Abies Embryogenic Cultures after Cryopreservation Using a Pregrowth-Dehydration Method. Plant Cell Tissue Organ Cult. PCTOC. 2013;113:303–313. doi: 10.1007/s11240-012-0270-2. [DOI] [Google Scholar]
- 137.Berjak P., Pammenter N.W. Cryostorage of Germplasm of Tropical Recalcitrant-Seeded Species: Approaches and Problems. Int. J. Plant Sci. 2014;175:29–39. doi: 10.1086/673303. [DOI] [Google Scholar]
- 138.Ballesteros D., Sershen , Varghese B., Berjak P., Pammenter N.W. Uneven Drying of Zygotic Embryos and Embryonic Axes of Recalcitrant Seeds: Challenges and Considerations for Cryopreservation. Cryobiology. 2014;69:100–109. doi: 10.1016/j.cryobiol.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 139.Xia K., Hill L.M., Li D.-Z., Walters C. Factors Affecting Stress Tolerance in Recalcitrant Embryonic Axes from Seeds of Four Quercus (Fagaceae) Species Native to the USA or China. Ann. Bot. 2014;114:1747–1759. doi: 10.1093/aob/mcu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Malik S., Choudhary R., Kaur S., Chaudhury R., Pritchard H. Storage Behavior and Cryopreservation of Citrus Cavaleriei, an Endangered, Cold-Resistant Species of Northeast India with Exceptionally Large Seeds. CryoLetters. 2020;41:281–290. [PubMed] [Google Scholar]
- 141.Gaidamashvili M., Khurtsidze E., Kutchava T., Lambardi M., Benelli C. Efficient Protocol for Improving the Development of Cryopreserved Embryonic Axes of Chestnut (Castanea Sativa Mill.) by Encapsulation-Vitrification. Plants. 2021;10:231. doi: 10.3390/plants10020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Srivastava V., Hajong S., Chandora R., Agrawal A. Desiccation and Freezing Tolerance of Recalcitrant Seeds and Embryonic Axes of Prunus Napaulensis (Ser.) Steud.: A Crop Wild Relative of Cherry. Genet. Resour. Crop Evol. 2022;69:1571–1583. doi: 10.1007/s10722-021-01320-3. [DOI] [Google Scholar]
- 143.N’Nan O., Hocher V., Verdeil J.-L., Konan J.-L., Ballo K., Mondeil F., Malaurie B. Cryopreservation by Encapsulation-Dehydration of Plumules of Coconut (Cocos nucifera L.) Cryoletters. 2008;29:339–350. [PubMed] [Google Scholar]
- 144.Chmielarz P., Michalak M., Pałucka M., Wasilenczyk U. Successful Cryopreservation of Quercus Robur Plumules. Plant Cell Rep. 2011;30:1405–1414. doi: 10.1007/s00299-011-1049-3. [DOI] [PubMed] [Google Scholar]
- 145.Plitta B.P., Michalak M., Naskręt-Barciszewska M.Z., Barciszewski J., Chmielarz P. DNA Methylation of Quercus robur L. Plumules Following Cryo-Pretreatment and Cryopreservation. Plant Cell Tissue Organ Cult. PCTOC. 2014;117:31–37. doi: 10.1007/s11240-013-0417-9. [DOI] [Google Scholar]
- 146.Dickie J.B., May K., Morris S.V.A., Titley S.E. The Effects of Desiccation on Seed Survival in Acer platanoides L. and Acer pseudoplatanus L. Seed Sci. Res. 1991;1:149–162. doi: 10.1017/S0960258500000829. [DOI] [Google Scholar]
- 147.Finchsavage W. Embryo Water Status and Survival in the Recalcitrant Species Quercus-Robur L—Evidence for a Critical Moisture-Content. J. Exp. Bot. 1992;43:663–669. doi: 10.1093/jxb/43.5.663. [DOI] [Google Scholar]
- 148.Tao D., Li P. Classification of Plant-Cell Cryoprotectants. J. Theor. Biol. 1986;123:305–310. doi: 10.1016/S0022-5193(86)80245-4. [DOI] [Google Scholar]
- 149.Sakai A., Engelmann F. Vitrification, Encapsulation-Vitrification and Droplet-Vitrification: A Review. Cryoletters. 2007;28:151–172. [PubMed] [Google Scholar]
- 150.Sakai A., Kobayashi S., Oiyama I. Cryopreservation of Nucellar Cells of Navel Orange (Citrus-Sinensis Osb Var Brasiliensis Tanaka) by Vitrification. Plant Cell Rep. 1990;9:30–33. doi: 10.1007/BF00232130. [DOI] [PubMed] [Google Scholar]
- 151.Zhao M.A., Zhu Y.Z., Dhital S.P., Khu D.M., Song Y.S., Wang M.H., Lim H.T. An Efficient Cryopreservation Procedure for Potato (Solanum tuberosum L.) Utilizing the New Ice Blocking Agent, Supercool X1000. Plant Cell Rep. 2006;25:164. doi: 10.1007/s00299-005-0107-0. [DOI] [PubMed] [Google Scholar]
- 152.Nishizawa S., Sakai A., Amano Y., Matsuzawa T. Cryopreservation of Asparagus (Asparagus officinalis L.) Embryogenic Suspension Cells and Subsequent Plant Regeneration by Vitrification. Plant Sci. 1993;91:67–73. doi: 10.1016/0168-9452(93)90189-7. [DOI] [Google Scholar]
- 153.Valencia-Quintana R., Gómez-Arroyo S., Waliszewski S.M., Sánchez-Alarcón J., Gómez-Olivares J.L., Flores-Márquez A.R., Cortés-Eslava J., Villalobos-Pietrini R. Evaluation of the Genotoxic Potential of Dimethyl Sulfoxide (DMSO) in Meristematic Cells of the Root of Vicia Faba. Toxicol. Environ. Health Sci. 2012;4:154–160. doi: 10.1007/s13530-012-0130-9. [DOI] [Google Scholar]
- 154.Galvao J., Davis B., Tilley M., Normando E., Duchen M.R., Cordeiro M.F. Unexpected Low-Dose Toxicity of the Universal Solvent DMSO. FASEB J. 2014;28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 155.Awan M., Buriak I., Fleck R., Fuller B., Goltsev A., Kerby J., Lowdell M., Mericka P., Petrenko A., Petrenko Y., et al. Dimethyl Sulfoxide: A Central Player since the Dawn of Cryobiology, Is Efficacy Balanced by Toxicity? Regen. Med. 2020;15:1463–1491. doi: 10.2217/rme-2019-0145. [DOI] [PubMed] [Google Scholar]
- 156.Krishnaswamy E.A., Hanson A., Communications E. Summary Report World Commission on Forests and Sustainable Development. World Commission on Forests & Sustainable Development; Winnipeg, MB, Canada: 1999. [Google Scholar]
- 157.Li D.-Z., Pritchard H.W. The Science and Economics of Ex Situ Plant Conservation. Trends Plant Sci. 2009;14:614–621. doi: 10.1016/j.tplants.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 158.Hay F.R., Probert R.J. Advances in Seed Conservation of Wild Plant Species: A Review of Recent Research. Conserv. Physiol. 2013;1:cot030. doi: 10.1093/conphys/cot030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Devi S.D., Kumaria S., Das M.C. Development of Cryopreservation Protocol for Aquilaria Malaccensis Lam., a Recalcitrant Seeded Tropical Tree Species. Cryoletters. 2019;40:18–27. [PubMed] [Google Scholar]
- 160.Nadarajan J., Staines H.J., Benson E.E., Marzalina M., Krishnapillay B., Harding K. Optimization of Cryopreservation Protocol for Sterculia Cordata Zygotic Embryos Using Taguchi Experiments. J. Trop. For. Sci. 2006;18:222–230. [Google Scholar]
- 161.Luis Z.G., Scherwinski-Pereira J.E. A Simple and Efficient Protocol for the Cryopreservation of Zygotic Embryos of Macaw Palm [Acrocomia Aculeata (Jacq.) Lodd. Ex Mart.], A Tropical Species with A Capacity for Biofuel Production. Cryoletters. 2017;38:7–16. [PubMed] [Google Scholar]
- 162.Hor Y.L., Stanwood P.C., Chin H.F. Effect of Dehydration on Freezing Characteristics and Survival in Liquid Nitrogen of Three Recalcitrant Seeds. Pertanika. 1990;13:309–314. [Google Scholar]
- 163.Normah M.N., Jamilah M.S., Serimala S.D. Viability Studies on Seeds and Embryonic Axes of Lansium Domesticum Corr. Malays. Appl. Biol. 1996;25:39–43. [Google Scholar]
- 164.Normah M.N., Ramiya S.D., Gintangga M. Desiccation Sensitivity of Recalcitrant Seeds—A Study on Tropical Fruit Species. Seed Sci. Res. 1997;7:179–183. doi: 10.1017/S0960258500003512. [DOI] [Google Scholar]
- 165.Groot S.P.C., de Groot L., Kodde J., van Treuren R. Prolonging the Longevity of Ex Situ Conserved Seeds by Storage under Anoxia. Plant Genet. Resour.-Charact. Util. 2015;13:18–26. doi: 10.1017/S1479262114000586. [DOI] [Google Scholar]
- 166.Roberts E., Ellis R. Water and Seed Survival. Ann. Bot. 1989;63:39–52. doi: 10.1093/oxfordjournals.aob.a087727. [DOI] [Google Scholar]
- 167.Abadia M.B., San Martino S., Bartosik R.E. Can Anoxic Atmospheres Protect the Quality of Maize Seeds during Storage? J. Stored Prod. Res. 2022;96:101927. doi: 10.1016/j.jspr.2021.101927. [DOI] [Google Scholar]
- 168.Han B., Fernandez V., Pritchard H.W., Colville L. Gaseous Environment Modulates Volatile Emission and Viability Loss during Seed Artificial Ageing. Planta. 2021;253:106. doi: 10.1007/s00425-021-03620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang R., Zhang L., Lu Q. Exploration of Mechanisms for Internal Deterioration of Wheat Seeds in Postharvest Storage and Nitrogen Atmosphere Control for Properties Protection. Crop Sci. 2018;58:823–836. doi: 10.2135/cropsci2017.08.0481. [DOI] [Google Scholar]
- 170.Moncini L., Simone G., Romi M., Cai G., Guerriero G., Whittaker A., Benedettelli S., Berni R. Controlled Nitrogen Atmosphere for the Preservation of Functional Molecules during Silos Storage: A Case Study Using Old Italian Wheat Cultivars. J. Stored Prod. Res. 2020;88:101638. doi: 10.1016/j.jspr.2020.101638. [DOI] [Google Scholar]
- 171.Xia F., Cheng H., Chen L., Zhu H., Mao P., Wang M. Influence of Exogenous Ascorbic Acid and Glutathione Priming on Mitochondrial Structural and Functional Systems to Alleviate Aging Damage in Oat Seeds. BMC Plant Biol. 2020;20:104. doi: 10.1186/s12870-020-2321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu Q.-J., Chen M.-X., Song T., Cheng C.-L., Tian Y., Hu J., Zhang J.-H. Spermidine Enhanced the Antioxidant Capacity of Rice Seeds during Seed Aging. Plant Growth Regul. 2020;91:397–406. doi: 10.1007/s10725-020-00613-4. [DOI] [Google Scholar]
- 173.Adetunji A.E., Adetunji T.L., Varghese B., Sershen , Pammenter N.W. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy. 2021;11:2369. doi: 10.3390/agronomy11122369. [DOI] [Google Scholar]
- 174.Das G., Dutta P. Effect of Nanopriming with Zinc Oxide and Silver Nanoparticles on Storage of Chickpea Seeds and Management of Wilt Disease. J. Agric. Sci. Technol. 2022;24:213–226. [Google Scholar]
- 175.Kaur R., Chandra J., Keshavkant S. Nanotechnology: An Efficient Approach for Rejuvenation of Aged Seeds. Physiol. Mol. Biol. Plants. 2021;27:399–415. doi: 10.1007/s12298-021-00942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ciacka K., Krasuska U., Staszek P., Wal A., Zak J., Gniazdowska A. Effect of Nitrogen Reactive Compounds on Aging in Seed. Front. Plant Sci. 2020;11:1011. doi: 10.3389/fpls.2020.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ciacka K., Staszek P., Sobczynska K., Krasuska U., Gniazdowska A. Nitric Oxide in Seed Biology. Int. J. Mol. Sci. 2022;23:14951. doi: 10.3390/ijms232314951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Elena Gonzalez-Benito M., Kremer C., Ibanez M.A., Martin C. Effect of Antioxidants on the Genetic Stability of Cryopreserved Mint Shoot Tips by Encapsulation-Dehydration. Plant Cell Tissue Organ Cult. 2016;127:359–368. doi: 10.1007/s11240-016-1056-8. [DOI] [Google Scholar]
- 179.Uchendu E.E., Leonard S.W., Traber M.G., Reed B.M. Vitamins C and E Improve Regrowth and Reduce Lipid Peroxidation of Blackberry Shoot Tips Following Cryopreservation. Plant Cell Rep. 2009;29:25. doi: 10.1007/s00299-009-0795-y. [DOI] [PubMed] [Google Scholar]
- 180.Sheng J., Liu T., Zhang D. Exogenous Dehydrin NnRab18 Improves the Arabidopsis Cryopreservation by Affecting ROS Metabolism and Protecting Antioxidase Activities. In Vitro Cell. Dev. Biol.-Plant. 2022;58:530–539. doi: 10.1007/s11627-022-10254-z. [DOI] [Google Scholar]
- 181.Manuchehrabadi N., Gao Z., Zhang J., Ring H.L., Shao Q., Liu F., McDermott M., Fok A., Rabin Y., Brockbank K.G.M., et al. Improved Tissue Cryopreservation Using Inductive Heating of Magnetic Nanoparticles. Sci. Transl. Med. 2017;9:eaah4586. doi: 10.1126/scitranslmed.aah4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Oliveira N.M., Reis R.L., Mano J.F. The Potential of Liquid Marbles for Biomedical Applications: A Critical Review. Adv. Healthc. Mater. 2017;6:1700192. doi: 10.1002/adhm.201700192. [DOI] [PubMed] [Google Scholar]
- 183.Saadeldin I.M., Khalil W.A., Alharbi M.G., Lee S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals. 2020;10:2281. doi: 10.3390/ani10122281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ren L., Deng S., Chu Y., Zhang Y., Zhao H., Chen H., Zhang D. Single-Wall Carbon Nanotubes Improve Cell Survival Rate and Reduce Oxidative Injury in Cryopreservation OfAgapanthus Praecoxembryogenic Callus. Plant Methods. 2020;16:130. doi: 10.1186/s13007-020-00674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kulus D., Tymoszuk A. Gold Nanoparticles Affect the Cryopreservation Efficiency of in Vitro-Derived Shoot Tips of Bleeding Heart. Plant Cell Tissue Organ Cult. 2021;146:297–311. doi: 10.1007/s11240-021-02069-4. [DOI] [Google Scholar]
- 186.Walters C., Ballesteros D., Vertucci V.A. Structural Mechanics of Seed Deterioration: Standing the Test of Time. Plant Sci. 2010;179:565–573. doi: 10.1016/j.plantsci.2010.06.016. [DOI] [Google Scholar]
- 187.Pence V.C., Bruns E.B., Meyer A., Pritchard H.W., Westwood M., Linsky J., Gratzfeld J., Helm-Wallace S., Liu U., Rivers M., et al. Gap Analysis of Exceptional Species—Using a Global List of Exceptional Plants to Expand Strategic Ex Situ Conservation Action beyond Conventional Seed Banking. Biol. Conserv. 2022;266:109439. doi: 10.1016/j.biocon.2021.109439. [DOI] [Google Scholar]
- 188.Mucha J., Szymańska A.K., Zadworny M., Tylkowski T., Michalak M., Suszka J. Effect of Seed Storage Temperature on Fine Root Development and Mycorrhizal Colonization of Young Populus Nigra Seedlings. Ann. For. Sci. 2015;72:539–547. doi: 10.1007/s13595-015-0470-0. [DOI] [Google Scholar]
- 189.Pirredda M., González-Benito M.E., Martín C., Mira S. Genetic and Epigenetic Stability in Rye Seeds under Different Storage Conditions: Ageing and Oxygen Effect. Plants. 2020;9:393. doi: 10.3390/plants9030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Plitta-Michalak B.P., Ramos A.A., Pupel P., Michalak M. Oxidative Damage and DNA Repair in Desiccated Recalcitrant Embryonic Axes of Acer pseudoplatanus L. BMC Plant Biol. 2022;22:40. doi: 10.1186/s12870-021-03419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rahman M., Liu L., Barkla B.J. A Single Seed Protein Extraction Protocol for Characterizing Brassica Seed Storage Proteins. Agronomy. 2021;11:107. doi: 10.3390/agronomy11010107. [DOI] [Google Scholar]
- 192.Fleming M.B., Richards C.M., Walters C. Decline in RNA Integrity of Dry-Stored Soybean Seeds Correlates with Loss of Germination Potential. J. Exp. Bot. 2017;68:2219–2230. doi: 10.1093/jxb/erx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Puchta M., Boczkowska M., Groszyk J. Low RIN Value for RNA-Seq Library Construction from Long-Term Stored Seeds: A Case Study of Barley Seeds. Genes. 2020;11:1190. doi: 10.3390/genes11101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Saighani K., Kondo D., Sano N., Murata K., Yamada T., Kanekatsu M. Correlation between Seed Longevity and RNA Integrity in the Embryos of Rice Seeds. Plant Biotechnol. 2021;38:277–283. doi: 10.5511/plantbiotechnology.21.0422a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Puchta M., Groszyk J., Małecka M., Koter M.D., Niedzielski M., Rakoczy-Trojanowska M., Boczkowska M. Barley Seeds MiRNome Stability during Long-Term Storage and Aging. Int. J. Mol. Sci. 2021;22:4315. doi: 10.3390/ijms22094315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tetreault H., Fleming M., Hill L., Dorr E., Yeater K., Richards C., Walters C. A Power Analysis for Detecting Aging of Dry-Stored Soybean Seeds: Germination versus RNA Integrity Assessments. Crop Sci. 2022 doi: 10.1002/csc2.20821. [DOI] [Google Scholar]
- 197.Arif M.A.R., Afzal I., Börner A. Genetic Aspects and Molecular Causes of Seed Longevity in Plants—A Review. Plants. 2022;11:598. doi: 10.3390/plants11050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ince A.G., Karaca M. Tissue and/or Developmental Stage Specific Methylation of NrDNA in Capsicum Annuum. J. Plant Res. 2021;134:841–855. doi: 10.1007/s10265-021-01287-3. [DOI] [PubMed] [Google Scholar]
- 199.Yalamalle V., Ithape D., Kumar A., Bhagat K., Ghosh S., Singh M. Seed Treatment with 5-Azacytidine Reduces Ageing-Induced Damage in Onion Seeds. Seed Sci. Technol. 2020;48:407–412. doi: 10.15258/sst.2020.48.3.09. [DOI] [Google Scholar]
- 200.Waterworth W.M., Bray C.M., West C.E. Seeds and the Art of Genome Maintenance. Front. Plant Sci. 2019;10:706. doi: 10.3389/fpls.2019.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.