Abstract
Introduction
We aimed to investigate the association of frailty with persistent and incident common mental disorders (CMD) in older adults during the pandemic.
Methods
We included 706 older adults who participated in the onsite wave of the ELSA-Brasil study (2017–2019) and the online COVID-19 assessment (May–July 2020). CMD were assessed in both waves by the Clinical Interview Schedule-Revised. Frailty was defined according to the physical phenotype and Frailty Index in the 2017–2019 wave. Logistic regression was used to investigate the association of frailty with persistent and incident CMD, adjusted for sociodemographics.
Results
Frailty according to both definitions were associated with persistent CMD (Frailty Index: OR = 8.61, 95 % CI = 4.08–18.18; physical phenotype: OR = OR = 23.67, 95 % CI = 7.08–79.15), and incident CMD (Frailty Index: OR = 2.79, 95 % CI = 1.15–6.78; physical phenotype OR = 4.37, 95 % CI = 1.31–14.58). The exclusion of exhaustion (that overlaps with psychiatric symptoms) from the frailty constructs did not change the association between frailty and persistent CMD, although the associations with indent CMD were no longer significant.
Limitations
Fluctuations in CMD status were not captured between both assessments.
Conclusion
Frailty status before the COVID-19 outbreak was associated with higher odds of persistent and incident CMD in older adults during the pandemic first wave. Identifying individuals at higher risk of mental burden can help prioritize resources allocation and management.
Keywords: COVID-19, Frailty, Mental disorders, Older adults
Abbreviations: CMD, common mental disorders
1. Introduction
Common mental disorders (CMD), such as anxiety and depression, are leading causes of the global burden of diseases, and their prevalence increased during the pandemic in some populations (COVID-19 Mental Disorders Collaborators, 2021). Mental disorders are more common in people with physical illnesses (Rosenblat et al., 2020), and their treatment is further complicated by the adverse effects, pharmacological interactions, and contraindications of antidepressant drugs (Carvalho et al., 2016). In addition, mental disorders are also associated with risk factors for medical illnesses, such as poor cardiovascular health (Szlejf et al., 2019a), and sarcopenia (Szlejf et al., 2019b).
Frailty is a major geriatric syndrome characterized by an increased vulnerability to stressors, in which minor insults can result in disproportionate changes in health. It develops as a consequence of the accumulation of molecular and cellular damage throughout life and it is associated with adverse outcomes in older adults (Clegg et al., 2013). Despite numerous definitions of the condition, two main frailty models prevail. The physical phenotype is based on the presence of at least three of the following criteria: unintentional weight loss, exhaustion, low energy expenditure, slowness, and weakness (Morley et al., 2013). The other model is based on the accumulation of age-related health deficits, such as symptoms, signs, diseases, and disabilities, that compose a frailty index, which is associated with higher mortality (Clegg et al., 2013).
The relationship between frailty and psychiatric comorbidities, such as depression, has been extensively investigated. Frail individuals have higher odds of prevalent and incident depression (Soysal et al., 2017) and depressed individuals have higher odds of prevalent and incident frailty (Soysal et al., 2017; Vaughan et al., 2015). Despite common symptomatology and sociodemographic predictors, studies have demonstrated that frailty and depression are distinct, but highly correlated constructs in middle-aged and older adults (Mezuk et al., 2013; Lohman et al., 2014, Lohman et al., 2016). However, the relationship between frailty and other mental disorders, beyond depression, have been little explored. Cross-sectional studies have demonstrated associations of anxiety symptoms with pre-frail status and frailty in different populations of older adults (Ní Mhaoláin et al., 2012; Mlynarska et al., 2018; Zhao et al., 2020).
The stressful environment generated by the COVID-19 pandemic has perpetuated and produced mental distress globally. Frail individuals may be at higher risk of CMD, which could potentiate adverse outcomes. Therefore, the aim of our study was to investigate the association of frailty status before the pandemic with prevalent and incident CMD in older adults living in São Paulo during the period that Brazil became the global epicenter of the pandemic.
2. Methods
2.1. Study design and participants
The ELSA-Brasil study is a prospective longitudinal cohort of active and retired civil servants from academic institutions located in six different Brazilian states (São Paulo, Rio de Janeiro, Minas Gerais, Espírito Santo, Bahia, and Rio Grande do Sul). The study aims to investigate the development and progression of clinical and subclinical chronic diseases in a population from a low-middle income country. The baseline assessment was conducted in 2008–2010 and included 15,105 participants aged between 35 and 74 years, followed by two posterior waves performed in 2012–2014 and 2017–2019 (Aquino et al., 2012; Schmidt et al., 2015). In all waves, information on sociodemographic factors, clinical conditions, and lifestyle were obtained from questionnaires; anthropometric measurements were performed; and laboratory tests were collected after an overnight fast. In the third wave, a battery of physical performance tests was applied, including handgrip strength (measured twice in the dominant hand with a Jamar hydraulic handheld dynamometer), gait speed (measurement of the time to walk 4 m in usual pace with the use of assistive devices when needed), and repeated chair stand test (measurement of the time to complete 5 repeated chair stands without using the arm).
In 2020, online assessments related to the COVID-19 pandemic were conducted with 2175 participants from the São Paulo center in three waves (between 18 May and 18 July; 20 July and 30 September; and 1 October and 22 December) to investigate psychiatric disorders and symptoms (Brunoni et al., 2021). The first, second, and third waves of the COVID-19 assessment corresponded, respectively, to the periods in which the most severe lockdown measures were implemented in the city of São Paulo, the exponential increase in cases and deaths in Brazil and paradoxically some flexibility in quarantine measures, and the moderated decline in the rate of daily deaths and cases and quarantine relaxation measures.
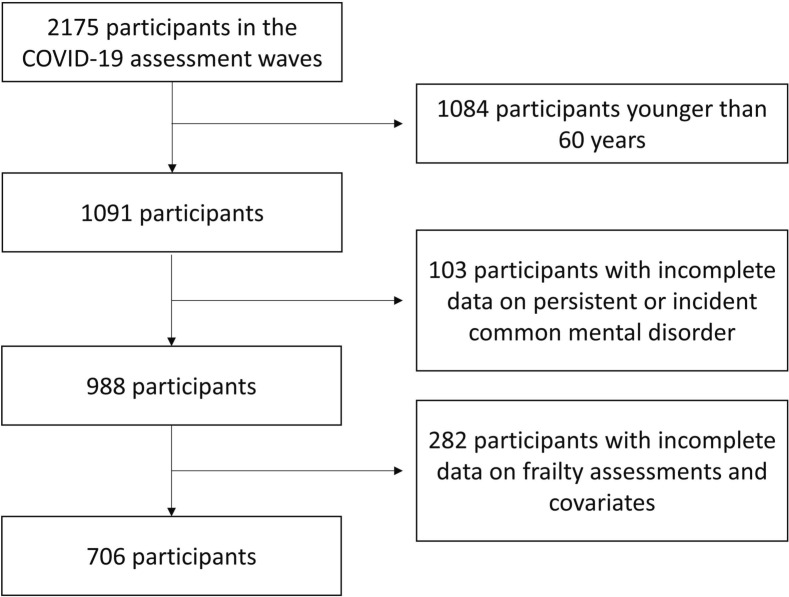
The present study included 706 participants aged 60 years and older who undertook the psychiatric examination both, in the onsite study wave (2017–2019) and in the first COVID-19 online wave and had complete information on frailty and covariates (Fig. 1 ). The study was approved by the local ethics review board and participants provided informed consent.
Fig. 1.
Flowchart of study sample.
2.2. Common mental disorders assessment
The validated Brazilian version of the Clinical Interview Schedule-Revised (CIS-R) was used to assess non-psychotic psychiatric morbidity (Lewis et al., 1992; Nunes et al., 2011). It is a structured interview that can be applied by trained lay interviewers that includes the assessment of 14 symptoms of CMD and 13 psychiatric disorders based on the International Classification of Diseases, 10th edition. The CIS-R sections are somatic complaints, fatigue, concentration and forgetfulness, sleep disturbance, irritability, worry about physical health, depression, depressive ideas, worry, anxiety, phobias, panic attacks, compulsions, and obsessions. Each section is scored from 0 to 4, except depressive ideas, which is scored from 0 to 5. The total score ranges from 0 to 57. A score ≥ 12 defines CMD (Lewis et al., 1992).
In the third on-site wave of the ELSA-Brasil study (2017–2019), the CIS-R was administered in person by trained interviewers. In the first wave of the online COVID-19 assessment (2020), due to quarantine measures, we used an electronic self-report version of the CIS-R, which has been validated (Lewis, 1994), and presented similar performance than the standard version (Head et al., 2013). We defined persistent CMD for participants who had a CIS-R score ≥ 12 in the onsite assessment (2017–2019) and in the first online COVID-19 assessment (2020), and incident CMD for participants who had a CIS-R score < 12 in the onsite assessment (2017–2019) and ≥ 12 in the first online COVID-19 assessment (2020).
2.3. Frailty assessment
Frailty was defined according to two different criteria: the cumulative deficit and the physical phenotype criteria (Clegg et al., 2013). The Frailty Index is based on the grounds that the likelihood of being frail is positively associated with the individual's accumulated deficits throughout life. The index counts deficits in health (symptoms, signs, disabilities, and diseases) and is expressed as the ratio of deficits present to the total number of deficits being considered, ranging from 0 to 1. Not every Frailty Index considers the same deficits. To build an index, it is recommended the inclusion of 30 to 40 deficits (Rockwood and Mitnitski, 2007; Searle et al., 2008). We included 36 health deficits measured in the first and third onsite study waves in the Frailty Index, which was calculated for all participants with complete data on at least 30 health deficits. The detailed description of each variable included can be seen in the Supplementary Material (Table S1). The index was calculated as the sum of the total score of deficits divided by the number of variables with complete information for each participant. As proposed by previous studies, we defined frailty as a Frailty Index ≥0.25 (Rockwood and Mitnitski, 2007).
The frailty phenotype is based on the presence of three of the following factors: unintentional weight loss, self-reported exhaustion, low energy expenditure, slow gait speed, and weak grip strength. The presence of one or two factors characterizes a pre-frail state (Fried et al., 2001). We adapted the criteria definitions adopted in the Cardiovascular Health Study (Fried et al., 2001) as described in the Supplementary Table S2.
2.4. Sociodemographic characteristics
Sociodemographic characteristics assessed were age at the third onsite wave (between 2017 and 2019), sex, education (college or more vs. high school or lower), self-reported race (black, brown, white, and other in accordance with the Brazilian Institute of Geography and Statistics recommendation).
2.5. Statistical analysis
Variables were presented as follows: continuous variables with normal distribution as mean and standard deviation, skewed continuous variables as median and interquartile range, and categorical variables as absolute and relative frequencies. Characteristics of participants according to frailty status defined according to the Frailty Index were compared using Student's t-test, Wilcoxon rank-sum test, chi-square test, and Fisher's exact-test for continuous variables with normal distribution, skewed continuous variables, categorical variables, and categorical variables with small expected values, respectively. Characteristics between robust, pre-frail, and frail participants according to the physical frailty phenotype were compared using one-way ANOVAs, Kruskal-Wallis tests, chi-square tests and Fisher's exact tests for continuous variables with normal distribution, skewed continuous variables, categorical variables, and categorical variables with small expected values, respectively.
Logistic regression models were used to investigate the association of frailty according to the Frailty Index and physical frailty phenotype with persistent and incident CMD. Robust status was considered reference for frailty according to the Frailty Index and for pre-frailty and frailty status according to the physical frailty phenotype. To investigate the associations of frailty with persistent CMD, we excluded individuals with incident CMD from the reference group. Meanwhile, to investigate the associations of frailty with incident CMD, we excluded from the reference group participants with CMD (CIS-R score ≥ 12) in the onsite assessment (2017–2019). Models were adjusted for age, sex, race, and education. Additionally, we investigated the association of the continuous Frailty Index with persistent and incident CMD. Because exhaustion is a symptom that frequently overlaps between frailty and depression, we conducted sensitivity analyses excluding exhaustion from the Frailty Index and the physical frailty phenotype. Finally, we tested whether age, sex and education were effect modifiers on the association of frailty status defined according to both criteria with persistent and incident CMD. We considered an interaction term p-value <0.10 to stratify these analyses. All statistical tests were two-tailed, and alfa was considered 0.05.
3. Results
The mean age of participants was 69.2 ± 5.4 years, and 54.7 % were female. A total of 42 participants (6.0 %) had persistent and 48 (6.8 %) had incident CMD. The Frailty Index ranged from 0 to 0.57, the mean value was 0.16 ± 0.08, and 75 participants (10.6 %) were classified as frail. According to the physical frailty phenotype, the status of participants was the following: 302 robust (42.8 %), 373 pre-frail (52.8 %), and 31 frail (4.4 %). The characteristics of participants according to frailty status defined by each frailty definition can be seen in Table 1 . The frequency of persistent CMD was higher among frail participants according to both definitions. However, the frequency of incident CMD did not differ according to frailty status in univariate analysis.
Table 1.
Characteristics of participants according to frailty status (n = 706).
| Frailty index |
Physical frailty phenotype |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Robust (n = 631) |
Frail (n = 75) |
p | Robust (n = 302) |
Pre-frail (n = 373) |
Frail (n = 31) |
p |
| Age (years), mean ± SD | 66.6 ± 5.4 | 68.4 ± 6.1 | 0.009a | 66.5 ± 5.4 | 66.9 ± 5.5 | 68.1 ± 5.8 | 0.758e |
| Female, n (%) | 342 (54.2) | 44 (58.7) | 0.463b | 166 (55.0) | 204 (54.7) | 16 (51.6) | 0.938b |
| Self-reported race, n (%) | 0.925c | 0.684c | |||||
| Black | 38 (6.0) | 5 (6.7) | 15 (5.0) | 24 (6.4) | 4 (12.9) | ||
| Brown | 67 (10.6) | 8 (10.7) | 32 (10.6) | 41 (11.0) | 2 (6.4) | ||
| White | 476 (75.5) | 55 (73.3) | 231 (76.5) | 278 (74.5) | 22 (71.0) | ||
| Other | 50 (7.9) | 7 (9.3) | 24 (7.9) | 30 (8.1) | 3 (9.7) | ||
| College education, n (%) | 462 (73.2) | 46 (61.3) | 0.030b | 240 (79.5) | 251 (67.3) | 17 (54.8) | <0.001b |
| Persistent common mental disorder, n (%)g | 26 (4.1) | 16 (21.3) | <0.001b | 7 (2.3) | 27 (7.2) | 8 (25.8) | <0.001c |
| Incident common mental disorder, n (%)h | 40 (6.3) | 8 (10.7) | 0.151c | 19 (6.3) | 24 (6.4) | 5 (16.1) | 0.108c |
| Handgrip strength (kg), median (IQR) | 26.5 (20.5–34) | 20.5 (17–28) | <0.001d | 30 (24–38) | 22 (19–31) | 18 (16.5–21) | <0.001f |
| Muscle weakness, n (%)i | 123 (19.5) | 29 (38.7) | <0.001b | 0 | 130 (34.9) | 22 (71.0) | <0.001b |
| Gait speed (m/s), median (IQR) | 1.0 (0.9–1.2) | 1.2 (1.0–1.4) | <0.001d | 1.0 (0.9–1.1) | 1.1 (0.9–1.2) | 1.3 (1.1–1.6) | <0.001f |
| Slow gait speed (< 0.8 m/s), n (%) | 29 (4.7) | 3 (4.5) | 1.000c | 17 (5.6) | 14 (3.8) | 1 (4.2) | 0.496c |
| Repeated chair stands (s), median (IQR) | 9.9 (8.5–11.5) | 12.0 (10.1–13.8) | <0.001d | 9.4 (8.2–11.1) | 10.4 (9.0–12.0) | 12.5 (9.7–14.1) | <0.001f |
| Repeated chair stands performance, n (%) | <0.001c | <0.001c | |||||
| Intermediate (between 12 and 15 s) | 87 (14.1) | 23 (35.9) | 29 (9.7) | 72 (20.1) | 9 (40.9) | ||
| Slow (> 15 s) | 28 (4.6) | 9 (14.1) | 12 (4.0) | 21 (5.9) | 4 (18.2) | ||
| Unintentional weight loss, n (%) | 31 (4.9) | 14 (18.7) | <0.001b | 0 | 31 (8.3) | 14 (45.2) | <0.001c |
| Exhaustion, n (%) | 27 (4.3) | 28 (33.3) | <0.001b | 0 | 39 (10.5) | 16 (51.6) | <0.001c |
| Low energy expenditure, n (%) | 219 (35.2) | 47 (56.0) | <0.001b | 139 (24.7) | 98 (87.5) | 27 (93.6) | <0.001b |
IQR: interquartile range; SD: standard deviation.
Student's t test.
Chi-square test.
Fisher's exact-test.
Wilcoxon ranksum test.
Oneway ANOVA.
Kruskal-Wallis test.
Defined as CIS-R score ≥ 12 at the ELSA-Brasil study third onsite wave and at the first COVID-19 assessment.
Defined as CIS-R score < 12 at the ELSA-Brasil study third onsite wave and ≥ 12 at the first COVID-19 assessment.
Muscle weakness defined as the lowest handgrip strength quintile according to sex and body mass index quartile.
The associations of frailty status with persistent and incident CMD are shown in Table 2 . After adjustment, frail participants according to both definitions had higher odds of persistent CMD when compared with robust individuals (Frailty Index: OR = 8.61, 95 % CI = 4.08–18.18, p < 0.001; physical phenotype: OR = 23.67, 95 % CI = 7.08–79.15, p < 0.001). The odds of persistent CMD were also higher among the pre-frail according to the physical phenotype (OR = 3.54, 95 % CI = 1.50–8.38, p = 0.004). Also, regardless of the definition, frailty was associated with incident CMD in the adjusted models (Frailty Index: OR = 2.79, 95 % CI = 1.15–6.78, p = 0.023; physical phenotype: OR = 4.37, 95 % CI = 1.31–14.58, p = 0.017). However, the association of pre-frailty with incident CMD was not significant.
Table 2.
Association of frailty status defined according to the Frailty Index and the physical frailty phenotype with persistent and incident common mental disorder.
| Persistent common mental disorderb (n = 658) | Incident common mental disorderc (n = 597) | |||
|---|---|---|---|---|
| Frailty Index | OR (95 % CI) | p | OR (95 % CI) | p |
| Frailtya | ||||
| Crude | 6.82 (3.44–13.53) | <0.001 | 2.77 (1.21–6.34) | 0.016 |
| Adjustedd | 8.61 (4.08–18.18) | <0.001 | 2.79 (1.15–6.78) | 0.023 |
| Physical frailty phenotype | ||||
| Pre-frailtya | OR (95 % CI) | p | OR (95 % CI) | p |
| Crude | 3.31 (1.42–7.71) | 0.006 | 1.19 (0.64–2.22) | 0.586 |
| Adjustedd | 3.54 (1.50–8.38) | 0.004 | 1.06 (0.55–2.02) | 0.872 |
| Frailtya | OR (95 % CI) | p | OR (95 % CI) | p |
| Crude | 17.52 (5.71–53.75) | <0.001 | 5.26 (1.70–16.32) | 0.004 |
| Adjustedd | 23.67 (7.08–79.15) | <0.001 | 4.37 (1.31–14.58) | 0.017 |
Reference: robust participants.
Reference: participants with CIS-R score < 12 in the COVID-19 assessment (2020).
Reference: participants with CIS-R score < 12 in the onsite assessment (2017–2019).
Model adjusted for age, sex, education, and self-reported race.
When we investigated the association of the Frailty Index as a continuous variable with the outcomes, we found that each 0.01 increment in the Frailty Index was associated with 12 % higher odds of persistent CMD (OR = 1.12, 95 % CI = 1.08–1.17, p < 0.001) and with 4 % higher odds of incident CMD (OR = 1.04, 95 % CI = 1.00–1.08, p = 0.036) after adjustment for sociodemographic factors. Additionally, the exclusion of exhaustion from the frailty constructs did not change the association between frailty and persistent CMD. However, after this exclusion, the associations of pre-frailty with persistent CMD and of frailty according to either definition with incident CMD were no longer significant (Table 3 ). Finally, age, sex, and education were not effect modifiers on the associations of frailty with persistent or incident CMD, regardless of the frailty definition (see Supplementary Table S3 for the interaction term p-values).
Table 3.
Association of frailty status according to the Frailty Index and the physical frailty phenotype excluding exhaustion from the definitions with persistent and incident common mental disorder.
| Persistent common mental disorderb (n = 658) | Incident common mental disorderc (n = 597) | |||
|---|---|---|---|---|
| Frailty Index | OR (95 % CI) | p | OR (95 % CI) | p |
| Frailtya | ||||
| Crude | 3.71 (1.80–7.62) | 0.001 | 2.19 (0.97–4.95) | 0.060 |
| Adjustedd | 4.52 (2.10–9.70) | <0.001 | 2.23 (0.94–5.27) | 0.068 |
| Physical frailty phenotype | ||||
| Pre-frailtya | OR (95 % CI) | p | OR (95 % CI) | p |
| Crude | 1.58 (0.78–3.17) | 0.202 | 1.29 (0.71–2.36) | 0.405 |
| Adjustedd | 1.68 (0.82–3.44) | 0.155 | 1.15 (0.62–2.15) | 0.664 |
| Frailtya | OR (95 % CI) | p | OR (95 % CI) | p |
| Crude | 10.08 (3.30–30.77) | <0.001 | 1.47 (0.18–12.16) | 0.723 |
| Adjustedd | 13.55 (4.06–45.25) | <0.001 | 1.10 (0.13–9.65) | 0.932 |
Reference: robust participants.
Reference: participants with CIS-R score < 12 in the COVID-19 assessment (2020).
Reference: participants with CIS-R score < 12 in the onsite assessment (2017–2019).
Model adjusted for age, sex, education, and self-reported race.
4. Discussion
This study demonstrated that older adults who were frail before the pandemic had higher odds of presenting persistent and incident CMD in the first pandemic wave. This association was significant for both frailty definitions adopted (the Frailty Index or the physical phenotype). Additionally, pre-frailty, an intermediate status defined according to the physical phenotype, was associated with persistent CMD. Finally, the exclusion of exhaustion of both frail definitions did not change the associations of frailty with persistent CMD, which occurred for incident CMD.
The pandemic global toll is undebatable. Individual immediate and long-term adverse outcomes from the disease itself, added to multiple societal impacts related to the burden on healthcare systems, social restriction measures, extreme changes in day-to-day life, loss of loved ones, and economic disruptions, compose a boiling cauldron of emotional distress. The mental health impact in the general population, reflected by higher frequencies of psychiatric symptoms and morbidity, have been demonstrated in multiple countries (COVID-19 Mental Disorders Collaborators, 2021; Cénat et al., 2021), including in Brazil (Goularte et al., 2021). Although younger age groups have been more affected (COVID-19 Mental Disorders Collaborators, 2021), studies conducted with older adults have also shown high prevalence of anxiety and depression (Sepúlveda-Loyola et al., 2020). From the perspective of frailty, the existing evidence focuses on the higher risk of mortality and adverse outcomes of frail older adults with COVID-19 (Dumitrascu et al., 2021; Aliberti et al., 2021), while little is known about the impact of the life-changing pandemic environment on frail older adults. Consequences of the social restriction measures in the older population, such as diminished physical activity, reduced mobility, and loneliness (Sepúlveda-Loyola et al., 2020; Leppä et al., 2021; Perracini et al., 2021) could predispose frail older adults to adverse health outcomes. Frail older adults who experienced restricted life-space mobility had higher odds of impact in quality of life than robust individuals (Saraiva et al., 2021).
In our study, frailty was associated with persistent and incident CMD during the pandemic. Findings from previous studies conducted before the pandemic were similar to ours. Cross-sectional studies have demonstrated associations between frailty and depression (Ní Mhaoláin et al., 2012; Zhao et al., 2020; Andrew and Rockwood, 2007; Chang et al., 2010; Uchmanowicz et al., 2020), whereas fewer studies have also evidenced its association with anxiety symptoms (Ní Mhaoláin et al., 2012; Mlynarska et al., 2018; Zhao et al., 2020). Moreover, results from longitudinal studies showed that frail older adults are more likely than robust individuals to have new depressive symptoms after different follow-up periods (Feng et al., 2014; Makizako et al., 2015). However, a study conducted during the pandemic did not reflect our findings. In a cohort of community-dwelling older adults, frailty was not associated with an increase in depressive and anxiety symptoms just after the first wave of the COVID-19 pandemic compared to previous years (van den Besselaar et al., 2021). These contrasting results might be explained by the different scales used for psychiatric assessments.
Our study has several strengths. We were able to consistently explore CMD before and during the pandemic using the CIS-R, a validated and reliable tool to assess psychiatric symptoms and disorders. Additionally, we assessed frailty by the two mainly used definitions that are based on different constructs, and our results were consistent independently of the approach used. Moreover, it is interesting to report that previous studies conducted with the ELSA-Brasil cohort that included younger adults in the analysis showed that CMD trajectories and rates were almost stable between pre-pandemic and pandemic assessments (Brunoni et al., 2021; Fatori et al., 2022). The fact that we were able to demonstrate associations of frailty with incident CMD in the pandemic highlights the need to systematically screen for frailty in older adults. Finally, we were able to show the robustness of the associations between frailty and persistent CMD after excluding exhaustion from the frailty constructs, a condition that overlaps with psychiatric symptoms.
Nevertheless, limitations should also be mentioned. First, the assessment of psychiatric symptoms was performed in a 1- to 3-year interval, and it is possible that fluctuations in psychiatric morbidity had occurred within this time frame. The CIS-R enquired about symptoms that have occurred in the 30 days preceding the interview, and if present, symptoms were further assessed in detail over the past week. It is likely that individuals who may have had CMD symptoms within the two measurement periods are misclassified as non-cases. Second, we used an online version of the CIS-R, due to the quarantine measures, equivalent to the one used in clinical interviews from on-site assessments. Notwithstanding, the online version was self-applied, whereas the onsite version was read by trained personnel. Still, previous studies have already validated and compared an electronic, self-applied CIS-R version with its standard format, showing that the electronic version presents valid and reliable performance (Lewis, 1994; Lewis et al., 1988). In fact, a validation study showed that the performance of both versions was similar (Head et al., 2013). Third, although the CIS-R allows the assessment of specific psychiatric diagnosis based on the ICD-10, the number of specific diagnoses was too small to investigate the associations of these diagnoses with frailty. Fourth, although we were able to assess 36 health deficits to compose the frailty index, a few variables were based on information collected in the ELSA-Brasil baseline assessment, conducted in 2008–2010, and the lack of up-to-date data may have led to some degree of health deficits misclassification However, we believe that this issue has been attenuated by the large number of variables composing the frailty index. Additionally, by adding variables collected almost 9 years apart to build the frailty index, we assumed that some factors remained unchanged between the baseline and the third onsite assessment (2017–2019). Fifth, we did not have data on individual information on social isolation and SARS-Cov-2 infection in relation to the timeline of CMD evaluations. Finally, <50 % of the São Paulo sample accepted to participate and were able to complete the online COVID-19 assessment. Selection bias favoring older individuals who were more cognitively and functionally apt may have occurred, while frailer older individuals denied participation in the study or were not able to properly answer the online questionnaire.
In conclusion, frailty status before the COVID-19 outbreak was associated with higher odds of persistent and incident CMD in older adults living in São Paulo during the first wave of the pandemic, when the strictest social distancing measures were applied. The world continuously faces viral dissemination in the form of consecutive waves and their accompanying mental health toll on the general population. The identification of individuals at higher risk of mental burden, such as frail older adults, can help prioritize resources allocation and implementation of strategies to prevent adverse outcomes.
CRediT authorship contribution statement
CS: Conceptualization, formal analysis, writing original draft; CKS: Conceptualization, supervision, writing – reviewing & editing; ACG: Writing – reviewing & editing; ISS: Writing – reviewing & editing; DF: Writing – reviewing & editing; LBR: Writing – reviewing & editing; MCV: Writing – reviewing & editing; PAL: Writing – reviewing & editing; IMB: Funding acquisition, writing – reviewing & editing; ARB: Conceptualization, data curation, methodology, supervision, writing – reviewing & editing.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgements
None.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo -FAPESP (20/05441-9) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (405547/2015-3). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jad.2023.01.028.
Appendix A. Supplementary data
Supplementary tables
References
- Aliberti M., Szlejf C., Avelino-Silva V.I., Suemoto C.K., Apolinario D., Dias M.B., Garcez F.B., Trindade C.B., Amaral J., de Melo L.R., de Aguiar R.C., Coelho P., Hojaij N., Saraiva M.D., da Silva N., Jacob-Filho W., Avelino-Silva T.J., COVID HCFMUSP Study Group COVID-19 is not over and age is not enough: using frailty for prognostication in hospitalized patients. J. Am. Geriatr. Soc. 2021;69:1116–1127. doi: 10.1111/jgs.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew M.K., Rockwood K. Psychiatric illness in relation to frailty in community-dwelling elderly people without dementia: a report from the Canadian Study of Health and Aging. Can. J. Aging. 2007;26:33–38. doi: 10.3138/8774-758w-702q-2531. [DOI] [PubMed] [Google Scholar]
- Aquino E.M., Barreto S.M., Bensenor I.M., Carvalho M.S., Chor D., Duncan B.B., Lotufo P.A., Mill J.G., Molina M., Mota E.L., Passos V.M., Schmidt M.I., Szklo M. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): objectives and design. Am. J. Epidemiol. 2012;175:315–324. doi: 10.1093/aje/kwr294. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Suen P., Bacchi P.S., Razza L.B., Klein I., Dos Santos L.A., Santos I.D.S., da Costa Lane Valiengo L., Gallucci-Neto J., Moreno M.L., Pinto B.S., de Cássia Silva Félix L., de Sousa J.P., Viana M.C., Forte P.M., de Altisent Oliveira Cardoso M.C., Bittencourt M.S., Pelosof R., de Siqueira L.L., Fatori D., Bellini H., Bueno P.V.S., Passos I.C., Nunes M.A., Salum G.A., Bauermeister S., Smoller J.W., Lotufo P.A., Benseñor I.M. Prevalence and risk factors of psychiatric symptoms and diagnoses before and during the COVID-19 pandemic: findings from the ELSA-Brasil COVID-19 mental health cohort. Psychol. Med. 2021:1–12. doi: 10.1017/S0033291721001719. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.F., Sharma M.S., Brunoni A.R., Vieta E., Fava G.A. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother. Psychosom. 2016;85:270–288. doi: 10.1159/000447034. [DOI] [PubMed] [Google Scholar]
- Cénat J.M., Blais-Rochette C., Kokou-Kpolou C.K., Noorishad P.G., Mukunzi J.N., McIntee S.E., Dalexis R.D., Goulet M.A., Labelle P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res. 2021;295 doi: 10.1016/j.psychres.2020.113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.S., Weiss C.O., Xue Q.L., Fried L.P. Patterns of comorbid inflammatory diseases in frail older women: the Women's Health and Aging Studies I and II. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Mental Disorders Collaborators Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrascu F., Branje K.E., Hladkowicz E.S., Lalu M., McIsaac D.I. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J. Am. Geriatr. Soc. 2021;69:2419–2429. doi: 10.1111/jgs.17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatori D., Suen P., Bacchi P., Afonso L., Klein I., Cavendish B.A., Lee Y.H., Liu Z., Bauermeister J., Moreno M.L., Viana M.C., Goulart A.C., Santos I.S., Bauermeister S., Smoller J., Lotufo P., Benseñor I.M., Brunoni A.R. Trajectories of common mental disorders symptoms before and during the COVID-19 pandemic: findings from the ELSA-Brasil COVID-19 Mental Health Cohort. Soc. Psychiatry Psychiatr. Epidemiol. 2022;1–11 doi: 10.1007/s00127-022-02365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Nyunt M.S., Feng L., Yap K.B., Ng T.P. Frailty predicts new and persistent depressive symptoms among community-dwelling older adults: findings from Singapore longitudinal aging study. J. Am. Med. Dir. Assoc. 2014;15:76.e7–76.e12. doi: 10.1016/j.jamda.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., McBurnie M.A., Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Goularte J.F., Serafim S.D., Colombo R., Hogg B., Caldieraro M.A., Rosa A.R. COVID-19 and mental health in Brazil: psychiatric symptoms in the general population. J. Psychiatr. Res. 2021;132:32–37. doi: 10.1016/j.jpsychires.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J., Stansfeld S.A., Ebmeier K.P., Geddes J.R., Allan C.L., Lewis G., Kivimäki M. Use of self-administered instruments to assess psychiatric disorders in older people: validity of the General Health Questionnaire, the Center for Epidemiologic Studies Depression Scale and the self-completion version of the revised clinical interview schedule. Psychol. Med. 2013;43:2649–2656. doi: 10.1017/S0033291713000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä H., Karavirta L., Rantalainen T., Rantakokko M., Siltanen S., Portegijs E., Rantanen T. Use of walking modifications, perceived walking difficulty and changes in outdoor mobility among community-dwelling older people during COVID-19 restrictions. Aging Clin. Exp. Res. 2021;33:2909–2916. doi: 10.1007/s40520-021-01956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G., Pelosi A.J., Glover E., Wilkinson G., Stansfeld S.A., Williams P., Shepherd M. The development of a computerized assessment for minor psychiatric disorder. Psychol. Med. 1988;18:737–745. doi: 10.1017/s0033291700008448. [DOI] [PubMed] [Google Scholar]
- Lewis G., Pelosi A.J., Araya R., Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol. Med. 1992;22:465–486. doi: 10.1017/s0033291700030415. [DOI] [PubMed] [Google Scholar]
- Lewis G. Assessing psychiatric disorder with a human interviewer or a computer. J. Epidemiol. Community Health. 1994;48:207–210. doi: 10.1136/jech.48.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman M., Dumenci L., Mezuk B. Sex differences in the construct overlap of frailty and depression: evidence from the Health and Retirement Study. J. Am. Geriatr. Soc. 2014;62:500–505. doi: 10.1111/jgs.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman M., Dumenci L., Mezuk B. Depression and frailty in late life: evidence for a common vulnerability. J. Gerontol. B Psychol. Sci. Soc. Sci. 2016;71:630–640. doi: 10.1093/geronb/gbu180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H., Shimada H., Doi T., Yoshida D., Anan Y., Tsutsumimoto K., Uemura K., Liu-Ambrose T., Park H., Lee S., Suzuki T. Physical frailty predicts incident depressive symptoms in elderly people: prospective findings from the Obu Study of Health Promotion for the Elderly. J. Am. Med. Dir. Assoc. 2015;16:194–199. doi: 10.1016/j.jamda.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Mezuk B., Lohman M., Dumenci L., Lapane K.L. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am. J. Geriatr. Psychiatry. 2013;21:560–569. doi: 10.1016/j.jagp.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarska A., Mlynarski R., Golba K.S. Anxiety, age, education and activities of daily living as predictive factors of the occurrence of frailty syndrome in patients with heart rhythm disorders. Aging Ment. Health. 2018;22:1179–1183. doi: 10.1080/13607863.2017.1348468. [DOI] [PubMed] [Google Scholar]
- Morley J.E., Vellas B., van Kan G.A., Anker S.D., Bauer J.M., Bernabei R., Cesari M., Chumlea W.C., Doehner W., Evans J., Fried L.P., Guralnik J.M., Katz P.R., Malmstrom T.K., McCarter R.J., Gutierrez Robledo L.M., Rockwood K., von Haehling S., Vandewoude M.F., Walston J. Frailty consensus: a call to action. J. Am. Med. Dir. Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ní Mhaoláin A.M., Fan C.W., Romero-Ortuno R., Cogan L., Cunningham C., Kenny R.A., Lawlor B. Frailty, depression, and anxiety in later life. Int. Psychogeriatr. 2012;24:1265–1274. doi: 10.1017/S1041610211002110. [DOI] [PubMed] [Google Scholar]
- Nunes M.A.A., Alves M.G.de M., Chor D., Schmidt M.I., Duncan B.B. Cross-cultural adaptation of CIS-R (clinical interview schedule-revised version) for the Portuguese in longitudinal study of adult health (ELSA) Ver. HCPA. 2011:487–490. [Google Scholar]
- Perracini M.R., de Amorim J., Lima C.A., da Silva A., Trombini-Souza F., Pereira D.S., Pelicioni P., Duim E., Batista P.P., Dos Santos R.B., de Lima M., REMOBILIZE Research Network (CANSORT-SCI) Impact of COVID-19 pandemic on life-space mobility of older adults living in Brazil: REMOBILIZE Study. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.643640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Rosenblat J.D., Kurdyak P., Cosci F., Berk M., Maes M., Brunoni A.R., Li M., Rodin G., McIntyre R.S., Carvalho A.F. Depression in the medically ill. Aust. N. Z. J. Psychiatry. 2020;54:346–366. doi: 10.1177/0004867419888576. [DOI] [PubMed] [Google Scholar]
- Saraiva M.D., Apolinario D., Avelino-Silva T.J., de Assis Moura Tavares C., Gattás-Vernaglia I.F., Marques Fernandes C., Rabelo L.M., Tavares Fernandes Yamaguti S., Karnakis T., Kalil-Filho R., Jacob-Filho W., Romero Aliberti M.J. The impact of frailty on the relationship between life-space mobility and quality of life in older adults during the COVID-19 pandemic. J. Nutr. Health Aging. 2021;25:440–447. doi: 10.1007/s12603-020-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.I., Duncan B.B., Mill J.G., Lotufo P.A., Chor D., Barreto S.M., Aquino E.M., Passos V.M., Matos S.M., Molina M., Carvalho M.S., Bensenor I.M. Cohort profile: longitudinal study of adult health (ELSA-Brasil) Int. J. Epidemiol. 2015;44:68–75. doi: 10.1093/ije/dyu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle S.D., Mitnitski A., Gahbauer E.A., Gill T.M., Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., Ganz F., Torralba R., Oliveira D.V., Rodríguez-Mañas L. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J. Nutr. Health Aging. 2020;24:938–947. doi: 10.1007/s12603-020-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soysal P., Veronese N., Thompson T., Kahl K.G., Fernandes B.S., Prina A.M., Solmi M., Schofield P., Koyanagi A., Tseng P.T., Lin P.Y., Chu C.S., Cosco T.D., Cesari M., Carvalho A.F., Stubbs B. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res. Rev. 2017;36:78–87. doi: 10.1016/j.arr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Szlejf C., Suemoto C.K., Brunoni A.R., Viana M.C., Moreno A.B., Matos S., Lotufo P.A., Benseñor I.M. Depression is associated with sarcopenia due to low muscle strength: results from the ELSA-Brasil study. J. Am. Med. Dir. Assoc. 2019;20:1641–1646. doi: 10.1016/j.jamda.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Szlejf C., Suemoto C.K., Santos I.S., Brunoni A.R., Nunes M.A., Viana M.C., Barreto S.M., Lotufo P.A., Benseñor I.M. Poorer cardiovascular health is associated with psychiatric comorbidity: results from the ELSA-Brasil Study. Int. J. Cardiol. 2019;274:358–365. doi: 10.1016/j.ijcard.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Uchmanowicz I., Lomper K., Gros M., Kałużna-Oleksy M., Jankowska E.A., Rosińczuk J., Cyrkot T., Szczepanowski R. Assessment of frailty and occurrence of anxiety and depression in elderly patients with atrial fibrillation. Clin. Interv. Aging. 2020;15:1151–1161. doi: 10.2147/CIA.S258634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Besselaar J.H., MacNeil Vroomen J.L., Buurman B.M., Hertogh C., Huisman M., Kok A., Hoogendijk E.O. Symptoms of depression, anxiety, and perceived mastery in older adults before and during the COVID-19 pandemic: results from the Longitudinal Aging Study Amsterdam. J. Psychosom. Res. 2021;151 doi: 10.1016/j.jpsychores.2021.110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L., Corbin A.L., Goveas J.S. Depression and frailty in later life: a systematic review. Clin. Interv. Aging. 2015;10:1947–1958. doi: 10.2147/CIA.S69632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Zhang Y., Liu X., Yue J., Hou L., Xia X., Zuo Z., Liu Y., Jia S., Dong B., Ge N. Comorbid depressive and anxiety symptoms and frailty among older adults: findings from the West China health and aging trend study. J. Affect. Disord. 2020;277:970–976. doi: 10.1016/j.jad.2020.08.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables