Abstract
This study aims to examine the relationships of dietary α-carotene and β-carotene intake with cognitive function. The data were selected from the National Health and Nutrition Examination Survey (NHANES) 2011–2014. A total of 2009 participants were included in this analysis. Dietary α-carotene and β-carotene intake were averaged by two 24-h dietary recalls. The Consortium to Establish a Registry for Alzheimer’s Disease Word Learning subset (CERAD W-L), Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST) were used to evaluate cognitive function. Logistic regression and restricted cubic spline models were applied to explore the associations of dietary α-carotene and β-carotene intake with cognitive performance. After adjusting for all confounding factors, compared with individuals in the lowest quartile of β-carotene dietary intake, those in the highest quartile had lower risks of both CERAD W-L decline [odds ratio (OR) = 0.63, 95% confidence interval (CI): 0.44–0.90] and AFT decline (OR = 0.66, 95% CI: 0.47–0.94). In addition, the third quartile of β-carotene dietary intake had a significantly decreased risk of lower DSST (OR = 0.67, 95% CI: 0.48–0.83). Compared with the lowest quartile of α-carotene intake, the OR of AFT decline in the highest intake quartile was 0.66 (95% CI: 0.46, 0.94). For males, both dietary α-carotene and β-carotene intake were associated with a decreased risk of AFT decline (OR = 0.42, 95% CI: 0.25–0.71; OR = 0.51, 95% CI: 0.30–0.85, respectively). For females, dietary α-carotene intake was associated with a decreased risk of CERAD W-L decline (OR = 0.55, 95% CI: 0.33–0.91) and dietary β-carotene intake was associated with decreased risks of both CERAD W-L and AFT decline (OR = 0.37, 95% CI: 0.21–0.64; OR = 0.58, 95% CI: 0.37–0.91, respectively). Our results suggested that higher dietary α-carotene and β-carotene intake had inverse effects on cognitive function decline among older adults.
Keywords: α-carotene, β-carotene, cognitive function, cross-sectional study
1. Introduction
With the increased life expectancy around the world, the number of elderly people with cognitive decline has been escalating, causing a burden for their families and governments. The decline in cognitive function is associated with various factors [1], including normal aging processes and neurological diseases. However, without any prevention measures to delay cognitive function decline, the decline in cognitive function will gradually develop into mild cognitive impairment (MCI) and Alzheimer’s disease [2]. The process of Alzheimer’s disease is irreversible, and medical treatment for this disease is still limited. According to statistics in 2021, there were approximately 6.2 million Alzheimer’s patients in America [3], and this number was estimated to rise to 13.8 million by the mid-century. Deaths resulting from Alzheimer’s disease increased by more than 145% between 2000 and 2019, which is far more serious than death from stroke, cancer, and heart disease [3]. Thus, it is important to explore the risk factors of Alzheimer’s disease and other cognitive impairments.
Previous studies have shown that lack of physical activity [4], obesity [5], low education level [6], smoking [7], and lack of nutrition [8], such as vitamin B12 and metals [9], are risk factors associated with cognitive function decline. The underlying mechanism of vitamin B12 on cognitive function is related to the activation of methylation reactions in the brain [10]. According to previous studies, vitamin A, an antioxidant in the central nervous system [11], also participates in cognitive function decline in older people [12]. Both α-carotene and β-carotene can be transformed into retinol, which will be converted into a long-chain fatty acid ester that is the main precursor of vitamin A in the human body [13,14]. Thus, α-carotene and β-carotene may have similar effects on neurocognitive decline. Some previous studies showed that higher levels of α-carotene and β-carotene in the plasma were associated with better cognitive function [15,16]. However, the relationships of dietary α-carotene and β-carotene consumption with cognitive function have not been well explored.
Therefore, we used the National Health and Nutrition Examination Survey (NHANES) to explore the association of dietary α-carotene and β-carotene intake with cognitive function in elderly people. In addition, we also investigated the dose-response relationships of dietary α-carotene and β-carotene intake with cognitive function decline.
2. Materials and Methods
2.1. Study Population
As a cross-sectional survey, the NHANES was administered by the Centers for Disease Control and Prevention (CDC), aiming to estimate the health and nutritional status of the U.S. population [17]. The protocols in the NHANES were approved by the Review Board of the National Center for Health Statistics Ethics (NCHS). All the participants provided informed consent before the survey.
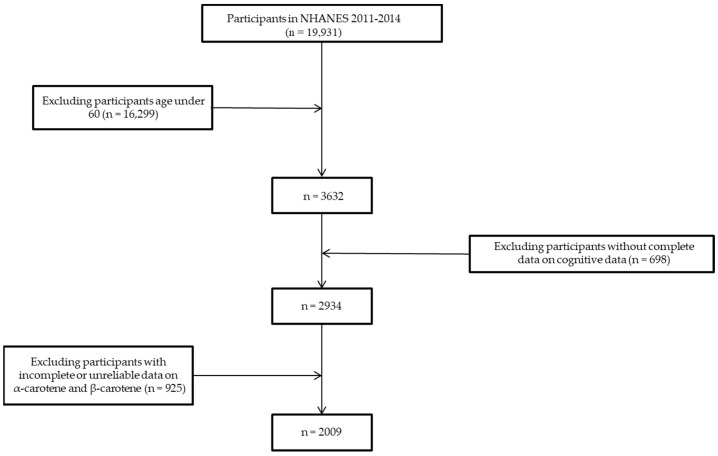
The data on dietary α-carotene and β-carotene intake and cognitive function measures were obtained from two cycles of the 2011–2012 and 2013–2014 NHANES. A total of 19,931 participants were recruited in the first round. We then excluded the participants who were under 60 years old (n = 16,229) and did not have complete data for cognitive function measurements (n = 2934). To reduce the influences of outliers on the analysis, participants with extreme values (dietary α-carotene intake > 1379.5 mcg/d and dietary β-carotene intake > 7876 mcg/d) (n = 410) and incomplete 24 h recall data for α-carotene or β-carotene intake (n = 515) were also excluded. Finally, 2009 participants were included in this analysis (Figure 1).
Figure 1.
Flow chart for the selection of eligible participants for this study.
2.2. Measurement of the Dietary α-Carotene and β-Carotene Intake
The dietary interview component in the NHANES was conducted by the U.S. Department of Agriculture (USDA) and the Department of Health and Human Services (DHHS). Under the cooperation, the survey sample design and data collection were processed by the NCHS. The dietary methodology was designed by the Food Surveys Research Group (FSRG). There were standardized investigation processes and strict quality controls in the survey.
Regarding previous studies, dietary information about α-carotene and β-carotene was obtained from 24 h dietary recall interviews [9,18]. The first day of diet recall was gathered in person during the visit and the next interview was conducted after 3–10 days through telephone. The detailed process of dietary data collection can be found on the NHANES website [19]. Nutrient intake profiles reported by individuals assembled detailed information about various food/beverage items. Information on recalls was acquired from “Total Nutrient Intakes Files”. In this study, the total α-carotene and β-carotene intake was calculated by the mean value of two dietary recalls. We categorized dietary α-carotene and β-carotene intake into quartiles according to published articles for further study (Q1: <25th percentile, Q2: ≥25th to 50th percentile, Q3: ≥50th to 75th percentile, Q4: ≥75th percentile with Q1 as the reference) [20].
2.3. Assessments of Cognitive Function
The NHANES survey contained three cognitive function tests including the Consortium to Establish Registry for Alzheimer’s disease (CERAD W-L), Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST). These cognitive function tests have been performed in large epidemiological and clinical studies [21,22,23]. For quality control, participants could choose their familiar language during the surveys, and the surveys were administered by two trained interviewers in the Mobile Examination Center. All tests were completed on the same day.
The CERAD W-L was separated into three successive learning trials and a delayed recall. The delayed word recall did not start until the other two cognitive tests were over. The score for each test ranged from 0 to 10 points. In the learning trials, participants were guided to read 10 unrelated words, and then they were asked to recall as many words as possible according to the sequence of the words. The sequence of words would be changed after each trial. The AFT was used to examine categorical verbal fluency, which is a component of executive function. In this test, participants were asked to name as many animals as possible in one minute, and a correct name was assigned one point. Participants in the DSST were required to finish the corresponding logograms in the 133 boxes in 2 min, and the final score was calculated by the total number of correct answers [24,25,26].
Until now, there were no standard cut-off points in the CERAD W-L, AFT, and DSST. According to previous papers, we classified the scores into quartiles and defined the minimum quartile for each test as the reference group [20,27,28]. Regarding the CERAD W-L, AFT, and DSST scores, the cut-off values were 20, 12, and 33, respectively. Participants whose scores were lower than the corresponding cut-off values in tests were assigned to the low cognitive function group, and other participants were assigned to the normal cognitive function groups.
2.4. Other Variables of Interest
The set of covariates was based on previous studies [18,29,30]. We selected socioeconomic status variables, including age, gender (male/female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other race), marital status (married, widowed, divorced, separated, never married, or living with a partner), education (<high school, high school, or >high school), poverty-income ratio, body mass index (BMI), health behavior variables such as smoking and drinking status, and health factors such as diabetes. The poverty ratio was a concept of social economics that was calculated by dividing family (or individual) income by the poverty guidelines specific to the survey year. Smoking status was divided into two groups (yes/no) based on the item “Have you smoked at least 100 cigarettes in your entire life”. Participants were characterized as alcohol drinkers if they consumed at least 12 alcoholic drinks per year. The history of diabetes was defined by the doctor’s diagnosis (yes, no, or borderline). For MCI/dementia participants, confounding factors were provided by family members in the NHANES project.
2.5. Statistical Analysis
In this study, both α-carotene and β-carotene were Ln-transformed due to the skewed distribution. Continuous variables and categorical variables are presented as the mean [standard deviation (SD)] and percentage (%), respectively. The differences between the two groups were examined using the Student’s t-test and the chi-squared test.
Furthermore, dietary α-carotene and β-carotene intake were modeled as both continuous (Ln-transformed) and categorical variables to explore the associations with cognitive function by logistic regression. In the logistic regression analysis, the crude model had no adjustment, while model I was adjusted for age, gender, race/ethnicity, and BMI. Model II was then adjusted for all potential confounding factors, including age, race/ethnicity, gender, BMI, education, marital status, poverty-income ratio, smoking status, alcohol intake, and diabetes.
We further performed a stratified analysis by gender to examine the associations of dietary α-carotene and β-carotene intake with the three cognitive function tests. Model II was adjusted for all potential covariates mentioned above.
Additionally, the restricted cubic spline (RCS) was utilized to explore the dose-response relationships of dietary α-carotene and β-carotene intake with cognitive function. There were four knots at the 5th, 25th, 75th, and 95th percentiles of the dietary α-carotene and β-carotene intake after adjusting for all covariates. SPSS (version 24.0) and R (vision 4.0.3, R Foundation for Statistical Computing) were used to analyze the data. A two-tailed p value less than 0.05 was considered statistically significant.
3. Results
Table 1 shows the basic characteristics of the participants from the NHANES 2011–2014 in three cognitive tests (CERAD W-L, AFT, and DSST), which were categorized into low cognition and normal cognition groups. In all cognitive tests, the participants in the low cognitive function group were older, tended to be non-black, be married, and have lower levels of poverty-income ratio, dietary β-carotene intake, and education. In the AFT, the participants in the low cognition group had lower dietary α-carotene intake than those in the normal cognition group. Moreover, in the CERAD W-L, participants in the low cognition group had lower BMI levels than those in the normal cognition group. Participants in the low cognition group from both the AFT and DSST were mostly alcohol drinkers and had no history of diabetes (all p values < 0.05). No significant differences in smoking status were observed between the two groups in all cognitive tests.
Table 1.
Baseline characteristics of participants classified by cognitive function status.
| CERAD W-L | AFT | DSST | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Cognitive Function | Normal Cognitive Function | p-Value | Low Cognitive Function | Normal Cognitive Function | p-Value | Low Cognitive Function | Normal Cognitive Function | p-Value | |
| Age a | 72.22 (6.84) | 68.56 (6.44) | <0.001 | 71.17 (6.82) | 68.95 (6.61) | <0.001 | 71.61 (6.81) | 68.79 (6.56) | <0.001 |
| Poverty-income ratio a | 2.23 (1.51) | 2.76 (1.59) | <0.001 | 2.16 (1.46) | 2.77 (1.59) | <0.001 | 1.78 (1.26) | 2.89 (1.58) | <0.001 |
| BMI a | 28.73 (6.40) | 29.70 (6.53) | 0.005 | 29.25 (6.39) | 29.53 (6.54) | 0.424 | 29.46 (6.52) | 29.47 (6.51) | 0.974 |
| Ln α-carotene a | 4.49 (1.67) | 4.60 (1.62) | 0.218 | 4.40 (1.59) | 4.62 (1.64) | 0.013 | 4.48 (1.63) | 4.60 (1.63) | 0.155 |
| Ln β-carotene a | 6.62 (1.23) | 6.92 (1.08) | <0.001 | 6.61 (1.22) | 6.91 (1.09) | <0.001 | 6.54 (1.23) | 6.94 (1.07) | <0.001 |
| Gender b | |||||||||
| male | 291 (60.4%) | 694 (45.4%) | <0.001 | 219 (49.8%) | 766 (48.8%) | 0.724 | 254 (55.0%) | 731 (47.3%) | 0.004 |
| female | 191 (39.6%) | 833 (54.6%) | 221 (50.2%) | 803 (51.2%) | 208 (45.0%) | 816 (52.7%) | |||
| Race/ethnicity b | |||||||||
| Mexican American | 52 (10.8%) | 113 (7.4%) | 0.035 | 34 (7.7%) | 131 (8.3%) | <0.001 | 61 (13.2%) | 104 (6.7%) | <0.001 |
| Other Hispanic | 55 (11.4%) | 134 (8.8%) | 48 (10.9%) | 141 (9.0%) | 81 (17.5%) | 108 (7.0%) | |||
| Non-Hispanic White | 239 (49.6%) | 804 (52.7%) | 162 (36.8%) | 881 (56.2%) | 152 (32.9%) | 891 (57.6%) | |||
| Non-Hispanic Black | 107 (22.2%) | 356 (23.3%) | 157 (35.7%) | 306 (19.5%) | 155 (33.5%) | 308 (19.9%) | |||
| Other Race | 29 (6.0%) | 120 (7.9%) | 39 (8.9%) | 110 (7.0%) | 13 (2.8%) | 136 (8.8%) | |||
| Marital status b | |||||||||
| Married | 256 (53.1%) | 869 (56.9%) | 0.004 | 224 (50.9%) | 901 (57.4%) | <0.001 | 214 (46.3%) | 911 (58.9%) | <0.001 |
| Widowed | 114 (23.7%) | 262 (17.2%) | 115 (26.1%) | 261 (16.6%) | 127 (27.5%) | 249 (16.1%) | |||
| Divorced | 53 (11.0%) | 234 (15.3%) | 58 (13.2%) | 229 (14.6%) | 55 (11.9%) | 232 (15.0%) | |||
| Separated | 16 (3.3%) | 44 (2.9%) | 17 (3.9%) | 43 (2.7%) | 29 (6.3%) | 31 (2.0%) | |||
| Never married | 25 (5.2%) | 84 (5.5%) | 19 (4.3%) | 90 (5.7%) | 25 (5.4%) | 84 (5.4%) | |||
| Living with a partner | 18 (3.7%) | 34 (2.2%) | 7 (1.6%) | 45 (2.9%) | 12 (2.6%) | 40 (2.6%) | |||
| Education b | |||||||||
| <High school | 187 (38.8%) | 275 (18.0%) | <0.001 | 158 (35.9%) | 304 (19.4%) | <0.001 | 240 (51.9%) | 222 (14.4%) | <0.001 |
| High school | 115(23.9%) | 368 (24.1%) | 128 (29.1%) | 355 (22.6%) | 114 (24.7%) | 369 (23.9%) | |||
| >High school | 180 (37.3%) | 884 (57.9%) | 154 (35.0%) | 910 (58.0%) | 108 (23.4%) | 956 (61.8%) | |||
| Smoking status b | |||||||||
| Yes | 243 (50.4%) | 803 (52.6%) | 0.405 | 232 (52.7%) | 814 (51.9%) | 0.753 | 238 (51.5%) | 808 (52.2%) | 0.787 |
| No | 239 (49.6%) | 724 (47.4%) | 208 (47.3%) | 755 (41.8%) | 224 (48.5%) | 739 (47.8%) | |||
| Alcohol intake b | |||||||||
| Yes | 334 (69.3%) | 1074 (70.3%) | 0.664 | 287 (65.2%) | 1121 (71.4%) | 0.012 | 293 (63.4%) | 1115 (72.1%) | <0.001 |
| No | 148 (30.7%) | 453 (29.7%) | 153 (34.8%) | 448 (28.6%) | 169 (36.6%) | 432 (27.9%) | |||
| Diabetes b,c | |||||||||
| Yes | 132 (27.4%) | 353 (23.1%) | 0.161 | 136 (30.9%) | 349 (22.2%) | 0.001 | 153 (33.1%) | 332 (21.5%) | <0.001 |
| No | 328 (68.0%) | 1101 (72.1%) | 286 (65.0%) | 1143 (72.8%) | 288 (62.3%) | 1141 (73.8%) | |||
| Borderline | 22 (6.4%) | 73 (4.8%) | 18 (4.1%) | 77 (4.9%) | 21 (4.5%) | 74 (4.8%) | |||
Note: a mean (SD), b n (%). c Diabetes variable was defined by whether a person had been told by a doctor or other health professional that they had diabetes or borderline diabetes.
The associations between dietary α-carotene and β-carotene intake and cognitive functions by the CERAD W-L, AFT, and DSST are depicted in Table 2. In the CERAD W-L, the odds ratio (OR) values in the crude model of cognitive function with β-carotene intake in the second quartile (Q2), third quartile (Q3), and fourth quartile (Q4) groups were 0.72 [95% confidence interval (CI): 0.53, 0.98], 0.66 (95% CI: 0.50, 0.88) and 0.60 (95% CI: 0.37, 0.69), respectively, compared to those in the first quartile (Q1) group. After adjusting for all covariates in model II, the OR value in the Q4 group for dietary β-carotene intake was 0.63 (95% CI: 0.44, 0.90) compared to the Q1 group.
Table 2.
Weighted odds ratio (95% confidence intervals) of low cognitive performance by quartiles of dietary α-carotene and β-carotene intake.
| CERAD W-L | AFT | DSST | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Model I a | Model II b | Crude | Model I a | Model II b | Crude | Model I a | Model II b | |
| α-carotene (mcg/d) | |||||||||
| Q1 (≤17.5) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (17.5 to ≤62.5) | 0.85 (0.62, 1.16) |
0.79 (0.56, 1.09) |
0.89 (0.63, 1.25) |
0.85 (0.62, 1.17) |
0.83 (0.60, 1.14) |
0.95 (0.68, 1.33) |
1.00 (0.72, 1.37) |
0.93 (0.67, 1.29) |
1.26 (0.87, 1.83) |
| Q3 (62.5 to ≤358) | 0.74 (0.55, 1.00) |
0.69 (0.50, 0.94) * |
0.82 (0.59, 1.14) |
0.86 (0.63, 1.16) |
0.81 (0.59, 1.10) |
0.98 (0.71, 1.35) |
0.84 (0.62, 1.13) |
0.77 (0.56, 1.06) |
1.16 (0.81, 1.65) |
| Q4 (>358) | 0.79 (0.58, 1.09) |
0.70 (0.50, 0.97) * |
0.84 (0.60, 1.19) |
0.57 (0.41, 0.80) * |
0.53 (0.38, 0.74) ** |
0.66 (0.46, 0.94) * |
0.80 (0.58, 1.11) |
0.73 (0.52, 1.02) |
1.15 (0.79, 1.69) |
| β-carotene (mcg/d) | |||||||||
| Q1 (≤338) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (338 to ≤819) | 0.72 (0.53, 0.98) * |
0.67 (0.49, 0.93) * |
0.84 (0.60, 1.18) |
0.65 (0.47, 0.88) * |
0.65 (0.47, 0.89) * |
0.80 (0.57, 1.10) |
0.64 (0.47, 0.87) * |
0.60 (0.44, 0.81) * |
0.89 (0.63, 1.26) |
| Q3 (819 to ≤2222.5) | 0.66 (0.50, 0.88) ** |
0.61 (0.46, 0.83) * |
0.81 (0.59, 1.11) |
0.51 (0.38, 0.69) ** |
0.49 (0.36, 0.66) ** |
0.65 (0.48, 0.89) * |
0.45 (0.34, 0.60) ** |
0.42 (0.31, 0.56) ** |
0.67 (0.48, 0.83) * |
| Q4 (>2222.5) | 0.60 (0.37, 0.69) ** |
0.46 (0.33, 0.64) ** |
0.63 (0.44, 0.90) * |
0.52 (0.38, 0.71) ** |
0.48 (0.35, 0.66) ** |
0.66 (0.47, 0.94) * |
0.42 (0.30, 0.57) ** |
0.39 (0.28, 0.54) ** |
0.73 (0.50, 1.06) |
a Model I was adjusted for age, gender, race/ethnicity, and BMI. b Model II was adjusted for age, gender, race/ethnicity, BMI, education, marital status, poverty-income ratio, smoking status, alcohol intake, and diabetes. * p < 0.05, ** p < 0.01.
In the AFT, the OR values in the crude model of cognitive function with β-carotene intake in the Q2, Q3, and Q4 groups were 0.65 (95% CI: 0.47, 0.88), 0.51 (95% CI: 0.38, 0.69), and 0.52 (95% CI: 0.38, 0.71), respectively, compared to those in the Q1 group. Furthermore, after adjusting for all confounding factors in model II, the OR value in the Q4 group for dietary β-carotene intake was 0.66 (95% CI: 0.47, 0.94) compared to those in the Q1 group.
In the DSST, the OR values in the crude model of cognitive function with β-carotene intake in the Q2, Q3, and Q4 groups were 0.64 (95% CI: 0.47, 0.87), 0.45 (95% CI: 0.34, 0.60), and 0.42 (95% CI: 0.30, 0.57), respectively, compared to those in the Q1 group. In model II, the OR value in the Q3 group for β-carotene intake was 0.67 (95% CI: 0.48, 0.83) compared to the Q1 group. No significant difference between the Q4 and Q1 groups were observed after adjusting for all covariates in the DSST.
There were no significant associations between dietary α-carotene intake and cognitive function by the CERAD W-L and DSST without or with adjustment for covariates. However, in the AFT, the OR of the cognitive function decline with α-carotene intake in the Q4 group was 0.57 (95% CI: 0.41, 0.80) in the crude model and 0.66 (95% CI: 0.46, 0.94) in the fully adjusted model II.
Stratified analyses were performed to investigate the associations of dietary α-carotene and β-carotene intake with the cognitive function measured by the CERAD W-L, AFT, and DSST among the male and female participants (Table 3). Among the male participants, there were significant associations between dietary α-carotene intake (OR: 0.42, 95% CI: 0.25, 0.71) and β-carotene intake (OR: 0.51, 95% CI: 0.30, 0.85) with the cognitive function measured by the AFT in model II. Among the female participants, the OR of cognitive function measured by the CERAD W-L with the Q3 group for α-carotene intake was 0.55 (95% CI: 0.33, 0.91) compared to those in the Q1 group. Furthermore, the OR value of the cognitive function measured by the CERAD W-L with the Q4 group for β-carotene intake was 0.37 (95% CI: 0.21, 0.64), while the OR of the cognitive function measured by the AFT with the Q3 group for β-carotene intake was 0.58 (95% CI: 0.37, 0.91) in model II. No significant associations were observed between dietary α-carotene and β-carotene intake with the cognitive function measured by the DSST.
Table 3.
Weighted odds ratio (95% confidence intervals) of low cognitive performance by quartiles of dietary α-carotene and β-carotene intake, stratified by gender.
| CERAD W-L | AFT | DSST | ||||
|---|---|---|---|---|---|---|
| Crude | Model II a | Crude | Model II a | Crude | Model II a | |
| Male | ||||||
| α-carotene (mcg/d) | ||||||
| Q1 (≤17.5) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (17.5 to ≤62.5) | 0.97 (0.64, 1.47) |
1.02 (0.66, 1.60) |
0.87 (0.57, 1.33) |
0.96 (0.61, 1.51) |
1.04 (0.69, 1.57) |
1.31 (0.81, 2.12) |
| Q3 (62.5 to ≤358) | 0.95 (0.64, 1.42) |
1.10 (0.71, 1.69) |
0.84 (0.55, 1.27) |
1.00 (0.64, 1.56) |
0.69 (0.46, 1.04) |
0.98 (0.61, 1.59) |
| Q4 (>358) | 0.89 (0.59, 1.34) |
0.98 (0.62, 1.53) |
0.37 (0.23, 0.60) ** |
0.42 (0.25, 0.71) ** |
0.62 (0.40, 0.96) * |
0.89 (0.54, 1.47) |
| β-carotene (mcg/d) | ||||||
| Q1 (≤338) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (338 to ≤819) | 0.84 (0.55, 1.26) |
1.05 (0.67, 1.63) |
0.72 (0.47, 1.10) |
0.95 (0.60, 1.50) |
0.61 (0.40, 0.92) * |
0.94 (0.58, 1.51) |
| Q3 (819 to ≤2222.5) | 0.79 (0.54, 1.17) |
0.99 (0.65, 1.51) |
0.57 (0.38, 0.85) ** |
0.73 (0.47, 1.13) |
0.46 (0.31, 0.68) ** |
0.68 (0.43, 1.07) |
| Q4 (>2222.5) | 0.71 (0.46, 1.08) |
0.94 (0.59, 1.51) |
0.41 (0.25, 0.65) ** |
0.51 (0.30, 0.85) ** |
0.35 (0.22, 0.55) ** |
0.64 (0.38, 1.09) |
| Female | ||||||
| α-carotene (mcg/d) | ||||||
| Q1 (≤17.5) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (17.5 to ≤62.5) | 0.74 (0.45, 1.21) |
0.70 (0.41, 1.20) |
0.85 (0.52, 1.39) |
0.94 (0.56, 1.57) |
1.01 (0.60, 1.71) |
1.22 (0.68, 2.23) |
| Q3 (62.5 to ≤358) | 0.61 (0.38, 0.97) * |
0.55 (0.33, 0.91) * |
0.91 (0.58, 1.43) |
0.98 (0.60, 1.58) |
1.15 (0.71, 1.86) |
1.39 (0.83, 2.41) |
| Q4 (>358) | 0.72 (0.44, 1.17) |
0.66 (0.39, 1.13) |
0.86 (0.53, 1.40) |
0.99 (0.59, 1.65) |
1.17 (0.71, 1.95) |
1.57 (0.87, 2.83) |
| β-carotene (mcg/d) | ||||||
| Q1 (≤338) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (338 to ≤819) | 0.59 (0.37, 0.94) * |
0.64 (0.39, 1.06) |
0.58 (0.37, 0.91) * |
0.65 (0.40, 1.04) |
0.67 (0.43, 1.06) |
0.83 (0.50, 1.40) |
| Q3 (819 to ≤2222.5) | 0.55 (0.36, 0.83) ** |
0.63 (0.40, 1.00) |
0.46 (0.31, 0.70) ** |
0.58 (0.37, 0.91) * |
0.45 (0.30, 0.69) ** |
0.66 (0.41, 1.08) |
| Q4 (>2222.5) | 0.33 (0.20, 0.54) ** |
0.37 (0.21, 0.64) ** |
0.63 (0.41, 0.97) * |
0.81 (0.50, 1.30) |
0.50 (0.32, 0.79) ** |
0.83 (0.49, 1.41) |
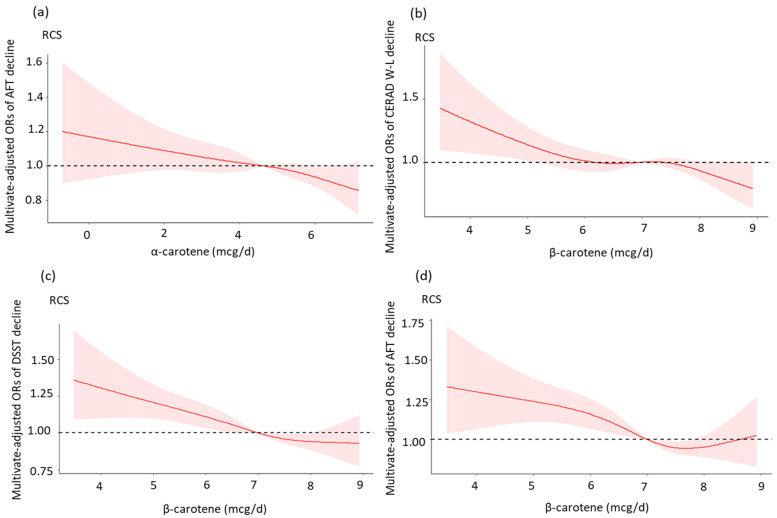
a Model II was adjusted for age, race/ethnicity, BMI, education, marital status, poverty-income ratio, smoking status, alcohol intake, and diabetes. * p < 0.05, ** p < 0.01. Figure 2 presents the results of the restricted cubic spline analysis. For the dietary α-carotene intake, there was a linear dose-response relationship between Ln-transformed α-carotene and the AFT decline. Ln-transformed dietary β-carotene intake was significantly associated with a decreased probability of CERAD W-L decline, showing a reduced possibility of AFT decline and DSST decline (all nonlinear p > 0.05). However, when the Ln-transformed dietary β-carotene intake reached 8.7 mcg/day, the relationship was no longer significant. Furthermore, there were no significant associations between dietary α-carotene intake and cognitive function assessed by the CERAD W-L and DSST, and the results are not shown in the following graph.
4. Discussion
With the growing aging population, dementia has become a worldwide problem [4]. There are many nutritional factors related to cognitive decline [31]. High-fat and high-sugar diets affect neurogenesis and neuroplasticity by decreasing hippocampal brain-derived neurotrophic factor (BDNF) [32,33], which is a vital mediator of long-term memory formation. Oxidative stress is another necessary factor in cellular injury and the activation of neuroinflammation during the aging process [34]. A previous study indicated that supplementation with folic acid improved cognitive function in older people [35]. In an animal experiment, folates along with vitamins B6 and B12 were associated with DNA methylation in neurons [36]. In a randomized, double-blind, placebo-controlled, multicenter trial, a diet with probiotic supplements could shift gut microbiota status, which was inversely associated with BDNF levels in the blood among older people [37]. To date, some studies have examined the associations of α-carotene and β-carotene with cognitive function. However, to our knowledge, most of the previous studies have mainly focused on the plasma level of carotenes with cognitive function [15,38,39]. Since the plasma concentrations are generally maintained within a certain range, it may be difficult to explore the effect of actual intake. Creatively, our study explored the associations of dietary α-carotene and β-carotene intake with cognitive performance using the CERAD W-L, AFT, and DSST.
In this research, we found that dietary α-carotene and β-carotene intake were inversely associated with cognitive function decline. β-carotene intake showed negative relationships with cognitive performance in all three cognitive function tests, while α-carotene intake was only negatively associated with cognitive performance in the ATF. In the RCS results, after adjusting for all potential covariates, there were approximately linear dose-response relationships of β-carotene intake with CERAD W-L, AFT, and DSST decline. In previous studies, we found that a high plasma level of α-carotene in patients was associated with higher cognition function scores [15]. A randomized trial study of 4052 participants reported that the participants had a higher global score in the β-carotene intake group than in the control group. In the verbal memory test, men with durable beta carotene replenishment also had significantly better scores than the control group [40]. Another survey of 298 participants also found that serum β-carotene was significantly associated with cognitive function [44]. Perrig et al. found that a higher β-carotene plasma level was associated with better memory performance in 442 participants aged 65–94 years [41]. However, this relationship in the DSST changed when the Ln-transformed dietary β-carotene intake exceeded 8.7 mcg/day, indicating that dietary β-carotene intake may not have positive effects on cognitive function decline. In a meta-analysis, an overdose of β-carotene intake enhanced the mortality of cancers and other diseases [42].
The mechanism of the effect of dietary carotene intake on cognitive decline remains unclear. In a previous paper, the progression of cognitive decline was related to vascular diseases [43]. Dietary carotene intake reduced the progression of atherosclerosis, stroke, and other oxidative impairments, which are risk factors for cognitive decline [43]. Carotenes are mainly obtained through daily food intake [15], so another hypothesis is related to the antioxidant function of plasma α-carotene and β-carotene through dietary intake, which can promote the formation of gap junctions between cells and can be converted to vitamin A [16]. In previous studies, vitamin A had positive effects on human health, including cognitive function and neurodevelopment [39,45,46]. In contrast, long-term vitamin A deficiency could cause cognitive function decline by affecting the nuclear receptors RXR and RAR (mainly present in the hippocampus, cortex, and caudate) to initiate target gene transcription [47]. Furthermore, a survey of 2983 participants indicated that carotenoid-rich dietary patterns containing α-carotene and β-carotene were associated with better cognitive function consequences in older people [38]. In addition, lutein and zeaxanthin, as parts of carotenoids, can also improve cognitive function [48,49] in either older or younger generations [50,51]. Moreover, dietary foods containing α-carotene and β-carotene are full of vitamin E, selenium, and flavonoids [52] which have benefits on cognitive function decline [53,54,55]. Thus, we cannot exclude the effects of other nutrients in food on cognitive decline without laboratory experiments.
We found differences in cognitive function between genders in our study, which may be related to hormones. A previous study found that levels of plasma β-carotene in men were lower than in women in each tertile of daily intake of fruits and vegetables [56]. In a previous animal experiment, the efficiency of β-carotene conversion to vitamin A in female rats was higher than that in male rats and the difference was related to hormone-regulated genes [57].
This study has several advantages. First, we used a large-scale sample of older adults in the United States. Second, the quality of data in the NHANES could be guaranteed in terms of survey methods and quality control. In addition, we controlled a wide range of potential confounders to estimate the associations of dietary α-carotene and β-carotene intake with cognitive function decline. Our study also contains several limitations. First, due to the cross-sectional nature of the study, we cannot confirm the causal relationships between dietary α-carotene and β-carotene intake and cognitive function. Moreover, although we controlled basic social confounding of carotenes and cognitive function, not all possible confounding factors were included. Furthermore, since the functions of dietary α-carotene on the CERAD W-L and DSST were not observed, further studies are still needed. Finally, the levels of dietary α-carotene and β-carotene intake were collected through the questionnaire survey rather than the measurement in the laboratory. An RCT study combined with laboratory confirmations is still needed to explore more accurate relationships of dietary α-carotene and β-carotene intake with cognitive function in the future.
5. Conclusions
In this study, our results reflected that dietary α-carotene and β-carotene intake might have inverse effects on cognitive function decline in older people. However, the excessive intake of dietary α-carotene and β-carotene may be a problem that needs special attention. Further longitudinal studies and laboratory confirmations are required to confirm these results.
Figure 2.
Dose–response relationships between dietary α-carotene and β-carotene intake and cognitive performance. (a) Restricted cubic spline model of the ORs of the AFT decline with Ln-transformed dietary α-carotene intake; (b) Restricted cubic spline model of the ORs of the CERAD W-L decline with Ln-transformed dietary β-carotene intake; (c) Restricted cubic spline model of the ORs of the DSST decline with Ln-transformed dietary β-carotene intake; (d) Restricted cubic spline model of the ORs of the AFT decline with Ln-transformed dietary β-carotene intake. All models were adjusted for age, gender, race/ethnicity, BMI, education, marital status, poverty-income ratio, smoking status, alcohol intake, and diabetes.
Author Contributions
Conceptualization, Q.Z.; data curation, Q.Z. and W.S.; validation, Q.Z.; formal analysis, Q.Z. and Y.Q.; methodology, Q.Z.; supervision, H.X.; writing—original draft preparation, Q.Z.; writing—review and editing, H.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the National Center for Health Statistics. No further ethical approval is required.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are publicly available on the NHANES website: https://www.cdc.gov/nchs/nhanes/Index.htm (accessed on 10 June 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by the Scientific Research Fund of Zhejiang Provincial Education Department (Y202249200) and the Qiuzhen Excellent Young Talent Program of Hangzhou Medical College (00004F1RCYJ2203). Special thanks to the NHANES for providing the publicly available data.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Soldan A., Pettigrew C., Cai Q., Wang J., Wang M.C., Moghekar A., Miller M.I., Albert M., Team B.R. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol. Aging. 2017;60:164–172. doi: 10.1016/j.neurobiolaging.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory J., Vengalasetti Y.V., Bredesen D.E., Rao R.V. Neuroprotective herbs for the management of Alzheimer’s disease. Biomolecules. 2021;11:543. doi: 10.3390/biom11040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association 2021 Alzheimers disease facts and figures. Alzheimer’s Dement. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 4.Karssemeijer E.G.A., Aaronson J.A., Bossers W.J., Smits T., Olde Rikkert M.G.M., Kessels R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017;40:75–83. doi: 10.1016/j.arr.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Dye L., Boyle N.B., Champ C., Lawton C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017;76:443–454. doi: 10.1017/S0029665117002014. [DOI] [PubMed] [Google Scholar]
- 6.Lovden M., Fratiglioni L., Glymour M.M., Lindenberger U., Tucker-Drob E.M. Education and cognitive functioning across the life span. Psychol. Sci. Public Interest. 2020;21:6–41. doi: 10.1177/1529100620920576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin X., He W., Zhang Y., Gong E., Niu Z., Ji J., Li Y., Zeng Y., Yan L.L. Association of APOE epsilon4 genotype and lifestyle with cognitive function among Chinese adults aged 80 years and older: A cross-sectional study. PLoS Med. 2021;18:e1003597. doi: 10.1371/journal.pmed.1003597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrattan A.M., McGuinness B., McKinley M.C., Kee F., Passmore P., Woodside J.V., McEvoy C.T. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 2019;8:53–65. doi: 10.1007/s13668-019-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S., Sun W., Zhang D. Association of zinc, iron, copper, and selenium intakes with low cognitive performance in older adults: A cross-sectional study from National Health and Nutrition Examination Survey (NHANES) J. Alzheimers Dis. 2019;72:1145–1157. doi: 10.3233/JAD-190263. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Liu K., Zhang X., Wang Y., Liu W., Wang T., Hao L., Ju M., Xiao R. The effect and mechanism of cholesterol and vitamin B12 on multi-domain cognitive function: A prospective study on Chinese middle-aged and older adults. Front. Aging Neurosci. 2021;13:707958. doi: 10.3389/fnagi.2021.707958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono K., Yamada M. Vitamin A and Alzheimer’s disease. Geriatr. Gerontol. Int. 2012;12:180–188. doi: 10.1111/j.1447-0594.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- 12.Woloszynowska-Fraser M.U., Kouchmeshky A., McCaffery P. Vitamin A and retinoic acid in cognition and cognitive disease. Annu. Rev. Nutr. 2020;40:247–272. doi: 10.1146/annurev-nutr-122319-034227. [DOI] [PubMed] [Google Scholar]
- 13.Polcz M.E., Barbul A. The role of vitamin A in wound healing. Nutr. Clin. Pract. 2019;34:695–700. doi: 10.1002/ncp.10376. [DOI] [PubMed] [Google Scholar]
- 14.Harrison E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta. 2012;1821:70–77. doi: 10.1016/j.bbalip.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Dhana K., Furtado J.D., Agarwal P., Aggarwal N.T., Tangney C., Laranjo N., Carey V., Barnes L.L., Sacks F.M. Higher circulating alpha-carotene was associated with better cognitive function: An evaluation among the MIND trial participants. J. Nutr. Sci. 2021;10:e64. doi: 10.1017/jns.2021.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai J.S., Cai S., Lee B.L., Godfrey K.M., Gluckman P.D., Shek L.P., Yap F., Tan K.H., Chong Y.S., Ong C.N., et al. Higher maternal plasma beta-cryptoxanthin concentration is associated with better cognitive and motor development in offspring at 2 years of age. Eur. J. Nutr. 2021;60:703–714. doi: 10.1007/s00394-020-02277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Health and Nutrition Examination Survey Questionnaires, Datasets, and Related Documentation. [(accessed on 8 December 2022)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
- 18.Wang A., Zhao M., Luo J., Zhang T., Zhang D. Association of dietary vitamin K intake with cognition in the elderly. Front. Nutr. 2022;9:900887. doi: 10.3389/fnut.2022.900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Health and Nutrition Examination Survey 2013–2014 Data Documentation, Codebook, and Frequencies. [(accessed on 22 October 2022)]; Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DR1IFF_H.htm.
- 20.Dong X., Li S., Sun J., Li Y., Zhang D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020;12:840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillenbaum G.G., van Belle G., Morris J.C., Mohs R.C., Mirra S.S., Davis P.C., Tariot P.N., Silverman J.M., Clark C.M., Welsh-Bohmer K.A., et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The first twenty years. Alzheimers Dement. 2008;4:96–109. doi: 10.1016/j.jalz.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark L.J., Gatz M., Zheng L., Chen Y.L., McCleary C., Mack W.J. Longitudinal verbal fluency in normal aging, preclinical and prevalent Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement. 2009;24:461–468. doi: 10.1177/1533317509345154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B., Burke J.R., Hurd M.D., Potter G.G., Rodgers W.L., et al. Prevalence of dementia in the United States, the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sotaniemi M., Pulliainen V., Hokkanen L., Pirttila T., Hallikainen I., Soininen H., Hanninen T. CERAD-neuropsychological battery in screening mild Alzheimer’s disease. Acta Neurol. Scand. 2012;125:16–23. doi: 10.1111/j.1600-0404.2010.01459.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosano C., Perera S., Inzitari M., Newman A.B., Longstreth W.T., Studenski S. Digit Symbol Substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing. 2016;45:688–695. doi: 10.1093/ageing/afw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long L.S., Wolpaw J.T., Leung J.M. Sensitivity and specificity of the animal fluency test for predicting postoperative delirium. Can. J. Anaesth. 2015;62:603–608. doi: 10.1007/s12630-014-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu X., Li H., Chen X., Cai J., Yao T., Song L., Cen M., Wu J. Associations between urinary caffeine and caffeine metabolites and cognitive function in older adults. Nutr. Neurosci. 2022 doi: 10.1080/1028415X.2022.2071809. [DOI] [PubMed] [Google Scholar]
- 28.Chen S.P., Bhattacharya J., Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135:963–970. doi: 10.1001/jamaophthalmol.2017.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J., Ma L., Zhao L., Sheng J., Xu Y., Chen J., Yu L., Sun Q., Zhou H., Zhu S., et al. Association between the prognostic nutritional index and cognitive function among older adults in the United States: A population-based study. J. Alzheimers Dis. 2021;83:819–831. doi: 10.3233/JAD-210141. [DOI] [PubMed] [Google Scholar]
- 30.Peeri N.C., Egan K.M., Chai W., Tao M.H. Association of magnesium intake and vitamin D status with cognitive function in older adults: An analysis of US national health and nutrition examination survey (NHANES) 2011 to 2014. Eur. J. Nutr. 2021;60:465–474. doi: 10.1007/s00394-020-02267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulose S.M., Miller M.G., Scott T., Shukitt-Hale B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv. Nutr. 2017;8:804–811. doi: 10.3945/an.117.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molteni R., Barnard R.J., Ying Z., Roberts C.K., Gomez-Pinilla F. A high fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/S0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 33.Park H.R., Park M., Choi J., Park K.Y., Chung H.Y., Lee J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain derived neurotrophic factor. Neurosci. Lett. 2010;482:235. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Simon M., Czeh B., Fuchs E. Age-dependent susceptibility of adult hip pocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049:244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Durga J., van Boxtel M.P., Schouten E.G., Kok F.J., Jolles J., Katan M.B., Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet. 2007;369:208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 36.Kronenberg G., Harms C., Sobol R.W., Cardozo-Pelaez F., Linhart H., Winter B., Balkaya M., Gertz K., Gay S.B., Cox D., et al. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J. Neurosci. 2008;28:7219–7230. doi: 10.1523/JNEUROSCI.0940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim C.S., Cha L., Sim M., Jung S., Chun W.Y., Baik H.W., Shin D.M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76:32–40. doi: 10.1093/gerona/glaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kesse-Guyot E., Andreeva V.A., Ducros V., Jeandel C., Julia C., Hercberg S., Galan P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br. J. Nutr. 2014;111:915–923. doi: 10.1017/S0007114513003188. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Liu S., Man Y., Li N., Zhou Y.U. Effects of vitamins E and C combined with beta-carotene on cognitive function in the elderly. Exp. Ther. Med. 2015;9:1489–1493. doi: 10.3892/etm.2015.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grodstein F., Kang J.H., Glynn R.J., Cook N.R., Gaziano J.M. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007;167:2184–2190. doi: 10.1001/archinte.167.20.2184. [DOI] [PubMed] [Google Scholar]
- 41.Perrig W.J., Perrig P., Stahelin H.B. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 42.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012;3:Cd007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jama J.W., Launer L.J., Witteman J.C.M., Den Breeijen J.H., Breteler M.M.B., Grobbee D.E., Hofman A. Dietary antioxidants and cognitive function in a population-based sample of older persons: The Rotterdam Study. Am. J. Epidemiol. 1996;144:275–280. doi: 10.1093/oxfordjournals.aje.a008922. [DOI] [PubMed] [Google Scholar]
- 44.Johnson E.J., Vishwanathan R., Johnson M.A., Hausman D.B., Davey A., Scott T.M., Green R.C., Miller L.S., Gearing M., Woodard J., et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013;2013:951786. doi: 10.1155/2013/951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Biyong E.F., Alfos S., Dumetz F., Helbling J.C., Aubert A., Brossaud J., Foury A., Moisan M.P., Laye S., Richard E., et al. Dietary vitamin A supplementation prevents early obesogenic diet-induced microbiota, neuronal and cognitive alterations. Int. J. Obes. 2021;45:588–598. doi: 10.1038/s41366-020-00723-z. [DOI] [PubMed] [Google Scholar]
- 47.González R.P., De la Cruz-Góngora V., Rodríguez A.S. Serum retinol levels are associated with cognitive function among community-dwelling older Mexican adults. Nutr. Neurosci. 2022;25:1881–1888. doi: 10.1080/1028415X.2021.1913315. [DOI] [PubMed] [Google Scholar]
- 48.Johnson E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014;72:605–612. doi: 10.1111/nure.12133. [DOI] [PubMed] [Google Scholar]
- 49.Johnson E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012;96:1161S–1165S. doi: 10.3945/ajcn.112.034611. [DOI] [PubMed] [Google Scholar]
- 50.Renzi-Hammond L.M., Bovier E.R., Fletcher L.M., Miller L.S., Mewborn C.M., Lindbergh C.A., Baxter J.H., Hammond B.R. Effects of a lutein and zeaxanthin intervention on cognitive function: A randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients. 2017;9:1246. doi: 10.3390/nu9111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceravolo S.A., Hammond B.R., Oliver W., Clementz B., Miller L.S., Renzi-Hammond L.M. Dietary carotenoids lutein and zeaxanthin change brain activation in older adult participants: A randomized, double-masked, placebo-controlled trial. Mol. Nutr. Food Res. 2019;63:e1801051. doi: 10.1002/mnfr.201801051. [DOI] [PubMed] [Google Scholar]
- 52.Semba R.D., Chang S.S., Sun K., Talegawkar S., Ferrucci L., Fried L.P. Serum carotenoids and pulmonary function in older community-dwelling women. J. Nutr. Health Aging. 2012;16:291–296. doi: 10.1007/s12603-012-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farina N., Llewellyn D., Isaac M.G.E.K.N., Tabet N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017;4:CD002854. doi: 10.1002/14651858.CD002854.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawada T. Selenium intake and cognitive function. Clin. Nutr. ESPEN. 2022;50:338–339. doi: 10.1016/j.clnesp.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Yeh T.S., Yuan C., Ascherio A., Rosner B.A., Willett W.C., Blacker D. Long-term dietary flavonoid intake and subjective cognitive decline in US men and women. Neurology. 2021;97:e1041–e1056. doi: 10.1212/WNL.0000000000012454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bacchetti T., Turco I., Urbano A., Morresi C., Ferretti G. Relationship of fruit and vegetable intake to dietary antioxidant capacity and markers of oxidative stress: A sex-related study. Nutrition. 2019;61:164–172. doi: 10.1016/j.nut.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 57.Borel P., Troadec R., Damiani M., Halimi C., Nowicki M., Guichard P., Margier M., Astier J., Grino M., Reboul E., et al. β-carotene bioavailability and conversion efficiency are significantly affected by sex in rats: First observation suggesting a possible hormetic regulation of vitamin A metabolism in female rats. Mol. Nutr. Food Res. 2021;65:e2100650. doi: 10.1002/mnfr.202100650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are publicly available on the NHANES website: https://www.cdc.gov/nchs/nhanes/Index.htm (accessed on 10 June 2022).