Abstract
Objectives:
To evaluate immunologic response to ART among HIV-infected Nigerian children (<36 months old) and to assess its association with early infant feeding pattern and nutritional status at treatment initiation
Design:
Mixed prospective and retrospective cohort study
Methods:
150 HIV-infected children were followed for 12 months from initiation of ART. CD4 count/CD4% was assessed at baseline and every 4–6 months. Nutritional status was assessed by height-for-age (HAZ), weight-for-age (WAZ) and weight-for-height (WHZ) z-scores using the 2006 WHO growth reference. Children were classified into 4 feeding groups - exclusively breast fed, predominantly breastfed, mixed fed and exclusively formula fed. Logistic regression was used to model odds of failure to reach CD4% of ≥25% at the 12 month follow-up. Linear random effects models were used to model the longitudinal change in CD4%.
Results:
There was a significant increase in CD4% for all children from 13.8% at baseline to 28.5% after 12 months (ΔCD4%=14.7%, 95% CI: 12.1%−17.4%). There was no association of feeding pattern with immunologic outcomes. In adjusted analyses, children who were underweight (WAZ<−2.0) or with CD4% <15% at baseline were 4.30 (95% CI: 1.16, 15.87; p<0.05) times and 3.41 (95% CI: 1.10, 10.52; p<0.05) times, respectively, more likely not to attain CD4% of ≥25% at 12 months.
Conclusion:
Baseline nutritional status and CD4% were independently associated with failure to reach CD4% ≥25% at 12-months among HIV-infected Nigerian children on ART. These results emphasize the importance of early screening and initiation of ART among children in resource-poor settings before malnutrition and severe immuno-suppression sets in.
Keywords: Immunologic outcomes, antiretroviral therapy, HIV-infected children, early infant feeding, nutritional status
In the absence of antiretroviral treatment (ART), there is a progressive decline in CD4 cells of HIV-infected individuals [1], and the immunodeficiency that results leads to increased morbidity from susceptibility to opportunistic infections [2]. In adults and older children, the CD4 cell count (and CD4 percentage in children < 5 years) is used to assess disease progression, the timing of treatment initiation, and for monitoring response to ART [3, 4]. The goal of ART is to slow down or stop disease progression by suppressing HIV replication and reversing immunodeficiency [5], thus immune reconstitution is an important outcome of successful treatment programs.
The vast majority (over 90%) of the world’s HIV-infected children live in resource-poor Sub-Saharan Africa [6], where access to pediatric HIV treatment is relatively new [7]. Despite severe immune suppression and advanced disease stage at treatment initiation common in these resource-poor settings [8], immunologic responses to ART that have been reported among African children are encouraging [9–17] and comparable to those obtained among children in developed countries [1, 5, 18–21]. Most of the children in previous African studies started ART treatment malnourished [11, 13–16], but there is little information on the impact of nutritional status at treatment initiation on immunologic treatment outcomes. Furthermore, an important determinant of nutritional status among children in resource-poor settings is feeding practice during the first six months of life, a factor which has been a dilemma for HIV-positive mothers with respect to prevention of mother-to-child transmission of HIV [22]. The challenge has been how to balance the benefits of breastfeeding with the risk of transmitting HIV through their milk to the infant [22]. The recently revised WHO HIV and infant feeding guidelines [23] recommend exclusive breastfeeding for the first 6 months of life and continued breastfeeding for the first 12 months of life for infants who are HIV uninfected or of unknown HIV status born to mothers known to be HIV-infected. For already infected infants, exclusive breastfeeding for the first six months of life and continued breastfeeding up to two years or beyond is strongly encouraged [23]. With ART now being provided earlier to these infected children [24], the impact of early feeding practice on immunologic treatment outcomes remains to be known.
In the present study, we sought to evaluate the immunologic response to ART over 12 months among HIV-infected Nigerian children under 36 months of age, and assess the association between early infant feeding practice, nutritional status at treatment initiation and immunologic outcomes.
METHODS
HIV-infected children below 36 months of age on ART were recruited from the pediatric HIV clinic at two hospitals in Nigeria - University of Benin Teaching Hospital, Benin City and University of Abuja Teaching Hospital, Abuja. Both sites are part of the AIDS Care and Treatment in Nigeria (ACTION) Project in Nigeria. The ACTION Project is funded by the United States’ President’s Emergency Plan for AIDS Relief (PEPFAR) and implemented by the Institute of Human Virology, Nigeria (IHVN), an affiliate of the University of Maryland’s Institute of Human Virology, Baltimore, Maryland.
This study was mixed prospective and retrospective cohort in design. The inclusion criteria for enrollment into the study were documented HIV infection, age less than 36 months, and mother/caregiver willing to give informed consent. Enrollment of study subjects was from August 2008 to June 2009. All children attending the pediatric HIV clinic at both sites during this time period who met the inclusion criteria were enrolled in the study. Study subjects were recruited from among those just initiating treatment and were followed-up for 12 months; and from among those already on treatment who were then followed from the point of enrollment up to 12 months post-treatment initiation. For children already on treatment, previously collected data at treatment initiation (baseline) and subsequent clinic visits were abstracted from medical records. Informed consent was obtained from the child’s mother or primary caregiver prior to enrollment into the study. Follow-up of study subjects was completed in June 2010.
Before initiation of treatment, children at the pediatric HIV clinic underwent a comprehensive clinical evaluation by a physician and were classified into a disease stage using the WHO staging criteria [3]. Children with advanced disease (stages 3 & 4) were eligible for treatment regardless of age according to the Nigerian national guideline at the time of this study. Age-specific recommendations for initiating treatment based on immunological markers were according to the WHO guidelines [3] in place at the time of data collection. Children <12 months of age with CD4% <25% or CD4 count <1500 cells/mm3 and children ages 12–35 months with CD4% <20% or CD4 count <750 cells/mm3 were eligible for treatment. Following initiation of treatment, children were routinely monitored at monthly or bimonthly clinic visits. All children in the treatment program received cotrimoxazole prophylaxis as part of routine HIV care.
Weight and height/length measurements were taken at treatment initiation (baseline) and during monthly/bimonthly clinic visits by trained staff as part of routine clinical examination. Weight measurements were taken using a Salter baby weighing scale (Model 180, Made in England) and recorded to the nearest 0.1 kg. Older children were weighed using a Seca Digital scale (Model 872). Recumbent length was measured in children <24 months in the supine position using an infant length measuring board/infantometer (Seca Model 416). Standing height was measured in children ≥ 24 months old using a Shorr stadiometer (Irwin Shorr, Olney, MD, USA). Measurements were recorded to the nearest 0.1 cm. Standardization of anthropometric measurements was achieved by taking multiple measurements on non-study subjects prior to commencement of data collection and repeated twice during follow-up to ensure quality of measurements taken. The reliability of height and weight measurements was ≥0.99, the total error of measurement for height ranged from 0.1–0.3 cm, while that for weight ranged from 0 – 0.05 kg.
Infant feeding information of study subjects was collected using an interviewer-administered questionnaire (pre-tested in pilot study). Questionnaire administration was conducted by research assistants and administered once to the mother or primary caregiver on the day of enrollment into the study. The questionnaire was designed to collect detailed information on feeding practices during the first 6 months of the child’s life. Three children were less than 6 months of age at enrollment [mean (SD) age: 4.9(0.3) months], for whom the questionnaire was re-administered when they became older than 6 months. Additional information collected during questionnaire administration include maternal age, parity, marital status, and socioeconomic status variables including level of education, type of employment, residence (rural vs. urban), and housing (own vs. rent).
CD4 cell count/CD4 percentage was assessed before initiation of treatment and every 4–6 months thereafter and abstracted from the child’s medical records. CD4 measurements were done using a Partec CyFlow® Counter (Partec GmbH, Made in Germany). For children already on treatment at enrollment (n=109), previously collected data at treatment initiation and subsequent clinic visits was abstracted from medical records. This included the child’s date of treatment initiation, date of birth, disease stage, CD4 cell count/CD4% and anthropometric measurements at initiation of treatment and at subsequent clinic visits. Data abstraction was done by trained research assistants. Data was manually abstracted from medical records and recorded onto data abstraction forms. The data on the abstraction forms were subsequently entered into an electronic database.
Ethical approvals were obtained from the Ethics and Research Committees of the University of Benin Teaching Hospital and University of Abuja Teaching Hospital, and from the Institutional Review Board of the University of Maryland, Baltimore MD, to which the Institute of Human Virology, Baltimore and Institute of Human Virology, Nigeria are affiliated.
Statistical methods
Height and weight measurements for each child were converted to age- and gender-specific z-scores to obtain height-for-age, weight-for-age and weight-for-height z-scores (HAZ, WAZ & WHZ) using the 2006 WHO Child Growth Standards [25]. Children with HAZ, WAZ and WHZ <−2 at baseline were classified as stunted, underweight and wasted, respectively. Extreme or biologically implausible z-scores at baseline [HAZ <−6 (13/129), WAZ <−6 (6/144) and WHZ <−5 (4/129)] were excluded from the analysis according to the WHO recommendation for data exclusion [25].
Children were grouped into feeding categories based on how they were fed during the first 6 months of life as follows - exclusively breast fed (EBF), predominantly breast fed (PBF), mixed-fed (MF) and exclusively formula fed (EFF). The exclusively breastfed children were those who received breast milk only and no other liquids or solids during the first 6 months of life. The predominantly breastfed children were those whose predominant source of nourishment during the first 6 months was breast milk, but also received non-milk liquids, e.g., water, juice. The mixed-fed group comprised the mixed breast fed (i.e. given breast milk + infant formula or cows’ milk or solids/semi-solids) and mixed formula fed children (i.e. not breastfed but given infant formula + other solids/semi-solids). The exclusively formula fed children received only infant formula during the first 6 months and represents those in line with the WHO replacement feeding recommendation (in avoidance of all breastfeeding) at the time the data for this study was collected [26]. Feeding pattern was coded as an indicator variable with EBF as the reference category.
Baseline characteristics of all children (n=150) were compared between feeding groups using one-way analysis of variance for continuous variables and chi-square tests for categorical variables. There were 2 main outcomes examined – change in CD4 percentage from baseline to 12 months and failure to reach CD4% of 25% or more by 12 months of ART. Only those children with both baseline and 12-month CD4% were included in this analysis (n=88). The mean change in CD4% from baseline to12 months was tested using the paired t-test. Differences in CD4% at baseline and at 12 months between groups were tested by one-way ANOVA. Univariate and multivariable logistic regression was used to model odds of failure to reach CD4% ≥25% at 12-months. Linear random effects modeling which takes into account the correlation between repeated measurements on the same child [27], was used to assess the association between feeding pattern, baseline nutritional status and longitudinal change in CD4% in univariate and multivariable regression analyses. Models were fit with a time interaction for each covariate to assess the rate of change in CD4% from baseline to 6 months, and from 6 months to 12 months. All adjusted analyses included potential confounders including age, gender, disease stage and baseline CD4% and variables that were associated univariately at p<0.10 level. Socioeconomic status variables and maternal demographics did not have any significant effect on the outcomes and were excluded from the regression models. Statistical analyses were conducted using STATA Version 11.1 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics
A total of 150 children were enrolled in the study whose baseline characteristics are summarized overall and by feeding pattern categories in Table, Supplemental Digital Content 1, http://links.lww.com/INF/B480. The mean (SD) age at treatment initiation among children was 14.3 (8.3) months, with 44.7% commencing treatment at less than 12 months of age. Children commenced treatment mostly severely immuno-compromised and malnourished. Their mean (SD) CD4 count at baseline was 778 (532) cells/μL, while mean (SD) CD4% at baseline was 13.7 (6.7) %. About 56% of the children had CD4% < 15%, and 66%, 57% and 27% were stunted, underweight and wasted, respectively. More than half (58%) had advanced disease (Stages 3&4) at treatment initiation. Those with advanced disease were 2.7 (95% CI: 1.1, 6.3; p<0.05), 4.9 (95% CI: 2.2, 10.7; p<0.001) and 6.6 (95% CI: 2.3, 18.9; p<0.001) times more likely to be stunted, underweight and wasted respectively, compared to those with mild disease (data not shown). There were no significant differences in baseline characteristics between feeding groups, except in age at treatment initiation (Table, Supplemental Digital Content 1, http://links.lww.com/INF/B480). In the course of the study, 2 children were transferred to other treatment sites at the request of their mothers, 4 died, and 11 others were lost to follow-up. Thus the attrition rate was 11% (17/150).
Change in CD4 percentage
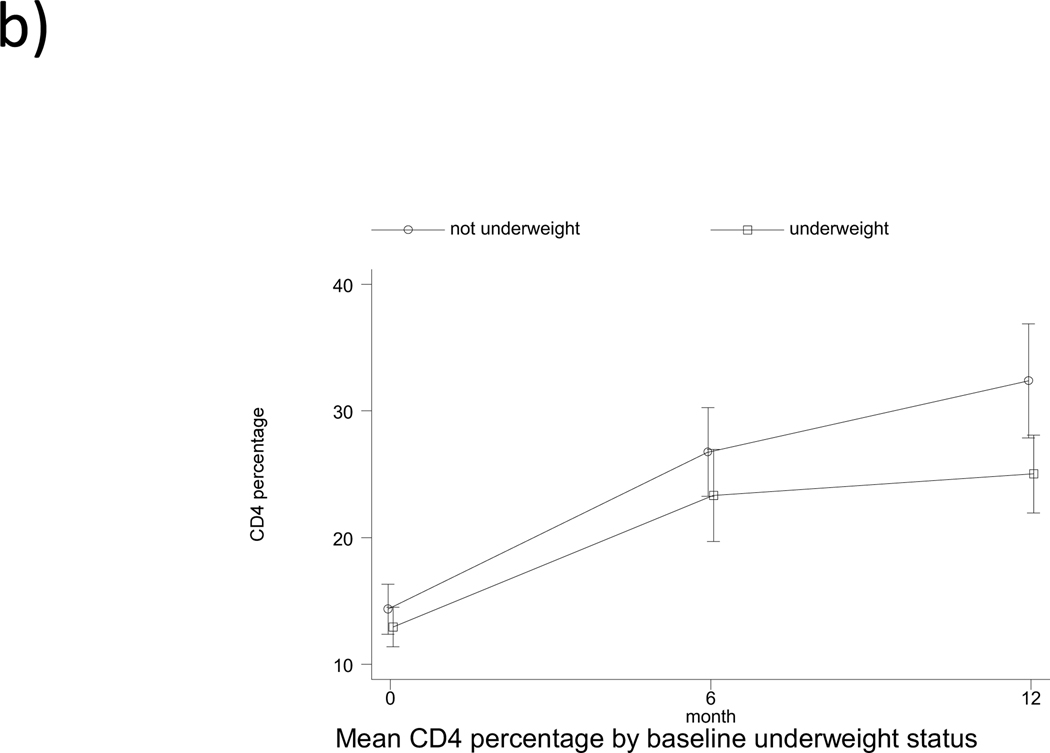
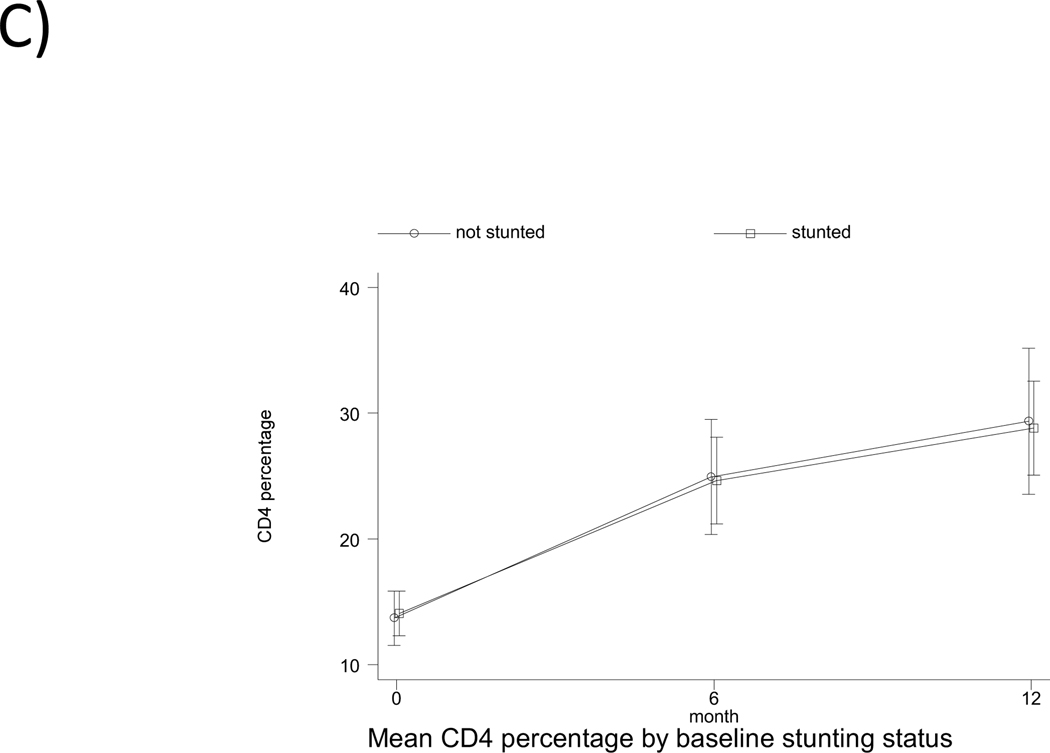
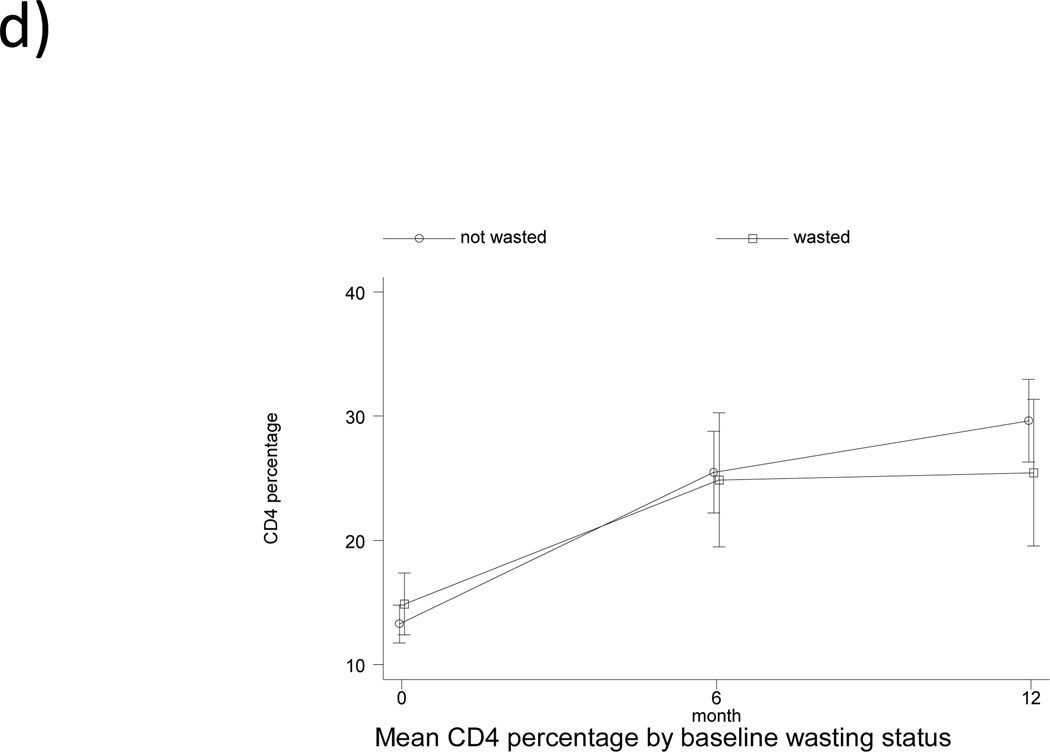
Mean CD4% increased from 13.8% at baseline to 28.5% at 12 months for all children included in the main analysis (n=88), an increase of 14.7% (p<0.001, Table 2). The mean change in CD4% from baseline to 12 months for EBF, PBF, MF and EFF children was 13.1% (p=0.023), 15.3% (p<0.001), 14.8% (p<0.001) and 14.6% (p=0.004), respectively, but there was no difference between feeding categories (p=0.984). Children who had CD4% <15% at baseline had an increase of 15.8%, while those with CD4% ≥15% at baseline had an increase of 13.3% (p<0.001). Children who were underweight at treatment initiation increased in CD4% from 11.9% at baseline to 24.3% at 12 months (p<0.001). Those who were stunted at baseline increased in CD4% from 13.9% at baseline to 28.2% at 12 months (p<0.001), while those who were wasted at baseline increased in CD4% from 14.1% to 23.5% (p<0.01). The increase in CD4% did not differ between groups. However, there was a significant difference in 12-month CD4% by baseline underweight status (p=0.006) and by baseline CD4% category (p=0.02) (Table 2).
Table 2.
Mean increase in CD4 percentage from baseline to 12 months according to child characteristics
| CD4 percentage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | ΔCD4% | ||||||
| N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | p1 | p2 | p3 | |
| All children | 88 | 13.8 (12.4, 15.1) | 88 | 28.5 (25.7, 31.3) | 14.7 (12.1, 17.4) | <0.001 | ||
| Feeding pattern | ||||||||
| EBF | 7 | 15.6 (12.4, 18.8) | 7 | 28.6 (19.5, 37.8) | 13.1 (2.6, 23.6) | 0.023 | 0.984 | 0.624 |
| PBF | 19 | 10.2 (7.7, 12.8) | 19 | 25.5 (20.6, 30.4) | 15.3 (10.8, 19.8) | <0.001 | ||
| MF | 55 | 14.2 (12.4, 16.0) | 55 | 29.0 (25.0, 33.0) | 14.8 (10.9, 18.7) | <0.001 | ||
| EFF | 7 | 18.1 (15.0, 21.3) | 7 | 32.7 (23.6, 41.8) | 14.6 (6.9, 22.3) | 0.004 | ||
| Age at treatment initiation | ||||||||
| <12 | 33 | 14.9 (12.3, 17.5) | 33 | 28.9 (24.4, 33.4) | 14.0 (9.9, 18.1) | <0.001 | 0.672 | 0.823 |
| ≥12 | 55 | 13.1 (11.5, 14.6) | 55 | 28.3 (24.6, 31.9) | 15.2 (11.6, 18.8) | <0.001 | ||
| Disease stage | ||||||||
| Stages 1&2 | 34 | 14.7 (12.2, 17.2) | 34 | 29.4 (23.7, 35.1) | 14.6 (9.1, 20.1) | <0.001 | 0.771 | 0.725 |
| Stage 3 | 38 | 14.1 (12.2, 16.0) | 38 | 26.8 (23.4, 30.3) | 12.7 (9.7, 15.7) | <0.001 | ||
| Stage 4 | 6 | 12.2 (7.4, 17.1) | 6 | 27.7 (14.2, 41.2) | 15.5 (1.1, 29.8) | 0.039 | ||
| Baseline CD4% | ||||||||
| ≥15 | 38 | 18.9 (17.2, 20.6) | 38 | 32.2 (28.2, 36.2) | 13.3 (9.4, 17.2) | <0.001 | 0.350 | 0.020 |
| <15 | 50 | 9.8 (8.7, 11.0) | 50 | 25.7 (21.9, 29.5) | 15.8 (12.1, 19.6) | <0.001 | ||
| Baseline underweight status | ||||||||
| WAZ ≥ −2.00 | 40 | 15.2 (13.0, 17.5) | 40 | 32.5 (27.8, 37.3) | 17.3 (12.5, 22.0) | <0.001 | 0.092 | 0.006 |
| WAZ < −2.00 | 39 | 11.9 (10.2, 13.6) | 39 | 24.3 (20.8, 27.7) | 12.4 (9.1, 15.7) | <0.001 | ||
| Baseline stunting status | ||||||||
| HAZ ≥ −2.00 | 24 | 14.7 (12.0, 17.4) | 24 | 30.0 (23.8, 36.1) | 15.2 (8.6, 21.9) | 0.0001 | 0.788 | 0.611 |
| HAZ < −2.00 | 43 | 13.9 (11.8, 15.9) | 43 | 28.2 (24.1, 32.2) | 14.3 (10.6, 18.0) | <0.001 | ||
| Baseline wasting status | ||||||||
| WHZ ≥ −2.00 | 60 | 13.5 (11.7, 15.2) | 60 | 29.4 (25.8, 33.0) | 15.9 (12.5, 19.4) | <0.001 | 0.106 | 0.152 |
| WHZ < −2.00 | 13 | 14.1 (10.8, 17.3) | 13 | 23.5 (17.4, 29.6) | 9.4 (2.9, 16.0) | 0.009 | ||
p-value for mean increase in CD4% from baseline to 12 months post-treatment initiation
p-value for difference in ΔCD4% between groups
p-value for difference in 12-month CD4% between groups
Abbreviations: EBF, exclusively breastfed; PBF, predominantly breastfed; MF, mixed-fed; EFF, exclusively formula fed; WAZ, weight-for-age z-scores; HAZ, height-for-age z-scores; and WHZ, weight-for-height z-scores.
Failure to attain CD4% ≥25% at 12 months
Overall, 37% of the children did not attain CD4% ≥25% in the first 12 months of ART. There was no significant association of feeding pattern with failure to attain CD4% ≥25% at 12 months in both unadjusted and adjusted analysis (Table 3). Those with CD4% <15% at baseline were 3.41 (95% CI: 1.10, 10.52; p<0.05) times more likely not to attain CD4% ≥25% compared to those with CD4% ≥15%, while those who were underweight (WAZ <−2) at baseline were 4.3 (95% CI: 1.16, 15.87; p<0.05) times more likely to experience failure to reach CD4% ≥25% compared to those not underweight, after adjusting for confounders (Table 3).
Table 3.
Unadjusted and adjusted odds ratio for failure to attain CD4% ≥25% at 12 months
| Factor | Odds Ratio | |
|---|---|---|
| Child characteristics | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Feeding | ||
| EBF | 1.00 | 1.00 |
| PBF | 1.82 (0.28, 11.86) | 1.28 (0.14, 11.52) |
| MF | 1.67 (0.30, 9.37) | 1.26 (0.18, 8.60) |
| EFF | 0.42 (0.03, 6.06) | 1.03 (0.05, 22.99) |
| Age at treatment initiation | ||
| <12 | 1.00 | 1.00 |
| ≥12 | 1.08 (0.44,2.64) | 1.07 (0.28, 4.13) |
| Gender | ||
| Male | 1.00 | 1.00 |
| Female | 0.73 (0.30,1.74) | 1.40 (0.43, 4.59) |
| Baseline CD4% | ||
| ≥15 | 1.00 | 1.00 |
| <15 | 3.75** (1.44, 9.76) | 3.41* (1.10, 10.52) |
| Disease stage | ||
| Stages 1&2 | 1.00 | 1.00 |
| Stage 3 | 0.94 (0.36,2.45) | 0.79 (0.22, 2.82) |
| Stage 4 | 1.62 (0.28,9.23) | 0.60 (0.07, 5.01) |
| Baseline underweight status | ||
| WAZ ≥ −2.00 | 1.00 | 1.00 |
| WAZ < −2.00 | 4.46** (1.68, 11.82) | 4.30* (1.16, 15.87) |
p<0.05
p<0.01
Abbreviations: EBF, exclusively breastfed; PBF, predominantly breastfed; MF, mixed-fed; EFF, exclusively formula fed; WAZ, weight-for-age z-scores.
Factors associated with change in CD4 percentage
The pattern of change in CD4% by feeding pattern and by baseline underweight, stunting and wasting status is shown in Fig. 1. The pattern observed is a rapid increase in CD4% within the first 6 months of ART (overall mean rate of change of 2.02% per month, followed by a slower increase from 6 to 12 months (overall mean rate of change of 0.51% per month. There was no significant association of feeding pattern with change in CD4% from baseline to 6 months, and from 6 to 12 months in both univariate and multivariable analysis (Table, Supplemental Digital Content 2, http://links.lww.com/INF/B481). Being underweight at baseline was associated with a significant 1.12% lower monthly change in CD4% (95% CI: −2.11,−0.14; p<0.05) from baseline to 6 months in the adjusted analysis. Age at treatment initiation and gender were independently associated with change in CD4% from 0–6 months, but not from 6–12 months. Initiating treatment at ≥12 months of age was associated with a 1.30% (95% CI: 0.28, 2.32; p<0.05) greater change in CD4% per month from 0–6 months, after adjusting for confounders (Table, Supplemental Digital Content 2, http://links.lww.com/INF/B481). Compared to male children, females had a 1.56% lower change in CD4% per month (95% CI: −2.52,−0.61; p<0.01) from 0–6 months. Socioeconomic status variables and maternal demographics did not have any significant effect on change in CD4% and were excluded from the analysis.
Fig. 1.
Pattern of change in CD4 percentage from baseline to 12 months according to (a) feeding pattern (b) baseline underweight status (c) baseline stunting status (d) baseline wasting status. EBF denoted exclusive breastfed; PBF, predominantly breastfed; MF, mixed-fed; and EFF, exclusively formula fed.
DISCUSSION
This study describes the immunological response to ART among HIV-infected Nigerian children, and our results confirm the positive impact of ART on immune reconstitution among children in a resource-constrained setting. Significant improvements in CD4% were observed for all children and occurred in all subgroups of feeding pattern, age at treatment initiation, gender, baseline disease stage, CD4% and nutritional status. The overall increase in CD4% among all children in our study and stratified by baseline immunological status are comparable to those reported in pooled analyses of results from other pediatric cohorts in similar resource-constrained settings [28, 29].
We observed that children with severe immuno-suppression (CD4% <15%) at baseline had more than 3 times the odds of failure to attain CD4% ≥25% at 12-months compared to those not severely immuno-suppressed, and only half of these children attained CD4% values above 25% after 12 months of ART. These results emphasize the importance of early initiation of ART prior to severe immuno-suppression [24]. A longer duration of follow-up may be required to determine whether children who commenced treatment with low CD4% are able to normalize their CD4 values, since children have an active thymus [18, 30, 31] and compared to adults have a greater recovery of naïve CD4+ T cells which promotes immune reconstitution when HIV replication is suppressed [32]. However, there is evidence to suggest that even from a long-term perspective, only about one-third of children with the most severe immuno-suppression at baseline will reach normal CD4 percentages, i.e. above 25% [33]. Thus, early initiation of ART is of utmost importance in achieving immune recovery.
The nutritional status of HIV-infected children has been shown to be associated with HIV disease progression [34]. Generally as disease progresses, nutritional status becomes impaired due to increased nutrient requirements, insufficient dietary intake, malabsorption and diarrhea, altered metabolism and impaired nutrient storage [35, 36]. While the beneficial impact of ART on nutritional status and growth of children is well documented [37–39], there is little information on the impact of nutritional status prior to treatment commencement on immunologic response to ART. One South African study reported significant improvements in CD4% among children who commenced treatment moderately and severely underweight, not significantly different from that of their better nourished peers after 24 months of treatment [40]. In our study, we observed a significant increase in CD4% for all children from baseline to 12 months whether or not they were underweight, wasted or stunted at baseline. However, while we found no association of baseline stunting and wasting status with 12-month immunologic outcomes, those who were underweight at baseline had a significantly lower CD4% at 12 months and ~4 times the odds of failure to attain CD4% ≥25% at 12-months compared to those not underweight and independent of CD% at baseline. Although it is possible that the observed association between baseline underweight status and CD4 response at 12 months may be due to under-dosing effect since physicians are trained on weight-based dosing for antiretrovirals, this effect may be greater in the early months following treatment initiation and diminish over time. It is also known that pathophysiological changes in the gut associated with malnutrition can alter pharmacokinetic processes but the exact mechanisms involved are unclear and deserves further study since a large proportion of children initiating treatment in resource-limited settings are underweight (57% in our study), and is likely to be an important risk factor for CD4 response. Thus our finding underscores the need to increase case finding of undernourished children in general and especially among pediatric ART patients. Furthermore, HIV testing and treatment should be integrated into programs treating both moderate and severe malnutrition. Nutrition counseling, including anthropometric and dietary assessment should be integrated into all HIV programs.
In addition to severe immuno-suppression, low WAZ or WHZ has been shown to be associated with poor survival among treated HIV-infected children [41–44]. Another study among untreated HIV-infected children showed rapid declines in CD4 counts after an episode of severe malnutrition [45]. Nutritional status is an important determinant of immune function. Malnutrition is usually a complex syndrome of multiple nutrient deficiencies, and a number of micronutrient deficiencies including vitamin A, B6, iron, copper, selenium and zinc have been shown to impair immune response in malnourished individuals [46]. Micronutrient deficiencies are common in HIV-infected individuals and are associated with a faster rate of disease progression and decline in CD4 counts [47–49]. One randomized placebo-controlled study among 40 HIV-infected adults on HAART reported micronutrient supplementation for 12 weeks significantly increased CD4 cell counts [50]. Possibly macro- and micronutrient supplementation may be beneficial as adjunct to therapy especially among undernourished and immuno-compromised HIV-infected children commencing treatment in resource-poor settings.
We found no association between early feeding pattern and immunologic outcomes suggesting that the significant gain in CD4 was largely driven by ART. It is important to note that the proportion of children exclusively breast or formula fed was small compared to those predominantly breastfed or mixed fed, and thus presents inadequate power to detect differences, if any, in immunologic outcomes between feeding groups. We found that children who initiated treatment when older than 12 months of age had a significantly greater change in CD4% during the first 6 months of treatment compared to those who initiated treatment less than 12 months of age, and that it took the younger children twice as long to achieve the same level of immune reconstitution as the older children, probably due to their more immature immune system [24]. Although the timing of infection for these children could not be ascertained, possibly those who initiated treatment <12 months of age had earlier infection (in utero or intrapartum) and had a faster rate of disease progression compared to the older children and less likely to have survived to one year. An interesting finding was that exclusively breastfed children initiated treatment significantly later compared to predominantly breastfed, mixed breastfed and exclusively formula fed children. This finding suggests that exclusively breastfed children remained healthier longer than those in other feeding groups, underscoring the importance of promoting optimal feeding practices among HIV-positive mothers during their infant’s first 6 months of life.
Our study has a number of limitations. There is the possibility of misclassification of feeding pattern due to recall bias for (mothers of) older children who had a longer time lapse since 6 months of age to enrollment in the study. There is also possible bias from using previously collected data abstracted from medical records in addition to prospective data collection. Furthermore, we did not assess adherence to therapy, an important determinant of treatment response, but difficult to measure in children. Also, given that the treatment regimen for all children in our study included nevirapine, there is the possibility of development of nevirapine-resistant virus and virologic treatment failure as a result of prior exposure to single dose nevirapine as demonstrated in a study among children from several African countries [51]. Nonetheless, the effect of these factors, though not collected, is more likely to be non-differential in relation to nutritional status and feeding pattern. The strength of this study is the younger age of our study population. Most previous studies in similar resource-poor settings have children starting treatment at older ages. Our study thus offers a unique opportunity to evaluate immunologic response to ART among young children in relation to nutritional status and early infant feeding.
In conclusion, this study adds to the growing body of evidence that demonstrates the effectiveness of ART in improving immune status of children in resource-poor settings, despite severe immune suppression and malnutrition at treatment initiation. However, there is an urgent need to scale up and improve linkages between mother-to-child HIV prevention programs, early infant diagnosis and pediatric ART programs so that HIV-infected children are identified early and initiate treatment before severe immune suppression and malnutrition occurs. Stronger integration between HIV care and nutrition care is also needed. Further studies with a longer duration of follow-up are required in similar resource-poor settings to determine the long-term effects of ART and durability of immunologic response in young children followed into adolescence, particularly among those who commence treatment with poor immune and nutritional status.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff at the pediatric HIV clinics of University of Benin Teaching Hospital, Benin City, and University of Abuja Teaching Hospital, Abuja, Nigeria for their help with this study. The authors are also grateful to the mothers and children that were enrolled in the study.
Research funding:
IHV-UM NIH Fogarty AIDS International Training Research Program (AITRP, D43 TW001041)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Resino S, Galan I, Perez A, et al. HIV-infected children with moderate/severe immune-suppression: changes in the immune system after highly active antiretroviral therapy. Clin Exp Immunol 2004; 137: 570 – 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant A. Clinical features of HIV disease in developing countries. Lepr Rev. 2002; 73: 197 – 205. [PubMed] [Google Scholar]
- 3.WHO 2006a. WHO Pediatric HIV Guidelines 2006. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. [Google Scholar]
- 4.WHO 2006b. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2006 Revision. Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf [PubMed]
- 5.Ghaffari G, Passalacqua DJ, Caicedo JL, Goodenow MM, Sleasman JW. Two-Year clinical and immune outcomes in human immunodeficiency virus-infected children who reconstitute CD4 T cells without control of viral replication after combination antiretroviral therapy. Pediatrics 2004; 114: 604 – 611. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS 2010. UNAIDS Report on the Global AIDS epidemic 2010. Available at: http://www.unaids.org/documents/20101123_GlobalReport_em.pdf
- 7.DeBaets AJ, Bulterys M, Abrams EJ, Kankassa C, Pazvakavambwa IE. Care and Treatment of HIV-infected African children: Issues and Challenges at the District Hospital Level. Pediatr Infect Dis J 2007; 26:163 – 173. [DOI] [PubMed] [Google Scholar]
- 8.De Baets AJ, Ramet J, Msellati P, Lepage P. The unique features of pediatric HIV-1 in sub-Saharan Africa. Curr HIV Res. 2008. Jun; 6(4):351–62. [DOI] [PubMed] [Google Scholar]
- 9.Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d’Ivoire. AIDS 2004; 18:1905 – 1913. [DOI] [PubMed] [Google Scholar]
- 10.Rouet F, Fassinou P, Inwoley A, et al. for the ANRS 1244/1278 Programme Enfants Yopougon. Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS 2006; 20: 2315 – 2319. [DOI] [PubMed] [Google Scholar]
- 11.Eley B, Davies M, Apolles P, et al. Antiretroviral treatment for children. S Afr Med J 2006; 96: 988 – 993. [PubMed] [Google Scholar]
- 12.Song R, Jelagat J, Dzombo D, et al. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics 2007; 120(4):e856–61. [DOI] [PubMed] [Google Scholar]
- 13.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr 2007; 45(3):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 2007; 298(16):1888–99. [DOI] [PubMed] [Google Scholar]
- 15.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr 2007;7:13 (Available at: http://www.biomedcentral.com/1471-2431/7/13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaspan HB, Berrisford AE, Boulle AM. Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J 2008; 27(11):993–8. [DOI] [PubMed] [Google Scholar]
- 17.Adjé-Touré C, Hanson DL, Talla-Nzussouo N, et al. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Côte d’Ivoire. AIDS Res Hum Retroviruses. 2008. Jul; 24(7):911–7. [DOI] [PubMed] [Google Scholar]
- 18.Gibb DM, Newberry A, Klein N, de Rossi A, Grosch-Woerner I, Babiker A, Pediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Immune repopulation after HAART in previously untreated HIV-1-infected children. Lancet 2000; 355: 1331–1332. [DOI] [PubMed] [Google Scholar]
- 19.Resino S, Resino R, Micheloud D, et al. on behalf of the Spanish Group of Pediatric HIV infection. Long-term effects of highly active antiretroviral therapy in pretreated, vertically HIV type 1-infected children: 6 years of follow-up. Clin Infect Dis 2006; 42: 862 – 869. [DOI] [PubMed] [Google Scholar]
- 20.Doerholt K, Duong T, Tookey P, et al. and the Collaborative HIV Pediatric Study (CHIPS). Outcomes for human immunodeficiency virus-1-infected infants in the United Kingdom and Republic of Ireland in the era of effective antiretroviral therapy. Pediatr Infect Dis J 2006; 25: 420 – 426. [DOI] [PubMed] [Google Scholar]
- 21.Chiappini E, Galli L, Tovo P, et al. and The Italian Register for HIV Infection in Children. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS 2006; 20: 207 – 215. [DOI] [PubMed] [Google Scholar]
- 22.Coutsoudis A. Infant feeding dilemmas created by HIV: South African Experiences. J Nutr 2005; 1315: 956–959. [DOI] [PubMed] [Google Scholar]
- 23.WHO 2010. Guidelines on HIV and infant feeding 2010. Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva, World Health Organization. [PubMed] [Google Scholar]
- 24.Violari A, Cotton MF, Gibb DM, et al. ; CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008. Nov 20; 359(21):2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO 2006c. WHO Multicentre Growth Reference Study Group (2006). WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; pp 312. (Available at: http://www.who.int/childgrowth/publications/en/) [Google Scholar]
- 26.WHO 2007. HIV and infant feeding: new evidence and programmatic experience: report of a technical consultation held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and their Infants, Geneva, Switzerland, 25–27 October 2006. [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38:963–974. [PubMed] [Google Scholar]
- 28.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis 2009. Dec 15; 49(12):1915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien DP, Sauvageot D, Olson D, et al. Treatment outcomes stratified by baseline immunological status among young children receiving nonnucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in resource-limited settings. Clin Infect Dis 2007; 44: 1245–8. [DOI] [PubMed] [Google Scholar]
- 30.Hainaut M, Ducarme M, Schandene L, et al. Age-related immune reconstitution during highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J 2003. Jan; 22(1):62–9. [DOI] [PubMed] [Google Scholar]
- 31.Walker AS, Doerholt K, Sharland M, Gibb DM for the Collaborative HIV Pediatric Study (CHIPS) Steering Committee. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Pediatric Study. AIDS 2004; 18: 1915 – 1924. [DOI] [PubMed] [Google Scholar]
- 32.Franco JM, León-Leal JA, Leal M, et al. CD4+ and CD8+ T lymphocyte regeneration after anti-retroviral therapy in HIV-1-infected children and adult patients. Clin Exp Immunol 2000. Mar; 119(3):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soh CH, Oleske JM, Brady MT, et al. ; Pediatric AIDS Clinical Trials Group.Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet 2003. Dec 20; 362(9401):2045–51. [DOI] [PubMed] [Google Scholar]
- 34.Berhane R, Bagenda D, Marum L, et al. Growth Failure as a Prognostic Indicator of Mortality in Pediatric HIV Infection. Pediatrics 1997; 100: 7–10. [DOI] [PubMed] [Google Scholar]
- 35.Fiore P, Donelli E, Boni S, Pontali E, Tramalloni R, Bassetti D. Nutritional status changes in HIV-infected children receiving combined antiretroviral therapy including protease inhibitors. Int. J. Antimicrob Agents 2000; 16: 365 – 369. [DOI] [PubMed] [Google Scholar]
- 36.Semba RD, Tang AM Micronutrients and the pathogenesis of human immunodeficiency virus infection. Br J Nutr 1999; 81: 181–189. [DOI] [PubMed] [Google Scholar]
- 37.Aurpibul L, Puthanakit T, Taecharoenkul S, Sirisanthana T, Sirisanthana V. Reversal of Growth Failure in HIV-Infected Thai Children Treated with Non-Nucleoside Reverse Transcriptase Inhibitor–Based Antiretroviral Therapy. AIDS Patient Care STDS 2009; 23(12):1067–71. [DOI] [PubMed] [Google Scholar]
- 38.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008; 22(3):245–51. [DOI] [PubMed] [Google Scholar]
- 39.Kekitiinwa A, Lee KJ, Walker AS, et al. ; Collaborative HIV Paediatric Study (CHIPS) Steering Committee; Mulago Cohort Team. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr 2008; 49(4):384–92. [DOI] [PubMed] [Google Scholar]
- 40.Naidoo R, Rennert W, Lung A, Naidoo K, McKerrow N. The influence of nutritional status on the response to HAART in HIV-infected children in South Africa. Pediatr Infect Dis J 2010; 29(6):511–3. [DOI] [PubMed] [Google Scholar]
- 41.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007; 298(16):1888–99. [DOI] [PubMed] [Google Scholar]
- 42.Bong CN, Yu JK, Chiang HC, et al. Risk factors for early mortality in children on adult fixed-dose combination antiretroviral treatment in a central hospital in Malawi. AIDS 2007. Aug 20; 21(13):1805–10. [DOI] [PubMed] [Google Scholar]
- 43.Rajasekaran S, Jeyaseelan L, Ravichandran N, Gomathi C, Thara F, Chandrasekar C. Efficacy of antiretroviral therapy program in children in India: prognostic factors and survival analysis. J Trop Pediatr 2009. Aug; 55(4):225–32. [DOI] [PubMed] [Google Scholar]
- 44.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART). Ethiop Med J 2010. Jan; 48(1):1–10. [PubMed] [Google Scholar]
- 45.Hughes SM, Amadi B, Mwiya M, et al. CD4 counts decline despite nutritional recovery in HIV-infected Zambian children with severe malnutrition. Pediatrics 2009. Feb; 123(2):e347–51. [DOI] [PubMed] [Google Scholar]
- 46.Chandra RK. Nutritional regulation of immunity and risk of illness. Indian J Pediatr 1989. Sep-Oct; 56(5):607–11. [DOI] [PubMed] [Google Scholar]
- 47.Baum MK, Shor-Posner G, Lu Y, et al. Micronutrients and HIV-1 disease progression. AIDS 1995. Sep; 9(9):1051–6. [DOI] [PubMed] [Google Scholar]
- 48.Tang AM, Graham NM, Chandra RK, Saah AJ. Low serum vitamin B-12 concentrations are associated with faster human immunodeficiency virus type 1 (HIV-1) disease progression. J Nutr 1997. Feb; 127(2):345–51. [DOI] [PubMed] [Google Scholar]
- 49.Bogden JD, Kemp FW, Han S, et al. Status of selected nutrients and progression of human immunodeficiency virus type 1 infection. Am J Clin Nutr 2000. Sep; 72(3):809–15. [DOI] [PubMed] [Google Scholar]
- 50.Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, Baum MK. Micronutrient Supplementation Increases CD4 Count in HIV-Infected Individuals on Highly Active Antiretroviral Therapy: A Prospective, Double-Blinded, Placebo-Controlled Trial. J Acquir Immune Defic Syndr 2006; 42:523–528. [DOI] [PubMed] [Google Scholar]
- 51.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010; Oct 14; 363(16):1510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.