Abstract
Asteraceae species Tanacetum balsamita L. (costmary) is renowned for its traditional usage as an aromatic, carminative and tonic plant. This work aimed at in-depth study of the phytochemical and in vitro biological profilings of methanol–aqueous extracts from the costmary leaves, flower heads and roots. An UHPLC-HRMS analysis revealed more than 100 secondary metabolites including 24 acylquinic acids, 43 flavonoid glycosides, aglycones and methoxylated derivatives together with 15 phenolic acids glycosides. For the first time, 91 compounds are reported in the costmary. The flower heads extract possessing the highest content of total phenolics and flavonoids, actively scavenged DPPH (84.54 ± 3.35 mgTE/g) and ABTS radicals (96.35 ± 2.22 mgTE/g), and showed the highest reducing potential (151.20 and 93.22 mg TE/g for CUPRAC and FRAP, respectively). The leaves extract exhibited the highest inhibition towards acetyl- and butyrylcholinesterase (2.11 and 2.43 mg GALAE/g, respectively) and tyrosinase (54.65 mg KAE/g). The root extract inhibited α-glucosidase (0.71 ± 0.07 mmol ACAE/g), α-amylase (0.43 ± 0.02 mmol ACAE/g) and lipase (8.15 ± 1.00 mg OE/g). At a concentration >2 µg/mL, a significant dose dependent reduction of cell viability towards THP-1 monocyte leukemic cells was observed. Costmary could be recommended for raw material production with antioxidant and enzyme inhibitory properties.
Keywords: Tanacetum balsamita, secondary metabolites, antioxidant properties, enzyme inhibitory activity, cytotoxic activity, UHPLC-HRMS
1. Introduction
The use of plants as sources of drugs and secondary metabolites has been attracting scientific attention over the past decades, considering not only the well-known medicinal species but also plants used in traditional medicines and a variety of edible plants. Tanacetum balsamita L. (costmary) is renowned for its traditional use as flavor, carminative and cardiotonic in the Mediterranean, Balkan and South American countries [1]. The species is distributed in the South-East of Europe and South-West of Asia but has also been widely naturalized throughout the whole world [2,3]. T. balsamita is commonly referred to as: costmary, balsam herb, alecost, sweet tongue and bible leaf. The plant is cultivated in Iran, Turkey, Romania, Germany, Italy, Spain and England [1]. It has a traditional usage as aromatic plant in Europe and Asia. Fresh and dried costmary leaves possess a strong lemony-minty flavor and a sweet astringent taste. The dried leaves have a long history of application as flavorings in soups and meats, sausages and cakes, as well as for making tonic tea. Costmary leaves have been used in ethnopharmacological approach as a hepatoprotective, tonic, sedative, pain relief and astringent agent [1]. The analgesic, anti-inflammatory, antimicrobial and antioxidant activity of the essential oil and extracts support the traditional claims regarding the costmary use for the alleviation of inflammatory diseases [4,5,6,7,8]. Venskutonis [7] and Hassanpouraghdam et al. [1] reviewed the phytochemistry and application of costmary and highlighted that it may be considered as a forgotten plant with potential for development of functional food ingredients.
Costmary is well known for the essential oil secreted in the glandular trichomes. Overall, a total of 186 compounds have been reported in the essential oils from aerial parts, flower heads and stems [7]. According to their main constituents, four chemotypes were distinguished: carvone, camphor, camphor–thujone, and camphor-α-thujone [1]. Moreover, bornyl acetate-pinocarvone chemotype of T. balsamita spp. balsamitoides was established by Jaimand and Rezaee [9]. A high level of carvone was evidenced in costmary leaves essential oils (51–80% in the total oil content) [6,8,10,11,12,13], while α-thujone and β-thujone reached 16% and 84%, respectively [11,14]. Even though the various protective effects of carvone, being the most abundant constituents in the majority of the costmary essential oils, it is worth noting that α-thujone and β-thujone are toxic monoterpenes. Thus, great care should be taken regarding the internal use of α-thujone and β-thujone rich essential oils of costmary. EMA (European Medicinal Agency) and EC (European Commission) recommended a maximum uptake between 3 and 7 mg thujone/day [15]. The cytotoxicity of T. balsamita spp. balsamita essential oil towards human fetal skin fibroblast (HFSF) and monkey kidney cells (Vero) expressed as IC50 was 2500 ± 1.9 µg/mL and 1250 ± 1.4 μg/mL, respectively [8].
Costmary contains caffeoylquinic acids, methoxylated flavonoids and sesquiterpene lactones [5,14,16,17]. For instance, chlorogenic acid, 3, 5-dicaffeoylquinic acid, 5, 7, 4’-trihydroxy-3’,8-dimethoxyflavon and 5,7,3’,4’-tetrahydroxy-3,8-dimethoxyflavonol were isolated from costmary aerial parts [17]. Benedec et al. [16] and Baczek et al. [14] determined by HPLC-MS/DAD several caffeic acid derivatives and flavonoids in the ethanol-aqueous extracts from aerial parts. Cichoric acid dominated the polyphenols, being present in 3.33 g/100 g dry extract [14], while rutin reached 8.04 mg/g dry material [16]. Diosmetin and acacetin glycosides were also reported [5]. Sesquiterpene lactones of eudesmanolide and C-9β-hydroxylated or esterified germacranolide type have been isolated from cultivated costmary [18,19].
In vitro antioxidant, antimicrobial and cytotoxic activity of costmary essential oils and extracts has been reported [5,7,8,14,16]. Overall, the authors concluded that costmary had a great potential for pharmaceutical and food industry, notably for nutraceuticals.
It is worth noting that the aforementioned studies highlight the essential oil composition and antimicrobial effect, while there is no in-depth metabolic profile of costmary leaves and flower heads by liquid chromatography-high-resolution mass spectrometry (LC-HRMS) integrated with an assessment of antioxidant, enzyme inhibitory and cytotoxic potential. Recently, metabolic and biological profilings of T. macrophyllum and T. vulgare integrated with multivariate data analysis provided a new insight into the taxa for designing health-promoting applications [20,21].
Species of the Tanacetum genus are of economic importance. T. parthenium, T. macrophyllum and T. vulgare are cultivated worldwide as ornamental plants and raw material for food, pharmaceutical and agricultural use [22,23,24,25].
As a part of our ongoing investigation on Tanacetum [20,21,25], in this study we aimed at an in-depth metabolite and biological profiling, including antioxidant, enzyme inhibitory and cytotoxic activity of methanol–aqueous extracts from T. balsamita leaves, flower heads and roots. Multivariate data analyses were applied to determine possible correlations between chemical compositions and results from the biological assays of the tested extracts.
2. Results and Discussion
2.1. Secondary Metabolite Profiling of Tanacetum Balsamita Extracts
Based on our previous study on the solvent efficiency in the phenolic and flavonoid extraction from T. vulgare [20], methanol–aqueous extracts from T. balsamita flower heads, leaves and roots were obtained and the total phenolic (TPC) and flavonoid (TFC) contents were determined (Table 1). The highest TPC and TFC were found in the flower heads (59.75 ± 0.66 mg GAE/g and 41.02 ± 0.50 mg QE, respectively), while the lowest values were determined in the leaves (30.82 ± 0.16 mg GAE/g for TPC) and roots (3.74 ± 0.07 mg QE for TFC). It is worth noting that the results revealed a higher amount in both studied classes compared to those in Chrysanthemum balsamita var. tanacetoides aerial parts, where 2.92 g GAE% and 1.19 g RE% were evidenced [16]. Overall, our results were in the same order of magnitude or lower than TPC and TFC reported in flower heads and aerial parts from T. poterifolim, T. macrophyllum, T. vulgare and T. parthenium with a Turkish and a Bulgarian provenance [20,21,25,26] and higher than those in T. corymbosum, T. vulgare and T. macrophyllum from Romanian origin [27]. In line with the results, the accumulation of phenolic compounds could be firmly connected to the plant ontogenetic development and they could be related differently to each plant organs [28]. Therefore, the diverse qualitative and quantitative content of the bioactive compounds reveal different potential and effects.
Table 1.
Total phenolic and flavonoid contents of the T. balsamita extracts.
| Samples | TPC (mg GAE/g) | TFC (mg QE/g) |
|---|---|---|
| Leaves | 30.82 ± 0.16 c | 18.97 ± 0.44 b |
| Roots | 43.41 ± 0.30 b | 3.74 ± 0.07 c |
| Flower heads | 59.75 ± 0.66 a | 41.02 ± 0.50 a |
Values are reported as mean ± SD of three parallel experiments. GAE: Gallic acid equivalent; QE: Quercetin equivalents; TE: Trolox equivalent. Different letters indicate significant differences in the tested extracts (p < 0.05).
As it was previously reported, Tanacetum species contain a wide range of hydroxycinnamic esters and flavonoid derivatives [20,21,24,25,26,29]. To assess the secondary metabolites, non-targeted metabolic profiling of the hydroxycinnamic acids, flavones, flavonols and flavanones of each methanol–aqueous extract was carried out by UHPLC-Orbitrap-HRMS. The parameters of Full-scan ddMS2 mode were adjusted to favor the formation of diagnostic fragment ions for the subclasses of phenolic acids derivatives, acylquinic acids (AQA) and flavonoids. Fragmentation patterns along with exact masses of precursor ions in negative and positive (for flavonoids) ionization mode were depicted in Table 2 and Table S1. Thus, based on the fragmentation patterns and characteristic ions and authentic standards, fragmentation keys for recognition of AQA and methoxylated flavonoids were generated.
Table 2.
Secondary metabolites in Tanacetum balsamita extracts assayed by UHPLC-ESI-MS/MS.
| No | Identified/Tentatively Annotated Compound |
Molecular Formula | Exact Mass [M-H]− |
Fragmentation Pattern in (-) ESI-MS/MS | tR (min) |
Δ ppm | Distribution |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic, Hydroxycinnamic and Acylquinic Acids, and Derivatives | |||||||
| 1 | protocatechuic acid-O-hexoside b | C13H16O9 | 315.0727 | 315.0724 (100), 153.0180 (26.8), 152.0101 (61.7), 123.0071 (3.4), 109.0287 (9.5), 108.0200 (92.3) | 1.72 | 0.840 | 1,2,3 |
| 2 | hydroxybenzoic acid-pentosylhexosideb | C18H23O12 | 431.1198 | 431.1198 (63.96), 137.0230 (100), 93.0329 (77.34) | 1.83 | 0.582 | 2 |
| 3 | protocatechuic acid a,b | C7H6O4 | 153.0182 | 153.0180 (17.8), 125.0228 (0.4), 109.0279 (100), 91.0174 (1.7), 81.0329 (1.8) | 2.05 | −8.574 | 1,2,3 |
| 4 | protocatechuic acid-O-hexoside isomer b | C13H16O9 | 315.0753 | 315.0729 (37.5), 153.0544 (100), 123.0436 (54.5), 109.0279 (34.8) | 2.15 | 0.840 | 1,2,3 |
| 5 | p-hydroxyphenylacetic acid 1-O-hexoside b | C14H18O8 | 313.0727 | 313.0941 (1.1), 151.0387 (100), 121.0281 (3.4), 107.0486 (98.6) | 2.17 | 4.022 | 1,2 |
| 6 | syringic acid a | C9H10O5 | 197.0455 | 197.0444 (25.1), 182.0210 (100), 166.9974 (26.1), 153.0547 (5.3), 138.0309 (16.7), 123.0072 (46.0), 95.0122 (15.4) | 2.29 | −5.819 | 1,2,3 |
| 7 | syringic acid 4-O-hexoside b | C15H20O10 | 359.0984 | 359.0986 (8.2), 197.0446 (100), 182.0210 (18.1), 153.0543 (14.5), 138.0308 (27.2), 123.0072 (29.5) | 2.30 | 0.362 | 2,3 |
| 8 | neochlorogenic (3-caffeoylquinic) acid a,b | C16H18O9 | 353.0867 | 353.0879 (43.1), 191.0550 (100), 179.0338 (62.8), 173.0442 (3.2), 161.0232 (3.5), 135.0437 (53.1), 93.0330 (4.9), 85.0278 (9.3) | 2.38 | 0.240 | 1,2,3 |
| 9 | caffeic acid- O-hexoside b | C15H18O9 | 341.0867 | 341.0867 (4.27), 179.0338 (100), 135.0436 (0.91), 107.0485 (0.91) | 2.42 | −3.153 | 1,2,3 |
| 10 | vanillyl-O-hexose b | C14H18O9 | 329.0875 | 329.0875 (100), 329.0674 (5.0), 209.0448 (32.0), 167.0338 (46.7), 152.0105 (4.8), 123.0433 (4.3) | 2.50 | −1.049 | 2,3 |
| 11 | gentisic acid-O-hexoside b | C13H16O9 | 315.0727 | 315.0723 (38.1), 153.0183 (68.4), 135.0071 (3.8), 109.0279 (100), 91.0174 (0.5), 65.0380 (6.4) | 2.58 | 0.555 | 2,3 |
| 12 | aesculetin-O-hexoside b | C15H15O9 | 339.0724 | 339.0723 (24.7), 177.0182 (100), 149.0227 (1.4), 133.0280 (10), 105.0330 (3.9), 89.0381 (1.9) | 2.69 | 0.781 | 1,2 |
| 13 | vanillic acid a,b | C8H8O4 | 167.0350 | 167.0337 (100), 137.0230 (4.9), 123.0437 (21.8), 152.0092 (0.2), 108.0201 (100), 95.0486 (1.9) | 3.03 | −7.735 | 1,2 |
| 14 | caffeoylgluconic acid b | C15H18O10 | 357.0827 | 357.0835 (16.6), 195.0501 (100), 179.0339 (45.0), 177.0394 (11.2), 165.0398 (0.5), 147.0284 (4.8), 135.0437 (42.24), 129.0180 (6.1), 87.0072 (8.7), 59.0123 (1.9) | 2.82 | 2.185 | 2,3 |
| 15 | O-caffeoyl hexose b | C15H18O9 | 341.0867 | 341.0875 (20.5), 281.0665 (77.4), 251.0557 (37.6), 221.0449 (31.7), 179.0338 (100), 161.0231 (45.3), 135.0437 (60.1), 111.0438 (8.7) | 2.83 | −1.012 | 1,2,3 |
| 16 | 4-hydroxybenzoic acid a,b | C7H6O3 | 137.0230 | 137.0230 (100), 119.0126 (1.8), 108.0202 (7.3), 93.0330 (3.7), 65.0380 (0.9) | 2.86 | −10.052 | 1,2,3 |
| 17 | p-hydroxyphenylacetic acid-O-hexoside isomer b | C14H18O8 | 313.0936 | 313.0934 (13.6), 151.0387 (100), 123.0070 (1.1), 109.0281 (2.7) | 3.01 | 1.754 | 2 |
| 18 | hydroxybenzoic acid-O-hexoside b | C13H16O8 | 299.0778 | 299.0779 (1.6), 137.0230 (100), 93.0330 (53.4) | 3.02 | 2.238 | 2,3 |
| 19 | dihydroxyphenylacetic acid-O-pentosylhexoside b | C22H21O11 | 461.1115 | 461.1115 (10.80), 281.0454 (15.56), 167.0337 (100), 149.0230 (82.64), 123.6436 (73.22), 108.0199 (29.20), 95.0486 (7.49) | 3.03 | 5.520 | 1,2 |
| 20 | caffeoylgluconic acid isomer b | C15H18O10 | 357.0827 | 357.0830 (1.7), 195.0502 (100), 179.0338 (6.6), 177.0398 (5.1), 161.0230 (4.0), 135.0437 (10.5), 129.0179 (14.8), 87.0072 (5.2), 59.0123 (3.4) | 3.07 | 0.812 | 2,3 |
| 21 | caffeic acid-O-hexoside isomer b | C15H18O9 | 341.0867 | 341.0879 (27.9), 179.0338 (100), 135.0437 (71.2), 107.0487 (1.0) | 3.12 | 0.160 | 1,2,3 |
| 22 | quinic acid b | C7H12O6 | 191.0561 | 191.0550 (100), 173.0446 (2.0), 155.0337 (0.3), 127.0386 (3.8), 111.0436 (1.8), 93.0330 (5.9), 85.0278 (19.8) | 3.19 | −5.921 | 1,2,3 |
| 23 | chlorogenic (5-caffeoylquinic) acid a | C16H18O9 | 353.0867 | 353.0880 (5.1), 191.0550 (100), 173.0444 (1.4), 161.0232 (1.7), 127.0385 (1.9), 111.0435 (1.0), 93.0330 (3.3), 85.0278 (8.7) | 3.19 | 0.495 | 1,2,3 |
| 24 | caffeic acid-O-hexoside b | C15H18O9 | 341.0867 | 341.0878 (10.4), 179.0338 (100), 135.0436 (73.3), 107.0488 (0.8) | 3.27 | −0.104 | 1,2,3 |
| 25 | coumaric acid-O-hexoside b | C15H18O8 | 325.0930 | 325.0930 (6.1), 163.0385 (53.5), 135.0435 (0.4), 119.0486 (100) | 3.32 | 0.305 | 1,2,3 |
| 26 | p-hydroxyphenylacetic acid-O-hexoside isomer b | C14H18O8 | 313.0934 | 313.0932 (5.1), 151.0386 (10.2), 107.0486 (100) | 3.31 | 0.988 | 2 |
| 27 | p-coumaric acid a | C9H8O3 | 163.0389 | 163.0387 (3.4), 135.0072 (1.1), 119.0486 (100) | 3.35 | −8.510 | 1,2 |
| 28 | 4-caffeoylquinic acid a,b | C16H18O9 | 353.0867 | 353.0880 (31.1), 191.0554 (45.9), 179.0341 (68.1), 173.0446 (100), 135.0439 (52.6), 111.0437 (2.8), 93.0332 (20.7), 85.0280 (8.0) | 3.36 | 0.551 | 1,2,3 |
| 29 | caffeoylgluconic acid isomer b |
C15H18O10 | 357.0827 | 357.0820 (5.0), 195.0652 (100), 179.0541 (0.2), 177.0410 (1.0), 135.0437 (601), 59.0123 (11.6) | 3.41 | −2.100 | 2,3 |
| 30 | 3-feruloylquinic acid b | C17H20O9 | 367.1035 | 367.1028 (22.3), 193.0496 (100), 191.0556 (2.5), 173.0443 (4.5), 134.0358 (48.1), 127.0389 (0.5), 93.0329 (1.8) | 3.43 | −1.921 | 2 |
| 31 | p-hydroxyphenylacetic acid a,b | C8H8O3 | 151.0401 | 151.0386 (100), 107.0486 (0.59), 136.0154 (0.48), 123.0072 (4.00) | 3.47 | −9.715 | 2 |
| 32 | caffeic acid a | C9H8O4 | 179.0338 | 179.0339 (21.1), 135.0436 (100), 117.0330 (0.6), 107.0487 (1.3) | 3.54 | −6.211 | 1,2,3 |
| 33 | gentisic acid a | C7H6O4 | 153.0182 | 153.0180 (84.5), 135.0073 (32.7), 125.0233 (0.4), 109.0279 (100), 91.0173 (6.1), 81.0331 (0.4), 65.0380 (18.9) | 3.84 | −8.901 | 2,3 |
| 34 | 5-p-coumaroylquinic acid b | C16H18O8 | 337.0929 | 337.0932 (9.3), 191.0549 (100), 173.0444 (7.1), 163.0388 (6.6), 119.0487 (5.3), 111.0436 (2.9), 93.0329 (17.5), 85.0278 (4.7) | 3.96 | 1.096 | 2,3 |
| 35 | 3-hydroxy-dihydrocaffeoyl-5-caffeoylquinic acid b | C25H26O13 | 533.1301 | 533.1298 (100), 371.0992 (19.1), 353.0880 (16.7), 191.0551 (84.2), 179.0339 (66.7), 161.0236 (4.9), 135.0437 (88.2), 93.0329 (15.5) | 4.05 | −0.570 | 2,3 |
| 36 | 5-feruloylquinic acid b | C17H20O9 | 367.1035 | 367.1035 (18.8), 191.0550 (100), 173.0443 (11.8), 155.0336 (0.5), 134.0360 (9.0), 111.0435 (4.1), 93.0329 (22.1) | 4.41 | −0.015 | 2,3 |
| 37 | dihydroxyphenylacetic acid b | C8H8O4 | 167.0341 | 167.0344 (1.3), 137.0230 (2.0), 123.0436 (19.3), 108.0200 (100) | 4.41 | −5.520 | 1,2,3 |
| 38 | 1-caffeoyl-3-hydroxy-dihydrocaffeoylquinic acid b | C25H26O13 | 533.1301 | 533.1313 (27.6), 371.0985 (49.1), 353.0902 (4.3), 335.0750(2.0), 191.0551 (13.4), 179.0342 (11.8), 173.0444 (23.0), 161.0232 (2.5), 135.0436 (100), 111.0436 (1.6), 93.0330 (8.0) | 4.45 | 2.412 | 2,3 |
| 39 | coumaric acid-O-hexoside isomer b | C15H18O8 | 325.0931 | 325.0930 (1.6), 163.0387 (100), 119.0487 (98.8) | 4.45 | 0.398 | 2,3 |
| 40 | m-coumaric acid a,b | C9H8O3 | 163.0389 | 163.0387(2.86), 135.0434 (11.34), 119.0487 (100) | 4.46 | −7.651 | 2,3 |
| 41 | o-coumaric acid a,b | C9H8O3 | 163.0389 | 163.0387 (170), 135.0436 (11.3), 119.0487 (100) | 4.56 | −8.142 | 2,3 |
| 42 | 5-p-coumaroylquinic acid isomer b | C16H18O8 | 337.0929 | 337.0932 (7.8), 191.0550 (100), 173.0444 (2.8), 163.0388 (1.9), 127.0385 (1.7), 119.0486 (1.5), 111.0433 (1.3), 93.0329 (5.2), 85.0278 (7.2) | 4.62 | 0.829 | 1,2 |
| 43 | 4-feruloylquinic acid b | C17H20O9 | 367.1035 | 367.1035 (96.7), 193.0496 (11.3), 191.0552 (0.7), 173.0446 (70.1), 134.0358 (24.5), 111.0435 (15.6), 93.0329 (100) | 4.68 | 0.122 | 2,3 |
| 44 | 3,5-dicaffeoylquinic acid-hexoside b | C31H34O17 | 677.1512 | 677.1538 (53.28), 515.1409 (100), 353.0878 (7.1), 341.0879 (14.5), 323.0774 (56.3), 335.0778 (4.1), 191.0551 (99.7), 179.0340 (44.8), 173.0446 (6.1), 161.0231 (44.2), 135.0437 (42.5), 127.0382 (2.1), 93.0329 (10.4) | 5.16 | 3.850 | 1,2,3 |
| 45 | 4,5-dicaffeoylquinic acid-hexoside b | C31H34O17 | 677.1512 | 677.1729 (100), 515.1287 (15.2), 353.0862 (24.8), 341.0890 (2.9), 323.0792 (19.4), 191.0553 (33.6), 179.0340 (60.5), 173.0443 (71.6), 161.0232 (26.3), 135.0438 (65.6), 93.0328 (17.1) | 5.56 | 0.779 | 1,2,3 |
| 46 | 3,4-dicaffeoylquinic acid a | C25H24O12 | 515.1195 | 515.1198 (100), 353.0880 (14.3), 335.0774 (5.9), 203.0340 (0.8), 191.0551 (29.5), 179.0339 (50.0), 173.0444 (62.9), 161.0230 (16.4), 135.0437 (50.0), 111.0436 (4.4), 93.0329 (15.6) | 5.70 | 0.487 | 1,2,3 |
| 47 | 3-dehydrocaffeoyl-5-caffeoylquinic acid b | C25H22O12 | 513.1038 | 513.1042 (61.9), 351.0724 (100), 335.0770 (10.0), 191.0551 (18.5), 179.0339 (42.8), 177.0182 (53.8), 173.0443 (35.9), 161.0231 (15.7), 135.0434 (47.9), 133.0280 (86.1), 93.0329 (18.9) | 5.85 | 0.898 | 2, 3 |
| 48 | 3,5-dicaffeoylquinic acid a | C25H24O12 | 515.1195 | 515.1199 (19.8), 353.0877 (98.3), 335.0765 (0.5), 191.0550 (100), 179.0338 (49.6), 173.0445 (3.6), 161.0232 (4.3), 135.0436 (49.1), 127.0385 (2.4), 111.0433 (1.7), 93.0330 (3.7), 85.0278 (7.3) | 5.87 | 0.137 | 1,2,3 |
| 49 | dihydroxyphenylacetic acid-O-dipentosyl-hexoside b | C27H29O15 | 593.1543 | 593.1543 (2.33), 461.1083 (0.84), 167.0338 (100), 149.0230 (5.91), 131.0699 (9.05), 123.0430 (39.75), 108.0200 (90.10) | 6.19 | 5.238 | 1,2 |
| 50 | 4,5-dicaffeoylquinic acid b | C25H24O12 | 515.1195 | 515.1198 (92.5), 353.0879 (54.9), 335.0771 (0.9), 191.0550 (36.1), 179.0337 (65.6), 173.0442 (100), 161.0230 (5.3), 135.0435 (64.3), 111.0435 (4.0), 93.0328 (25.2) | 6.23 | 0.390 | 1,2,3 |
| 51 | shikimic acid b | C7H10O5 | 173.0455 | 173.0443 (100), 155.0337 (1.6), 127.0381 (1.3), 111.0434 (9.3), 93.0329 (61.6) | 6.22 | −7.147 | 2,3 |
| 52 | rosmarinic acid a | C18H16O8 | 359.0778 | 359.0778 (16.3), 197.0447 (29.2), 179.0341 (12.8), 161.0231 (100), 135.0437 (16.0) | 6.33 | 1.781 | 2 |
| 53 | 3-feruloyl-4-caffeoylquinic acid b | C26H26O12 | 529.1351 | 529.1352 (100), 367.1038 (3.8), 353.0878 (6.2), 335.0771 (11.3), 193.0496 (52.2), 191.0552 (7.1), 179.0340 (35.7), 173.0444 (39.5), 161.0233 (20.3), 135.0439 (30.8), 134.0359 (38.6), 111.0436 (5.9), 93.0331 (10.3) | 6.50 | 0.096 | 2,3 |
| 54 | 3-p-coumaroyl-5-caffeoylquinic acid b | C25H24O11 | 499.1246 | 499.1254 (31.7), 353.0872 (0.4), 337.0931 (75.6), 335.0769 (2.1), 191.0550 (9.7), 173.0443 (8.4), 163.0388 (100.0), 135.0437 (2.9), 119.0487 (37.5), 93.0330 (4.1) | 6.52 | 1.694 | 1,2,3 |
| 55 | 1-p-coumaroyl-5-caffeoylquinic acid b | C25H24O11 | 499.1246 | 499.1235 (36.8), 353.0880 (45.8), 337.0934 (59.7), 191.0551 (100), 179.0337 (33.7), 173.0444 (18.6), 163.0388 (49.4), 135.0436 (39.6), 119.0484 (25.8) | 6.80 | −2.173 | 1,2,3 |
| 56 | 3-feruloyl-5-caffeoylquinic acid b | C26H26O12 | 529.1351 | 529.1354 (54.2), 367.1034 (97.1), 335.0782 (2.3), 193.0497 (100), 191.0546 (11.6), 173.0443 (53.4), 161.0230 (22.3), 135.0441 (10.8), 134.0358 (86.4), 111.0437 (3.3), 93.0330 (13.9) | 6.82 | 0.190 | 2,3 |
| 57 | 4-feruloyl-5-caffeoyl quinic acid b | C26H26O12 | 529.1351 | 529.1354 (92.8), 367.1034 (100), 353.0876 (5.6), 193.0496 (8.9), 191.0546 (10.5), 179.0338 (44.9), 173.0444 (65.7), 161.0231 (20.4), 135.0437 (56.2), 134.0358 (22.6), 111.0437 (11.2), 93.0329 (75.0) | 7.02 | 0.549 | 2,3 |
| 58 | 4-caffeoyl-5-feruloylquinic acid b | C26H26O12 | 529.1351 | 529.1359 (7.2), 367.1042 (12.0), 353.0875 (49.7), 193.0486 (1.4), 191.0551 (58.1), 179.0337 (61.9), 173.0444 (83.7), 161.0230 (21.8), 135.0437 (65.7), 134.0360 (2.6), 111.0436 (5.0), 93.0330 (30.6) | 7.18 | 1.343 | 2,3 |
| 59 | 4-caffeoyl-5-p-coumaroylquinic acid b | C25H24O11 | 499.1246 | 499.1253 97.66), 353.0868 (76.7), 337.0949 (7.9), 191.0549 (73.8), 179.0338 (75.5), 173.0442 (100), 161.0233 (7.6), 135.0437 (89.6), 111.0437 (8.9), 93.0329 (29.0) | 7.63 | 1.453 | 2 |
| 60 | 3,4,5-tricaffeoylquinic acid b | C34H30O15 | 677.1512 | 677.1517 (94.2). 515.1199 (31.6), 353.0879 (55.7), 335.0774 (14.1), 299.0594 (1.3), 255.0676 (1.7), 203.0349 (3.9), 191.0551 (47.7), 179.0338 (76.8), 173.0443 (100), 161.0232 (28.7), 135.0436 (82.0), 111.0435 (5.6), 93.0330 (24.3) | 7.78 | 0.748 | 1,2,3 |
| Flavonoids | |||||||
| 61 | naringenin 6, 8 diC-hexoside b | C27H32O15 | 595.1678 | 595.1680 (100), 475.1255 (3.8), 457.1151 (2.5), 415.1039 (11.2), 385.0930 (30.4), 355.0826 (37.6), 271.0618 (0.6), 163.0027 (1.4), 151.0017 (1.0), 119.0487 (15.2), 107.0123 (3.3) | 3.64 | 1.994 | 1,2 |
| 62 | apigenin 6, 8-diC-hexoside b | C27H29O15 | 593.1512 | 593.1518 (100), 503.1208 (4.7), 473.1090 (16.0), 413.0892 (2.0), 395.0779 (1.9), 383.0775 (18.6), 353.0669 (32.6), 325.0706 (2.4), 297.0767 (10.9), 161.0233 (2.0), 117.0329 (3.2) | 4.04 | 0.905 | 2 |
| 63 | homoorientin (luteolin 6-C-glucoside) a,b | C21H20O11 | 447.0933 | 447.0930 (100), 369.0610 (2.5), 357.0614 (39.3), 339.0497 (2.4), 327.0514 (53.7), 311.0537 (1.7), 299.0573 (3.5), 298.0487 (3.3), 297.0411 (14.0), 285.0405 (3.8), 133.0280 (11.4), 175.0376 (2.9) | 4.54 | 0.225 | 1, 2,3 |
| 64 | luteolin O-hexuronosyl-O-hexoside b | C27H28O17 | 623.1264 | 623.1263 (66.0), 447.0930 (2.6), 285.0403 (100), 257.0454 (0.5), 243.0290 (0.9), 217.0499 (1.5), 199.0393 (2.5), 175.0391 (2.5), 151.0025 (3.9), 133.0280 (7.5), 107.0125 (2.6) | 4.72 | 1.457 | 2 |
| 65 | rutin a | C27H30O16 | 609.1464 | 609.1467 (100), 301.0346 (30.2), 300.0274 (79.6), 271.0247 (39.9), 255.0296 (17.3), 243.0294 (8.7), 227.0345 (2.3), 211.0391 (0.4), 178.9976 (2.7), 163.0022 (1.4), 151.0023 (5.5), 121.0277 (0.3), 107.0121 (2.3) | 5.08 | 0.972 | 1,2 |
| 66 | luteolin O-pentosylhexoside b | C26H28O15 | 579.1360 | 579.1364 (83.4), 447.0879 (0.5), 285.0404 (100), 256.0366 (1.3), 241.0502 (0.6), 227.0341 (0.8), 175.0385 (2.0), 151.0024 (5.0), 133.0280 (3.7), 107.0124 (2.3) | 5.09 | 1.394 | 2 |
| 67 | isoquercitrin a | C21H20O12 | 463.0886 | 463.0887 (100), 343.0472 (0.5), 301.0346 (37.6), 300.0274 (82.0), 271.0248 (32.5), 255.0296 (13.3), 243.0296 (8.2), 227.0344 (2.6), 211.0398 (0.5), 178.9979 (2.30), 163.0033 (1.5), 151.0024 (9.1), 121.0275 (1.0), 107.0124 (3.8) | 5.18 | 1.103 | 1,2,3 |
| 68 | hyperoside a,b | C21H20O12 | 463.0887 | 463.0829 (100), 301.0352 (12.6), 300.0272 (21.7), 271.0245 (10.2), 255.0283 (4.4), 243.0284 (2.2), 179.0331 (1.8), 175.0245 (8.0), 163.0372 (1.5), 151.0023 (50.4), 135.0438 (40.4), 107.0123 (12.3) | 5.29 | 1.218 | 1,2,3 |
| 69 | nepetin O-pentosylhexoside b | C27H30O16 | 609.1468 | 609.1468 (100), 315.0516 (63.35), 301.0354 (7.20), 300.0279 (32.54), 299.0202 (8.60), 285.0401 (5.50), 271.0251 (1.33), 133.0282 (6.77) | 5.35 | −5.123 | 1,2 |
| 70 | luteolin 7-O-rutinoside b | C27H30O15 | 593.1512 | 593.1518 (83.0), 285.0403 (100), 256.0372 (0.6), 243.0290 (0.6), 229.0499 (0.4), 217.0492 (0.8), 175.0391 (2.2), 151.0023 (4.7), 133.0281 (4.6), 107.0119 (1.8) | 5.22 | 1.006 | 1,2 |
| 71 | luteolin 7-O-glucoside a | C21H20O11 | 447.0933 | 447.0935 (100), 285.0404 (43.0), 284.0324 (49.0), 255.0291 (0.7), 227.0349 (3.2), 211.0394 (2.7), 161.0230 (1.9), 151.0025 (3.8), 133.0280 (4.1), 107.0122 (2.3) | 5.31 | 0.437 | 1, 2,3 |
| 72 | luteolin O-hexuronide b | C21H18O12 | 461.0736 | 461.0730 (54.1), 285.0403 (100), 267.0295 (0.3), 243.0297 (0.8), 229.0491 (0.5), 217.0503 (0.7), 199.0393 (2.8), 151.0023 (4.7), 133.0280 (8.7), 107.0122 (2.2) | 5.38 | 0.978 | 2 |
| 73 | isorhamnetin O-hexuronide b | C22H20O13 | 491.0832 | 491.0836 (72.0), 387.0720 (0.4), 357.0628 (0.7), 315.0511 (100), 300.0275 (52.0), 272.0325 (8.1), 255.0290 (0.2), 243.0295 (0.3), 229.6530 (0.2), 215.0344 (0.3) 175.0232 (1.0), 151.0025 (1.6), 107.0118 (1.0) | 5.47 | 0.970 | 2 |
| 74 | kaempferol 7-O-rutinoside b | C27H30O15 | 593.1520 | 593.1519 (100), 285.0403 (74.9), 284.0325 (44.5), 255.0297 (36.6), 227.0344 (24.1), 211.0394 (1.7), 163.0022 (1.7), 151.0020 (1.5), 107.0117 (1.3) | 5.65 | 1.124 | 1,2 |
| 75 | nepetin O-hexoside b | C22H22O12 | 477.1038 | 477.1041 (100), 315.0486 (33.8), 300.0268 (16.2), 299.0198 (19.1), 285.0407 (2.6), 271.0243 (2.5), 255.0304 (1.1), 243.0290 (3.2), 227.0341 (3.1), 199.0391 (8.8), 136.9868 (0.9), 133.0281 (10.1) | 5.67 | −0.253 | 1,2,3 |
| 76 | axillarin O- pentosylhexoside b | C28H32O17 | 639.1567 | 639.1567 (100), 345.0616 (73.7), 330.0387 (21.0), 315.0145 (7.7), 287.0190 (4.8) | 5.74 | 0.012 | 2 |
| 77 | apigenin O-pentosylhexoside b | C26H28O14 | 563.1406 | 563.1412 (32.9), 269.0453 (100), 239.0337 (0.3), 225.0561 (1.1), 151.0022 (0.9), 117.0330 (3.6), 107.0122 (1.8) | 5.75 | 0.961 | 2 |
| 78 | apigenin 7-O-rutinosideb | C27H30O14 | 577.1570 | 577.1570 (48.7), 269.0454 (100), 457.1350 (1.5), 239.0348 (0.3), 225.0556 (1.5), 163.0388 (6.6), 119.0486 (10.2), 117.0330 (3.3), 107.0124 (1.9) | 5.82 | 1.250 | 2 |
| 79 | isorhamnetin 3-O-glucoside a,b | C22H22O12 | 477.1042 | 477.1038 (100), 315.0493 (12.8), 314.0432 (56.2), 299.0200 (4.3), 271.0246 (23.1), 257.0453 (5.4), 243.0293 (24.6), 227.0343 (3.3), 215.0341 (3.3), 199.0391 (4.0),178.9975 (0.6), 151.0023 (3.2), 107.0122 (0.8) | 5.90 | 0.253 | 1,2,3 |
| 80 | hispidulin O-pentosylhexoside b | C27H30O15 | 593.1512 | 593.1523 not fragmented * | 5.93 | 1.832 | 2 |
| 81 | isorhamnetin O-pentoside b | C21H19O11 | 447.0935 | 447.0935 (100), 315.0486 (7.4), 314.0436 (43.6), 300.0276 (20.4), 285.0415 (6.3), 271.0247 (23.2), 255.0304 (2.1), 243.0294 (15.6), 227.0340 (2.6), 151.0020 (2.0) | 6.02 | 0.437 | 1, 2 |
| 82 | chrysoeriol O-pentosylhexoside b | C27H30O15 | 593.1512 | 593.1530 not fragmented * | 6.04 | 2.962 | 2 |
| 83 | apigenin O-hexuronide b | C21H18O11 | 445.0787 | 445.6779 (29.6), 269.0453 (100), 225.0550 (1.8), 213.0537 (0.1), 197.0596 (1.2), 183.0440 (1.3), 175.0237 (15.2), 151.0024 (2.1), 117.0330 (6.6), 107.0123 (2.9) | 6.13 | 0.484 | 2 |
| 84 | kaempferol 3-O-glucoside a,b | C21H19O11 | 447.0935 | 447.0935 (100), 285.0393 (15.8), 284.0326 (51.3), 255.0296 (36.5), 227.0344 (37.4), 211.0395 (1.4), 151.0023 (2.3) | 6.21 | 0.504 | 1, 2 |
| 85 | jaceosidin O-hexuronide b | C23H22O13 | 505.0988 | 505.0994 (95.1), 371.0758 (0.8), 329.0667 (100), 314.0433 (18.018), 299.0197 (35.648), 285.0405 (2.0), 271.0247 (36.4), 243.0306 (0.4), 227.0341 (1.0), 175.0236 (11.1), 161.0227 (0.9), 113.0227 (31.0), 85.0278 (19.0) | 6.33 | −2.731 | 2,3 |
| 86 | chrysoeriol O-hexuronide b | C22H20O12 | 475.0882 | 475.0883 (87.3), 299.0560 (100), 284.0325 (68.0), 256.0374 (7.3), 239.0351 (0.3), 227.0356 (1.7), 211.0387 (0.9), 175.0236 (15.1), 151.0021 (2.1), 139.0015 (0.3), 107.0125 (2.8) | 6.34 | 0.254 | 2 |
| 87 | jaceosidin O-hexoside | C23H24O12 | 491.1195 | 491.1199 (100), 329.0667 (4.8), 328.0586 (8.9), 313.0356 (35.8), 298.0136 (9.2), 285.0400 (4.3), 270.0179 (15.6), 136.9867 (1.0) | 6.50 | 0.877 | 2 |
| 88 | eupatilin O-hexoside | C24H26O12 | 505.1351 | 505.1356 not fragmented * | 7.49 | 0.932 | 2 |
| 89 | luteolin a | C15H10O6 | 285.0405 | 285.0403 (100), 217.0495 (1.0), 199.0394 (1.8), 175.0391 (1.9), 151.0023 (4.3), 133.0280 (24.1), 121.0279 (1.1), 107.0121 (4.5) | 7.58 | −0.636 | 2,3 |
| 90 | quercetin a | C15H10O7 | 301.0354 | 301.0354 (100), 273.0409 (2.5), 257.0482 (0.7), 178.9975 (25.3), 151.0023 (42.4), 121.0279 (12.1), 107.0123 (12.8) | 7.63 | −0.019 | 1,2 |
| 91 | patuletin (6-methoxyquercetin) b | C16H12O8 | 331.0464 | 331.0460 (100), 316.0223 (64.6), 287.0198 (7.4), 271.0245 (6.2), 259.0246 (3.6), 243.0292 (2.2), 181.0134 (5.5),165.9895 (17.5), 139.0023 (11.3), 136.9863 (1.2), 121.0280 (3.1), 109.9994 (10.6) | 7.72 | 0.149 | 2,3 |
| 92 | nepetin (6-methoxyluteolin) b | C16H12O7 | 315.0514 | 315.0514 (73.4), 300.0278 (100), 272.0317 (0.4), 255.0307 (0.6), 243.0306 (1.5), 227.0348 (1.7), 165.9895 (0.8), 139.0029 (0.7), 136.9868 (10.1), 133.0287 (2.7), 109.9997 (1.6) | 7.75 | 1.251 | 1,2,3 |
| 93 | spinacetin b | C17H14O8 | 345.0616 | 345.0613 (100), 330.0380 (94.95), 315.0148 (30.00), 287.0196 (24.64), 259.0245 (14.99), 243.0296 (2.13), 231.0292 (2.05), 215.0341 (5.30), 187.0390 (3.78), 175.0388 (0.16), 165.9890 (0.52), 163.0387 (1.08), 149.0230 (2.98), 139.0022 (1.05), 136.9864 (1.81) | 7.85 | −0.726 | 2,3 |
| 94 | axillarin b | C17H14O8 | 345.0616 | 345.0615 (100), 330.0381 (99.2), 315.0147 (48.3), 287.0196 (14.5), 271.0241 (1.6), 259.0245 (3.6), 243.0294 (3.5), 231.0293 (4.8), 215.0341 (4.2), 175.0026 (3.2), 165.9894 (5.6), 149.0230 (10.0), 139.0386 (2.9), 136.9867 (1.1), 121.0281 (1.5), 109.9994 (3.4) | 8.25 | −0.205 | 2,3 |
| 95 | apigenin a | C15H10O5 | 269.0457 | 269.0454 (100), 225.0551 (1.0), 201.0541 (0.4), 151.0022 (5.3), 121.0124 (1.1), 117.0330 (18.4), 107.0124 (4.4) | 8.62 | −1.942 | 2 |
| 96 | hispidulin (scutellarein-6-methyl ether) a,b | C16H12O6 | 299.0563 | 299.0560 (65.36), 284.0323 (100), 255.0299 (1.50), 227.0340 (3.52), 211.0393 (2.15), 165.9894 (0.86), 136.9865 (15.57), 117.0329 (1.85) | 8.84 | −0.372 | 2,3 |
| 97 | quercetagetin-3,6,3’(4’)-trimethyl ether b | C18H16O8 | 359.0772 | 359.0776 (100), 344.0539 (85.8), 329.0305 (41.5), 314.0068 (2.8), 301.0356 (10.3), 286.0123 (7.3), 258.0169 (4.0), 242.0218 (15.0), 230.0207 (2.5), 214.0267 (9.1), 186.0303 (1.7), 163.0381 (1.7), 161.0223 (1.3), 109.9985 (0.4) | 9.08 | 1.112 | 2,3 |
| 98 | isorhamnetina,b | C16H12O7 | 315.0512 | 315.0514 (100), 300.0278 (48.1), 271.0254 (3.3), 255.0296 (2.4), 243.0300 (1.4), 227.0340 (1.8), 211.0388 (0.5), 163.0025 (3.0), 151.0025 (8.4), 107.0124 (8.0) | 9.11 | −0.551 | 1,2 |
| 99 | jaceosidin (6-hydroxyluteolin-6,3’-dimethyl ether) a,b | C17H14O7 | 329.0677 | 329.0667 (87.3), 314.0433 (100), 299.0197 (20.2), 271.0249 (33.3), 255.0288 (0.6), 243.0296 (3.0), 227.0346 (2.9), 215.0347 (1.9), 199.163.0021 (1.9), 136.9868 (2.3), 135.0076 (0.6), 133.0279 (4.5) | 9.15 | 0.073 | 2,3 |
| 100 | cirsiliolb | C17H14O7 | 329.0677 | 329.0670 (100), 314.0436 (85.7), 299.0198 (35.2), 271.0250 (62.0), 243.0301 (1.0), 199.0393 (0.9), 161.0231 (0.8), 151.0028 (0.5) | 9.47 | 0.954 | 2,3 |
| 101 | quercetagetin-3,6,3’(4’)-trimethyl ether b | C18H16O8 | 359.0772 | 359.0777 (100), 344.0539 (42.5), 329.0306 (63.0), 314.0073 (14.7), 301.0346 (1.6), 286.0122 (3.5), 258.0172 (4.3), 230.0216 (1.5), 214.0269 (0.3), 202.0258 (1.2), 165.9889 (0.4), 163.0391 (7.6), 148.0153 (10.2), 139.0019 (0.5), 136.9864 (1.2) | 9.66 | 1.196 | 2,3 |
| 102 | cirsimaritin (6-hydroxyapigenin-6,7-dimethyl ether) a,b | C17H14O6 | 313.0719 | 313.0718 (100), 298.0480 (38.6), 283.0248 (12.6), 269.0455 (12.8), 255.0295 (35.2), 227.0337 (0.4), 151.0017 (0.4), 107.0122 (0.5) | 10.39 | 0.059 | 2,3 |
| 103 | eupatilin/santin b | C18H16O7 | 343.0812 | 343.0824 (100), 328.0591 (69.9), 313.0358 (47.5), 298.0117 (15.9), 285.0411 (2.4), 270.0173 (11.6), 242.0221 (4.8), 214.0266 (2.1), 163.0029 (4.4), 147.0438 (4.7), 136.9864 (2.3), 132.0203 (4.1), 109.9997 (0.4) | 10.68 | −0.047 | 2,3 |
a Compared to a reference standard; b reported for the first time; 1-T. balsamita flower heads; 2-T. balsamita leaves; 3-T. balsamita roots; * annotation was done in (+) ESI-MS/MS (see Supplementary Materials Table S1).
2.1.1. Hydroxybenzoic and Hydroxycinnamic Acids and Their Derivatives
Based on the comparison with the fragmentation patterns and retention times of reference standards, 5 hydroxybenzoic acids (3, 6, 13, 16 and 33) and 4 hydroxycinnamic acids (27, 32, 40 and 41) together with p-hydroxyphenylacetic (31) and rosmarinic acid (52) were identified in the extracts (Table 2 and Figure S1).
A variety of hydroxybenzoic and hydroxycinnamic acid glycosides was tentatively identified including hexosides (1, 4, 5, 7, 9, 11, 17, 18, 21, 24, 25, 26 and 39) along with pentosylhexoside of hydroxybenzoic acid (2) and dihydroxyphenylacetic acid (19). MS/MS spectra of sugar esters vanillyl-hexose (10) and caffeoyl-hexose (15) were acquired. In contrast to the corresponding hexosides, fragment ions resulting from the sugar cross ring cleavages were registered in the sugar esters as follows: 0,4Hex (−60 Da), 0,3Hex (−90 Da) and 0,2Hex (−120 Da) [20]. In addition, three caffeoylgluconic acid isomers (14, 21 and 29) at m/z 357.084 [M-H]− were deduced from the prominent ions at m/z 195.050 [gluconic acid (GA)-H]− supported by m/z 177.040 [GA-H-H2O]−, 165.040 [GA-H-CH2O]−, 147.028 [GA-H-CH2O-H2O]−, 129.018 [GA-H-CH2O-2H2O]−, 87.007 [GA-H-C3H8O4]− and 59.012 [GA-H-C3H8O4-CO]− (Table 2). Within this group, 25 was the main compound in the leaves extract, together with 6, 1, 22 and 46, while roots and flower heads were dominated by 22 (Figure S1). Even though hydroxybenzoic and hydroxycinnamic acids were present in their free form, herein a large number of phenolic acid glycosides were revealed in costmary for the first time.
Previously, cichoric acid was determined as prevailing compound in the costmary aerial parts, being present at 3.33 g/100 g extract [14]. In contrast, cichoric acid was not found in this study. Ethanol-aqueous extracts of T. vulgare leaves and flower heads were especially rich in protocatechuic acid and its hexoside along with caffeic and salicylic acid and caffeic acid-O-(salicyl)-hexoside [20]. In line with these findings, T. parthenium aerial parts were rich in p-hydroxyphenylacetic and caffeic acid, being present in 280.4 and 129.8 mg/kg extract, respectively [25]. On the other hand, T. macrophyllum was distinguished by the phenylpropanoid glycosides caffeic acid-O-(hydroxybutanoyl)-hexoside and vanillic/gentisic acid-O-(caffeoyl)-hexoside, together with two caffeoyl-(syringic) acid isomers.
2.1.2. Acylquinic Acids
Overall, 8 monoAQA, 13 diAQA and 1 triAQA, together with two diAQA-hexosides, were identified/annotated in the assayed extracts (Table 2 and Figure S2). The systematic investigation on the fragmentation patterns and diagnostic fingerprints in the MS/MS spectra of AQA in Asteraceae taxa allowed for the differentiation of the AQA subclasses [20,21,30]. The AQA annotation was based on the preferential fragmentation resulting in relevant ions corresponding to each subclass AQA. Thus, 23, 34/42 and 36 were ascribed as 5-caffeoyl-, 5-coumaroyl- and 5-feruloylquinic acid, respectively, by the base peak at m/z 191.055 [quinic acid-H]−. Compounds 8 and 30 were identified as 3-caffeoyl- and 3- feruloylquinic acid, while 28 and 43 were assigned to the respective 4-substituted monoAQA as suggested the base peak corresponding to the dehydrated ion of quinic acid at 173.045 (Table 2).
Five commonly found diAQA subclasses were identified/annotated: di-caffeoylquinic acid isomers (diCQA) at m/z 515.120 [M-H]−, feruloyl-caffeolylquinic acids (FCQA) at m/z 529.135, p-coumaroyl-caffeoylquinic acids (p-CoCQA) at m/z 499.125, hydroxydihydrocaffeoyl-caffeolylquinic acids (HC-CQA) at m/z 533.130 and dehydrocaffeoyl-caffeoylquinic acid (DC-CQA) at m/z 513.104 (Table 2).
Compounds 46, 50, 53, 57, 58 and 59 were consistent with vicinal diAQA yielding prominent ions at m/z 173.044 (base peak) and 135.044 [caffeic acid-H-CO2]−. The dehydrated ion at m/z 335.077 [CQA-H-H2O]− clearly defined 3,4-diCQA (46) and 3F-4CQA (53). The assignment of FCQA was confirmed by the prominent fragment ions at m/z 367.103 [M-H-caffeoyl]− and 134.036 [ferulic acid-H-CH3-CO2]−. The lack of ion at m/z 335 (or its negligible abundance) together with the chromatographic behavior on the reverse phase support (the most lipophilic diAQA isomer within the subclass) evidenced 4,5-diCQA (50), 4F-5CQA (57), 4C-5FQA (58) and 4C-5-p-CoQA (59). This assignment was supported by the abundant ions at m/z 367.103 [M-H-caffeoyl]− (100%) (57), 353.088 [M-H-feruloyl]− (49.7%) (58) and 353.087 [M-H-p-coumaroyl]− (76.7%) (59) (Table 2). Generally, 3,5-diAQAs easily cleaves the acyl moiety at C-5, compared to C-3. Thus, 48 (3,5- diCQA) produced a base peak at m/z 191.055 accompanied with abundant ions at m/z 179.034 [caffeic acid-H]− and 135.044, while the relevant ions at m/z 367.103 [M-H-caffeoyl]− (97.1%) and 193.050 [ferulic acid-H]− (base peak) suggested 3F-5CQA (56) (Table 2). In the same manner, 54 was ascribed as 3-p-Co-5CQA by the base peak at m/z 163.039 [p-coumaric acid-H]− and diagnostic ions at m/z 337.093 [M-H-caffeoyl]− (75.6%) and 119.049 [p-coumaric acid-H-CO2]− (37.5%) (Table 2). 3-DC-5-CQA (47) was deduced from the distinctive fragments at m/z 351.074 [M-H-caffeoyl]− (100%), 177.018 [dehydrocaffeic acid-H]− (53.8%) and 133.028 [dehydrocaffeic acid-H-CO2]− (86.1%), while 3-HC-5-CQA (35) was evidenced by ions at m/z 371.099 [M-H-caffeoyl]−, 191.055 and 135.044.
Peaks 44 and 45 gave a precursor ion at m/z 677.173(4) (C31H33O17), together with the transitions at m/z 677.173→515.141→353.088→191.055 (44) resulting from the losses of two caffeoyl moieties and a hexose unit, respectively (Table 2). 3,5-disubstituted quinic acid skeleton (44) was discernible by the ions at m/z 191.055 (99.7%), 179. 034 (44.8%) and 135.044 (42.5%). Thus, 44 was ascribed as 3,5-dicaffeoylquinic acid-hexoside. In the same way, 4,5-dicaffeoylquinic acid-hexoside (45) was deduced from the ions at m/z 173.044 (71.6%) and 179.034 (60.5%), together with m/z 341.089 and 323.079.
Compound 60 with [M-H]− at m/z 677.152 (consistent with C34H29O15) was the most hydrophobic AQA. It afforded prominent fragment ions at m/z 515.120 [M-H-caffeoyl]−, 353.089 [M-H-2caffeoyl]− and 191.055 [M-H-3caffeoyl]− indicating triCQA. 3,4,5- triCQA was discernible from the fragment ions at m/z 173.044 (100%), 135.044 (82%) and 179.034 (76.8%) [31].
Overall, 23, 28 and 36 were the major monoAQA together with diAQA 46, 48 and 50 (Figure S2). These results were consistent with the AQAs evidenced in T. vulgare and T. parthenium aerial parts extracts where chlorogenic acid was found between 2.6 mg/g dw and 925 mg/100 g extract, and 487.8 mg/kg extract, respectively [14,25,27]. T. macrophyllum was discernible by 1,5 diCQA, isomeric pairs 4-p-Co-5-CQA/4C-5-p-CoQA and 1C-5FQA/1C-3FQA, together with a variety of HC-CQA and DC-CQA [20,21].
2.1.3. Flavones, Flavonols and Flavanones
MS/MS spectra of 61 and 62 at m/z 595.168 [M-H]− and 593.152, respectively, were acquired (Table 2 and Table S1, Figure S3). A typical diC-hexosyl flavonoid fragmentation pathway was observed including a series of the following transitions: [M-H-120]− at m/z 475.126 (61) and 473.109 (62), [M-H-120-60]− at m/z 415.104 and 413.089, [M-H-120-90]− at m/z 385.093 and 383.078, and [M-H-2 × 120]− at m/z 355.083 and 353.067, respectively (Table 2). Additionally, the aglycone naringenin in 61 was evidenced by the deprotonated molecule at m/z 271.062 supported by the RDA ions at m/z 163.003 (0,2A−), 151.002 (1,3A−), 119.049 (1,3B−) and 107.012. (0,4A−). Concerning 62, the aglycone apigenin was discernible by the prominent ions at m/z 325.071 [(M-H)-2 × 120-CO]−, 297.077 [(M-H)-2 × 120-2CO]− and 117.033 (1,3B−) in (-) ESI-MS/MS supported by m/z 177.018 [1,3A+-H2O (0,2X0/0,2X1)] and 121.028 [0,2B+ (0,2X0/0,2X1)] (Table 2 and Table S1). Thus, 61 and 62 were ascribed as 6,8-diC-hexosyl-naringenin and 6,8-diC-hexosyl-apigenin (vicenin-2), respectively. In the same way, 6-C-hexosyl-luteolin was confirmed by the diagnostic ions 0,3X− at m/z 357.061 (39.3%) and 0,2X− at m/z 327.051 (53.7%); luteolin ([Lu-H]− at m/z 285.041) was deduced from RDA ions 1,3B− at m/z 133.028 and 1,4A− at m/z 175.038. Based on the comparison with reference standards, 62 and 63 were unambiguously identified as vicenin-2 [32] and homoorientin, respectively.
The sugar chain of 66, 69, 76, 77, 80 and 81 was consistent with pentosylhexoside (294 Da, C16H18O9) (Table 2 and Table S1, Figure S3). Exemplified by 76, in (-) ESI, the precursor ion at m/z 639.157 [M-H]− gave the prominent ion at m/z 345.062 corresponding to the deprotonated aglycone, while in (+) ESI [M+H]+ at m/z 641.167 exhibited successive losses of pentose moiety at m/z 509.127 (31.5%) and hexosyl at m/z 347.076 (100%). On the other hand, the aglycone (Agl) was discernible by the consecutive methyl radical losses at m/z 330.039 [Agl-H-•CH3]−, 315.015 [M-H-2•CH3]−, 287.019 [M-H-2•CH3-CO]− in (-) ESI supported by the transitions 347.076→331.045→289.046 in (+) ESI. Thus, 76 was referred to quercetagetin-dimethyl ether-O-pentosylhexoside.
Compounds 65, 70, 74 and 78 were closely associated to the same fragmentation pattern giving characteristic fragment ions at m/z 301.035 (65), 285.040 (70, 74) and 269.045 (78) [(M-H)-308]−, indicating deoxyhexosylhexosides (Table 2). In (+) ESI, an interglycosidic linkage breakdown occurred, yielding [M-H-146] at m/z 465.102 (65), 449.108 (70, 74) and 433.112 (78) (Table S1), as was observed in case of 1→6 linkage [33,34]. Thus, aforementioned compounds were assigned as quercetin/luteolin/kaempferol/apigenin-O-rutinosides.
Compounds 72, 73, 83, 85, and 86 presented similar fragmentation patterns yielding base peaks at m/z 285.040 (72), 315.051 (73), 269.045 (83), 329.067 (85) and 299.056 (86) [(M-H)-HexA]−, respectively, indicating flavonoid hexuronides (Table 2 and Table S1). Compounds 72, 73 and 83 were consistent with luteolin-, isorhamnetin- and apigenin-O-hexuronide, respectively. Compound 86 was ascribed to chrysoeriol-O-hexuronide (1,3A− at m/z 151.002, 0,4A− at m/z 107.013), while 85 was assigned as jaceosidin-O-hexuronide [35].
Isoquercitrin (67), hyperoside (68), luteolin 7-glucoside (71), isorhamnetin 3-glucoside (79) and kaempferol 3-glucoside (84) were unambiguously identified by comparison with reference standards.
Hexuronides of luteolin, isorhamnetin, apigenin, chrysoeriol and jaceosidin were exclusively produced by T. balsamita leaves (Figure S3), Flavonoid glycosides profile of both leaves and flower heads extracts were dominated by rutin; the former was also characterized by luteolin/jaceosidin-hexuronide, while the latter was rich in isoquercitrin and hyperoside. Previously, apigenin- and luteolin 7-glucoside were determined in T. balsamita aerial parts, being present in 1099.3 and 725.7 mg/100 g extract [14].
2.2. Methoxylated Flavonoids
Methoxyflavonoids annotation was based on the characteristic fragment ions delineated in previous studies on Tanacetum sp. [21,30] and Achillea sp. [30]. Generally, the initial RDA ions 1,3A− and 1,3B− were not observed [35,36]. In (-) ESI, a series of RDA ions originating from 1,3A− were produced including [1,3A-•CH3]−, [1,3A-H2O-CH2]−, [1,3A-CO-CH2]− and [1,3A-CO-CH4]−, while [1,3B-•CH3-CH2]−, [1,3B-CH4]− and [1,3B-CH2]− suggested methoxylation in a B ring (Table 2). In (+) ESI, a commonly found fragment at m/z 168.005 [1,3A-•CH3]+ suggested methoxylation in an A ring (Table S1).
MS/MS spectra of five quercetagetin derivatives (91, 93, 94, 97 and 101) were acquired (Figure S4). The methoxylated derivative 91 was deduced from the fragment ions at m/z 316.022 [M-H-•CH3]− and 287.020 [M-H-•CH3-CHO•]−; 6-methoxylation in a ring A was revealed by the prominent ions in (-) ESI at m/z 181.0134 [1,3A]−, 165.990 [1,3A-•CH3]−, 139.002 [1,3A-CO-CH2]−, 136.986 [1,3A-CO-CH4]− and 109.999 [1,3A-•CH3-2CO]−, supported by m/z 168.005 [1,3A-•CH3]+ in (+) ESI (Table 2 and Table S1). Compounds 93 and 94 shared the same [M-H]− at m/z 345.061, indicating an additional methyl group compared with 91. Regarding 94, RDA ions generated from [1,3A]− were consistent with those in 91 (Table 2). Fragment ions at m/z 121.028 [1,2B]− and 137.023 [0,2B]+, evidenced the lack of methoxy group in a ring B. In line with this assumption, methoxy group at C-3 was suggested; accordingly, 94 was assigned as quercetagetin-3, 6-dimethyl ether (axillarin), previously reported in Tanacetum sp. [20,21]. Concerning 93, a methoxylated B ring was discernible by m/z 161.023 [1,3B]− as was observed in spinacetin.
Both isomers 97 and 101 at m/z 359.078 [M-H]− generated prominent ions at m/z 344.054, 329.031 and 314.007, resulting from the consecutive losses of three methyl radicals •CH3. They were referred to quercetagetin 3, 6, 3’(4’)- trimethyl ether; two methoxy groups in RDA ion [1,3B]− were evidenced by the typical losses at m/z 148.015 [1,3B-•CH3-CH2]−, 161.022 [1,3B-CH4]− and 163.039 (1,3B−-CH2) (Table 2).
In the same way, four closely associated 6-methoxyluteolin derivatives (92, 99, 100 and 103) were described (Figure S4). Among them, 99 and 100 shared the same [M-H]− at m/z 329.067. Jaceosidin (6-hydroxyluteolin-6, 3’-dimethylether) (99) was discernible by the RDA ions in (-) ESI at m/z 163.002 (1,3A−-H2O), 136.988 [1,3A-CH4-CO]−, 135.008 (1,3A−-H2O-CO-CH2) and 133.028 [1,3B-CH2]−, supported by m/z 168.005 [1,3A-CH3]+ in (+) ESI (Table 2 and Table S1). Additionally, 99 was confirmed by comparison with reference standard. On the other hand, 100 afforded RDA fragments in (-) ESI at m/z 161.023 [1,3A−-CH4-H2O]− and 151.003 [1,3A−-CH4-CO]−.together with m/z 136.015 [1,3A-CH3-H2O-CO]+.and 137.022 [0,2B]+ in (+) ESI (Table 2 and Table S1). Accordingly, 100 could be associated with cirsiliol (6-hydroxyluteolin-6, 7-dimethylether). Compound 103 afforded prominent fragment ions at m/z 328.059, 313.036 and 298.012, resulting from the consequent losses of 3 methyl radicals (Table 2). A methoxylated ring A was evidenced by RDA ions at m/z 136.987 (1,3A−-CO-CH4) and 163.002 (1,3A−-H2O), while m/z 132.0203 (1,3B−-•CH3-CH2) indicated 2 methoxy groups either in C-3, C-4′ or C-3′, C-4′, as was observed in santin and eupatilin, respectively [20,37].
Two scutellarein (6-hydroxyapigenin) derivatives 94 and 96 were discernible from the prominent ions in (-) ESI at m/z 165.990 [1,3A-CH3]− and 136.987 [1,3A-CH3-CO]− (96), and m/z 151.002 [1,3A-CO-CH4]− and 107.012 [0,4A-CO-CH4]− (94) (Table 2). Thus, 94 was assigned as cirsimaritin, while 96 was referred to hispidulin, additionally confirmed by comparison with reference standard. The aforementioned assumption was supported by fragments at m/z 168.005 [1,3A-CH3]+ (96) and 153.018 [1,3A-CH4-CO]+ (94) in (+) ESI (Table S1).
Methoxylated flavones and flavonols are widespread in Asteraceae species. Generally, nepetin (6-methoxy luteolin) and jaceosidin (6-hydroxyluteolin 3’,6-dimethyl ether) appeared to be characteristic for costmary leaves and root extracts; in contrast, flower heads were the richest in quercetin and isorhamnetin (Figures S2 and S3).
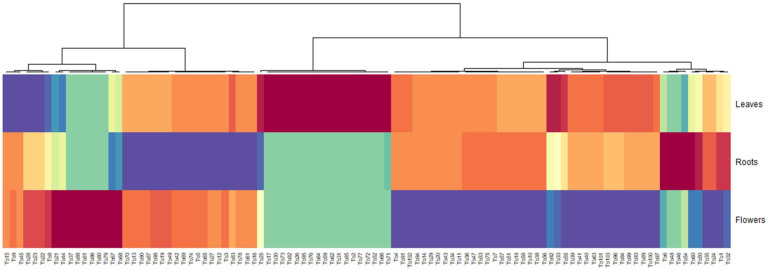
2.3. Heatmap Analysis
To gain an intuitive viewing of the metabolite contrast among the different extracts of Tanacetum balsamita, a heatmap was generated. Figure 1 displays the outcomes and highlights the arrangements of the groups of metabolites characterizing each extract. The red and blue color in the plot specify higher and lower metabolite amounts than the mean, respectively. As observed, the metabolites within group C were abundant in the leaves extract. In contrast, the lowest concentration of the metabolites within group A1 was exhibited by the leaves extract. Similarly, higher concentrations of the group A2 metabolites were found in the flower heads extract, while the group E metabolites were found at lower levels. On the other hand, the metabolites of the root were of low concentrations and were consolidated in the group B. A few studies have reported that the concentration of metabolites is varying in the different parts of the same species. High concentration of phenolics in leaves versus that of roots may be resulted to the presence or absence of light that impacts the phenolic contents of organs [38]. Furthermore, variation in the amount of various phenolic molecules in plants during its phenological cycle is reported by Çirak et al. [39].
Figure 1.
Global overview of the metabolite’s contrasts among Tanacetum balsamita samples (Clustered Image Map).
2.4. Antioxidant Properties
Antioxidants impair the oxidative damage in foods and herbs by delaying or inhibiting oxidation, and expand the shelf-life and quality of these foods [40]. Thus, their consumption could be of help in the treatment of diseases correlated with oxidative damage, as cardiac vascular diseases, inflammations, diabetes and cancer [41]. Therefore, in the present work, the in vitro antioxidant properties of T. balsamita extracts were assayed, and the results are depicted in Table 3.
Table 3.
Antioxidant activity of the T. balsamita extracts.
| Samples | PMD Assay (mmol TE/g) |
DPPH (mg TE/g) |
ABTS (mg TE/g) |
CUPRAC (mg TE/g) |
FRAP (mg TE/g) |
Metal Chelating (mg EDTAE/g) |
|---|---|---|---|---|---|---|
| Leaves | 1.09 ± 0.03 c | 43.87 ± 0.26 b | 65.64 ± 1.77 c | 86.71 ± 0.72 c | 57.34 ± 0.08 b | 36.16 ± 0.36 a |
| Roots | 1.48 ± 0.01 a | 44.87 ± 0.08 b | 91.52 ± 0.76 b | 137.08 ± 0.55 b | 92.21 ± 2.05 a | 33.00 ± 1.18 b |
| Flower heads | 1.20 ± 0.03 b | 84.54 ± 3.35 a | 96.35 ± 2.22 a | 151.20 ± 0.22 a | 93.22 ± 1.59 a | 17.43 ± 1.87 c |
Values are reported as mean ± SD of three parallel experiments. TE: Trolox equivalent; EDTAE: EDTA equivalent. Different letters indicate significant differences in the tested extracts (p < 0.05).
The collected data are consistent with the highest values of TPC and TFC (Table 1). The total antioxidant capacity (TAC) of the T. balsamita extracts was evaluated by the phosphomolybdenum assay, where the highest values, up to 1.48 ± 0.01 mmol TE/g were detected in the root extract, with the highest values, followed by flower heads and leaf extracts (Table 3). Regarding TAC, our results were comparable to those obtained in T. poterifolim, T. macrophyllum, T. vulgare and T. parthenium [20,21,25,26]. Additional antioxidant assays were carried out to provide insights into the antioxidant properties of the assayed T. balsamita extracts. The flower heads extract had the most pronounced radical scavenging activity in DPPH (84.54 ± 3.35 mg TE/g) and ABTS (96.35 ± 2.22 mg TE/g) assays. Reducing power is an important way to evaluate electron-donating ability of antioxidants. Thus, the reducing power of the assayed extracts was investigated by FRAP (from Fe3+ to Fe2+) and CUPRAC (from Cu2+ to Cu+) assays. The highest reducing power in both assays was found in the flower heads extract (93.22 ± 1.59 mg TE/g for FRAP and 151.20 ± 0.22 mg TE/g for CUPRAC). The results compare favorably with our previous study on Tanacetum species using the same assays. Generally, the received data for radical scavenging activity are consistent with those previously recorded in T. parthenium, T. poteriifolium and T. vulgare extracts and substantially lower in comparison with T. macrophyllum extracts [20,21,25,26]. The aforementioned Tanacetum species revealed higher reducing power activity than costmary extracts. T. balsamita leaves extract possessed strong chelating ability being more potent compared to T. parthenium, T. poteriifolium and T. vulgare extracts.
2.5. Enzyme Inhibitory Activity
The inhibitory ability of extracts prepared from the T. balsamita flower heads, leaves and roots against enzymes targeted in the management of type II diabetes mellitus, Alzheimer’s disease, lipid metabolism and skin hyperpigmentation problems were investigated.
The highest AChE and BChE inhibitory potential were observed for the leaf extract (2.11 ± 0.04 mg GALAE/g and 2.43 ± 0.04 mg GALAE/g, respectively) (Table 4).
Table 4.
Enzyme inhibitory activity of the T. balsamita extracts.
| Samples | AChE (mg GALAE/g) |
BChE (mg GALAE/g) |
Tyrosinase (mg KAE/g) |
α-Amylase (mmol ACAE/g) |
α-Glucosidase (mmol ACAE/g) | Lipase (mg OE/g) |
|---|---|---|---|---|---|---|
| Leaves | 2.11 ± 0.04 a | 2.43 ± 0.04 a | 54.65 ± 1.30 a | 0.44 ± 0.01 a | 0.19 ± 0.05 c | 4.02 ± 0.67 b |
| Roots | 2.00 ± 0.03 b | 1.33 ± 0.20 b | 51.43 ± 0.66 b | 0.43 ± 0.02 a | 0.71 ± 0.07 a | 8.15 ± 1.00 a |
| Flower heads | 1.83 ± 0.08 c | na | 45.49 ± 1.11 c | 0.28 ± 0.02 b | 0.50 ± 0.03 b | na |
Values are reported as mean ± SD of three parallel experiments. GALAE: Galantamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; OE: Orlistat equivalent; na: not active. Different letters indicate significant differences in the tested extracts (p < 0.05).
The same sample was found to have the highest tyrosinase and α-amylase inhibitory activity (54.65 ± 1.30 mg KAE/g and 0.44 ± 0.01 mmol ACAE/g, respectively). α-Glucosidase inhibitory potential did not exceed 0.71 ± 0.07 mmolACAE/g, while lipase inhibition was up to 8.15 ± 1.00 mg OE/g, whre both were for the root extract (Table 4). Aforementioned results were consistent with those previously recorded in T. vulgare except for the lower α-glucosidase and the higher tyrosinase inhibitory potential [20]. It’s worth noting that T. poteriifolium aerial parts demonstrated remarkable tyrosinase and α-glucosidase inhibition among the assayed Tanacetum species [26].
These findings could be related to the extracts chemical profiling (Table 2). For instance, flavonoids and acylquinic acids have been shown as inhibitors of the studied enzymes. However, the enzyme inhibitory potential is not directly related to TPC and TFC as seen in T. vulgare and T. macrophyllum [20,21]. It appears that the enzyme inhibition could be ascribed to the sesquiterpene lactones. Hence, it may be assumed that sesquiterpene lactones act in a synergistic way in AChE related disorders [42]. Furthermore, the germacranolide parthenolid and monoterpene thujone have been already reported as cholinesterase inhibitors [43,44,45]. Orhan et al. (2015) hypothesized that parthenolode plays a role in AChE inhibition in a synergistic manner together with other compounds (monoterpenes). Thus, the leaves extract of Tanacetum argenteum subsp. flabellifolium had the highest AChE inhibitory effect (96.68 ± 0.35%). C-flavonoid glycoside homoorientin, identified in costmary leaves extract, was previously reported to inhibit AChE in an in silico and in vivo study [46]. At 100 mg/kg for 3 weeks homoorientin inhibited the activity of AChE in rats with experimentally induced Alzheimer’s disease. Hispidulin, identified in the Phyla nodiflora extracts, was previously reported to inhibit tyrosinase with an IC50 value of 146 µM [47]. In addition, chlorogenic acid and its derivatives have potential as cholinesterase and glucosidase inhibitors [48,49,50]. Thus, chlorogenic acid inhibited AChE and BChE and pro-oxidant-induced lipid peroxidation in rat brain in vitro (IC50 value of 8.01 mg/mL and 6.3 mg/mL, respectively) [49]. At 5 mg/kg 3,5-dicaffeoylquinic acid reduced significantly the blood glucose levels and ameliorate the oxidative stress biomarkers reduced glutathione, malondialdehyde and serum biochemical parameters [50].
2.6. PLS-DA Analysis
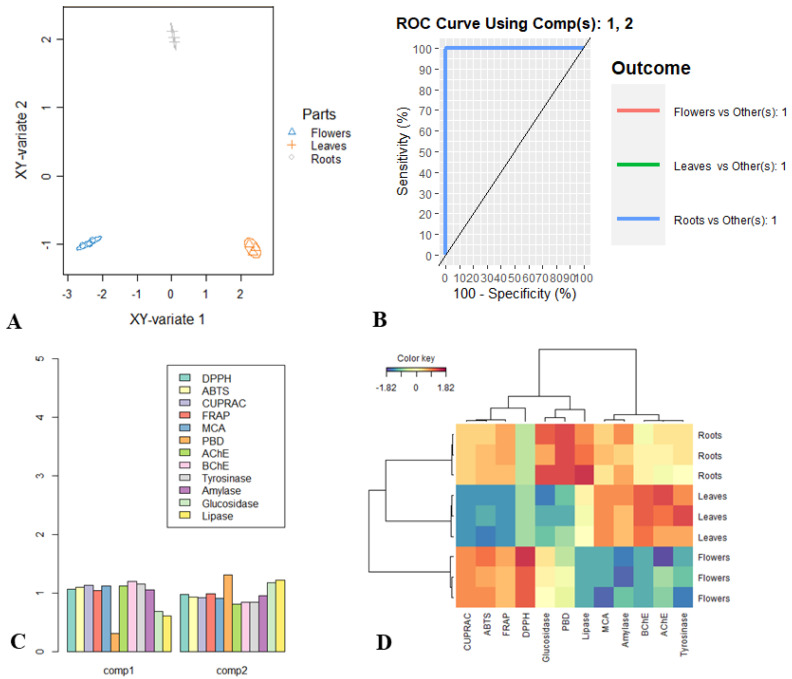
Based on the antioxidant, enzyme inhibitory, a supervised partial least square discriminant analysis (PLS-DA) was simulated considering parts as class membership criteria, and the outcomes are summarized in Figure 2.
Figure 2.
Partial Least-Squares Discriminant analysis graphical outputs on the biological activities of Tanacetum balsamita. (A) Samples plot. (B) ROC curve and AUC averaged using one-vs.-all comparisons. (C) The most discriminant biological activities identifying though VIP score calculation. (D) Clustered Image map (Ward linkage, Euclidean distance).
The discriminant analysis resulted in a good segregation of the three parts (Figure 2A). Figure 2B. showed the performance of the model evaluated through the Area Under the Curve (AUC) average using one-vs-all comparisons. An AUC value of 1 was obtained when taking account 2 function, suggesting the great segregation between the three parts along the first two function. By referring to Figure 2C, it appears that the first function separated the samples based on DPPH, ABTS, CUPRAC, FRAP, MCA, AChE, BChE, tyrosinase and amylase activities, while the second function separated the samples according to PBD, glucosidase and lipase activities. Regarding Figure 2D, the strongest activity recorded by the flower heads extract were DDPH, FRAP, CUPRAC and ABTS. Similarly, the roots proved to be the most effective plant part to give better PBD, anti-glucosidase and anti-lipase properties, while the higher anti-BChE, anti-AChE and anti-tyrosinase activity were recorded by the leaves extract. Furthermore, the contribution of the metabolites in the biological activities was evaluated through the Pearson’s correlation analysis. As reported in Tables S2 and S3, several metabolites seem to be involved in various biological activities, since a positive Pearson coefficient higher than 0.7 was obtained. Some metabolites are well known in the literature for their various properties; protocatechuic acid, syringic acid, isorhamnetin and quercetin have been reported for potential action, such as antioxidant activity [51,52,53,54]. In addition, neochlorogenic was reported to be the predominant antioxidant compound in Polygonum cuspidatum leaves [55]. Further, experimental studies support the effectiveness of protocatechuic acid and vanillic acid in the prevention of diabetes diseases and neurodegenerative processes, including Alzheimer’s [56,57]. In addition, p-coumaric acid is well known for its antioxidant activity, prevention and improvement of diabetes and neuroprotection [58].
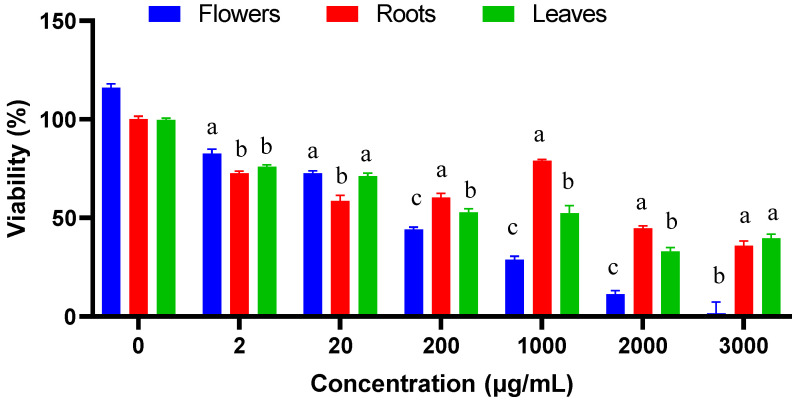
2.7. Cytotoxicity Assay
To assess the cytotoxicity of the extracts, we used the common THP-1 cells, a human monocytic cell line that mimics the behavior of the costmary extracts towards the immune system (Figure 3). After 24 h of incubation of macrophage cell line THP-1 with flower heads, roots, and leaves extracts, we observe 25% toxicity for the three extracts at concentration of 2 µg mL−1. A toxicity of 50% was reached at 200 µg mL−1 for flower heads extract, 1000 µg mL−1 and 2000 µg mL−1 for leaves and roots extracts, respectively, which may be regarded as a very high concentration, without any biological meaning. At a concentration of 3000 µg mL−1, a 100% toxicity for flower heads extract was observed.
Figure 3.
Cytotoxicity of T. balsamita extracts towards THP-1 cells (one-way ANOVA, different letters indicate significant difference between plant parts in the same concentration (a, b and c), p ≤ 0.05).
3. Materials and Methods
3.1. Plant Material
The leaves, flower heads and roots of T. balsamita were collected from herbal garden (Belopoptsi village, Gorna Malina region) in Bulgaria at 700 m a.s.l. (42.67° N 23.77° E), during the full flowering stage in July 2021. The seedlings were provided by the greenhouse “Zelena prolet” (Sofia, Bulgaria). The plant was identified by one of us (V. B.) according to Kuzmanov [59]. The voucher specimen was deposited at Herbarium Academiae Scientiarum Bulgariae (SOM 177 806). Seven plant samples were separate into roots, leaves and flower heads and dried at room temperature.
3.2. Sample Extraction
Air-dried powdered leaves, roots and flower heads (10 g) were extracted with 80% MeOH (1:20 w/v) by sonication (80 kHz, ultrasound bath Biobase UC-20C) for 15 min (×2) at room temperature. The extracts were concentrated in vacuo and lyophilized (lyophilizer Biobase BK-FD10P) (Jinan, China) to yield crude extracts as follows: flower heads 1.08 g, leaves 1.93 g and roots 0.68 g. The lyophilized extracts were dissolved in 80% methanol (0.1 mg/mL). An aliquot (2 mL) of each extract solution was filtered through a 0.45 μm syringe filter (Polypure II, Alltech, Lokeren, Belgium) and subjected to UHPLC–HRMS analyses.
3.3. Chemicals
Acetonitrile (hypergrade for LC–MS), formic acid (for LC-MS) and methanol (analytical grade) were purchased from Merck (Merck, Bulgaria). The authentic standards used for compound identification were obtained from Extrasynthese (Genay, France) for protocatechuic, syringic, vanillic gentisic, p-coumaric, m-coumaric, o-coumaric acid, quercetin, apigenin, luteolin, apigenin 7-O-glucoside, kaempferol 3-O-glucoside, isorhamnetin 3-O-glucoside, luteolin 7-O-glucoside and rutin, and kaempferol 3-O-rutinoside, homoorientin, isoquercitrin, hyperoside, nepetin. Caffeic acid, neochlorogenic acid, 3,4-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, jaceosidin and hispidulin were supplied from Phytolab (Vestenbergsgreuth, Germany). Chlorogenic acid, isorhamnetin and cirsimaritin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.4. Ultra High-Performance Liquid Chromatography—High Resolution Mass Spectrometry (UHPLC—HRMS)
Mass spectrometry analyses were carried out on a Q Exactive Plus mass spectrometer (ThermoFisher Scientific, Inc., Waltham, MA, USA) equipped with a heated electrospray ionization (HESI-II) probe (ThermoScientific). The mass spectrometer was operated in negative and positive ESI modes within the m/z range from 100 to 1000. The other parameters were as follows: spray voltage 3.5 kV (+) and 2.5 kV (−); sheath gas flow rate 38; auxiliary gas flow rate 12; spare gas flow rate 0; capillary temperature 320 °C; probe heater temperature 320 °C; S-lens RF level 50 and scan mode: full MS (resolution 70,000) and MS/MS (17,500). The chromatographic separation was performed on a reversed-phase column Kromasil EternityXT C18 (1.8 µm, 2.1 × 100 mm) at 40 °C. The chromatographic analyses were run using 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) as the mobile phases. The flow rate was 0.3 mL/min. The run time was 33 min. The following gradient elution program was used: 0–1 min, 0–5% B; 1–20 min, 5–30% B; 20–25 min, 30–50% B; 25–30 min, 50–70% B; 30–33 min, 70–95% and 33–34 min 95–5% B. Equilibration time was 4 min [21]. Data were processed by Xcalibur 4.2 (ThermoScientific) instrument control/data handling software. Metabolite profiling using MZmine 2 software was applied to the UHPLC–HRMS raw files of the studied T. balsamita extracts. The areas under the curve (AUC) for each identified/annotated compound were plotted and used for further statistical analysis in 3.10.
3.5. Data Filtering for Annotation of Target Compounds
Raw (ThermoFisher Scientific) mass spectrometric files were converted to .ms1 (MS1 data) and .mgf (MS2 data) by MSConvertGUI 3.1 (ProteoWizard) and manipulated under the R programming language (version 4.2.1, 2022-06-23, Funny-Looking Kid). The MS2 spectra were screened for the presence of the available target (hydroxybenzoic acid derivatives and flavonoids) compounds. The screening was achieved by selecting spectra based on the following criteria: m/z error of the molecular ion <15 ppm, retention time error <2%, number of fragment ions match >2/3, absolute error of the percentage intensity of matched fragment ions <15. Spectra identified as the same reference compound found in the same chromatographic peak were grouped, i.e., the spectra were summed, the m/z were adjusted by weight averaging where is the recalculated m/z value and inti are the m/z and the intensity of the ith fragment ion, respectively.
3.6. Total Phenolic and Flavonoid Contents
Total phenols and flavonoids were measured as gallic acid (GAE) and rutin (RE) equivalents respectively, through validated spectrophotometric methods. The experiments were carried out as reported in previous studies [60,61,62]. The detailed protocols are given in Supplementary Materials.
3.7. Determination of Antioxidant and Enzyme Inhibitory Effects
Intrinsic scavenging/reducing properties of the extracts (0.2–1 mg/mL) were determined through colorimetric assays [21]. Additionally, extracts (0.2–1 mg/mL) were assayed for evaluating enzyme inhibition effects towards tyrosinase, α-amylase, α-glucosidase and cholinesterases and lipase. Detailed protocols were reported elsewhere [21,62,63,64]. The detailed antioxidant and enzyme inhibitory assays are given in Supplementary Materials.
3.8. Cell Line and Culture
The human monocytic THP-1 (TIB-202) cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells grown in RPMI 1640 were supplemented with 10% fetal bovine serum, 1% glutamine, 1% penicillin/streptomycin and 0.5% Amphotericin B. Cells were cultured in a humidified atmosphere at 37 °C under a 5% CO2 atmosphere.
3.9. Cytotoxicity Assay
THP-1 cells (at 1.105 cells/mL) in RPMI medium (Thermo-Fisher) supplemented with 10% FBS were seeded in each well of a 48-well plate (n = 4). Cells were permitted to adhere for 24 h, and then treated with roots, flowers and leaves extracts of T. balsamita in a medium for 24 h. Then, 40 µL of WST-1 testing solutions (Sigma-Aldrich) was added to each well and the plate incubated at 37 °C for 2 h. The contents of each well were laid down in 3 wells of a 96-well plate [65]. The absorbances were measured at 350 and 630 nm with an Omega StarLab spectrophotometer (Omega, Ortenberg, Germany).
3.10. Statistical Analysis
In the antioxidant and enzyme inhibitory assays, the values are expressed as mean ± SD of three parallel experiments.
In terms of antioxidant and enzyme inhibitory abilities, one way ANOVA with Tukey’s assay was performed to determine differences between the tested extracts. The statistical analysis was performed using XlStat 16.0 software. Clustered Image Maps (CIM) were used to visualize metabolite variation among the extracts. Prior to CIM analyses, data were normalized and centered. Afterwards, a supervised Partial Least-Square discriminant analysis (PLS-DA) was done to discriminate the different parts regarding their biological activities. Then, CIM was applied on PLS-DA outcomes to characterize each extract. Lastly, Pearson’s correlation coefficients were calculated to evaluate the relationship between secondary metabolites and the biological activities, respectively.
4. Conclusions
More than 100 secondary metabolites, including methoxylated flavonols and flavones, acylquinic acids analogues, hydroxybenzoic and hydroxycinnamic acids derivatives, and their glycosides, were annotated/dereplicated in the costmary leaves, flower heads and roots extracts. Ninety-one compounds are reported in the species for the first time. Chlorogenic, 3, 5-diCQA and 4, 5-diCQA acid dominated the leaves and roots extracts profiles. Despite the previously published data on the high concentration of cichoric acid in the costmary aerial parts extract, we were not able to confirm the presence of either cichoric acid or any esters of tartaric acid and hydroxycinnamic acid. According to this study, the presence of 6-methoxylated flavones and flavonols, dicaffeoylquinic acids and their hexosides and phenolic acids glycosides could be considered significant in the chemotaxonomy of the Tanacetum genus. To understand the relationship between plant parts and biological activity, multivariate statistical analyses were performed. The strongest antioxidant activity (DDPH, FRAP, CUPRAC and ABTS) of the flower heads extract could be related to the presence of rutin, isoquercitrin and hyperoside, and the corresponding aglycone. A variety of acylquinic acids, flavoneshexuronides and methoxylated aglycones in the leaves extract could be associated with its anti-BChE, anti-AChE and anti-tyrosinase activity. Phenolic acidshexosides, di- and tri- caffeoylquinic acids accounted for the stronger α-glucosidase and α-lipase inhibitory activity of the roots extracts. The assayed extracts expressed low cytotoxicity towards THP-1 viability. In addition to evoking an antioxidant response, costmary extracts display in vitro enzyme inhibitory effects, which generate interest in the plant as a valuable herbal drug. Moreover, this study advocates further work geared towards additional in vivo studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12010022/s1: Figure S1: Extracted ion chromatogram of hydroxybenzoic and hydroxycinamic acids and derivatives in negative ion mode of methanol–aqueous extracts from Tanacetum balsamita. Figure S2: Extracted ion chromatogram of acylquinic acids in negative ion mode of methanol–aqueous extracts from Tanacetum balsamita. Figure S3: Extracted ion chromatogram of flavonoid glycosides in negative ion mode of methanol–aqueous extracts from Tanacetum balsamita. Figure S4: Extracted ion chromatogram of flavonoid aglycones in the negative ion mode of methanol–aqueous extracts from Tanacetum balsamita. Table S1: Flavonoids in Tanacetum balsamita extracts assayed by UHPLC-ESI-MS/MS in positive ion mode. Assays for the total phenolic and flavonoid content. Determination of antioxidant and enzyme inhibitory effects. Table S2: Correlation antioxidant activity. Table S3: Correlation enzyme inhibition.
Author Contributions
Conceptualization, R.G., G.Z., Y.V. and O.J.; methodology, R.G., G.Z., Y.V., D.Z.-D., K.I.S. and O.J.; software, K.I.S. and Y.V.; validation, R.G., G.Z. and D.Z.-D.; formal analysis, G.Z. and M.K.; investigation, D.Z.-D., V.B., M.K. and O.J.; resources, R.G. and O.J.; data curation, R.G., G.Z., D.Z.-D. and Y.V.; writing—original draft preparation, R.G., G.Z. and O.J.; writing—review and editing, R.G., G.Z., V.B. and O.J.; visualization, G.Z.; supervision, R.G. and O.J.; project administration, R.G. and O.J. and funding acquisition, R.G. and O.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by a French–Bulgarian bilateral project: Grant KП-06-Rila/5, 15 December 2021, Bulgarian National Science Fund and Hubert Curien PHC RILA 48057WC.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hassanpouraghdam M.-B., Tabatabaie S.-J., Nazemiyeh H., Vojodi L., Aazami M.-A., Shoja A.M. Chrysanthemum balsamita (L.) Baill.: A forgotten medicinal plant. FU Med. Biol. 2008;15:119–124. [Google Scholar]
- 2.Bremer K. Asteraceae: Cladistics and Classification, 34–35. Timber Press; Portland, OR, USA: 1994. [Google Scholar]
- 3.Oberprieler C., Himmelreich S., Källersjö M., Joan Vallès J., Linda E., Watson L., Vogt R. Anthemideae. In: Funk V.A., Susanna A., Stussey T.F., Bayer R.J., editors. Systematics, Evolution, and Biogeography of Compositae. IAPT; Vienna, Austria: 2009. pp. 631–666. [Google Scholar]
- 4.Bagci E., Kursat M., Kocak A., Gur S. Composition and antimicrobial activity of the essential oils of Tanacetum balsamita L. subsp. balsamita and T. chiliophyllum (Fisch. et Mey.) Schultz Bip. var. chiliophyllum (Asteraceae) from Turkey. J. Essent. Oil-Bear Plants. 2008;11:476–484. doi: 10.1080/0972060X.2008.10643656. [DOI] [Google Scholar]
- 5.Ivashchenko I. Antimicrobial properties of Tanacetum balsamita L.(Asteraceae) introduced in Ukrainian Polissya. Ukr. J. Ecol. 2017;7:52–57. doi: 10.15421/20176. [DOI] [Google Scholar]
- 6.Sharif M., Najafizadeh P., Asgarpanah J., Mousavi Z. In vivo analgesic and anti-inflammatory effects of the essential oil from Tanacetum balsamita L. Braz. J. Pharm. Sci. 2020;56:e18357. doi: 10.1590/s2175-97902019000418357. [DOI] [Google Scholar]
- 7.Venskutonis P.R. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; Amsterdam, The Netherlands: 2016. Costmary (Chrysanthemum balsamita) Oils; pp. 365–375. [Google Scholar]
- 8.Yousefzadi M., Ebrahimi S.N., Sonboli A., Miraghasi F., Ghiasi S., Arman M., Mosaffa N. Cytotoxicity, antimicrobial activity and composition of essential oil from Tanacetum balsamita L. subsp. balsamita. Nat. Prod. Comm. 2009;4:1934578X0900400126. doi: 10.1177/1934578X0900400126. [DOI] [PubMed] [Google Scholar]
- 9.Jaimand K., Rezaee M. Chemical constituents of essential oils from Tanacetum balsamita L. ssp. balsamitoides (Schultz-Bip.) Grierson. from Iran. J. Essent. Oil. Res. 2005;17:565–566. doi: 10.1080/10412905.2005.9698996. [DOI] [Google Scholar]
- 10.Başer K.H.C., Demirci B., Tabanca N., Özek T., Gören N. Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch. & Mey.) Schultz Bip. var. chiliophyllum and T. haradjani (Rech. fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flavour Fragr. J. 2001;16:195–200. [Google Scholar]
- 11.Bylaitė E., Venskutonis R., Roozen J.P., Posthumus M.A. Composition of essential oil of costmary [Balsamita major (L.) Desf.] at different growth phases. J. Agric. Food Chem. 2000;48:2409–2414. doi: 10.1021/jf990245z. [DOI] [PubMed] [Google Scholar]
- 12.Gallori S., Flamini G., Bilia A.R., Morelli I., Landini A., Vincieri F.F. Chemical composition of some traditional herbal drug preparations: Essential oil and aromatic water of costmary (Balsamita suaveolens Pers.) J. Agric. Food Chem. 2001;49:5907–5910. doi: 10.1021/jf0107656. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Alonso M., Velasco-Negueruela A., Burzaco A. Tanacetum balsamita L.: A Medicinal Plant from Guadalajara (Spain); International Symposium on Medicinal and Aromatic Plants, XXIII IHC. Acta Hortic. 1992;306:188–193. doi: 10.17660/ActaHortic.1992.306.19. [DOI] [Google Scholar]
- 14.Bączek K.B., Kosakowska O., Przybył J.L., Pióro-Jabrucka E., Costa R., Mondello L., Gniewosz M., Synowiec A., Węglarz Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.) Ind. Crops Prod. 2017;102:154–163. doi: 10.1016/j.indcrop.2017.03.009. [DOI] [Google Scholar]
- 15.EMA, (European Medicines Agency) Public Statement on the Use of Herbal Medicinal Products Containing Thujone EMA/HMPC/732886/2010. May 22, 2012. [(accessed on 24 November 2022)]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/public-statement-use-herbal-medicinal-products-containing-thujone-revision-1_en.pdf.
- 16.Benedec D., Filip L., Vlase L., Bele C., Sevastre B., Raita O., Olah N.-K., Hanganu D. In vitro study of antioxidant activity and phenolic content of Chrysanthemum balsamita varieties. Pak. J. Pharm. Sci. 2016;29:1359–1364. [PubMed] [Google Scholar]
- 17.Pukalskas A., Venskutonis P.R., Dijkgraaf I., van Beek T.A. Isolation, identification and activity of natural antioxidants from costmary (Chrysanthemum balsamita) cultivated in Lithuania. Food Chem. 2010;122:804–811. doi: 10.1016/j.foodchem.2010.03.064. [DOI] [Google Scholar]
- 18.Samek Z., Holub M., Herout V., Błoszyk E., Drożdż B. Isoerivanin and dehydroisoerivanin-Minor sesquiterpene lactone components from Balsamita major DESF. Collect. Czechoslov. Chem. Commun. 1979;44:1468–1474. doi: 10.1135/cccc19791468. [DOI] [Google Scholar]
- 19.Todorova M.N., Ognyanov I.V. Sesquiterpene lactones in a population of Balsamita major cultivated in Bulgaria. Phytochemistry. 1989;28:1115–1117. doi: 10.1016/0031-9422(89)80194-3. [DOI] [Google Scholar]
- 20.Ak G., Gevrenova R., Sinan K.I., Zengin G., Zheleva D., Mahomoodally M.F., Senkardes I., Brunetti L., Leone S., Di Simone S.C. Tanacetum vulgare L.(Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021;153:112268. doi: 10.1016/j.fct.2021.112268. [DOI] [PubMed] [Google Scholar]
- 21.Gevrenova R., Zheleva-Dimitrova D., Balabanova V., Voynikov Y., Sinan K.I., Mahomoodally M.F., Zengin G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch. Bip. and Telekia speciosa (Schreb.) Baumg.(Asteraceae) Ind. Crops Prod. 2020;155:112817 [Google Scholar]
- 22.Abad M., Bermejo P., Villar A. An approach to the genus Tanacetum L.(Compositae): Phytochemical and pharmacological review. Phytother. Res. 1995;9:79–92. doi: 10.1002/ptr.2650090202. [DOI] [Google Scholar]
- 23.Radulović N.S., Blagojević P.D., Skropeta D., Zarubica A.R., Zlatković B.K., Palić R.M. Misidentification of tansy, Tanacetum macrophyllum, as yarrow, Achillea grandifolia: A health risk or benefit? Nat. Prod. Comm. 2010;5:1934578X1000500129. doi: 10.1177/1934578X1000500129. [DOI] [PubMed] [Google Scholar]
- 24.Williams C.A., Harborne J.B., Eagles J. Variations in lipophilic and polar flavonoids in the genus Tanacetum. Phytochemistry. 1999;52:1301–1306. doi: 10.1016/S0031-9422(99)00425-2. [DOI] [Google Scholar]
- 25.Zengin G., Cvetanović A., Gašić U., Stupar A., Bulut G., Şenkardes I., Dogan A., Sinan K.I., Uysal S., Aumeeruddy-Elalfi Z. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020;146:112202. doi: 10.1016/j.indcrop.2020.112202. [DOI] [Google Scholar]
- 26.Zengin G., Sieniawska E., Senkardes I., Picot-Allain M.C.N., Sinan K.I., Mahomoodally M.F. Antioxidant abilities, key enzyme inhibitory potential and phytochemical profile of Tanacetum poteriifolium Grierson. Ind. Crops Prod. 2019;140:111629. doi: 10.1016/j.indcrop.2019.111629. [DOI] [Google Scholar]
- 27.Ivănescu B., Tuchiluș C., Corciovă A., Lungu C., Mihai C.T., Gheldiu A.-M., Vlase L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia. 2018;66:282–288. [Google Scholar]
- 28.Chepel V., Lisun V., Skrypnik L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants. 2020;9:926. doi: 10.3390/plants9080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venditti A., Frezza C., Sciubba F., Serafini M., Bianco A., Cianfaglione K., Lupidi G., Quassinti L., Bramucci M., Maggi F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.) Ind. Crops Prod. 2018;118:225–235. doi: 10.1016/j.indcrop.2018.03.056. [DOI] [Google Scholar]
- 30.Gevrenova R., Zengin G., Sinan K.I., Yıldıztugay E., Zheleva-Dimitrova D., Picot-Allain C., Mahomoodally M.F., Imran M., Dall’Acqua S. UHPLC-MS Characterization and biological insights of different solvent extracts of two Achillea species (A. aleppica and A. santolinoides) from Turkey. Antioxidants. 2021;10:1180. doi: 10.3390/antiox10081180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clifford M.N., Wu W., Kirkpatrick J., Kuhnert N. Profiling the chlorogenic acids and other caffeic acid derivatives of herbal Chrysanthemum by LC− MS n. J. Agric. Food Chem. 2007;55:929–936. doi: 10.1021/jf062314x. [DOI] [PubMed] [Google Scholar]
- 32.Gevrenova R., Zheleva-Dimitrova D., Ruseva S., Denkov N., Konstantinov S., Lozanov V., Mitev V. Cytotoxic and hepatoprotective effects of Bupleurum flavum flavonoids on hepatocellular carcinoma HEP-G2 cells. J. Pharmaceut. Res. Int. 2016;11:1–8. doi: 10.9734/BJPR/2016/25785. [DOI] [Google Scholar]
- 33.Cuyckens F., Claeys M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectr. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 34.Faustino M.V., Pinto D.C., Gonçalves M.J., Salgueiro L., Silveira P., Silva A.M. Calendula L. species polyphenolic profile and in vitro antifungal activity. J. Funct. Foods. 2018;45:254–267. doi: 10.1016/j.jff.2018.04.013. [DOI] [Google Scholar]
- 35.Ren D., Ran L., Yang C., Xu M., Yi L. Integrated strategy for identifying minor components in complex samples combining mass defect, diagnostic ions and neutral loss information based on ultra-performance liquid chromatography-high resolution mass spectrometry platform: Folium Artemisiae Argyi as a case study. J. Chrom. A. 2018;1550:35–44. doi: 10.1016/j.chroma.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Justesen U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectr. 2001;36:169–178. doi: 10.1002/jms.118. [DOI] [PubMed] [Google Scholar]
- 37.Abd-Alla H.I., Shalaby N.M., Hamed M.A., El-Rigal N.S., Al-Ghamdi S.N., Bouajila J. Phytochemical composition, protective and therapeutic effect on gastric ulcer and α-amylase inhibitory activity of Achillea biebersteinii Afan. Arch. Pharm. Res. 2016;39:10–20. doi: 10.1007/s12272-014-0544-9. [DOI] [PubMed] [Google Scholar]
- 38.Achakzai A.K.K., Achakzai P., Masood A., Kayani S.A., Tareen R.B. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak. J. Bot. 2009;41:2129–2135. [Google Scholar]
- 39.Çirak C., Radušienė J., Ivanauskas L., Janulis V. Variation of bioactive secondary metabolites in Hypericum origanifolium during its phenological cycle. Acta Physiol. Plant. 2007;29:197–203. doi: 10.1007/s11738-007-0024-7. [DOI] [Google Scholar]
- 40.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajner-Czopek A., Gertchen M., Rytel E., Kita A., Kucharska A.Z., Sokół-Łętowska A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants. 2020;9:412. doi: 10.3390/antiox9050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel M.B., Amin D. Sphaeranthus indicus flower derived constituents exhibits synergistic effect against acetylcholinesterase and possess potential antiamnestic activity. J. Complement. Integr. Med. 2012;9:23. doi: 10.1515/1553-3840.1618. [DOI] [PubMed] [Google Scholar]
- 43.Bajic V., Essack M. Combination Comprising Parthenolide for Use in the Treatment of Alzheimer’s Disease and Other Neurodegenerative Disorders. US 20150164858 A1. U.S. Patent. 2015 June 18;
- 44.Orhan I.E., Tosun F., Gülpınar A.R., Kartal M., Duran A., Mihoglugil F., Akalgan D. LC–MS quantification of parthenolide and cholinesterase inhibitory potential of selected Tanacetum L.(Emend. Briq.) taxa. Phytochem. Lett. 2015;11:347–352. doi: 10.1016/j.phytol.2014.10.003. [DOI] [Google Scholar]
- 45.Savelev S.U., Okello E.J., Perry E.K. Butyryl-and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004;18:315–324. doi: 10.1002/ptr.1451. [DOI] [PubMed] [Google Scholar]
- 46.Rasool M., Malik A., Waquar S., Tul-Ain Q., Jafar T.H., Rasool R., Kalsoom A., Ghafoor M.A., Sehgal S.A., Gauthaman K. In-silico characterization and in-vivo validation of Albiziasaponin-A, Iso-Orientin, and Salvadorin using a rat model of alzheimer’s disease. Front. Pharmacol. 2018;9:730. doi: 10.3389/fphar.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin F.-J., Yen F.-L., Chen P.-C., Wang M.-C., Lin C.-N., Lee C.-W., Ko H.-H. HPLC-fingerprints and antioxidant constituents of Phyla nodiflora. Sci. World J. 2014;2014:528653. doi: 10.1155/2014/528653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon S.-H., Lee H.-K., Kim J.-A., Hong S.-I., Kim H.-C., Jo T.-H., Park Y.-I., Lee C.-K., Kim Y.-B., Lee S.-Y. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010;649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Oboh G., Agunloye O.M., Akinyemi A.J., Ademiluyi A.O., Adefegha S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013;38:413–419. doi: 10.1007/s11064-012-0935-6. [DOI] [PubMed] [Google Scholar]
- 50.Simeonova R., Vitcheva V., Zheleva-Dimitrova D., Balabanova V., Savov I., Yagi S., Dimitrova B., Voynikov Y., Gevrenova R. Trans-3, 5-dicaffeoylquinic acid from Geigeria alata Benth. & Hook. f. ex Oliv. & Hiern with beneficial effects on experimental diabetes in animal model of essential hypertension. Food Chem. Toxicol. 2019;132:110678. doi: 10.1016/j.fct.2019.110678. [DOI] [PubMed] [Google Scholar]
- 51.Baghel S.S., Shrivastava N., Baghel R.S., Agrawal P., Rajput S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharmaceutical. Sci. 2012;1:146–160. [Google Scholar]
- 52.Gong G., Guan Y.-Y., Zhang Z.-L., Rahman K., Wang S.-J., Zhou S., Luan X., Zhang H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020;128:110301. doi: 10.1016/j.biopha.2020.110301. [DOI] [PubMed] [Google Scholar]
- 53.Kakkar S., Bais S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Notices. 2014;2014:952943. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C., Kumar C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 55.Kurita S., Kashiwagi T., Ebisu T., Shimamura T., Ukeda H. Identification of neochlorogenic acid as the predominant antioxidant in Polygonum cuspidatum leaves. Ital. J. Food Sci. 2016;28:25–31. [Google Scholar]
- 56.Krzysztoforska K., Mirowska-Guzel D., Widy-Tyszkiewicz E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019;22:72–82. doi: 10.1080/1028415X.2017.1354543. [DOI] [PubMed] [Google Scholar]
- 57.Sharma N., Tiwari N., Vyas M., Khurana N., Muthuraman A., Utreja P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020;20((Suppl. 2)):3053–3059. [Google Scholar]
- 58.Guan X.-Q. Research progress on pharmacological effects of p-coumaric acid. Chin. Tradit Herb. Drugs. 2018;24:4162–4170. [Google Scholar]
- 59.Kuzmanov B. Tanacetum balsamita L. In: Peev L.D., editor. Flora Republicae Bulgaricae: Asteraceae. Volume XI. Drinov M.; Sofia, Bulgaria: 2012. p. 382. [Google Scholar]
- 60.Slinkard K., Singleton V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977;28:49–55. [Google Scholar]
- 61.Zengin G., Nithiyanantham S., Locatelli M., Ceylan R., Uysal S., Aktumsek A., Selvi P.K., Maskovic P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016;8:286–292. doi: 10.1016/j.eujim.2015.12.004. [DOI] [Google Scholar]
- 62.Uysal S., Zengin G., Locatelli M., Bahadori M.B., Mocan A., Bellagamba G., De Luca E., Mollica A., Aktumsek A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017;8:290. doi: 10.3389/fphar.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grochowski D.M., Uysal S., Aktumsek A., Granica S., Zengin G., Ceylan R., Locatelli M., Tomczyk M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017;20:365–372. doi: 10.1016/j.phytol.2017.03.005. [DOI] [Google Scholar]
- 64.Saqib S., Nazeer A., Ali M., Zaman W., Younas M., Shahzad A., Sunera, Nisar M. Catalytic potential of endophytes facilitates synthesis of biometallic zinc oxide nanoparticles for agricultural application. Biometals. 2022;35:967–985. doi: 10.1007/s10534-022-00417-1. [DOI] [PubMed] [Google Scholar]
- 65.Asghar M., Younas M., Arshad B., Zaman W., Ayaz A., Rasheed S., Shah A., Ullah F., Saqib S. Bioactive potential of cultivated Mentha arvensis l. for preservation and production of health-oriented food. J. Anim. Plant Sci. 2022;32:835–844. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.