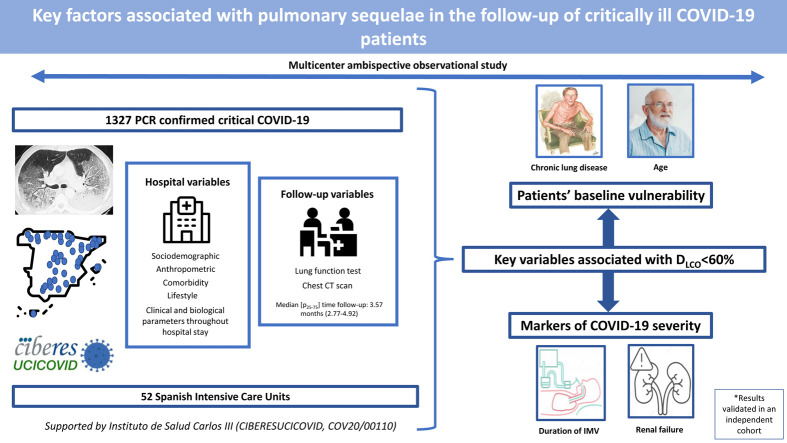
Graphical abstract
Keywords: COVID-19, CT abnormalities, ICU, Lung function, Sequelae
Abstract
Introduction
Critical COVID-19 survivors have a high risk of respiratory sequelae. Therefore, we aimed to identify key factors associated with altered lung function and CT scan abnormalities at a follow-up visit in a cohort of critical COVID-19 survivors.
Methods
Multicenter ambispective observational study in 52 Spanish intensive care units. Up to 1327 PCR-confirmed critical COVID-19 patients had sociodemographic, anthropometric, comorbidity and lifestyle characteristics collected at hospital admission; clinical and biological parameters throughout hospital stay; and, lung function and CT scan at a follow-up visit.
Results
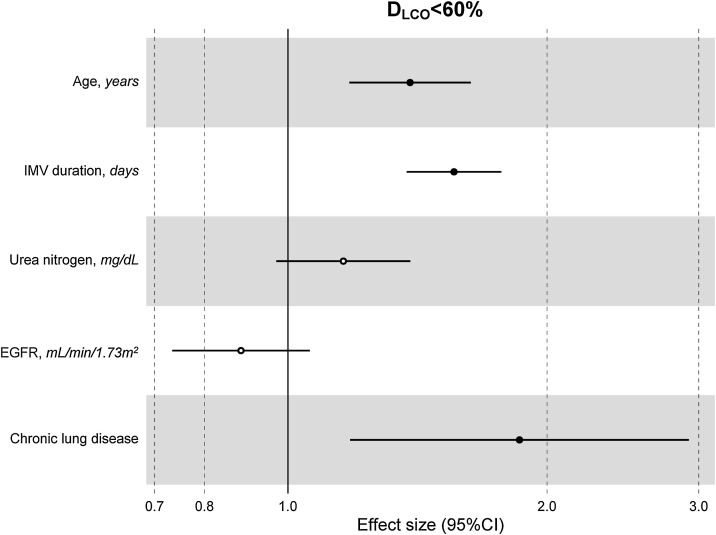
The median [p25–p75] time from discharge to follow-up was 3.57 [2.77–4.92] months. Median age was 60 [53–67] years, 27.8% women. The mean (SD) percentage of predicted diffusing lung capacity for carbon monoxide (DLCO) at follow-up was 72.02 (18.33)% predicted, with 66% of patients having DLCO < 80% and 24% having DLCO < 60%. CT scan showed persistent pulmonary infiltrates, fibrotic lesions, and emphysema in 33%, 25% and 6% of patients, respectively. Key variables associated with DLCO < 60% were chronic lung disease (CLD) (OR: 1.86 (1.18–2.92)), duration of invasive mechanical ventilation (IMV) (OR: 1.56 (1.37–1.77)), age (OR [per-1-SD] (95%CI): 1.39 (1.18–1.63)), urea (OR: 1.16 (0.97–1.39)) and estimated glomerular filtration rate at ICU admission (OR: 0.88 (0.73–1.06)). Bacterial pneumonia (1.62 (1.11–2.35)) and duration of ventilation (NIMV (1.23 (1.06–1.42), IMV (1.21 (1.01–1.45)) and prone positioning (1.17 (0.98–1.39)) were associated with fibrotic lesions.
Conclusion
Age and CLD, reflecting patients’ baseline vulnerability, and markers of COVID-19 severity, such as duration of IMV and renal failure, were key factors associated with impaired DLCO and CT abnormalities.
Introduction
As of 21st December 2022, more than 650 million COVID-19 cases have been confirmed globally, and more than 6.6 million people have died.1 The clinical spectrum of SARS-CoV-2 pneumonia ranges from mild to critically ill cases, with a proportion of 20–30% of hospitalized patients resulting in acute respiratory distress syndrome (ARDS).2 This has generated a surge of patients who require respiratory support with invasive or noninvasive mechanical ventilation (IMV and NIMV), overburdening intensive care units (ICU) worldwide.3, 4
Patients who survive critical COVID-19 have the highest prevalence (56–89%) of pulmonary involvement represented by an abnormal diffusing lung capacity for carbon monoxide (D LCO) and chest computed tomography (CT) scan after hospital discharge.5, 6, 7, 8 With COVID-19 continuing to be a public health emergency and the enormous global disease burden of surviving patients, it is crucial to understand the key factors associated with pulmonary sequelae after critical COVID-19 hospital discharge and plan the follow-up accordingly.
Some predictors of pulmonary involvement after COVID-19 have been described in the literature, the most important being the severity of the disease in the acute phase7, 9 and its respiratory management,10 sex,7, 11, 12 age,12 and previous comorbidities.11, 12 These studies are descriptive cohorts of patients in which the primary objective was to assess pulmonary sequelae during the follow-up. In addition, only one of these studies10 focused on critically ill survivors. In this regard, there is a lack of studies aiming to determine the key factors associated with respiratory sequelae after hospital discharge that have a representative sample of critically ill COVID-19 patients with the required characterization and follow-up.
Our main objective was to assess the key factors associated with an altered D LCO at a follow-up visit after hospital discharge using data from a large ambispective and multicentric cohort of patients who needed ICU admission due to COVID-19. Additionally, we intended to evaluate key factors associated with abnormalities in chest CT and the involvement of other spirometry values.
Materials and methods
Study design
The current manuscript is based on data from the CIBERESUCICOVID study,13 which is an observational, pragmatic, multicenter, ambispective study including critically ill COVID-19 patients admitted to the ICUs of 55 Spanish hospitals. CIBERESUCICOVID was registered in ClinicalTrials.gov with the identifier NCT04457505. The study collected retrospective data from patients admitted to participating ICUs before May 2020 and prospective data from then onward. CIBERESUCICOVID included a follow-up within the first year after hospital discharge of the maximum number of patients that the pandemic situation allowed in each participating hospital, without a specific protocol and regardless of whether the patients presented symptoms or not.
Study population
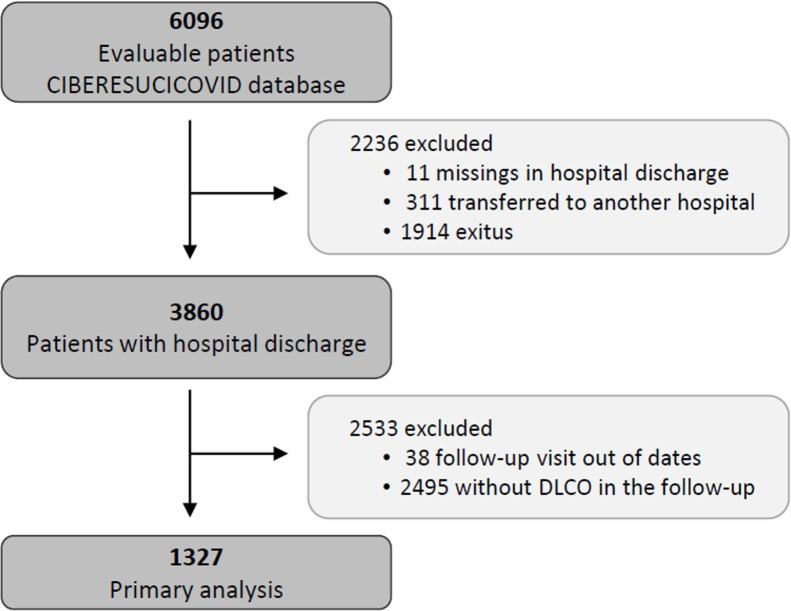
The data for the current analyses correspond to consecutive COVID-19 patients admitted to 52 Spanish ICUs from March 2020 to August 2021. All included patients had a confirmed COVID-19 diagnosis (positive nasopharyngeal swab polymerase chain reaction (PCR) test for SARS-CoV-2) and were admitted to the ICU. Patients not surviving the hospital stay or patients transferred to other hospitals during or after ICU admission were not considered eligible. Patients lacking a follow-up visit with lung function test after discharge was excluded from the analyses. Additionally, patients receiving palliative care or with severe mental disability precluding pulmonary function tests after discharge were also excluded. The study flow chart is shown in Fig. 1 .
Fig. 1.
Flowchart of the study.
Additionally, an external cohort consisting of 200 critically ill COVID-19 patients participating in the Post-COVID study5 held at the University Hospital Arnau de Vilanova and Santa Maria in Lleida, Spain, was used as a validation cohort.
Measures
Sociodemographic, anthropometric, comorbidity and lifestyle variables were collected using a large predetermined checklist at hospital admission (see Comorbidity checklist in the online supplement). Detailed information from the ICU stay included arterial blood gas test and complete blood test (at ICU admission and through ICU stay), including estimated glomerular filtration rate (EGFR) by means of the 2021 Chronic Kidney Disease Epidemiology Collaboration creatinine equation14; medical procedures before and during ICU stay, including ventilatory support; pharmacological treatment; and in-hospital complications such as ARDS, infections, thrombotic events or acute organ failure. Sequelae were objectively assessed at a follow-up visit by means of a thoracic CT scan (persistent infiltrates, emphysema and fibrotic lesions) and a lung function test (forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and D LCO).
Primary and secondary outcomes
The primary outcome of this study was D LCO as measured at a follow-up visit. Secondary outcomes were also assessed at the same visit and included CT scan findings and other parameters of the lung function test (FEV1 and FVC).
Ethics and data protection
The participating hospitals obtained ethical approval from the corresponding governing board. Study number: HCB/2020/0370; date of approval: 14/05/2020; original project title: “Factores de riesgo, pronóstico personalizados y seguimiento a un año de los enfermos ingresados en las unidades de Cuidados Intensivos Españolas infectados por el virus COVID19: CIBERESUCICOVID” (Risk factors, personalized prognosis and one-year follow-up of patients admitted to Spanish Intensive Care units infected with the COVID19 virus: CIBERESUCICOVID); governing board granting approval: Clinical Research Ethics Committee of Hospital Clínic de Barcelona. Participants or their relatives provided informed consent when possible or, when unfeasible, an informed consent waiver was authorized by the ethics board. Procedures were followed in accordance with the ethical standards of the Clinical Research Ethics Committee of Hospital Clínic de Barcelona and with the Helsinki Declaration of 1975 and its most recent amendments. Data were pseudonymized and stored in a REDCap database hosted in the Centro de Investigación Biomédica en Red (CIBER) premises in Madrid, Spain. The study complied with national and international law on data protection.
Similarly, the Post-COVID study, used as a validation cohort, was approved by the Clinical Research Ethics Committee of the University Hospital Arnau de Vilanova and Santa Maria (ref.: CEIC/2273; Title: “Factores de riesgo, pronósticos personalizados y seguimiento a un año de los enfermos ingresados en las unidades de Cuidados Intensivos Españolas infectados por el virus COVID19: ESTUDIO CIBERESUCICOVID” (Risk factors, personalized prognoses and one-year follow-up of patients admitted to Spanish Intensive Care units infected with the COVID19 virus: CIBERESUCICOVID STUDY); date: 02/06/2020); which was conducted in accordance with the ethical standards of the Clinical Research Ethics Committee of Hospital Arnau de Vilanova and Santa Maria and with the Helsinki Declaration of 1975 and its most recent amendments, and complied with national and international law on data protection.
Statistical analyses
Descriptive statistics were used to summarize the characteristics of the study population. Absolute and relative frequencies were used for qualitative data. The means (SD) or medians (25–75th percentile) were estimated for quantitative variables with normal and non-normal distributions, respectively. Normal distributions were assessed by the Shapiro–Wilk test.
Clinical data during the hospital stay were compared between surviving patients with and without follow-up using a t test (or Wilcoxon signed-rank test for variables with nonnormal distribution) for continuous variables and a chi-squared test (or Fisher exact test when the expected frequencies were less than 5 in some cells) for qualitative variables.
Hospital factors were compared to D LCO severity (categorized as: D LCO < 80%, 60% < D LCO < 80% and D LCO < 60%) using Mantel–Haenszel test of trend for categorical factors and Pearson test (or Spearman test in non-normal distribution) for continuous variables.
The missingness mechanism was assumed to be missing at random (MAR). In multivariable analyses, missing values were handled with multiple imputation by chained equations (MICE).15 Primary and secondary outcomes were included in the imputation models but were not imputed. The MICE procedure created 10 complete datasets. A minimum threshold of absolute correlation of 0.15 was used to select predictors in the imputation models. The predictors included in final multivariable model for each outcomes were selected using multiple imputation grouped adaptive least absolute shrinkage and selection operator (LASSO).16 The lambda value was set to the sparsest model within one standard error of the minimum 5-fold cross-validation error. Finally, final multivariable models were based on a logistic model (or linear model for continuous outcomes) for each outcome with variables selected (in LASSO regression) as predictors. The results across the multiply imputed datasets were combined using Rubin's rules.17 Additionally, a sensitivity analysis was carried out by fitting the final multivariable models in the population of complete cases.
The individual association between the primary outcome and the selected important variables was represented by violin plots or bar charts for dichotomous outcomes, and representations of generalized additive models for continuous outcomes.
Odds ratios were estimated to assess the direction and magnitude of the associations between the selected factors and the primary outcome in an independent cohort. This was used as a validation of the main results.
R statistical software, version 4.0.1 (R Project for Statistical Computing), was used for all analyses.
Results
Baseline characteristics of the cohort
From a total of 3860 severe COVID-19 patients discharged from participating hospitals, 1327 had D LCO measured in a follow-up visit after discharge and were included in the current analyses (Fig. 1). A comparison of patients with and without follow-up visits is shown in the online supplement (eTable 1), showing that both groups of patients were similar and had no striking or clinically relevant differences a part from the higher use of hydroxychloroquine among the included patients. The included patients had a median [p 25; p 75] age of 60 [53; 67] years, 27.8% were women, and 59.9% were never smokers. The most common comorbidities were hypertension (44.8%), obesity (35.8%) and diabetes mellitus (17.6%). The median hospital stay was 27 [17; 43] days. The median [p 25; p 75] time from hospital discharge to the first follow-up visit was 3.57 [2.77; 4.92] months (eFigure 1).
Primary outcome
The mean (SD) percentage of predicted diffusion capacity at the follow-up visit was 72.02 (18.33)% predicted. A total of 877 (66%) patients showed an impairment of diffusion capacity (D LCO < 80%), and 318 (24%) had moderate to severe impairment (D LCO < 60%).
A univariate analysis was performed to evaluate the dose–response association between hospital factors and D LCO impairment using p for trend. Briefly, age, comorbidities (except obesity), duration of IMV, and most hospital complications increased according to the level of D LCO impairment (Table 1, Table 2 ).
Table 1.
Univariate analyses and dose–response relations between sociodemographic data, comorbidities, and treatment at hospitalization and degree of DLCO impairment at the follow-up.
| Variables | Global | ≥80% | 60% ≤ x < 80% | <60% | p for trend | N |
|---|---|---|---|---|---|---|
| n = 1327 | n = 450 | n = 559 | n = 318 | |||
| Median [p25; p75] or n(%) | Median [p25; p75] or n(%) | Median [p25; p75] or n(%) | Median [p25; p75] or n(%) | |||
| Sociodemographic data | ||||||
| Sex, woman | 369 (27.8%) | 100 (22.2%) | 175 (31.3%) | 94 (29.6%) | 0.013 | 1327 |
| Age, years | 60.0 [53.0;67.0] | 57.0 [48.0;64.0] | 61.0 [53.0;67.0] | 64.0 [58.0;70.0] | <0.001 | 1327 |
| Smoking history | 0.004 | 1282 | ||||
| Former | 457 (35.6%) | 114 (26.6%) | 213 (39.4%) | 130 (41.5%) | ||
| Non smoker | 768 (59.9%) | 302 (70.6%) | 304 (56.2%) | 162 (51.8%) | ||
| Current | 57 (4.45%) | 12 (2.80%) | 24 (4.44%) | 21 (6.71%) | ||
| Comorbidities | ||||||
| Body mass index | 0.004 | 1246 | ||||
| Normal/overweight | 744 (59.7%) | 232 (55.5%) | 310 (59.2%) | 202 (66.4%) | ||
| Obese Class I | 338 (27.1%) | 128 (30.6%) | 134 (25.6%) | 76 (25.0%) | ||
| Obese Class II | 164 (13.2%) | 58 (13.9%) | 80 (15.3%) | 26 (8.55%) | ||
| Obesity | 475 (35.8%) | 160 (35.6%) | 215 (38.5%) | 100 (31.4%) | 0.333 | 1327 |
| Hypertension | 595 (44.8%) | 162 (36.0%) | 258 (46.2%) | 175 (55.0%) | <0.001 | 1327 |
| Diabetes mellitus (Type I/II) | 233 (17.6%) | 62 (13.8%) | 101 (18.1%) | 70 (22.0%) | 0.003 | 1327 |
| Chronic renal disease | 58 (4.37%) | 9 (2.00%) | 22 (3.94%) | 27 (8.49%) | <0.001 | 1327 |
| Chronic lung disease | 98 (7.39%) | 20 (4.44%) | 35 (6.26%) | 43 (13.5%) | <0.001 | 1327 |
| Rheumatic disease | 67 (5.05%) | 15 (3.33%) | 31 (5.55%) | 21 (6.60%) | 0.035 | 1327 |
| Hematology disorders | 50 (3.77%) | 13 (2.89%) | 23 (4.11%) | 14 (4.40%) | 0.254 | 1327 |
| Treatment | ||||||
| Antivirals | 1011 (76.2%) | 357 (79.5%) | 427 (76.4%) | 227 (71.4%) | 0.010 | 1326 |
| Antibiotics | 1244 (93.8%) | 421 (93.8%) | 520 (93.0%) | 303 (95.3%) | 0.459 | 1326 |
| Corticosteroids | 1104 (83.4%) | 362 (80.6%) | 474 (85.3%) | 268 (84.3%) | 0.134 | 1323 |
| Anticoagulant | 1288 (97.2%) | 435 (96.9%) | 539 (96.6%) | 314 (98.7%) | 0.161 | 1325 |
| NIMV duration, days | 0.00 [0.00;2.00] | 0.00 [0.00;1.00] | 0.00 [0.00;2.00] | 0.00 [0.00;2.00] | <0.001 | 1315 |
| IMV duration, days | 8.00 [0.00;20.0] | 5.00 [0.00;13.0] | 9.00 [0.00;21.0] | 14.0 [0.00;31.0] | <0.001 | 1318 |
| Prone position | 726 (54.9%) | 224 (49.9%) | 312 (56.1%) | 190 (59.9%) | 0.005 | 1322 |
| Prone position duration, days | 12.0 [0.00;48.0] | 0.00 [0.00;43.0] | 15.0 [0.00;50.0] | 19.0 [0.00;59.8] | 0.001 | 1293 |
| Lung recruitment maneuvers | 19 (1.43%) | 6 (1.33%) | 6 (1.07%) | 7 (2.20%) | 0.376 | 1327 |
| Renal replacement therapy | 60 (4.52%) | 11 (2.44%) | 25 (4.48%) | 24 (7.55%) | 0.001 | 1326 |
| Ionotropic/Vasoconstrictor drugs | 741 (56.2%) | 211 (47.3%) | 330 (59.2%) | 200 (63.5%) | <0.001 | 1318 |
| Neuromuscular-blocking drugs | 731 (55.4%) | 205 (45.9%) | 324 (58.4%) | 202 (63.7%) | <0.001 | 1319 |
Abbreviations: DLCO, lung diffusing capacity; NIMV, non-invasive mechanic ventilation; IMV, invasive mechanic ventilation. Note: significant p-values are shown in bold. Univariate analysis was performed with available data.
Table 2.
Univariate analyses and dose–response relations between hospital complications, laboratory data at ICU admission, arterial blood gas at ICU admission, worst arterial blood gas during ICU admission, and scores at hospitalization and degree of DLCO impairment at the follow-up.
| Variables | Global | ≥ 80% | 60% ≤ x < 80% | <60% | p for trend | N |
|---|---|---|---|---|---|---|
| n = 1327 | n = 450 | n = 559 | n = 318 | |||
| Median [p25; p75] or n(%) | Median [p25; p75] or n(%) | Median [p25; p75] or n(%) | Median [p25; p75] or n(%) | |||
| Hospital complications | ||||||
| Bacterial pneumonia | 339 (25.6%) | 73 (16.2%) | 141 (25.3%) | 125 (39.3%) | <0.001 | 1325 |
| ARDS | 1001 (75.4%) | 314 (69.8%) | 431 (77.1%) | 256 (80.5%) | <0.001 | 1327 |
| Pulmonary embolism | 171 (13.0%) | 47 (10.6%) | 85 (15.3%) | 39 (12.3%) | 0.340 | 1315 |
| Bacteremia | 324 (24.4%) | 82 (18.2%) | 139 (24.9%) | 103 (32.5%) | <0.001 | 1326 |
| Acute renal failure | 266 (20.0%) | 68 (15.1%) | 111 (19.9%) | 87 (27.4%) | <0.001 | 1327 |
| Liver dysfunction | 389 (29.3%) | 141 (31.3%) | 162 (29.0%) | 86 (27.0%) | 0.192 | 1327 |
| Hyperglycemia | 905 (68.2%) | 274 (60.9%) | 383 (68.5%) | 248 (78.0%) | <0.001 | 1327 |
| Infectious complications | 602 (45.4%) | 146 (32.4%) | 267 (47.8%) | 189 (59.4%) | <0.001 | 1326 |
| Laboratory data at ICU admission | ||||||
| Lymphocyte count, ×109/L | 0.70 [0.50;1.00] | 0.70 [0.50;1.00] | 0.73 [0.50;1.00] | 0.68 [0.44;0.98] | 0.106 | 1306 |
| Neutrophil count, ×109/L | 7.24 [5.10;10.1] | 7.23 [5.22;9.96] | 7.17 [4.91;10.3] | 7.31 [5.12;10.2] | 0.939 | 1302 |
| Platelet count, ×109/L | 237 [189;309] | 238 [195;311] | 237 [191;312] | 231 [174;303] | 0.090 | 1314 |
| International Normalized Ratio | 1.14 [1.07;1.24] | 1.15 [1.06;1.24] | 1.14 [1.07;1.25] | 1.13 [1.07;1.23] | 0.483 | 1173 |
| D-dimer, mg/L | 800 [429;1635] | 748 [414;1359] | 790 [404;1685] | 941 [516;1964] | 0.016 | 1170 |
| C-reactive protein, mg/dL | 137 [70.0;225] | 134 [62.4;214] | 136 [75.5;230] | 148 [65.8;230] | 0.207 | 1273 |
| Bilirubin, mg/dL | 0.60 [0.43;0.84] | 0.60 [0.43;0.84] | 0.60 [0.40;0.87] | 0.60 [0.47;0.82] | 0.615 | 1131 |
| Urea, mg/dL | 41.0 [29.4;54.0] | 38.0 [28.0;49.0] | 41.0 [29.0;53.5] | 47.0 [32.3;63.0] | <0.001 | 1167 |
| Blood urea nitrogen, mg/dL | 19.1 [13.6;25.2] | 17.7 [13.1;22.9] | 19.1 [13.6;24.8] | 21.9 [15.0;29.4] | <0.001 | 1164 |
| EGFR, mL/min/1.73 m2 | 98.4 [82.5;107] | 102 [91.6;111] | 97.9 [79.8;107] | 94.0 [71.5;103] | <0.001 | 1316 |
| Procalcitonine, ng/mL | 0.18 [0.09;0.39] | 0.16 [0.09;0.29] | 0.20 [0.09;0.46] | 0.18 [0.10;0.42] | 0.018 | 920 |
| Ferritin, log | 3.09 [2.84;3.28] | 3.11 [2.90;3.28] | 3.09 [2.82;3.29] | 3.04 [2.81;3.26] | 0.064 | 767 |
| Arterial blood gas at ICU admission | ||||||
| Partial pressure of oxygen, daily, mmHg | 76.0 [62.2;98.0] | 75.9 [64.0;97.0] | 75.2 [62.0;97.0] | 78.0 [61.0;101] | 0.953 | 1134 |
| Partial pressure of carbon dioxide, daily, mmHg | 37.6 [33.0;44.0] | 37.0 [33.0;44.0] | 37.9 [33.0;44.0] | 38.0 [32.8;44.8] | 0.963 | 1136 |
| PaO2to FiO2ratio | 115 [84.0;166] | 110 [83.2;163] | 117 [86.2;170] | 119 [83.0;173] | 0.229 | 1122 |
| pH, daily | 7.43 [7.37;7.46] | 7.44 [7.38;7.47] | 7.43 [7.37;7.47] | 7.42 [7.36;7.45] | 0.006 | 1191 |
| Bicarbonate, daily, mmol/L | 24.5 [22.1;27.0] | 24.8 [22.9;27.0] | 24.6 [22.2;27.0] | 24.0 [21.3;26.8] | 0.005 | 1130 |
| Respiratory rate, rpm | 25.0 [22.0;31.0] | 26.0 [22.0;31.0] | 25.0 [22.0;31.0] | 25.0 [21.0;31.0] | 0.272 | 1242 |
| Worst arterial blood gas during ICU admission | ||||||
| PaO2to FiO2ratio (min) | 105 [76.6;143] | 100 [75.1;136] | 107 [77.7;146] | 103 [75.0;146] | 0.363 | 1216 |
| Partial pressure of oxygen, mmHg (min) | 64.0 [54.1;73.4] | 64.0 [54.5;73.3] | 64.0 [54.6;73.2] | 64.0 [54.0;74.0] | 0.839 | 1257 |
| Partial pressure of carbon dioxide, mmHg (max) | 45.3 [39.0;52.0] | 45.0 [39.4;51.0] | 45.7 [38.9;52.0] | 46.0 [40.0;53.0] | 0.494 | 1254 |
| pH (min) | 7.38 [7.33;7.43] | 7.39 [7.34;7.43] | 7.38 [7.33;7.43] | 7.37 [7.32;7.42] | 0.002 | 1284 |
| Bicarbonate, mmol/L (min) | 23.1 [21.0;25.3] | 23.4 [21.4;25.3] | 23.1 [21.0;25.2] | 22.7 [20.4;25.2] | 0.004 | 1254 |
| Scores | ||||||
| APACHE score | 10.0 [8.00;13.0] | 9.50 [7.00;12.0] | 11.0 [8.00;14.0] | 11.0 [9.00;15.0] | <0.001 | 855 |
| SOFA score | 4.00 [3.00;7.00] | 4.00 [3.00;6.00] | 4.00 [3.00;7.00] | 5.00 [3.00;7.00] | 0.004 | 972 |
Abbreviations: DLCO, lung diffusing capacity; ARDS, acute respiratory distress syndrome; EGFR, Estimated Glomerular Filtration Rate. Note: significant p-values are shown in bold. Univariate analysis was performed with available data.
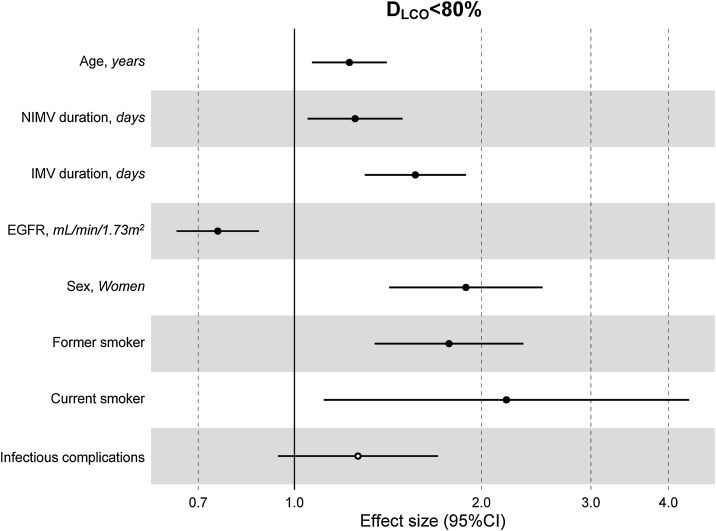
The multivariate model, which included the most relevant variables associated with D LCO, was based on LASSO models. The most relevant variables associated with D LCO < 80% in the multivariate analyses were age (OR[per 1 SD] (95% CI): 1.23 (1.07–1.41)), female sex (1.89 (1.42–2.51)), current smoking (2.19 (1.11–4.32)), duration of IMV (1.57 (1.30–1.89)), duration of NIMV (1.25 (1.05–1.49)), EGFR at ICU admission (0.75 (0.67–0.88)) and hospital infectious complications (1.27 (0.94–1.70)) (Fig. 2 , Table 3 , and eFig. 2). Regarding moderate/severe impairment diffusion capacity (D LCO < 60%), the multivariate model included age (1.39 (1.18–1.63)), chronic lung disease (CLD) (1.86 (1.18–2.92)), duration of IMV (1.56 (1.37–1.77)), urea at ICU admission (1.16 (0.97–1.39)) and EGFR at ICU admission (0.88 (0.73–1.06)) (Fig. 3 , Table 3, and eFig. 2). Furthermore, age and duration of IMV and EGFR at ICU admission showed a linear dose–response association with D LCO in a multivariate linear regression model (eFigs. 2 and 3, and Table 3). Most of these results were validated using an external cohort, with the exception of female sex, which had a different prognostic value in each of the two cohorts (eTable 2). Similarly, the inclusion of time since hospital discharge as a confounder in sensitivity analyses of the associations between clinical parameters at hospitalization and lung diffusion capacity impairment, CT scan findings and spirometry parameters at the follow-up visit did not affect the magnitude of the associations (eTable 3).
Fig. 2.
Hospital factors related to diffusion capacity impairment (DLCO < 80%) at the follow-up visit. Logistic LASSO regression. Abbreviations: DLCO: lung diffusing capacity; NIMV, non-invasive mechanic ventilation; IMV, invasive mechanic ventilation; EGFR, Estimated Glomerular Filtration Rate; LASSO, least absolute shrinkage and selection operator.
Table 3.
Associations between clinical parameters at hospitalization and lung diffusion capacity impairment at the follow-up visit.
| DLCO | >80% (n = 450) |
<80% (n = 450) |
OR (univariable) | OR (complete cases) | OR (multiple imputation) |
|---|---|---|---|---|---|
| Predictors | |||||
| Age, years | 56.0 (11.2) | 60.8 (10.9) | 1.53 (1.37–1.73, p < 0.001) | 1.17 (1.02–1.35, p = 0.030) | 1.23 (1.07–1.41, p = 0.004) |
| Sex, woman | 100 (22.2%) | 269 (30.7%) | 1.55 (1.19–2.02, p = 0.001) | 1.87 (1.40–2.50, p < 0.001) | 1.89 (1.42–2.51, p < 0.001) |
| Smoking history | |||||
| Non smoker | 302 (70.6%) | 466 (54.6%) | – | – | – |
| Former | 114 (26.6%) | 343 (40.2%) | 1.95 (1.51–2.53, p < 0.001) | 1.81 (1.37–2.40, p < 0.001) | 1.77 (1.35–2.34, p < 0.001) |
| Current | 12 (2.8%) | 45 (5.3%) | 2.43 (1.31–4.88, p = 0.008) | 2.27 (1.17–4.68, p = 0.020) | 2.19 (1.11–4.32, p = 0.023) |
| NIMV duration, days | 1.1 (3.2) | 1.9 (4.9) | 1.30 (1.11–1.55, p = 0.002) | 1.32 (1.10–1.61, p = 0.004) | 1.25 (1.05–1.49, p = 0.012) |
| IMV duration, days | 8.4 (11.4) | 15.8 (18.6) | 1.79 (1.54–2.11, p < 0.001) | 1.61 (1.33–1.97, p < 0.001) | 1.57 (1.30–1.89, p < 0.001) |
| Infectious complication | 146 (32.4%) | 456 (52.1%) | 2.26 (1.79–2.87, p < 0.001) | 1.20 (0.88–1.63, p = 0.242) | 1.27 (0.94–1.70, p = 0.120) |
| EGFR, mL/min/1.73 m2 | 98.9 (18.5) | 89.7 (23.5) | 0.62 (0.54–0.71, p < 0.001) | 0.73 (0.62–0.86, p < 0.001) | 0.75 (0.65–0.88, p < 0.001) |
| DLCO | >60% (n = 1009) |
<60% (n = 318) |
OR (univariable) | OR (complete cases) | OR (multiple imputation) |
|---|---|---|---|---|---|
| Predictors | |||||
| Age, years | 58.0 (11.2) | 62.9 (10.4) | 1.63 (1.41–1.88, p < 0.001) | 1.36 (1.14–1.62, p = 0.001) | 1.39 (1.18–1.63, p < 0.001) |
| Chronic lung disease | 55 (5.5%) | 43 (13.5%) | 2.71 (1.77–4.12, p < 0.001) | 2.02 (1.25–3.25, p = 0.004) | 1.86 (1.18–2.92, p = 0.007) |
| IMV duration, days | 11.0 (13.9) | 20.5 (22.3) | 1.66 (1.47–1.88, p < 0.001) | 1.57 (1.38–1.79, p < 0.001) | 1.56 (1.37–1.77, p < 0.001) |
| Urea at ICU admission, mg/dL | 42.9 (22.6) | 53.8 (31.9) | 1.48 (1.30–1.69, p < 0.001) | 1.18 (0.98–1.42, p = 0.087) | 1.16 (0.97–1.39, p = 0.106) |
| EGFR, mL/min/1.73 m2 | 95.1 (20.8) | 85.5 (25.2) | 0.67 (0.59–0.75, p < 0.001) | 0.89 (0.73–1.08, p = 0.223) | 0.88 (0.73–1.06, p = 0.182) |
| DLCO (Continuous) | Coefficient (univariable) | Coefficient (complete cases) | Coefficient (multiple imputation) |
|---|---|---|---|
| Predictors | |||
| Age, years | −4.23 (−5.19 to −3.27, p < 0.001) | −2.49 (−3.54 to −1.43, p < 0.001) | −2.51 (−3.55 to −1.46, p < 0.001) |
| IMV duration, days | −4.85 (−5.81 to −3.89, p < 0.001) | −4.22 (−5.16 to −3.28, p < 0.001) | −4.13 (−5.06 to −3.19, p < 0.001) |
| EGFR, mL/min/1.73 m2 | 4.39 (3.43–5.36, p < 0.001) | 2.64 (1.59–3.70, p < 0.001) | 2.61 (1.56–3.67, p < 0.001) |
Abbreviations: DLCO, lung diffusing capacity; NIMV, non-invasive mechanic ventilation; IMV, invasive mechanic ventilation; EGFR, Estimated Glomerular Filtration Rate; ICU, intensive care unit. Note: odds ratios are presented for the 1-SD change of continuous variable. In descriptive data, mean (SD) or n(%) accordingly.
Fig. 3.
Hospital factors related to diffusion capacity impairment (DLCO < 60%) at the follow-up visit. Logistic LASSO regression. Abbreviations: DLCO: lung diffusing capacity; IMV, invasive mechanic ventilation; EGFR, Estimated Glomerular Filtration Rate; LASSO, least absolute shrinkage and selection operator.
Secondary outcomes
The CT scan exploration at the follow-up visit showed prevalent lung damage in the cohort. The prevalence of the assessed CT scan abnormalities was as follows: persistent pulmonary infiltrate (n: 322 (32.59%) patients), fibrotic lesions (244 (24.69%)), and emphysema (56 (5.66%)).
Table 4 shows, on the one hand, the key variables associated with CT findings at the follow-up visit. Briefly, the duration of ventilatory support (NIMV (1.23 (1.06–1.42)) and IMV (1.21 (1.01–1.45)), bacterial pneumonia (1.62 (1.11–2.35)) and the duration of prone positioning (1.17 (0.98–1.39)) were associated with fibrotic lesions. Acute respiratory distress syndrome (OR: 1.93 (1.33–2.79)), need for a prone position (OR: 1.98 (1.44–2.71)) and antiviral treatment (1.66 (1.14–2.40)), APACHE score (1.13 (0.95–1.33)), neutrophil (1.13 (0.98–1.30)) and platelet count at ICU admission (1.17 (1.02–1.35)), and the partial pressure of carbon dioxide (1.09 (0.94–1.26)) were associated with pulmonary infiltrates. Finally, emphysema was only determined by smoking status (former: 7.04 (3.56–13.92); current: 3.20 (0.67–15.11)). On the other hand, Table 4 shows the key variables associated with lung function parameters (FEV1 and FVC) measured at the follow-up visit. In this regard, the combination of baseline chronic lung or renal disease, renal function at ICU admission and the duration of ventilatory procedures were the main determinants of FEV1 and FVC.
Table 4.
Associations between clinical parameters at hospitalization and CT scan findings and spirometry parameters.
| CT findings (n = 988) | |||||
|---|---|---|---|---|---|
| Persistent pulmonary infiltrate |
|||||
| Predictors | No (n = 666) | Yes (n = 322) | OR (univariable) | OR (complete cases) | OR (multiple imputation) |
| Antiviral treatment | 506 (76.0%) | 275 (85.4%) | 1.85 (1.30–2.67, p = 0.001) | 1.43 (0.94–2.21, p = 0.099) | 1.66 (1.14–2.40, p = 0.008) |
| Prone position | 328 (49.6%) | 227 (70.5%) | 2.43 (1.83–3.23, p < 0.001) | 1.72 (1.17–2.54, p = 0.006) | 1.98 (1.44–2.71, p < 0.001) |
| ARDS complication | 472 (70.9%) | 276 (85.7%) | 2.47 (1.74–3.55, p < 0.001) | 1.35 (0.89–2.10, p = 0.165) | 1.93 (1.33–2.79, p = 0.001) |
| Neutrophil count at ICU admission, ×109/L | 7.9 (4.9) | 8.9 (4.5) | 1.22 (1.07–1.39, p = 0.002) | 1.17 (0.96–1.43, p = 0.118) | 1.13 (0.98–1.30, p = 0.103) |
| Platelet count at ICU admission, ×109/L | 251.4 (106.7) | 270.9 (103.3) | 1.19 (1.05–1.36, p = 0.007) | 1.18 (0.99–1.40, p = 0.057) | 1.17 (1.02–1.35, p = 0.030) |
| APACHE score | 11.1 (4.9) | 12.3 (5.7) | 1.26 (1.07–1.49, p = 0.006) | 1.14 (0.96–1.37, p = 0.143) | 1.13 (0.95–1.33, p = 0.161) |
| Partial pressure of carbon dioxide, mmHg (max) | 46.6 (11.1) | 49.8 (13.6) | 1.29 (1.13–1.47, p < 0.001) | 1.08 (0.88–1.32, p = 0.444) | 1.09 (0.94–1.26, p = 0.258) |
| Emphysema |
|||||
|---|---|---|---|---|---|
| Predictors | No (n = 932) | Yes (n = 56) | OR (univariable) | OR (complete cases) | OR (multiple imputation) |
| Smoking history | |||||
| Non smoker | 558 (61.9%) | 10 (18.2%) | – | – | – |
| Former | 313 (34.7%) | 43 (78.2%) | 7.67 (3.96–16.35, p < 0.001) | 7.67 (3.96–16.35, p < 0.001) | 7.04 (3.56–13.92, p < 0.001) |
| Current | 31 (3.4%) | 2 (3.6%) | 3.60 (0.54–14.42, p = 0.108) | 3.60 (0.54–14.42, p = 0.108) | 3.20 (0.67–15.11, p = 0.141) |
| Fibrotic lesions |
|||||
|---|---|---|---|---|---|
| Predictors | No (n = 744) | Yes (n = 244) | OR (univariable) | OR (complete cases) | OR (multiple imputation) |
| NIMV duration, days | 1.6 (3.5) | 2.7 (6.7) | 1.24 (1.08–1.43, p = 0.002) | 1.25 (1.08–1.46, p = 0.003) | 1.23 (1.06–1.42, p = 0.006) |
| IMV duration, days | 12.3 (15.7) | 19.5 (19.8) | 1.46 (1.28–1.67, p < 0.001) | 1.18 (0.97–1.41, p = 0.087) | 1.21 (1.01–1.45, p = 0.036) |
| Prone duration, hours | 28.9 (44.5) | 46.6 (62.0) | 1.41 (1.21–1.64, p < 0.001) | 1.18 (0.99–1.41, p = 0.068) | 1.17 (0.98–1.39, p = 0.076) |
| Bacterial pneumonia | 166 (22.3%) | 97 (39.9%) | 2.31 (1.69–3.15, p < 0.001) | 1.71 (1.16–2.51, p = 0.006) | 1.62 (1.11–2.35, p = 0.012) |
| Spirometry (n = 1319) | |||||
|---|---|---|---|---|---|
| FEV1 |
|||||
| Predictors | >80 (n = 886) | <80 (n = 433) | OR (univariable) | OR (complete cases) | OR (multiple imputation) |
| Chronic renal disease | 28 (2.8%) | 30 (9.3%) | 3.51 (2.06–6.00, p < 0.001) | 2.69 (1.48–4.87, p = 0.001) | 2.99 (1.71–5.24, p < 0.001) |
| Chronic lung disease | 58 (5.9%) | 40 (12.3%) | 2.27 (1.47–3.45, p < 0.001) | 1.83 (1.14–2.92, p = 0.011) | 1.9 (1.22–2.97, p = 0.005) |
| IMV duration, days | 12.0 (15.1) | 17.4 (20.9) | 1.33 (1.18–1.50, p < 0.001) | 1.33 (1.18–1.51, p < 0.001) | 1.3 (1.15–1.47, p < 0.001) |
| Urea at ICU admission, mg/dL | 43.9 (23.9) | 50.8 (30.0) | 1.28 (1.13–1.45, p < 0.001) | 1.13 (0.99–1.30, p = 0.076) | 1.12 (0.98–1.28, p = 0.101) |
| FVC |
|||||
|---|---|---|---|---|---|
| Predictors | >80 (n = 991) | <80 (n = 324) | OR (univariable) | OR (complete cases) | OR (multiple imputation) |
| Chronic renal disease | 22 (2.5%) | 36 (8.3%) | 3.56 (2.08–6.22, p < 0.001) | 3.37 (1.94–5.96, p < 0.001) | 3.47 (1.99–6.06, p < 0.001) |
| NIMV duration, days | 1.4 (3.6) | 2.3 (5.6) | 1.23 (1.10–1.40, p = 0.001) | 1.22 (1.08–1.39, p = 0.002) | 1.20 (1.06–1.37, p = 0.004) |
| IMV duration, days | 11.3 (14.3) | 17.3 (20.4) | 1.42 (1.26–1.59, p < 0.001) | 1.41 (1.26–1.59, p < 0.001) | 1.42 (1.26–1.60, p < 0.001) |
Abbreviations: CT, chest thorax; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; NIMV, non-invasive mechanic ventilation; IMV, invasive mechanic ventilation; FEV1, forced expiratory volume in 1 second; FVC, Forced vital capacity. Note: odds ratios are presented for the 1-SD change of continuous variable. In descriptive data, mean (SD) or n(%) accordingly.
Discussion
In this ambispective multicentric cohort of patients who needed an ICU admission due to COVID-19, the most important factors related to a moderate/severe impairment in D LCO (<60%) after hospital discharge were: age, baseline CLD, duration of IMV, and renal function in terms of urea and EGFR at ICU admission. Similarly, renal function, duration of ventilatory procedures and baseline chronic renal disease were associated with other parameters of spirometry (FEV1 and FVC). Finally, fibrotic lesions in the chest CT were also associated with the length of IMV and NIMV, prone positioning and bacterial complications. The identified factors showed similar associations with the outcome in an independent external cohort.
One of the most well-known prognostic factors regarding pulmonary sequelae after COVID-19 is age.5, 7 Tissue repair and remodeling responses to a lung injury such a severe COVID-19 infection may be altered by the aging process and cellular senescence.18 These processes are associated with a decline in the immune system and promote inflammation,19 in addition to generating more oxidative stress18 and a deterioration in the repair capacity of damaged cells.18 Therefore, it is plausible that these age-related limitations imply worse clinical outcomes with more lung damage in older subjects. In this line, patients with CLD, especially COPD, also show an ineffective repair response to lung damage (most commonly caused by toxic inhalants)20 which could explain the worse outcomes in both, the acute phase21, 22, 23, 24 and in follow-up.11, 12, 25 Moreover, patients with COPD also have a higher expression of ACE-2 receptors in the bronchial epithelium26, 27 and impairment of immune response.28 Importantly, patients with CLD probably have worse baseline pulmonary function before COVID-19 infection.
Another key variable associated with decreased D LCO is the length of IMV. Clearly, the respiratory management of these patients is crucial, and factors such as the timing of intubation10 or indices such as ventilatory ratio29 have important implications in terms of mortality and pulmonary sequelae. However, developing ARDS and the duration of IMV are directly related to a more severe disease that often involves more complications, such as ventilator-induced lung injury and ventilator-associated pneumonia, leading to more mechanical stress and lung damage.30, 31 In addition, and as our study highlights, survivors of more severe COVID-19 who developed ARDS and need to be intubated have already been associated with the presence of chest CT abnormalities (such as fibrotic lesions or persistent pulmonary infiltrates) or with the involvement of other respiratory parameters, such as FEV1 and FVC, during follow-up.32, 33, 34
A similar phenomenon surrounds the link between renal and pulmonary involvement. Acute renal failure could be caused directly by SARS-CoV-2 or secondary to end-organ damage in severe COVID-19 patients with hemodynamic instability, inflammatory cytokines and consequences of ICU therapies.35 In this way, renal failure could also be seen as a marker of the severity of COVID-19, involving more global vascular damage with important prognostic values in the acute phase.36 This global vascular damage could be related to the distinctive vascular features found in COVID-19 patients, with severe endothelial injury associated with widespread thrombosis and microangiopathy,37 contributing to the deterioration of D LCO after discharge.
Our results have relevant clinical consequences, as a substantial proportion of survivors of critical COVID-19 will face mid- to long-term respiratory or functional sequelae. The identification of key variables associated with a moderate/severe D LCO impairment or chest CT abnormalities after hospital discharge allows a more personalized planning of the patients’ follow-up, while also helping to estimate the health resources that should be allocated to its monitoring. We know from other respiratory diseases the importance of presenting a moderate/severe deterioration of D LCO for patients, generating more symptoms, worse exercise performance and quality of life.38 This work contributes to laying the foundations for planning the follow-up of critically ill COVID-19 patients and highlights the importance of the pulmonologists who will follow up with the patients having access to and accounting for the data collected during the critical stage of the illness. For all these reasons, and knowing the high proportion of patients (up to 30%) who continue to present abnormal D LCO values one year after hospital discharge,39 it should be mandatory to carry out a complete study in a first follow-up.
This study has some key strengths: (i) the availability of a huge amount of information at different time points of the COVID-19 course; (ii) the fact that all data were thoroughly revised and validated, in contrast to registry-based studies; (iii) the representativeness of our study population, including multiple sites and pandemic waves; and, (iv) the validation of our results in an independent cohort of critically ill COVID-19 patients, which, furthermore, included different pandemic waves thus making the current results timeless. In this regard, female sex, which was the only variable that was not validated, has been reported as a key factor determining full recovery one year after discharge in other large cohorts.40 On the other hand, some limitations must be acknowledged: (i) the observational design; (ii) the pragmatic design, adapting to the different pandemic scenarios in each participating hospital and producing uneven follow-up; (iii) the lack of information on the period between hospital discharge and the follow-up visit, especially in terms of treatment, rehabilitation and other procedures; (iv) the high number of patients lacking a D LCO measure in the follow-up and consequently not included in our analyses, although this was mitigated by the large study cohort and confirmed by the lack of clinically significant difference between included and non-included patients; and, (v) the short-term follow-up. In this regard, we have divided the cohort into mild D LCO deterioration (<80%) and moderate/severe deterioration (<60%) to try to identify more significant lung damage that requires at least a first short-term follow-up.
Conclusions
In this cohort of critically ill COVID-19 patients, we identified key factors directly associated with worse D LCO and chest CT abnormalities at a postdischarge follow-up visit. They include nonmodifiable factors such as age and CLD, reflecting a more vulnerable population with poor host response to viral infection and poor lung repair, and markers of a more severe disease, such as duration of IMV and renal function. Physicians should consider all of these variables to plan the follow-up of critically ill COVID-19 survivors.
Authors’ contributions
Conceptualization (JG, JdB, IDB, FB), data curation (IDB, CG-P, AM-M), formal analysis (IDB), funding acquisition (AT, FB), investigation (all), methodology (IDB, JdB, JG, AT, FB), project administration (AT, FB), supervision (AT, FB), writing – original draft (JG, JdB, IDB), and writing – review & editing (all). All authors provided final approval of the version submitted for publication.
Funding/support
Financial support was provided by Instituto de Salud Carlos III (CIBERESUCICOVID, COV20/00110), co-funded by Fondo Europeo de Desarrollo Regional (FEDER), “Una manera de hacer Europa”; Centro de Investigación Biomédica en Red – Enfermedades Respiratorias (CIBERES); Donation Program “estar preparados”, UNESPA, Madrid, Spain; and, Fundación Francisco Soria Melguizo (Madrid, Spain). JdB acknowledges receiving financial support from Instituto de Salud Carlos III (ISCIII; Miguel Servet 2019: CP19/00108), co-funded by the European Social Fund (ESF), “Investing in your future”. DdGC acknowledges receiving financial support from Instituto de Salud Carlos III (ISCIII; Miguel Servet 2020: CP20/00041), co-funded by the European Social Fund (ESF), “Investing in your future”. AC acknowledges receiving financial support from Instituto de Salud Carlos III (ISCIII; Sara Borrell 2021: CD21/00087). None of the funding sources had a role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of interests
None declared.
Acknowledgments
The authors are indebted to Maricel Arbonés, Maria Arguimbau, Raquel Campo, Natalia Jarillo, Javier Muñoz, Silvia Ortega and Manuel Sanchez for their extensive support in project management and article preparation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.arbres.2022.12.017.
Contributor Information
on behalf of the CIBERESUCICOVID Project:
María Aguilar Cabello, Victoria Alcaraz-Serrano, Cesar Aldecoa, Cynthia Alegre, Ángela Algaba Calderón, Sergio Álvarez, Antonio Álvarez Ruiz, Ruth Andrea, Maria de Alba Aparicio, Marta Arrieta, J. Ignacio Ayestarán, Joan Ramon Badia, Mariona Badía, Orville Báez Pravia, Ana Balan Mariño, Begoña Balsera, Laura Barbena, Enric Barbeta, Tommaso Bardi, Patricia Barral Segade, Marta Barroso, José Ángel Berezo García, Jesus Bermejo, Judit Bigas, Rafael Blancas, María Luisa Blasco Cortés, María Bodi Saera, Neus Bofill, María Teresa Bouza Vieiro, Leticia Bueno, Juan Bustamante-Munguira, Cecilia del Busto Martínez, David Campi Hermoso, Sandra Campos Fernández, Iosune Cano, Joan Canseco, Pablo Cardinal Fernández, Laura Carrión García, Sulamita Carvalho, Manuel Castellà, Andrea Castellví, Pedro Castro, María José Centelles-Serrano, Ramon Cicuendez Ávila, Catia Cillóniz, Luisa Clar, Cristina Climent, Jordi Codina, Pamela Conde, Sofía Contreras, Raul de Frutos Parra, Raul de Pablo Sánchez, Diego De Mendoza, Yolanda Díaz, María Digna Rivas Vilas, Cristina Dólera Moreno, Irene Dot, Pedro Enríquez Giraudo, Inés Esmorís Arijón, Teresa Farre Monjo, Javier Fernández, Carlos Ferrando, Albert Figueras, Lorena Forcelledo Espina, Enric Franquesa, Àngels Furro, Albert Gabarrus, Cristóbal Galbán, Felipe García, Beatriz García, Emilio García Prieto, Carlos García Redruello, Amaia García Sagastume, Maria Luisa Gascón Castillo, Gemma Gomà, Vanesa Gómez Casal, Silvia Gómez, Carmen Gómez Gonzalez, Federico Gordo, Maria Pilar Gracia, María José Gutierrez Fernández, Alba Herraiz, Rubén Herrán-Monge, Mercedes Ibarz, Silvia Iglesias, Maria Teresa Janer, Gabriel Jiménez, Mar Juan Díaz, Karsa Kiarostami, Juan I. Lazo Álvarez, Miguel León, Alexandre López-Gavín, Desiree Macias Guerrero, Nuria Mamolar Herrera, Rafael Mañez Mendiluce, Cecilia L. Mantellini, Gregorio Marco Naya, Iris Marco Barcos, Pilar Marcos, Enrique Marmol Peis, Marta Martín Cuadrado, María Cruz Martin Delgado, Paula Martín Vicente, María Martínez, Carmen Eulalia Martínez Fernández, Maria Dolores Martínez Juan, Basilisa Martínez Palacios, Juan Fernando Masa Jimenez, Joan Ramon Masclans, Emilio Maseda, Eva María Menor Fernández, Priscila Metora Banderas, Olga Minguez, Mar Miralbés, Josman Monclou, Juan Carlos Montejo-González, Neus Montserrat, María Mora Aznar, Dulce Morales, Sara Guadalupe Moreno Cano, David Mosquera Rodríguez, Rosana Muñoz-Bermúdez, José María Nicolás, Ramon Nogue Bou, Rafaela Nogueras Salinas, Marta Ocón, Ana Ortega, Sergio Ossa, Pablo Pagliarani, Francisco Parrilla, José Pedregosa-Díaz, Leire Pérez Bastida, Purificación Pérez, Felipe Pérez-García, Gloria Pérez Planelles, Eva Pérez Rubio, David Pestaña Laguna, Javier Prados, Andrés Pujol, Núria Ramon Coll, Gloria Renedo Sanchez-Giron, Ferran Roche-Campo, Laura Rodriguez, Felipe Rodríguez de Castro, Silvia Rodríguez, Covadonga Rodríguez Ruiz, Jorge Rubio, Alberto Rubio López, Ángela Leonor Ruiz-García, Miriam Ruiz Miralles, Pablo Ryan Murúa, Eva Saborido Paz, Ana Salazar Degracia, Miguel Sanchez, Ana Sánchez, Susana Sancho Chinesta, Bitor Santacoloma, Miguel Sanchez, Maria Teresa Sariñena, Marta Segura Pensado, Lidia Serra, Mireia Serra-Fortuny, Ainhoa Serrano Lázaro, Lluís Servià, Laura Soliva, Carla Speziale, Adrián Tormos, Mateu Torres, Celia Tranque-Liberal, Sandra Trefler, Javier Trujillano, Rafaela Vaca, Estela Val, Luis Valdivia Ruiz, Montserrat Vallverdú, Maria Van der Hofstadt Martin-Montalvo, Sabela Vara Adrio, Nil Vázquez, Javier Vengoechea, Clara Vilà-Vilardel, Judit Vilanova, Tatiana Villada Warrington, Hua Yang, Minlan Yang, and Ana Zapatero
Appendix A. CIBERESUCICOVID collaborators
María Aguilar Cabello, Victoria Alcaraz-Serrano, Cesar Aldecoa, Cynthia Alegre, Ángela Algaba Calderón, Sergio Álvarez, Antonio Álvarez Ruiz, Ruth Andrea, Maria de Alba Aparicio, Marta Arrieta, J Ignacio Ayestarán, Joan Ramon Badia, Mariona Badía, Orville Báez Pravia, Ana Balan Mariño, Begoña Balsera, Laura Barbena, Enric Barbeta, Tommaso Bardi, Patricia Barral Segade, Marta Barroso, José Ángel Berezo García, Jesus Bermejo, Judit Bigas, Rafael Blancas, María Luisa Blasco Cortés, María Bodi Saera, Neus Bofill, María Teresa Bouza Vieiro, Leticia Bueno, Juan Bustamante-Munguira, Cecilia del Busto Martínez, David Campi Hermoso, Sandra Campos Fernández, Iosune Cano, Joan Canseco, Pablo Cardinal Fernández, Laura Carrión García, Sulamita Carvalho, Manuel Castellà, Andrea Castellví, Pedro Castro, María José Centelles-Serrano, Ramon Cicuendez Ávila, Catia Cillóniz, Luisa Clar, Cristina Climent, Jordi Codina, Pamela Conde, Sofía Contreras, Raul de Frutos Parra, Raul de Pablo Sánchez, Diego De Mendoza, Yolanda Díaz, María Digna Rivas Vilas, Cristina Dólera Moreno, Irene Dot, Pedro Enríquez Giraudo, Inés Esmorís Arijón, Teresa Farre Monjo, Javier Fernández, Carlos Ferrando, Albert Figueras, Lorena Forcelledo Espina, Enric Franquesa, Àngels Furro, Albert Gabarrus, Cristóbal Galbán, Felipe García, Beatriz García, Emilio García Prieto, Carlos García Redruello, Amaia García Sagastume, Maria Luisa Gascón Castillo, Gemma Gomà, Vanesa Gómez Casal, Silvia Gómez, Carmen Gómez Gonzalez, Federico Gordo, Maria Pilar Gracia, María José Gutierrez Fernández, Alba Herraiz, Rubén Herrán-Monge, Mercedes Ibarz, Silvia Iglesias, Maria Teresa Janer, Gabriel Jiménez, Mar Juan Díaz, Karsa Kiarostami, Juan I Lazo Álvarez, Miguel León, Alexandre López-Gavín, Desiree Macias Guerrero, Nuria Mamolar Herrera, Rafael Mañez Mendiluce, Cecilia L Mantellini, Gregorio Marco Naya, Iris Marco Barcos, Pilar Marcos, Enrique Marmol Peis, Marta Martín Cuadrado, María Cruz Martin Delgado, Paula Martín Vicente, María Martínez, Carmen Eulalia Martínez Fernández, Maria Dolores Martínez Juan, Basilisa Martínez Palacios, Juan Fernando Masa Jimenez, Joan Ramon Masclans, Emilio Maseda, Eva María Menor Fernández, Priscila Metora Banderas, Olga Minguez, Mar Miralbés, Josman Monclou, Juan Carlos Montejo-González, Neus Montserrat, María Mora Aznar, Dulce Morales, Sara Guadalupe Moreno Cano, David Mosquera Rodríguez, Rosana Muñoz-Bermúdez, José María Nicolás, Ramon Nogue Bou, Rafaela Nogueras Salinas, Marta Ocón, Ana Ortega, Sergio Ossa, Pablo Pagliarani, Francisco Parrilla, José Pedregosa-Díaz, Leire Pérez Bastida, Purificación Pérez, Felipe Pérez-García, Gloria Pérez Planelles, Eva Pérez Rubio, David Pestaña Laguna, Javier Prados, Andrés Pujol, Núria Ramon Coll, Gloria Renedo Sanchez-Giron, Ferran Roche-Campo, Laura Rodriguez, Felipe Rodríguez de Castro, Silvia Rodríguez, Covadonga Rodríguez Ruiz, Jorge Rubio, Alberto Rubio López, Ángela Leonor Ruiz-García, Miriam Ruiz Miralles, Pablo Ryan Murúa, Eva Saborido Paz, Ana Salazar Degracia, Miguel Sanchez, Ana Sánchez, Susana Sancho Chinesta, Bitor Santacoloma, Miguel Sanchez, Maria Teresa Sariñena, Marta Segura Pensado, Lidia Serra, Mireia Serra-Fortuny, Ainhoa Serrano Lázaro, Lluís Servià, Laura Soliva, Carla Speziale, Adrián Tormos, Mateu Torres, Celia Tranque-Liberal, Sandra Trefler, Javier Trujillano, Rafaela Vaca, Estela Val, Luis Valdivia Ruiz, Montserrat Vallverdú, Maria Van der Hofstadt Martin-Montalvo, Sabela Vara Adrio, Nil Vázquez, Javier Vengoechea, Clara Vilà-Vilardel, Judit Vilanova, Tatiana Villada Warrington, Hua Yang, Minlan Yang, Ana Zapatero.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard n.d. [accessed 28.12.22].
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/JAMA.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González J., Benítez I.D., Carmona P., Santisteve S., Monge A., Moncusí-Moix A., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160:187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Gassel R.J.J., Bels J.L.M., Raafs A., van Bussel B.C.T., van de Poll M.C.G., Simons S.O., et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203:371–374. doi: 10.1164/rccm.202010-3823le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y., Liang W., Li Y., He J., Guan W. The short- and long-term clinical, radiological and functional consequences of COVID-19. Arch Bronconeumol. 2022;58:32–38. doi: 10.1016/J.ARBRES.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco J.R., Cobos-Ceballos M.J., Navarro F., Sanjoaquin I., Arnaiz de las Revillas F., Bernal E., et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect. 2021;27:892–896. doi: 10.1016/j.cmi.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González J., Benítez I.D., de Gonzalo-Calvo D., Torres G., de Batlle J., Gómez S., et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: a prospective cohort study. Crit Care. 2022;26:18. doi: 10.1186/s13054-021-03882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellan M., Soddu D., Balbo P.E., Baricich A., Zeppegno P., Avanzi G.C., et al. Respiratory and psychophysical sequelae among patients with covid-19 four months after hospital discharge. JAMA Netw Open. 2021;4:1–12. doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57:2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres A., Arguimbau M., Bermejo-Martín J., Campo R., Ceccato A., Fernandez-Barat L., et al. CIBERESUCICOVID: a strategic project for a better understanding and clinical management of COVID-19 in critical patients. Arch Bronconeumol. 2021;57:1–2. doi: 10.1016/J.ARBRES.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker L.A., Eneanya N.D., Coresh J., Tighiouart H., Wang D., Sang Y., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021:385. doi: 10.1056/nejmoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Buuren S., Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 16.Du J., Boss J., Han P., Beesley L.J., Kleinsasser M., Goutman S.A., et al. Variable selection with multiply-imputed datasets: choosing between stacked and grouped methods. J Comput Graph Stat. 2022;31:1063–1075. doi: 10.1080/10618600.2022.2035739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin D.B. Inference and missing data. Biometrika. 1976;63:581–592. doi: 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- 18.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol. 2021;11:571416. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockley R.A., Halpin D.M.G., Celli B.R., Singh D. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med. 2019;199:1195–1204. doi: 10.1164/rccm.201810-1860SO. [DOI] [PubMed] [Google Scholar]
- 21.Wu F., Zhou Y., Wang Z., Xie M., Shi Z., Tang Z., et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. J Thorac Dis. 2020;12:1811–1823. doi: 10.21037/jtd-20-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan W., Liang W., Zhao Y., Liang H.-R., Chen Z-SZ, Li Y.-M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLOS ONE. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibila O., Albacar N., Perea L., Faner R., Torralba Y., Hernandez-Gonzalez F., et al. Lung function sequelae in COVID-19 patients 3 months after hospital discharge. Arch Bronconeumol. 2021;57:59–61. doi: 10.1016/j.arbres.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs M., Van Eeckhoutte H.P., Wijnant S.R.A., Janssens W., Joos G.F., Brusselle G.G., et al. Increased expression of ACE2, the SARS-CoV-2 entry receptor, in alveolar and bronchial epithelium of smokers and COPD subjects. Eur Respir J. 2020;56:2002378. doi: 10.1183/13993003.02378-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhat T.A., Panzica L., Kalathil S.G., Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;vol. 12:S169–S175. doi: 10.1513/AnnalsATS.201503-126AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres A., Motos A., Riera J., Fernández-Barat L., Ceccato A., Pérez-Arnal R., et al. The evolution of the ventilatory ratio is a prognostic factor in mechanically ventilated COVID-19 ARDS patients. Crit Care. 2021;25:1–13. doi: 10.1186/s13054-021-03727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 32.Guler S.A., Ebner L., Aubry-Beigelman C., Bridevaux P.O., Brutsche M., Clarenbach C., et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerum T.V., Aaløkken T.M., Brønstad E., Aarli B., Ikdahl E., Lund K.M.A., et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57:2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baricich A., Borg M.B., Cuneo D., Cadario E., Azzolina D., Balbo P.E., et al. Midterm functional sequelae and implications in rehabilitation after coVid-19: a cross-sectional study. Eur J Phys Rehabil Med. 2021;57:199–207. doi: 10.23736/S1973-9087.21.06699-5. [DOI] [PubMed] [Google Scholar]
- 35.Ye W., Chen G., Li X., Lan X., Ji C., Hou M., et al. Dynamic changes of D-dimer and neutrophil–lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21:169. doi: 10.1186/s12931-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 37.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balasubramanian A., MacIntyre N.R., Henderson R.J., Jensen R.L., Kinney G., Stringer W.W., et al. Diffusing capacity of carbon monoxide in assessment of COPD. Chest. 2019;156:1111–1119. doi: 10.1016/J.CHEST.2019.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González J., Zuil M., Benítez I.D., de Gonzalo-Calvo D., Aguilar M., Santisteve S., et al. One year overview and follow-up in a post-COVID consultation of critically ill patients. Front Med. 2022;9:897990. doi: 10.3389/fmed.2022.897990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans R.A., Leavy O.C., Richardson M., Elneima O., McAuley H.J.C., Shikotra A., et al. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. 2022;10:761–775. doi: 10.1016/S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.