Abstract
We examined the mechanism of resistance against reactivation of infection with Toxoplasma gondii in the brain. BALB/c-background gamma interferon (IFN-γ)-knockout (IFN-γ−/−) and control mice were infected and treated with sulfadiazine beginning 4 days after infection for 3 weeks. After discontinuation of treatment, IFN-γ−/− mice succumbed to toxoplasmic encephalitis (TE) and died, whereas control animals did not develop TE and survived. Adoptive transfer of immune spleen cells from infected control mice did not prevent development of TE or mortality in the IFN-γ−/− mice. To examine whether the failure of the cell transfer to protect against TE is unique to IFN-γ−/− mice, athymic nude and SCID mice that lack T cells were infected and injected with the immune spleen or T cells in the same manner as IFN-γ−/− mice. Whereas control nude and SCID mice that had not received the immune cells developed severe TE and died after discontinuation of sulfadiazine, those that had received the cells did not develop TE and survived. Before cell transfer, IFN-γ mRNA was detected in brains of infected nude and SCID but not in brains of IFN-γ−/− mice. IFN-γ mRNA was also detected in brains of infected SCID mice depleted of NK cells by treatment with anti-asialo GM1 antibody, and such animals did not develop TE after receiving immune T cells. Thus, IFN-γ production by non-T cells, in addition to T cells, is required for prevention of reactivation of T. gondii infection in the brain. The IFN-γ-producing non-T cells do not appear to be NK cells.
Cytokines, peptide hormones, and neurotransmitters, as well as their receptors, are endogenous to the brain, endocrine, and immune system (1, 9, 27), indicating that the immune and neuroendocrine system represents an integrated information circuit. In contrast, the healthy brain is believed to be an immunological privileged site since the brain is able to exclude components of the immune system by the blood-brain barrier (1, 9, 27). Since the brain has specialized cells that produce cytokines and execute immunological effector functions, the brain appears to have a unique mechanism of immune responses. When infection occurs in the brain, lymphocytes infiltrate the organ. Therefore, it is possible that lymphoid cells, the regular components of the immune system, and the specialized cells in the brain for immune responses cooperate in host defense against infection in this organ. However, the mechanism of host defense in the brain remains to be defined.
Toxoplasma gondii, an obligate intracellular protozoan parasite, has provided an excellent model for investigating the mechanism of host defense in the brain. During the acute stage of infection, tachyzoites proliferate within various cells; thereafter, the parasite forms cysts in the brain and establishes a chronic (latent) infection. Persistence of the chronic infection in the brain has been shown to require the host immune system. In immunocompromised individuals, reactivation of the infection may occur and result in life-threatening toxoplasmic encephalitis (TE) (21, 43). However, the mechanism by which the immune system maintains a latent chronic T. gondii infection in the brain remains unclear. Murine models have been used to study the mechanism of host resistance against development of TE. Immune responses mediated by gamma interferon (IFN-γ) (13, 33, 34, 37), tumor necrosis factor alpha (4, 13, 35, 44), and inducible nitric oxide synthase (17, 31) have been shown to be required for prevention of TE, although antibodies are also involved in the resistance (11, 22).
However, each of these studies was performed in strains of mice (C57BL/6 and CBA/Ca) that are genetically susceptible to development of TE and eventually develop progressive and ultimately fatal TE without immunosuppressive treatment (2, 35, 36). In these animals, active infection with tachyzoites appears to continue in the brain during the entire period of infection. Therefore, these animals do not appear to be ideal models for analyzing the mechanism of host resistance to prevent reactivation of a latent (cyst) infection.
In contrast to genetically susceptible strains, genetically resistant strains (e.g., BALB/c) are able to control T. gondii infection in the brain and develop a latent chronic infection as do immunocompetent humans (2, 35, 36). Recently, we developed a murine model of reactivation of T. gondii infection in the brain using infected, sulfadiazine-treated BALB/c-background IFN-γ-knockout (IFN-γ−/−) mice (37). In the present study, we investigated the mechanism of prevention of reactivation of T. gondii infection by using adoptive transfer of immune T cells into infected, sulfadiazine-treated immunodeficient animals including athymic nude, SCID, and IFN-γ−/− mice. We found that the IFN-γ production by non-T cells, in addition to T cells, play a critical role in prevention of reactivation of T. gondii infection in the brain.
MATERIALS AND METHODS
Mice.
Female BALB/c-background IFN-γ−/−, athymic nude, SCID, and control BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, Main). Female Swiss Webster mice were from Taconic Farms (Germantown, N.Y.). Mice were 6 to 8 weeks old when used. There were three to six mice in each experimental group.
Infection with T. gondii.
Cysts of the ME49 strain were obtained from brains of Swiss Webster mice that had been infected intraperitoneally with 10 cysts for 2 to 3 months. Mice were sacrificed by asphyxiation with CO2, and their brains were removed and triturated in phosphate-buffered saline (pH 7.2) (39). An aliquot of the brain suspension was examined for numbers of cysts, and after appropriate dilution in phosphate-buffered saline, animals were infected with 10 cysts perorally by gavage. Mice were treated with sulfadiazine in drinking water (400 mg/liter) beginning 4 days (for IFN-γ−/− mice) or 7 days (for nude and SCID mice) after infection for 3 weeks.
Histopathology.
At 5 or 7 days after discontinuation of treatment with sulfadiazine, mice were euthanized by asphyxiation with CO2. Their brains were removed and immediately fixed in a solution containing 10% formalin, 70% ethanol, and 5% acetic acid. Two to four 5-μm-thick sagittal sections (50 or 100 μm between sections) of the brain from each mouse were stained with hematoxylin and eosin. Immunoperoxidase staining using rabbit immunoglobulin G antibody against tachyzoite-specific SAG2 was used for detection of tachyzoites (3, 23, 37). The specificity of the antibody was described previously (28). Sections stained with hematoxylin and eosin were evaluated for inflammatory changes. Sections stained by the immunoperoxidase method were evaluated for the numbers of areas of inflammation associated with tachyzoites.
Transfer of immune spleen cells and T cells.
Control BALB/c mice infected perorally with 10 cysts for 2 to 3 months were challenged intraperitoneally with 100 cysts. One week after the challenge infection, spleen cells were obtained from two or three mice, suspended in Hanks' balanced salt solution, and pooled. A total of 2 × 107 immune spleen cells were injected intravenously from a tail vein to recipient IFN-γ−/−, nude, or SCID mice at 9 and 2 days before discontinuation of treatment with sulfadiazine. CD4+ and CD8+ T-cell subsets of the immune spleen cells were purified by treating the spleen cells with either magnetic beads-conjugated anti-mouse CD4 (GK 1.5) or anti-mouse CD8 (53-6.7) monoclonal antibody (MAbs) (Miltenyi Biotec, Sunnyvale, Calif.). The purity of the T-cell subset in each of the purified preparations was >98%. Total T-cell population was purified from immune spleen cells by applying both anti-CD4 and anti-CD8 MAbs together. For adoptive transfer of the purified T cells, 107 of the cells were injected intravenously to nude mice in the same manner as a transfer of immune spleen cells. In some experiments, immune spleen cells were obtained from IFN-γ−/− mice that had been infected and treated with sulfadiazine for 3 weeks.
Depletion of NK cells.
Infected SCID mice were injected intraperitoneally with 100 μl of rabbit anti-asialo GM1 antiserum (Wako Pure Chemical Industries Ltd., Osaka, Japan) every 3 days beginning 10 day before discontinuation of sulfadiazine treatment. As a control, mice were injected with normal rabbit serum in the same manner.
Flow cytometry.
At the last day of treatment with sulfadiazine or 7 days after discontinuation of the treatment, mononuclear cells infiltrated into brains or spleen cells of infected SCID mice treated or untreated with anti-asialo GM1 antibody were obtained as described previously (40). The mononuclear cells (106) were pretreated on ice for 10 min with 10 μl of a predetermined optimal concentration of anti-FcγII/III receptors (2.4G2) to block non-antigen-specific binding of antibodies to the FcγII/III receptors. Thereafter, the cells were incubated on ice for 30 min with 10 μl of optimal concentrations of phycoerythrin-conjugated anti-pan NK cell MAb (clone DX5; PharMingen, San Diego, Calif.). Analysis of stained cells was performed with a FACScan (Becton Dickinson, Mountain View, Calif.). Dead cells were gated out on the basis of propidium iodide staining.
Semiquantitative reverse transcription-PCR (RT-PCR) for detection of mRNA for IFN-γ.
At the last day of sulfadiazine treatment, RNA was isolated from brains of infected mice by using RNA STAT-60 (TEL-TEST “B”, Inc., Friendswood, Tex.) as instructed by the manufacturer. cDNA was synthesized using the RNA as described previously (35, 42). PCR for β-actin and IFN-γ was performed with 5 μl of 1:10 dilution of the original cDNA reaction mixture with a GeneAmp 9700 thermocycler (Perkin-Elmer, Emeryville, Calif.), using 30 cycles to produce an amount of DNA within a linear range as described previously (35, 42). This number of cycles was determined in preliminary studies using different amounts of cDNA of the sample. Specific primers for β-actin and IFN-γ (Clontech, Palo Alto, Calif.) designed to span at least one intron allowed differentiation of amplified target DNA derived from either cDNA or genomic DNA in the PCR.
Homology of PCR products to the predicted transcript sequence was examined by Southern blot analysis. Ten-microliter aliquots of the final PCR mixtures were electrophoresed at 100 V for 1 h on a 1.5% agarose gel and denatured (35, 42). The DNA was then transferred to a Duralon-UV membrane (Stratagene, La Jolla, Calif.) by the standard blotting procedure (30) and UV cross-linked. Oligonucleotide probes for β-actin and IFN-γ (Clontech) which hybridize to the PCR products wholly within the region amplified by the primers were end labeled as described for the 3′-end labeling and signal amplification system for a FluorImager (Amersham, Little Chalfont, England), and hybridization was detected by scanning of the membranes with a FluorImager Storm 860 (Molecular Dynamics, Sunnyvale, Calif.) as described previously (40). Quantification of mRNA was performed by densitometry analysis with the FluorImager and normalized to the β-actin level.
Statistical analysis.
Levels of significance for numbers of areas associated with tachyzoites in the brain were determined by the Student t, alternate Welch t, or Wilcoxon rank sum test. The alternative Welch t test was applied when standard deviations were significantly different between groups tested. The Wilcoxon rank sum test was applied when the standard deviation was zero. Levels of significance for mortality in mice were determined using Fisher's exact test. Differences which provided P values of less than 0.05 were considered significant.
RESULTS
Effect of adoptive transfer of immune spleen cells on mortality and development of TE in infected IFN-γ−/− mice.
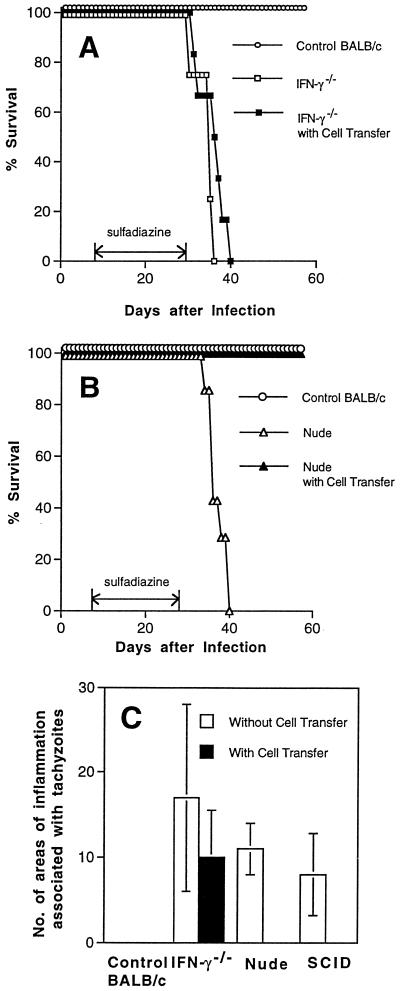
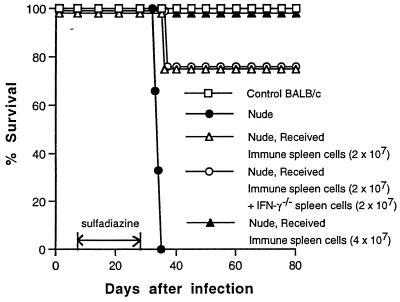
We recently reported that IFN-γ−/− mice that had been infected and treated with sulfadiazine developed severe TE and died after discontinuation of treatment with sulfadiazine (37). We examined whether adoptive transfer of immune spleen cells from infected control mice prevents mortality and development of TE in the IFN-γ−/− mice. Immune spleen cells (2 × 107) were injected intravenously at 9 and 2 days before discontinuation of sulfadiazine. Transfer of the immune spleen cells failed to prevent the development of TE in these animals. Mice that had received the cell transfer all died after discontinuation of sulfadiazine treatment as early as did mice that had not received the cell transfer (Fig. 1A). Many areas of inflammation associated with tachyzoites were observed in brains of both animals with and without the cell transfer (Fig. 1C). The brain was the only organ in which large numbers of T. gondii tachyzoites were detected in the animals with or without the cell transfer, although small numbers of the parasite were detectable in their hearts and lungs (data not shown).
FIG. 1.
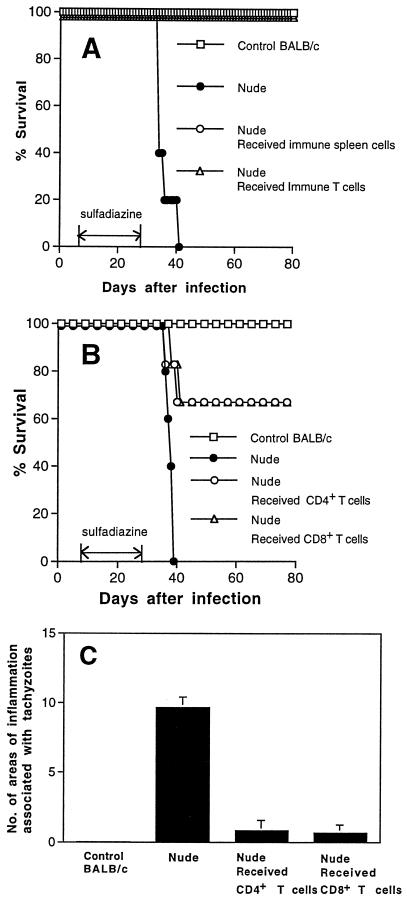
Mortality (A and B) and development of TE (C) in T. gondii-infected, sulfadiazine-treated IFN-γ−/−, nude, and SCID mice with and without adoptive transfer of immune spleen cells. Mice (A, six mice in each group; B, seven mice in each group; C, four mice in each group) were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 4 days (IFN-γ−/−) or 7 days (nude and SCID) after infection. Nine and 2 days before discontinuation of sulfadiazine treatment, mice received an intravenous injection of 2 × 107 immune spleen cells from infected, control BALB/c mice (see Materials and Methods). Histological studies were performed 5 days (IFN-γ−/− mice) or 7 days (nude and SCID mice) after discontinuation of treatment with sulfadiazine. Two to four sagittal sections (distance between sections of 50 μm) of the brain were stained with immunoperoxidase stain by using tachyzoite-specific SAG2 antibody and evaluated for counting. The mean value from these sections for each mouse was calculated as the number per section. The data shown are pooled from two independent experiments (A and B) or representative of two separate experiments (C).
Effect of adoptive transfer of immune spleen cells on mortality and development of TE in infected athymic nude and SCID mice.
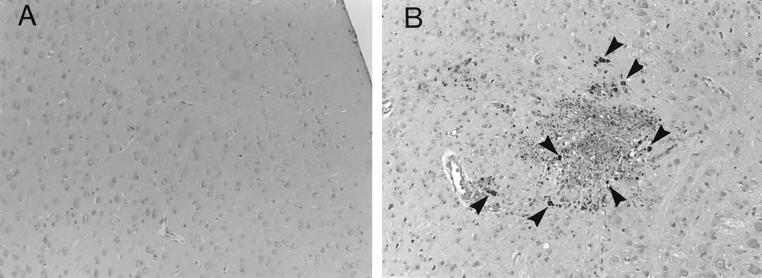
Since the failure of adoptive transfer of immune spleen cells to prevent the development of TE in IFN-γ−/− mice was unexpected, we examined whether the cell transfer confers resistance to development of TE in other immunodeficient strains of mice. Athymic nude and SCID mice were infected, treated with sulfadiazine, and injected with immune spleen cells. Nude mice that had not received the cell transfer developed necrotizing TE and died after discontinuation of sulfadiazine treatment (Fig. 1B, 1C and 2B). In contrast, nude mice that had received the immune spleen cells did not develop TE and survived (P < 0.001 for mortality and P < 0.05 for histology [Fig. 1B, 1C, and 2A]). SCID mice that had received the immune spleen cells also did not develop TE, whereas control SCID mice that had not received the cells developed severe TE (P < 0.05 [Fig. 1C]). Thus, adoptive transfer of immune spleen cells can confer resistance against development of TE in infected nude and SCID mice but not in IFN-γ−/− mice. These results suggest that IFN-γ production by non-T cells in the recipient animals is required for passively transferred immune spleen cells to demonstrate their protective activity against TE.
FIG. 2.
Histological changes in brains of T. gondii-infected, sulfadiazine-treated athymic nude mice with (A) or without (B) adoptive transfer of immune spleen cells. Mice were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 7 days after infection. Nine and 2 days before discontinuation of sulfadiazine treatment, mice received an intravenous injection of 2 × 107 immune spleen cells from infected, control BALB/c mice (see Materials and Methods). Histological studies were performed 7 days after discontinuation of treatment with sulfadiazine. Sections were stained with immunoperoxidase stain with tachyzoite-specific anti-SAG2 antibody (dark-stained dots [some are marked by arrowheads] in panel B indicate collections of tachyzoites). The experiments were performed three times, and there were three or four mice in each group in each experiment.
Expression of IFN-γ mRNA in brains of infected nude and SCID mice.
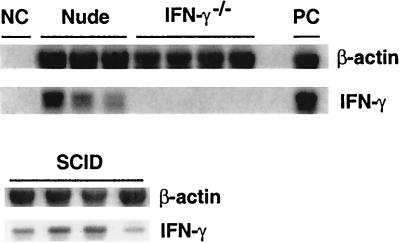
We examined whether IFN-γ was expressed in the brains of infected, sulfadiazine-treated nude and SCID mice. Amounts of IFN-γ mRNA in total RNA fractions obtained from brains of mice were measured by semiquantitative RT-PCR at the last day of sulfadiazine treatment without adoptive transfer of immune spleen cells. mRNA for IFN-γ was detected in brains of either of these animals (Fig. 3). In contrast, IFN-γ mRNA was not detected, as expected, in brains of infected IFN-γ−/− mice (Fig. 3), nor was it detectable in brains of uninfected nude or SCID mice (data not shown). Thus, adoptive transfer of immune spleen cells prevented TE in immunodeficient animals that express IFN-γ in the brain before receiving the immune spleen cells but not in those that did not express IFN-γ.
FIG. 3.
Expression of IFN-γ mRNA in brains of T. gondii-infected, sulfadiazine-treated IFN-γ−/−, nude, and SCID mice. Mice were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 4 days (IFN-γ−/−) or 7 days (nude and SCID) after infection. At the last day of the treatment, the brains were analyzed for amounts of β-actin and IFN-γ mRNAs (see Materials and Methods). NC, negative control; PC, positive control. There were three or four mice in each experimental group. The data shown are representative of two separate experiments.
IFN-γ mRNA levels in brains of infected IFN-γ−/− and nude mice after adoptive transfer of immune spleen cells.
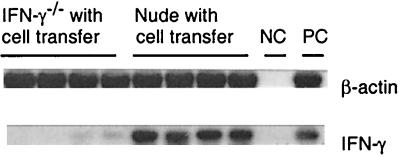
At 5 days after discontinuation of sulfadiazine treatment, expression of IFN-γ mRNA was examined in brains of infected IFN-γ−/− and nude mice that had received immune spleen cells. Whereas large amounts of IFN-γ mRNA were detected in brains of the nude mice, no mRNA or only trace amounts were detected in brains of the IFN-γ−/− mice (Fig. 4). These results suggest that passively transferred immune spleen cells which produce IFN-γ had hardly infiltrated into the brains of the IFN-γ−/− mice.
FIG. 4.
Expression of IFN-γ mRNA in brains of T. gondii-infected, sulfadiazine-treated IFN-γ−/− and nude mice after adoptive transfer of immune spleen cells. Mice were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 4 days (IFN-γ−/−) or 7 days (nude) after infection. Nine and 2 days before discontinuation of sulfadiazine treatment, mice received an intravenous injection of 2 × 107 immune spleen cells from infected, control BALB/c mice (see Materials and Methods). At 5 days after discontinuation of sulfadiazine, the brains were analyzed for amounts of β-actin and IFN-γ mRNAs (see Materials and Methods). NC, negative control; PC, positive control. There were four mice in each experimental group.
Inability of spleen cells of infected IFN-γ−/− mice to inhibit the protective activity of immune spleen cells.
The immune responses in IFN-γ−/− mice might have been altered due to the absence of IFN-γ, and such altered immune response, in addition to the absence of IFN-γ, might have contributed to the failure to prevent the development of TE following adoptive transfer of immune spleen cells in these animals. To assess this possibility, we examined whether spleen cells of infected IFN-γ−/− mice can inhibit the protective effect of immune spleen cells. Immune spleen cells from infected control mice were mixed with the same number of spleen cells from infected IFN-γ−/− mice, and 4 × 107 mixed spleen cells were transferred into infected, sulfadiazine-treated nude mice. Nude mice that had received the mixed spleen cells survived after discontinuation of sulfadiazine, as did nude mice that had received only immune spleen cells (either 2 × 107 or 4 × 107 cells) from the control mice (P < 0.05 [Fig. 5]). Control nude mice that received no immune spleen cells all died (Fig. 5). These results indicate that spleen cells of infected IFN-γ−/− mice do not inhibit the protective activity of the immune spleen cells. Thus, lack of IFN-γ production appears to be the major cause of the failure of IFN-γ−/− mice to prevent reactivation of T. gondii infection even after adoptive transfer of immune spleen cells.
FIG. 5.
Mortality in T. gondii-infected, sulfadiazine-treated nude mice that received immune spleen cells with and without addition of spleen cells from infected IFN-γ−/− mice. Nude mice were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 7 days after infection. Spleen cells were obtained from infected IFN-γ−/− mice that had been treated with sulfadiazine for 3 weeks beginning at 4 days after infection. Their spleen cells were mixed with the same number of immune spleen cells obtained from infected, control BALB/c mice, and a total of 4 × 107 cells were injected intravenously into infected nude mice at 9 and 2 days before discontinuation of sulfadiazine. Other groups of infected nude mice received either 2 × 107 or 4 × 107 immune spleen cells only. There were four to six mice in each experimental group.
T cells as the protective component of immune spleen cells.
NK and B cells do not appear to be the protective components of immune spleen cells which prevent development of TE in the recipient animals, since nude mice, without adoptive transfer of immune spleen cells, have these cells but develop TE. To examine whether T cells are the protective cells among the immune spleen cells, T cells were purified from immune spleen cells and transferred into infected, sulfadiazine-treated nude mice. The animals that had received the purified T cells survived after discontinuation of sulfadiazine, as did those that had received whole immune spleen cells (P < 0.01 [Fig. 6A]). Furthermore, an adoptive transfer of either CD4+ or CD8+ T-cell populations of immune spleen cells prevented mortality and development of TE in infected nude mice (P < 0.01 for mortality and P < 0.001 for histology [Fig. 6B and C]).
FIG. 6.
Mortality (A and B) and development of TE (C) in T. gondii-infected, sulfadiazine-treated nude mice with adoptive transfer of immune T cells. Nude mice (A, five mice in each group; B, seven or eight mice in each group; C, three or four mice in each group) were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 7 days after infection. Nine and 2 days before discontinuation of sulfadiazine treatment, mice received an intravenous injection of 107 immune T cells (A) or either CD4+ or CD8+ subsets of immune T cells (B and C) purified from immune spleen cells (see Materials and Methods). The data shown are representative of two separate experiments (A and C) or pooled from two independent experiments (B).
Effect of NK cell depletion on the development of TE in SCID mice that received immune spleen cells.
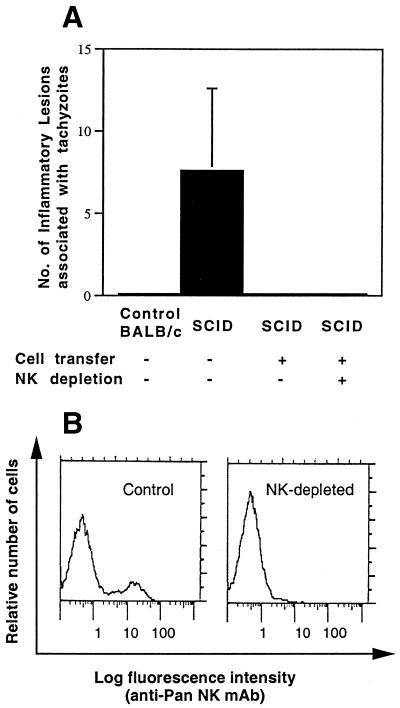
Next, we examined whether IFN-γ-producing non-T cells that are required in the recipient mice for prevention of TE are NK cells, since NK cells have been reported to produce IFN-γ during the acute stage of T. gondii infection (15, 20, 32). In this experiment, we used SCID mice, instead of nude mice, as the recipients because the brains of nude mice may have small numbers of thymus-independent T cells. Infected SCID mice were treated with sulfadiazine and received immune spleen cells in combination with treatment with anti-asialo GM1 antibody to deplete NK cells. Histological study of the brains was performed 7 days after discontinuation of sulfadiazine. Many areas of inflammation associated with tachyzoites were observed in brains of control SCID mice that had not received immune spleen cells (Fig. 7A). In contrast, brains of SCID mice that had received immune spleen cells with or without treatment with anti-asialo GM1 antibody exhibited no inflammatory changes or tachyzoites (P < 0.05 [Fig. 7A]). Depletion of NK cells in the treated mice was confirmed by the absence of NK cells in their spleens 7 days after discontinuation of sulfadiazine (Fig. 7B). Thus, it appears that NK cells are not required for prevention of reactivation of T. gondii infection in the brain.
FIG. 7.
(A) Effect of depletion of NK cells on development of TE in T. gondii-infected, sulfadiazine-treated SCID mice with adoptive transfer of immune spleen cells. SCID mice were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 7 days after infection. Nine and 2 days before discontinuation of sulfadiazine treatment, mice received an intravenous injection of 2 × 107 immune spleen cells from infected, control BALB/c mice (see Materials and Methods). To deplete NK cells, mice were injected intraperitoneally with 100 μl of rabbit anti-asialo GM1 antiserum every 3 days beginning 1 day before the first transfer of immune spleen cells. Histological studies were performed 7 days after discontinuation of treatment with sulfadiazine. Two to four sagittal sections (distance between sections of 50 μm) of the brain were stained with an immunoperoxidase stain by using tachyzoite-specific SAG2 antibody and evaluated for counting. The mean value from these sections for each mouse was calculated as the number per section. There were four mice in each experimental group. The data are representative of two independent experiments. (B) Absence of NK cells in the spleens of NK cell-depleted SCID mice with adoptive transfer of immune spleen cells. SCID mice were infected, treated with sulfadiazine, and injected with immune spleen cells as described above. With or without treatment with anti-asialo GM1 antiserum as described above, their spleens were examined for presence or absence of NK cells by flow cytometry at 7 days after discontinuation of sulfadiazine treatment (see Materials and Methods). Essentially identical results were obtained in each of four mice in each experimental group. The data are representative of two independent experiments.
Expression of IFN-γ in brains of infected, NK cell-depleted SCID mice.
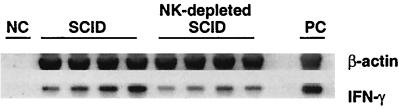
Since adoptive transfer of immune T cells conferred resistance to development of TE in NK-depleted SCID mice, we examined whether NK-depleted SCID mice express IFN-γ in the brain before receiving immune T cells. Infected, sulfadiazine-treated SCID mice were treated with anti-asialo GM1 antibody to deplete NK cells. NK cells were undetectable by flow cytometry in brains of the NK-depleted animals at the last day of sulfadiazine treatment (proportion of NK cells in lymphocyte preparations isolated from brains of the treated versus untreated animals, < 0.1% [n = 8] versus 2.0% ± 1.1% [n = 8]; P < 0.005). Despite the absence of NK cells in the anti-asialo GM1-treated mice, significant amounts of mRNA for IFN-γ were detected in their brains. The amounts of the RNA did not significantly differ between the treated and untreated mice, although the mRNA detected tended to be less in the treated than untreated animals (IFN-γ/β-actin ratio, 0.13 ± 0.05 versus 0.30 ± 0.18; P = 0.16) (Fig. 8). These results suggest that cells that are neither T nor NK cells express significant amounts of IFN-γ in the brains of infected SCID mice.
FIG. 8.
Detection of IFN-γ mRNA in brains of T. gondii-infected, NK-depleted SCID mice. SCID mice were infected with 10 cysts of the ME49 strain perorally and treated with sulfadiazine for 3 weeks beginning 7 days after infection. To deplete NK cells, mice were injected intraperitoneally with 100 μl of rabbit anti-asialo GM1 antiserum every 3 days beginning 10 days before discontinuation of sulfadiazine treatment. Their brains were examined for amounts of mRNA for β-actin and IFN-γ at the last day of sulfadiazine treatment. NC, negative control; PC, positive control.
DISCUSSION
This study revealed that T cells require non-T cells that produce IFN-γ to prevent reactivation of T. gondii infection in the brains in BALB/c mice. Adoptive transfer of immune spleen or T cells prevented development of TE in either athymic nude or SCID mice that had been infected and treated with sulfadiazine but not in IFN-γ−/− mice that had been infected and treated with sulfadiazine in the same manner. Nude and SCID mice that lack T cells have previously been shown to have T-cell-independent IFN-γ production during the acute stage of T. gondii infection, and such IFN-γ production confers a partial resistance to the infection (15, 19, 36). In the present study, large amounts of IFN-γ mRNA were detected in brains of infected nude and SCID mice that had not received adoptive transfer of immune T cells but not in brains of infected IFN-γ−/− mice. Our experiments indicate that the adoptive transfer of immune T cells conferred resistance against development of TE only in the recipient mice that express IFN-γ in the brain before receiving the cell transfer. Thus, a combination of T cells with non-T cells that produce IFN-γ is required for prevention of reactivation of infection in the brain. This is the first evidence of the importance of IFN-γ production by non-T cells for resistance to T. gondii during the chronic stage of infection and for prevention of reactivation of infection.
In regard to the failure of IFN-γ−/− mice to prevent the development of TE, this study demonstrated that spleen cells of infected IFN-γ−/− mice did not inhibit the protective activity of the immune spleen cells. Therefore, the failure to prevent the development of TE by adoptive transfer of immune T cells in IFN-γ−/− mice is due mostly, if not entirely, to lack of activity to produce IFN-γ in these animals. In infected IFN-γ−/− mice that had received immune T cells, the brain was the only organ in which large amounts of T. gondii tachyzoites were detected after discontinuation of sulfadiazine. Thus, IFN-γ- production by non-T cells that is required for T cells to prevent TE is most likely within the brain itself.
NK cells have previously been shown to produce IFN-γ during acute infection with T. gondii (15, 20, 32), and depletion of NK cells in SCID or immunocompetent mice by treatment with anti-asialo GM1 antibody resulted in early mortality following an acute lethal infection (15, 19). In contrast to these results in the acute stage of infection, the present study demonstrated that depletion of NK cells in SCID mice by treatment with the antibody did not reduce their resistance to reactivation of chronic infection when they received immune T cells. In these studies, NK cells were undetectable by fluorescence-activated cell sorting (FACS) in the depleted animals. Thus, it appears that the roles of NK cells in resistance to T. gondii infection differ between the acute and chronic stages and that NK cells are not required for prevention of reactivation of chronic infection in the brain.
This study demonstrated the presence of significant amounts of IFN-γ mRNA by semiquantitative RT-PCR in brains of infected, NK-depleted SCID mice in which NK cells were undetectable by FACS. Furthermore, the amounts of the RNA detected did not significantly differ between the NK-depleted and control SCID mice, in which 2% of inflammatory cells infiltrated into the brain were NK cells. Although the mRNA detected in the NK-depleted mice may include those derived from extremely small numbers of NK cells that were undetectable by FACS, these results suggest that cells that are neither T nor NK cells express significant amounts of IFN-γ mRNA in brains of infected SCID mice. Since NK-depleted SCID mice prevented the development of TE after receiving immune T cells, IFN-γ expressed by such non-T, non-NK cells seems to be sufficient for collaboration with T cells to prevent reactivation of infection in the brain. The identity of the IFN-γ-producing non-T cells in this study is unclear. Microglia and astrocytes may produce IFN-γ, since these cells obtained from neonatal rats have been shown to express IFN-γ mRNA in vitro (7). Neumann et al. (25) reported that cultured fetal rat dorsal root ganglion neurons produce IFN-γ in vitro. Neurons may be a source of IFN-γ in brains of infected mice. Dendritic cells have also been reported to produce IFN-γ in vitro (12, 26). Fischer et al. (10) recently reported the presence of dendritic-type cells in brains of T. gondii-infected mice.
There are two possible mechanisms by which T cells require non-T cells that produce IFN-γ in the brain to prevent TE. First, immune T cells need to collaborate with IFN-γ-producing non-T cells within the brain to control the parasite. We have previously reported the requirement of IFN-γ for genetic resistance of BALB/c mice against development of TE (37). A combination of both T and non-T cells may be required to provide sufficient amounts of IFN-γ to control the parasite in the brain. T cells may have other functions (e.g., cytotoxic activity) besides IFN-γ production that aid in the prevention of TE, in addition to IFN-γ production by non-T cells. In this regards Denkers et al. (6) reported that cytotoxic activity of T cells mediated by perforin plays only a limited role in resistance to T. gondii infection in C57BL/6 mice genetically susceptible to TE. However, it is possible that the cytotoxic activity of T cells plays a more critical role in prevention of TE in genetically resistant BALB/c mice than in susceptible C57BL/6 mice, hence BALB/c mice are resistant to TE.
The second possibility is that IFN-γ production by non-T cells in the brain is required for T cells to infiltrate the brain. Adhesion molecules on T cells and their endothelial ligands play a critical role in regulating T-cell trafficking into the organs (16, 24, 29). IFN-γ has been shown to induce or enhance expression of adhesion molecules on the vascular endothelial cells in vitro (8, 18). Deckert-Schlüter et al. (5) recently reported that IFN-γ plays an important role in induction of ICAM-1 and VCAM-1 on cerebral vascular endothelial cells in C57BL/6 mice infected with T. gondii. IFN-γ production by non-T cells within the brain may be crucial for induction and upregulation of the adhesion molecules, required for infiltration of the protective T cells, on the cerebral vascular endothelial cells. In relation to this possibility, in the present study we detected low amounts of IFN-γ mRNA in the brains of IFN-γ−/− mice even after adoptive transfer of immune spleen cells. It appears that passively transferred immune cells that produce IFN-γ hardly infiltrated into brains of the recipient IFN-γ−/− mice. In contrast, numerous inflammatory cells were detected in areas associated with tachyzoites in brains of these animals. It may be that the mechanisms of infiltration of T cells into the brain differ among their subsets or subpopulations in the infected mice and that IFN-γ production by non-T cells within the brain is important for induction of infiltration of the protective subpopulation(s) of T cells.
This study revealed the importance of two different types of cells, T cells and IFN-γ-producing non-T cells, for genetic resistance of BALB/c mice to reactivation of T. gondii infection in the brain. In regard to responder cells to IFN-γ, Yap et al. (45) recently reported that both hematopoietic and nonhematopoietic cells need to be activated by this cytokine for controlling persistent infection with T. gondii in genetically susceptible C57BL/6 mice. Thus, host resistance against development of TE acts in concert with multiple types of cells involving at least T cells, IFN-γ-producing non-T cells, and two types of effector cells activated by IFN-γ.
ACKNOWLEDGMENTS
This work was supported by University of California Universitywide AIDS Research Program R00-PAM-015 and Public Health Service grant AI04717.
We thank Jack S. Remington for his support and helpful suggestions, and we thank Edgar Gufwoli and Pauline Chu for excellent technical assistance with histological studies.
REFERENCES
- 1.Blalock J E. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–511. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 2.Brown C R, Hunter C A, Estes R G, Beckmann E, Forman J, David C, Remington J S, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 3.Conley F K, Jenkins K A, Remington J S. Toxoplasma gondii infection of the central nervous system: use of the peroxidase-antiperoxidase method to demanstrate toxoplasma in formalin-fixed paraffin embedded tissue section. Hum Pathol. 1981;12:690–698. doi: 10.1016/s0046-8177(81)80170-0. [DOI] [PubMed] [Google Scholar]
- 4.Deckert-Schlüter M, Bluethmann H, Rang A, Hof H, Schlüter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- 5.Deckert-Schlüter M, Bluethmann H, Kaefer N, Rang A, Schlüter D. Interferon-γ receptor-mediated but not tumor necrosis factor receptor type 1- or type 2-mediated signaling is crucial for the activation of cerebral blood vessel endothelial cells and microglia in murine toxoplasma encephalitis. Am J Pathol. 1999;154:1549–1561. doi: 10.1016/s0002-9440(10)65408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkers E Y, Yap G, Scharton-Kersten T, Charest H, Butcher B A, Casper P, Heiny S, Sher A. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol. 1997;159:1903–1908. [PubMed] [Google Scholar]
- 7.De Simone R, Levi G, Aloisi F. Interferon gamma gene expression in rat central nervous system glia cells. Cytokine. 1998;10:418–422. doi: 10.1006/cyto.1997.0314. [DOI] [PubMed] [Google Scholar]
- 8.Elfont R M, Griffin D E, Goldstein G W. Enhanced endothelial cell adhesion of human cerebrospinal fluid lymphocytes. Ann Neurol. 1995;38:405–413. doi: 10.1002/ana.410380310. [DOI] [PubMed] [Google Scholar]
- 9.Fabry Z, Raine C S, Hart M N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994;15:218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 10.Fischer H-G, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–4834. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 11.Fukao T, Matsuda S, Koyasu S. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-γ production by dendritic cells. J Immunol. 2000;164:64–71. doi: 10.4049/jimmunol.164.1.64. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel J K, Taylor D W. Toxoplasmosis in immunoglobulin M-suppressed mice. J Immunol. 1982;38:360–367. doi: 10.1128/iai.38.1.360-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 14.Gazzinelli R T, Hakim F T, Hieny S, Sheaper G M, Sher A. Synergistic role of CD4+ and CD8+ T-lymphocyte in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 15.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanninen A, Taylor C, Streeter P R, Stark L S, Sarte J M, Shimizu J A, Simell O, Michie S A. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Investig. 1993;92:2509–2525. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi S, Chan C, Gazzinelli R, Roberge F G. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- 18.Hughes C C, Male D K, Lantos P L. Adhesion of lymphocytes to cerebral microvascular cells: effect of interferon-γ, tumor necrosis factor and interleukin-1. Immunology. 1988;64:677–681. [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter C A, Candolfi E, Subauste C, Van Cleave V, Remington J S. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology. 1995;84:16–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter C A, Subauste C S, Van Cleave V H, Remington J S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israelski D M, Remington J S. Toxoplasmosis in the non-AIDS immunocompromised host. Curr Top Infect Dis. 1993;13:322–356. [PubMed] [Google Scholar]
- 22.Kang H, Remington J S, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-γ, TNF-α, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 23.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T cell-dependent, interferon-γ-mediated necrosis of small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobb R, Hession C, Osborn L. Vascular cell adhesion molecule-1. In: Cochrane C G, Gimbrone M A Jr, editors. Cellular and molecular mechanisms of inflammation. Vol. 2. San Diego, Calif: Academic Press; 1991. pp. 151–170. [Google Scholar]
- 25.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon γ gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, Koyasu S. Interleukin 12-dependent interferon γ production by CD8α+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens T, Renno T, Taupin V, Krakowski M. Inflammatory cytokines in the brain: does the CNS shape immune responses? Immunol Today. 1994;15:566–571. doi: 10.1016/0167-5699(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 28.Parmley S F, Yang S, Harth G, Sibley L D, Scharczuk A, Remington J S. Molecular characterization of a 65-kilodalton Toxoplasma gondii antigen expressed abundantly in the matrix of tissue cysts. Mol Biochem Parasitol. 1994;66:283–296. doi: 10.1016/0166-6851(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 29.Rothlein R, Barton R W, Winquist R. The role of intercellular adhesion molecule-1 (ICAM-1) in the inflammatory response. In: Cochrane C G, Gimbrone M A Jr, editors. Cellular and molecular mechanisms of inflammation. Vol. 2. San Diego, Calif: Academic Press; 1991. pp. 171–181. [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sher A, Oswald I O, Hieny S, Gazzinelli R T. Toxoplasma gondii induces a T-independent IFN-γ response in NK cells which requires both adherent cells and TNF-α. J Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 33.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 34.Suzuki Y, Joh K. Effect of the strain of Toxoplasma gondii on the development of toxoplasmic encephalitis in mice treated with antibody to interferon-gamma. Parasitol Res. 1994;80:125–130. doi: 10.1007/BF00933779. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Joh K, Kwon O-C, Yang Q, Conley F K, Remington J S. MHC class I gene(s) in the D/L region but not the TNF-α gene determines development of toxoplasmic encephalitis in mice. J Immunol. 1994;153:4649–4654. [PubMed] [Google Scholar]
- 36.Suzuki Y, Joh K, Orellana M A, Conley F K, Remington J S. A gene(s) within H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991;74:732–739. [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki Y, Kang H, Parmley S, Lim S, Park D. Induction of tumor necrosis factor-α and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-γ in genetically resistant BALB/c mice. Microbes Infect. 2000;2:455–462. doi: 10.1016/s1286-4579(00)00318-x. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki Y, Kobayashi A, Ohtomo H. Presence of interferon-gamma-mediated resistance against Toxoplasma gondii in T cell-deficient mice. Jpn J Parasitol. 1993;42:507–510. [Google Scholar]
- 39.Suzuki Y, Orellana M A, Wong S-Y, Conley F K, Remington J S. Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection. Infect Immun. 1993;61:2284–2288. doi: 10.1128/iai.61.6.2284-2288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen T A, Dalrymple S A, Murray R, Remington J S. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997;65:2339–2345. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki Y, Remington J S. Effect of anti-IFN-γ antibody on protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 42.Suzuki Y, Yang Q, Yang S, Nguyen N, Lim S, Lisnfeld O, Kojima T, Remington J S. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–2569. [PubMed] [Google Scholar]
- 43.Wong S Y, Remington J S. Toxoplasmosis in the setting of AIDS. In: Broder S, Merigan T C Jr, Bolognesi D, editors. Textbook of AIDS medicine. Baltimore, Md: Williams & Wilkins; 1994. pp. 223–257. [Google Scholar]
- 44.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]
- 45.Yap G S, Sher A. Effector cells of both nonhematopoietic and hematopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1091. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]