Abstract
Litomosoides sigmodontis is the only filaria which develops from infective larvae into microfilaria-producing adults in immunocompetent laboratory mice. In this study we report that interleukin-4 knockout (IL-4 KO) mice have an up to 100-fold-higher and a significantly prolonged microfilaremia compared to wild-type BALB/c mice, as well as 20 times more microfilariae in the thoracic cavity, the site of infection. While worm development and adult worm persistence were equivalent in IL-4 KO and wild-type mice, the fertility and length of adult female worms in IL-4 KO mice was clearly enhanced. The high susceptibility to microfilariae in IL-4 KO mice required the presence of adult worms in a full infection cycle since microfilariae loads did not differ much between IL-4 KO and wild-type mice when purified microfilariae were injected into mice. In addition, we found that eosinophilia was diminished and immunoglobulin E (IgE) was absent in IL-4 KO mice. IgE, however, does not seem to be the essential factor for microfilarial containment since microfilaremia was not elevated in B-cell KO mice. In conclusion, IL-4 is shown for the first time to be essential for the control of microfilarial loads but not of adult worm loads in a fully permissive murine filarial infection. IL-4 dependent effector pathways seem to operate on adult worms rather than directly on microfilariae.
Filariasis is an arthropode-borne parasitism which affects more than 120 million people in the tropics and confronts them with debilitating outcomes such as blindness, e.g., in onchocerciasis or elefantiasis. Infective third-stage larvae (L3 larvae) are injected into the host during a blood meal of the arthropod vector and develop into adult worms which release microfilariae into either skin (e.g., onchocerciasis) or blood (e.g., lymphatic filarioses).
Experimental studies with Brugia species in mice (20) have shown that intraperitoneally (i.p.) injected microfilariae elicit a TH1-type response. In contrast, infective third-stage larvae, as well as adult worms, induced a TH2-type response. In addition, the strong TH2-type response to adult, especially female, worms was able to override the TH1-type response to emerging microfilariae. However, interleukin-4 knockout (IL-4 KO) mice showed no alteration of parasitic loads in this model (18). In contrast, in another study where the infection of BALB/c and C57BL/6 mice with Brugia malayi was partially permissive, IL-4 deficiency resulted in prolonged worm survival (5). Thus, there remains some uncertainty as to whether TH2 responses are indeed host protective.
L. sigmodontis in BALB/c mice is the only model of filariasis which allows the observation of the complete development in an immunocompetent mouse (32). All developmental stages of the worm can be examined during one course of infection, in contrast to non- or semipermissive models. In addition, in nonpermissive models immune reactions can arise that differ from those in a permissive system because the parasite cannot reach its proper sanctuary (28).
Therefore, in the present study we compared the course of L. sigmodontis infection in IL-4 KO mice and BALB/c wild-type mice. IL-4-deficient mice showed a massive elevation of the microfiliarial burden in the blood and in the thoracic cavity, accompanied by a significantly prolonged microfilaremia. Thus, IL-4 is a major factor of microfilarial control in filarial infection.
(This work formed part of a doctoral study by L.V. at The University of Hamburg, Hamburg, Germany.)
MATERIALS AND METHODS
Animal maintenance and infection of mice with L. sigmodontis.
BALB/c-IL-4<tm2Nt> mice (hereafter referred to as IL-4 KO mice) were obtained from The Jackson Laboratory (Bar Harbor, Maine). B-less mice (BKO mice) backcrossed to a BALB/c background were a kind gift of A. O'Garra, DNAX, Calif. The KO strain was housed under specific-pathogen-free conditions in micro-isolator cages. KO offspring and wild-type BALB/c mice (originally from Charles River Laboratories, Sulzfeld, Germany) and cotton rats were bred at the animal facilities of the Bernhard Nocht Institute. Natural infections of mice with L. sigmodontis were performed as described previously (1, 3). L. sigmodontis lives naturally in the cotton rat (Sigmodon hispidus), which can survive very high blood microfilaria levels (up to 10,000 microfilariae [MF]/μl of blood). During the blood meal on an infected rat, the arthropod intermediate host (Ornithonyssus bacoti mite) ingests the MF, which molt twice and develop to the L3 larvae within 10 days. In a subsequent blood meal the mites transmit the L3 larvae onto cotton rats. Similarly, in the case of mice infection, the L3 larvae containing mites are allowed to take blood from mice and thereby transmit the L3 larvae. In the rodent host the L3 larvae migrate to the thoracic cavity and reach sexual maturity within 25 to 33 days. After copulation, the viviparous female worms start producing MF, which are detectable in the blood after day 50. At least five mice were used for each treatment group.
Parasite and inflammatory nodule recovery.
The number of adult worms was counted at days 28 and 80 postinfection (p.i.). To this, the thoracic cavity, the representative site for assessment of worm numbers (3, 25), was flushed with 10 ml of phosphate-buffered saline (PBS)–1% fetal calf serum (FCS), and the worms were allowed to sediment. The sediment was also used to determine the number of inflammatory nodules. Microfilaremia was determined in EDTA-treated peripheral blood after being stained with Hinkelmann's solution (0.5% [wt/vol] eosin Y, 0.5% [wt/vol] phenol, and 0.185 [vol/vol] formaldehyde in distilled water) as described earlier (3).
For the examination of uterine MF loads adult female worms were cleared in lactophenol and examined under the microscope (at magnifications of from ×20 to ×100). Thirteen female worms isolated from IL-4 KO mice and twenty-six female worms isolated from BALB/c mice were examined. The stages of developing eggs and MF were identified, and their uterine densities were quantified from zero (absent) to four crosses (+) (6). The lengths of females were measured with a rule. Each female worm was examined from the posterior extremity to the vulva in the anterior part (24).
Purification of peripheral blood MF.
MF were purified from the peripheral blood of cotton rats on a Percoll gradient as described elsewhere (7). Iso-osmotic Percoll (IOP) was prepared by mixing 9 parts of Percoll (density, 1.130 g/ml) with 1 part of 2.5 M sucrose. Various dilutions of the IOP in 0.25 M sucrose were made to obtain 25, 30, and 35% solutions. The gradient dilutions were layered in 15-ml tubes, and then twofold-diluted peripheral blood was pipetted onto the top of the Percoll layers. The tubes were then centrifuged at 400 × g for 30 min at room temperature. Recovered MF (between the 25 and the 30% layers) were washed with RPMI 1640–1% FCS, and 1.5 × 105 viable MF were injected i.p. into naive mice.
MF recovery from the peritoneal cavity.
The number of MF in the peritoneal cavity was counted 7, 14, and 21 days after the i.p. injection. To this the peritoneal cavity was flushed with 10 ml of PBS plus 1% FCS. MF burdens were examined after staining with Hinkelmann's solution.
Determination of proportions of inflammatory cells in thoracic cavity fluid.
For cytospin preparations, 200 μl of thoracic cavity cells (2 × 105/ml in PBS–1% FCS) were centrifuged against a glass slide with absorptive filter paper using a Shandon cytocentrifuge. Cytospin preparations were then stained using the Wright-Giemsa stain (Sigma, Taufkirchen, Germany) and neutrophils (NP), eosinophils (EP), macrophages (MP), and lymphocytes were differentially enumerated.
Cell culture.
The culture of thoracic cavity MP was carried out after adhesion on 96-well culture plates (Greiner, Frickenkausen, Germany) in RPMI 1640–5% FCS at 37°C and 5% CO2 for 2 h. We first did a fluorescence-activated cell sorter analysis to determine the relative proportion of MP in the thoracic cavity cells. According to these data, the cell input was calculated such that 40,000 MP/well were allowed to adhere. The nonadherent cells were removed by washing them three times with PBS. The purity was >95% (not shown). MP were cultured for 48 h in the presence of medium, 100 μg of adult worm antigen (AWA) per ml, or 20 ng of gamma interferon (IFN-γ; Pharmingen, Heidelberg, Germany) and 20 ng of lipopolysaccharide (E. coli O55; Sigma) per ml. Supernatants were then removed for cytokine determination.
NO determination.
Nitric oxide (NO) was assessed in supernatants of MP cultures by determination of the amounts of NO2− and NO3− (Griess reaction) as described previously (35).
Cytokine assays.
Concentrations of the cytokines IFN-γ, IL-5, IL-10, IL-12p70, and tumor necrosis factor alpha (TNF-α) in the pleura or peritoneal exudates (thoracic and peritoneal wash) were determined by specific two-site enzyme-linked immunosorbent assays (ELISAs) using standard protocols. The antibody pairs for capture and detection (biotinylated) were purchased from Pharmingen in the combinations recommended. Recombinant cytokines (from Pharmingen and from R&D Systems, Wiesbaden, Germany) were used as standards. Mouse KC (a homologue of human IL-8) was determined using affinity-purified goat IgG as capture (R&D catalog no. MAB453) and as biotinylated detector antibody (catalog no. BAF453). Recombinant chemokine (catalog no. 453-KC-010) was used as standard. All ELISAs were developed after incubation with streptavidin-peroxidase complex (1:10,000; Boehringer, Mannheim, Germany), using 3,5,3′,5′-tetramethylbenzidine as a substrate (Roth, Karlsruhe, Germany) dissolved at 6 mg/ml in dimethyl sulfoxide. The sensitivity was 20 pg/ml.
Total serum IgE.
The same ELISA standard procedure as for KC was used to determine the total IgE level. Monoclonal antibody (MAb) pairs (catalog no. R53-72, capture antibody; catalog no. R53-92, biotinylated detector antibody) were purchased from Pharmingen and used at 2 μg/ml for capture antibody and at 1 μg/ml for detector antibody. Murine IgE protein (MAb clone SPE-7, anti-DNP; Sigma) were used as standards. The sensitivity was 0.05 μg/ml.
NP chemotaxis assay.
Casein-elicited peritoneal cells (30) and thoracic cavity cells from L. sigmodontis-infected mice at day 80 p.i. were collected and resuspended in PBS–0.5 mM MgCl2–0.5 mM CaCl2–0.1% bovine serum albumin (BSA). The relative proportion of NP was determined by cytospin. NP chemotactic activity was assayed as described elsewhere (37). Briefly, in the lower compartment of blind-well Boyden chambers (Bio-Rad, Munich, Germany), either medium or the chemotaxin platelet-activating factor (PAF) or formyl-methionyl-leucyl phenylalanine (fFLP) was introduced in 100 μl at a 10−6 M concentration; this dilution had been found in pilot experiments to give optimal results. The chambers were covered with a polyvinylpyrrolidone-free polycarbonate filter (pore size, 3 μm; Nuclepore, Tübingen, Germany). Then, 105 cells were layered in 100 μl on top of this filter in the upper part of the chamber, and the NP were allowed to migrate within 1 h at 37°C in 5% CO2. Thereafter, the remaining cells in the upper compartment and the filters were removed. The migrated cells present in the lower chamber were lysed with Triton X-100 (0.1% [vol/vol]) in order to quantify them by assessment of NP-specific β-glucuronidase. To this, the lysates were incubated for 18 h with 100 μl of p-nitrophenyl-β-d-glucuronide (Sigma) (at 0.01 M in 0.1 M sodium acetate buffer [pH 4.0]) as a substrate. The enzymatic reaction was stopped by the addition of 100 μl of 0.4 M glycine buffer (pH 10), and the yellow color was measured photometrically at 405 nm. For the calculation of the number of migrated cells, a calibration curve was generated with 1 × 103 to 2 × 105 cells lysed by 0.1% (vol/vol) Triton X-100 and incubated with p-nitrophenyl-β-d-glucuronide. All chemotaxis experiments were performed in duplicate. Parallel to the enzyme testing before cell lysis, the migrated cells in the lower compartment of the chamber were checked for NP purity by Giemsa-Wright staining.
Phagocytosis assay.
The phagocytosis capacity of NP was measured using an assay described elsewhere (23), with modifications for casein-elicited NP and L. sigmodontis infection. A total of 2 × 105 cells from the peritoneal (casein induction) or the thoracic cavities (day 77 after L. sigmodontis infection) were suspended in 200 μl of RPMI 1640, and 0.9-μm-diameter fluorescein isothiocyanate-labeled latex beads (Sigma) were added at 4.5 × 107 beads/well. After incubation for 1 h at 37°C in 5% CO2, the cells were washed with 10 ml of RPMI 1640, layered over cold FCS, and spun at 1,500 rpm for 5 min to remove the uningested beads. Cells were then fixed with 1.0% paraformaldehyde in PBS. Next, 200 μl of the cell suspension described above containing approximately 2 × 104 cells was centrifuged against a glass slide covered with absorptive filter paper using a Shandon cytocentrifuge at 800 rpm for 5 min. These filter papers allowed the adsorption of the solution and therefore the flattened adherence of the cells to the surface of the glass slide. Cells were then dried for 10 min in the darkness at room temperature. The following staining procedures were performed avoiding light exposure, and all antibodies were diluted in PBS–1% BSA (Sigma). At first, nonspecific antibody binding via FcγRII/III was blocked by prior incubation of slides with an anti-CD16/CD32 MAb (5 μg/ml; Pharmingen) for 60 min at room temperature. Slides were then washed in PBS and incubated for 2 h at room temperature with anti-NP MAb NIMP-R14 (rat IgG 2b [2]; 100 μl of a hybridoma culture SN containing 100 μg of protein per ml) as a primary antibody. After the slides were washed, they were incubated for 1 h with phycoerythrin-conjugated anti-rat-kappa antibodies (1 μg/ml; Pharmingen). Slides were washed, dried, and treated with mounting medium. Double staining was analyzed using a double filter fluorescence microscope (Zeiss, Oberkochen, Germany).
RESULTS
IL-4 deficiency does not affect early worm development nor adult worm survival during patency.
IL-4 deficient mice showed no altered worm load compared to infected wild-type mice, neither during development from L3 larvae to adult worms in the first 28 days p.i. nor in the late stage of infection, as analyzed at day 77 p.i. (Table 1). This indicates that the development from L3 larvae to adult worms, i.e., worm establishment, as well as adult worm persistence during the microfilaremic phase, is not drastically influenced by the lack of IL-4.
TABLE 1.
Worm loads in L. sigmodontis-infected wild-type and IL-4 KO micea
| Days p.i. | Mean no. of worms ± SD in:
|
|
|---|---|---|
| IL-4 KO mice | Wild-type mice | |
| 28 | 10.3 ± 10.4 | 9.0 ± 3.0 |
| 77 | 95.1 ± 55.2 | 144.4 ± 61.1 |
One of two consistent experiments performed per time point is shown. The difference in worm load between day 28 and day 77 p.i. reflects the different infection loads in the day 28 versus day 77 experiments.
Lack of IL-4 leads to a massive increase of MF.
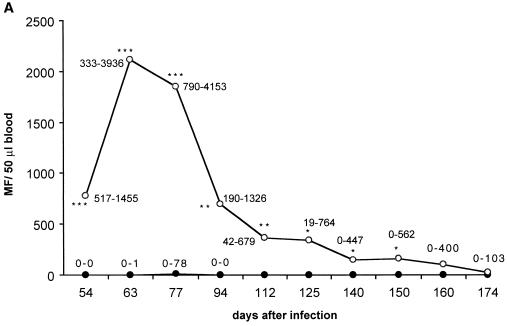
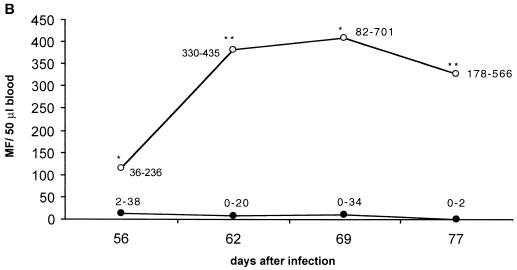
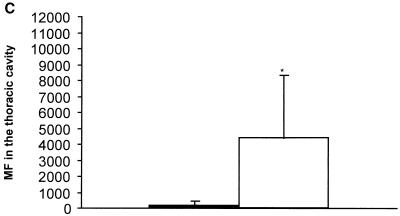
IL-4 had a very strong effect on the survival of MF, as examined during the whole period of patency in one experiment and from 54 days p.i. until 77 days p.i. in two other experiments. Microfilaremia was up to 100-fold higher in IL-4 KO mice and lasted more than 14 weeks longer compared to BALB/c mice (Fig. 1A). In the second and third experiments mice were sacrificed at day 77 p.i. to determine thoracic-cavity MF loads. The numbers of MF found in IL-4 KO mice were 20-fold higher than in control mice (Fig. 1B and C).
FIG. 1.
(A) Microfilaremia in infected IL-4 KO (○) or wild-type (●) mice observed over the whole time of patency. Numbers in the graph denote the range. (B) Microfilaremia in infected mice, observed in a second (and representative for a third consistent) experiment until day 77 p.i. (C) MF levels in the thoracic cavity at day 77 p.i. The number of MF is up to 200-fold higher in IL-4 KO mice (white bar) compared to the wild-type mice (black bar). Significant differences are denoted by asterisks as follows: ∗, P < 0.05; ∗∗, P < 0.03; ∗∗∗, P < 0.01 (Mann-Whitney U test).
Diminished EP and increased NP accumulation in the thoracic cavity in IL-4 KO mice.
EP accumulation in the thoracic cavity was diminished in both early and late stages of infection, while the NP numbers were elevated (Table 2). Consistent with increased NP accumulation in the thoracic cavity is that levels of the chemotactic factor KC (the murine homologue of IL-8) were enhanced in IL-4-deficient mice (532.5 ± 221.9 pg/ml) compared to concentrations in control mice (199.5 ± 81.6 pg/ml) (P < 0.04, Mann-Whitney test). IL-5 levels in the thoracic cavity in IL-4 KO mice (367.5 ± 135 pg/ml) were equivalent to those of control mice (138.8 ± 241.3 pg/ml).
TABLE 2.
Quantity of various cell types in the thoracic cavity of L. sigmodontis-infected IL-4 KO and wild-type mice 72 days p.i.
| Cell population | Mean no. of cells (%) ± SDa in:
|
|
|---|---|---|
| IL-4 KO mice | Wild-type mice | |
| EP | 6.3 ± 2.1∗ | 37.5 ± 6.0 |
| NP | 40.0 ± 7.0∗ | 18.5 ± 6.6 |
| Lymphocytes | 31.0 ± 1.0∗ | 13.3 ± 3.0 |
| MP | 22.7 ± 9.1 | 30.5 ± 10.3 |
| Mast cells | 0.3 ± 0.5 | 0 ± 0 |
Total cell numbers were (22.3 ± 14.0) × 106 in IL-4 KO mice and (23.9 ± 17.8) × 106 in wild-type mice. Cells were counted on cytospins which were stained with Wright solution. ∗, P < 0.05 compared with wild-type mice (Mann-Whitney U test).
In addition, NP functions were not impaired in IL-4 KO mice as examined in NP chemotaxis assays using fMLP and PAF as chemotactic substances and phagocytosis assays assessing the digestion of latex beads. In the chemotaxis assay 42.5% ± 2.1% of NP from IL-4 KO mice migrated when stimulated with fMLP compared to NP from control mice, in which 40.1% ± 2.8% migrated. When stimulated with PAF, 41.5% ± 9.2% of NP from IL-4 KO mice migrated, compared to 42.9% ± 2.8% of NP from wild-type mice. The background migration of NP was 21.4% ± 0.7% in IL-4 KO mice compared to 20.5% ± 0.7% in control mice. In the phagocytosis assay, 31.9% ± 2.8% of NP of IL-4 KO mice digested latex beads compared to 34.1% ± 1.4% of NP of control mice.
MP from IL-4 KO mice show an increased NO release.
MP were seeded in microtiter plates and stimulated with adult worm antigen or with IFN-γ plus lipopolysaccharide. MP of IL-4 KO mice showed a significantly higher NO production in response to both stimulants compared to MP from infected wild-type littermates: in response to AWA, MP of IL-4 KO mice produced 24.8 ± 5.2 μmol of NO and MP of BALB/c mice produced 8.7 ± 0.6 μmol of NO (P < 0.05, Mann-Whitney test); in response to LPS and IFN-γ the MP of IL-4 KO mice produced 30.3 ± 7.3 μmol of NO compared to the MP of BALB/c mice, which produced 10.5 ± 0.9 μmol of NO (P < 0.05, Mann-Whitney test). NO was not detectable in the sera of infected IL-4 KO or wild-type mice.
Impaired IgE production in IL-4-deficient mice does not explain the high levels of microfilaremia.
IgE was undetectable in the sera and in the thoracic cavity of IL-4-deficient mice, while high IgE levels in the infected wild-type littermates were found both in the sera (13,247 ± 2,362.9 ng/ml) and in the thoracic cavity (1,062.5 ± 1,027.4 ng/ml).
However, this difference in antibody production cannot explain the increased and extended microfilaremic phase given that, in separate experiments with BKO mice, microfilaremia, as well as the MF burden, in the thoracic cavity was equivalent to that of the control mice (Table 3).
TABLE 3.
Parasite loads of worms 80 days p.i. and MF in the blood and in the thoracic cavity in BALB/c and BKO micea
| Mouse group | Mean no. ± SD
|
Mean MF load ± SD in serum at (day p.i.):
|
Mean MF load ± SD in the thoracic cavity | ||||
|---|---|---|---|---|---|---|---|
| Worms | Nodules | 52 | 59 | 66 | 73 | ||
| BALB/c | 13.8 ± 11.1 | 2.6 ± 2.5 | 0.2 ± 0.4 | 2.2 ± 2.4 | 5.0 ± 5.6 | 3.8 ± 6.6 | 190.0 ± 221.9 |
| BKO | 11.3 ± 2.6 | 1.8 ± 0.5 | 0 ± 0 | 1.0 ± 1.6 | 2.0 ± 3.1 | 1.0 ± 1.1 | 212.0 ± 85.4 |
Microfilaremia was examined in 50 μl of peripheral blood. Results are representative for three experiments.
Adult female worm fertility is strongly enhanced in the absence of IL-4.
In order to differentiate whether the increased MF loads could be explained by increased adult worm fertility or by reduced MF killing, worm fertility and survival of MF stages in vivo were analyzed. Adult worms were obtained after mice were sacrificed at day 77 p.i. Up to 60% of the female worms harbored high uterine MF loads in IL-4 KO mice, whereas no MF were found in the uteri of worms isolated from BALB/c mice, a finding in line with earlier data that MF production ceases at this time in normal BALB/c mice (24). The lengths of female worms isolated from IL-4 KO mice were increased (67.6 ± 3.1 mm) compared to female worms isolated from BALB/c mice (43.4 ± 3.7 mm; P < 0.01, Student's t test). These data indicate that adult female worm fertility is strongly enhanced in the absence of IL-4.
In a further series of experiments, MF were injected i.p. into naive BALB/c, IL-4 KO, and BKO mice, and the MF loads in the peritoneal cavity and the blood were examined at 7-day intervals for 3 weeks. Compared to BALB/c mice, IL-4 KO mice and BKO mice showed a higher microfilaremia at 14 days and 21 days p.i. (Table 4). This experiment was repeated for BALB/c and IL-4 KO mice two more times, with no differences observed in the MF loads (not shown). In the first experiment the cytokine levels (IFN-γ, IL-5, IL-10, IL-12p70, and TNF-α) in the sera and peritoneal cavity were examined, but no significant differences were detected. Together, these data suggest that IL-4 has a limited direct effect on MF survival.
TABLE 4.
MF loads in the peritoneal cavity and in 50 μl of peripheral blood in BALB/c mice, IL-4 KO mice, and BKO mice 7, 14, and 21 days after injection of MF i.p. into naive mice
| Location and day p.i. | No. of MFa in:
|
||
|---|---|---|---|
| BALB/c mice | IL-4 KO mice | BKO mice | |
| Peritoneal cavity | |||
| 7 | 30,200–37,900 | 9,300–45,200 | 17,800–43,200 |
| 14 | 9,200–80,400 | 30,000–65,600 | 30,100–51,300 |
| 21 | 300–35,800 | 3,800–33,400 | 15,000–41,000 |
| Blood (50 μl) | |||
| 7 | 6.4 ± 15.3 | 4.8 ± 6.6 | 2.3 ± 2.9 |
| 14 | 3.4 ± 3.6 | 6.9 ± 4.2∗ | 4.8 ± 2.5 |
| 21 | 1.5 ± 1.3 | 7.6 ± 5.7∗ | 9.8 ± 4.6∗∗ |
The numbers of MF in the peritoneal cavity and in blood are given as a range and as the mean ± the standard deviation, respectively. Statistical analysis was performed using the Student's t test: ∗∗, P < 0.03; ∗, P < 0.04.
DISCUSSION
The existence of immunity to larvae and adults of filarial nematodes is a highly debated issue. TH2 lymphocyte responses are frequently associated with protective responses to parasitic helminths. Studies on gastrointestinal nematodes have shown an essential role for IL-4 in protective immunity (9), whereas immunity to several tissue-dwelling nematodes has been shown to depend on IL-5 (16, 40).
Studies of murine infection with L. sigmodontis so far have not addressed the role of IL-4 but have been confined to IL-5: it was shown that ablation or the genetic KO of IL-5 does not affect L3-larvae-to-adult-worm development in a primary infection (2, 21, 26) but leads to higher adult worm persistence during patency (2); IL-5, however, is essential for protection by vaccination with irradiated L3 larvae (2, 21, 26); there, it needs to be depleted not during the priming of the immune response but only shortly before challenge, since short-lived EP migrating to the tissue are the major effector cells in this type of immunity (26). Conversely, IL-5 overproduction in transgenic mice leads to shorter adult worm survival (27).
In this study we analyzed the role of IL-4 in infection with L. sigmodontis. We used IL-4 KO mice which were backcrossed to a BALB/c background in comparison to BALB/c wild-type mice. Our data show that IL-4 is essential for MF containment. MF levels were 50- to 100-fold higher on average. The maximal difference was 2,000-fold (Fig. 1A) at day 63 p.i. IL-4 did not affect worm development from L3 larvae to adult worms, nor did it affect adult worm persistence. The examination of female worms isolated from IL-4 KO mice and from BALB/c mice showed that worms from the KO mice had higher MF loads in the uterus. In conclusion, IL-4 KO mice had a higher and extended period of fertility. In studies in which MF were injected i.p., we could show that microfilaremia, compared to the drastic difference observed in full infection, was only moderately higher in IL-4 KO and BKO mice compared to BALB/c mice.
Our results differ from those obtained in the semipermissive B. malayi system (5), where the lack of IL-4 did not lead to such an increase in the MF load compared to wild-type mice as seen in our study. This feature is likely due to the semipermissivity of the B. malayi model and may also explain the somewhat contradictory results generated earlier in this model (18, 31). Anti-IL-4 treatment in a nonpermissive model, where chambers containing L3 larvae were implanted in mice, abolished vaccine-induced killing of Onchocerca L3 and L4 larval stages (17). Equivalent results were obtained in C57BL/6 IL-4 KO mice (13). However, conclusions on the role of IL-4 for full protection, i.e., complete adult worm development, could not be drawn in this model, since the larvae did not develop to adults. In previous studies using C57BL/6 mice, it was shown that Onchocerca lienalis MF are killed even faster in IL-4 KO mice, independently of IL-4 (11, 12). In that system there were no adult worm stages which might have had a suppressive effect on the immune defense mechanisms of the infected mice (20).
Taken together, these reports illustrate some of the difficulties encountered in trying to draw conclusions on the effects of cytokines for parasite control in non- or semipermissive models. In such models, immune reactions can develop which do not occur in permissive infections, e.g., because infective organisms get trapped at improper sites (28). In contrast, in a fully permissive system cytokine-sensitive and -insensitive phases of parasite development can be distinguished.
In our experiments we found that the accumulation of EP at the site of infection was reduced in IL-4 KO mice. It was shown that IL-4 has chemotactic functions on EP (13, 29). Induction of EP in our model could be one reason for the higher MF load in IL-4 KO mice, given that the EP levels have been diminished. However, in an earlier study by our group, ablation of IL-5 produced a phenotype different (2) from the one seen in IL-4 KO mice, so the effect of IL-4 is likely to be not only EP mediated.
In contrast to EP, NP numbers were elevated in IL-4 KO mice (Table 2). The enhanced accumulation of NP in the present study is probably due to the elevated levels of the NP chemotactic KC (36) in the thoracic cavity. The fact that NP did accumulate at the site of infection in both BALB/c and IL-4 KO mice may explain that adult worm killing is not impaired in IL-4 KO mice, given that NP are strongly involved in adult worm containment (2). Worm containment was found to be associated with encapsulation by inflammatory tissue, of which the innermost cell layer around the worm is made of NP; NP accumulation was found to be dependent on IL-5 (2). Thus, while IL-5 has been shown to affect adult worm survival, mediating NP-dependent adult worm encapsulation, IL-4 is primarily targeted at MF. This implies that the two different cytokines act differently against different stages of the worm. In addition, IL-5 is regulated independently of IL-4 in filarial infection, as shown earlier in experiments with O. volvulus in IL-4 KO mice (13). In our study, IL-4 KO mice did not show reduced IL-5 levels in the blood and in the thoracic cavity.
NO was elevated in IL-4 KO mice, but it did not seem to be a parameter responsible for adult worm or MF killing in the present study. This is in accordance with earlier experiments in which mice were treated with aminoguanidine, a potent inhibitor of the inducible NO synthase, and in which the killing of adult worms and MF was not affected (33, 38). However, the attack of L3 larvae in vivo has been demonstrated to be dependent on NO: a study with B. malayi showed enhanced adult worm development when NO was blocked (34). Apart from the fact that in the latter study, again, a semipermissive model was used, NO susceptibility may be also a species-dependent phenomenon.
The question arose as to whether diminished IgE levels were responsible for the massive increase in MF accumulation in the blood and thoracic cavity, given that B. malayi MF, in contrast to earlier reports, do induce high IgE levels when injected not i.p. but intravenously (19). Although in our study, IgE was induced in wild-type mice as a consequence of infection, the fact that BKO mice did not show reduced worm killing and had an unaltered microfilaremia during a full infection cycle (Table 3) would argue against IL-4 acting via IgE as an important mediator of MF containment.
MF killing in vivo was shown to be antibody dependent (10, 14). This was partly confirmed in the present study when MF injected into BKO mice showed a longer persistence in peripheral blood (Table 4). In contrast, BKO mice undergoing the full infection cycle did not have increased microfilaremia (Table 3). This apparent contradiction with experiments in the present study (Table 4) and in other reports (10, 14) using isolated MF may be explained by the fact that, in a full infection cycle, MF which are being born by adult female worms arrive in an environment “prepared” by their mothers via immunosuppression (4, 8, 15, 22, 39, 41), where antibody-mediated killing plays only a minor role.
In this regard, it is of great importance that the major mode of action of IL-4 on MF containment apparently does not lie in antibody-mediated MF killing but in the limitation of the fertility of adult female worms: while MF injected i.p. into IL-4 KO mice only in one of three experiments showed a slightly increased persistence in the blood (factor 4, Table 4), MF loads in the full infection of IL-4 KO mice were much more drastically enhanced (up to factor 2,000; Fig. 1A), and worm fertility was clearly prolonged (note the high percentage of fertile adult female worms at day 77 p.i.). Thus, it is likely that the mechanisms of immunosuppression exerted by adult worms are more efficient under IL-4 KO conditions.
In conclusion, this study demonstrates for the first time the essential role of IL-4 for MF control in a fully permissive filarial infection. The detection of a hitherto-unknown function of IL-4 in worm fertility control will be a focus of further investigations.
ACKNOWLEDGMENTS
We are indebted to T. Schueler and other staff for their help with the maintenance of the parasite life cycle, to B. Richter for animal genotyping, and to T. Liman for help with part of the experiment. We also appreciate the cooperation of N. Brattig and F. Geisinger in performing the phagocytosis and chemotaxis assays.
This study received financial support from the Deutsche Forschungsgemeinschaft (grant Ho 2009/1-3, 1-4 to A. H.) and from the Edna McConnell Clark Foundation (to A.H.).
REFERENCES
- 1.Al-Qaoud K M, Fleischer B, Hoerauf A. The Xid defect imparts susceptibility to experimental murine filariosis—association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int Immunol. 1998;10:17–25. doi: 10.1093/intimm/10.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qaoud K M, Pearlman E, Klukowski J, Hartung T, Fleischer B, Hoerauf A. A new mechanism for IL-5 dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int Immunol. 2000;12:899–908. doi: 10.1093/intimm/12.6.899. [DOI] [PubMed] [Google Scholar]
- 3.Al-Qaoud K M, Taubert A, Zahner H, Fleischer B, Hoerauf A. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: role of CD4+ T cells in controlling larval development. Infect Immun. 1997;65:2457–2461. doi: 10.1128/iai.65.6.2457-2461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen J E, Lawrence R A, Maizels R M. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. doi: 10.1093/intimm/8.1.143. [DOI] [PubMed] [Google Scholar]
- 5.Babu S, Ganley L M, Klei T R, Shultz L D, Rajan T V. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infect Immun. 2000;68:3034–3035. doi: 10.1128/iai.68.5.3034-3035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breton B, Diagne M, Wanji S, Bugnoux M E, Chandre F, Marechal P, Petit G, Vuong P N, Bain O. Ivermectin and moxidectin in two filarial systems: resistance of Monanema martini: inhibition of Litomosoides sigmodontis insemination. Parassitologia. 1997;39:19–28. [PubMed] [Google Scholar]
- 7.Chandrashekar R, Rao R U, Rajasekariaf R G, Subrahmanyam D. Separation of viable microfilariae free of blood cells on percol gradient. J Helminthol. 1984;58:69–70. doi: 10.1017/s0022149x00028078. [DOI] [PubMed] [Google Scholar]
- 8.Doetze A, Satoguina J, Burchard G, Rau T, Löliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in generalized onchocerciasis is mediated by Th3/Tr1-type cytokines IL-10 and TGF-beta but not by a Th1 to Th2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 9.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 10.Folkard S G, Jenkins R E, Bianco A E. Vaccination generates serum-mediated protection against Onchocerca lienalis microfilariae in the mouse. Trop Med Int Health. 1996;1:359–362. doi: 10.1046/j.1365-3156.1996.d01-48.x. [DOI] [PubMed] [Google Scholar]
- 11.Hogarth P G, Folkard S G, Taylor M, Bianco A E. Accelerated clearance of Onchocerca microfilariae and resistance to reinfection in interleukin-4 gene knockout mice. Parasite Immunol. 1995;17:653–657. doi: 10.1111/j.1365-3024.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 12.Hogarth P J, Taylor M J, Bianco A E. IL-5-dependent immunity to microfilariae is independent of IL-4 in a mouse model of onchocerciasis. J Immunol. 1998;160:5436–5440. [PubMed] [Google Scholar]
- 13.Johnson E H, Schynder-Candrian S, Rajan T V, Nelson F K, Lustigman S, Abraham D. Immune responses to third stage larvae of Onchocerca volvulus in interferon-gamma and interleukin-4 knockout mice. Parasite Immunol. 1998;20:319–324. doi: 10.1046/j.1365-3024.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- 14.Kazura J W, Davis R S. Soluble Brugia malayi microfilarial antigens protect mice against challenge by an antibody-dependent mechanism. J Immunol. 1982;128:1792–1796. [PubMed] [Google Scholar]
- 15.King C L, Mahanty S, Kumaraswami V, Abrams J S, Regunathan J, Jayaraman K, Ottesen E A, Nutman T B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Investig. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korenaga M, Hitoshi Y, Yumaguchi N, Sato Y, Takatsu K, Tada I. The role of IL-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991;72:502–507. [PMC free article] [PubMed] [Google Scholar]
- 17.Lange A M, Yutanawiboonchai W, Scott P, Abraham D. IL-4 and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 18.Lawrence R A, Allen J A, Gregory W F, Kopf M, Maizels R M. Infection of IL-4-deficient mice with the parasite nematode Brugia malayi demonstrates that host resistance is not dependent on a T helper 2-dominated immune response. J Immunol. 1995;154:5995–6001. [PubMed] [Google Scholar]
- 19.Lawrence R A, Allen J E, Gray C A. Requirements for in vivo IFN-gamma induction by live microfilariae of the parasitic nematode, Brugia malayi. Parasitology. 2000;120:631–640. doi: 10.1017/s003118209900596x. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence R A, Allen J E, Osborne J, Maizels R A. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. J Immunol. 1994;153:1216–1224. [PubMed] [Google Scholar]
- 21.Le Goff L, Loke P N, Ali H F, Taylor D W, Allen J E. IL-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect Immun. 2000;68:2513–2517. doi: 10.1128/iai.68.5.2513-2517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald A S, Maizels R M, Lawrence R A, Dransfield I, Allen J E. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J Immunol. 1998;160:4124–4132. [PubMed] [Google Scholar]
- 23.MacIvor D M, Shapiro S D, Pham C T, Belaaouaj A, Abraham S N, Ley T J. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–4293. [PubMed] [Google Scholar]
- 24.Marechal P, Goff L, Hoffmann W, Rapp J, Oswald I P, Ombrouck C, Taylor D W, Bain O, Petit G. Immune response to the filaria Litomosoides sigmodontis in susceptible and resistant mice. Parasite Immunol. 1997;19:273–279. doi: 10.1046/j.1365-3024.1997.d01-209.x. [DOI] [PubMed] [Google Scholar]
- 25.Marechal P, Goff L, Petit G, Diagne M, Taylor D W, Bain O. The fate of the filaria Litomosoides sigmodontis in susceptible and naturally resistant mice. Parasite. 1996;3:25–31. doi: 10.1051/parasite/1996031025. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Al-Qaoud K M, Bain O, Paehle K, Fleischer B, Hoerauf A. IL-5 is essential for protection after immunization against murine filariasis but not during primary infection. Med Microbiol Immunol. 2000;189:67–74. doi: 10.1007/pl00008258. [DOI] [PubMed] [Google Scholar]
- 27.Martin C, Le Goff L, Ungeheuer M N, Vuong P N, Bain O. Drastic reduction of a filarial infection in eosinophilic IL-5 transgenic mice. Infect Immun. 2000;68:3651–3656. doi: 10.1128/iai.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeusen E N, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 29.Moser R, Groscurth P, Carballido J M, Bruijnzeel P L, Blaser K, Heusser C H, Fehr J. Interleukin-4 induces tissue eosinophilia in mice: correlation with its in vitro capacity to stimulate the endothelial cell-dependent selective transmigration of human eosinophils. J Lab Clin Med. 1993;122:567–575. [PubMed] [Google Scholar]
- 30.Padgett E L, Pruett S B. Rat, mouse and human neutrophils stimulated by a variety of activating agents produce much less nitrite than rodent macrophages. Immunology. 1995;84:135–141. [PMC free article] [PubMed] [Google Scholar]
- 31.Pearlman E, Heinzel F P, Hazlett F E, Kazura J W. IL-12 modulation of T helper responses to the filarial helminth, Brugia malayi. J Immunol. 1995;154:4658–4664. [PubMed] [Google Scholar]
- 32.Petit G, Diagne M, Marechal P, Owen D, Taylor D, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice: comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp. 1992;67:144–150. doi: 10.1051/parasite/1992675144. [DOI] [PubMed] [Google Scholar]
- 33.Pfaff A W, Schulz-Key H, Soboslay P T, Geiger S M, Hoffmann W H. The role of nitric oxide in the innate resistance to microfilariae of Litomosoides sigmodontis in mice. Parasite Immunol. 2000;22:397–405. doi: 10.1046/j.1365-3024.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- 34.Rajan T V, Porte P, Yates J A, Keefer L, Shultz L D. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun. 1996;64:3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockett K A, Awburn M M, Rockett E J, Cowden W B, Clark I A. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994;16:243–249. doi: 10.1111/j.1365-3024.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 36.Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 37.Rubio de Krömer M T, Krömer M, Luersen K, Brattig N W. Detection of a chemotactic factor for neutrophils in extracts of female Onchocerca volvulus. Acta Trop. 1998;71:45–56. doi: 10.1016/s0001-706x(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 38.Saeftel, M., L. Volkmann, S. Korten, N. Brattig, K. M. Al-Qaoud, B. Fleischer, and A. Hoerauf. Lack of IFN-γ confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect., in press. [DOI] [PubMed]
- 39.Sartono E, Kruize Y C, Kurniawan A, Maizels R M, Yazdanbakhsh M. Depression of antigen-specific interleukin-5 and interferon-gamma responses in human lymphatic filariasis as a function of clinical status and age. J Infect Dis. 1997;175:1276–1280. doi: 10.1086/593701. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 41.Soboslay P T, Lüder C G, Hoffmann W H, Michaelis I, Helling G, Heuschkel C, Dreweck C M, Blanke C H, Pritze S, Banla M, Schulz-Key H. Ivermectin-facilitated immunity in onchocerciasis: activation of parasite-specific Th1-type responses with subclinical Onchocerca volvulus infection. Clin Exp Immunol. 1994;96:238–244. doi: 10.1111/j.1365-2249.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]