Abstract
Enhanced prostaglandin production during fungal infection could be an important factor in promoting fungal colonization and chronic infection. Host cells are one source of prostaglandins; however, another potential source of prostaglandins is the fungal pathogen itself. Our objective was to determine if the pathogenic yeasts Cryptococcus neoformans and Candida albicans produce prostaglandins and, if so, to begin to define the role of these bioactive lipids in yeast biology and disease pathogenesis. C. neoformans and C. albicans both secreted prostaglandins de novo or via conversion of exogenous arachidonic acid. Treatment with cyclooxygenase inhibitors dramatically reduced the viability of the yeast and the production of prostaglandins, suggesting that an essential cyclooxygenase like enzyme may be responsible for fungal prostaglandin production. A PGE series lipid was purified from both C. albicans and C. neoformans and was biologically active on both fungal and mammalian cells. Fungal PGEx and synthetic PGE2 enhanced the yeast-to-hypha transition in C. albicans. Furthermore, in mammalian cells, fungal PGEx down-modulated chemokine production, tumor necrosis factor alpha production, and splenocyte proliferation while up-regulating interleukin 10 production. These are all activities previously documented for mammalian PGE2. Thus, eicosanoids are produced by pathogenic fungi, are critical for growth of the fungi, and can modulate host immune functions. The discovery that pathogenic fungi produce and respond to immunomodulatory eicosanoids reveals a virulence mechanism that has potentially great implications for understanding the mechanisms of chronic fungal infection, immune deviation, and fungi as disease cofactors.
Prostaglandins are potent regulators of host immune responses. They are produced following the action of a cyclooxygenase on dihomo-γ-linolenic, arachidonic, or eicosanopentaenoic acid (20). The activities of prostaglandins on mammalian cells are numerous. Prostaglandins can inhibit Th1-type immune responses, chemokine production, phagocytosis, and lymphocyte proliferation (1, 11, 16, 18, 20, 23, 26, 27). Prostaglandins can also promote Th2-type responses and tissue eosinophilia (4, 16, 20, 24). In the context of anti-fungal immunity, chronic or disseminating fungal infections will result if the Th1-Th2 balance of cellular immunity is shifted away from Th1- toward Th2-type responses (21). The role of prostaglandins in promoting fungal virulence remains to be determined. However, elevated prostaglandin levels have been observed in chronic Candida albicans infections (28). Thus, enhanced prostaglandin production during fungal infection could be an important factor in promoting fungal colonization and chronic infection.
Host cells are one source of prostaglandins during fungal infection; however, another potential source of prostaglandins is the fungal pathogen itself. There have been reports in the literature of eicosanoid production by slime molds and soil fungi (12). Our objective was to determine if the pathogenic yeasts Cryptococcus neoformans and C. albicans produce prostaglandins and, if so, to begin to define the role of these bioactive lipids in yeast biology and disease pathogenesis.
MATERIALS AND METHODS
Determination of prostaglandin concentration by ELISA.
C. neoformans strains 24067E and H99 and C. albicans strain CHN1 (a clinical isolate) were grown to stationary phase (72 h) at 25°C in Sabouraud dextrose broth (SDB) (1% neopeptone, 2% dextrose; Difco, Detroit, Mich.) or asparagine broth (AspB) (0.1% asparagine, 0.05% MgSO4 · 7H2O, 0.3% glucose, 0.0001% thiamine; Sigma Chemical Co., St. Louis, Mo.) with shaking. The culture supernatants were analyzed for prostaglandin production using a monoclonal PGE2 enzyme-linked immunosorbant assay (ELISA) (Cayman Chemicals, Ann Arbor, Mich.) or a prostaglandin screening enzyme immunoassay kit (the specificity is described in Results; Cayman Chemicals).
HPLC analysis of arachidonic acid metabolites secreted by C. neoformans.
Strain 24067E was grown to stationary phase (72 h) in SDB, and 5 × 106 CFU was incubated with 0.5 μCi of [3H]arachidonic acid (DuPont-NEN, Boston, Mass.) in 1 ml of SDB at 37°C for 30 min. Lipid residues were extracted using Sep-Pac C18 cartridges (Waters Associates, Milford, Mass.), evaporated under nitrogen, and stored at −70°C. The dehydrated samples were then dissolved in 1 ml of acetonitrile-water-trifluoroacetic acid and analyzed by reverse-phase high performance liquid chromatography (HPLC) on a 5-μm Bondapak C18 column (30 by 0.4 cm; Waters Associates) using a mobile phase of acetonitrile-water-trifluoroacetic acid at a flow rate of 2 ml/min. Arachidonic acid metabolites were eluted during a series of linear gradient increases of acetonitrile from initial conditions of 50:50:0.1 (vol/vol/vol) to 73:27:0.1 (vol/vol/vol) at 7 min, then to 85:15:0.1 (vol/vol/vol) at 9 min, and finally to 100:0:0.1 (vol/vol/vol) at 15 min. The eluate was continuously monitored for UV absorbance, and peaks were identified on the basis of their coelution with authentic standards. Radioactivity was quantitated by liquid scintillation counting.
Cyclooxygenase inhibitor assay.
C. neoformans strain H99 and C. albicans strain CHN1 were grown in SDB for 24 h at 25°C. Indomethacin (Sigma Chemical Co.) was dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 100 mM. Indomethacin was added to the yeast cultures to give a final concentration of 1.0 mM, while the control cultures contained DMSO alone. The cultures were incubated with shaking for an additional 24 h at 25°C.
Purification of fungal PGEx.
C. neoformans strain H99 and C. albicans strain CHN1 were grown to stationary phase (72 h) in SDB at 25°C. The culture supernatants were loaded onto a PGE2 affinity column (Cayman Chemicals), washed, and eluted according to the manufacturer's instructions. The eluates were dried and resuspended in buffer, and the PGEx concentrations were determined.
C. albicans germ tube assay.
A standard germ tube assay was performed in which C. albicans was resuspended in 100% fetal calf serum (FCS) (Sigma Chemical Co.), purified PGEx was added to the cell suspension to give final concentrations of 0.33 nM PGE2 and 66% FCS, and the cells were incubated at 37°C for 2 h. Samples were removed in duplicate, and 400 cells were counted under 200× power using phase-contrast microscopy. The mean numbers of budding yeast forms and germ tube forms were determined.
Mitogen-induced lymphocyte proliferation and cytokine production.
Splenocytes were harvested from CBA/J mice and plated in 96-well tissue culture plates at 5 × 105/well with a 0.65 nM (250 pg/ml) final concentration of purified fungal PGEx or commercially available PGE2 (Cayman Chemicals) and 5 μg of conconavalin A (ConA) (Sigma Chemical Co.)/ml. The cultures were incubated for 48 h at 37°C and pulsed with 5 μCi of [3H]thymidine/ml for an additional 16 h at 37°C. The cells were harvested on paper filters, and the amount of [3H]thymidine incorporated was measured by liquid scintillation counting. For cytokine production, cell supernatants from ConA-stimulated splenocyte cultures were harvested after 24 h of incubation at 37°C, and cytokines were measured by ELISA for interleukin 10 (IL-10) and tumor necrosis factor alpha (TNF-α; BD PharMingen, San Diego, Calif.).
Cytokine production by human epithelial cells.
A549 human epithelial cells were trypsinized and plated on 12-well tissue culture plates at 105/well. The cells were grown to confluency (24 h) and treated with a 0.65 nM (250 pg/ml) final concentration of purified fungal PGEx or commercially available PGE2 (Cayman Chemicals). For IL-6 induction, TNF-α (10 ng/ml) was added to the cultures. The culture supernatants were harvested after 24 h, and the cytokines were analyzed by ELISA for IL-8 and IL-6 (BD PharMingen).
Statistical analysis.
Student's t test (two tailed; unequal variance) was used to analyze the significance of differences between experimental groups. Data with a P value of 0.05 or less were considered to be significant.
RESULTS
To determine whether C. neoformans could produce prostaglandins, broth cultures of strain 24067E were assayed by monoclonal anti-PGE2 ELISA. The different classes of prostanoids (PGA, PGB, PGD, PGF, and TXB) have the same basic molecular structure, differing only in the oxygen substitution of the five-membered ring portion of the lipid. The PGE2 ELISA utilized in these experiments exhibits cross-reactivity with prostaglandins of the E class only, which includes PGE1 (19%), PGE2 (100%), and PGE3 (43%). The PGE2 ELISA does not recognize prostanoids of the PGA, PGB, PGD, PGF, or TXB class or isomers of PGE2. Thus, we will refer to the prostaglandins detected as PGEx. The yeasts were grown to stationary phase (72 h) in AspB, a defined minimal medium of salts, glucose, thiamine, and asparagine. PGEx was detected in C. neoformans AspB culture supernatants (34.2 ± 8.2 pg/ml). We also detected PGEx in culture supernatants from C. neoformans grown in SDB, an undefined nutrient-rich broth containing glucose and neopeptone (117.4 ± 29.2 pg/ml). Prostaglandin production was detected at both 25 and 37°C, with slightly higher levels of prostaglandins detected at 25°C (data not shown). Lipids were also extracted from the C. neoformans broth samples and assayed for PGEx. Significant levels of PGEx were detected in the extracts, confirming the lipid nature of these molecules (data not shown). These results demonstrate that C. neoformans can produce and secrete PGEx into its surroundings and can synthesize PGEx without an exogenous source of arachidonic acid (AspB).
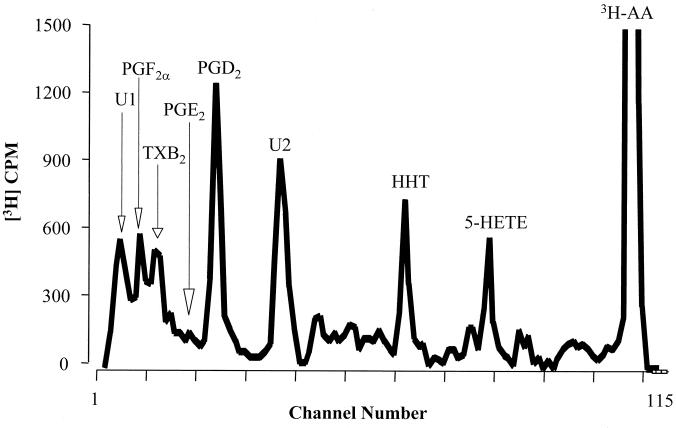
We next examined whether C. neoformans could utilize exogenous sources of arachidonic acids to produce eicosanoids. Cryptococci were incubated with [3H]-arachidonic acid to generate labeled eicosanoids for HPLC analysis. The major arachidonic acid metabolite produced by C. neoformans coeluted with an authentic PGD2 standard. Other cryptococcal eicosanoids coeluted with authentic HHT, 5-HETE, PGF2, TXB2, and PGE2 (Fig. 1). Interestingly, we also detected large amounts of two other eicosanoids, U1 and U2 (Fig. 1). A similar pattern of peaks was observed from HPLC analysis of C. albicans culture supernatant, with the exception that C. albicans has an additional peak that coelutes with 15-HETE (data not shown). Given the heterogeneity of prostaglandin products detected by HPLC, a polyclonal prostaglandin-screening ELISA was used to analyze total prostaglandin levels. This ELISA detects PGE2, PGD2, and TXB2 along with PGE1, PGE3, PGF1α, PGF2α, and PGF3α. It does not detect the PGA class, PGB1, 15-keto PGE2, 13,14-dihydro-15-keto PGF2α, or misopristol. For C. neoformans, we detected higher levels of prostaglandins with the prostaglandin ELISA (>1,500 pg/ml) than with the monoclonal PGE2 ELISA (∼120 pg/ml), confirming the results of the HPLC analysis (Fig. 1). Thus, C. neoformans can utilize exogenous sources of arachidonic acid to produce a number of different eicosanoids.
FIG. 1.
Secretion of arachidonic acid metabolites by C. neoformans. Strain 24067E was grown to stationary phase (72 h) and was incubated with 0.5 μCi of [3H]arachidonic acid (3H-AA) in 1 ml of SDB at 37°C for 30 min. The supernatant was analyzed by HPLC, and peaks were identified on the basis of their coelution with authentic standards. Pc, prostacyclin.
To determine whether other pathogenic yeasts also produce prostaglandins, we assayed the prostaglandin levels in broth cultures of a number of C. albicans and C. neoformans strains. C. albicans strain CHN1 secreted significant amounts of prostaglandins (>1,800 pg/ml), including PGEx (∼130 pg/ml, comparable to the levels produced by C. neoformans strain 24067E PGEx [∼120 pg/ml]). A number of clinical and laboratory strains of C. neoformans (H99, 145, 184, and 602) and C. albicans (28366 and 36082) were analyzed, and all produced significant amounts of prostaglandins in culture, although there was strain-to-strain variation in the levels even when they were normalized to numbers of CFU (data not shown). Similar to C. neoformans, C. albicans synthesized prostaglandins de novo (in AspB) and in greater quantities if the medium was supplemented with exogenous arachidonic acid (data not shown). Altogether, these results demonstrate by three independent assays that the pathogenic yeasts C. neoformans and C. albicans synthesize and secrete prostaglandins, including a PGE series compound (PGEx).
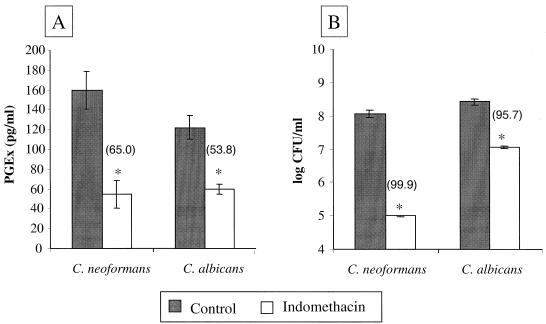
We next tested the effect of cyclooxygenase inhibitors on fungal PGEx production and fungal growth (Fig. 2). Cyclooxygenases are the enzymes that catalyze the conversion of arachidonic acid into the prostaglandins in mammalian cells. The cyclooxygenase inhibitor indomethacin was added to exponentially growing cultures of C. neoformans and C. albicans and incubated with shaking for an additional 24 h. Indomethacin inhibited C. neoformans and C. albicans PGEx production by 65 and 54%, respectively (Fig. 2A). Strikingly, indomethacin treatment also sharply reduced the viability of these fungi. After 24 h, 99.9% of C. neoformans and 95.7% of C. albicans cells were killed (Fig. 2B). Similar decreases in viability were observed with the cyclooxygenase inhibitors etodolac and piroxicam (data not shown). Therefore, the decrease in yeast viability and PGEx production was not due to a nonspecific toxicity of indomethacin for the yeast cells. Thus, cyclooxygenase plays a critical role in fungal metabolism (including PGEx production), and inhibition of cyclooxygenase activity decreases the viability of C. neoformans and C. albicans.
FIG. 2.
Effect of cyclooxygenase inhibitors on prostaglandin production and survival of C. neoformans and C. albicans. Cultures of C. neoformans strain H99 and C. albicans strain CHN1 were grown for 24 h at 25°C. Indomethacin (1.0 mM dissolved in DMSO) was added, and the cultures were then incubated for an additional 24 h. Control cultures contained DMSO alone. The numbers in parentheses represent percent inhibition of PGE2 compared to control (A) and percent killing compared to initial CFU per milliliter measured before the addition of inhibitor (B). The results represent the averages (± standard errors) of two independent experiments. ∗, P < 0.05 compared to control.
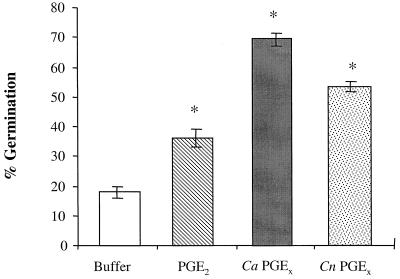
To assess the activities of fungal prostaglandins on fungal biology, we examined the effects of purified fungal PGEx on germ tube formation in C. albicans (Fig. 3). Germ tube formation is the initial step in the yeast-to-hypha phase transition. Using an affinity purification system with the same monoclonal PGE2 antibody used in the ELISA, we specifically isolated PGEx from both C. albicans and C. neoformans and compared it to commercially available PGE2. The addition of affinity-purified Candida PGEx to C. albicans yeast cells enhanced germ tube formation (Fig. 3). Affinity-purified Cryptococcus PGEx and commercial PGE2 also enhanced germ tube formation in C. albicans (Fig. 3). The addition of indomethacin completely blocked germ tube formation induced by serum alone (data not shown). However, no viable Candida yeasts were recovered after indomethacin incubation (data not shown), confirming the results shown in Fig. 2B indicating that cyclooxygenase activity is required for yeast viability. Thus, both host PGE2 and fungal PGEx can enhance yeast-to-hypha phase transition in C. albicans.
FIG. 3.
Effects of exogenous prostaglandins on C. albicans germ tube formation. C. albicans was incubated in FCS with either buffer, commercially available PGE2, or affinity-purified fungal PGEx. Ca, C. albicans; Cn, C. neoformans. The results represent the averages (± standard errors) of three independent experiments. ∗, P < 0.05 compared to buffer alone.
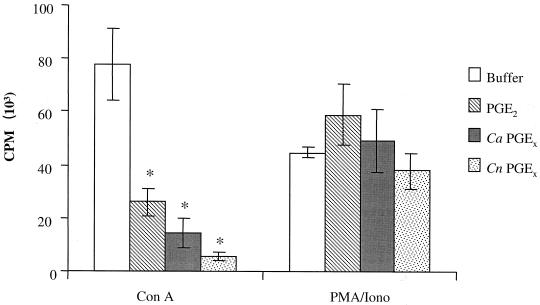
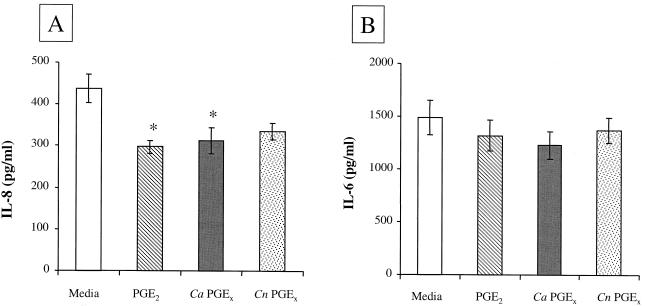
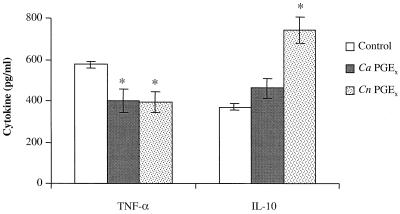
To assess the activity of fungal prostaglandins on mammalian cell biology, we addressed whether fungal PGEx can modulate mammalian-cell cytokine production and proliferation as has been reported for mammalian PGE2. The four assays used were (i) inhibition of mitogen-induced lymphocyte proliferation, (ii) inhibition of TNF-α production, (iii) enhancement of IL-10 production, and (iv) inhibition of chemokine production (11, 23, 26, 27). We chose to test the biological activity of the fungal PGEx throughout these studies at a concentration (0.65 nM) that might be found in tissue at a site of infection (in vitro experiments typically use between 10 and 1,000 nM). Murine splenocytes were stimulated with mitogen (ConA) in the presence or absence of 0.65 nM commercially available PGE2 or PGEx purified from C. albicans or C. neoformans. Fungal PGEx and PGE2 both suppressed ConA-induced lymphocyte proliferation (Fig. 4). The human epithelial cell line A549 constitutively produces the CXC chemokine IL-8. Fungal PGEx and PGE2 both reduced IL-8 production from the bronchial epithelial cell line (Fig. 5A). While the inhibition of IL-8 production was not dramatic (23 to 32%), this low dose of fungal PGEx did produce a measurable effect. PGEx from both C. albicans and C. neoformans also inhibited TNF-α production and enhanced IL-10 production by mitogen-stimulated splenocytes (Fig. 6). The PGEx preparations used in these assays did not affect phorbol myristate acetate-ionomycin-induced proliferation of the splenocytes or IL-6 induction by the A549 cells, thereby verifying that the preparations did not contain a toxic contaminant (Fig. 4 to 6). These experiments demonstrate that even at low levels fungal PGEx is biologically active on mammalian cells and has activity similar to that reported for mammalian PGE2 (23, 26).
FIG. 4.
Effect of fungal PGE2 on mitogen-induced lymphocyte proliferation in murine splenocytes. Ca, C. albicans; Cn, C. neoformans. Splenocytes were harvested from CBA/J mice and cultured in the presence of mitogen and either medium, commercially available PGE2, or affinity-purified fungal PGEx. The results shown are the means (± standard errors) from two or three experiments. ∗, P < 0.05 compared to buffer alone.
FIG. 5.
Effect of fungal PGE2 on chemokine production by a human bronchial epithelial cell line. Ca, C. albicans; Cn, C. neoformans. Human epithelial cell line A549 was cultured in the presence of either medium, PGE2, or affinity-purified fungal PGEx for 24 h. For IL-6 induction, TNF-α (10 ng/ml) was added to the cultures. The IL-8 and IL-6 concentrations were measured by ELISA. The results shown are the means (± standard errors) from two or three experiments. ∗, P < 0.05 compared to buffer alone.
FIG. 6.
Effect of fungal PGE2 on mitogen-induced cytokine production in murine splenocytes. Ca, C. albicans; Cn, C. neoformans. Splenocytes were harvested from CBA/J mice and cultured in the presence of ConA and either medium, PGE2, or affinity-purified fungal PGEx. The supernatants were harvested after 24 h, and the cytokines were measured by ELISA. The results shown are the means from two or three experiments. ∗, P < 0.05 compared to buffer alone.
DISCUSSION
These studies have identified a group of extracellular signal molecules in fungi which are biologically active on both fungi and mammalian cells and can modulate host defenses. We have concentrated on the activity of one of these bioactive lipids, PGEx. It is tempting to assume that the identity of our purified fungal PGEx is truly PGE2 because the PGE2 monoclonal antibody used to detect (by ELISA) and affinity purify fungal PGEx binds PGE2 with the highest affinity. However, the antibody does cross-react with prostaglandins of the E class (PGE1 [19%], PGE2 [100%], and PGE3 [43%]). The PGE2 monoclonal antibody does not recognize isomers of PGE2 or members of the PGA, PGB, PGD, PGF, or TXB class. Eicosanoids are biologically active lipids derived from dihomo-γ-linolenic acid, arachidonic acid, and eicosanopentaenoic acid (C20 polyunsaturated fatty acids [PUFA] which differ only in the number of cis double bonds). Prostanoids are a subfamily of eicosanoids, characterized by an enzymatically generated ring structure, which includes the prostaglandins and thromboxanes. The different classes of prostanoids are denoted by a letter representing the oxygen substitution on the ring structure and by a number representing the number of cis double bonds in the lipid. In mammals and other higher eukaryotes, the initial step of eicosanoid biosynthesis is catalyzed by cyclooxygenase. We have demonstrated that prostaglandin production by pathogenic yeasts also involves a cyclooxygenaselike pathway and that the yeasts can metabolize exogenous arachidonic acid to form eicosanoids that coelute in HPLC analysis with known prostanoids. Future studies (including mass spectrometry) will determine whether PGEx is PGE1, PGE2, PGE3, or a mixture of E series prostaglandins.
Fungal prostaglandin production occurred de novo, but greater quantities were produced via metabolism of exogenous arachidonic acid or other fatty acids (found in SDB). Arachidonic acid-derived eicosanoid production has been reported in nonpathogenic yeasts (3, 9, 12, 13). The nature of the eicosanoids produced by these fungi varies from species to species, and most require exogenous substrates. Arachidonic acid is common and abundant in protistan fungi and certain true fungi, such as the oomycetes and zygomycetes. However, the occurrence of arachidonic acid in other true fungi, including pathogenic yeasts, is sporadic (7). Arachidonic acid was detected among the very long chain fatty acids (C19–25) of the yeast Rhodotorula (19). The occurrence of arachidonic acid in C. neoformans and C. albicans has not been verified (6, 15). It is possible that the growth conditions and/or methods of fatty acid extraction and identification were not optimized for detecting very long chain fatty acids. Our studies and a recently published report have demonstrated that C. albicans can take up and metabolize exogenous arachidonic acid (5). Thus, both C. neoformans and C. albicans can utilize exogenous sources of arachidonic acid for eicosanoid synthesis. This opens up the possibility that host-derived arachidonic acid may be used by fungi for prostaglandin synthesis. It is also possible that the fungal prostaglandins are not derived from arachidonic acid but are produced from shorter-chain PUFA. We have also identified that phospholipase B mutants of C. neoformans have defects in prostaglandin synthesis (M. C. Noverr, unpublished observation), suggesting that phospholipase may participate in PUFA release. The identity of the C20 PUFA determines the type of prostanoid produced (i.e., dihomo-γ-linolenic acid, arachidonic acid, and eicosanopentaenoic acid serve as the substrates for PG1, PG2, and PG3 series prostanoids, respectively). Future studies will focus on identifying the endogenous substrates for prostaglandin synthesis. Overall, our data demonstrate that fungal eicosanoids can be synthesized from exogenous (host?) or endogenous (fungal) substrates.
We have demonstrated that cyclooxygenase plays a critical role in fungal metabolism. In mammals and other higher eukaryotes, the initial step of eicosanoid biosynthesis is catalyzed by cyclooxygenase. Although a fungal cyclooxygenase has not been identified, the cyclooxygenase inhibitors indomethacin, etodolac, and piroxicam all block PGEx production by C. neoformans and C. albicans. We cannot rule out the possibility that decreases in prostaglandin production caused by these inhibitors are due to decreases in viability (as opposed to inhibition of cyclooxygenase activity). In preliminary studies, we have identified a cyclooxygenase-cross-reactive protein in C. neoformans cell lysates by Western blotting with an anti-rat cyclooxygenase antibody (data not shown). Cyclooxygenase inhibitors have also been shown to arrest the growth or prevent the sexual maturation of some species of environmental fungi (2, 10). The data shown in Fig. 2 suggest not only that a cyclooxygenase-dependent metabolic pathway is found in C. albicans and C. neoformans but that blocking this pathway kills the yeast. Thus, these signal molecules (prostaglandins and other eicosanoids) appear to be generated via a conserved biochemical pathway that is important in both fungal metabolism and host defense.
The most striking aspect of these studies is that fungal PGEx exhibits the same activities reported for mammalian PGE2 in the five assays tested in this report. Fungal PGEx was active on mammalian cells and could down-modulate chemokine production and TNF-α production and proliferation while up-regulating IL-10 production. Furthermore, fungal PGEx could also promote Candida germ tube formation. These are all activities previously documented for mammalian PGE2 (8, 11, 23, 26, 27). This observation offers a potential mechanism to explain the recent reports that chemokine production by endothelial cells is inhibited by live, but not killed, cryptococci (17). In addition, the observation that C. albicans produced and responded to PGEx (Fig. 2 and 3) provides a microbiological explanation for the ability of human PGE2 to enhance C. albicans germ tube formation (8). The germinated hyphal form of C. albicans is better able to adhere to and penetrate host tissues than the yeast form (22, 25). Thus, it is likely that fungal PGEx is important in C. albicans pathogenesis, and host PGE2 could actually exacerbate the infection.
Given the biological activity of the purified fungal eicosanoids, they have the potential to mediate host-pathogen “cross talk,” in which the pathogen can down-regulate (local) host immune responses at the site of infection and the host can inadvertently augment pathogen virulence. The result of such interactions could be an immunological stalemate, i.e., chronic low-grade infection or parasitism. The most common feature of fungal infections is their chronic persistence within tissues of otherwise healthy individuals, ranging in severity from athlete's foot to chronic vaginitis to pulmonary granulomas (14). Prostaglandins can also modulate the Th1-Th2 balance of a response and promote tissue eosinophilia, a feature of some chronic fungal infections (1, 16, 20, 24). While we do not have isogenic strains of prostaglandin knockout yeast, we have identified a laboratory strain of C. neoformans (2E-TuC) that produces less prostaglandin than other clinical isolates (e. g., 24067E, 145, and H99). Interestingly, this low-prostaglandin strain does not induce the pulmonary eosinophilia in genetically susceptible (but immunologically normal) C57BL/6 mice seen with other C. neoformans strains (data not shown). In conclusion, the discovery that pathogenic fungi produce and respond to immunomodulatory eicosanoids reveals a virulence mechanism that has potentially great implications for understanding the mechanisms of chronic fungal infection, immune deviation, and fungi as disease cofactors.
ACKNOWLEDGMENTS
We wish to thank Dierdra Williams for her work on the chemokine assays.
This work was supported by a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs-Wellcome Fund (G.B.H.). M.C.N. is supported by NIH-NIAID training grant T32AI07528.
REFERENCES
- 1.Betz M, Fox B S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- 2.Botha A, Kock J, Coetzee D, Linde N, van Dyk M. Yeast eicosanoids. II. The influence of non-steroidal anti-inflammatory drugs on the life cycle of Dipodascopsis. Syst Appl Microbiol. 1992;15:155–160. [Google Scholar]
- 3.Botha A, Kock J, Nigam S. The production of eicosanoid precusors by mucoralean fungi. In: Sinzinger H, Samuelsson B, Vane J, Paoletti R, Ramwell P, Wong P, editors. Recent advances in prostaglandin, thromboxane, and leukotriene Research. New York, N.Y: Plenum Press; 1998. [Google Scholar]
- 4.Demeure C E, Yang L-P, Desjardins C, Raynauld P, Delespesse G. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol. 1997;27:3526–3531. doi: 10.1002/eji.1830271254. [DOI] [PubMed] [Google Scholar]
- 5.Deva R, Ciccoli R, Schewe T, Kock J L, Nigam S. Arachidonic acid stimulates cell growth and forms a novel oxygenated metabolite in Candida albicans. Biochim Biophys Acta. 2000;1486:299–311. doi: 10.1016/s1388-1981(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 6.Gangopadhyay P, Thadepalli H, Roy I, Ansari A. Identification of species of Candida, Cryptococcus, and Torulopsis by gas-liquid chromatography. J Infect Dis. 1979;140:952–958. doi: 10.1093/infdis/140.6.952. [DOI] [PubMed] [Google Scholar]
- 7.Herman R P. Oxylipin production and action in fungi and related organisms. In: Rowley A F, Kuhn H, Schewe T, editors. Eicosanoids and related compounds in plants and animals. Princeton, N.J: Princeton University Press; 1998. [Google Scholar]
- 8.Kalo-Klein A, Witkin S. Prostaglandin E2 enhances and gamma interferon inhibits germ tube formation in Candida albicans. Infect Immun. 1990;58:260–262. doi: 10.1128/iai.58.1.260-262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kock J, Coetzee D, van Dyk M, Truscott M, Cloete P, van Wyk V, Augustyn O. Evidence for pharmacologically active prostaglandins in yeasts. S Afr J Sci. 1991;87:73–76. [Google Scholar]
- 10.Krystofova S, Vareka L, Vollek V, Grimova J, Bettina V. Growth and conidiation of Tricoderma viride are affected by non-steroidal antiinflammatory agents. Folia Microbiol. 1994;39:44–48. doi: 10.1007/BF02814528. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel S L, Spengler M, May M A, Spengler R, Larrick J, Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988;263:5380–5384. [PubMed] [Google Scholar]
- 12.Lamacka M, Sajbidor J. The occurence of prostaglandins and related compounds in lower organisms. Prostaglandins Leukot Essent Fatty Acids. 1995;52:357–364. doi: 10.1016/0952-3278(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 13.Lodewyk J, Kock J, Venter P, Botha A, Coetzee D, Botes P, Nigam S. The production of biologically active eicosanoids by yeasts. Adv Exp Med Biol. 1997;433:217–219. doi: 10.1007/978-1-4899-1810-9_46. [DOI] [PubMed] [Google Scholar]
- 14.Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. [Google Scholar]
- 15.Marumo K, Aoki Y. Disciminant analysis of cellular fatty acids of Candida species, Torulopsis glabrata, and Cryptococcus neoformans determined by gas-liquid chromatography. J Clin Microbiol. 1990;28:1509–1513. doi: 10.1128/jcm.28.7.1509-1513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2019. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian N, Casadevall A, Berman J W. Inhibition of human endothelial cell chemokine production by the opportunistic fungal pathogen Cryptococcus neoformans. J Immunol. 2000;165:1541–1547. doi: 10.4049/jimmunol.165.3.1541. [DOI] [PubMed] [Google Scholar]
- 18.Murphy J W, Zhou A, Wong S C. Direct interactions of human natural killer cells with Cryptococcus neoformans inhibit granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha production. Infect Immun. 1997;65:4565–4571. doi: 10.1128/iai.65.11.4564-4571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrier V, Dubreucq E, Galzy P. Fatty acid and carotenoid composition of Rhodotorula strains. Arch Microbiol. 1995;164:173–179. doi: 10.1007/BF02529968. [DOI] [PubMed] [Google Scholar]
- 20.Peters-Golden M. Lipid mediator synthesis by lung macrophages. In: Lipscomb M F, Russell S W, editors. Lung macrophages and dendritic cells in health and disease. Vol. 102. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 151–182. [Google Scholar]
- 21.Romani L, Kaufmann S H. Immunity to fungi: editorial overview. Res Immunol. 1998;149:277–281. doi: 10.1016/s0923-2494(98)80751-7. [DOI] [PubMed] [Google Scholar]
- 22.Sandin R L, Rogers R J, Patterson R J, Beneke E S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982;35:79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sergeeva M G, Gonchar M V, Mevkh A T, Varfolomeyev S D. Prostaglandin E2 biphasic control of lymphocyte proliferation inhibition by picomolar concentrations. FEBS Lett. 1997;418:235–238. doi: 10.1016/s0014-5793(97)01388-4. [DOI] [PubMed] [Google Scholar]
- 24.Snijdewint F G, Kalinski P, Wierenga E A, Bos J D, Kapsenberg M L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- 25.Sobel J D, Muller G, Buckley H R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984;44:576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Standiford T J, Kunkel S L, Rolfe M W, Evanoff H L, Allen R M, Strieter R M. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992;6:75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witkin S S, Jeremias J, Ledger W J. A localized vaginal allergic response in women with recurrent vaginitis. J Allergy Clin Immunol. 1988;81:412–416. doi: 10.1016/0091-6749(88)90909-8. [DOI] [PubMed] [Google Scholar]