Abstract
Background
To evaluate the incidence of lymph node degeneration and its association with nodal metastatic pattern in prostate cancer.
Methods
A retrospective analysis of the submitted lymph node specimen of 390 prostatectomies in 2011 was performed. All lymph nodes were histologically re-evaluated and the degree of lymph node degeneration e.g. lipomatous atrophy, capsular and framework fibrosis, and calcifications as well as the lymph node size were recorded. Lymph node degeneration was compared in the anatomic regions of the pelvis as well as in lymph nodes with and without metastases of prostatic cancer.
Results
Eighty-one of 6026 lymph nodes demonstrated metastases. Complete histologic examination with analysis of a complete cross-section was possible in 5173 lymph nodes including all lymph nodes with metastases. The incidence of lymph node degeneration was different across the various landing sites. Lymph node metastases were primarily detected in less degenerative and therefore more functional lymph nodes. In metastatic versus non-metastatic lymph nodes low lipomatous atrophy was reported in 84.0% versus 66.7% (p = 0.004), capsular fibrosis in 14.8% versus 35.4% (p < 0.001), calcifications in 35.8% versus 46.1% (p = 0.072) and framework fibrosis in 69.8% versus 75.3% (p = 0.53). Metastases were also identified more frequently in larger than in smaller lymph nodes (63.0% vs. 47.5%; p = 0.007).
Conclusions
Degenerative changes in pelvic lymph nodes are commonly detectable but occur with variable frequency in the various nodal landing sites in the pelvis. The degree of lymph node degeneration of single lymph nodes has a significant influence on whether a lymph node is infiltrated by tumor cells and may harbour metastases.
Keywords: Pelvic lymph nodes, Degeneration, Prostate carcinoma, Skip metastasis, Lymph node dissection
Background
Lymph node (LN) staging in prostatic cancer (PCa) is an essential tool in the management paradigm. The presence of LN metastasis alters the treatment concept from a local and curative therapy to an adjuvant androgen-deprivation therapy with or without radiotherapy [1, 2]. In addition, there is increasing evidence for positive therapeutic effects of pelvic lymph node dissection (PLND) [3–6]. In radical prostatectomy (RP) the extended pelvic lymph node dissection (ePLND) is recommended by the European Association of Urology (EAU) guidelines for high-risk and intermediate-risk patients with a risk for positive LN over 5% [2]. It provides relevant information for staging and prognosis which cannot be achieved by any imaging method [7]. To date, PLND still represents the most accurate and reliable staging procedure for the detection of LN invasion in PCa [8]. However, the role of PLND and its extensions remains controversial, e.g., because of associated worse intra- and peri-operative outcomes [9].
The concept of sentinel lymph node (SLN) identification and examination in PCa was introduced by Wawroschek et al. in 1999 [10]. Therefore technetium-99m colloid was applied sonographically-guided directly into the prostate 1-day prior RP with PLND. The authors were able to demonstrate a high staging accuracy for LN metastases [11–14] with a low morbidity [15]. Meanwhile, different other techniques provide a high diagnostic accuracy in LN detection, particularly the PSMA-ligand-PET imaging [16].
The presence of skip metastases in prostate cancer has been commonly explained with the multidirectional lymphatic drainage from the prostate with a high individual variability due to anatomical variations [17]. However, degeneration of lymphatic tissue accompanied by hyalinization, fibrosis and lipomatous changes is also suspected to cause skip metastases [18]. Due to these architectural disturbances, which can cause cortical gaps in the LN cortex, the LN might lose its ability to filter malignant cells or microorganisms as a result of changed intranodal homeostasis [19, 20]. The purpose of this study was to investigate the morphological changes of pelvic LN and their effect on the occurrence of metastasis.
Methods
We retrospectively reviewed the dissected LN of 390 patients examined in our institute, who underwent RP with PLND between January and December 2011. The resected tissue of all patients was completely embedded in 4% buffered formalin fixed for histological evaluation to identify all resected LN. The study was approved by the institutional review committee (Witten/Herdecke University, No. 20/2016).
Out of 6026 detected LN, 5173 were histological reevaluated (D.G., C.W. and S.S.) on hematoxylin and eosin stained step sections for degenerative changes such as lipomatous atrophy, capsular or framework fibrosis, and calcifications as a morphological correlate for degenerative changes. Lipomatous atrophy was documented as a percentage of the incised area in stages of ≤ 30%, 31–60%, and ≥ 61%. Changes in framework fibrosis were recorded as absent, low, moderate, or severe. Capsular fibrosis and calcifications were classified as detectable or undetectable. Furthermore, the LN size (greatest diameter in millimeter) as well as the occurrence of metastases was documented. Finally, the extent of degenerative changes and LN size was compared in positive LN (with metastasis) and negative LN (without metastasis) as well for the different pelvic anatomical regions. The variables were described using absolute number and percentages. Analytic statistics was performed using the chi-square test where necessary. Results were reported as statistically significant whenever p < 0.05. The data were analyzed using the Statistics Package for Social Sciences version 25 (SPSS, IMB Corp, Armonk, NY, USA).
Results
In total of 390 male patients aged 44 to 79 years (median: 68, IQR: 62–71 years) were analyzed retrospectively. In 35 patients (3.9%) aged 46 to 79 years a total of 81 metastasis out of 6026 examined LN (1.6%) were found. No age group was preferred (< 60 years: 1.5%, 61–70 years: 1.7%, 71–80 years: 1.6%; p = 0.601). In 15 patients only a single LN metastasis was identified, whereas in 20 patients 2–6 positive LN with metastasis could be detected. The positive LN were located in all pelvic anatomical regions but mostly in the obturator fossa in both sides. Metastasis diameter ranged between 0.5 and 18 mm (median 4, IQR 2–6 mm) occupying between 1 and 90% (median 45.5, IQR 10–70%) of the LN volume. Only six metastases demonstrated transcapsular growth. The metastatic PCa showed a broad range in both the T stage and the Gleason grading (Table 1).
Table 1.
Metastatic prostatic carcinomas—T-Stage (UICC), Gleason Grade, and anatomical localization of metastases
| T-Stage | n (%) | Gleason/ISUP | n (%) | Localization | n (%) |
|---|---|---|---|---|---|
| pT2a | 1 (2.8%) | 3 + 4/2 | 5 (14.2%) | OR | 19 (23.5%) |
| pT2c | 4 (11.4%) | 4 + 3/3 | 8 (22.9%) | IER | 4 (4.9%) |
| pT3a | 6 (17.1%) | 4 + 4/4 | 10 (28.6%) | IIR | 8 (9.9%) |
| pT3b | 23 (65.7%) | 4 + 5/5 | 12 (34.3%) | OL | 16 (19.8%) |
| pT4 | 1 (2.8%) | IEL | 8 (9.9%) | ||
| IIL | 15 (18.5%) | ||||
| EBR/L | 11 (13.5%) |
OR Right obturator fossa, IER right external iliac vessels, IIR right iliac internal vessels, OL left obturator fossa, IEL left iliac external vessels, IIL left iliac internal vessels, EBR/L en bloc right/left without detailed allocation
Degenerative changes in LN structure and size could be detected in all 5173 histologically examined LN. Of them, 47.8% had a minimal diameter of 10 mm, while 52.2% were smaller than 10 mm. Almost all LN showed a lipomatous atrophy in varying degrees (Fig. 1—top left): 67.0% showed a lipomatous remodeling in ≤ 30%, 13.1% in 31–60%, and 19.9% in ≥ 61% respectively. LN with every quantity of lipomatous atrophy were found in all sizes. Most of the LN showed little (23.7%) or no evidence (69.9%) of framework fibrosis with a diffuse expression form the cortex to the medulla with a nodular, later confluent enlargement of the connective tissue. A predominantly sector-shaped fibrosis with widening of the capsule appeared in 35.1% of all LN. Capsular fibrosis was more common with higher grades of framework fibrosis (Fig. 1—top right & bottom right). The association with framework fibrosis was even more pronounced for calcifications (Fig. 1—bottom left): 45.9% of the LN showed calcifications especially those LN with framework fibrosis.
Fig. 1.
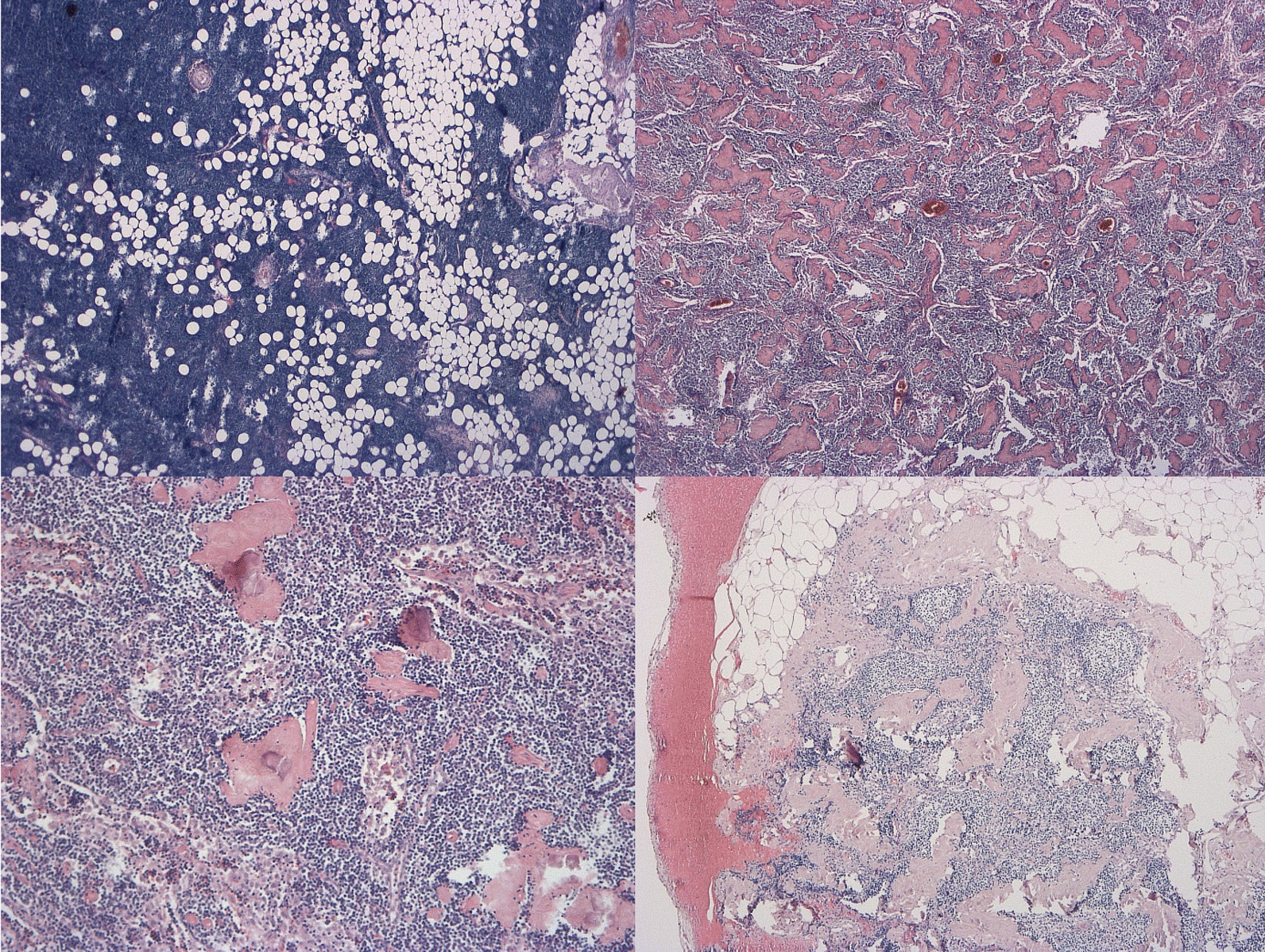
Top left—LN with lipomatous atrophy in 31–60% (H&E, 25 ×), top right—LN with high framework fibrosis (H&E, 25 ×), bottom left—LN with calcifications and moderate framework fibrosis (H&E, 100 ×), bottom right—LN with capsular fibrosis, moderate framework fibrosis and calcifications (H&E, 50 ×)
Although the degenerative changes between LNs of the right and left side (LN size (p = 0.667), lipomatous atrophy (p = 0.143), capsular (p = 0.504) or framework fibrosis (p = 0.552), and calcifications (p = 0.143)) show no significant differences, LN of the different pelvic anatomical regions (internal and external iliac vessels and the obturator fossa) show differently pronounced, patchy, and uneven but partly significant degenerative changes (LN size (p = 0.012), framework fibrosis (p = 0.005), and calcifications (p = 0.032). Details are displayed in Table 2.
Table 2.
Morphological changes in LN in different anatomical regions of the pelvis
| Total 5173 LN | OR | IER | IIR | EBR | OL | IEL | IIL | EBL |
|---|---|---|---|---|---|---|---|---|
| 919 (17.8%) | 667 (12.9%) | 677 (13.1%) | 318 (6.2%) | 882 (17.1%) | 724 (14.0%) | 714 (13.8%) | 272 (5.2%) | |
| LN size | ||||||||
| < 10 mm | 481 (17.8%) | 338 (12.5%) | 363 (13.4%) | 191 (7.0%) | 464 (17.2%) | 386 (14.3%) | 351 (13.0%) | 127 (4.8%) |
| ≥ 10 mm | 438 (17.7%) | 329 (13.3%) | 314 (12.7%) | 127 (5.1%) | 418 (16.9%) | 338 (13.7%) | 363 (14.7%) | 145 (5.9%) |
| p = 0.012 | ||||||||
| Lipomatous atrophy (%) | ||||||||
| ≤ 30 | 607 (17.5%) | 427 (12.3%) | 486 (14.0%) | 242 (7.0%) | 570 (16.5%) | 483 (13.9%) | 464 (13.4%) | 185 (5.4%) |
| 31–60 | 128 (18.9%) | 87 (12.8%) | 85 (12.5%) | 26 (3.8%) | 123 (18.1%) | 100 (14.7%) | 99 (14.6%) | 30 (4.4%) |
| ≥ 61 | 184 (17.8%) | 153 (14.8%) | 106 (10.3%) | 50 (5.0%) | 189 (18.3%) | 141 (13.7%) | 151 (14.6%) | 57 (5.7%) |
| p = 0.011 | ||||||||
| Framework fibrosis | ||||||||
| Non | 637 (17.6%) | 473 (13.1%) | 460 (12.7%) | 229 (6.4%) | 624 (17.3%) | 528 (14.6%) | 487 (13.4%) | 176 (4.9%) |
| Low | 224 (18.3%) | 157 (12.8%) | 171 (14.0%) | 74 (6.1%) | 194 (15.8%) | 145 (11.8%) | 182 (14.9%) | 77 (6.3%) |
| Moderate | 54 (17.5%) | 34 (11.0%) | 41 (13.3%) | 15 (4.8%) | 57 (18.5%) | 48 (15.6%) | 40 (13.2%) | 19 (6.1%) |
| High | 4 (14.8%) | 3 (11.1%) | 5 (18.5%) | 0 (0%) | 7 (25.9%) | 3 (11.1%) | 5 (18.6%) | 0 (0%) |
| p = 0.005 | ||||||||
| Capsular fibrosis | ||||||||
| With | 289 (15.9%) | 243(13.4%) | 264 (14.6%) | 111 (6.1%) | 301 (16.6%) | 254 (14.0%) | 270 (14.9%) | 207 (6.2%) |
| Without | 630 (18.8%) | 424 (12.6%) | 413 (12.3%) | 82 (4.5%) | 581 (17.3%) | 470 (14.0%) | 444 (13.2%) | 190 (5.6%) |
| p = 0.060 | ||||||||
| Calcifications | ||||||||
| With | 426 (17.9%) | 298 (12.5%) | 315 (13.3%) | 129 (5.4%) | 411 (17.3%) | 335 (14.1%) | 334 (14.1%) | 128 (5.4%) |
| Without | 493 (17.6%) | 369 (13.2%) | 362 (12.9%) | 189 (6.7%) | 471 (16.8%) | 389 (13.9%) | 380 (13.6%) | 144 (5.3%) |
| p = 0.032 | ||||||||
No significant differences in the occurrence of LN metastases between right and left side were found (right: 37, left 44; p = 0.262). Concerning the positive lymph nodes, 63.0% measured more than 10 mm in diameter (vs. 47.5% in negative LN) with an overall LN size ranging from 3 to 50 mm in diameter (median 11, IQR 8–17 mm). 9 LN with metastases were less than 5 mm in size. The structural changes of LN architecture in positive LN were less pronounced in comparison with negative LN: 84.0% of LN with metastasis showed only little lipomatous atrophy (vs. 66.7% in negative LN, p = 0.004) and a capsular fibrosis was seen only in 14.8% versus 35.4% respectively (p < 0.001). Although the results for calcifications were not statistically significant, the absolute numbers show a tendency towards less degenerative changes in positive LN. No significant difference could be shown for framework fibrosis (Table 3).
Table 3.
Morphological changes in LN with and without metastases
| Metastasized LN | Non-metastasized LN | |
|---|---|---|
| Lymph node size | ||
| < 10 mm | 30 (37.0%) | 2671 (52.5%) |
| ≥ 10 mm | 51 (63.0%) | 2421 (47.5%) |
| p = 0.007 | ||
| Lipomatous atrophy (%) | ||
| ≤ 30 | 68 (84.0%) | 3396 (66.7%) |
| 31–60 | 7 (8.6%) | 671 (13.2%) |
| ≥ 61 | 6 (7.4%) | 1025 (20.1%) |
| p = 0.004 | ||
| Framework fibrosis | ||
| Non | 61 (75.3%) | 3553 (69.8%) |
| Low | 15 (18.5%) | 1209 (23.7%) |
| Moderate | 4 (4.9%) | 304 (6.0%) |
| High | 1 (1.2%) | 26 (0.5%) |
| p = 0.53 | ||
| Capsular fibrosis | ||
| With | 12 (14.8%) | 1802 (35.4%) |
| Without | 69 (85.2%) | 3290 (64.6%) |
| p < 0.001 | ||
| Calcifications | ||
| With | 29 (35.8%) | 2347 (46.1%) |
| Without | 52 (64.2%) | 2745 (53.9%) |
| p = 0.072 | ||
Discussion
The staging of pelvic LN in PCa allows a statement with regards to prognosis and treatment options and therefore LN dissection in PCa is recommended for intermediate and high-risk patients [21]. Lymphatic drainage from the prostate has high individual variability, and direct drainage outside the pelvic area is observed rarely [17, 22]. Despite advances in imaging modalities the gold standard in LN is the histological evaluation of an ePLND [23, 24]. Due to a up to three-fold higher complication rates compared with a limited pelvic LN dissection [25] the role of ePLND remains controversial, especially since PLND failed to improve oncological outcomes, including survival [9].
Because of prostate specific antigen relapse, symptomatic progression and tumor related death are significantly affected by the number of positive LN [6, 26, 27] the detection rate for positive LN must be optimized. To achieve this goal, a better understanding of the lymphogenic metastatic behavior of PCa in pelvic LN is required. The present series is the first histological analysis based on a large cohort that focuses on degenerative changes in pelvic LN in connection with pelvic LN localization and the occurrence of metastases in PCa. Our histological evaluation revealed significant morphological differences between pelvic LN in different anatomical localizations as well in LN with or without metastases of prostatic cancer.
Changes in LN morphology have been described in connection with the aging process as well as with chronic inflammatory diseases. Pan et al. [28] proposed a generating-degenerating circle of LN affecting all compartments including the lymphatic tissue, the medulla, and the architecture. Sato et al. described similar changes in LN of older patients with formation of gaps and fragmentation of the superficial cortex. These degenerative changes are less pronounced in central like gastric LN than in peripheral like para-aortic and pelvic LN [19]. Furthermore, lipomatous atrophy is described in other studies as a basin-specific degenerative change of peripheral LN usually subjected to little antigenic stimulation [18, 20]. We were able to prove, that degenerative changes in pelvic LN are always detectable in older male patients but occur inconsistently within the different anatomical locations of the pelvis. These findings suggest the idea that degenerative changes may be somewhat random in their occurrence. The grade of the degenerative changes is associated with the anatomical localization of the LN and may be caused by its exposure to more or less antigenic stimulation. Degenerative changes may have an effect on the metastatic pattern found in lymphadenectomy specimens [28] and are possibly caused by the altered immune status in the elderly [20, 29, 30].
Conclusions
Degenerative changes in pelvic LN are commonly detectable but occur with variable frequency in the various nodal landing sites in the pelvis. We were able to demonstrate that metastases are predominantly detected in larger and less degeneratively altered LN in the pelvis. The degree of LN degeneration of single LN has a significant influence on whether a LN is infiltrated by tumor cells and may harbour metastases. This, in addition to multidirectional lymphatic drainage, is an additional explanation for the occurrence of skip metastases in prostate cancer, resulting in an unpredictable metastasis pattern.
Acknowledgements
Not applicable.
Abbreviations
- LN
Lymph node
- PCa
Prostatic cancer
- PLND
Pelvic lymph node dissection
- RP
Radical prostatectomy
- ePLND
Extended pelvic lymph node dissection
- EAU
European Association of Urology
- SLN
Sentinel lymph node
- IQR
Interquartile range
- OR
Right obturator fossa
- IER
Right external iliac vessels
- IIR
Right iliac internal vessels
- EBR
En bloc right
- OL
Left obturator fossa
- IEL
Left iliac external vessels
- IIL
Left iliac internal vessels
- EBL
En bloc left
Author contributions
Study concept: DG, SD, SS. Design: DG, SD, FCR, SS. Data Collection: DG, CW, RK, SS. Data analysis: KG, DG, CW, NMD, SS. Manuscript draft: DG, SD. Revised the manuscript: SS, SK, HMK, FCR. All authors have read and approved this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and statement of informed consent
The study was conducted according to the Declaration of Helsinki, and approved by the ethics commission of the Witten/Herdecke University (Witten/Herdecke University, ref. No. 20/2016). All patients were treated in academic hospitals. Upon admission to the hospital, all patients were asked to give their general consent for the scientific analysis of disease-specific data (except for genetic analyses) in anonymized form. Patients also had the opportunity to refuse their consent. Therefore, the ethics committee of the University of Witten/Herdecke approved the present study without renewed patient consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial or commercial interests related to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel Gödde and Stephan Degener have equally contributed to this work.
References
- 1.Jegadeesh N, Liu Y, Zhang C, Zhong J, Cassidy RJ, Gillespie T, et al. The role of adjuvant radiotherapy in pathologically lymph node-positive prostate cancer. Cancer. 2017;123(3):512–520. doi: 10.1002/cncr.30373. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Choo MS, Kim M, Ku JH, Kwak C, Kim HH, Jeong CW. Extended versus standard pelvic lymph node dissection in radical prostatectomy on oncological and functional outcomes: a systematic review and meta-analysis. Ann Surg Oncol. 2017;24(7):2047–2054. doi: 10.1245/s10434-017-5822-6. [DOI] [PubMed] [Google Scholar]
- 4.Withrow DR, DeGroot JM, Siemens DR, Groome PA. Therapeutic value of lymph node dissection at radical prostatectomy: a population-based case-cohort study. BJU Int. 2011;108(2):209–216. doi: 10.1111/j.1464-410X.2010.09805.x. [DOI] [PubMed] [Google Scholar]
- 5.Winter A, Henke RP, Wawroschek F. Targeted salvage lymphadenectomy in patients treated with radical prostatectomy with biochemical recurrence: complete biochemical response without adjuvant therapy in patients with low volume lymph node recurrence over a long-term follow-up. BMC Urol. 2015;15:10. doi: 10.1186/s12894-015-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiler R, Studer UE, Tschan K, Bader P, Burkhard FC. Removal of limited nodal disease in patients undergoing radical prostatectomy: long-term results confirm a chance for cure. J Urol. 2014;191(5):1280–1285. doi: 10.1016/j.juro.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Briganti A, Blute ML, Eastham JH, Graefen M, Heidenreich A, Karnes JR, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55(6):1251–1265. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Fossati N, Willemse PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017;72(1):84–109. doi: 10.1016/j.eururo.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Wawroschek F, Vogt H, Weckermann D, Wagner T, Harzmann R. The sentinel lymph node concept in prostate cancer—first results of gamma probe-guided sentinel lymph node identification. Eur Urol. 1999;36(6):595–600. doi: 10.1159/000020054. [DOI] [PubMed] [Google Scholar]
- 11.Winter A, Kneib T, Henke RP, Wawroschek F. Sentinel lymph node dissection in more than 1200 prostate cancer cases: rate and prediction of lymph node involvement depending on preoperative tumor characteristics. Int J Urol: Off J Jpn Urol Assoc. 2014;21(1):58–63. doi: 10.1111/iju.12184. [DOI] [PubMed] [Google Scholar]
- 12.Holl G, Dorn R, Wengenmair H, Weckermann D, Sciuk J. Validation of sentinel lymph node dissection in prostate cancer: experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging. 2009;36(9):1377–1382. doi: 10.1007/s00259-009-1157-2. [DOI] [PubMed] [Google Scholar]
- 13.Winter A, Kneib T, Wasylow C, Reinhardt L, Henke RP, Engels S, et al. Updated nomogram incorporating percentage of positive cores to predict probability of lymph node invasion in prostate cancer patients undergoing sentinel lymph node dissection. J Cancer. 2017;8(14):2692–2698. doi: 10.7150/jca.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wit EMK, Acar C, Grivas N, Yuan C, Horenblas S, Liedberg F, et al. Sentinel node procedure in prostate cancer: a systematic review to assess diagnostic accuracy. Eur Urol. 2017;71(4):596–605. doi: 10.1016/j.eururo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Grivas N, Wit E, Pos F, de Jong J, Vegt E, Bex A, et al. Sentinel Lymph node dissection to select clinically node-negative prostate cancer patients for pelvic radiation therapy: effect on biochemical recurrence and systemic progression. Int J Radiat Oncol Biol Phys. 2017;97(2):347–354. doi: 10.1016/j.ijrobp.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Muteganya R, Goldman S, Aoun F, Roumeguere T, Albisinni S. Current imaging techniques for lymph node staging in prostate cancer: a review. Front Surg. 2018;5:74. doi: 10.3389/fsurg.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bonilla-Damia A, Roberto Brouwer O, Meinhardt W, Valdes-Olmos RA. Lymphatic drainage in prostate carcinoma assessed by lymphoscintigraphy and SPECT/CT: its importance for the sentinel node procedure. Rev Esp Med Nucl Imagen Mol. 2012;31(2):66–70. doi: 10.1016/j.remn.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi O, McCall JL, Stringer MD. Does senescence affect lymph node number and morphology? A systematic review. ANZ J Surg. 2013;83(9):612–618. doi: 10.1111/ans.12067. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, Taniguchi I, Fujiwara D, Ichikawa H, Suzuki M, Nawata S, et al. Gaps and fragmentation of the superficial cortex in the abdominal and pelvic lymph nodes of elderly Japanese. Anat Sci Int. 2003;78(4):211–222. doi: 10.1046/j.0022-7722.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 20.Luscieti P, Hubschmid T, Cottier H, Hess MW, Sobin LH. Human lymph node morphology as a function of age and site. J Clin Pathol. 1980;33(5):454–461. doi: 10.1136/jcp.33.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winter A, Engels S, Reinhardt L, Wasylow C, Gerullis H, Wawroschek F. Magnetic marking and intraoperative detection of primary draining lymph nodes in high-risk prostate cancer using superparamagnetic iron oxide nanoparticles: additional diagnostic value. Molecules. 2017;22(12):2192. doi: 10.3390/molecules22122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JJ, Zhu ZS, Zhu YY, Shi HQ. Applied anatomy of pelvic lymph nodes and its clinical significance for prostate cancer: a single-center cadaveric study. BMC Cancer. 2020;20(1):330. doi: 10.1186/s12885-020-06833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prendeville S, van der Kwast TH. Lymph node staging in prostate cancer: perspective for the pathologist. J Clin Pathol. 2016;69(12):1039–1045. doi: 10.1136/jclinpath-2016-203643. [DOI] [PubMed] [Google Scholar]
- 24.Berney DM, Wheeler TM, Grignon DJ, Epstein JI, Griffiths DF, Humphrey PA, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 4: seminal vesicles and lymph nodes. Mod Pathol. 2011;24(1):39–47. doi: 10.1038/modpathol.2010.160. [DOI] [PubMed] [Google Scholar]
- 25.Briganti A, Chun FK, Salonia A, Suardi N, Gallina A, Da Pozzo LF, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol. 2006;50(5):1006–1013. doi: 10.1016/j.eururo.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003;169(3):849–854. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 27.Briganti A, Karnes JR, Da Pozzo LF, Cozzarini C, Gallina A, Suardi N, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol. 2009;55(2):261–270. doi: 10.1016/j.eururo.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 28.Pan WR, Suami H, Taylor GI. Senile changes in human lymph nodes. Lymphat Res Biol. 2008;6(2):77–83. doi: 10.1089/lrb.2007.1023. [DOI] [PubMed] [Google Scholar]
- 29.Pahlavani MA, Vargas DM, Guo Z, Richardson A. Normal immune function in young and old DNA polymerase-beta deficient mice. Immunol Lett. 2000;72(1):17–21. doi: 10.1016/S0165-2478(00)00159-0. [DOI] [PubMed] [Google Scholar]
- 30.Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001;26(7):608–612. doi: 10.1046/j.1365-2230.2001.00898.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


