Abstract
Objective:
To analyze the relationship of electrode array (EA) type and position on hearing preservation longevity following cochlear implantation.
Study Design:
Retrospective chart review.
Setting:
Tertiary referral center.
Patients:
Adult cochlear implant recipients between 2013 and 2019 with hearing preserved postoperatively and postoperative CT scans.
Interventions:
CT scan analysis of EA position. Stepwise regression to determine influence of EA position, EA type, and patient demographics on postoperative low frequency hearing.
Main Outcome Measures:
Low frequency pure tone average (LFPTA), LFPTA shift, angular insertion depth, base insertion depth, scalar position, mean perimodiolar distance.
Results:
Of 792 cochlear implant recipients, 121 had preoperative LFPTA ≤80 dB HL with 60 of the 121 (49.6%) implanted with straight, 32 (26.4%) with precurved, styletted, and 29 (24.0%) implanted precurved, nonstyletted EA. Mean follow up was 28.6 months (range 1–103). There was no statistically significant difference in activation, 6- and 12-month, and last follow-up LFPTA (125, 250, and 500 Hz) shift based on EA type (straight p = 0.302, precurved, styletted p = 0.52, precurved, nonstyletted p = 0.77). Preoperative LFPTA and age of implantation were significant predictors of LFPTA shift at activation, accounting for 30.8% of variance (F[2, 113] = 26.603, p<0.0001). LFPTA shift at activation, scalar position, and base insertion depth were significant predictors of variability and accounted for 39.1% of variance in LFPTA shift at 6 months (F[3, 87] = 20.269, p<0.0001). Only LFPTA shift at 12 months was found to be a significant predictor of LFPTA shift at last follow up, accounting for 41.0% of variance (F[1, 48] = 32.653, p<0.0001).
Conclusions:
Patients had excellent long-term residual hearing regardless of EA type. Age, preoperative acoustic hearing, and base insertion depth may predict short term preservation, while 12-month outcomes significantly predicted long-term hearing preservation.
Keywords: Cochlear implant, Electrode position, Hearing longevity, Hearing preservation
INTRODUCTION
Many factors are known to influence cochlear implant (CI) outcomes, including (but certainly not limited to) duration of deafness, hearing aid (HA) use, age of implantation, and neurocognition (1–6). Expanded indications for cochlear implantation to include patients with residual low-frequency hearing unveiled new variables that may influence hearing preservation and speech perception outcomes. Initially shorter electrode arrays (EA) were favored to minimize trauma to the cochlear apex where residual hearing is more likely to be present (7–11). Further investigations revealed that straight EA, often referred to as lateral wall EA, are less likely to deviate from scala tympani (ST), presumably reducing intracochlear trauma and preserving residual hearing (12–14). Round window versus cochleostomy insertion, in addition to straight EA, demonstrated improved hearing preservation and speech outcomes (15).
Prior research has reported that straight EA have superior hearing preservation and postoperative speech performance when compared to precurved EA (12,14,16). This is likely due to the fact that precurved EA are designed to hug the modiolus and have a higher incidence of translocation from ST to scala vestibuli (SV) (16–18). As EA design has advanced, longer straight and precurved EA are now used routinely in patients with residual hearing where hearing preservation is desired, particularly with the introduction of the nonstyletted precurved EA with external sheath. Recent studies have demonstrated successful hearing preservation with this EA and outcomes comparable to, or superseding straight EA (19–21). Yet the longevity of hearing preservation amongst EA types within a large cohort, including the nonstyletted precurved EA, has not been established.
The relationship of perimodiolar distance and angular insertion depth with speech outcomes has been established for conventional CI, yet the impact and stability of residual low-frequency hearing remains to be investigated. In addition to EA type, EA position within the cochlea is significant for speech perception outcomes (12,22,23). Chakravorti et al. demonstrated a deeper angle of insertion predicted improved speech outcomes for straight EA, while a decreased distance from the modiolus and shallower depth of insertion in precurved EA predicted improved performance.
There were two aims of this study: (1) to compare the longevity of hearing preservation with varying EA type in patients under-going hearing preservation cochlear implantation, and (2) to further investigate the impact of EA positioning on long-term hearing preservation and speech outcomes.
METHODS AND MATERIALS
Following approval from the Institutional Review Board, a retrospective chart review was used to identify adult patients who underwent cochlear implantation at our tertiary referral center between January 2013 and March 2019. Patients older than 18 years of age, with preserved low-frequency hearing postoperatively (LFPT ≤80 dB HL at 250 Hz) and those with postoperative computed topography (CT) scanning for EA localization were included in the analysis. LFPTA was calculated by averaging the audiometric and/or vibrotactile thresholds at 125, 250, and 500 Hz. Exclusion criteria included revision cases, cochlear anomalies, and loss of residual hearing after surgery (LFPt >80 dB HL at 250 Hz). If a patient had under gone bilateral implantation, each ear was accounted for individually. After the cohort was identified, further retrospective chart review was used to obtain demographic information, CI manufacturer and EA type, and surgical approach (round window vs. cochleostomy). Patients were categorized into groups based on EA type: straight (a.k.a. lateral wall), styletted precurved, and nonstyletted precurved.
Patients were implanted with devices from all three U. S. Food and Drug Administration (FDA) approved manufacturers: Cochlear (Syndey, South Wales, Australia), MED-EL (Innsbruck, Austria), and Advanced Bionics (Valencia, CA). Device selection was based on patient preference, while EA selection was dictated by enrollment in a clinical trial or surgeon preference.
The methods for postoperative CT scan analysis of EA position have previously been published and are mentioned here in brevity (22). Cochlear anatomy was segmented in the clinical CT scans using a nonrigid shape model of intracochlear structures defined using high resolution μCTs of 16 cadaveric cochlear specimens. Intracochlear structures were localized with mean errors of approximately 0.1 mm (24,25). The EA were localized within the postoperative cochleas using an additional automatic algorithm with mean localized errors of approximately 0.13 mm (26,27). Rigid co-registration of the pre- and postoperative images allows localization of the implanted electrode with reference to the cochlea. Multiple positional measurements are obtained, including mean closest distance from each electrode to the modiolous (mean perimodiolar distance), scalar position ((ST only, SV only, or translocation from ST to SV or vice-versa), angular depth of the tip of the array, and basal electrode insertion depth from the round window (basal insertion depth). These positional measurements are independent of EA type or number of stimulating electrodes.
Hearing preservation was defined as having postoperative audiometric and/or vibrotactile thresholds ≤ 80 dB HL at 250 Hz. To quantify the maximum amount of hearing loss at a frequency, 120 dB HL was used when no response was obtained at the limit of the audiometer. LFPTA “threshold shift” was calculated by subtracting the LFPTA at activation, 6- and 12-months and at last follow up from the preoperative LFPTA.
To better understand the influence of EA type, demographic variables, and positional measurements on hearing preservation outcomes, a stepwise multiple regression was used with forward selection forced entry model (F 0.05 entry, 0.10 removal) of strongly correlated factors using SPSS version 26 (Armonk, NY). For all variables that did not fit a normal distribution, median and interquartile ranges (IQR) are reported. Ordinary one-way ANOVA was performed using Graph Pad Prism 8.3 (GraphPad Software, La Jolla, CA) to compare LFPTA, LFPTA shifts, and positional measurements between EA types.
RESULTS
Out of 792 postlingually deafened adult CI patients in our REDCap clinical outcomes database implanted from 2013 to 2018, a total of 121 ears were identified to be candidates for hearing preservation with a preoperative LFPTA ≤80 dB HL and postoperative CT scanning (Fig. 1). The average age of implantation was 63.3 years (range 23.0–95.8), with 56 (47%) female, and 51 (42.1%) implanted in the left ear. Patients were implanted with all three manufactures’ devices with straight and precurved EA including 39 Advanced Bionics (Valencia, CA), 61 Cochlear (Sydney, Australia), and 21 MED-EL (Innsbruck, Austria) CI. Sixty ears (49.6%) were implanted with straight EA, 32 ears (26.4%) were implanted with precurved EA, and 29 ears (24.0%) were implanted with a precurved, nonstyletted electrode. A round window insertion was used for 86.9% of cases (n = 106). Mean length of surgery date to last follow up was 28.6 months (range 1–103).
FIG. 1.

Flowchart of patient cohort. CI indicates cochlear implant; CT, computed topography; HP, hearing preservation, LFPTA, low frequency pure tone average.
Electrode Positional Measurements
For all EA, the median angle of insertion was 385.4° (IQR 357.3–431.7), base insertion depth was 1.88 mm (IQR 0.90–2.61), and median perimodiolar distance was 0.80mm (IQR 0.36–1.06) (Fig. 2A and C). Precurved, nonstyletted EA had a significantly smaller perimodiolar distance (median 0.31, IQR 0.24–0.38), followed by the precurved styletted EA (median 0.53, IQR 0.35–0.65), and straight EA (median 1.07, IQR 0.98–1.16) (Fig. 1A). Eight EA (8.3%) were identified as translocated while the remaining were completely within ST. Six of which were precurved, while the remaining two were straight EA. For these eight patients, median activation LFPTA shift was 21.7 dB HL (IQR 14.2–35.4). There was no significant difference in base insertion depth between EA (Fig. 1B). Straight EA had a significantly deeper angle of insertion (median 414.1°, IQR 371.5–504.1) when compared to both precurved styletted (median 367.5°, IQR 347.1–391.0), and precurved nonstyletted EA (median 379.9°, IQR 255.9–412.4) (Fig. 2C).
FIG. 2.
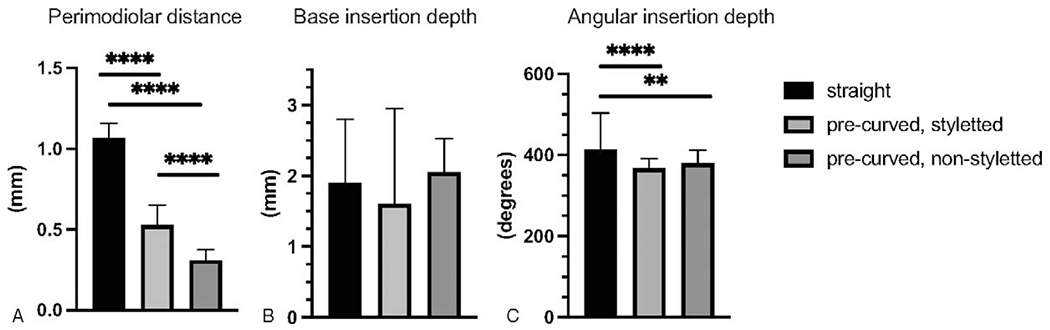
Electrode position measurements for each electrode type. A, Perimodiolar distance was significantly greater for straight and precurved styletted EA compared to precurved, nonstyletted EA ( p<0.0001). B, There was no statistically significant difference between EA types in base insertion depth ( p>0.05). C, Angular insertion depth was significantly greater for straight EA ( p<0.0001 and p=0.0017).
Hearing Preservation
The median preoperative LFPTA for all arrays was 50.0 dB HL (IQR 37.3–60.0). Patients implanted with straight EA had significantly better preoperative hearing with lower preoperative LFPTA (median 46.7 dB HL, IQR 32.1–61.3) when compared to the precurved, styletted EA (median 53.3, IQR 43.8–61.3, p=0.0258) (Fig. 2A). The median LFPTA significantly deteriorated to 70.0 dB HL (IQR 63.3–80.0, p<0.0001) at activation, to 71.7 dB HL (iQR 61.7–88.3) at 6-months, 71.7 dB HL (IQR 63.3–85.0, p<0.0001) at 12-months, and to 78.3 dB HL (IQR 63.8–95.8, p<0.0001) at last follow-up. Results of LFPTA and LFPTA shift from activation to last follow-up are presented in Figures 3 and 4. When separated by type of EA (i.e., straight vs. precurved, styletted vs. precurved nonstyletted) all had significant increases in LFPTA at 6, 12-months, and last follow-up when compared to preoperative LFPTA (Fig. 3A). There was no statistically significant difference in LFPTA following activation for both the straight and precurved, nonstyletted EA; however, patients implanted with precurved, styletted EA had a significant difference between activation LFPTA and last follow up LFPTA ( p = 0.0365) (Fig. 3B). Both cohorts implanted with the straight and precurved nonstyletted EA had significantly lower LFPTA at both 6 months postoperatively ( p = 0.0046 and 0.047, respectively) and last follow-up ( p = 0.0016) when compared to the precurved, styletted EA cohort (Fig. 3B).
FIG. 3.

Low frequency pure tone averages (LFPTA) (125–500 Hz) preoperatively and at subsequent follow up intervals. A, Although postop LFPTA significantly increased when compared to pre-op ( p<0.0001), there was no statistically significant change following activation for straight and precurved, nonstyletted EA. B, Preoperative LFPTA was significantly lower for straight EA ( p=0.0258). Six- and 12-month LFPTA was significantly higher for precurved, styletted EA ( p=0.0046, 0.0477, and 0.0016).
FIG. 4.

Low frequency pure tone average (LFPTA) shifts at activation and at subsequent follow up intervals. A, There was no statistically significant difference between LFPTA shifts for each EA type ( p>0.05). B, There was no statistically significant difference in LFPTA shifts across follow up intervals between EA types ( p>0.05).
The median threshold shift at activation for all arrays was 20.0 dB HL (IQR 11.7–28.3). LFPTA remained stable between 6 and 12-months postoperatively with 6- and 12-month threshold shifts of 21.7 dB HL (IQR 9.6–38.3) and 20.0 dB HL (IQR 11.3–35.8), respectively. The mean threshold shift significantly deteriorated by 28.3 dB HL (IQR 13.3–41.7) at last follow-up compared to activation for all arrays ( p = 0.015). There was no significant difference in activation, 6- and 12-month, and last follow up LFPTA shift when the cohort was separated by EA (straight p = 0.30, precurved, styletted p = 0.52, precurved, nonstyletted p = 0.77) (Fig. 4A). When comparing the LFPTA shift at activation, 6-month and 12-months postoperatively, and last follow-up between EA types (straight vs. precurved, styletted vs. precurved nonstyletted), there was no statistically significant difference (Fig. 4B).
Stepwise Regression
The change in LFPTA between activation and last follow up was also analyzed as a function of EA type (straight, precurved nonstyletted vs. styletted), scalar position, angular insertion depth, base insertion depth, mean perimodiolar distance, age of implantation, and biological sex (Table 1). Preoperative LFPTA and age of implantation was a significant predictor of LFPTA shift at activation, accounting for 30.8% of variance (F[2, 113] = 26.603, p<0.0001). LFPTA shift at activation, scalar position, and base insertion depth were significant predictors of variability and accounted for 39.1% of variance in LFTA shift at 6 months (F[3, 87] = 20.269, p<0.0001). Data was limited at the 12-month postoperative interval (n = 50), therefore a model could be not generated. Only LFPTA shift at 12 months was found to be a significant predictor of LFPTA shift at last follow up, accounting for 41.0% of variance (F[1, 48] = 32.653, p < 0.0001).
TABLE 1.
Manufacturer and electrode arrays
| Straight | Precurved | Precurved, nonstyletted | Total | |
|---|---|---|---|---|
| Cochlear | 22 | 10 | 29 | 61 |
| MED-EL | 21 | NA | NA | 21 |
| Advanced Bionics | 17 | 22 | NA | 39 |
| Total | 60 | 32 | 29 |
DISCUSSION
Over the last decade, significant advances in electrode design and surgical technique has afforded patients the benefit of preserving residual low-frequency hearing. Cochlear implant recipients fit with combined electric and acoustic stimulation (EAS) demonstrate improved speech perception in noise, improved sound localization, and music appreciation compared to electrical stimulation alone (28–30). Given such benefit, the opportunity for hearing preservation should be optimized for each patient. Not infrequently, despite the best effort of the surgeon, residual hearing is unintentionally lost. Both to make this undesirable outcome less common and to predict progression of residual hearing loss over time, it is important to understand variables which impact residual hearing. Within this study, we report the influence of patient demographics, EA type and position on the longevity of serviceable hearing over an average 29 months following cochlear implantation.
Within our cohort, LFPTA shift postoperatively did not differ between EA types. When compared to previous reports at our institution, Wanna et al. reported higher rates of short and long-term hearing preservation (mean PTA shifts) in straight and “mid-scala” (styletted precurved EA from Advanced Bioinics) EA compared to traditional styletted precurved EA (n = 225) (14). This pattern was also reported by Sweeney et al. (16). They reported styletted precurved EA were associated with a greater PTA shift when compared to straight EA (n = 16). The mean preoperative, activation and last follow up LFPTA reported by Wanna et al. was 63, 88, and 97 dB HL, respectively noting that not all of these patients had hearing preserved postoperatively as in our cohort. The mean preoperative, activation, and last follow-up LFPTA reported by Sweeney et al. was 57.5, 74.3, and 82.5 dB HL, respectively all of which were higher thresholds than our cohort. Such differences in results reported herein may be due to improved surgical techniques over time regardless of EA type including immediate postoperative CT scanning presented to the surgeon for nearly all CI recipients at our institution (31).
Within our study, precurved, styletted electrodes did have a significantly deteriorated LFPTA between activation and last follow-up, but this pattern was not significant when comparing LFPTA shift. We suspect that these data were likely influenced by selection bias due to surgeons favoring straight and nonstyletted EA for patients with the lowest (i.e., best) preoperative audiometric thresholds.
Preoperative LFPTA and age of implantation were both significant factors for initial and 6-month hearing preservation rates. This is consistent with prior studies (32), although Sweeney et al. and O’Connell et al. found no correlation between PTA and patient age (12,14). Younger age and lower preoperative LFPTA may be a marker of cochlear and/or neural health. Younger patients or those with residual hearing may be more resistant to cochlear trauma or the acute inflammatory process caused by surgical trauma (33–35).
Our results also suggest that EA position may influence hearing preservation. As expected, straight EA had a greater distance from the modiolus compared to the precurved EA. A deeper base insertion depth was associated with decreased LFPTA shift at 6-months postoperatively which at first glance appears unexpected. However, EA with base insertion depth <1.0 mm have a shallower insertion than recommended by the visible markers on the EA. Such shallow insertion—particularly those with base insertion depth <1.0 mm—may result from resistance met during insertion implying that the surgeon is choosing to stop advancing the electrode in an effort to avoid cochlear trauma, which could result in greater LFPTA shift observed.
Additionally, we did not observe a trend between angle of insertion and perimodiolar distance with short- and long-term hearing preservation. O’Connell et al. reported deeper insertion of straight EA was associated with worse short-term hearing preservation, but this did not reach statistical significance (12). Our results are similar to Lee et al., who similarly did not observe a difference in angular insertion depth between those with and without hearing preserved postoperatively (32). Overall, insertion depth can impact cochlear trauma, but may be overcome with soft surgical technique.
Surprisingly, eight patients in our cohort had interscalar translocation with hearing preserved postoperatively. The raw CT scans and segmented images were further reviewed and translocation was clear in each case. We hypothesize at least three potential mechanisms for the hearing preservation. First, as proposed by Wright and Roland, electrode arrays may translocate by dissecting between the bone and soft tissue of the spiral ligament theoretically preserving the separation of perilymph and endolymph (36). Second, if dissection through the basilar membrane occurs, the electrode array could function as a tight stopper again preventing the mixing of perilymph and endolymph. Finally, scar tissue might effectively seal around the electrode array. Hearing preservation following translocation will be reported from the same institution in four subjects (one overlapped with our cohort), suggesting that although rare, it is possible ( publication in submission).
Our results indicate that hearing preservation may stabilize by 12 months. Hearing preservation at last follow up appeared to be most influenced by the 12-month outcomes, demonstrating that insertion trauma and associated inflammatory processes are likely most relevant for early hearing preservation outcomes such as those occurring in the first months following cochlear implantation (37).
As a field we continue to seek innovations that could provide understanding of the variables contributing to the stability of acoustic hearing following cochlear implantation. Intraoperative electrocochleography and impedance fluctuations show promise as potential markers for post-operative residual hearing (38–41). These advances may provide intra-operative feedback for the surgeon to aid in hearing preservation electrode insertion. Limitations of this study include its retrospective nature and lack of power in our 12-month regression analysis. The variability in steroid regimen and administration between surgeons limits analysis of it as a potentially cofounding variable.
CONCLUSION
This study reported the relationship between EA type, intracochlear position, and patient demographics in patients with preserved residual low frequency hearing following cochlear implantation. Patients had reasonable (median threshold shift of 28.3 dB HL [IQR 13.3–41.7] at last follow up) long-term residual hearing regardless of EA type. Age and preoperative acoustic hearing may be predictive of short-term hearing preservation, while electrode position, insertion trauma, and associated inflammatory processes do not appear to influence hearing preservation beyond 12 months.
Acknowledgments
PI Dr René Gifford and the work presented here was supported by NIH NIH NIDCD R01 DC009404. She is a consultant for Advanced Bionics and Cochlear Americas; Clinical advisory board for Frequency Therapeutics; Board of Directors for the American Auditory Society.
Footnotes
Co-author Robert Labadie, MD, PhD is a consultant for Advanced Bionics and Spiral Therapeutics.
Co-author David Haynes MD is a consultant for MED-EL and Cochlear Americas and serves on the Advanced Bionics surgical advisory board.
The authors disclose no conflicts of interest.
REFERENCES
- 1.Anagiotos A, Hamdan N, Lang-Roth R, et al. Young age is a positive prognostic factor for residual hearing preservation in conventional cochlear implantation. Otol Neurotol 2015;36:28–33. [DOI] [PubMed] [Google Scholar]
- 2.Blamey P, Artieres F, Baskent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol Neurootol 2013;18:36–47. [DOI] [PubMed] [Google Scholar]
- 3.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013;34:342–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: Effects of implantation criteria. Am J Otol 1999;20:445–52. [PubMed] [Google Scholar]
- 5.Zhan KY, Lewis JH, Vasil KJ, et al. Cognitive functions in adults receiving cochlear implants: Predictors of speech recognition and changes after implantation. Otol Neurotol 2020;41:e322–9. [DOI] [PubMed] [Google Scholar]
- 6.Moberly AC, Castellanos I, Mattingly JK. Neurocognitive factors contributing to cochlear implant candidacy. Otol Neurotol 2018;39:e1010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gantz BJ, Hansen MR, Turner CW, et al. Hybrid 10 clinical trial: Preliminary results. Audiol Neurootol ;14 (Suppl 1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn CC, Oleson J, Parkinson A, et al. Nucleus Hybrid S12: Multicenter Clinical Trial Results. Laryngoscope 2020;130:e548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gantz BJ, Dunn C, Oleson J, et al. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope 2016;126:962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenarz T, James C, Cuda D, et al. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. Int J Audiol 2013;52:838–48. [DOI] [PubMed] [Google Scholar]
- 11.Roland JT Jr, Gantz BJ, Waltzman SB,et al. Long-term outcomes of cochlear implantation in patients with high-frequency hearing loss. Laryngoscope 2018;128:1939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell BP, Hunter JB, Gifford RH, et al. Electrode location and audiologic performance after cochlear implantation: A comparative study between nucleus CI422 and CI512 electrode arrays. Otol Neurotol 2016;37:1032–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanna GB, Noble JH, Gifford RH, et al. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: Preliminary results. Otol Neurotol 2015;36:1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanna GB, O’Connell BP, Francis DO, et al. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope 2018;128:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Friedmann DR, Treaba C, et al. Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope 2015;125:966–71. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney AD, Hunter JB, Carlson ML, et al. Durability of hearing preservation after cochlear implantation with conventional-length electrodes and scala tympani insertion. Otolaryngol Head Neck Surg 2016;154:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014;124 (Suppl 6):S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer E, Karkas A, Attye A, et al. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol 2015;36:422–9. [DOI] [PubMed] [Google Scholar]
- 19.Holder JT, Yawn RJ, Nassiri AM, et al. Matched cohort comparison indicates superiority of precurved electrode arrays. Otol Neurotol 2019;40:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassiri AM, Yawn RJ, Holder JT, et al. Hearing preservation outcomes using a precurved electrode array inserted with an external sheath. Otol Neurotol 2020;41:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodson E, Smeal M, Nelson RC, et al. Slim perimodiolar arrays are as effective as slim lateral wall arrays for functional hearing preservation after cochlear implantation. Otol Neurotol 2020;41:e674–679. [DOI] [PubMed] [Google Scholar]
- 22.Chakravorti S, Noble JH, Gifford RH, et al. Further evidence of the relationship between cochlear implant electrode positioning and hearing outcomes. Otol Neurotol 2019;40:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connell BP, Hunter JB, Haynes DS, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope 2017;127:2352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reda FA, McRackan TR, Labadie RF, et al. Automatic segmentation of intra-cochlear anatomy in post-implantation CT of unilateral cochlear implant recipients. Med Image Anal 2014;18:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reda FA, Noble JH, Labadie RF, et al. An artifact-robust, shape library-based algorithm for automatic segmentation of inner ear anatomy in post-cochlear-implantation CT. Proc SPIE Int Soc Opt Eng 2014;9034:90342V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Dawant BM, Labadie RF, et al. Automatic localization of cochlear implant electrodes in CT. Med Image Comput Comput Assist Interv 2014;17:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Chakravorti S, Labadie RF, et al. Automatic graph-based method for localization of cochlear implant electrode arrays in clinical CT with sub-voxel accuracy. Med Image Anal 2019;52:1−12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adunka OF, Dillon MT, Adunka MC, et al. Hearing preservation and speech perception outcomes with electric-acoustic stimulation after 12 months of listening experience. Laryngoscope 2013;123:2509–15. [DOI] [PubMed] [Google Scholar]
- 29.Dorman MF, Gifford RH. Speech understanding in complex listening environments by listeners fit with cochlear implants. J Speech Lang Hear Res 2017;60:3019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gifford RH, Davis TJ, Sunderhaus LW, et al. Combined electric and acoustic stimulation with hearing preservation: Effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear 2017;38:539–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labadie RF, Schefano AD, Holder JT, et al. Use of intraoperative CT scanning for quality control assessment of cochlear implant electrode array placement. Acta Otolaryngol 2020;140:206–11. [DOI] [PubMed] [Google Scholar]
- 32.Lee G, Lee S, Chung JH, et al. Preimplant hearing threshold: An important predictor of hearing preservation in cochlear implantation with lateral wall electrodes. Otol Neurotol 2020;42:e145–152. [DOI] [PubMed] [Google Scholar]
- 33.Eshraghi AA, Yang NW, Balkany TJ. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope 2003;113:415–9. [DOI] [PubMed] [Google Scholar]
- 34.Eshraghi AA. Prevention of cochlear implant electrode damage. Curr Opin Otolaryngol Head Neck Surg 2006;14:323–8. [DOI] [PubMed] [Google Scholar]
- 35.Kamakura T, Nadol JB Jr. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res 2016;339:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charles G, Wright PSR. Cochlear Anatomy via Microdissection with Clinical Implications. Springer; 2018. [Google Scholar]
- 37.Eshraghi AA, Polak M, He J, et al. Pattern of hearing loss in a rat model of cochlear implantation trauma. Otol Neurotol 2005;26:442–7. discussion 7. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary S, Briggs R, Gerard JM, et al. Intraoperative observational real-time electrocochleography as a predictor of hearing loss after cochlear implantation: 3 and 12 month outcomes. Otol Neurotol 2020;41:1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalbert A, Rohner P, Roosli C, et al. Correlation between electrocochleographic changes during surgery and hearing outcome in cochlear implant recipients: A case report and systematic review of the literature. Otol Neurotol 2020;41:318–26. [DOI] [PubMed] [Google Scholar]
- 40.Giardina CK, Brown KD, Adunka OF, et al. Intracochlear electrocochleography: Response patterns during cochlear implantation and hearing preservation. Ear Hear 2019;40:833–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson NJ, Dillon MT, Buss E, et al. Electrode array type and its impact on impedance fluctuations and loss of residual hearing in cochlear implantation. Otol Neurotol 2020;41:186–91. [DOI] [PubMed] [Google Scholar]


