Abstract
Nontypeable Haemophilus influenzae (NTHi) is a major pathogen causing otitis media (OM). One of the outer membrane proteins of NTHi, P6, is a common antigen to all strains and is considered a candidate for mucosal vaccine. We have previously reported that intranasal immunization with P6 and cholera toxin (CT) could induce P6-specific immunoglobulin A (IgA) antibodies in the middle ear. In the present study, we assessed the effect of intranasal immunization for the protection against NTHi-induced OM. Mice were immunized intranasally with P6 and CT as an adjuvant on days 0, 7, and 14. Control mice were given phosphate-buffered saline (PBS) without antigen. One week after the final immunization, a suspension of live NTHi (107 CFU) was injected into the tympanic cavity to induce experimental OM. On days 3 and 7 after bacterial challenge, mice were killed and middle ear effusions (MEEs) were collected. All immunized mice showed elevated titers of P6-specific antibodies in MEEs. The rank order of specific antibody included, from highest to lowest levels, IgG, IgA, and IgM. In addition, immunized mice showed enhanced clearance of NTHi from the middle ear and the number of NTHi in MEEs of immunized mice was reduced by 97% on day 3 and by 92% on day 7 after bacterial challenge relative the number in the MEEs of control mice. The protective effect of intranasal immunization on the incidence of NTHi-induced experimental OM was evident on day 7 after challenge. By day 7, the number of MEEs in immunized mice was 64% less than that in control mice and the incidence of NTHi culture-positive MEEs in immunized mice was 56% less than that in control mice. Less stimulation of tumor necrosis factor alpha (TNF-α) production in the middle ear was evident on day 3 after challenge. Immunized mice showed lower concentrations of TNF-α in MEEs. These results indicate that intranasal immunization affords protection against experimental OM as evidenced by enhanced clearance of NTHi and less stimulation of TNF-α production in the middle ear. These findings suggest that a nasal vaccine might be useful for preventing OM.
Otitis media (OM) is one of the most common infectious diseases in children, and the peak incidence of this disease occurs in early childhood. Nontypeable Haemophilus influenzae (NTHi) is a major causative pathogen of OM and is often isolated from middle ear effusions (MEEs) and the nasopharynx (12). Because of the increased incidence of antibiotic-resistant strains of NTHi in recent years, the development of a vaccine against this bacterium is considered an important goal for public health (14, 15). Recent efforts to develop an effective vaccine candidate against NTHi have focused on P6, an outer membrane protein of NTHi and a common antigen to all strains (32, 33).
The middle ear mucosa is capable of producing local and systemic responses after an appropriate antigenic stimulus (30). Local immunity in the middle ear, in conjunction with systemic immunity, plays an important role in OM. Antigen-specific antibodies appear in MEE during acute OM and seem to play a major role in the resolution of acute OM (20, 46). Thus, vaccination leading to elevation of the levels of NTHi antigen-specific antibodies is likely to prevent acute OM.
The mucosal immune system is considered a separate functional entity quite independent of the systemic immune system because the mucosal immune system possesses unique anatomic features and is composed of specialized subsets of lymphoid cells (29). At the mucosal surface, secretory immunoglobulin A (IgA) plays a major role in protective immunity. We previously demonstrated that intranasal immunization was an effective regimen to induce mucosal IgA immune responses in the upper respiratory tract and that the nasal mucosal IgA immune responses induced by intranasal immunization were effective for the clearance of bacteria in the nasopharynx (25, 35). In addition, we recently reported that the middle ear mucosa is characterized as a mucosal effector site and that intranasal immunization with P6 and cholera toxin (CT) could induce P6-specific IgA responses in the middle ear (22). Thus, prevention of OM by intranasal immunization with P6 is an important area of investigation into the development of the vaccine. Since intranasal immunization enhances NTHi clearance from the nasopharynx, it is also likely to enhance NTHi clearance from the middle ear cavity. Moreover, it is also likely that the same mechanisms for clearing bacteria from the respiratory tract mucosa are used in the middle ear mucosa because the mucosal effector sites are similar (22).
The middle ear mucosa can secrete high levels of inflammatory cytokines in response to microbial or endotoxin stimulation (31), and tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) are present in large proportions of the MEEs (34, 38). These cytokines are known to contribute to the formation and maintenance of OM (19), and less intense stimulation of their production may decrease inflammation and pathologic changes associated with OM.
While the protective effect of mucosal IgA antibodies against mucosal surface infection is well documented, the underlying mechanisms are not entirely understood. Local immunity in the middle ear has been ignored largely because of technical difficulties associated with analyzing small quantities of MEE. Chinchillas are often used as animal models for NTHi-induced OM. However, this model is inadequate due to its lack of genetic uniformity and our lack of knowledge of its immune responses. Since correlation between inflammatory cytokines and mucosal immunoglobulin production may help elucidate the mechanism of protection against NTHi infection, we devised a method to analyze inflammatory and immune responses on a single murine MEE sample. In this study, we tested the efficacy of intranasal vaccination with P6 and CT for enhancing the clearance of NTHi from the middle ear, reducing the stimulation of middle ear mucosal inflammatory cytokine production, and preventing OM in a murine model.
MATERIALS AND METHODS
Animals.
BALB/c mice were purchased from Charles River Japan (Atsugi, Japan). The mice were maintained under specific-pathogen-free conditions. A total of 92 healthy adult mice between 6 and 8 weeks of age were used in the experiment.
Immunization.
P6 was purified from NTHi (strain 76) in our laboratory by a previously reported method (22, 23). Sixty-four mice were immunized intranasally three times, on days 0, 7, and 14, with 10 μl of phosphate-buffered saline (PBS) containing a mixture of 10 μg of P6 and 1 μg of CT (Sigma, St. Louis, Mo.) as a mucosal adjuvant (P6+CT-immunized mice) or 10 μl of PBS containing 1 μg of CT alone (CT-immunized mice). Twenty-eight control mice were given PBS without antigen.
Middle ear challenge with live NTHi and induction of experimental OM.
The same strain of NTHi used for the preparation of P6 was cultured on chocolate agar plates overnight at 37°C in 5% CO2, removed by scraping, and resuspended in PBS (109 CFU/ml) for middle ear challenge. One week after the third immunization, the bacterial suspension was injected into the right tympanic cavity. Briefly, after a submandibular skin incision was made, the right inferior bulla was exposed and two microholes were made in the bulla with a 27-gauge needle. A micropipette was inserted into one of the microholes, and 10 μl (109 CFU) of the live NTHi suspension was injected slowly. The mice were monitored by otomicroscopic observation to confirm the presence of MEE and tympanic membrane changes. Fourteen mice from each group were killed on days 3 and 7 after the operation. The rates of NTHi clearance from the middle ear and the incidence of experimental OM were measured.
Sampling.
Mice were anesthetized by intraperitoneal injection with 0.1 ml of PBS containing 2 mg of ketamine (Sigma) and 0.2 mg of xylazine (Sigma), and blood, ear wash, and MEE samples were collected. Blood samples were collected by orbital puncture 1 week after the third immunization and on days 3 and 7 after bacterial challenge. Samples were stored at −20°C prior to being assayed for antibodies and cytokines. One week after the third immunization, ear wash samples were collected, the left tympanic cavity was washed three times with 10 μl of cold PBS, and then the 30 μl of PBS wash was pooled and adjusted to a final volume of 50 μl with PBS. Care was taken to avoid contamination of ear wash samples with blood. On days 3 and 7 after injection with NTHi, the mice were killed and perfused transcardially with PBS to avoid contamination of MEEs with peripheral blood. To collect MEE samples, the tympanic cavity was washed three times with 10 μl of cold PBS immediately after sacrifice. The MEE samples and the 30 μl of PBS wash were pooled and adjusted to a final volume of 50 μl with PBS. Suspensions were centrifuged, an aliquot from each supernatant was serially diluted 10-fold, and 10-μl aliquots of the diluted samples were spread on chocolate agar plates to determine the concentration of live bacteria. After overnight incubation at 37°C in 5% CO2, NTHi was identified by standard bacteriologic techniques, including Gram staining and determination of their V and X growth factor requirements, and the colonies were counted (24). The concentration of NTHi was expressed as CFU per milliliter of ear wash. The remaining supernatants (approximately 36 to 41 μl) were then frozen at −20°C and stored prior to before being assayed for antibodies and cytokines. Due to the small size of the samples, we could not assay each MEE specimen for all antibodies (IgA, IgM, and IgG) and inflammatory cytokines (IL-1β and TNF-α). MEE samples in control and immunized groups were assayed for IgA, IL-1β and TNF-α (34 μl) or for IgG, IL-1β, and TNF-α (34 μl). The remaining MEEs were assayed for IgA and IgG (26 μl). The remaining supernatants of the MEEs from each group of mice were pooled (67 μl) and assayed for IgM. The ear samples were not run in duplicate due to the small volume of samples obtained from each ear. The cell pellet was diluted with PBS and processed for enumeration of inflammatory cells with a hemocytometer (the number of cells per cubic millimeter was given) and differential cell enumeration with Wright stain (Wright stain solution; Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Detection of P6-specific antibodies by ELISA.
P6-specific antibody titers in ear wash, MEE, and serum samples were determined by an enzyme-linked immunosorbent assay (ELISA) as described previously (36). Briefly, 96-well plates (Nunc, Roskilde, Denmark) were coated with an optimal concentration of P6 (100 μl of a 5-μg/ml concentration of P6) in PBS. The plates were incubated overnight at 4°C in a humidified atmosphere and washed three times with PBS. The wells were each blocked for 1 h at 37°C with 200 μl of PBS containing 1% bovine serum albumin (Gibco BRL, Gaithersburg, Md.). After extensive washing, serial twofold dilutions of ear wash, MEE, and serum were added and incubated for 4 h at room temperature. After incubation and washing, 100 μl of a 1:1,000-dilution of biotinylated goat anti-mouse IgM, IgG, or IgA (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was added to each well. A detection solution containing a 1:2,000 dilution of horseradish peroxidase-conjugated streptavidin (Gibco BRL) was added. The plates were incubated overnight at 4°C. After the plates were washed, the color reaction was developed at room temperature with 100 μl of 1.1 mM ABTS [2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid)] in 0.1 M citrate-phosphate buffer (pH 4.2) containing 0.01% H2O2 (Moss, Inc., Pasadena, Calif.) after a 15-min incubation. End-point titers were expressed as the reciprocal log2 of the last dilution that had an optical density at 414 nm (OD414) of >0.1 OD unit above the OD414 value of negative control samples obtained from control mice.
Detection of cytokines in MEE by ELISA.
Assays for IL-1β and TNF-α were performed with commercially available mouse ELISA kits (R&D Systems, Inc., Minneapolis, Minn.). ELISAs were performed as specified by the manufacturer. All samples were run with negative and positive controls. Standard curves were generated from known concentrations of cytokine provided by the manufacturer. The sensitivity of these assays, i.e., the lowest concentration of cytokine detectable in MEEs, was less than 5.1 pg/ml for TNF-α and less than 3.0 pg/ml for IL-1β. Samples were diluted such that the concentration of cytokine was within the range of the assay standards. The optical densities were read with a microtiter ELISA plate reader (Sanko Junyaku Co., Ltd., Tokyo, Japan).
Statistical analysis.
The mean volume and variability of volume within each MEE group were similar between control and immunized mice. Thus, variations in volume and dilution of MEE were unlikely to affect the results. The bacterial concentrations (in CFU per milliliter) were analyzed for statistical significance by the Mann-Whitney test. Specific antibody titers (in reciprocal log2), the cytokine concentrations (in picograms per milliliter), and inflammatory cell numbers (cells per cubic millimeter) were analyzed for statistical significance by Student's paired t test. Differences at P < 0.05 were considered significant. The correlations of bacterial concentration (in CFU per milliliter), IgA and IgG titers, and cytokine concentration were analyzed by Pearson's product moment method (null hypothesis: H0:P = 0; alternative hypothesis: H1:P < 0).
RESULTS
P6-specific antibody assay.
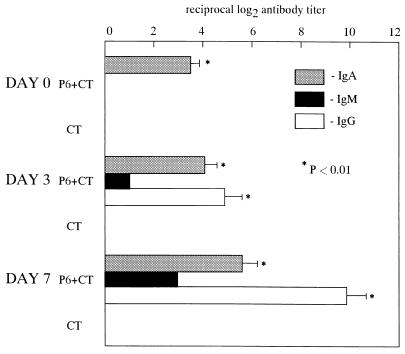
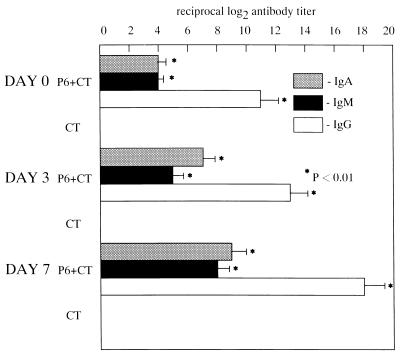
The levels of P6-specific IgA antibodies in ear wash were significantly elevated after intranasal immunization with P6 and CT (P < 0.01) but not after immunization with CT alone. After middle ear challenge with NTHi, P6-specific IgG, IgA, and IgM antibodies were detected in MEEs of P6+CT-immunized mice as early as day 3, and their titers were further increased by day 7 (P < 0.01). The rank order of specific immunoglobulin included, from highest to lowest levels, IgG, IgA, and IgM. However, the increase of the P6-specific antibodies was not observed in MEEs of CT-immunized mice (Fig. 1). Since antibodies in MEEs during the acute period might represent passive diffusion from serum, serum antibodies were also examined. P6-specific IgG, IgA, and IgM antibodies were detected in serum (Fig. 2), and the levels of these antibodies directly correlated with those in MEEs. The levels of IgG, IgA, and IgM antibodies in serum were also higher in P6+CT-immunized mice than in CT-immunized mice. Thus, intranasal immunization with P6 and CT followed by intratympanic inoculation of NTHi resulted in antigen-specific middle ear mucosal and systemic immunological responses.
FIG. 1.
The levels of P6-specific IgA antibodies in ear washes were significantly elevated after intranasal immunization with P6 and CT (P < 0.01) but not after immunization with CT alone. After middle ear challenge with NTHi, P6-specific IgG, IgA, and IgM antibodies were detected in MEEs of P6+CT-immunized mice as early as day 3 and their titers were further increased by day 7 (P < 0.01). However, P6-specific antibody levels were not elevated in MEEs of CT-immunized mice. These results are expressed as the mean and standard error and were obtained from a total of three experiments with 14 mice per group.
FIG. 2.
The levels of P6-specific antibodies were elevated in serum after intranasal immunization with P6 and CT (day 0 [1 week after the final immunization]) and further increased after middle ear challenge with NTHi (postchallenge days 3 and 7). Isotype patterns and increases in the titers of P6-specific antibodies were the same in serum and MEE. The levels of P6-specific antibodies were not elevated in serum after intranasal immunization with CT alone. These results are expressed as the mean and standard error and were obtained from a total of three experiments with 14 mice per group.
Intranasal immunization enhances the clearance of NTHi from middle ear.
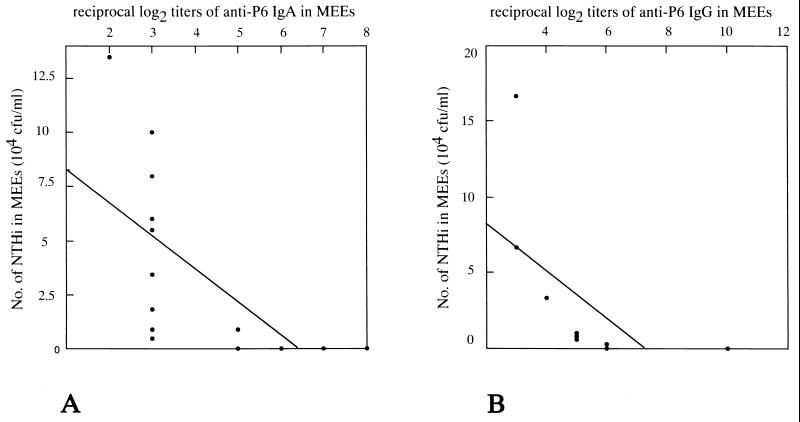
Control mice, CT-immunized mice, and P6+CT-immunized mice showed different rates of clearance of bacteria from the middle ear as evidenced by the number of NTHi cultured from the MEEs (Table 1). P6+CT-immunized mice had smaller NTHi numbers in MEEs than did control mice, and this difference was significant throughout the experiment (from days 3 to 7 after middle ear challenge with live NTHi: day 3, P = 0.0006; day 7, P = 0.0346). On postchallenge day 3, the number of NTHi in MEE was 97% smaller than that of the controls, and on postchallenge day 7, the number of NTHi in MEE was 92% smaller than that of the controls. The numbers of NTHi showed significant negative correlation with the titers of anti-P6 IgA (r = −0.638, P = 0.007) and anti-P6 IgG (r = −0.605, P = 0.042) in MEEs of P6+CT-immunized mice on postchallenge day 3 (Fig. 3). However, the enhanced clearance of NTHi from the middle ear was not observed in CT-immunized mice. The rates of clearance of bacteria from the middle ear in CT-immunized mice were not different from those in control mice throughout the experiment (P > 0.05). These results indicate that intranasal immunization with P6 plus CT enhanced the clearance of NTHi from middle ear; the protective effect was associated with P6-specific IgA and IgG antibody titers in MEEs.
TABLE 1.
Bacterial clearance and occurrence of OM with effusion
| Vaccination and day postchallengea | No. of NTH: cultured from MEEs (CFU/ml) (range)b | Presence of MEEs (no. of MEEs/total no. of ears) (%) | No. of culture-positive MEEs/total no. of ears (%) | No. of tympanic membrane changes/total no. of ears (%)f |
|---|---|---|---|---|
| Day 3 | ||||
| Control | 8.6 × 105 (0–2.8 × 106) | 14/14 (100) | 13/14 (92.8) | 13/14 (92.8) |
| CT | 6.4 × 105 (2.1 × 104–3.5 × 106)c | 14/14 (100) | 14/14 (100) | 13/14 (92.8) |
| P6+CT | 2.6 × 104 (0–1.3 × 105)d | 14/14 (100) | 11/14 (78.5) | 12/14 (85.7) |
| Day 7 | ||||
| Control | 4.4 × 104 (0–1.3 × 105) | 11/14 (78.6) | 9/14 (64.2) | 3/14 (21.4) |
| CT | 4.7 × 104 (0–3.4 × 105)c | 12/14 (85.7) | 9/14 (64.2) | 2/14 (14.2) |
| P6+CT | 3.7 × 103 (0–2.7 × 104)e | 4/14 (28.5) | 4/14 (28.5) | 0/14 (0) |
Results were obtained from a total of three experiments with 14 mice per group.
Results are expressed as the mean concentration (minimum and maximum values). The ratio of the mean of bacterial numbers in the control and P6+CT-immunized groups was 33:1 on postchallenge day 3 compared to 12:1 on postchallenge day 7.
P > 0.05 compared with the control group.
P < 0.0006 compared with the control group.
P < 0.0346 compared with the control group.
Otoscopical signs of otitis media, including dilated vessels, increased thickness and reduced translucency. Number of ears with tympanic membrane changes/total number of ears.
FIG. 3.
Correlation between the number of NTHi and the P6-specific IgA antibody titers (A) and between the number of NTHi and the P6-specific IgG antibody titers (B) in MEEs of P6+CT-immunized mice on postchallenge day 3. The number of NTHi showed a significant negative correlation with the P6-specific IgA antibody titers (r = −0.638, P = 0.007) and with the P6-specific IgG antibody titers (r = −0.605, P = 0.042).
Intranasal immunization reduces the incidence of NTHi-induced experimental OM.
Experimental OM was determined by the presence of MEE and tympanic membrane changes, including dilated vessels, increased thickness, and reduced translucency. On postchallenge day 3, NTHi-positive MEE was evident in both control and immunized mice (Table 1). On postchallenge day 7, the number of MEEs in P6+CT-immunized mice was 64% smaller than that in control mice and the incidence of NTHi culture-positive MEEs in P6+CT-immunized mice was 56% lower than that in control mice. However, intranasal immunization with CT showed no effect on the incidence of OM and the duration of culture-positive MEEs.
The tympanic membrane changes were shown in most of the control and immunized mice on postchallenge day 3; by postchallenge day 7, there were minor differences in the numbers of immunized and control mice with tympanic membrane changes. Thus, intranasal immunization with P6 and CT afforded a reduction in the incidence of OM and in the duration of culture-positive MEEs in response to infection with NTHi.
Quantitative determination of inflammatory cytokines in MEEs by ELISA.
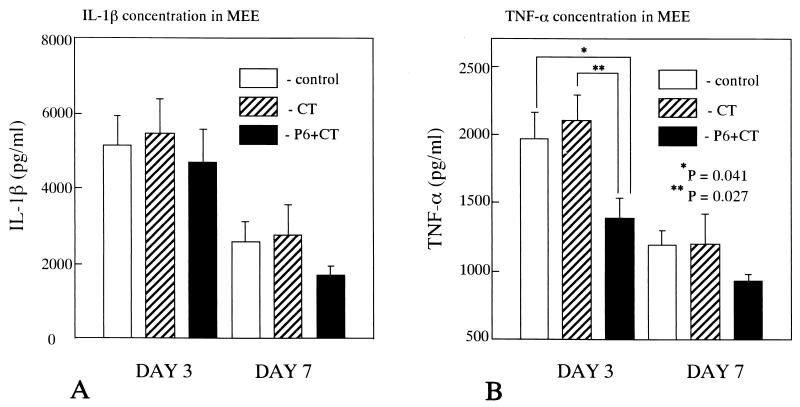
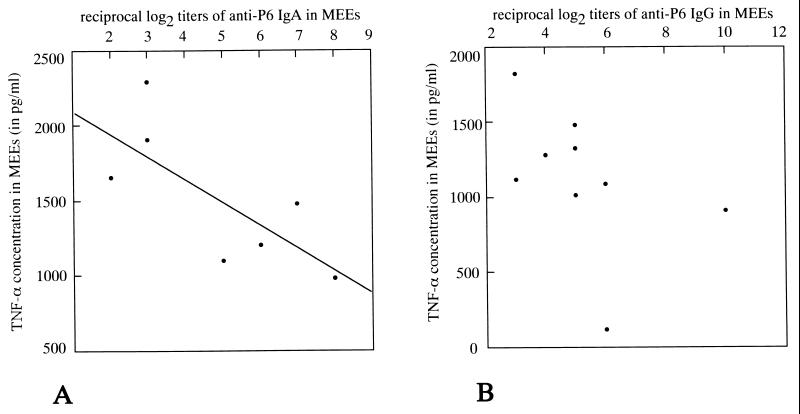
ELISA of MEE samples from mice injected with NTHi revealed significant levels of TNF-α and IL-1β. Throughout the experiment, the mean concentration of TNF-α and IL-1β in MEEs tended to be lower in P6+CT-immunized mice than in control mice (Fig. 4). On postchallenge day 3, the concentrations of TNF-α in MEEs of P6+CT-immunized mice were significantly lower than those in control mice (P = 0.041) and CT-immunized mice (P = 0.027). On postchallenge day 7, the concentrations of TNF-α in MEEs of P6+CT-immunized mice were lower than those in control mice, but no statistical significance was demonstrated (P = 0.089). On postchallenge day 3, the concentration of TNF-α in MEE was positively correlated with the number of NTHi in MEE (immunized, r = 0.716 and P = 0.035; controls, r = 0.647 and P = 0.058) and negatively correlated with the IgA titer (r = −0.784, P = 0.018) (Fig. 5). There was no significant correlation between the concentration of TNF-α and the IgG titer (r = −0.463, P = 0.104). The concentrations of IL-1β in MEEs of P6+CT-immunized mice were also lower than those in control mice, but no statistical significance in IL-1β concentration was detected due to large within-groups variation (day 3, P = 0.732; day 7, P = 0.189). The TNF-α and IL-1β concentrations in MEEs of CT-immunized mice were not different from those in MEEs of control mice throughout the experiment (P > 0.05). Negligible levels of TNF-α and IL-1β were detected in serum. These results indicate that there was less stimulation of TNF-α but not IL-1β production in the middle ear after intranasal immunization with P6 and CT.
FIG. 4.
(A) IL-1β concentrations in MEEs. (B) TNF-α concentrations in MEEs. The IL-1β levels in MEEs of P6+CT-immunized mice was slightly lower than those in MEEs of control mice (day 3, P = 0.732; day 7, P = 0.189). The TNF-α concentrations in MEEs of P6+CT-immunized mice were significantly less than those in MEEs of control mice (P = 0.041) and CT-immunized mice (P = 0.027) on postchallenge day 3. On postchallenge day 7, statistical significance was not demonstrated between P6+CT-immunized and control mice (P = 0.089). The TNF-α and IL-1β concentrations in MEEs of CT-immunized mice were not different from those in MEEs of control mice throughout the experiment (P > 0.05). These results are expressed as the mean ± SE and were obtained from a total of three experiments with 14 mice per group.
FIG. 5.
Correlation between TNF-α concentrations and P6-specific antibody titers in MEEs of P6+CT-immunized mice on postchallenge day 3. (A) Correlation between TNF-α concentrations and P6-specific IgA antibody titers. A significant negative correlation was detected (r = −0.784, P = 0.018). (B) Correlation between TNF-α concentrations and P6-specific IgG antibody titers. There was no significant correlation (r = −0.463, P = 0.104).
The mean number of inflammatory cells was slightly but not significantly smaller in MEEs from P6+CT-immunized mice (day 3, 6,012 cells/mm3; day 7, 1,039 cells/mm3) than in MEEs from control mice (day 3, 7,942 cells/mm3; day 7, 1,589 cells/mm3) or CT-immunized mice (day 3, 7,464 cells/mm3; day 7, 1,180 cells/mm3). No correlation was found between the number of inflammatory cells in MEEs and the rate of bacterial clearance or P6-specific antibody titers in the middle ear. Patterns of differential cell counts were the same for control and immunized mice throughout the experiment. Cell counts in MEEs on postchallenge days 3 and 7 consisted mainly of neutrophils (84 to 96%), lymphocytes (2 to 14%), and a few macrophages/monocytes (1 to 3%).
DISCUSSION
Here we have reported on the protective effect of intranasal immunization against middle ear infection by NTHi. We directly demonstrated protective immunity against NTHi-induced OM in mice intranasally immunized with P6 and CT. Moreover, our study partially elucidated the mechanism by which intranasal vaccination protects the middle ear by analyzing immune and inflammatory responses after NTHi challenge.
Intranasal immunization is known to elicit a local secretory IgA response in addition to considerable serum IgG, IgA, and IgM antibody levels (5). Secretory IgA plays a major role in eradicating the organism from the mucosal surface, and the protective effect of IgA is associated with adhesive and agglutinative activities (42). The effect of inflammation on the transfer of circulating IgG antibodies into MEE during OM is well documented (21). It is likely that some of the protective effects observed in the middle ear of immunized animals after challenge may result from the IgG antibody produced. Like IgA, IgG may limit the entry of mucosal pathogens into the organism and their subsequent multiplication and thus prevent systemic infection and promote bactericidal mechanisms (39). Serum-derived IgG antibodies would probably be protective in accordance with the principle of “pathotopic potentiation” of local mucosal immunity described in experimental animals (6). As in acute OM (11, 47), we found that MEEs of infected mice contained a relatively higher proportion of P6-specific IgG than of IgA. The increase in the levels of specific IgG, IgA, and IgM antibodies in serum was similar to that in MEE but was higher in serum, suggesting that these antibodies migrated from serum to MEE. However, in comparison with the specific IgA, IgM, and IgG antibodies in serum, middle ear washes from P6+CT-immunized mice contained only specific IgA antibodies prior to bacterial challenge. It has been reported that specific antibody-producing cells in the middle ear mucosa (with the dominant IgA isotype) and elevated levels of specific IgA antibodies in ear washes were detected after intranasal immunization (22). These results suggest that IgA antibody-producing cells within the middle ear mucosa are important sources of antigen-specific IgA in ear wash. Therefore, it is more likely that during the course of OM, P6-specific IgA antibodies in MEEs of P6+CT-immunized mice represented both local antibody production and transudation from serum. In contrast, P6-specific IgG and IgM antibodies in MEEs seem to be derived mainly from serum.
In several different animal models, protection against NTHi-induced middle ear infection was demonstrated after mucosal (27) or systemic (1, 2, 16) presentation of antigen. Intestinal immunization with killed NTHi enhanced the clearance of NTHi from the middle ear in rats, but the protective antigen and protective immune responses were not shown in that study (27). Enhanced clearance of NTHi from the middle ear was observed in chinchillas after systemic immunization with a mixture of outer membrane proteins, and the protection was associated with antigen-specific bactericidal antibodies (1, 2, 16). Enhanced NTHi clearance from the mucosal surface generally correlates well with antigen-specific mucosal IgA titer but not with serum IgA and IgG antibody levels after mucosal immunization (24). The present study showed that intranasal immunization with P6 and CT effectively enhanced the clearance of NTHi from the murine middle ear. Since a significant negative correlation between NTHi numbers and anti-P6 IgA titer was found in MEEs, we speculate that intranasal immunization produced mucosal immunity, thereby inhibiting the adherence of NTHi to the middle ear mucosa. The systemic immunity elicited by this route of immunization also probably contributed to protection against NTHi infection, as evidenced by the significant negative correlation between NTHi numbers and the anti-P6 IgG titer in MEEs. Intestinal immunization with killed NTHi or P6 enhanced the clearance of NTHi from the lungs, and the clearance mechanism involved rapid recruitment of phagocytic cells (26, 40). Here, using our murine OM infection model, we demonstrate that antibody-mediated immunity but not recruitment of neutrophils plays a major role in clearance of NTHi from the middle ear.
DeMaria et al. (10) demonstrated that systemic immunization with P6 reduced the incidence of NTHi-induced OM in chinchillas, and this effect was associated with elevated levels of bactericidal antibody in serum. Our present study indicates that intranasal immunization with P6 and CT also provides a significant degree of protection against experimental OM as assessed by the incidence of NTHi-induced OM and the presence of P6-specific antibodies in MEE and serum.
The unregulated release of large amounts of inflammatory cytokines accounts for the majority of toxic effects of endotoxin. TNF-α and IL-1β are pivotal inflammatory cytokines, and their role in the pathogenesis of OM is well documented (3, 19). Hypothetically, suppression of key inflammatory mediators of the acute-phase response will effectively prevent or reduce MEE and inflammation (17). Both cytokines are produced in the middle ear and are present in MEE (9, 37). Neutrophils are the principal source of IL-1β at sites of inflammation (18, 28), whereas mucosal macrophages are the principal source of TNF-α (4).
IgA antibodies prevent potentially harmful local and systemic inflammatory reactions. Yanagita et al. (48) reported that fimbria-specific IgA antibodies produced in saliva after intranasal immunization inhibited bacterial attachment to and reduced subsequent inflammatory cytokine production by epithelial cells in vitro. In vitro studies showed that IgA downregulates TNF-α release in human monocytes (43, 45). Yellon et al. (49) assayed MEEs from children with OM and did not find any significant correlation between the concentrations of inflammatory cytokines and immunoglobulins. We showed that inflammatory-cytokine release was tempered after intranasal immunization and that concentrations of TNF-α were significantly lower (P = 0.041) in MEEs of P6+CT-immunized mice than those in control mice on postchallenge day 3. Moreover, a significant negative correlation (r = −0.784, P = 0.018) was observed between the TNF-α concentration and IgA titer on postchallenge day 3 despite the small number of MEE specimens. IgA may reduce the stimulation of TNF-α production by enhancing the clearance of NTHi from the middle ear. Since antigen-specific IgA possesses the ability to inhibit bacterial attachment to epithelial cells, it prevents bacterial invasion, macrophage activation, and the subsequent inflammatory response. The present study is the first demonstration of less strong stimulation of TNF-α production after intranasal immunization in a murine model of OM. In control mice, increased numbers of bacteria invade the middle ear mucosa and stimulate macrophages to produce TNF-α. Elevation of the local levels of TNF-α activates and maintains neutrophil phagocytosis but simultaneously increases inflammatory effects in the middle ear. Our study showed the relationship between the TNF-α concentration and bacterial numbers in MEEs, and this relationship may explain why the difference in TNF-α concentrations between the immunized and control groups was more obvious on postchallenge day 3 than on day 7 (Table 1).
In the present study, we demonstrated protection against NTHi-induced OM after intranasal immunization. This was not due to the effects of CT as a mucosal immunogen, since the numbers of NTHi and concentrations of TNF-α in MEEs were comparable to those in MEEs of control mice. These results indicate that intranasal immunization with P6 and CT, but not with CT alone, provides a protective immunity against NTHi-induced OM.
The impact of intranasal immunization on IL-1β production is less clear. We failed to show less stimulation of IL-1β production in MEEs of immunized mice. Macrophages are the central effector cell type in endotoxin-mediated host responses (13). Moreover, monocytes are more likely to modulate IL-1β production since, in humans, interaction of these cells with IgA stimulates the release of the IL-1 receptor antagonist (44). The delay in the appearance of macrophages/monocytes in MEEs (7) and the middle ear mucosa (8) during OM is well documented and may explain the discrepancy between the downregulatory effect of IgA on IL-1β release in vitro and the results of our study.
Endotoxin is not readily eradicated by local host defense mechanisms and remains in the middle ear after the elimination of viable bacteria, continually stimulating the production of TNF-α and IL-1β (41). This observation is supported by our finding that on postchallenge day 13 middle ear washes were culture negative but contained detectable levels of inflammatory cytokines in both groups (data not shown). The concentrations of TNF-α and IL-1β were significantly lower in MEEs of P6+CT-immunized mice than in MEEs of control mice, and this is probably a result of stimulation by a lower concentration of endotoxin. In the present study, high levels of TNF-α and IL-1β were detected in MEE but not in serum, suggesting high local production of inflammatory cytokines in the middle ear.
In summary, our present findings demonstrate that intranasal immunization with P6 and CT induces P6-specific middle ear mucosal and systemic responses and affords protection in murine experimental OM, as evidenced by enhanced clearance of NTHi and reduced stimulation of TNF-α production in MEE. These findings suggest that nasal vaccine might be useful for the prevention of OM.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Basic Scientific Research (A) from the Ministry of Education, Science, Sports and Culture of Japan (10307040) and a grant from the Society for Promotion of International Oto-Rhino-Laryngology (SPIO).
We thank Y. Kurono (Department of Otolaryngology, Kagoshima University), K. Sato (Department of Otolaryngology, Niigata University), N. Eshima (Department of Medical Information Analysis, Oita Medical University), and I. Ichimiya, K. Yoshida, K. Maeda, and Y. Takenaka (in our department) for invaluable assistance.
REFERENCES
- 1.Bakaletz L O, Kennedy B J, Novotny L A, Duquesne G, Gohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun. 1999;67:2746–2762. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modified experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Cachetin: more than a tumor necrosis factor. N Engl J Med. 1987;316:379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- 4.Beutler B, Cerami A. The biology of cachectin/TNF-α primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Haneberg B. Role of nasal-associated lymphoid tissue in the human mucosal immune system. Mucosal Immunol Update. 1997;5:4–8. [Google Scholar]
- 6.Brandzaeg P, Jahnsen F L, Farstad I N. Immune functions and immunopathology of the mucosa of the upper respiratory pathways. Acta Otolaryngol. 1996;116:149–159. doi: 10.3109/00016489609137812. [DOI] [PubMed] [Google Scholar]
- 7.DeMaria T F, Briggs B R, Lim D J, Okazaki N. Experimental otitis media with effusion following middle ear inoculation of nonviable H. influenzae. Ann Otol Rhinol Laryngol. 1984;93:52–56. doi: 10.1177/000348948409300113. [DOI] [PubMed] [Google Scholar]
- 8.DeMaria T F, Yamaguchi T, Lim D J. Quantitiative cytologic and histologic changes in the middle ear after the injection of nontypeable Haemophilus influenzae endotoxin. Am J Otolaryngol. 1989;10:261–266. doi: 10.1016/0196-0709(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 9.DeMaria T F, Murwin D M. Tumor necrosis factor during experimental lipopolysaccharide-induced otitis media. Laryngoscope. 1997;107:369–372. doi: 10.1097/00005537-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 10.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faden H, Bernstein J, Brodsky L, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children. I. The systemic immune response to nontypeable Hemophilus influenzae. J Infect Dis. 1989;160:999–1004. doi: 10.1093/infdis/160.6.999. [DOI] [PubMed] [Google Scholar]
- 12.Faden H, Brodsky L, Bernstein J, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children: local immune responses to nontypeable Haemophilus influenzae. Infect Immun. 1989;57:3555–3559. doi: 10.1128/iai.57.11.3555-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freudenberg M A, Keppler D, Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986;51:891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giebink G S. Preventing pneumococcal disease in children: recommendations for using pneumococcal vaccine. Pediatr Infect Dis. 1985;4:343–348. doi: 10.1097/00006454-198507000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Giebink G S, Shiffman G, Petty K, Quie P G. Modification of otitis media following vaccination with the capsular polysaccharide of Streptococcus pneumoniae in chinchillas. J Infect Dis. 1978;138:480–487. doi: 10.1093/infdis/138.4.480. [DOI] [PubMed] [Google Scholar]
- 16.Green B A, Vazquez M E, Zlotnick G W, Quigley-Peape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebda P A, Alper C M, Doyle W J, Burckart G J, Diven W F, Zeevi A. Upregulation of messenger RNA for inflammatory cytokines in middle ear mucosa in a rat model of acute otitis media. Ann Otology Rhinol Laryngol. 1998;107:501–507. doi: 10.1177/000348949810700608. [DOI] [PubMed] [Google Scholar]
- 18.Himi T, Suzuki T, Kodama H, Takezawa H, Kataura A. Immunologic characteristics of cytokines in otitis media with effusion. Ann Otol Rhinol Laryngol. 1992;157:21–25. doi: 10.1177/0003489492101s1006. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M D, Fitzgerald J E, Leonard G, Burleson J A, Kreutzer D L. Cytokines in experimental otitis media with effusion. Laryngoscope. 1994;104:191–196. doi: 10.1288/00005537-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Karjalainen H, Koskela M, Luotonen J, Herva E, Sipilä P. Occurrences of antibodies against Streptococcus pneumonia, Haemophilus influenzae and Branhamella catarrhalis in middle ear effusion and serum during the course of acute otitis media. Acta Otolaryngol. 1991;111:112–119. doi: 10.3109/00016489109137361. [DOI] [PubMed] [Google Scholar]
- 21.Keithley E M, Krekorian T D, Sharp P A, Harris J P, Ryan A F. Comparison of immune-mediated models of acute and chronic otitis media. Eur Arch Otorhinolaryngol. 1990;247:247–251. doi: 10.1007/BF00178996. [DOI] [PubMed] [Google Scholar]
- 22.Kodama S, Suenaga S, Hirano T, Suzuki M, Mogi G. Induction of specific immunoglobulin A and Th2 immune responses to P6 outer membrane protein of nontypeable Haemophilus influenzae in middle ear mucosa by intranasal immunization. Infect Immun. 2000;68:2294–2300. doi: 10.1128/iai.68.4.2294-2300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama H, Faden H. Cellular immunity to the P6 outer membrane protein of nontypeable Haemophilus influenzae. Infect Immun. 1995;63:2467–2472. doi: 10.1128/iai.63.7.2467-2472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurono Y, Shigemi H, Kodama S, Mogi G. Effects of oral and systemic immunization on nasopharyngeal clearance of nontypeable Haemophilus influenzae in BALB/c mice. Laryngoscope. 1996;106:614–618. doi: 10.1097/00005537-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, McGhee J R, Kiyono H. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180:122–132. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 26.Kyd J M, Cripps A W. Modulation of antigen-specific T and B cell responses influence bacterial clearance of non-typeable Haemophilus influenzae from the lung in a rat model. Vaccine. 1996;14:1471–1478. doi: 10.1016/s0264-410x(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 27.Kyd J M, Cripps A W. Killed whole bacterial cells, a mucosal delivery system for the induction of immunity in the respiratory tract and middle ear: an overview. Vaccine. 1999;17:1775–1781. doi: 10.1016/s0264-410x(98)00441-1. [DOI] [PubMed] [Google Scholar]
- 28.Matsukawa A, Yoshinaga M. Neutrophils as a source of cytokines in inflammation. Histol Histopathol. 1999;14:511–516. doi: 10.14670/HH-14.511. [DOI] [PubMed] [Google Scholar]
- 29.McGhee J R, Lam M E, Strober W. Mucosal immune responses. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1999. pp. 485–506. [Google Scholar]
- 30.Mogi G, Bernstein J M. Immune mechanisms in otitis media with effusion. In: Bernstein J M, Ogra P L, editors. Immunology of the ear. New York, N.Y: Raven Press; 1987. pp. 279–299. [Google Scholar]
- 31.Morrison D C, Ryan J L. Endotoxins and disease mechanism. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 32.Munson R S, Jr, Granoff D M. Purification and partial characterization of outer membrane protein P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985;49:544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy T F, Apicella M A. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune responses to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Ondrey F G, Juhn S K, Adams G L. Early-response cytokine expression in adult middle ear effusions. Otolaryngol Head Neck Surg. 1998;119:342–345. doi: 10.1016/S0194-5998(98)70075-0. [DOI] [PubMed] [Google Scholar]
- 35.Sabirov A, Kodama S, Hirano T, Suzuki M, Mogi G. Th1/Th2 nasal immune responses to P6 outer membrane protein of nontypeable Haemophilus influenzae. Jpn J Rhinol. 2000;39:58–60. doi: 10.1128/iai.68.4.2294-2300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto N, Kurono Y, Suzuki M, Kerakawauchi H, Mogi G. Immune responses of adenoidal lymphocytes specific to Haemophilus influenzae in the nasopharynx. Laryngoscope. 1998;108:1036–1041. doi: 10.1097/00005537-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Liebeler C L, Quartey M K, Le C T, Giebink G S. Middle ear fluid cytokine and inflammatory cell kinetics in the chinchilla otitis media model. Infect Immun. 1999;67:1943–1946. doi: 10.1128/iai.67.4.1943-1946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Nonomura N, Kawana M, Nakano Y. Course of IL-1β, IL-6, IL-8, and TNF-α in the middle ear fluid of the guinea pig otitis media model induced by nonviable Haemophilus influenzae. Ann Otol Rhinol Laryngol. 1999;108:559–563. doi: 10.1177/000348949910800606. [DOI] [PubMed] [Google Scholar]
- 39.Underdown B J, Plotkin S A. The induction of mucosal protection by parenteral immunization. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1999. pp. 719–728. [Google Scholar]
- 40.Wallace F J, Witt C S, Clancy R L, Cripps A W. Protection against nontypable Haemophilus influenzae following sensitization of gut associated lymphoid tissue: role of specific antibody and phagocytes. Immunol Cell Biol. 1995;73:258–265. doi: 10.1038/icb.1995.42. [DOI] [PubMed] [Google Scholar]
- 41.Willet D N, Rezaee R P, Billy J M, Tighe M B, DeMaria T F. Relationship of endotoxin to tumor necrosis factor-alpha and interleukin-1 beta in children with otitis media with effusion. Ann Otol Rhinol Laryngol. 1998;107:28–33. doi: 10.1177/000348949810700106. [DOI] [PubMed] [Google Scholar]
- 42.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- 43.Wolf H M, Eibl M M. The anti-inflammatory effect of an oral immunoglobulin (IgA-IgG) preparation and its possible relevance for the prevention of necrotizing enterocolitis. Acta Paediatr. 1994;396:37–40. doi: 10.1111/j.1651-2227.1994.tb13240.x. [DOI] [PubMed] [Google Scholar]
- 44.Wolf H M, Hauber I, Gulle H, Samstag A, Fisher M B, Ahmad R U, Eibl M M. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin Exp Immunol. 1996;105:537–543. doi: 10.1046/j.1365-2249.1996.d01-793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf H M, Fisher M B, Pühringer H, Samstag A, Vogel E, Eibl M M. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-α, interleukin-6) in human monocytes. Blood. 1994;83:1278–1288. [PubMed] [Google Scholar]
- 46.Yamaguchi T, DeMaria T F, Lim D J. Antibody response in experimental Haemophilus influenzae otitis media. Arch Otolaryngol Head Neck Surg. 1986;112:554–557. doi: 10.1001/archotol.1986.03780050078014. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka N, Faden H. Local antibody response to P6 of nontypable Haemophilus influenzae in otitis-prone and normal children. Acta Otolaryngol. 1993;113:524–529. doi: 10.3109/00016489309135857. [DOI] [PubMed] [Google Scholar]
- 48.Yanagita M, Hiroi T, Kitagaki N, Hamada S, Ito H, Shimauchi H, Murakami S, Okada H, Kiyono H. Nasopharyngeal-associated lymphoreticular tissue (NALT) immunity: fimbriae-specific Th1 and Th2 cell-regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokine production. J Immunol. 1999;162:3559–3565. [PubMed] [Google Scholar]
- 49.Yellon R F, Doyle W J, Whiteside T L, Diven W F, March A R, Fireman P. Cytokines, immunoglobulins, and bacterial pathogens in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1995;121:865–869. doi: 10.1001/archotol.1995.01890080033006. [DOI] [PubMed] [Google Scholar]