Abstract
Background
Prevention of pregnancy-related alloimmunization and the management of hemolytic disease of the fetus and newborn (HDFN) has significantly improved over the past decades. Considering improvements in HDFN care, the objectives of this systematic literature review were to assess the prenatal treatment landscape and outcomes of Rh(D)- and K-mediated HDFN in mothers and fetuses, to identify the burden of disease, to identify evidence gaps in the literature, and to provide recommendations for future research.
Methods
We performed a systematic search on MEDLINE, EMBASE and clinicaltrials.gov. Observational studies, trials, modelling studies, systematic reviews of cohort studies, and case reports and series of women and/or their fetus with HDFN caused by Rhesus (Rh)D or Kell alloimmunization. Extracted data included prevalence; treatment patterns; clinical outcomes; treatment efficacy; and mortality.
Results
We identified 2,541 articles. After excluding 2,482 articles and adding 1 article from screening systematic reviews, 60 articles were selected. Most abstracted data were from case reports and case series. Prevalence was 0.047% and 0.006% for Rh(D)- and K-mediated HDFN, respectively. Most commonly reported antenatal treatment was intrauterine transfusion (IUT; median frequency [interquartile range]: 13.0% [7.2–66.0]). Average gestational age at first IUT ranged between 25 and 27 weeks. weeks. This timing is early and carries risks, which were observed in outcomes associated with IUTs. The rate of hydrops fetalis among pregnancies with Rh(D)-mediated HDFN treated with IUT was 14.8% (range, 0–50%) and 39.2% in K-mediated HDFN. Overall mean ± SD fetal mortality rate that was found to be 19.8%±29.4% across 19 studies. Mean gestational age at birth ranged between 34 and 36 weeks.
Conclusion
These findings corroborate the rareness of HDFN and frequently needed intrauterine transfusion with inherent risks, and most births occur at a late preterm gestational age. We identified several evidence gaps providing opportunities for future studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-022-05329-z.
Keywords: Hemolytic disease of the fetus and newborn, Fetal therapy, Fetal anemia, Intrauterine transfusion
Background
Despite advances in the prevention of pregnancy-related red blood cell immunization and management and treatment of pregnancies affected by hemolytic disease of the fetus and newborn (HDFN) over recent decades, the disease still poses a significant risk in affected pregnancies [1, 2]. HDFN is caused by maternal alloimmunization through exposure to incompatible red blood cell antigens of the fetus or through incompatible blood transfusion [1, 3]. The then-formed immunoglobulin G (IgG) antibodies are actively transported across the placenta and can cause fetal hemolysis and anemia. When untreated, progressive fetal anemia results in hydrops fetalis and ultimately fetal demise. If the fetus survives, persistent hemolysis causes neonatal anemia and hyperbilirubinemia, which—when untreated—ultimately leads to a severe cerebral condition (“kernicterus”).
No cure exists for HDFN. Hence, interventions have focused on its prevention and minimizing adverse effects of associated complications [1, 4]. Through transfusing women within the reproductive ages with Kell-negative donor blood, if possible, and through the introduction of Rhesus (Rh) immunoglobulin prophylaxis, the occurrence of red blood cell alloimmunization and the prevalence of Rh(D)- and K-mediated HDFN has decreased [1, 4–6]; however, the gap between anti-Rh(D) supply and demand is large in low-income countries and is below the optimal threshold in high-income countries [7]. Additionally, the disease still poses a significant risk for mortality and morbidity in developing countries, whereas it is considered treatable with good outcomes in developed countries. Serological monitoring, ultrasonography, and Doppler imaging decreased the need for risky and invasive diagnostic procedures [3, 8–12]. Antenatal treatment, however, still relies predominantly on (often serial) intrauterine transfusion (IUT)—an invasive procedure that carries maternal and fetal risks [13, 14].
Considering improvements in HDFN care, the objectives of this systematic literature review were to assess the prenatal treatment landscape and outcomes of Rh(D)- and K-mediated HDFN in mothers and fetuses to identify the burden of disease, to identify evidence gaps in the literature, and to provide recommendations for future research. Secondarily, we aim to determine the humanistic and economic burden of HDFN.
Methods
Search strategy
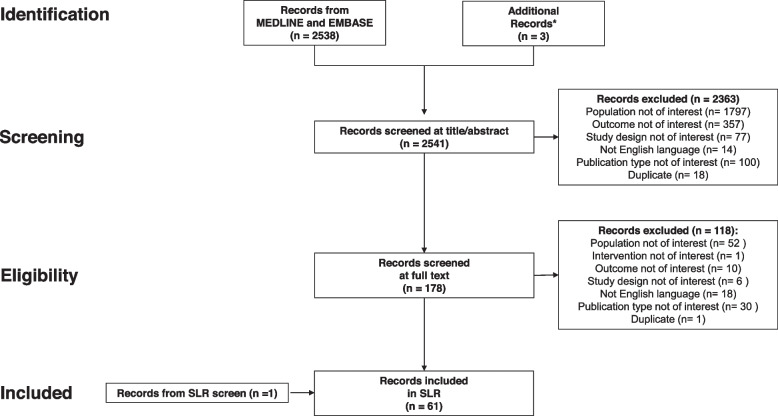
We conducted a systematic literature review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15] and MOOSE Reporting Guidelines for Meta-Analysis of Observational Studies [16] to address prespecified research questions (Table S2). To assess the treatment landscape, articles published between January 1, 2005, and March 10, 2021 were searched (Additional file 2: Appendix S1) in the MEDLINE and EMBASE databases and ClinicalTrials.gov using ProQuest (Fig. 1). The search strategy included descriptions of the disease, possible interventions and clinical outcomes. No limitations were set on studies reporting on cases managed before January 1, 2005. Searches for clinical outcomes were performed for journal articles and conference abstracts indexed in EMBASE. Duplicates were removed automatically. We also manually searched reference lists of pertinent systematic literature reviews of cohort studies and our personal libraries for potentially relevant articles.
Fig. 1.
Flowchart of the Article Selection Process. SLR, systematic literature review. *From authors’ personal library. †From eligible SLRs of cohort studies
Study selection
Two independent reviewers (D.P.D.W. and A.K.) (Table S3) [17] reviewed the titles/abstracts in Rayyan (https://rayyan.ai/) and then full texts in Microsoft Excel. Citations were independently evaluated to determine whether or not studies fulfilled inclusion and exclusion criteria. The project director (D.O.) and the project team adjudicated decisions. Randomized or nonrandomized trials; retrospective or prospective observational studies, including cohort, case-control, or cross-sectional studies; modelling studies; systematic reviews of cohort studies (to identify primary studies only); and case reports and case series of women and/or their fetuses, infants, or children experiencing or having experienced Rh(D)- and/or K-mediated HDFN were included. Studies or patient groups within studies where HDFN was caused by alloimmunization to antigens other than Rh(D) only and/or K only, such as c, e, E, Duffy (Fy), Kidd (Jk), MNS (S), or Gerbich, were excluded as the risk of prenatal disease is regarded as relatively low. Non–English-language articles were excluded, as were notes, editorials, and commentaries; nonsystematic reviews; reports of populations, interventions, outcomes, or study designs not of interest; publication types not of interest; indexed conference abstracts; and reports of animal or preclinical studies. The review was registered with PROSPERO before data were abstracted.
Two independent reviewers (D.P.D.W. and A.K.) abstracted data (i.e., study reference; study design; patient characteristics; HDFN treatment patterns; clinical outcomes [eg, fetal anemia, hydrops fetalis, and adverse events]; intravenous immunoglobulin [IVIG] efficacy; mortality; and prevalence) from studies that fulfilled inclusion and exclusion criteria. All abstracted data underwent quality control by the project director (D.O.), who screened 10% of included/excluded articles. The methodological quality (risk of bias) of the selected studies was assessed by 2 independent reviewers (D.P.D.W. and A.K.) using the JBI Critical Appraisal Checklist for Case Reports [18], the JBI Critical Appraisal Checklist for Case Series [19], the Newcastle-Ottawa Scale for retrospective and prospective cohort studies [20], the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) for questionnaires [21], and lastly the NICE checklist for randomized controlled trials (RCTs).
Analyses
Data from eligible studies were characterized as representative, which included data from studies that accurately reflected the characteristics of the larger group (e.g., larger case series, retrospective or prospective studies, RCTs), or were characterized as nonrepresentative, which included data from studies that reflected a small proportion of the characteristics of the larger group (e.g., case reports or small case series, or studies in a subset of the larger group, such as cases treated with IUT or only cases with hydrops fetalis). When possible, we aggregated information reported in a similar manner. For unique outcomes, we highlighted information from generalizable studies. Where appropriate, data were summarized as percentage (mean ± standard deviation [SD] or range) or median (interquartile range [IQR]) for patient groups or patient populations (e.g., Rh[D] or Kell, Rh[D] treated with IVIG or Rh[D] not treated with IVIG). Case reports and case series were excluded from prevalence analyses.
An assessment of the available findings was conducted to identify evidence gaps, and recommendations to fill unmet needs were formulated. Results pertinent to mothers and fetuses are reported herein. Neonatal outcomes will be reported in a separate article.
Humanistic and economic burden
A separate objective of this systematic review is to determine the humanistic and economic burden of HDFN. We conducted a systematic search using the same criteria as previously mentioned (Additional file 2: Appendix S2). We selected studies reporting on quality of life, humanistic burden, economic burden, health care resource use, and direct and indirect costs. The review process was performed according to the PRISMA and MOOSE guidelines and similar as previously stated.
Results
Data sources
In addition to the 2,538 articles identified through searches of MEDLINE and EMBASE, we identified 3 articles from our personal libraries (Fig. 1). The search on ClinicalTrials.gov did not yield any additional results to the search on MEDLINE and EMBASE. Overall, 2,363 of the 2,541 total articles were excluded on the basis of title and abstract review, and 119 were excluded on the basis of full-text review. Besides the 59 articles that remained, we identified 1 article from a review of applicable systematic reviews of cohort studies.
Study characteristics
Among the 60 eligible studies that were included in our analysis (Table S1), [2, 22–80] nearly half were retrospective cohort studies (n = 27 [45%]), followed by case reports and case series (n = 21 [35%]), prospective cohort studies (n = 7 [12%]), observational cohort studies (n = 3 [5%]), and RCTs and questionnaires (n = 1 [2%] each). More studies included patients with only Rh(D)-mediated HDFN (n = 26) than only K-mediated HDFN (n = 7); 27 studies included patients with Rh(D)- or K-mediated HDFN. Studies were conducted across 25 countries, most commonly The Netherlands (n = 12), followed by Turkey and the United States (n = 6 each). The 60 studies comprised 146 patient groups, including mothers, neonates, and fetuses. Of these patient groups, 46 were single patients extracted from case reports. Mean (range) group size, including case reports, was 36.5 (SD ± 68.7, range 1.0–334.0). The reported patient groups included cases managed between 1985 and 2019.
Methodological quality of the studies
Of the 14 included case reports, 7 received a perfect score (8/8) using the JBI Critical Appraisal Checklist for Case Reports. Median score among case reports was 7.5 [IQR: 6.0–8.0]. Two of the seven included case series received a perfect score among the applicable questions. Median score among case series was 5.0 [IQR: 5.0–8.5]. Twenty-one of 30 retrospective cohort studies were rated as good quality, 8 as fair, and 1 as poor. All 8 included prospective studies were rated as good quality. The randomized controlled trial by Santos et al. was rated as having low risk of bias in all 4 domains—selection bias, performance bias, attrition bias, and detection bias. Tables S4a-e contains a detailed overview of the methodological quality of the selected studies.
Diagnostic testing
Diagnostic testing data were available for 59 of the 60 studies. The most commonly reported diagnostic testing method was ultrasound (n = 20; median [IQR]: 100% [100–100%]), followed by percutaneous umbilical cord sampling (n = 17; 100% [100–100%]); anti-D and/or anti-K antibody titer (n = 16; 100% [100–100%]); fetal hemoglobin (n = 15; 100% [100–100%]); Coombs/antiglobulin testing (n = 15; 100%); cell-free DNA testing (n = 8; 100% [80–100%]); amniocentesis (n = 7; 100% [50–100%]); free antibody testing, antibody release testing, and gel card technique (n = 2; 100%); and magnetic resonance imaging (n = 2; 100% [56.5–100%]).
Prevalence of D- and K-mediated HDFN
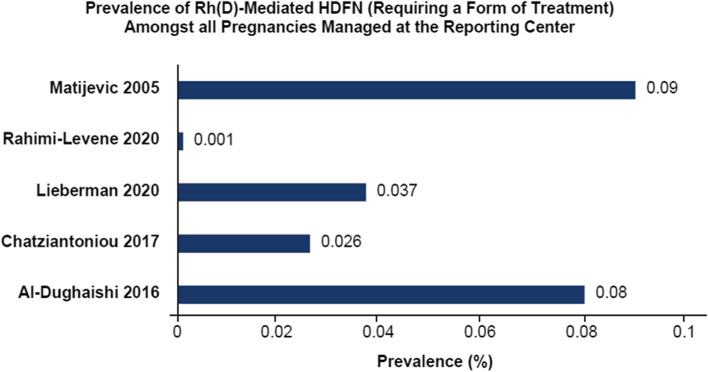
The mean ± SD prevalence of Rh(D)-mediated HDFN (requiring any form of treatment) as reported in 5 studies [23, 29, 45, 47, 54] was 0.047%±0.037% among all pregnancies that were managed and delivered in the centers of the 5 selected studies (Fig. 2). The reported prevalence of K-mediated HDFN (requiring any form of treatment) among all pregnancies managed and delivered in the centers reporting in 2 retrospective studies was 0.006% [45, 47]. No data were available for the prevalence of early-onset HDFN (requiring intervention before 24 weeks of pregnancy) in the selected studies. The gestational age at first IUT was between 25 and 27 weeksand the mean gestational age at birth between 34 and 36 weeks (Table 1) amongst all selected studies [2, 22–80].
Fig. 2.
Prevalence of Rh(D)-mediated HDFN (Requiring a Form of Treatment) Among All Referred Pregnancies [18, 24, 40, 42, 49]. D, Rh(D); HDFN, hemolytic disease of the fetus and newborn; Rh, Rhesus; SD, standard deviation
Table 1.
Patient Characteristics Among 155 HDFN Groups, Including Mothers, Neonates, and Fetuses, From 60 Included Studis
| Characteristic | Mean (± SD, Range) | Patient Groups (n) | No. of Studies (References) |
|---|---|---|---|
| Patient group size, n | 36.5 (± 68.7, 1.0–334.0) | 146a | 60 [2, 22–80] |
| Gestational age at birth, weeksb | |||
| Mean gestational age | 35.1 (± 2.1, 33.0–37.4) | 13 | 9 [23, 26, 28, 47, 53, 63, 65, 70, 72] |
| Median gestational age | 35.4 (± 2.1, 28.0–37.0) | 24 | 12 [31, 35, 42, 45, 51, 59, 60, 62, 63, 75, 76] |
| Exact gestational age | 34.1 (± 2.9, 28.1–38.0) | 18 | 14 [22, 24, 27, 34, 39, 43, 44, 46, 50, 52, 55, 66, 74, 77] |
| Gestational age at first IUT, weeks | |||
| Mean gestational age | 26.6 (± 0.1, 26.6–26.7) | 4 | 2 [28, 53] |
| Median gestational age | 25.9 (± 3.4, 21.7–36.3) | 27 | 13 [31, 51, 59–61, 63, 64, 69, 73, 75, 79, 80] |
| Exact gestational age | 24.8 (± 3.9, 20.0–30.1) | 8 | 5 [44, 52, 66, 68, 76] |
Means, medians, and exact gestational age as reported in each study were used to calculate mean (range)
HDFN Hemolytic disease of the fetus and newborn, IUT Intrauterine transfusion
aOf the 155 patient groups, 46 (29.7%) groups were single patients from case reports
bDoes not include 1 study [2] in which the reported percentage of the patient group fell within gestational age ranges (i.e., < 259 days and 259–294 days)
Frequencies of antenatal management strategies
Antenatal treatment data were available for 24 studies (Table S8) [24, 25, 29, 30, 33–37, 42, 43, 48, 51, 52, 56, 58–63, 65, 71, 80]. The most commonly reported antenatal treatment across these studies was IUT (n = 9 with representative data [25, 29, 33, 36, 37, 56, 59, 60, 65]; n = 3 with nonrepresentative data [35, 58, 62].
Intrauterine transfusions
Among the 9 studies with representative data, IUTs were given at a median frequency of 13.0% (IQR: 7.2–66.0) among pregnancies with a positive anti-Rh(D) screening that were monitored prenatally with ultrasonography. Three of these studies monitored pregnancies with a positive anti-Rh(D) screening and a risk-stratification using the antibody titers and/or antibody-dependent cellular cytotoxicity values above cut-off value, and might therefore overestimate the frequency of IUTs [56, 59, 60]. In these three studies, the frequency of IUTs was 64.7% (range 59.2–66) [56, 59, 60]. Number of IUTs was reported by only two of these studies with a median of 2 (range 0–4) [56, 59]. IUTs were required in 76.8% of pregnancies, as reported in 63/82 collective cases [36, 56, 60].
The frequency of IUTs in pregnancies with a positive anti-Rh(D) screening monitored without serological cut-off values was 11.2% (range, 4.5–58.6) in the collective cases (42/376) reported by 5 studies [25, 29, 33, 37, 65]. The number of IUTs required was only reported by 1 study with a mean of 2.4 (SD not reported) [33]. Data on the need for IUTs in pregnancies with K-alloimmunization without serological cut-offs were reported by 1 study and was 12.5% in 1/8 reported cases [25].
Alternative management strategies
Use of IVIG alone was reported in 1 study with representative data [48]. In this case series of 3 severely affected pregnancies, 2 (Rh[D], n = 1; Kell, n = 1) resulted in live births without IUT; the third (Rh[D] + anti-C) was treated with IUT but resulted in a post-procedure intrauterine death.
Use of IUT + IVIG was reported in 4 studies (n = 1 with representative data[61]; n = 3 with nonrepresentative data [35, 42, 62]). In the one study with representative data, 3.2% of the Rh(D) alloimmunization cases and 4.3% of the Kell alloimmunization cases were treated with IUT + IVIG [61].
Use of other treatments (therapeutic plasma exchange [TPE]; maternal plasma exchange ± high-dose IVIG; TPE + IVIG + IUT; TPE + immunoadsorption + IVIG + IUT; plasmapheresis + IUT; and plasmapheresis + IVIG + IUT) were reported in 10 studies with a total of 38 cases [24, 30, 34, 36, 43, 51, 52, 63, 71, 80]. Plasmapheresis + IVIG + IUT was the most commonly reported treatment regimen across these 10 studies.Two of these studies did not report gestational age at start of the treatment, at first IUT (if applicable) and at birth [36, 80]. The remaining 8 studies, including 20 cases total, reported the mean gestational age at treatment initiation (13.0 ± 5.7 weeks). 17/20 cases required an IUT for fetal anemia. The mean gestational age at first IUT was 24.2 ± 3.1 weeks, with a median of 4 IUTs (range 1–8) administered. Gestational age at birth was 34.4 ± 3.1 weeks [30, 34, 43, 51, 52, 63, 71]. In the series of 20 cases, one patient received plasmapheresis, which was started a week after the first IUT at a gestational age of 27 weeks [43]. In all other cases, the alternative treatment was started prior to the occurrence of fetal anemia. The indications to start the alternative treatment option in the 20 cases were previous intrauterine fetal death (n = 11), neonatal hydrops fetalis and/or death (n = 4), marked elevation in antibody titer (n = 4), and suspected fetal anemia after initial IUT (n = 1).
Clinical outcomes of mothers and fetuses
The most commonly reported maternal/fetal clinical outcome across studies was hydrops fetalis (n = 19 with representative data [23, 28, 31, 32, 41, 42, 47, 49, 51, 53, 59, 64, 66, 67, 69, 75, 76, 79, 80] (Table S8); n = 10 with nonrepresentative data [24, 39, 40, 44, 46, 52, 55, 68, 70, 74]). The rate of hydrops fetalis among pregnancies with Rh(D)-mediated HDFN treated with IUT was 14.9% (range, 0–50%) in 72/483 reported cases [31, 32, 49, 53, 66, 76, 79]. The rate of hydrops fetalis among pregnancies with K-mediated HDFN treated with IUT was 39.2% in 49/125 reported cases [69, 75, 76, 79]. Five studies reported on the rate of hydrops fetalis in all pregnancies monitored for Rh(D)- and or K-alloimmunization, with or without the need for antenatal treatment. The rate of hydrops fetalis in these studies was 7.3% in 17/232 collective cases.
Severe fetal anemia was reported in 1 study with representative data [28] (Table S8) and 11 studies with nonrepresentative data [30, 39, 42–44, 46, 52, 63, 68, 74, 80]. In the 1 cohort study with representative data, 100% of 22 successful IUTs performed in Rh(D)- or K-mediated HDFN cases within 20 weeks of gestation were considered severely anemic (≥ 5 SDs from the fetal hemoglobin reference value of 15 g/dL; 1 SD = 1 g/dL difference from reference value) [28]. Adverse events or procedure-related complications were commonly reported after IUTs or other treatments for Rh(D)- and/or K-mediated HDFN (n = 11 studies with representative data [28, 33, 47, 51, 53, 63, 64, 66, 69, 72, 80] (Table S8); n = 2 studies with nonrepresentative data [68, 73]). Bradycardia was the most frequently reported post-IUT complication per procedure, and adverse serological outcomes were the most frequently reported post-IUT complication per fetus, although adverse serological outcomes were reported in only 1 study [33] (Fig. S2).
IVIG efficacy
Assessment of IVIG efficacy in women and fetuses affected by HDFN was based on treatment response in 6 studies [24, 30, 34, 48, 62, 80] and associated mortality in 3 studies [35, 42, 63] (Table S8). Collectively, findings indicate that IVIG delayed or prevented IUT. IVIG-associated fetal mortality ranged from 0 to 50% across the 3 studies reporting this outcome [35, 42, 63].
Fetal mortality
The overall mean ± SD fetal mortality rate was 19.8% ± 29.4% across 19 studies, including representative case reports [28, 31, 35, 42, 43, 45, 47, 49, 53, 63, 64, 66, 68, 69, 73, 75, 76, 78, 80] (Table S8). Head-to-head comparison of mortality rates between the different treatment strategies is limited by variation in potential patient characteristics between the groups. Employed treatment strategies for HDFN take into account previous obstetrical history, for example 75% of cases in the “IUT + other” group had a history of fetal or neonatal death due to HDFN. Together, these will influence the outcomes of HDFN in the current pregnancy and limit our capability of mortality rate comparison.
Humanistic and economic burden
In addition to the 1457 articles identified in the systematic search, one additional article was identified from personal libraries (Fig. S2). Based on the title/abstract screening 1435 articles were excluded. Full-text screening was performed in the remaining 23 records of which 22 were excluded. Healthcare utilization was reported by only one study with a median of duration of phototherapy of 4-4.5 days and a median length of stay of 6.5–7.5 days [65].
Discussion
Main findings
We found that the prenatal burden and need for treatment remains relatively high – we estimated that 13% of pregnancies monitored for Rh(D) or K-alloimmunization required one or more IUTs – despite advances in the identification and care for pregnancies at risk of HDFN. Strikingly, the rate of hydrops fetalis in pregnancies requiring an IUT was found to be 14.9% for Rh(D)-mediated HDFN and 39.2% in K-mediated HDFN. As the occurrence of hydrops fetalis was previously found to be associated with impaired neurodevelopmental outcomes [81] still much is to gain in the timely identification of pregnancies at-risk and the timely detection and treatment of fetal anemia to prevent hydrops fetalis. The average gestational age at first IUT was 27 weeks, which was possibly delayed by using IVIG and/or plasmapheresis although evidence on this in the included studies is limited. Although IUT is a regarded as a relatively safe procedure in experienced hands, its invasive nature still poses serious risks to the mother and fetus. Fetal loss rate increases when procedures need to be done early in gestation (i.e., < 22 weeks) [13]. It is also noteworthy that the average gestational age at birth in the present analyses was approximately 35 weeks for Rh(D)- and/or K-mediated HDFN, which is considered late preterm and might also represent that early delivery is frequently employed in the management of pregnancies at risk of fetal anemia although we were unable to extract data on this from the included studies. But, late preterm birth has the potential for serious consequences, such as increased risk for short- or long-term respiratory issues [82–84], readmission [82], death [82, 84, 85], and neurocognitive impairment in late adulthood [86, 87].
Strengths and limitations
A strength of this systematic review and corresponding analyses is the minimal limitation on study design criteria. Overall, 35% of the studies included in our analyses were case reports or case series, validating the rarity of HDFN. By including case reports and case series, we were able to identify and aggregate data on treatment types (e.g., plasmapheresis and plasma exchange) that were not typically reported in larger cohort studies. But, inclusion of case reports and case series might also be regarded as a limitation as it may skew and overestimate the results as, given the rarity of HDFN, the most severe cases are generally reported in literature. Our estimates might therefore not truly mirror the population level data.
Also, we were unable to quantify heterogeneity (using e.g. the I2-statistic) due to the descriptive nature of this systematic review and consequent lack of reported comparisons between interventions. However, a certain level of heterogeneity may be expected due to differences in available management options, treatment protocols, prevalence of Rh(D)- and K-negativity, geographical location and sociodemographic differences. These differences may be approached by the varying frequencies of, for instance, IUTs and rate of hydrops fetalis between included studies.
Our analyses are further limited by the strict prespecified inclusion of only Rh(D)- or K-mediated HDFN populations. By applying this criterion, 7 studies were excluded from analyses—despite high quality of evidence and outcomes of interest—because Rh(c) populations were mixed with Rh(D) or Kell populations, or the population was not well defined [88–94]. We were unable to stratify the data per alloimmunization type with the data provided in these articles. One of these studies represented the only prospective study with long-term outcomes [89], thereby also indicating the lack of data on long-term outcomes and the need for further research on the topic.
Interpretation
Almost all studies included in these analyses were conducted in high-income countries, which have adequate resources for screening, prophylaxis and preventative measures for alloimmunization, and referral to specialized fetal therapy centers. Outcome data on HDFN-complicated pregnancies from less privileged or less organized societies are lacking and, if analyzed, likely are less favorable. This well-known bias in outcome reporting indicates an important evidence gap and signifies the need for international collaboration to gain a better understanding of the global burden of HDFN and to pave the way for potential wide-spread improvements. To add to that, we also found that the evidence for frequency of use and effectiveness of alternative treatment options such as IVIG, plasmapheresis, and plasma exchange on disease severity and the prevention of fetal anemia is limited in the included studies. Also, as previously mentioned it is likely that the most severe cases are reported in literature due to the rarity of the disease. Taken together, future research should aim to gain more exact insight into the employed treatment options and its efficacy and clinical outcomes of mothers, fetuses, and neonates affected by HDFN through an international retrospective and/or prospective registry through the collection of data on diagnostics, antenatal and postnatal treatments and short- and long-term clinical outcomes of mothers, fetuses, and neonates. Such an international effort will pave the way for long sought after answers.
A separate objective of this systematic review, as previously mentioned, was to ascertain the economic and humanistic burden of HDFN. However, through the systematic approach only one study reporting on healthcare utilization was included. This dearth of information indicates another major gap in knowledge, particularly as it relates to the impact of HDFN on a pregnant individual’s quality of life and the potential downstream consequences of high-risk pregnancy on family planning decisions, as well as on the healthcare system.
Conclusion
To conclude, we found that the clinical burden of Rh(D)- and K-mediated HDFN remains relatively high, with 13% of pregnancies monitored for Rh(D)- or K-alloimmunization requiring an IUT and most births occurring at a late preterm gestational age. We identified several important evidence gaps that provide opportunities for future studies to further improve the clinical care of HDFN.
Supplementary Information
Additional file 1: Table S1. General Characteristics of Studies and Patient Groups. Table S2. Research Questions Relevant to Mothers and Fetuses. Table S3. Inclusion and Exclusion Criteriaa. Table S4a. Methodologic Quality of Selected Case Reports. Table S4b. Methodologic Quality of Selected Case Series. Table S4c. Methodologic Quality of Selected Retrospective Cohort Studies. Table S4d. Methodologic Quality of Selected Prospective Cohort Studies. Table S4e. Methodologic Quality of Bennardello et al (25). Table S5. Antenatal Treatments Reported in Studies With Representative Data. Table S6. Hydrops Fetalis, Severe Fetal Anemia, and Adverse Events/Complications Reported in Studies With Representative Data. Table S7. IVIG Treatment Response and Associated Mortality. Table S8. Overall Fetal Mortality Associated With HDFN. Figure S1. Mean Rate of Procedure-related Complications After IUT (Per Procedure [A] and Per Fetus [B]).12,26,30,32,43,44,46,49,52,60 CS, cesarean section; IUT, intrauterine transfusion; PROM, preterm rupture of membranes. aAdverse serological outcome was defined as the development of additional maternal antibodies to anti-D and/or a ≥4-fold enhancement of antibody titer.12. Figure S2. Flowchart of the Article Selection Process for the Humanistic and Economic Burden. SLR, systematic literature review. *From authors’ personal library. †From eligible SLRs of cohort studies.
Additional file 2: Appendix S1. Search Strategy. Appendix S2. Search Strategy for the Humanistic and Economic Burden.
Acknowledgements
Dipen Patel and Chandrasekhar Boya of OPEN Health contributed to this work. Medical writing and editorial support were provided by Maribeth Bogush, PhD, of Peloton Advantage, LLC, an OPEN Health company, in accordance with Good Publication Practice (GPP3) guidelines, and funded by Janssen Pharmaceuticals.
Systematic review registration
PROSPERO 2021 CRD42021234940 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021234940.
Abbreviations
- HDFN
Hemolytic disease of the fetus and newborn
- CHERRIES
Checklist for Reporting Results of Internet E-Surveys
- DNA
Deoxyribonucleic acid
- IQR
Interquartile range
- IUT
Intrauterine transfusion
- IVIG
Intravenous immunoglobulins
- JBI
Joanna Briggs Institute
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- Rh
Rhesus
- SD
Standard deviation
- TPE
Therapeutic plasma exchange
- USA
United States of America
Authors’ contributions
DPDW, AK, ML, and DO contributed to the study design. AK conducted the search. DPDW and AK independently reviewed eligible studies, extracted data, evaluated the methodological quality, and performed analyses. The manuscript was written by DPDW. DPDW, AK, ML, and DO were involved in revising the manuscript. All authors approved the final version for publication.
Funding
Funding for this systematic literature review was provided by Janssen Pharmaceuticals (Raritan, NJ, USA). The authors had access to relevant aggregated study data and other information (e.g., study protocol, analytic plan and report, and validated data tables) required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors’ personal interests, financial or non-financial, relating to this research and its publication have been disclosed.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Derek P. de Winter: PhD program funded by Momenta Pharmaceuticals, Inc., which was acquired by Johnson & Johnson; coordinating investigator for a phase 2 trial (NCT03842189) of a new drug for the treatment of HDFN, which is sponsored by Janssen Pharmaceuticals.
Allysen Kaminski: Former employee of OPEN Health, which was retained by Janssen Pharmaceuticals to conduct the study.
May Lee Tjoa: Employee and stockholder of Janssen Pharmaceuticals.
Dick Oepkes: Former principal investigator for a phase 2 trial (NCT03842189) of a new drug for the treatment of HDFN, which is sponsored by Janssen Pharmaceuticals.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jackson ME, Baker JM. Hemolytic disease of the Fetus and Newborn: historical and current state. Clin Lab Med. 2021;41(1):133–51. doi: 10.1016/j.cll.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Raguz MJ, Prce Z, Bjelanovic V, Bjelanovic I, Dzida S, Mabic M. 20 years of follow-up alloimmunization and hemolytic disease in Newborn: has anything changed in the Field over the years? Klin Padiatr. 2020;232(6):314–20. doi: 10.1055/a-1248-2329. [DOI] [PubMed] [Google Scholar]
- 3.Dziegiel MH, Krog GR, Hansen AT, Olsen M, Lausen B, Nørgaard LN, et al. Laboratory Monitoring of Mother, Fetus, and Newborn in Hemolytic Disease of Fetus and Newborn. Transfus Med Hemother. 2021;48(5):306–15. doi: 10.1159/000518782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legler TJ. RhIg for the prevention rh immunization and IVIg for the treatment of affected neonates. Transfus Apher Sci. 2020;59(5):102950. doi: 10.1016/j.transci.2020.102950. [DOI] [PubMed] [Google Scholar]
- 5.Pollack W, Gorman JG, Freda VJ, Ascari WQ, Allen AE, Baker WJ. Results of clinical trials of RhoGAM in women. Transfusion. 1968;8(3):151–3. doi: 10.1111/j.1537-2995.1968.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 6.Koelewijn JM, de Haas M, Vrijkotte TG, Bonsel GJ, van der Schoot CE. One single dose of 200 microg of antenatal RhIG halves the risk of anti-D immunization and hemolytic disease of the fetus and newborn in the next pregnancy. Transfusion. 2008;48(8):1721–9. doi: 10.1111/j.1537-2995.2008.01742.x. [DOI] [PubMed] [Google Scholar]
- 7.Pegoraro V, Urbinati D, Visser GHA, Di Renzo GC, Zipursky A, Stotler BA, et al. Hemolytic disease of the fetus and newborn due to Rh(D) incompatibility: a preventable disease that still produces significant morbidity and mortality in children. PLoS ONE. 2020;15(7):e0235807-e. doi: 10.1371/journal.pone.0235807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Haas M, Thurik FF, Koelewijn JM, van der Schoot CE. Haemolytic disease of the fetus and newborn. Vox Sang. 2015;109(2):99–113. doi: 10.1111/vox.12265. [DOI] [PubMed] [Google Scholar]
- 9.Dukler D, Oepkes D, Seaward G, Windrim R, Ryan G. Noninvasive tests to predict fetal anemia: a study comparing doppler and ultrasound parameters. Am J Obstet Gynecol. 2003;188(5):1310–4. doi: 10.1067/mob.2003.265. [DOI] [PubMed] [Google Scholar]
- 10.Oepkes D, Brand R, Vandenbussche FP, Meerman RH, Kanhai HH. The use of ultrasonography and doppler in the prediction of fetal haemolytic anaemia: a multivariate analysis. Br J Obstet Gynaecol. 1994;101(8):680–4. doi: 10.1111/j.1471-0528.1994.tb13184.x. [DOI] [PubMed] [Google Scholar]
- 11.Oepkes D, Seaward PG, Vandenbussche FP, Windrim R, Kingdom J, Beyene J, et al. Doppler ultrasonography versus amniocentesis to predict fetal anemia. N Engl J Med. 2006;355(2):156–64. doi: 10.1056/NEJMoa052855. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman R, Carpenter RJ, Jr, Durig P, Mari G. Longitudinal measurement of peak systolic velocity in the fetal middle cerebral artery for monitoring pregnancies complicated by red cell alloimmunisation: a prospective multicentre trial with intention-to-treat. BJOG. 2002;109(7):746–52. doi: 10.1111/j.1471-0528.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 13.Zwiers C, van Kamp I, Oepkes D, Lopriore E. Intrauterine transfusion and non-invasive treatment options for hemolytic disease of the fetus and newborn - review on current management and outcome. Expert Rev Hematol. 2017;10(4):337–44. doi: 10.1080/17474086.2017.1305265. [DOI] [PubMed] [Google Scholar]
- 14.Snelgrove JW, D’Souza R, Seaward PGR, Windrim R, Kelly EN, Ryan G. Predicting Intrauterine Transfusion interval and perinatal outcomes in Alloimmunized Pregnancies: Time-to-event Survival Analysis. Fetal Diagn Ther. 2019;46(6):425–32. doi: 10.1159/000499972. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156(8):787–8. doi: 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- 17.Centre for Reviews and Dissemination . Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009. [Google Scholar]
- 18.Moola S, Munn Z, Tufanaru C, Aromartis E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, et al., editors. JBI manual for evidence synthesis. 2020. [Google Scholar]
- 19.Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evidence Synt. 2020;18(10):2127. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Newcastle-Ottawa quality assessment scale cohort studies. University of Ottawa; 2014. https://www.ncbi.nlm.nih.gov/books/NBK115843/bin/appe-fm3.pdf.
- 21.Eysenbach G. Improving the quality of web surveys: the Checklist for reporting results of internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6(3):e34-e. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akdağ A, Erdeve O, Uraş N, Simşek Y, Dilmen U. Hydrops Fetalis due to Kell Alloimmunization: a Perinatal Approach to a rare case. Turk J Haematol. 2012;29(1):72–5. doi: 10.5505/tjh.2012.37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Dughaishi T, Al Harrasi Y, Al-Duhli M, Al-Rubkhi I, Al-Riyami N, Al Riyami A, et al. Red cell alloimmunization to Rhesus Antigen among pregnant women attending a Tertiary Care Hospital in Oman. Oman Med J. 2016;31(1):77–80. doi: 10.5001/omj.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bek SG, Eren N, Uzay A, Bakirdogen S. Rh (D) alloimmunization treated by double filtration plasmapheresis. Transfus Apher Sci. 2019;58(1):83–6. doi: 10.1016/j.transci.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Bennardello F, Curciarello G. Survey on the prevention and incidence of haemolytic disease of the newborn in Italy. Blood Transfus. 2013;11(4):518–27. doi: 10.2450/2013.0179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi SH, Jiang LL, Dai LY, Zheng H, Zhang J, Wang LL, et al. Rh-incompatible hemolytic disease of the newborn in Hefei. World J Clin Cases. 2019;7(20):3202–7. doi: 10.12998/wjcc.v7.i20.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brumbaugh JE, Morgan S, Beck JC, Zantek N, Kearney S, Bendel CM, et al. Blueberry muffin rash, hyperbilirubinemia, and hypoglycemia: a case of hemolytic disease of the fetus and newborn due to anti-kpa. J Perinatol. 2011;31(5):373–6. doi: 10.1038/jp.2010.161. [DOI] [PubMed] [Google Scholar]
- 28.Canlorbe G, Macé G, Cortey A, Cynober E, Castaigne V, Larsen M, et al. Management of very early fetal anemia resulting from red-cell alloimmunization before 20 weeks of gestation. Obstet Gynecol. 2011;118(6):1323–9. doi: 10.1097/AOG.0b013e318235e3bb. [DOI] [PubMed] [Google Scholar]
- 29.Chatziantoniou V, Heeney N, Maggs T, Rozette C, Fountain C, Watts T, et al. A descriptive single-centre experience of the management and outcome of maternal alloantibodies in pregnancy. Transfus Med. 2017;27(4):275–85. doi: 10.1111/tme.12430. [DOI] [PubMed] [Google Scholar]
- 30.Colpo A, Tison T, Gervasi MT, Vio C, Vicarioto M, De Silvestro G, et al. Personalized treatment with immunoadsorption and intravenous immunoglobulin in a case of severe rh alloimmunization during pregnancy unresponsive to plasma - exchange. Transfus Apher Sci. 2017;56(3):480–3. doi: 10.1016/j.transci.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Craparo FJ, Bonati F, Gementi P, Nicolini U. The effects of serial intravascular transfusions in ascitic/hydropic RhD-alloimmunized fetuses. Ultrasound Obstet Gynecol. 2005;25(2):144–8. doi: 10.1002/uog.1833. [DOI] [PubMed] [Google Scholar]
- 32.de Assunção RA, Liao AW, Brizot Mde L, Francisco RP, Zugaib M. Changes in fetal myocardial performance index following intravascular transfusion: preliminary report. J Matern Fetal Neonatal Med. 2016;29(16):2697–702. doi: 10.3109/14767058.2015.1101757. [DOI] [PubMed] [Google Scholar]
- 33.Dubey A, Sonker A, Chaudhary R. Enhancement of antibody Titre and Development of additional red cell alloantibodies following intrauterine transfusion. Indian J Hematol Blood Transfus. 2016;32(1):92–4. doi: 10.1007/s12288-013-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández Alba JJ, León R, González-Macías C, Paz A, Prado F, Moreno LJ, et al. Treatment of D alloimmunization in pregnancy with plasmapheresis and intravenous immune globulin: case report. Transfus Apher Sci. 2014;51(1):70–2. doi: 10.1016/j.transci.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Fox C, Martin W, Somerset DA, Thompson PJ, Kilby MD. Early intraperitoneal transfusion and adjuvant maternal immunoglobulin therapy in the treatment of severe red cell alloimmunization prior to fetal intravascular transfusion. Fetal Diagn Ther. 2008;23(2):159–63. doi: 10.1159/000111599. [DOI] [PubMed] [Google Scholar]
- 36.Gottvall T, Filbey D. Alloimmunization in pregnancy during the years 1992–2005 in the central west region of Sweden. Acta Obstet Gynecol Scand. 2008;87(8):843–8. doi: 10.1080/00016340802268880. [DOI] [PubMed] [Google Scholar]
- 37.Gudlaugsson B, Hjartardottir H, Svansdottir G, Gudmundsdottir G, Kjartansson S, Jonsson T, et al. Rhesus D alloimmunization in pregnancy from 1996 to 2015 in Iceland: a nation-wide population study prior to routine antenatal anti-D prophylaxis. Transfusion. 2020;60(1):175–83. doi: 10.1111/trf.15635. [DOI] [PubMed] [Google Scholar]
- 38.Haider M, Memon S, Tariq F, Fatima S, Hameed A. Rhesus Isoimmunization: late-onset hemolytic disease of the Newborn without Jaundice. Cureus. 2020;12(1):e6559. doi: 10.7759/cureus.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper DC, Swingle HM, Weiner CP, Bonthius DJ, Aylward GP, Widness JA. Long-term neurodevelopmental outcome and brain volume after treatment for hydrops fetalis by in utero intravascular transfusion. Am J Obstet Gynecol. 2006;195(1):192–200. doi: 10.1016/j.ajog.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Hassan MZ, Iberahim S, Abdul Rahman WSW, Zulkafli Z, Bahar R, Ramli M, et al. Severe anti-D haemolytic disease of fetal and newborn in rhesus D negative primigravida. Malays J Pathol. 2019;41(1):55–8. [PubMed] [Google Scholar]
- 41.Karagol BS, Zenciroglu A, Okumus N, Karadag N, Dursun A, Hakan N. Hemolytic disease of the newborn caused by irregular blood subgroup (Kell, C, c, E, and e) incompatibilities: report of 106 cases at a tertiary-care centre. Am J Perinatol. 2012;29(6):449–54. doi: 10.1055/s-0032-1304826. [DOI] [PubMed] [Google Scholar]
- 42.Kriplani A, Malhotra Singh B, Mandal K. Fetal intravenous immunoglobulin therapy in rhesus hemolytic disease. Gynecol Obstet Invest. 2007;63(3):176–80. doi: 10.1159/000097661. [DOI] [PubMed] [Google Scholar]
- 43.Lakhwani S, Machado P, Pecos P, Coloma M, Rebollo S, Raya JM. Kell hemolytic disease of the fetus. Combination treatment with plasmapheresis and intrauterine blood transfusion. Transfus Apher Sci. 2011;45(1):9–11. doi: 10.1016/j.transci.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Levy-Zauberman Y, Mailloux A, Kane A, Castaigne V, Cortey A, Carbonne B. Massive fetomaternal hemorrhage secondary to intrauterine intravascular transfusion. Obstet Gynecol. 2011;118(2 Pt 2):439–42. doi: 10.1097/AOG.0b013e318212f935. [DOI] [PubMed] [Google Scholar]
- 45.Lieberman L, Callum J, Cohen R, Cserti-Gazdewich C, Ladhani NNN, Buckstein J, et al. Impact of red blood cell alloimmunization on fetal and neonatal outcomes: a single center cohort study. Transfusion. 2020;60(11):2537–46. doi: 10.1111/trf.16061. [DOI] [PubMed] [Google Scholar]
- 46.Manoura A, Korakaki E, Hatzidaki E, Saitakis E, Maraka S, Papamastoraki I, et al. Use of recombinant erythropoietin for the management of severe hemolytic disease of the newborn of a K0 phenotype mother. Pediatr Hematol Oncol. 2007;24(1):69–73. doi: 10.1080/08880010601001453. [DOI] [PubMed] [Google Scholar]
- 47.Matijevic R, Grgic O, Klobucar A, Miskovic B. Diagnosis and management of Rh Alloimmunization. Fetal Diagn Ther. 2005;20(5):393–401. doi: 10.1159/000086819. [DOI] [PubMed] [Google Scholar]
- 48.Mayer B, Hinkson L, Hillebrand W, Henrich W, Salama A. Efficacy of Antenatal Intravenous Immunoglobulin Treatment in Pregnancies at High Risk due to alloimmunization to Red Blood cells. Transfus Med Hemotherapy. 2018;45(6):429–36. doi: 10.1159/000490154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meraj B, Mobusher I, Waheed S, Waseem M, Rashid Y. Role of intrauterine blood transfusions in management of Rh-isoimmunized pregnancies. Pakistan J Medical Health Sci. 2015;9:318–21. [Google Scholar]
- 50.Navarro M, Negre S, Matoses ML, Golombek SG, Vento M. Necrotizing enterocolitis following the use of intravenous immunoglobulin for haemolytic disease of the newborn. Acta Paediatr. 2009;98(7):1214–7. doi: 10.1111/j.1651-2227.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 51.Nwogu LC, Moise KJ, Jr, Klein KL, Tint H, Castillo B, Bai Y. Successful management of severe red blood cell alloimmunization in pregnancy with a combination of therapeutic plasma exchange, intravenous immune globulin, and intrauterine transfusion. Transfusion. 2018;58(3):677–84. doi: 10.1111/trf.14453. [DOI] [PubMed] [Google Scholar]
- 52.Palfi M, Hildén JO, Matthiesen L, Selbing A, Berlin G. A case of severe rh (D) alloimmunization treated by intensive plasma exchange and high-dose intravenous immunoglobulin. Transfus Apher Sci. 2006;35(2):131–6. doi: 10.1016/j.transci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Phung TV, Houfflin-Debarge V, Ramdane N, Ghesquière L, Delsalle A, Coulon C, et al. Maternal red blood cell alloimmunization requiring intrauterine transfusion: a comparative study on management and outcome depending on the type of antibody. Transfusion. 2018;58(5):1199–205. doi: 10.1111/trf.14542. [DOI] [PubMed] [Google Scholar]
- 54.Rahimi-Levene N, Chezar J, Yahalom V. Red blood cell alloimmunization prevalence and hemolytic disease of the fetus and newborn in Israel: a retrospective study. Transfusion. 2020;60(11):2684–90. doi: 10.1111/trf.15987. [DOI] [PubMed] [Google Scholar]
- 55.Rahimi-Sharbaf F, Niromanesh S, Talebzadeh Z, Kaveh M, Nayary F. Rh alloimmunization and term delivery. Arch Iran Med. 2007;10(1):111–3. [PubMed] [Google Scholar]
- 56.Rath ME, Smits-Wintjens VE, Lindenburg IT, Brand A, van Kamp IL, Oepkes D, et al. Exchange transfusions and top-up transfusions in neonates with Kell haemolytic disease compared to Rh D haemolytic disease. Vox Sang. 2011;100(3):312–6. doi: 10.1111/j.1423-0410.2010.01408.x. [DOI] [PubMed] [Google Scholar]
- 57.Rath ME, Smits-Wintjens VE, Oepkes D, van Zwet EW, van Kamp IL, Brand A, et al. Thrombocytopenia at birth in neonates with red cell alloimmune haemolytic disease. Vox Sang. 2012;102(3):228–33. doi: 10.1111/j.1423-0410.2011.01539.x. [DOI] [PubMed] [Google Scholar]
- 58.Rath ME, Smits-Wintjens VE, Oepkes D, Walther FJ, Lopriore E. Iron status in infants with alloimmune haemolytic disease in the first three months of life. Vox Sang. 2013;105(4):328–33. doi: 10.1111/vox.12061. [DOI] [PubMed] [Google Scholar]
- 59.Rath ME, Smits-Wintjens VE, Lindenburg IT, Folman CC, Brand A, van Kamp IL, et al. Postnatal outcome in neonates with severe Rhesus c compared to rhesus D hemolytic disease. Transfusion. 2013;53(7):1580–5. doi: 10.1111/j.1537-2995.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- 60.Ree IMC, de Haas M, Middelburg RA, Zwiers C, Oepkes D, van der Bom JG, et al. Predicting anaemia and transfusion dependency in severe alloimmune haemolytic disease of the fetus and newborn in the first 3 months after birth. Br J Haematol. 2019;186(4):565–73. doi: 10.1111/bjh.15962. [DOI] [PubMed] [Google Scholar]
- 61.Ree IMC, Lopriore E, Zwiers C, Böhringer S, Janssen MWM, Oepkes D, et al. Suppression of compensatory erythropoiesis in hemolytic disease of the fetus and newborn due to intrauterine transfusions. Am J Obstet Gynecol. 2020;223(1):119.e1–.e10. doi: 10.1016/j.ajog.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 62.Ree IMC, de Grauw AM, Bekker V, de Haas M, Te Pas AB, Oepkes D, et al. Necrotizing enterocolitis in haemolytic disease of the newborn: a retrospective cohort study. Vox Sang. 2020;115(2):196–201. doi: 10.1111/vox.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruma MS, Moise KJ, Jr, Kim E, Murtha AP, Prutsman WJ, Hassan SS, et al. Combined plasmapheresis and intravenous immune globulin for the treatment of severe maternal red cell alloimmunization. Am J Obstet Gynecol. 2007;196(2):138.e1-6. doi: 10.1016/j.ajog.2006.10.890. [DOI] [PubMed] [Google Scholar]
- 64.Sainio S, Nupponen I, Kuosmanen M, Aitokallio-Tallberg A, Ekholm E, Halmesmäki E, et al. Diagnosis and treatment of severe hemolytic disease of the fetus and newborn: a 10-year nationwide retrospective study. Acta Obstet Gynecol Scand. 2015;94(4):383–90. doi: 10.1111/aogs.12590. [DOI] [PubMed] [Google Scholar]
- 65.Santos MC, Sá C, Gomes SC, Jr, Camacho LA, Moreira ME. The efficacy of the use of intravenous human immunoglobulin in brazilian newborns with rhesus hemolytic disease: a randomized double-blind trial. Transfusion. 2013;53(4):777–82. doi: 10.1111/j.1537-2995.2012.03827.x. [DOI] [PubMed] [Google Scholar]
- 66.Şavkli A, Çetin BA, Acar Z, Özköse Z, Behram M, Çaypinar SS, et al. Perinatal outcomes of intrauterine transfusion for foetal anaemia due to red blood cell alloimmunisation. J Obstet Gynaecol. 2020;40(5):649–53. doi: 10.1080/01443615.2019.1647521. [DOI] [PubMed] [Google Scholar]
- 67.Sikkel E, Klumper FJ, Oepkes D, Teunissen AK, Meerman RH, Le Cessie S, et al. Fetal cardiac contractility before and after intrauterine transfusion. Ultrasound Obstet Gynecol. 2005;26(6):611–7. doi: 10.1002/uog.1996. [DOI] [PubMed] [Google Scholar]
- 68.Simonazzi G, Bernabini D, Curti A, Bisulli M, Pilu G, Brill CB, et al. Fetal cerebellar damage in fetuses with severe anemia undergoing intrauterine transfusions. J Matern Fetal Neonatal Med. 2016;29(3):389–92. doi: 10.3109/14767058.2014.1001973. [DOI] [PubMed] [Google Scholar]
- 69.Somerset DA, Moore A, Whittle MJ, Martin W, Kilby MD. An audit of outcome in intravascular transfusions using the intrahepatic portion of the fetal umbilical vein compared to cordocentesis. Fetal Diagn Ther. 2006;21(3):272–6. doi: 10.1159/000091355. [DOI] [PubMed] [Google Scholar]
- 70.Takcı S, Alarcon-Martinez T, Bozkaya D, Yiğit Ş, Korkmaz A, Yurdakök M. Cholestasis in infants with immune hydrops fetalis. Turk J Pediatr. 2013;55(6):616–9. [PubMed] [Google Scholar]
- 71.Tara F, Maleki A, Taheri N, Moein Darbari S. A case of D alloimmunization in pregnancy: successfully treated solely with therapeutic plasma exchange (TPE) J Blood Med. 2019;10:251–3. doi: 10.2147/JBM.S204128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Temel Yüksel İ, Acar D, Turhan U, Aslan Çetİn B, Köroğlu N, Şenol G, et al. Assessment of fetal right ventricular myocardial performance index changes following intrauterine transfusion. J Maternal-Fetal Neonatal Med. 2021;34(18):3046–9. doi: 10.1080/14767058.2019.1677595. [DOI] [PubMed] [Google Scholar]
- 73.Tiblad E, Kublickas M, Ajne G, Bui TH, Ek S, Karlsson A, et al. Procedure-related complications and perinatal outcome after intrauterine transfusions in red cell alloimmunization in Stockholm. Fetal Diagn Ther. 2011;30(4):266–73. doi: 10.1159/000328683. [DOI] [PubMed] [Google Scholar]
- 74.Urutherakumar V, Welsh A, Henry A. Short-term outcomes following intrauterine transfusions for fetal anaemia: a retrospective cohort study. Aust N Z J Obstet Gynaecol. 2020;60(5):738–45. doi: 10.1111/ajo.13155. [DOI] [PubMed] [Google Scholar]
- 75.van den Akker ES, Klumper FJ, Brand A, Kanhai HH, Oepkes D. Kell alloimmunization in pregnancy: associated with fetal thrombocytopenia? Vox Sang. 2008;95(1):66–9. doi: 10.1111/j.1423-0410.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- 76.Walsh CA, Russell N, McAuliffe FM, Higgins S, Mahony R, Carroll S, et al. Relationship between maternal antibody type and antenatal course following intrauterine transfusion for red cell alloimmunisation. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):235–9. doi: 10.1016/j.ejogrb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Xu W. A case of severe rh (D) alloimmunization pregnant woman delivery an infant with limited treatment. Transfus Apher Sci. 2013;49(2):168–70. doi: 10.1016/j.transci.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 78.Zwiers C, Lindenburg ITM, Klumper FJ, de Haas M, Oepkes D, Van Kamp IL. Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound Obstet Gynecol. 2017;50(2):180–6. doi: 10.1002/uog.17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zwiers C, Oepkes D, Lopriore E, Klumper FJ, de Haas M, van Kamp IL. The near disappearance of fetal hydrops in relation to current state-of-the-art management of red cell alloimmunization. Prenat Diagn. 2018;38(12):943–50. doi: 10.1002/pd.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zwiers C, van der Bom JG, van Kamp IL, van Geloven N, Lopriore E, Smoleniec J, et al. Postponing early intrauterine transfusion with intravenous immunoglobulin treatment; the PETIT study on severe hemolytic disease of the fetus and newborn. Am J Obstet Gynecol. 2018;219(3):291.e1–.e9. doi: 10.1016/j.ajog.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Lindenburg IT, Smits-Wintjens VE, van Klink JM, Verduin E, van Kamp IL, Walther FJ, et al. Long-term neurodevelopmental outcome after intrauterine transfusion for hemolytic disease of the fetus/newborn: the LOTUS study. Am J Obstet Gynecol. 2012;206(2):141.e1-8. doi: 10.1016/j.ajog.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 82.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30(1):28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Natile M, Ventura ML, Colombo M, Bernasconi D, Locatelli A, Plevani C, et al. Short-term respiratory outcomes in late preterm infants. Ital J Pediatr. 2014;40(1):52. doi: 10.1186/1824-7288-40-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitsommart R, Janes M, Mahajan V, Rahman A, Seidlitz W, Wilson J, et al. Outcomes of late-preterm infants: a retrospective, single-center, canadian study. Clin Pediatr (Phila) 2009;48(8):844–50. doi: 10.1177/0009922809340432. [DOI] [PubMed] [Google Scholar]
- 85.Santos IS, Matijasevich A, Silveira MF, Sclowitz IK, Barros AJ, Victora CG, et al. Associated factors and consequences of late preterm births: results from the 2004 Pelotas birth cohort. Paediatr Perinat Epidemiol. 2008;22(4):350–9. doi: 10.1111/j.1365-3016.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- 86.Heinonen K, Eriksson JG, Lahti J, Kajantie E, Pesonen AK, Tuovinen S, et al. Late preterm birth and neurocognitive performance in late adulthood: a birth cohort study. Pediatrics. 2015;135(4):e818-25. doi: 10.1542/peds.2014-3556. [DOI] [PubMed] [Google Scholar]
- 87.Martínez-Nadal S, Bosch L. Cognitive and learning outcomes in late Preterm Infants at School Age: a systematic review. Int J Environ Res Public Health. 2020;18(1):74. doi: 10.3390/ijerph18010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deka D, Dadhwal V, Sharma AK, Shende U, Agarwal S, Agarwal R, et al. Perinatal survival and procedure-related complications after intrauterine transfusion for red cell alloimmunization. Arch Gynecol Obstet. 2016;293(5):967–73. doi: 10.1007/s00404-015-3915-7. [DOI] [PubMed] [Google Scholar]
- 89.van Klink JM, van Veen SJ, Smits-Wintjens VE, Lindenburg IT, Rijken M, Oepkes D, et al. Immunoglobulins in neonates with Rhesus Hemolytic Disease of the Fetus and Newborn: long-term outcome in a Randomized Trial. Fetal Diagn Ther. 2016;39(3):209–13. doi: 10.1159/000434718. [DOI] [PubMed] [Google Scholar]
- 90.van den Akker ES, de Haan TR, Lopriore E, Brand A, Kanhai HH, Oepkes D. Severe fetal thrombocytopenia in Rhesus D alloimmunized pregnancies. Am J Obstet Gynecol. 2008;199(4):387.e1-4. doi: 10.1016/j.ajog.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Smits-Wintjens VE, Rath ME, van Zwet EW, Oepkes D, Brand A, Walther FJ, et al. Neonatal morbidity after exchange transfusion for red cell alloimmune hemolytic disease. Neonatology. 2013;103(2):141–7. doi: 10.1159/000343261. [DOI] [PubMed] [Google Scholar]
- 92.Smits-Wintjens VE, Walther FJ, Rath ME, Lindenburg IT, te Pas AB, Kramer CM, et al. Intravenous immunoglobulin in neonates with rhesus hemolytic disease: a randomized controlled trial. Pediatrics. 2011;127(4):680–6. doi: 10.1542/peds.2010-3242. [DOI] [PubMed] [Google Scholar]
- 93.Ree IMC, Besuden CFJ, Wintjens V, Verweij J, Oepkes D, de Haas M, et al. Exchange transfusions in severe Rh-mediated alloimmune haemolytic disease of the foetus and newborn: a 20-year overview on the incidence, associated risks and outcome. Vox Sang. 2021;116(9):990–7. doi: 10.1111/vox.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koelewijn JM, Vrijkotte TG, van der Schoot CE, Bonsel GJ, de Haas M. Effect of screening for red cell antibodies, other than anti-D, to detect hemolytic disease of the fetus and newborn: a population study in the Netherlands. Transfusion. 2008;48(5):941–52. doi: 10.1111/j.1537-2995.2007.01625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. General Characteristics of Studies and Patient Groups. Table S2. Research Questions Relevant to Mothers and Fetuses. Table S3. Inclusion and Exclusion Criteriaa. Table S4a. Methodologic Quality of Selected Case Reports. Table S4b. Methodologic Quality of Selected Case Series. Table S4c. Methodologic Quality of Selected Retrospective Cohort Studies. Table S4d. Methodologic Quality of Selected Prospective Cohort Studies. Table S4e. Methodologic Quality of Bennardello et al (25). Table S5. Antenatal Treatments Reported in Studies With Representative Data. Table S6. Hydrops Fetalis, Severe Fetal Anemia, and Adverse Events/Complications Reported in Studies With Representative Data. Table S7. IVIG Treatment Response and Associated Mortality. Table S8. Overall Fetal Mortality Associated With HDFN. Figure S1. Mean Rate of Procedure-related Complications After IUT (Per Procedure [A] and Per Fetus [B]).12,26,30,32,43,44,46,49,52,60 CS, cesarean section; IUT, intrauterine transfusion; PROM, preterm rupture of membranes. aAdverse serological outcome was defined as the development of additional maternal antibodies to anti-D and/or a ≥4-fold enhancement of antibody titer.12. Figure S2. Flowchart of the Article Selection Process for the Humanistic and Economic Burden. SLR, systematic literature review. *From authors’ personal library. †From eligible SLRs of cohort studies.
Additional file 2: Appendix S1. Search Strategy. Appendix S2. Search Strategy for the Humanistic and Economic Burden.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.