Abstract
Porphyromonas gingivalis has been implicated as a causative pathogen in periodontitis. Immunotherapeutic approaches have recently been suggested to aid in the clearance of P. gingivalis from disease sites. Because antibody-Fc receptor (FcR) interactions play a role in the effector functions of polymorphonuclear neutrophils (PMN), we evaluated which FcR on PMN from gingival crevicular fluid (GCF) serves as an optimal target molecule for FcR-directed immunotherapy. GCF PMN and peripheral blood (PB) PMN from adult periodontitis patients were analyzed for their immunoglobulin G (IgG) and IgA FcR (FcγR and FcαR, respectively) expression and function by studying IgG- and IgA-mediated elimination of P. gingivalis. GCF PMN exhibited higher FcαRI and FcγRI levels and lower FcγRIIa and FcγRIIIb levels than PB PMN. Functional studies revealed that GCF PMN exhibited less of a capacity to phagocytose and kill IgG1-opsonized P. gingivalis than PB PMN. IgA1-mediated phagocytosis and killing capacity was, however, comparable between GCF PMN and PB PMN. In summary, these in vitro results document that FcαRI represents a candidate target for FcR-directed immunotherapy for the clearance of P. gingivalis.
Porphyromonas gingivalis has been implicated as an etiological agent of periodontitis (22). P. gingivalis has also been suggested to play an important role in the pathogenesis of refractory or recurrent periodontitis (5). Moreover, periodontal bacterial infection with P. gingivalis has recently been proposed to constitute an increasing problem for certain patients with coronary heart disease (2, 11, 12). P. gingivalis infection can cause local gingival inflammation, leading to the ulceration of gingival epithelium and an increased vascularization of connective tissues in the periodontium. Conventional periodontal therapies, including plaque control, scaling, and root planing, have therefore been suggested to induce transient (but repeated) bacteremia (10, 32), which may represent a risk factor for atherosclerosis (2).
Phagocytes and, in particular, polymorphonuclear neutrophils (PMN) are essential for an effective antibacterial host response. Neutropenia and PMN dysfunction are thus critical risk factors for susceptibility to periodontitis (36). Antibody-Fc receptor (FcR) interactions are important for optimal phagocytosis and killing of pathogenic bacteria by PMN. In particular, antibody opsonization is necessary for the clearance of P. gingivalis because of its ability to withstand phagocytosis by PMN owing to immunoglobulin G (IgG) and C3 proteases and capsular polysaccharide (8, 9, 54). In patients with periodontitis, PMN constitute the predominant component (approximately 90%) of immunocompetent cellular infiltrate in gingival crevicular fluid (GCF) (46), wherein increased levels of IgG and IgA antibodies against P. gingivalis are observed (7, 56). Moreover, periodontal lesions have been shown to contain significant levels of P. gingivalis-specific IgG and IgA subclass antibody-secreting cells (42). Therefore, receptors for IgG (FcγR) or IgA (FcαR) expressed on GCF PMN may play a crucial role in the elimination of P. gingivalis.
Human PMN constitutively express three different FcRs: FcγRIIa (CD32), FcγRIIIb (CD16), and FcαRI (CD89). FcγRI (CD64) expression on PMN can be induced by granulocyte colony-stimulating factor in vivo (29) and by gamma interferon (IFN-γ) in vivo and in vitro (21). We have previously shown that the surface expression of FcγRIIa and FcγRIIIb on GCF PMN is decreased, resulting in diminished IgG-mediated phagocytosis by PMN in the periodontal pocket (37). However, the role of FcαRI and FcγRI in the pathogenesis of periodontitis remains unclear. As an approach to enhancing anti-P. gingivalis PMN function or to inhibiting colonization, major attention has been focused on local passive immunization with polyclonal antibodies or monoclonal antibodies (MAb) (3, 28, 47). It is therefore important to clarify the relative contributions of IgG and IgA receptors in triggering the anti-P. gingivalis function of GCF PMN. In this study, we assessed which FcR on GCF PMN could serve as a target for FcR-directed immunotherapy for the clearance of P. gingivalis.
MATERIALS AND METHODS
Study subjects.
Twenty-one Japanese patients who had moderate to severe adult periodontitis (11 males and 10 females; age range, 36 to 70 years; mean age, 52.0 years) and who were referred to the Periodontal Clinic of Niigata University Dental Hospital participated in this study. None of the participants had a history or current signs of systemic disease, nor had they used any medication for 3 months prior to this study. Clinical assessment of GCF sampling sites was performed as previously reported (37, 53); details are listed in Table 1. Informed consent was obtained from all participants with a format that was previously reviewed and approved by the ethical committee for the use of human subjects in research, Niigata University Faculty of Dentistry.
TABLE 1.
Clinical characteristics of patients and sampling sitesa
| Patient | Gender | Age (yr) | Smoking | GI | PPD (mm) | PAL (mm) | BL (%) |
|---|---|---|---|---|---|---|---|
| P1 | M | 38 | + | 1.3 | 4.8 | 5.0 | 43.9 |
| P2 | F | 57 | − | 1.2 | 2.7 | 2.7 | 26.1 |
| P3 | F | 50 | + | 1.0 | 2.9 | 3.9 | 36.6 |
| P4 | F | 59 | − | 1.8 | 3.5 | 3.4 | 24.3 |
| P5 | M | 39 | + | 1.3 | 5.2 | 5.2 | 56.2 |
| P6 | F | 44 | − | 1.5 | 4.9 | 5.5 | 53.2 |
| P7 | F | 48 | + | 1.0 | 4.4 | 4.6 | 41.7 |
| P8 | M | 70 | − | 1.5 | 4.2 | 4.7 | 37.8 |
| P9 | M | 39 | − | 1.4 | 3.9 | 3.8 | 32.8 |
| P10 | F | 43 | − | 0.5 | 5.3 | 6.9 | 55.8 |
| P11 | M | 67 | − | 0.1 | 2.7 | 3.4 | 18.5 |
| P12 | M | 36 | + | 1.7 | 6.1 | 6.8 | 62.0 |
| P13 | M | 39 | + | 0.7 | 4.2 | 4.6 | 36.9 |
| P14 | F | 68 | − | 1.4 | 3.5 | 3.5 | 22.2 |
| P15 | M | 65 | − | 1.0 | 4.2 | 6.4 | 49.4 |
| P16 | M | 62 | − | 0.7 | 2.9 | 2.9 | 44.1 |
| P17 | F | 40 | − | 1.6 | 6.1 | 7.0 | 77.1 |
| P18 | F | 67 | − | 1.1 | 2.2 | 3.3 | 38.0 |
| P19 | M | 44 | + | 1.0 | 4.4 | 5.1 | 42.3 |
| P20 | F | 57 | − | 1.5 | 3.3 | 3.6 | 13.5 |
| P21 | M | 59 | − | 1.8 | 5.1 | 5.6 | 42.7 |
| Mean ± SE | 52.0 ± 2.6 | 1.2 ± 0.1 | 4.1 ± 0.2 | 4.7 ± 0.3 | 40.7 ± 3.3 |
Each value for the clinical parameters, (i.e., GI, PPD, PAL, and BL) represents the mean score. GI, gingival index; PPD, probing pocket depth; PAL, probing attachment level; BL, bone loss. M, male; F, female. +, patient smoked; −, patient did not smoke.
Collection and isolation of GCF PMN and PB PMN.
GCF was sampled from periodontal pockets with a probing depth of more than 4 mm around 6 to 20 teeth, from the first incisor to the second premolar, as detailed before (37, 53). Briefly, periodontal pockets were washed for 30 min with a flow of Ca2+- and Mg2+-free Hanks' balanced salt solution containing heparin (10 IU/ml) by use of a 22-gauge flexible Teflon catheter tip at flow speeds of 10 ml/h. GCF-containing Hanks' balanced salt solution was pooled in a tube at 4°C and then passed through a 48-μm-pore-size stainless steel grid to remove plaque and tissue debris. The cellular constituents were isolated by centrifugation at 230 × g for 5 min at 4°C and resuspended in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) solution. Peripheral blood (PB) was obtained by venipuncture in the presence of heparin.
PMN were isolated from GCF or PB using a double density gradient purification method (Histopaque 1077 and 1119; Sigma, St Louis, Mo.) (13). Remaining erythrocytes were removed by adding ice-cold hypotonic lysis solution (10 mM Tris, 10 mM KCl, 1 mM MgCl2 [pH 7.4]). Purified PMN were washed twice, resuspended in PBS, and used immediately. The cellular samples were analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.) and were found to consist of >97% PMN for PB and >96% for GCF. The viability of PMN routinely exceeded 98% for PB and 89% for GCF, as determined by trypan blue exclusion.
MAb.
Fluorescein isothiocyanate (FITC)-labeled anti-FcγRI (CD64) MAb 22 (mouse IgG1) (20), anti-FcγRII (CD32) MAb IV.3 (IgG2b) (33), anti-FcγRIII (CD16) MAb 3G8 (mouse IgG1) (17), and anti-FcαRI (CD89) MAb A77 (mouse IgG1) (38) and unlabeled anti-FcγRI MAb 197 (mouse IgG2a) (20), anti-FcγRII MAb IV.3 Fab fragments (48), anti-FcγRIII MAb 3G8 F(ab′)2 fragments (48), and anti-FcαRI MAb My43 (mouse IgM) (50) were obtained from Medarex (Annandale, N.J.). Phycoerythrin (PE)-conjugated MAb CD11b was obtained from Becton Dickinson and used to label human PMN in phagocytosis assays. FITC-labeled mouse IgG was obtained from Coulter (Hialeah, Fla.).
FcR expression.
Levels of surface expression of FcγR and FcαR were analyzed by indirect immunofluorescence using a panel of MAb as described previously (37). In short, PB and GCF samples were divided into aliquots of 2 × 105 PMN per tube and incubated with PE-conjugated MAb CD11b for 30 min at 4°C. After being washed with ice-cold PBS twice, samples were incubated with FITC-labeled MAb A77, MAb 22, MAb IV.3, and MAb 3G8 or isotype-matched FITC-labeled mouse IgG for 30 min at 4°C. Following incubation, the mixture was washed twice with ice-cold PBS containing 0.2% EDTA and 0.1% NaN3 and analyzed with a FACScan flow cytometer and CELLQuest software (Becton Dickinson). Ten thousands cells were counted per sample tube by gating according to their characteristic forward and side scatter patterns. FITC or PE fluorescence intensity was expressed as mean log fluorescence.
FcR mRNA levels.
A total RNA sample was prepared from PB PMN and GCF PMN by the acid guanidinium thiocyanate extraction method (ISOGEN-LS; Nippon Gene, Toyama, Japan) (6). After extraction, RNA concentrations were determined by measurement of absorbances at 260 and 280 nm. First-strand cDNA was synthesized as previously described (52). Briefly, 0.5 μg of total RNA was reverse transcribed in 40-μl reaction mixtures containing 20 U of Moloney murine leukemia virus reverse transcriptase (RT; Toyobo, Osaka, Japan), 25 mM each deoxynucleoside triphosphate (Takara Shuzo, Shiga, Japan), 80 U of RNase inhibitor (Stratagene, La Jolla, Calif.), 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, and 1 μg of oligo(dT)18 (Stratagene) by incubation at 37°C for 60 min, followed by heating at 95°C for 5 min.
PCR amplifications were performed with 10 μl of cDNA, 15 mM Tris-HCl (pH 8.0), 50 mM KCl, 2.5 mM MgCl2, 40 nM each deoxynucleoside triphosphate, 2.5 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.), 200 nM each oligonucleotide primer encompassing the entire CD89 coding region (sense: 5′-ATG GAC CCC AAA CAG ACC-3′; anti-sense: 5′-TCC AGG TGT TTA CTT GCA GAC AC-3′) (39), and β-actin-specific primers (forward: 5′-GCG AGA AGA TGA CCC AGA TCA TGT T-3′; reverse: 5′-GCT TCT CCT TAA TGT CAC GCA CGA T-3′) (44) in a 50-μl reaction volume. PCR conditions were as follows: 95°C for 10 min, followed by 35 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min, with an extension step at 72°C for 10 min. PCR products were electrophoresed on 2% agarose gels and visualized by ethidium bromide staining. The end products of FcαR and β-actin were 873 and 300 bp, respectively. The density of amplified bands was analyzed with National Institutes of Health image software. The transcript levels for FcαR relative to β-actin were calculated by a modification of the formula described by Chelly et al. (4).
Bacteria.
P. gingivalis 381 was cultured in Trypticase soy broth (BBL, Cockeysville, Md.) supplemented with 0.5% yeast extract (Difco, Detroit, Mich.), hemin (5 μg/ml), and menadione (0.5 μg/ml) in an anaerobic chamber (Anaerobox; Hirasawa, Tokyo, Japan) with an atmosphere of 80% N2–10% H2–10% CO2 at 37°C to the midlogarithmic phase (55). Bacteria were harvested by centrifugation at 10,000 × g for 10 min, washed twice, and resuspended in PBS (2 × 108 CFU/ml). For phagocytosis assays, the bacteria were killed by heating at 60°C for 30 min and were labeled with FITC (Molecular Probes, Eugene, Oreg.) at a concentration of 0.5 mg/ml in 0.1 M sodium carbonate buffer (pH 9.6) at 37°C. Following incubation for 30 min, the bacteria were washed three times with PBS, divided into aliquots, and stored at −20°C until use.
Affinity purification of IgG1 and IgA1 antibodies.
IgG1 antibody was isolated from the serum of a patient with adult periodontitis and with a high level of anti-P. gingivalis serum IgG1 antibody (i.e., more than 10 times the mean enzyme-linked immunosorbent assay [ELISA] unit in 15 other patients) as previously described (30). Briefly, the IgG fraction prepared by protein G column chromatography (HiTrap protein G; Pharmacia, Uppsala, Sweden) was applied to a HiTrap NHS-activated Sepharose gel column (Pharmacia) coupled to an anti-human IgG1 MAb (HP6069; Zymed, San Francisco, Calif.). After washing and deactivation of reactive groups, IgG1 antibody bound to columns was eluted with 0.05 M glycine-HCl (pH 3.0), and the pH was neutralized immediately with 1 M Tris-HCl (pH 9.0).
IgA1 antibody was affinity purified from IgG-depleted serum isolated from the same patient with high IgA1 titers to P. gingivalis (i.e., more than three times the mean ELISA unit in 15 other patients) using agarose-bound jacalin (Pierce, Rockford, Ill.) (43). IgA1 was eluted from columns under conditions similar to those used for IgG1.
Following dialysis against PBS, the concentrations of affinity-purified IgG1 and IgA1 were determined to be 863 and 127 μg/ml, respectively, by IgG1 (human IgG subclass profile kit; Zymed) and IgA1 (human IgA ELISA quantitation kit; Bethyl, Montgomery, Tex.) subclass ELISAs.
Phagocytosis assay.
The phagocytic activity of PMN was analyzed by flow cytometry as described previously (30). FITC-labeled P. gingivalis was opsonized with purified IgG1 (20 μg/ml) or IgA1 (100 μg/ml) by incubation for 30 min at 37°C. After three washes, opsonized bacteria (2 × 106) were resuspended in PBS and incubated with isolated PMN (105) at a ratio of 20:1 for various times (0, 5, 15, or 25 min) at either 4 or 37°C in the absence of complement. In some experiments, PMN were preincubated for 30 min at 4°C with anti-FcγRI MAb 197 (20 μg/ml), Fab fragments of anti-FcγRII MAb IV.3 (20 μg/ml), F(ab′)2 fragments of anti-FcγRIII MAb 3G8 (20 μg/ml), or anti-FcαRI MAb My43 (20 μg/ml). Phagocytosis of P. gingivalis was quantified as the FITC fluorescence intensities of PMN using a FACScan flow cytometer and CELLQuest software. PE-conjugated CD11b MAb served as a marker for PMN.
To confirm that the uptake of P. gingivalis by PMN was true phagocytosis, experiments were repeated either at 4°C or in the presence of 4 μg of cytochalasin D (Sigma)/ml. Because both of these conditions revealed no phagocytosis, the FITC fluorescence intensities of cells maintained at 4°C throughout served as a control for bacterial binding (i.e., 0% phagocytosis). The percentage of PMN that phagocytosed P. gingivalis was defined as follows: percentage of phagocytosing PMN at 37°C − percentage of phagocytosing PMN at 4°C. Results are expressed as means and standard errors (SE).
Bacterial killing assay.
A CFU assay was performed by the method of Amano et al. (1). Briefly, freshly grown P. gingivalis (4 × 105), opsonized with purified IgG1 (20 μg/ml) or IgA1 (100 μg/ml) by incubation for 30 min at 37°C, was added to suspensions of PMN (2 × 105). Mixtures were incubated at 37°C in a 5% CO2 incubator. At various times (0, 30, 60, or 120 min), sample tubes were centrifuged at 150 × g for 10 min. Supernatant fractions were removed for determination of the number of extracellular bacteria, and the remaining PMN were lysed by incubation with sterile distilled water for 10 min. Triplicate preparations of supernatant fluids and disrupted PMN were diluted and plated on Trypticase soy agar plates supplemented with 5% sheep blood, 1 mg of yeast extract/ml, 5 μg of hemin/ml, and 1 μg of menadione/ml. Following incubation at 37°C for 7 days in an anaerobic chamber, the number of black-pigmented colonies was enumerated. In some experiments with GCF PMN, P. gingivalis colonies were identified by using the An-IDENT system (Analytab Products, Plainview, N.Y.) (40). The killing index was expressed as follows: [(Ncont − Nextra − Nintra)/(Ncont − Nextra)] × 100, where Nextra and Nintra are the numbers of viable extracellular and intracellular bacteria, respectively, and Ncont is the number of viable bacteria without PMN.
Statistical analyses.
Differences in FcR expression on the protein, in mRNA levels, and in the number of FcR-positive cells between PB PMN and GCF PMN were assessed by paired t tests. The same test was used to compare anti-P. gingivalis functions between PB PMN and GCF PMN. Significance was set at 5% (P < 0.05).
RESULTS
PMN FcR expression.
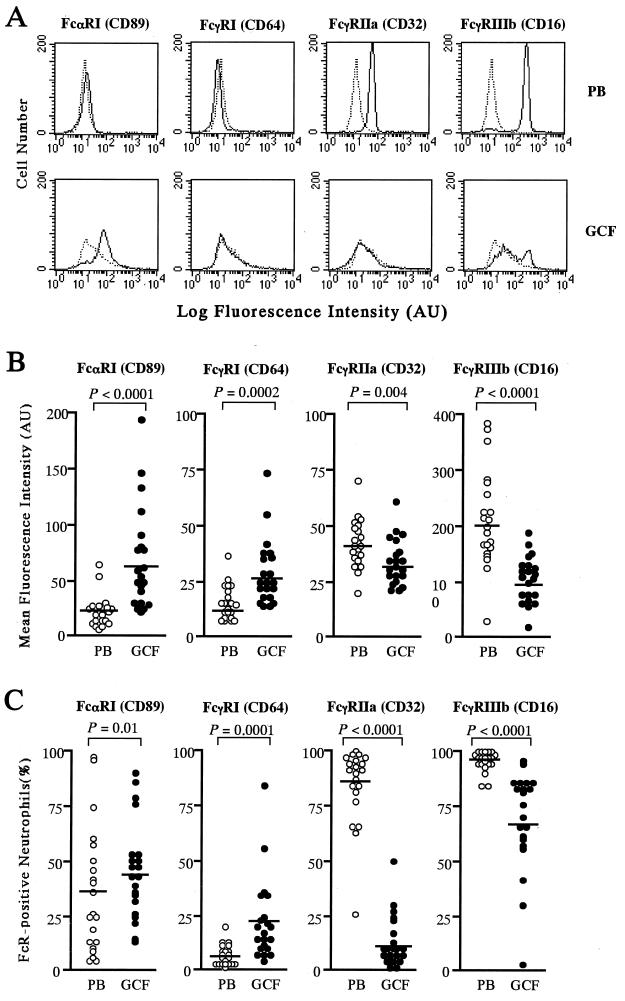
Figure 1A shows representative histograms of levels of FcR expression on PB PMN and GCF PMN obtained from a patient with adult periodontitis and analyzed by flow cytometry. The mean fluorescence intensities of GCF PMN labeled with anti-FcαRI and anti-FcγRI MAb were significantly higher than those of PB PMN (Fig. 1B). In addition, the percentages of FcαRI- and FcγRI-positive PMN were also higher in GCF than in PB (Fig. 1C).
FIG. 1.
Expression of FcR on PMN from adult periodontitis patients. (A) Representative flow cytometric histograms showing the expression of FcR (FcαRI [CD89], FcγRI [CD64], FcγRIIa [CD32], and FcγRIIIb [CD16]) on PMN from PB and GCF (solid lines) in a patient with adult periodontitis. The isotype-matched control profiles are shown by broken lines. AU, arbitrary units. (B) Expression of FcR on PB PMN and GCF PMN from 21 patients with adult periodontitis. Results are presented as mean fluorescence intensities on an arbitrary scale. Horizontal bars indicate the means for each group. (C) Percentages of FcR-positive PMN from PB and GCF in 21 patients with adult periodontitis. Results are presented as percentages of PMN showing specific fluorescence after labeling with anti-CD89, -CD64, -CD32, or -CD16 MAb. Horizontal bars indicate the means for each group. The P values reflect differences between the PB and GCF PMN groups, identified by paired t tests.
Levels of surface expression of FcγRIIa and FcγRIIIb on GCF PMN were significantly lower than those on PB PMN (Fig. 1B). The percentages of FcγRIIa- and FcγRIIIb-positive cells were also markedly lower in GCF PMN than in PB PMN (Fig. 1C).
Compared to FcγRI or FcγRIIa, FcαRI and FcγRIIIb were expressed at significantly higher levels on GCF PMN (the P value was <0.0001 for all comparisons). There was a similar trend in the number of MAb-positive GCF cells (for FcαRI versus FcγRI, the P value was 0.0023; the P value was <0.0001 for other comparisons).
PMN FcR mRNA levels.
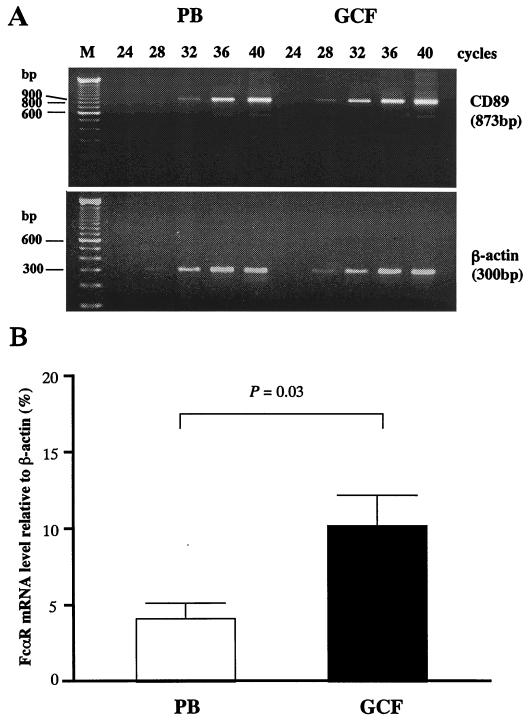
To characterize the mechanism leading to the changed FcR expression pattern, we next performed semiquantitative analyses of FcR mRNA by RT-PCR. Figure 2A shows typical FcαRI mRNA profiles determined by RT-PCR analyses. FcαRI mRNA levels of GCF PMN were higher than those of PB PMN, whereas β-actin levels were comparable (Fig. 2A). FcαRI mRNA levels relative to those of β-actin were significantly higher in GCF PMN than in PB PMN (Fig. 2B).
FIG. 2.
FcαRI mRNA levels in GCF PMN. (A) mRNA profiles for FcαRI determined by RT-PCR analysis. mRNA for β-actin was amplified as an internal control. Lane M, DNA molecular size markers; numbers over lanes indicate PCR amplification cycles. (B) Levels of transcripts of FcαRI relative to β-actin. Results are expressed as the percentage (mean and SE) of experiments with three different patients. The P value indicates the significance of the difference between the PB and GCF PMN groups, assessed by paired t tests.
IgG1- and IgA1-mediated phagocytosis by PMN.
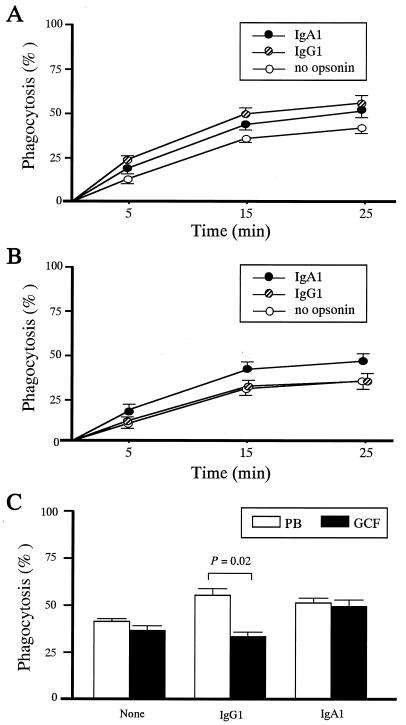
To determine the relative contributions of IgG and IgA receptors in triggering anti-P. gingivalis function, we compared the phagocytosis of IgG1- or IgA1-opsonized P. gingivalis between PB PMN and GCF PMN. The kinetics of phagocytosis of P. gingivalis by PB and GCF PMN throughout a 25-min incubation are shown in Fig. 3A and B, respectively. The phagocytosis of P. gingivalis by PB or GCF PMN was maximal after 25 min of incubation, irrespective of opsonin (Fig. 3A and B). P. gingivalis was significantly less effectively phagocytosed by GCF PMN than by PB PMN when incubated for 25 min with IgG1 (Fig. 3C). Notably, GCF PMN exhibited levels of phagocytosis of IgA1-opsonized P. gingivalis identical to those of PB PMN (Fig. 3C). IgA1-mediated phagocytosis by GCF PMN was strongly inhibited by anti-FcαRI MAb My43 but not by anti-FcγRI MAb 197, anti-FcγRII MAb IV.3, and anti-FcγRIII MAb 3G8, which block IgG1-mediated phagocytosis effectively (48, 58) (percentages of inhibition [mean and SE]: 86.1% ± 1.9% for anti-FcαRI, 1.9% ± 0.5% for anti-FcγRI, 8.7% ± 1.8% for anti-FcγRII, and 5.5% ± 2.0% for anti-FcγRIII). IgG1-mediated phagocytosis by GCF PMN was inhibited by anti-FcγRI, anti-FcγRII, and anti-FcγRIII MAb but not by anti-FcαRI MAb (percentages of inhibition: 0.8% ± 1.3% for anti-FcαRI, 11.8% ± 1.5% for anti-FcγRI, 65.8% ± 4.0% for anti-FcγRII, and 56.9% ± 2.3% for anti-FcγRIII).
FIG. 3.
Phagocytosis of IgG1- or IgA1-opsonized P. gingivalis by GCF PMN. (A) Phagocytosis of opsonized P. gingivalis by PB PMN over time. The percentage of phagocytosis was determined as defined in Materials and Methods. Mean and SE are indicated for each opsonin group (n = 3). (B) Phagocytosis of opsonized P. gingivalis by GCF PMN over time. The percentage of phagocytosis was determined as defined in Materials and Methods. Mean and SE are indicated for each opsonin group (n = 2). (C) Phagocytosis of IgG1- or IgA1-opsonized P. gingivalis by PB PMN and GCF PMN. Mean and SE are indicated for each opsonin group (n = 4). The P value indicates the significance of the difference between the PB and GCF PMN groups, identified by paired t tests.
IgG1- and IgA1-mediated bacterial killing by PMN.
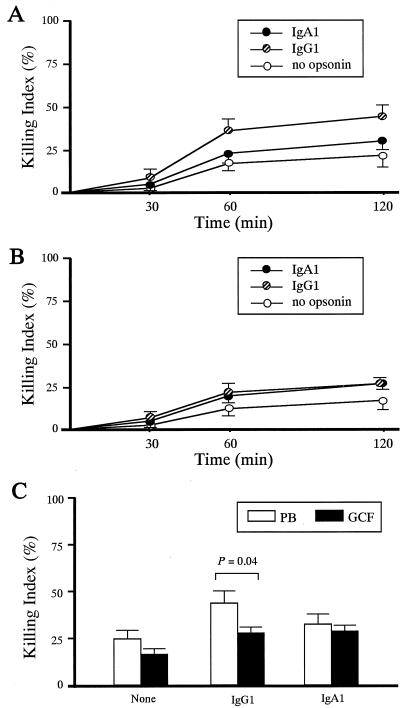
The kinetics of intracellular killing of opsonized P. gingivalis by PB PMN and GCF PMN are shown in Fig. 4A and B, respectively. Bactericidal activity was found to be maximal at 120 min of incubation, irrespective of opsonin (Fig. 4A and B). A significant difference was observed in the killing of IgG1-opsonized P. gingivalis between GCF and PB PMN, a result which was consistent with phagocytosis data (Fig. 4C). Bactericidal activity triggered by FcαRI, however, proved comparable between PB and GCF PMN.
FIG. 4.
Intracellular killing of IgG1- or IgA1-opsonized P. gingivalis by GCF PMN. (A) Intracellular killing of opsonized P. gingivalis by PB PMN over time. The killing index was determined as described in Materials and Methods. Mean and SE are indicated for each opsonin group (n = 3). (B) Intracellular killing of opsonized P. gingivalis by GCF PMN over time. The killing index was determined as described in Materials and Methods. Mean and SE are indicated for each opsonin group (n = 2). (C) Intracellular killing of IgG1- or IgA1-opsonized P. gingivalis by PB PMN and GCF PMN. Mean and SE are indicated for each opsonin group (n = 4). The P value indicates the significance of the difference between the PB and GCF PMN groups, identified by paired t tests.
DISCUSSION
We examined FcR expression and function of GCF PMN from adult periodontitis patients to identify target molecules as a first approach to the development of FcR-directed immunotherapy for the clearance of P. gingivalis. GCF PMN were found to exhibit higher levels of FcαRI and FcαRI-positive cells than PB PMN. These findings were in accordance with the results of immunohistochemical work showing that periodontal pocket areas were heavily infiltrated with FcαRI-expressing neutrophils (62). FcαRI expression on human PMN has been shown to be up-regulated by interleukin 8 (IL-8) (41), tumor necrosis factor alpha (TNF-α) (26), and FMLP (25). IL-8 and TNF-α mRNA-expressing cells have also been detected in inflamed gingival tissues of periodontitis patients (16, 34). Furthermore, patient GCF has been reported to contain high levels of IL-8 (27, 57), TNF-α (45, 51), and bacterial products (15, 23). It is therefore conceivable that the up-regulation of FcαRI levels is (at least partly) induced by cytokines and bacterial stimuli.
With regard to the mechanism underlying an increased level of FcαRI expression on activated PMN, this study documents higher FcαRI mRNA levels on GCF PMN than on PB PMN. This finding implies increased de novo synthesis of FcαRI transcripts. To our knowledge, this is the first study in which evidence for increased in vivo FcαRI transcript levels has been presented. However, it does not rule out the possibility that translocation of presynthesized FcαRI to PMN cell surfaces also occurs (25).
FcγRI levels increase on PMN after activation. Our results indicated that levels of surface expression of FcγRI were higher on GCF PMN than on PB PMN. Additionally, GCF contained significant numbers of FcγRI-positive cells, whereas PB did not. Earlier studies indicated that adult PB PMN expressed very low levels of FcγRI (35), a result consistent with our data. The up-regulation of FcγRI expression on PMN was observed for patients with febrile bacterial infections (31) and was shown to be induced by IFN-γ (21, 50). Human inflamed gingival tissue contains γδ T cells expressing IFN-γ mRNA (34, 61). Although we did not study IFN-γ levels in GCF in the present work, it does not seem too far-fetched to propose that this cytokine is (partly) responsible for the up-regulation of FcγRI on GCF PMN.
The levels of FcγRIIa and FcγRIIIb expression were down-regulated on GCF PMN. In particular, the numbers of double FcγR-positive GCF cells were profoundly decreased, a result consistent with previous data (37, 53). The decreased expression of both FcγRIIa and FcγRIIIb on GCF PMN may have been attributable to proteolytic cleavage by bacterial products, such as trypsin-like protease derived from P. gingivalis (55), or to insufficient intracellular pools. Neutrophil FcγRIIIb expression levels also decrease during the process of apoptosis (24).
Because GCF PMN exhibited higher levels of FcαRI (and FcγRIIIb) expression and larger numbers of FcαRI-positive GCF cells, we further examined the relative contributions of both receptors in triggering antibacterial function by using P. gingivalis opsonized with purified IgG1 and IgA1. Antibody opsonization is necessary for the clearance of P. gingivalis because of its ability to withstand phagocytosis by PMN due to IgG and C3 proteases and capsular polysaccharide (8, 9, 54). IgG1 is capable of interacting with all leukocyte FcγRs and therefore represents a good isotype for studying FcγR function (58). Our results showed that GCF PMN were less efficient in the IgG1-mediated clearance of P. gingivalis than PB PMN. These finding are consistent with the results of previous work, in which diminished phagocytosis of IgG-opsonized microspheres was linked to decreased levels of FcγRIIa and FcγRIIIb expression on GCF PMN (37).
The IgA1-mediated clearance of P. gingivalis by GCF PMN proved identical to the clearance by PB PMN. This results supports effective antibacterial function under physiological concentrations of IgA1 in vivo. Earlier studies indicated that neutrophil function in response to IgA correlated with FcαR expression levels (14, 25). Therefore, the highly expressed FcαR may induce anti-P. gingivalis function of GCF PMN to a level similar to that of PB PMN. Consistently, mucosal phagocytes have been shown to exhibit an increased capacity to bind and ingest IgA-opsonized targets (14, 25). Indeed, GCF PMN are immunocompetent cells that move from the bloodstream to mucosal sites (periodontal pocket), where subgingival plaque bacteria are coated with IgA (62). FcαR is expressed exclusively on phagocytes, whereas FcγR is more widely expressed on leukocytes (39). IgA-mediated functions may therefore be more effective for the elimination of periodontal pathogens than those initiated by IgG.
The concentration of IgA in GCF has been shown to negatively correlate with the severity of periodontitis (18, 19), suggesting that enhanced levels of IgA contribute to a decreased risk of periodontitis. In this study, we used polyclonal IgA1 antibodies for FcαR targeting. Conventional antibodies may, however, be limited for use in immunotherapy since many FcRs are ligand saturated in vivo (60). As a novel approach, we developed bispecific antibodies directed against both P. gingivalis and FcαR in a region other than the ligand-binding domains to improve the function of GCF PMN. The bispecific antibodies proved very effective in the elimination of other microorganisms (59) and may represent a novel immunotherapeutic approach for periodontitis.
In conclusion, GCF PMN exhibited increased FcαRI expression and concomitantly enhanced IgA-mediated anti-P. gingivalis function. These results support FcαRI as a suitable target for immunotherapy for the clearance of P. gingivalis.
ACKNOWLEDGMENTS
We are grateful to Marjolein van Egmond (Immunotherapy Laboratory, Department of Immunology, University Medical Center Utrecht) for valuable scientific comments.
This work was supported by grant-in aids for scientific research (12557191 and 12672032) from the Ministry of Education, Science, Sports and Culture, Tokyo, Japan, and by the Fund for Scientific Promotion of Tanaka Industries Co. Ltd., Niigata, Japan.
REFERENCES
- 1.Amano A, Ishimoto T, Tamagawa H, Shizukuishi S. Role of superoxide dismutase in resistance of Porphyromonas gingivalis to killing by polymorphonuclear leukocytes. Infect Immun. 1992;60:712–714. doi: 10.1128/iai.60.2.712-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 3.Booth V, Ashley F P, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996;64:422–427. doi: 10.1128/iai.64.2.422-427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelly J, Kaplan J-C, Maire P, Gautron S, Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissues. Nature. 1988;333:858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- 5.Choi J-I, Nakagawa T, Yamada S, Takazoe I, Okuda K. Clinical, microbiological and immunological studies on recurrent periodontal disease. J Clin Periodontol. 1990;17:426–434. [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Condorelli F, Scalia G, Cali G, Ressetti B, Nicoletti G, Lo Bue A M. Isolation of Porphyromonas gingivalis and detection of immunoglobulin A specific to fimbrial antigen in gingival crevicular fluid. J Clin Microbiol. 1998;36:2322–2325. doi: 10.1128/jcm.36.8.2322-2325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler C W, Arnold R R, Schenkein H A. Inhibition of C3 and IgG proteolysis enhances phagocytosis of Porphyromonas gingivalis. J Immunol. 1993;151:7016–7029. [PubMed] [Google Scholar]
- 9.Cutler C W, Kalmar J R, Arnold R R. Phagocytosis of virulent Porphyromonas gingivalis by human polymorphonuclear leukocytes requires specific immunoglobulin G. Infect Immun. 1991;59:2097–2104. doi: 10.1128/iai.59.6.2097-2104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly C, Mitchell D, Grossberg D, Highfield J, Stewart D. Bacteremia caused by periodontal probing. Aust Dent J. 1997;42:77–80. doi: 10.1111/j.1834-7819.1997.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande R G, Khan M B, Genco C A. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorn B R, Dunn W A, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–5798. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ericson S G, Zhao Y, Gao H, Miller K L, Gibson L F, Lynch J P, Landreth K S. Interleukin-6 production by human neutrophils after Fc-receptor cross-linking or exposure to granulocyte colony-stimulating factor. Blood. 1998;91:2099–2107. [PubMed] [Google Scholar]
- 14.Fanger M W, Goldstine S N, Shen L. Cytofluorographic analysis of receptors for IgA on human polymorphonuclear cells and monocytes and the correlation of receptor expression with phagocytosis. Mol Immunol. 1983;20:1019–1027. doi: 10.1016/0161-5890(83)90043-3. [DOI] [PubMed] [Google Scholar]
- 15.Fine D H, Mendieta C, Barnett M L, Furgang D, Naini A, Vincent J W. Endotoxin levels in periodontally healthy and diseased sites: correlation with levels of gram-negative bacteria. J Periodontol. 1992;63:897–901. doi: 10.1902/jop.1992.63.11.897. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald J E, Kreutzer D L. Localization of interleukin-8 in human gingival tissues. Oral Microbiol Immunol. 1995;10:297–303. doi: 10.1111/j.1399-302x.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 17.Fleit H B, Wright S D, Unkeless J C. Human neutrophil Fcγ receptor distribution and structure. Proc Natl Acad Sci USA. 1982;79:3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grbic J T, Lamster I B, Fine J B, Lam K S, Celenti R S, Herrera-Abreu M, Singer R E. Changes in gingival crevicular fluid levels of immunoglobulin A following therapy: association with attachment loss. J Periodontol. 1999;70:1221–1227. doi: 10.1902/jop.1999.70.10.1221. [DOI] [PubMed] [Google Scholar]
- 19.Grbic J T, Singer R E, Jans H H, Celenti R S, Lamster I B. Immunoglobulin isotypes in gingival crevicular fluid: possible protective role of IgA. J Periodontol. 1995;66:55–61. doi: 10.1902/jop.1995.66.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Guyre P M, Graziano R F, Vance B A, Morganelli P M, Fanger M W. Monclonal antibodies define two epitopes which trigger human FcγRI. J Immunol. 1989;143:1650–1655. [PubMed] [Google Scholar]
- 21.Guyre P M, Morganelli P M, Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Investig. 1983;72:393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haffajee A D, Socransky S S. Microbiological etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirose K, Isogai E, Miura H, Ueda I. Levels of Porphyromonas gingivalis fimbriae and inflammatory cytokines in gingival crevicular fluid from adult human subjects. Microbiol Immunol. 1997;41:21–26. doi: 10.1111/j.1348-0421.1997.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 24.Homburg C H, de Haas M, von dem Borne A E G K, Verhoeven A J, Reutelingsperger C P, Roos D. Human neutrophils lose their surface FcγRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–540. [PubMed] [Google Scholar]
- 25.Hostoffer R W, Krukovets I, Berger M. Increased FcαR expression and IgA-mediated function on neutrophils induced by chemoattractants. J Immunol. 1993;150:4532–4540. [PubMed] [Google Scholar]
- 26.Hostoffer R W, Krukovets I, Berger M. Enhancement by tumor necrosis factor-α of Fcα receptor expression and IgA-mediated superoxide generation and killing of Pseudomonas aeruginosa by polymorphonuclear leukocytes. J Infect Dis. 1994;170:82–87. doi: 10.1093/infdis/170.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Jin L, Söder B, Corbet E F. Interleukin-8 and granulocyte elastase in gingival crevicular fluid in relation to periodontopathogens in untreated adult periodontitis. J Periodontol. 2000;71:929–939. doi: 10.1902/jop.2000.71.6.929. [DOI] [PubMed] [Google Scholar]
- 28.Katoh M, Saito S, Takiguchi H, Abiko Y. Bactericidal activity of a monoclonal antibody against a recombinant 40-kDa outer membrane protein of Porphyromonas gingivalis. J Periodontol. 2000;71:368–375. doi: 10.1902/jop.2000.71.3.368. [DOI] [PubMed] [Google Scholar]
- 29.Kerst J M, van de Winkel J G J, Evans A H, de Haas M, Slaper-Cortenbach I C, de Wit T P, von dem Borne A E, van der Schoot C E, van Oers R H. Granulocyte colony-stimulating factor induces hFcγRI (CD64 antigen)-positive neutrophils via an effect on myeloid precursor cells. Blood. 1993;81:1457–1464. [PubMed] [Google Scholar]
- 30.Kobayashi T, van der Pol W-L, van de Winkel J G J, Hara K, Sugita N, Westerdaal N A C, Yoshie H, Horigome T. Relevance of IgG receptor IIIb (CD16) polymorphism to handling of Porphyromonas gingivalis: implications for the pathogenesis of adult periodontitis. J Periodontal Res. 2000;35:65–73. doi: 10.1034/j.1600-0765.2000.035002065.x. [DOI] [PubMed] [Google Scholar]
- 31.Leino L, Sorvajärvi K, Katajisto J, Laine M, Lilius E-M, Pelliniemi T-T, Rajamäki A, Silvoniemi P, Nikoskelainen J. Febrile infection changes the expression of IgG Fc receptors and complement receptors in human neutrophils in vivo. Clin Exp Immunol. 1997;107:37–43. doi: 10.1046/j.1365-2249.1997.d01-899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lofthus J E, Waki M Y, Jolkovsky D L, Otomo-Corgel J, Newman M G, Flemmig T, Nachnani S. Bacteremia following subgingival irrigation and scaling and root planing. J Periodontol. 1991;62:602–607. doi: 10.1902/jop.1991.62.10.602. [DOI] [PubMed] [Google Scholar]
- 33.Looney R J, Abraham G N, Anderson C L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986;136:1641–1647. [PubMed] [Google Scholar]
- 34.Lundqvist C, Baranov V, Teglund S, Hammarström S, Hammarström M-L. Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–2312. [PubMed] [Google Scholar]
- 35.Maeda M, van Schie R C A A, Yüksel B, Greenough A, Fanger M W, Guyre P M, Lydyard P M. Differential expression of Fc receptors for IgG by monocytes and granulocytes from neonates and adults. Clin Exp Immunol. 1996;103:343–347. doi: 10.1046/j.1365-2249.1996.d01-615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyasaki K T. The neutrophil: mechanisms of controlling periodontal bacteria. J Periodontol. 1991;62:761–774. doi: 10.1902/jop.1991.62.12.761. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki A, Kobayashi T, Suzuki T, Yoshie H, Hara K. Loss of Fcγ receptor and impaired phagocytosis of polymorphonuclear leukocytes in gingival crevicular fluid. J Periodontal Res. 1997;32:439–446. doi: 10.1111/j.1600-0765.1997.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 38.Monteiro R C, Cooper M D, Kubagawa H. Molecular heterogeneity of Fcα receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992;148:1764–1770. [PubMed] [Google Scholar]
- 39.Morton H C, Schiel A E, Janssen S W J, van de Winkel J G J. Alternatively spliced forms of the human myeloid Fcα receptor (CD89) in neutrophils. Immunogenetics. 1996;43:246–247. doi: 10.1007/BF00587311. [DOI] [PubMed] [Google Scholar]
- 40.Murray P R, Weber C J, Niles A C. Comparative evaluation of three identification systems for anaerobes. J Clin Microbiol. 1985;22:52–55. doi: 10.1128/jcm.22.1.52-55.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolova E B, Russell M W. Dual function of human IgA antibodies: inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J Leukoc Biol. 1995;57:875–882. doi: 10.1002/jlb.57.6.875. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa T, McGhee M L, Moldoveanu Z, Hamada S, Mestecky J, McGhee J R, Kiyono H. Bacteroides-specific IgG and IgA subclass antibody-secreting cells isolated from chronically inflamed gingival tissues. Clin Exp Immunol. 1989;76:103–110. [PMC free article] [PubMed] [Google Scholar]
- 43.Payne N R, Concepcion N F, Anthony B F. Opsonic effect of jacalin and human immunoglobulin A on type II group B streptococci. Infect Immun. 1990;58:3663–3670. doi: 10.1128/iai.58.11.3663-3670.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponte P, Ng S-Y, Engel J, Gunning P, Kedes L. Evolutionary conversation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984;12:1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossomando E F, Kennedy J E, Hadjimichael J. Tumor necrosis factor alpha in gingival crevicular fluid as a possible indicator of periodontal disease in humans. Arch Oral Biol. 1990;35:431–434. doi: 10.1016/0003-9969(90)90205-o. [DOI] [PubMed] [Google Scholar]
- 46.Saito I, Komiyama K, Moro I, Akachi K, Shiomi N, Ito K, Murai S, Umemura S. Ultrastructural and immunocytochemical characterization of polymorphonuclear leukocytes from gingival crevice in man. J Periodontol. 1987;58:493–497. doi: 10.1902/jop.1987.58.7.493. [DOI] [PubMed] [Google Scholar]
- 47.Saito S, Hayakawa M, Takiguchi H, Abiko Y. Opsonophagocytic effect of antibody against recombinant conserved 40-kDa outer membrane protein of Porphyromonas gingivalis. J Periodontol. 1999;70:610–617. doi: 10.1902/jop.1999.70.6.610. [DOI] [PubMed] [Google Scholar]
- 48.Sanders L A M, Feldman R G, Voorhorst-Ogink M M, de Haas M, Rijkers G T, Capel P J A, Zegers B J M, van de Winkel J G J. Human immunoglogulin G (IgG) Fc receptor IIA (CD32) polymorphism and IgG2-mediated bacterial phagocytosis by neutrophils. Infect Immun. 1995;63:73–81. doi: 10.1128/iai.63.1.73-81.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen L, Guyre P M, Fanger M W. Polymorphonuclear leukocyte function triggered through the high affinity Fc receptor for monomeric IgG. J Immunol. 1987;139:534–538. [PubMed] [Google Scholar]
- 50.Shen L, Lasser R, Fanger M W. My43, a monoclonal antibody that reacts with human myeloid cells, inhibits monocyte IgA binding and triggers function. J Immunol. 1989;143:4117–4122. [PubMed] [Google Scholar]
- 51.Stashenko P, Jandinski J J, Fujiyoshi P, Rynar J, Socransky S S. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 52.Sugita N, Kimura A, Matsuki Y, Yamamoto T, Yoshie H, Hara K. Activation of transcription factors and IL-8 expression in neutrophils stimulated with lipopolysaccharide from Porphyromonas gingivalis. Inflammation. 1998;22:253–267. doi: 10.1023/a:1022344031223. [DOI] [PubMed] [Google Scholar]
- 53.Sugita N, Suzuki T, Yoshie H, Yoshida N, Adachi M, Hara K. Differential expression of CR3, FcεRII and FcγRIII on polymorphonuclear leukocytes in gingival crevicular fluid. J Periodontal Res. 1993;28:363–372. doi: 10.1111/j.1600-0765.1993.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 54.Sundqvist G, Bloom G D, Enberg K, Johansson E. Phagocytosis of Bacteroides melaninogenicus and Bacteroides gingivalis in vitro by human neutrophils. J Periodontal Res. 1982;17:113–121. doi: 10.1111/j.1600-0765.1982.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 55.Tai H, Kobayashi T, Hara K. Changes in complement and immunoglobulin G receptor expression on neutrophils associated with Porphyromonas gingivalis-induced inhibition of phagocytosis. Infect Immun. 1993;61:3533–3535. doi: 10.1128/iai.61.8.3533-3535.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tew J G, Marshall D R, Burmeister J A, Ranney R R. Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect Immun. 1985;49:487–493. doi: 10.1128/iai.49.3.487-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonetti M S, Imboden M A, Lang N P. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–1147. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 58.van de Winkel J G J, Capel P J A. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 59.van Spriel A B, van den Herik-Oudijk I E, van Sorge N M, Vilé H A, van Strijp J A G, van de Winkel J G J. Effective phagocytosis and killing of Candida albicans via targeting FcγRI (CD64) or FcαRI (CD89) on neutrophils. J Infect Dis. 1999;179:661–669. doi: 10.1086/314643. [DOI] [PubMed] [Google Scholar]
- 60.van Spriel A B, van Ojik H H, van de Winkel J G J. Immunotherapeutic perspective for bispecific antibodies. Immunol Today. 2000;21:391–397. doi: 10.1016/s0167-5699(00)01659-5. [DOI] [PubMed] [Google Scholar]
- 61.Yamazaki K, Nakajima T, Hara K. Immunohistological analysis of T cell functional subsets in chronic inflammatory periodontal disease. Clin Exp Immunol. 1995;99:384–391. doi: 10.1111/j.1365-2249.1995.tb05562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Z-N, Gjermo P, Helgeland K, Schenck K. Fcα receptor I (CD89) on neutrophils in periodontal lesions. J Clin Periodontol. 2000;27:489–493. doi: 10.1034/j.1600-051x.2000.027007489.x. [DOI] [PubMed] [Google Scholar]