Abstract
Hand, foot, and mouth disease (HFMD) is a common infectious disease in children. Enterovirus A71 (EV-A71) is one of the main pathogens, and coxsackievirus A6 (CVA6) has gradually become the dominant pathogen of HFMD in recent years. This study was conducted mainly to assess the serological prevalence of EV-A71 and CVA6 antibodies in people of different ages, sexes, and regions through a systematic review and meta-analysis. A comprehensive study was performed based on the EV-A71 and CVA6 serological literature published before May 2022. Heterogeneity analysis (Cochrane's Q test and the I2 statistic) and random effect models were adopted. Subgroup and meta-regression analyses were used to identify potential sources of heterogeneity in the data, and all analysis was performed using STATA version 16.0. This study included 71 studies involving 55,176 people from 13 countries that met the inclusion criteria. The serological prevalence of EV-A71 antibody in different studies was 4.31-88.8%, and that of CVA6 antibody was 40.8-80.9%. Meta-analysis results showed that the serum positive rate for EV-A71 antibody was 45.9% (95% CI: 37.6-54.1%). The rate in the Chinese population was 47.8% (95% CI: 42.4-53.2%), and in the other countries, it was 38% (95% CI: 23-55%). The serum positive rate for CVA6 antibody was 58.3% (95% CI: 46.5-70.2%). The rate in the Chinese population was 49.1% (95% CI: 38.3-59.9%), and in the other countries, it was 68% (95% CI: 51-83%). Subgroup analysis was also conducted. The seroprevalence of EV-A71 and CVA6 antibodies is related to age rather than gender or region. The rates of EV-A71 and CVA6 seropositivity are considerably lower in children younger than five years of age. However, the rates gradually increase with age. The findings of this study suggest that children under five years of age may be susceptible to EV-A71 and CVA6. Thus, safety education and vaccination should be strengthened accordingly. This study provides a basis for understanding the risk factors for EV-A71 and CVA6 infection in China and for deciding how to formulate standard preventive measures to prevent the spread of the virus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00705-022-05642-0.
Introduction
Hand, foot, and mouth disease (HFMD) is a common infectious disease caused by a variety of enteroviruses that mainly infects infants [1]. Most patients have mild symptoms, with fever and rash or herpes on the hands, feet, and mouth as the main clinical signs [2]. Aseptic meningitis, encephalitis, acute flaccid paralysis, neurogenic pulmonary oedema, and myocarditis may occur in a small number of patients, and the disease progresses rapidly in some children with severe symptoms, which may lead to death [3]. HFMD is an important public health problem that endangers children's health worldwide. Every year, the occurrence of HFMD in children results in economic losses for many families. In 2008, the disease was listed as a class C infectious disease by the Ministry of Health of China [4].
HFMD can be caused by a variety of enteroviruses [2]. Before 2012, enterovirus A71 (EV-A71) and coxsackievirus A type 16 (CVA16) were the most common pathogens [5]. Since 2013, the proportion of HFMD caused by CVA6 infection has increased significantly, and CVA6 has gradually become the dominant pathogen of HFMD in many countries worldwide [6].
EV-A71 virus particles are icosahedral with a diameter of 24-30 nm and contain a single plus-stranded RNA [7]. Since the virus was first reported in the 1970s, epidemics of EV-A71 have been reported in many countries and regions [8, 9]. Humans are the only natural hosts of EV-A71. The main sources of EV-A71 infection are patients and asymptomatic carriers of the virus. Most infected people are under 10 years old, with infection being most common in children under 5 years old, but adult cases have occasionally been reported [1].
CVA6 is one of the serotypes of enterovirus coxsackievirus group A [10]. Previous studies have shown that CVA6 was mostly associated with the occurrence of herpetic pharyngitis. Since the outbreak of HFMD caused by CVA6 in Finland was first reported by Osterback et al. in 2009, attention has been given to HFMD caused by CVA6 worldwide [10]. Analysis of the epidemiology and aetiology of HFMD in China from 2013 to 2017 showed that CVA6 has become the dominant pathogen of HFMD in most regions of China [11]. The epidemiology of CVA6 has also been reported in some countries in recent years. However, due to the lack of continuous and systematic monitoring data for CVA6, the current incidence of CVA6 does not accurately reflect the true prevalence of CVA6. There is currently no effective vaccine against the virus, and targeted treatment is mainly achieved via pharmacotherapy. Therefore, it is of great importance to understand the characteristics of the seroepidemiological distribution of CVA6 for the formulation of intervention strategies for HFMD-susceptible populations.
Because HFMD infections can range from asymptomatic to fatal, despite surveillance efforts in numerous places, and given the large number of asymptomatic or subclinical cases, the actual number of people exposed or infected has been underestimated [12]. Serological screening is an important adjunct to PCR-based detection/diagnosis as well as an important means of assessing the cumulative rate of HFMD-associated virus infection. Such screening provides insight into the dynamic monitoring of specific antibody responses during and after transmission of the virus and can inform health authorities and policy-makers about seroprevalence at specific stages of an outbreak. The prevalence of specific serum antibodies to EV-A17 and CVA6 can provide reliable indicators of population exposure to the associated viruses and even indicate the immune status of an individual or population.
Serological analysis can help determine the susceptibility and immunity of people of different ages, sexes, and races, providing a reference and a theoretical basis for the prevention of virus-induced diseases as well as the implementation of immunization protocols. In previous serological studies of EV-A17 and CVA6, seropositivity rates have varied widely. In addition, due to differences in serological methods, sample sizes and regions among these studies, it is not possible to accurately assess the serological prevalence of EV-A17 and CVA6 infection. In this study, published studies on the seroepidemiology of EV-A71 and CVA6 antibodies were comprehensively collected and analysed. Accordingly, this study attempts to predict the immune dynamics of susceptible and general populations based on serological data to provide a theoretical basis for the prevention of virus-related diseases. It is hoped that this study can provide other researchers with reliable estimates of the true transmission rates of EV-A71 and CVA6, accurately assess the possible risk factors for the transmission of EV-A71 and CVA6, and provide a theoretical basis for virus prevention and control.
Methods
Search strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Supplementary Table S3) [13]. During literature retrieval, the following four databases were utilized: China National Knowledge Infrastructure (CNKI), WanFang, PubMed, and the Web of Science (Supplementary Tables S1 and S2). Serology-related retrospective or cross-sectional studies of EV-A71 and CVA6 published before May 2022 were systematically searched in the corresponding databases. The following subject words or free words were used for the search: TS = (HFMD OR Hand, Foot, Mouth Disease) AND TS = (Coxsackievirus A6/Enterovirus 71) AND TS = (Seroprevalence OR Seroepidemiological Study). To avoid omissions, each database was also searched manually to ensure the integrity of the analysis.
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were considered in the assessment of titles, abstracts, and texts of relevant literature. The inclusion criteria were as follows: (1) the research paper was published before May 2022; (2) the positive rate for EV-A71 or CVA6 antibodies was obtained accurately via direct or indirect methods; and (3) the study was a cross-sectional or retrospective study. The exclusion criteria were as follows: (1) the study was a review or conference paper and (2) the data were incomplete or contained obvious errors.
Literature screening and data extraction
By reading the corresponding studies, valid information from each study, including first author, sampling time, publication date, investigation area, sample size, test method, threshold for defining seropositivity, and age of the study population, was extracted into standardized tables. Seropositivity was defined as the proportion of people who tested positive for EV-A71 or CVA6 antibodies in the total population who provided blood samples. For statistical information, we preclassified sampling methods. “Random sampling” refers to random selection of research samples. “Conditional sampling” refers to selection of samples meeting certain criteria or the use of residual serum samples collected for other research purposes. “Physical examination” refers to selection of serum samples based on physical examination. Two evaluators (SY and CP) preliminarily screened the titles and abstracts of the studies and excluded obviously irrelevant studies independently. Two researchers (BY and XX) further read the full texts, determined the final inclusion results, independently screened the valid studies, and extracted and verified the results independently. When two investigators disagreed on the data extraction, a third investigator (LY) was involved in the data extraction and decision-making (Table 1).
Table 1.
Basic information about the literature included in this analysis
| First author | Sampling year | Publication year | Country | Sample size | No. positive | Assay method | Positive threshold | Age range | Group factors | Language | AHRQ score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CVA6 | |||||||||||
| Li Wei Ang | 2008-2010 | 2015 | Singapore | 700 | 439 | Neutralization test | ≥1:8 | 1-17y | Age | ENG | 7 |
| FanGao | 2012 | 2016 | China | 180 | 75 | CPE | ≥1:8 | 6-35m | Age | ENG | 7 |
| Rui Zhu | 2016 | 2018 | China | 515 | 203 | Microneutralization test | ≥ 1:16 | 5m-83y | Age | ENG | 8 |
| Fan Gao | 2012-2014 | 2018 | China | 181 | 74 | Neutralization test | ≥1:8 | 6-35m | Age | CHN | 8 |
| Dan Song | 2016~2019 | 2020 | China | 171 | 107 | Neutralization test | ≥1:8 | All ages | Age | CHN | 8 |
| C. Q. Hoang | 2014 | 2020 | Southern Vietnam | 336 | 106 | Microneutralization test | ≥1:8 | All ages | Age | ENG | 7 |
| Fan Gao | 2018 | 2021 | China | 488 | 298 | Microneutralization test | ≥1:8 | 2-83m | Age | ENG | 7 |
| Everlyn Kamau | 2006, 2011, 2017 | 2021 | United Kingdom | 1508 | 1220 | Microneutralization test | ≥ 1:16 | All ages | Age | ENG | 7 |
| EV-A71 | |||||||||||
| Ceyla M.O. CASTRO | 1998-2001 | 2005 | Brazil | 389 | 222 | Neutralization test | 1:8 | 0-15y | Age | ENG | 7 |
| Fan Gao | 2018 | 2021 | China | 401 | 127 | Neutralization test | 1:8 | 2-83 m | Age | ENG | 7 |
| Chun-Yi Lu | 1994-1999 | 2002 | China | 1705 | 740 | Microneutralization assay | 1:8 | All ages | Age, year | ENG | 8 |
| Luan-Yin Chang | 1992 | 2002 | China | 539 | 242 | Neutralizing antibody test | ≥8 | All ages | Age | ENG | 9 |
| Shili Zhou | 1999- 2003 | 2007 | China | 584 | 207 | ELISA | OD450 | All ages | Age | CHN | 7 |
| Xuebin Guo | 2005 | 2009 | China | 371 | 164 | Neutralization test | ≥8 | 1-6y | Age | CHN | 7 |
| Shu-Ting Luo | 2006- 2008 | 2009 | China | 618 | 158 | Neutralization test | ≥8 | 0-6 m | Age | ENG | 7 |
| MAO Qun-ying | 2007-2009 | G | China | 399 | 159 | Neutralization test | ≥8 | 0-6 m | Age | ENG | 8 |
| Dongxiao Zhang | 2011 | 2011 | China | 382 | 257 | ELISA | ≥0.16 | 0-59y | Age | CHN | 6 |
| Shengcang Zhao | 2009 | 2011 | China | 181 | 68 | Neutralization test | ≥8 | 1-6y | Age | CHN | 6 |
| Ruiling Guo | 2009 | 2011 | China | 856 | 546 | ELISA | OD450 | 0-15y | Age, sex | CHN | 6 |
| Qiang Ding | 2010 | 2011 | China | 420 | 178 | Neutralization test | ≥8 | 0-15y | Age | CHN | 6 |
| Lu Kuang | 2010 | 2011 | China | 819 | 239 | ELISA | OD450/630 | 0-14y | Age | CHN | 7 |
| Haiyang Yu | 2006–2007,2010 | 2011 | China | 472 | 247 | Microneutralization assay | ≥8 | 0-15y | Age, year | ENG | 7 |
| Hongbin Hou | 2010 | 2012 | China | 436 | 152 | ELISA | OD450 | 1m-28y | Age, sex | CHN | 7 |
| Jingmei Li | 2012 | 2012 | China | 528 | 241 | ELISA | A450 | 0-5y | Age | CHN | 6 |
| Huiling Deng | 2010 | 2012 | China | 312 | 108 | ELISA | A450 | 1-4y | Age | CHN | 6 |
| Feng-Cai Zhu | 2007 | 2012 | China | 715 | 404 | Neutralization test | ≥8 | 0-38 m | Age | ENG | 7 |
| Mei Zeng | 2010~2011 | 2012 | China | 614 | 122 | Microneutralization assay | ≥8 | 0-5y | Age | ENG | 7 |
| Hong Ji | 2010 | 2012 | China | 680 | 233 | Microneutralization assay | ≥8 | 0-15y | Age | ENG | 7 |
| Wen-Chan Huang | 2006~2007 | 2012 | China | 228 | 36 | Neutralization test | ≥8 | 2-5y | Age | ENG | 7 |
| Hongxia Ni | 2011 | 2012 | China | 258 | 138 | Neutralization test | ≥8 | All ages | Age, region | ENG | 7 |
| Menghua Xu | 2010-2011 | 2012 | China | 201 | 111 | Microneutralization assay | CPE | All ages | Age | CHN | 8 |
| Miaosen Cai | 2012 | 2013 | China | 240 | 106 | ELISA | ≥0.16 | 3m-62y | Age | CHN | 6 |
| Wei Li | 2007~2009,2010 | 2013 | China | 1458 | 742 | Neutralization test | ≥8 | 1-9y | Age, year | ENG | 9 |
| Wenguo Xu | 2006 | 2013 | China | 252 | 83 | Neutralizing antibody assay | ≥8 | 1-5y | Age | CHN | 7 |
| Xiaoqin Chen | 2010 | 2013 | China | 420 | 192 | Neutralizing antibody assay | ≥8 | 0-15y | Age | CHN | 8 |
| Ying Xiong | 2010 | 2013 | China | 1144 | 855 | Neutralizing antibody assay | ≥8 | all ages | Age | CHN | 8 |
| Xiang Wang | 2012 | 2014 | China | 391 | 335 | Neutralization tests | ≥10 | 18-71y | Age, gender, region | ENG | 9 |
| Yuling Xu | 2010-2012 | 2014 | China | 254 | 175 | Neutralizing antibody assay | ≥8 | 0-6y | Age | CHN | 8 |
| Juanjuan Gui | 2008~2012 | 2015 | China | 549 | 274 | Neutralization test | ≥8 | 0-20y | Age, year | ENG | 9 |
| Xiaoming Tu | 2010 | 2015 | China | 420 | 169 | ELISA | S/N≥2.1 | 0-20y | Age, gender, region | ENG | 9 |
| Fan Gao | 2012 | 2016 | China | 180 | 75 | Cytopathogenic effect (CPE) method | CPE | 6-35m | year | ENG | 6 |
| Paul F. Horwood | 2000-2011 | 2016 | China | 1707 | 1516 | Microneutralization assay | ≥1:8 | 2–15y | Age, year, region | ENG | 8 |
| Jian-xing Wang | 2010 | 2016 | China | 1378 | 1030 | Microneutralization test | ≥1:8 | All ages | Age, gender, region | ENG | 8 |
| Chuanxi Fu | 2013~2014 | 2016 | China | 224 | 185 | Modified cytopathogenic effect assay | CPE | mother-infant | Age | ENG | 7 |
| Xingui Tian | 2014 | 2016 | China | 96 | 71 | Neutralization tests | ≥1:16 | 20–49y | Age, gender | ENG | 7 |
| Dingmei Zhang | 2014-2015 | 2017 | China | 197 | 117 | Microneutralization test | ≥1:8 | 1–5y | Age | ENG | 7 |
| Jiayu Wang | 2014-2016 | 2018 | China | 1230 | 621 | Microneutralization test | ≥1:8 | 0–18y | Age, gender | ENG | 8 |
| Rui Zhu | 2016 | 2018 | China | 515 | 194 | Microneutralization test | ≥1:16 | 5m-83y | Age, district | ENG | 9 |
| Xianglin Wei | 2013-2018 | 2021 | China | 1066 | 705 | Neutralization assays | ≥1:16 | 2-36m | Age | ENG | 9 |
| Qunying Mao | 2004 | 2009 | China | 349 | 128 | Micro-CPE method | ≥1:8 | 7-30m | Age | CHN | 8 |
| Zhen Zhu | 2005 | 2010 | China | 900 | 288 | Neutralization assays | ≥1:8 | 5y | District | ENG | 8 |
| Jiameng Li | 2009 2010 | 2011 | China | 1611 | 1076 | Neutralization assays | ≥ 1: 4 | 0-50y | Age | CHN | 8 |
| Wen Zhu | 2011 | 2013 | China | 93 | 54 | Microneutralization test | ≥ 1: 8 | 0-8y | Age, gender | CHN | 8 |
| Hanna Honkanen | 1994-2010 | 2013 | Finland | 5686 | 2 | Neutralization assays | CPE | <11 | Age | ENG | 6 |
| Sabine Diedrich | 1997-2007 | 2009 | Germany | 436 | 263 |
Neutralization test |
≥ 1: 4 | 10m-75y | Age | ENG | 7 |
| Holger F | 2006 | 2010 | Germany | 696 | 30 | Microneutralization test | ≥1:10 | ≥1y | Age | ENG | 8 |
| Mahsa Javadi | 2015 | 2021 | Iran | 547 | 310 | Neutralization assay | ≥1:16 | All ages | Age | ENG | 8 |
| Sol Kim | 2013-2018 | 2020 | Korea | 220 | 141 | Neutralization test | ≥1: 4 | 7m-15y | Age | ENG | 8 |
| NMN NikNadia | 1995~2012 | 2016 | Malaysia | 1425 | 932 | Neutralization test | ≥1: 8 | 1-12y | Age, year | ENG | 8 |
| Jing Li | 2017 | 2019 | China | 6513 | 4015 | qRT-PCR | - | All ages | Age, year | ENG | 8 |
| Sabine M.G. van der Sanden | 2010~2014 | 2016 | Netherlands | 122 | 50 | Neutralization test | ≥1:16 | ≥5 y | Age, year | ENG | 8 |
| Ludmila V. Akhmadishina | 2007~2008 | 2014 | Russia | 826 | 241 | Neutralization test | ≥1:8 | 1-5y | Age | ENG | 8 |
| Eng-Eong Ooi | 1996-1997 | 2002 | Singapore | 856 | 297 | Neutralization test | ≥1:8 | ≥12 y | Age | ENG | 8 |
| Li-Wei Ang | 2008-2010 | 2011 | Singapore | 1883 | 526 | Neutralization assay | ≥ 8 | 1-17y | Age, gender | ENG | 8 |
| C. Q. Hoang | 2014 | 2020 | Southern Vietnam | 84 | 40 | Neutralization test | ≥1:8 | All ages | Age | ENG | 7 |
| Huang | 2006-2007 | 2012 | China (Taiwan) | 228 | 36 | Neutralization test | ≥1:8 | 2-7y | Age, year | ENG | 7 |
| Fang-Lin Kuo | 2012-2013 | 2020 | China (Taiwan) | 553 | 301 | Neutralization test | ≥1:8 | All ages | Age | ENG | 7 |
| Piyada Linsuwanon | 2009-2012 | 2014 | Thailand | 1050 | 92 | ELISA | ≥1:8 | 0-54y | Age | ENG | 7 |
| Hatairat Lerdsamran | 2013 | 2018 | Thailand | 579 | 285 | Microneutralization assay | ≥1:10 | 0-60y | Age, year | ENG | 9 |
| Jiratchaya Puenpa | 2015-2020 | 2020 | Thailand | 100 | 19 | Microneutralization assay | ≥1:16 | 0-4y | Age, year | ENG | 9 |
| Everlyn Kamau | 2006,2011,2017 | 2021 | UK | 1558 | 1152 | Neutralization Assays | ≥1:8 | All ages | Age, year | ENG | 9 |
| Chau Bich Nguyen Tran | 2006-2007 | 2011 | Viet Nam | 1035 | 116 | Neutralization assay | CPE | 0-38y | Age | ENG | 7 |
m, month; y, year; ENG, English; CHN, Chinese
Quality assessment
In the literature quality evaluation, a cross-sectional study was conducted with reference to the evaluation criteria of the Agency for Healthcare Research and Quality (AHRQ). The standard includes a total of 11 items, and the literature was evaluated against this standard. The evaluation result was “yes” (score 1 point), “no” (score 0 point), or “unclear” (scored 0 point)”, and the scores of each study were successively sorted. Scores of 0-3, 4-7, and 8-11 represented low-, medium-, and high-quality studies, respectively. In this study we used the Cochrane Risk of Bias Assessment tool. In the Cochrane Systematic review, risk of bias was assessed independently and repeatedly by two authors (SY and CP), both of which have a good understanding of the methodology. If the results of the two evaluations were different, a third author (LY) was consulted if there was still a difference of opinion after consultation.
Statistical analysis
STATA 16.0 was used for statistical processing. Descriptive analysis and single-group variable analysis were used. For data that did not conform to a normal distribution, the Freeman-Tukey double arcsine method was used for data conversion, whereas transformed data were used for the meta-analysis. Cochran’s Q test was used to analyse the heterogeneity among the included results, and I2 was used to judge heterogeneity quantitatively. If the results of Cochran's Q test were P < 0.1 and I2 ≤ 50 %, the fixed-effects model was chosen for the meta-analysis. Conversely, if statistical heterogeneity was noted between the results, the random-effects model was chosen. Seropositivity rates and 95% confidence intervals (CIs) were then calculated for the different subgroups. The relevant risk factors assessed in this study also included sampling methods, sample collection season, age, sex, and language. In the sensitivity analysis, the stability of the meta-analysis results was evaluated using a case-by-case exclusion study. Publication bias was evaluated by Egger's linear regression analysis and Begg's funnel plot analysis.
Results
Study search results
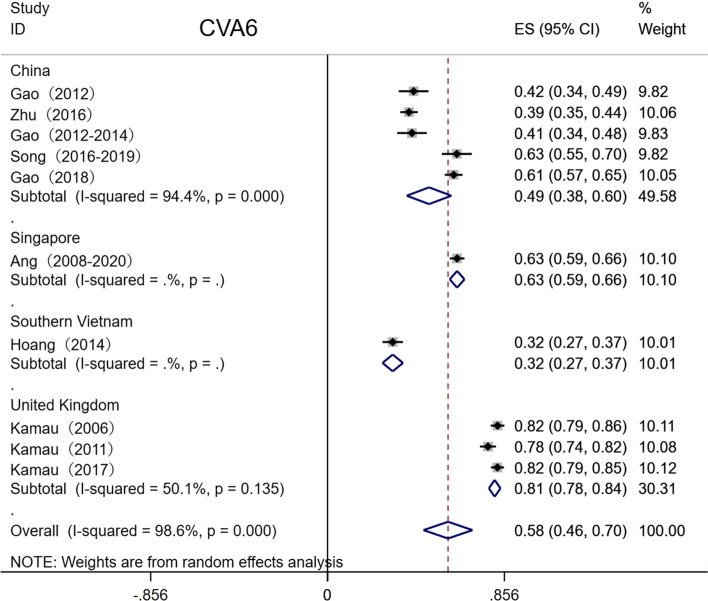
Two authors of this study searched two English-language databases and two Chinese-language databases using standard retrieval strategies (Supplementary Tables S1 and S2) [14–58]. Subsequently, the literature was screened independently with reference to pre-established inclusion and exclusion criteria, and valid data were extracted and verified. After excluding invalid and duplicate studies, 71 eligible studies were included in the meta-analysis [6, 8, 9, 59–73]. These 71 studies included a total of 55,176 samples, 23,297 of which were positive for EV-A71 neutralizing antibody. Thus, the total serum EV-A71 antibody positivity rate ranged from 37.6% to 54.1%. The serum CVA6 positivity rate was 58.3%. Among the 71 studies, there were 53 from China, three each from Singapore, Thailand, and Vietnam, two each from the United Kingdom and Germany, and one each from the Netherlands, Finland, Iran, Korea, and Russia (Table 1). Literature quality was also evaluated based on the evaluation criteria of AHRQ. In total, 36 of the included studies were found to be of medium quality, and 35 were of high quality (Fig. 1).
Fig. 1.
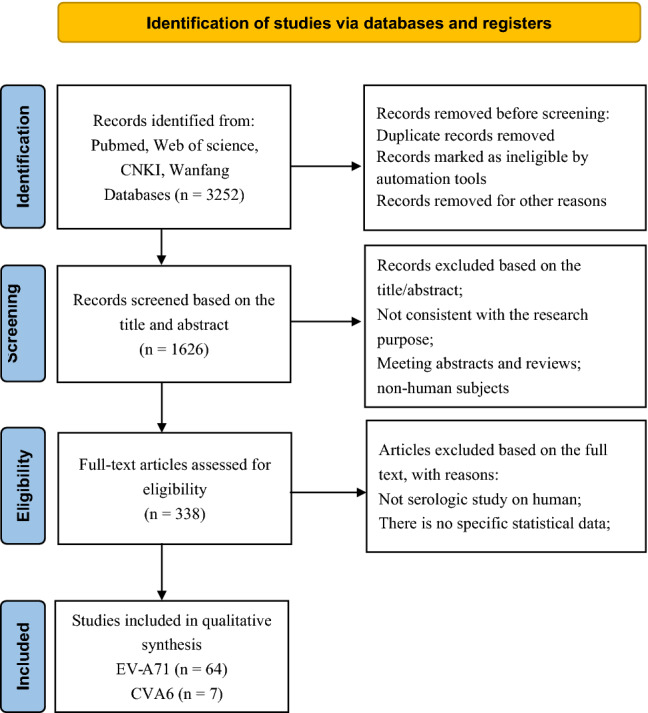
Flow chart of the literature selection process
Seroprevalence of EV-A71 antibody
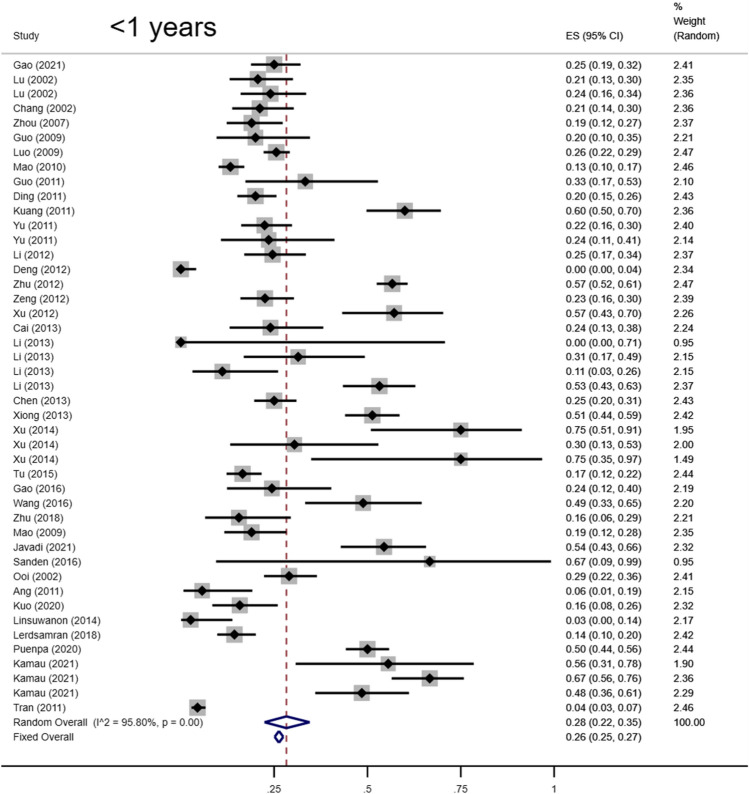
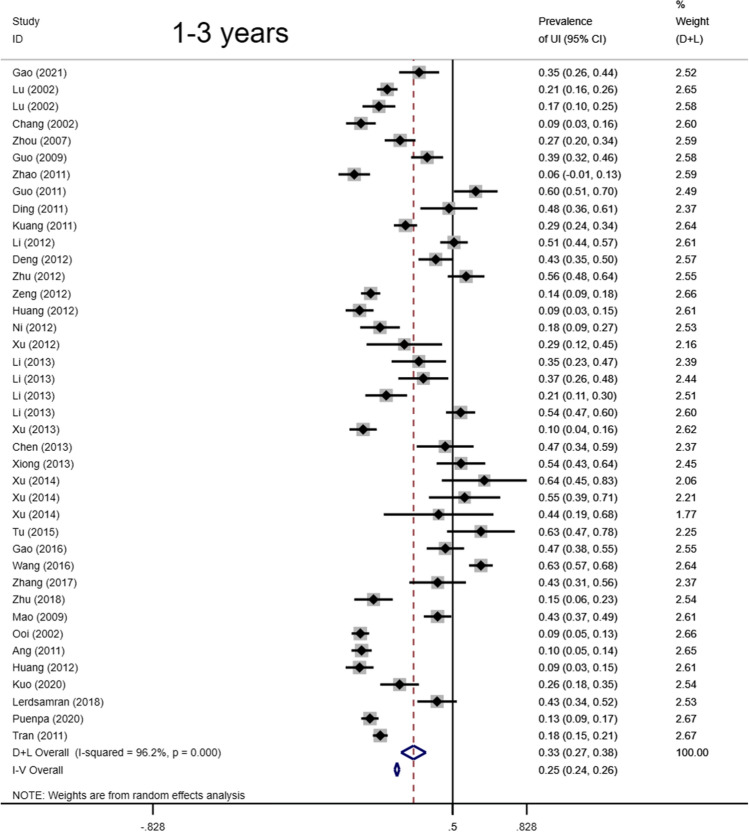
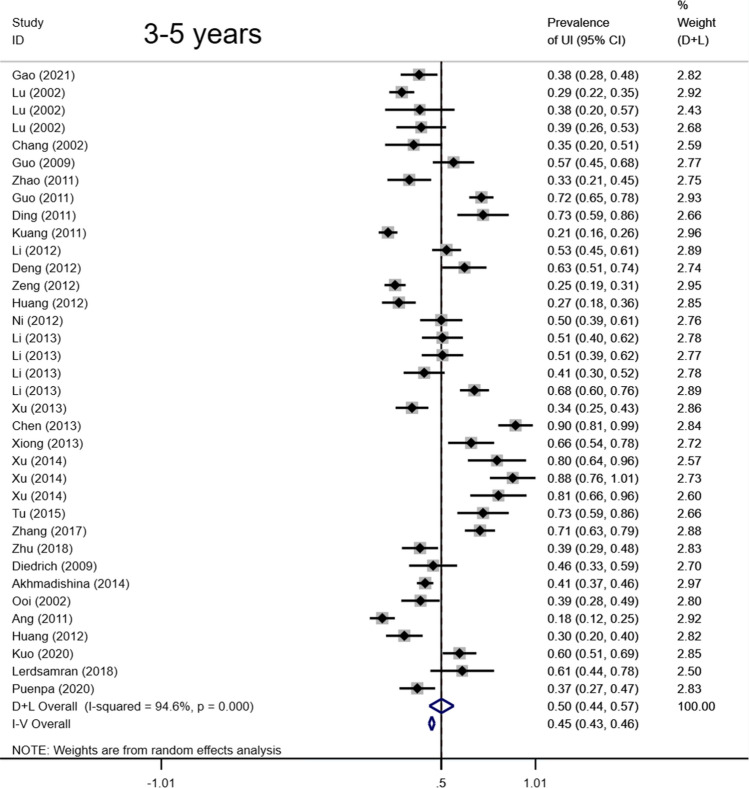
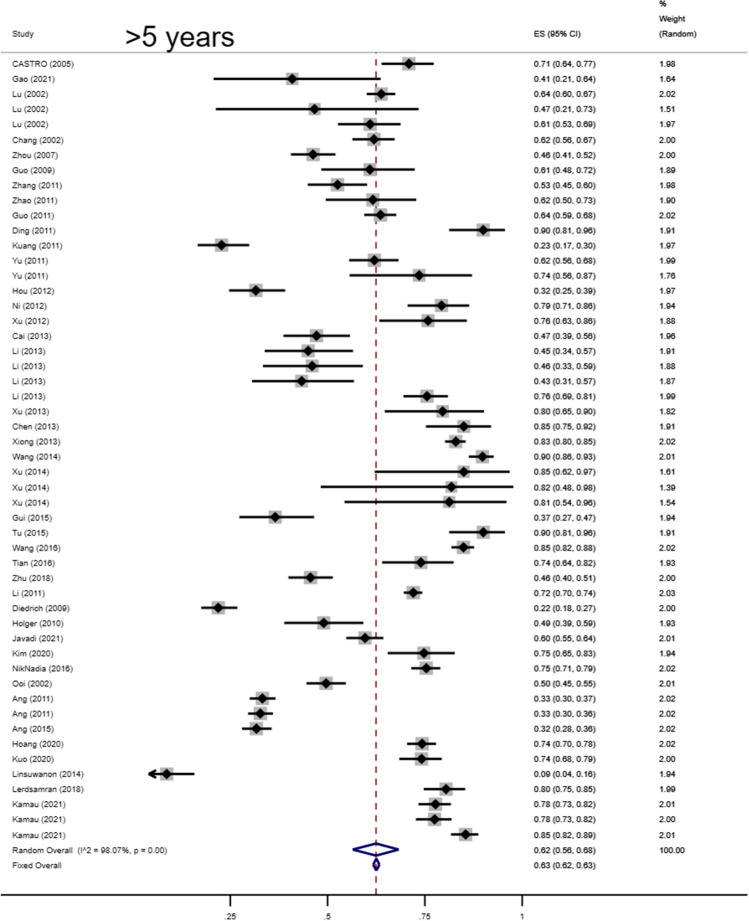
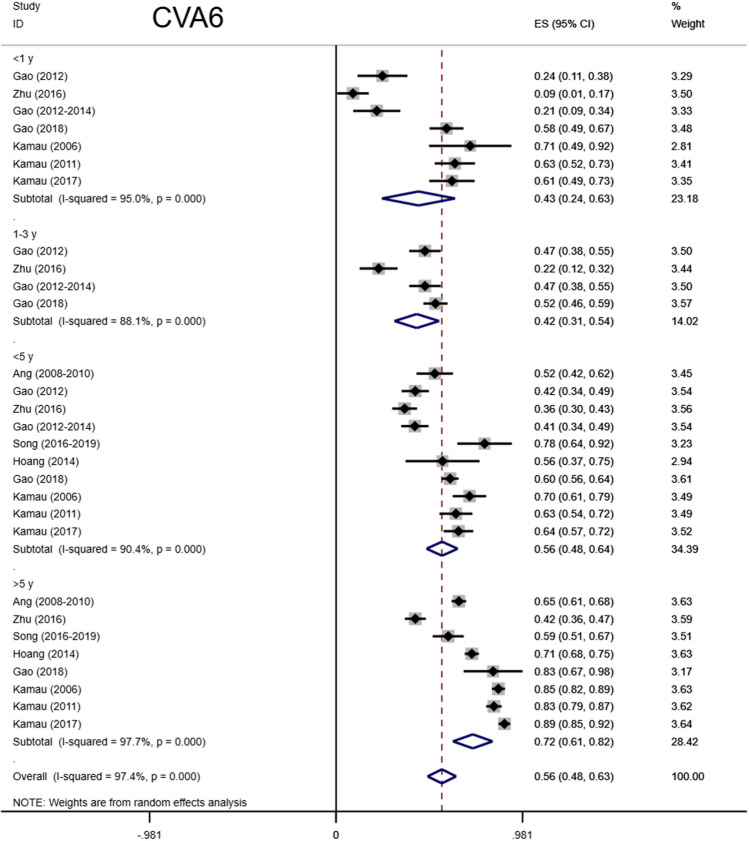
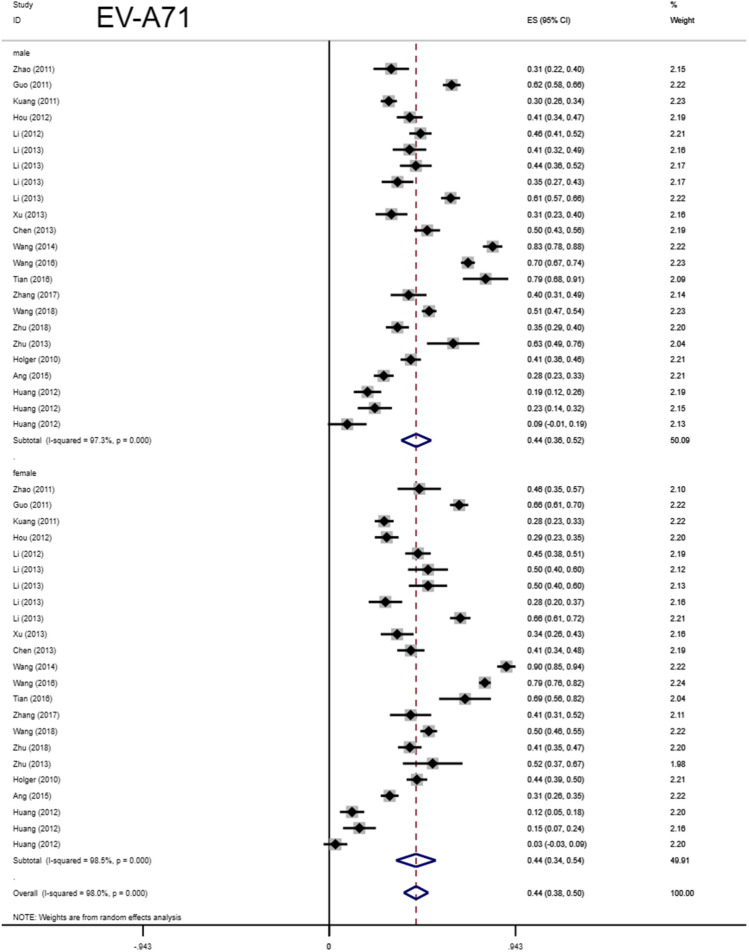
Total seropositivity rates were analysed and stratified by age, sex, sampling method, seasonality, publication language, and ethnicity (Tables 1,2, 3). Forest plots of the total seropositive rate of CVA6 are shown in Figure 2, and an overall summary of the results is shown in Table 2. The seroprevalence of EV-A71 antibody was 45.9% (95% CI: 37.6%-54.1%) in the overall population, 47.8% (95% CI: 42.4%-53.2%) in the Chinese population, and 38% (95% CI: 23%-55%) in other countries (Table 3). The seroprevalence of CVA6 antibody was 58.3% (95% CI: 46.5%-70.2%) in the overall population, 49.1% (95% CI: 38.3%-59.9%) in the Chinese population, and 68% (95% CI: 51%-83%) in other countries (Table 2). Moreover, age-based subgroup analysis showed that the seroprevalence of EV-A71 antibody was 28 % (22%-35%) in the < 1-year-old age group, 32.7 % (27.1%-38.2%) in the 1- to 3-year-old age group, 36.3% (95% CI: 32%-40.6%) in the 0- to 5-year-old age group, and 62 % (95% CI: 56%-68%) in the 5 years and older age group (Tables 3, 4, Figs. 4, 5, 6, 7). The seroprevalence of CVA6 antibody was 43% (24%-63%) in the < 1-year-old age group, 42.5 % (30.7%-54.2%) in the 1- to 3-year-old age group, 55.8% (95% CI: 47.6%-64%) in the 0- to 5-year-old age group, and 72 % (95% CI: 61%-82%) in the 5 years and older age group (Table 2, Fig. 3). Subgroup analysis based on sex showed that the seroprevalence of EV-A71 antibody was 44% (95% CI: 36.5%-51.6%) in males and 43.9% (95% CI: 33.8%-54%) in females (Fig. 8). The seroprevalence of CVA6 antibody was 54.1% (95% CI: 32.8%-75.4%) in males and 55.4% (95% CI: 42.5%-68.3%) in females.
Table 2.
Seroprevalence in different groups (CVA6)
| Variable | Ref (n) | n | Events | Transformation method | Seroprevalence (%) | 95% CI (%) | I2 (%) | Heterogeneity (P-value) |
Begg’s (P-value) |
Egger’s (P-value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 8 | 2522 | 4079 | Random-effects model | 0.583 | 0.465, 0.702 | 98.6 | <0.001 | 0.016 | 0.056 |
| Age | ||||||||||
| <1 y | 5 | 192 | 406 | Freeman-Tukey double arcsine | 0.43 | 0.24, 0.63 | 95.01 | <0.001 | ||
| 1-3 y | 4 | 269 | 582 | Random-effects model | 0.425 | 0.307, 0.542 | 88.1 | <0.001 | ||
| 3-5 y | 2 | 143 | 207 | Random-effects model | 0.686 | 0.490, 0.882 | 89.9 | <0.001 | ||
| <5 y | 8 | 839 | 1564 | Random-effects model | 0.558 | 0.476, 0.640 | 90.4 | <0.001 | ||
| >5 y | 6 | 1989 | 2760 | Freeman-Tukey double arcsine | 0.72 | 0.61, 0.82 | 97.74 | <0.001 | ||
| Gender | ||||||||||
| Female | 3 | 415 | 759 | Random-effects model | 0.554 | 0.425, 0.683 | 91.7 | <0.001 | ||
| Male | 3 | 334 | 627 | Random-effects model | 0.541 | 0.328, 0.754 | 96.5 | <0.001 | ||
| Sampling method | ||||||||||
| Physical examination | 2 | 546 | 871 | Fixed- effects model | 0.627 | 0.595, 0.659 | 0.0 | 0.973 | ||
| Random sampling | 2 | 501 | 1003 | Random-effects model | 0.502 | 0.290, 0.715 | 98.0 | <0.001 | ||
| Conditional sampling | 4 | 1475 | 2205 | Random-effects model | 0.595 | 0.421, 0.770 | 99.0 | <0.001 | ||
| Seasonality | ||||||||||
| Seasonal | 4 | 362 | 868 | Random-effects model | 0.440 | 0.310, 0.571 | 93.7 | <0.001 | ||
| Non-seasonal | 1 | 439 | 700 | Random-effects model | 0.627 | 0.591, 0.663 | - | - | ||
| Unknown | 3 | 1721 | 2511 | Freeman-Tukey double arcsine | 0.69 | 0.52, 0.84 | 98.75 | <0.001 | ||
| Publication language | ||||||||||
| English | 6 | 2341 | 3727 | Random-effects model | 0.599 | 0.465, 0.733 | 98.9 | <0.001 | ||
| Chinese | 2 | 181 | 352 | Random-effects model | 0.517 | 0.305, 0.730 | 94.3 | <0.001 | ||
| Ethnicity | ||||||||||
| Chinese | 5 | 757 | 1535 | Random-effects model | 0.491 | 0.383, 0.599 | 94.4 | <0.001 | ||
| Other | 3 | 1765 | 2544 | Freeman-Tukey double arcsine | 0.68 | 0.51, 0.83 | 98.78 | <0.001 |
Table 3.
Seroprevalence in different groups (EV-A71)
| Variable | Ref(n) | n | Event | Transformation Method | Seroprevalence (%) | 95% CI (%) | I2 (%) | Heterogeneity (P-value) |
Begg’s (P-value) |
Egger’s (P-value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 78 | 52654 | 23297 | Random-effects model | 0.459 | 0.376,0.541 | 99.9 | < 0.001 | 0.0001 | 0.271 |
| Age | ||||||||||
| <1 yrs | 36 | 5980 | 1716 | Freeman-Tukey double arcsine | 0.28 | 0.22,0.35 | 95.80 | < 0.001 | ||
| 1-3 yrs | 34 | 5570 | 1766 | Random-effects model | 0.327 | 0.271,0.382 | 96.2 | < 0.001 | ||
| 3-5 yrs | 29 | 3580 | 1601 | Random-effects model | 0.502 | 0.436,0.568 | 94.6 | < 0.001 | ||
| <5 yrs | 56 | 6713 | 19777 | Random-effects model | 0.363 | 0.320,0.406 | 98.1 | < 0.001 | ||
| >5 yrs | 40 | 8866 | 14387 | Freeman-Tukey double arcsine | 0.62 | 0.56,0.68 | 98.07 | < 0.001 | ||
| Gender | ||||||||||
| Female | 17 | 2511 | 5026 | Random-effects model | 0.439 | 0.338,0.540 | 98.5 | < 0.001 | ||
| Male | 17 | 2712 | 5640 | Random-effects model | 0.44 | 0.365,0.516 | 97.3 | < 0.001 | ||
| Sampling method | ||||||||||
| Physical examination | 9 | 2380 | 6961 | Random-effects model | 0.367 | 0.299,0.434 | 97.4 | < 0.001 | ||
| Random sampling | 41 | 12542 | 24843 | Random-effects model | 0.478 | 0.397,0.559 | 99.6 | < 0.001 | ||
| Conditional sampling | 13 | 3428 | 12912 | Freeman-Tukey double arcsine | 0.43 | 0.21,0.67 | 99.84 | < 0.001 | ||
| Seasonality | ||||||||||
| Season | 35 | 11141 | 21373 | Random-effects model | 0.476 | 0.393,0.558 | 99.5 | < 0.001 | ||
| Non-season | 8 | 3094 | 8673 | Freeman-Tukey double arcsine | 0.36 | 0.21,0.52 | 99.58 | < 0.001 | ||
| Unknown | 19 | 4115 | 14670 | Random-effects model | 0.450 | 0.300,0.599 | 99.8 | < 0.001 | ||
| Publication language | ||||||||||
| English | 46 | 18649 | 43952 | Random-effects model | 0.442 | 0.347,0.536 | 99.9 | < 0.001 | ||
| Chinese | 19 | 4940 | 9453 | Random-effects model | 0.502 | 0.429,0.575 | 98.2 | < 0.001 | ||
| Ethnicity | ||||||||||
| Chinese | 49 | 18256 | 33962 | Random-effects model | 0.478 | 0.424,0.532 | 99.2 | < 0.001 | ||
| Others | 17 | 5041 | 18692 | Freeman-Tukey double arcsine | 0.38 | 0.23,0.55 | 99.79 | < 0.001 |
Fig. 2.
Forest plots for the seroprevalence of CVA6 antibody in the overall population
Table 4.
The results of meta-regression (EV-A71)
| Covariate | Coefficient | 95% CI | t | P | Adjusted R2 (%) |
|---|---|---|---|---|---|
| Age | 29.58% | ||||
| <1 y | - | - | - | - | |
| 1-3 y | -.1894914 | -.2742778 -.104705 | -4.40 | 0.000 | |
| 3-5 y | -.1741073 | -.2596863 -.0885282 | -4.01 | 0.000 | |
| < 5 y | -.13795 | -.2150069 -.0608931 | -3.53 | 0.001 | |
| >5 y | .1171723 | .036483 .1978616 | 2.86 | 0.005 | |
| Gender | -1.53% | ||||
| Female | - | - | - | - | |
| Male | -.0013413 | -.1044165 .1017338 | -0.03 | 0.979 | |
| Sampling method | 1.39% | ||||
| Physical examination | - | - | - | - | |
| Random sampling | .1104207 | -.0156826 .2365241 | 1.75 | 0.085 | |
| Conditional sampling | .0836351 | -.0691683 .2364385 | 1.09 | 0.279 | |
| Seasonality | 0.47% | ||||
| Unknown | - | -- | - | - | |
| Seasonal | .1089901 | -.0323517 .2503319 | 1.54 | 0.129 | |
| Non-seasonal | .0829203 | -.072398 .2382386 | 1.06 | 0.291 | |
| Publication language | 0.47% | ||||
| English | - | - | - | - | |
| Chinese | .0610441 | -.0415077 .163596 | 1.19 | 0.240 | |
| Ethnicity | 1.28% | ||||
| Others | - | - | - | - | |
| Chinese | .0723176 | -.0303551 .1749904 | 1.40 | 0.165 |
The first line of every covariate represented reference; adjusted R2 was used to indicate the degree of heterogeneity explained by study characteristics.
Fig. 4.
Forest plots for the seroprevalence of EV-A71 antibody in the less-than-one-year age group
Fig. 5.
Forest plots for the seroprevalence of EV-A71 antibody in the 1-3 years age group
Fig. 6.
Forest plots for the seroprevalence of EV-A71 antibody in the 3-5 years age group
Fig. 7.
Forest plots for the seroprevalence of EV-A71 antibody in the older-than-five-years age group
Fig. 3.
Forest plots for the seroprevalence of CVA6 antibody in different age groups
Fig. 8.
Forest plots for the seroprevalence of EV-A71 antibody in males and females
Sensitivity analysis and publication bias
Univariate analysis showed that age and seasonality significantly affected the heterogeneity of the meta-analysis results (Tables 3, 4). A sensitivity analysis was also conducted on the serum prevalence of the CVA6 and EV-A71 antibodies (Supplementary Figs. S1-S2). Based on Begg’s and Egger’s tests, a publication bias plot was drawn, which demonstrated that the distribution of the prevalence in each study was not symmetrical (Figs. 9-10). Furthermore, the results indicated a possibility of publication bias.
Fig. 9.
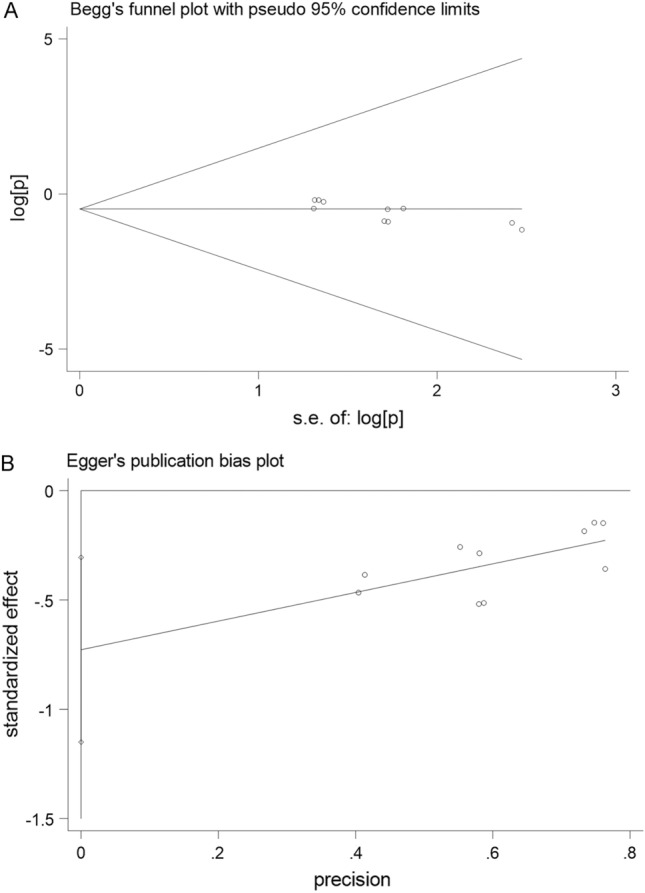
Begg’s and Egger’s publication bias plot for the seroprevalence of CVA6 antibody
Fig. 10.
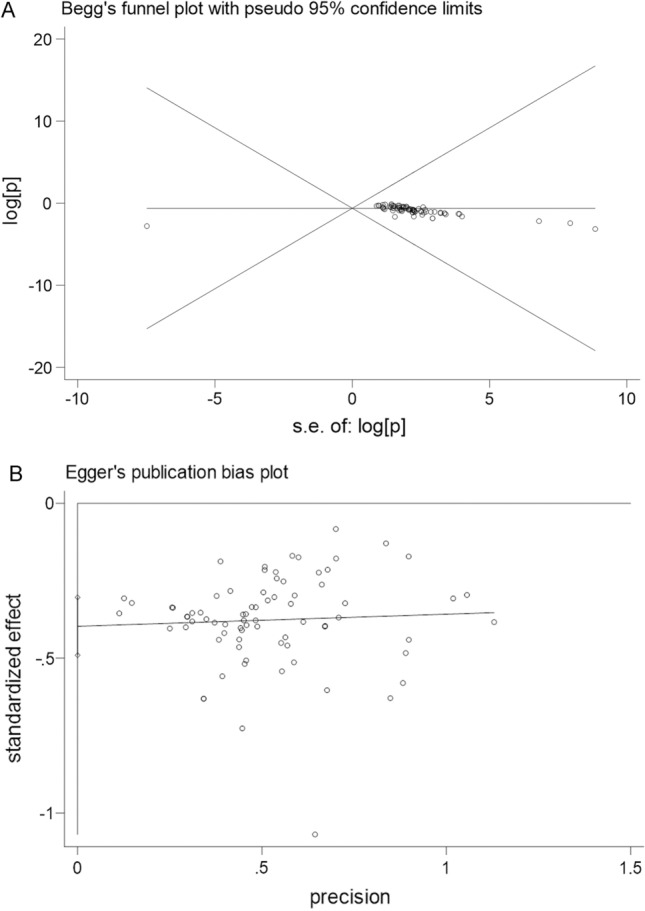
Begg’s and Egger’s publication bias plot for the seroprevalence of EV-A71 antibody
Discussion
HFMD caused by enterovirus infection is a disease that threatens children's health [8]. In the past decade, numerous outbreaks have occurred worldwide, especially in the Asia-Pacific region. EV-A71 and CVA6 are the main pathogens of HFMD and are occasionally associated with severe neurological complications [2]. Since the approval of an inactivated EV-A71 vaccine in China in 2016, the number of deaths from HFMD in China has decreased significantly, and the EV-A71 epidemic has been effectively controlled [74]. In this meta-analysis, most of the EV-A71 studies assessed the prevalence of the virus before 2016 [5]. Since the EV-A71 vaccine was widely administered in 2016, other enterovirus serotypes have replaced EV-A71 as the main cause of severe HFMD for the first time [75]. Due to the lack of cross-protection by inactivated monovalent EV-A71 vaccine against other enteroviruses, enteroviruses such as CVA16 and CVA6 remain relatively common, highlighting the need for attention to be given to these enteroviruses in future epidemic prevention and control strategies. In addition, serotype identification and vaccine development for other enteroviruses should be strengthened.
In a number of studies performed in China, the prevalence of HFMD showed obvious seasonality, with two main epidemic peaks: one in late spring and early summer and one in late autumn and winter. The factors causing this epidemic and seasonal pattern may be related to geographical location, population density, and environmental conditions, which need to be evaluated further [1].
Maternal antibodies play an important role in protecting children from HFMD. In a study in Thailand, serum samples were collected from children at birth (0 months of age) and 2, 7, 18, 24, 36, and 48 months of age, and the titre of neutralizing antibodies against EV-A71 was measured. The results showed that the serum protection rates (NT antibody 1:16) of children at 0, 2, 7, 18, 24, 36, and 48 months were 81.0%, 60.0%, 9.0%, 10.0%, 13.0%, 17.0%, and 37.1%, respectively. These findings indicated that the antibody titre was very high at birth. However, the antibody titre decreased significantly in the first year of life and reached its lowest value at approximately 7 months of age [70]. In another sero-epidemiological investigation of enteroviruses in 488 healthy subjects aged 2-83 months, maternally derived neutralizing antibodies to EV-A71 and CVA6 in neonates decreased to their lowest levels (11.11% and 10.14%) at approximately 6 months of age and increased thereafter [72]. In China, antibody levels in preschool children are low, and kindergarten children have a significantly increased risk of contracting the virus. A separate report detailing the seroepidemiology of EV-A71 in children in Singapore also noted that most infections occurred in preschool children at an age when children are concentrated in classrooms, sharing toys and teaching tools [43].
The seroprevalence rate of HFMD virus varies greatly in different countries and regions. In a prospective study of healthy children in Finland, EV-A71 was detected in only 0.3% of fecal samples and two serum samples, and the positive rate for neutralizing antibodies was only 1.6% [76]. In a Norwegian study of 1,255 stool samples tested by RT-PCR, the rate of EV-A71 infection was only 1.4% [77]. In Germany, 27% of healthy children under 4 years of age had neutralizing antibodies against EV-A71, and 75% of people aged 20-40 years had neutralizing antibodies against EV-A71 [50, 53]. A study in the United Kingdom found that the seroprevalence of EV-A71 and CVA6 increased from 32% and 54% at 6-11 months of age to >75% at 10 years of age. EV-A71 was most commonly found in stool, followed by cerebrospinal fluid, respiratory tract samples, vesicle or skin swabs, and blood. CVA6 was most frequently detected in vesicles or skin swabs, followed by respiratory specimens, stool, cerebrospinal fluid, and blood [8]. In this study, the lowest serum positive rate of EV-A71 in children less than 1 year old was 28%, and the serum positive rate gradually increased with age. The positive rate of EV-A71 was 32.7% in the 1- to 3-year-old group, 50.2% in the 3- to 5-year-old group, and 62% in the over 5-year-old group. The serological survey of CVA6 showed a similar trend. In another serological meta-analysis, the seropositivity of CVA16 was lowest in children under one year of age and increased with age. No significant differences were observed between males and females in the serum positivity rates for EV-A71 and CVA6. The EV-A71 positivity rate in China was higher than that in other countries, whereas the CVA6 positivity rate in China was lower than that in other countries [78].
In previous studies, most HFMD cases occurred in children, with a lower proportion of symptomatic infections in adults. However, a survey in Vietnam involving household contact transmission by subjects of all ages showed that the EV-A71 infection rate by household contact was 47.6%, the CV-A6 infection rate was 31.5%, and only 6.20% of household contacts developed symptoms. Symptoms of EV-A71 infection occurred in less than 10% of the 25-29, 30-34, and 45-49 age groups, and symptoms of CV-A6 infection occurred in less than 10% of the 25-29, 50-54, and 60-plus age groups. These results suggest that serologically detectable infections continue to occur in middle-aged and older adults at rates similar to those in children, but only a few individuals develop clinical symptoms. Therefore, specimen collection from family members of positive patients is strongly recommended, and the home environment is one of the targets of HFMD intervention [69]. At the same time, in the field of basic research, we should further clarify the main causes of susceptibility differences between children and adults and for symptoms after infection [69]. Molecular epidemiological surveillance of epidemic strains should be strengthened to prevent large-scale epidemics of mutant strains.
Given the increase in the incidence and severity of cases of HFMD caused by CVA6, it is necessary to strengthen the monitoring of HFMD and research on related vaccines and to actively educate the population about HFMD prevention and control before the epidemic peak years and peak seasons to improve public awareness. In view of the prevalence of HFMD, the accuracy of virus detection needs to be improved to prevent missed diagnosis and misdiagnosis. Early detection, diagnosis, and treatment are necessary to reduce the number of severe cases and deaths.
Most of the data in this study showed relatively high heterogeneity. Thus, for most of the analyses, random effects models were used or double-arcsine transformation was performed, and most of the variation could be explained by variables contained in the meta-regression model. The unexplained variability may be due to some other unmeasured factors in the study, such as possible differences in the time of specimen collection, serum storage time and methods, or virus strains selected in laboratory tests.
Our study has some limitations. First, the estimates we obtained were mainly obtained from China and other Southeast Asian countries, which are densely populated. Therefore, these findings may not objectively reflect the global seroepidemiological situation. Second, a standardized protocol for the detection of neutralizing antibodies is lacking, and laboratory techniques and reagents used by different laboratories differ greatly, which may lead to differences in the titre and serological prevalence of neutralizing antibodies. Third, relatively high heterogeneity was observed among the seroprevalence estimates in this study, which could not be fully explained in the meta-regression. Thus, there may be other factors influencing seroprevalence that were not analysed in this meta-analysis. Numerous retrospective and cross-sectional studies were included in this meta-analysis, and a number of studies used previously preserved serum samples. Antibody titres may decrease over time during storage, leading to deviations in the test results.
Conclusions
EV-A71 is the main pathogen of HFMD in infants and young children and is capable of causing neurological complications. Due to widespread vaccination with the EV-A71 vaccine, CVA6 has gradually become the dominant virus causing HFMD. Because only a subset of EV-A71- and CVA6-infected individuals experience clinical symptoms, the available data do not accurately reflect the prevalence of the virus. In this study, the seroepidemiology of EV-A71 and CVA6 was analysed systematically. The results showed that infants and young children had higher antibody levels at birth, which decreased to the lowest level within the first year and then gradually increased thereafter. EV-A71 and CVA6 serum antibody levels were correlated most closely with age, followed by sampling season and sampling method.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
The authors thank the authors of all of the studies used in the meta-analysis for providing original data.
Author contributions
Yingying Shi and Yongjuan Liu designed the project. Yingying Shi, Peiqing Chen, Yijing Bai, and Xuan Xu performed statistical analysis. Yingying Shi, Peiqing Chen, Yijing Bai, Xuan Xu, and Yongjuan Liu interpreted and wrote the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the National Natural Sciences Foundation of China (No. 82202494), the Natural Science Foundation of Hubei Province (No. 2018CFB254), the Wuhan COVID-19 Emergency Research Project (No. EX20D04), and the Natural Science Foundation of Jiangsu Province (No. BK20180269).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest relevant to this study.
Ethical approval
Ethical approval was not needed because this is a meta-analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, et al. Hand, foot, and mouth disease in china, 2008–12: an epidemiological study. Lancet Infect Dis. 2014;14(4):308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox B, Levent F. Hand, foot, and mouth disease. JAMA. 2018;320(23):2492. doi: 10.1001/jama.2018.17288. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez G, Carr MJ, Kobayashi M, Hanaoka N, Fujimoto T. Enterovirus-associated hand-foot and mouth disease and neurological complications in japan and the rest of the world. Int J Mol Sci. 2019;20(20):5201. doi: 10.3390/ijms20205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi Z, Pei S, Suo W, Wang X, Huang Z, Yi A, Wang B, He Z, Wang R, Li Y, et al. Epidemiological characteristics, routine laboratory diagnosis, clinical signs and risk factors for hand, -foot -and -mouth disease: a systematic review and meta-analysis. PLoS ONE. 2022;17(4):e267716. doi: 10.1371/journal.pone.0267716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Ren M, Chen S, Nie T, Cui J, Ran L, Li Z. Chang Z (2020) Pathogen spectrum of hand, foot, and mouth disease based on laboratory surveillance - China. China CDC Wkly. 2018;2(11):167–171. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Bae KS, Kim JH, Kang JH, Choi UY. Seroprevalence of neutralizing antibodies against candidate serotypes of enterovirus vaccines among Korean children. Viral Immunol. 2021;34(2):62–67. doi: 10.1089/vim.2020.0073. [DOI] [PubMed] [Google Scholar]
- 7.Diarimalala RO, Hu M, Wei Y, Hu K. Recent advances of enterovirus 71 formula: see text] targeting inhibitors. Virol J. 2020;17(1):173. doi: 10.1186/s12985-020-01430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamau E, Nguyen D, Celma C, Blomqvist S, Horby P, Simmonds P, Harvala H. Seroprevalence and virologic surveillance of enterovirus 71 and coxsackievirus a6, United Kingdom, 2006–2017. Emerg Infect Dis. 2021;27(9):2261–2268. doi: 10.3201/eid2709.204915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Teng Z, Cui X, Li C, Pan H, Zheng Y, Mao S, Yang Y, Wu L, Guo X, et al. Epidemiological and serological surveillance of hand-foot-and-mouth disease in Shanghai, China, 2012–2016. Emerg Microbes Infect. 2018;7(1):8. doi: 10.1038/s41426-017-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian L, Wang Y, Yao X, Mao Q, Xu M, Liang Z. Coxsackievirus a6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13(9):1061–1071. doi: 10.1586/14787210.2015.1058156. [DOI] [PubMed] [Google Scholar]
- 11.Kimmis BD, Downing C, Tyring S. Hand-foot-and-mouth disease caused by coxsackievirus a6 on the rise. Cutis. 2018;102(5):353–356. [PubMed] [Google Scholar]
- 12.Jiao M, Apostol LN, de Quiroz-Castro M, Jee Y, Roque VJ, Mapue MN, Navarro FM, Tabada CF, Tandoc AR. Non-polio enteroviruses among healthy children in the Philippines. BMC Public Health. 2020;20(1):167. doi: 10.1186/s12889-020-8284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;10:372n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai MS, Ren Y. Investigation on hfmd recessive infection in healthy population in some community of Shenzhen in 2012. Zhongguo Wei Sheng Jian Yan. 2013;23(10):2355–2357. [Google Scholar]
- 15.Li W, Yi L, Su J, Lu J, Zeng H, Guan D, Ma C, Zhang W, Xiao H, Li H, et al. Seroepidemiology of human enterovirus71 and coxsackievirusa16 among children in Guangdong province China. Bmc Infect Dis. 2013;13:13322. doi: 10.1186/1471-2334-13-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Yi L, Su J, Lu J, Ke C, Zeng H, Guan D, Ma C, Zhang W, Xiao H, et al. Seroprevalence of human enterovirus 71 and coxsackievirus a16 in Guangdong, China, in pre- and post-2010 hfmd epidemic period. PLoS ONE. 2013;8(12):e80515. doi: 10.1371/journal.pone.0080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W, Wang Y. Seroepidemiological study of enterovirus 71 infection in children aged 1 through 6 years in Changzhou in 2006. Pract Prev Med. 2013;20:179–180. [Google Scholar]
- 18.Chen XQ, Zhang ZY, Pang HY. Monitoring and analysis of the levels of neutralizing antibody against enterovirus 71 in individuals under the age of 15 in Donghai county. Xian Dai Yu Fang Yi Xue. 2013;40(20):3875–3877. [Google Scholar]
- 19.Xiong Y, Gong T, Shi Y, Zhou J, Liu LP, Zeng YW. Seroepidemiology investigation on hev71 in the population in Nanchang in early 2010. Xian Dai Yu Fang Yi Xue. 2013;40(1):7–10. [Google Scholar]
- 20.Zhu W, Ju L, Jiang L, Shen H, Wang Q, Jiang Q. Serum levels of antibody against enterovirus 71 in healthy children at Shanghai in 2011. Chin J Infect Dis. 2013;34:650–653. [Google Scholar]
- 21.Linsuwanon P, Puenpa J, Huang SW, Wang YF, Mauleekoonphairoj J, Wang JR, Poovorawan Y. Epidemiology and seroepidemiology of human enterovirus 71 among Thai populations. J Biomed Sci. 2014;21:2116. doi: 10.1186/1423-0127-21-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Xing M, Zhang C, Yang Y, Chi Y, Tang X, Zhang H, Xiong S, Yu L, Zhou D. Neutralizing antibody responses to enterovirus and adenovirus in healthy adults in China. Emerg Microbes Infect. 2014;3(5):e30. doi: 10.1038/emi.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akhmadishina LV, Eremeeva TP, Trotsenko OE, Ivanova OE, Mikhailov MI, Lukashev AN. Seroepidemiology and molecular epidemiology of enterovirus 71 in Russia. PLoS ONE. 2014;9(5):e97404. doi: 10.1371/journal.pone.0097404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.XU Yu-ling YLQJ Seroepidemiological study of enterovirus 71 among healthy children during 2010 to 2012 in Henan province of China. Chin J Viral Dis. 2014;2(4):141–143. [Google Scholar]
- 25.Yu H, Wang M, Chang H, Lu J, Lu B, Li J, Chen W, Tang R, Gan L, Zhao J, et al. Prevalence of antibodies against enterovirus 71 in children from Lu'an city in central China. Jpn J Infect Dis. 2011;64(6):528–532. [PubMed] [Google Scholar]
- 26.Ang LW, Phoon MC, Wu Y, Cutter J, James L, Chow VT. The changing seroepidemiology of enterovirus 71 infection among children and adolescents in Singapore. Bmc Infect Dis. 2011;11:11270. doi: 10.1186/1471-2334-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran CB, Nguyen HT, Phan HT, Tran NV, Wills B, Farrar J, Santangelo JD, Simmons CP. The seroprevalence and seroincidence of enterovirus71 infection in infants and children in ho Chi Minh city, Viet nam. PLoS ONE. 2011;6(7):e21116. doi: 10.1371/journal.pone.0021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Q, Zhu F, Li L, Li D, Liu Y. Serum epidemiology survey of hev 71 among children in Ganyu county. Jiangsu J Prev Med. 2011;22:224–306. [Google Scholar]
- 29.Lu KWCLZ. Investigation on enterovirus 71 antibody levels among children in Guangzhou area. Chin J Evid Based Pediatr. 2011;3(6):211–214. [Google Scholar]
- 30.Guo R, Deng J, Li Y, Ma Y. Serum epidemiological study of hfmd in handan, 2009. J Pathog Biol. 2011;6:848–850. [Google Scholar]
- 31.Zhang DX, Yang F, Wang B, Yue LJ, He YX, Song P. Subclinical infection of hand-foot-mouth disease in Shenzhen city. Zhongguo Re Dai Yi Xue. 2011;11(11):1332–1333. [Google Scholar]
- 32.Sheng-cang Z, Shi-jie Z, Jian-ning Y. Seroepidemiologic investigation of hev 71 among children in Xi’ning city. Chin J Public Healt. 2011;27(3):361–362. [Google Scholar]
- 33.Li J, Zhang Y, Gao L, Liu H, Li L, Lü LK, Yang D. Seroepidemiology investigation of neutralizing antibody against enterovirus 71 among healthy people in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32(6):568–570. [PubMed] [Google Scholar]
- 34.Zhu FC, Liang ZL, Meng FY, Zeng Y, Mao QY, Chu K, Song XF, Yao X, Li JX, Ji H, et al. Retrospective study of the incidence of hfmd and seroepidemiology of antibodies against ev71 and coxa16 in prenatal women and their infants. PLoS ONE. 2012;7(5):e37206. doi: 10.1371/journal.pone.0037206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng M, El KN, Tu S, Ren P, Xu S, Zhu Q, Mo X, Pu D, Wang X, Altmeyer R. Seroepidemiology of enterovirus 71 infection prior to the 2011 season in children in Shanghai. J Clin Virol. 2012;53(4):285–289. doi: 10.1016/j.jcv.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Ji H, Li L, Liu Y, Ge H, Wang X, Hu J, Wu B, Fu J, Zhang Z, Chen X, et al. Seroepidemiology of human enterovirus71 and coxsackievirusa16 in Jiangsu province, China. Virol J. 2012;9:9248. doi: 10.1186/1743-422X-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang WC, Huang LM, Kao CL, Lu CY, Shao PL, Cheng AL, Fan TY, Chi H, Chang LY. Seroprevalence of enterovirus 71 and no evidence of crossprotection of enterovirus 71 antibody against the other enteroviruses in kindergarten children in Taipei city. J Microbiol Immunol Infect. 2012;45(2):96–101. doi: 10.1016/j.jmii.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 38.HongBin H, HaiTao Z, HuaShu Z, RunLi C. Igg antibody levels in healthy people of Futian district of Shenzhen city. Occupation and Health. 2012;28(24):3107–3108. [Google Scholar]
- 39.Li JM, Xu YJ, Wang QY. Investigation of enterovirus 71 antibody levels among children in the Longgang district, city of Shenzhen. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi. 2012;7(12):924–926. [Google Scholar]
- 40.Deng HL, Ma SW, Mi B, Wu R, Wang J, Li JB. Survey on enterovirus subclinical infection of healthy children in Xi'an. Yi Xue Lin Chuang Yan Jiu. 2012;29(2):204–208. [Google Scholar]
- 41.Ni H, Yi B, Yin J, Fang T, He T, Du Y, Wang J, Zhang H, Xie L, Ding Y, et al. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Ningbo, China, 2008–2011. J Clin Virol. 2012;54(4):342–348. doi: 10.1016/j.jcv.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Xu MH. Pathogen composition causing hand foot and mouth disease (hfmd) and seroepidemiology of enterovirus 71 infection in shanghai, china. Fudan University; 2012. [Google Scholar]
- 43.Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002;8(9):995–997. doi: 10.3201/eid0809.10.3201/eid0809.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu CY, Lee CY, Kao CL, Shao WY, Lee PI, Twu SJ, Yeh CC, Lin SC, Shih WY, Wu SI, et al. Incidence and case-fatality rates resulting from the 1998 enterovirus 71 outbreak in Taiwan. J Med Virol. 2002;67(2):217–223. doi: 10.1002/jmv.2210. [DOI] [PubMed] [Google Scholar]
- 45.Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, Huang YC, Shih SR, Chiou ST, Chen PY, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109(6):e88. doi: 10.1542/peds.109.6.e88. [DOI] [PubMed] [Google Scholar]
- 46.Castro CM, Cruz AC, Silva EE, Gomes ML. Molecular and seroepidemiologic studies of enterovirus 71 infection in the state of para, Brazil. Rev Inst Med Trop Sao Paulo. 2005;47(2):65–71. doi: 10.1590/s0036-46652005000200002. [DOI] [PubMed] [Google Scholar]
- 47.Zhou SL, Li LL, He YQ. Serological epidemiology investigation of enterovirus type 71 infection in Shenzhen. J Trop Med. 2007;7(1):66–67. [Google Scholar]
- 48.Guo X, Zhu S, Wang D. Seroepidemiology investigation of hev71 in healthy children of 1–6 years old in three counties of China in 2005. Zhongguo Yi Miao He Mian Yi. 2009;15(2):141–144. [PubMed] [Google Scholar]
- 49.Luo ST, Chiang PS, Chao AS, Liou GY, Lin R, Lin TY, Lee MS. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis. 2009;15(4):581–584. doi: 10.3201/1504.081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diedrich S, Weinbrecht A, Schreier E. Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch Virol. 2009;154(7):1139–1142. doi: 10.1007/s00705-009-0413-x. [DOI] [PubMed] [Google Scholar]
- 51.Mao QY, Yang ZW, Yu X, Jing Q, He P. Epidemic tendency of neutralizing antibody against enterovirus 71 and coxsackievirus a 16 in infants in rural area of Kaifeng city, Henan province, China. Chin J Biol. 2009;22(9):911–913. [Google Scholar]
- 52.Mao QY, Liao XY, Yu X, Li N, Zhu FC, Zeng Y, Liang ZL, Li FX, Wang JZ, Lu FM, et al. Dynamic change of mother-source neutralizing antibodies against enterovirus 71 and coxsackievirus a16 in infants. Chin Med J (Engl) 2010;123(13):1679–1684. [PubMed] [Google Scholar]
- 53.Rabenau HF, Richter M, Doerr HW. Hand, foot and mouth disease: seroprevalence of coxsackie a16 and enterovirus 71 in Germany. Med Microbiol Immunol. 2010;199(1):45–51. doi: 10.1007/s00430-009-0133-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Z, Zhu S, Guo X, Wang J, Wang D, Yan D, Tan X, Tang L, Zhu H, Yang Z, et al. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus a16 circulated wildly in central and southern china before large-scale outbreaks from 2008. Virol J. 2010;7:7300. doi: 10.1186/1743-422X-7-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gui J, Liu Z, Zhang T, Hua Q, Jiang Z, Chen B, Gu H, Lv H, Dong C. Epidemiological characteristics and spatial-temporal clusters of hand, foot, and mouth disease in Zhejiang province, China, 2008–2012. PLoS ONE. 2015;10(9):e139109. doi: 10.1371/journal.pone.0139109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ang LW, Tay J, Phoon MC, Hsu JP, Cutter J, James L, Goh KT, Chow VT. Seroepidemiology of coxsackievirus a6, coxsackievirus a16, and enterovirus 71 infections among children and adolescents in Singapore, 2008–2010. PLoS ONE. 2015;10(5):e127999. doi: 10.1371/journal.pone.0127999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu X, Wang Y, Zhu F, Chen F. Two models for changes of ev71 immunity in infants and young children. Hum Vaccin Immunother. 2015;11(6):1429–1433. doi: 10.1080/21645515.2015.1016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.NikNadia N, Sam IC, Rampal S, WanNorAmalina W, NurAtifah G, Verasahib K, Ong CC, MohdAdib M, Chan YF. Cyclical patterns of hand, foot and mouth disease caused by enterovirus a71 in Malaysia. PLoS Negl Trop Dis. 2016;10(3):e4562. doi: 10.1371/journal.pntd.0004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Sanden SM, Koen G, van Eijk H, Koekkoek SM, de Jong MD, Wolthers KC. Prediction of protection against asian enterovirus 71 outbreak strains by cross-neutralizing capacity of serum from Dutch donors, the Netherlands. Emerg Infect Dis. 2016;22(9):1562–1569. doi: 10.3201/eid2209.151579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian X, Jiang Z, Ma Q, Liu Q, Lu X, Liu W, Liao X, Zhou R, Su X, Luo Q. Prevalence of neutralizing antibodies to common respiratory viruses in intravenous immunoglobulin and in healthy donors in southern China. J Thorac Dis. 2016;8(5):803–812. doi: 10.21037/jtd.2016.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao F, Mao QY, Chen P, Bian LL, Yao X, Li JX, Zhu FC, Liang ZL. Seroepidemiology of coxsackievirus a6, coxsackievirus a16, and enterovirus 71 infections in infants and children: a prospective cohort study in Jiangsu, China. J Infect. 2016;73(5):509–512. doi: 10.1016/j.jinf.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Horwood PF, Andronico A, Tarantola A, Salje H, Duong V, Mey C, Ly S, Dussart P, Cauchemez S, Buchy P. Seroepidemiology of human enterovirus 71 infection among children, Cambodia. Emerg Infect Dis. 2016;22(1):92–95. doi: 10.3201/eid2201.151323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang JX, Zhu SL, Wang J, Lin Y, Pei YW, Sun DP, Zhang Y, Wang XJ, Xu WB, Ding SJ. Seroprevalence of enterovirus a71 and coxsackievirus a16 in healthy people in Shandong province, China. PLoS ONE. 2016;11(9):e162373. doi: 10.1371/journal.pone.0162373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guerra JA, Waters A, Kelly A, Morley U, O'Reilly P, O'Kelly E, Dean J, Cunney R, O'Lorcain P, Cotter S, et al. Seroepidemiological and phylogenetic characterization of neurotropic enteroviruses in ireland, 2005–2014. J Med Virol. 2017;89(9):1550–1558. doi: 10.1002/jmv.24765. [DOI] [PubMed] [Google Scholar]
- 65.Zhang D, Chen Y, Chen X, He Z, Zhu X, Hao Y. Enterovirus 71 neutralizing antibodies seroepidemiological research among children in Guangzhou, China between 2014 and 2015: a cross-sectional study. Int J Environ Res Public Health. 2017;14(3):319. doi: 10.3390/ijerph14030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu R, Cheng T, Yin Z, Liu D, Xu L, Li Y, Wang W, Liu J, Que Y, Ye X, et al. Serological survey of neutralizing antibodies to eight major enteroviruses among healthy population. Emerg Microbes Infect. 2018;7(1):2. doi: 10.1038/s41426-017-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lerdsamran H, Prasertsopon J, Mungaomklang A, Klinmalai C, Noisumdaeng P, Sangsiriwut K, Tassaneetrithep B, Guntapong R, Iamsirithaworn S, Puthavathana P. Seroprevalence of antibodies to enterovirus 71 and coxsackievirus a16 among people of various age groups in a northeast province of Thailand. Virol J. 2018;15(1):158. doi: 10.1186/s12985-018-1074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Yang Z, Wang Z, Xu Y, Luo S, Yu X, Liu J, Zhou Y, Tong W, Zeng P. The surveillance of the epidemiological and serotype characteristics of hand, foot, mouth disease in Neijiang city, China, 2010–2017: a retrospective study. PLoS ONE. 2019;14(6):e217474. doi: 10.1371/journal.pone.0217474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoang CQ, Nguyen HD, Ho NX, Vu T, Pham T, Nguyen KT, Nguyen HT, Hoang LT, Clapham H, Nguyen T, et al. Incidence of infection of enterovirus 71 and coxsackieviruses a6 and a16 among household contacts of index cases in dong Thap province, southern Vietnam. Biomed Res Int. 2020 doi: 10.1155/2020/9850351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puenpa J, Chansaenroj J, Auphimai C, Srimuan D, Thatsanathorn T, Poovorawan Y, Wanlapakorn N. Neutralizing antibody against enterovirus-a71 in thai children: a longitudinal study from birth to age 4 years. Vaccine. 2020;38(48):7638–7644. doi: 10.1016/j.vaccine.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Kuo FL, Khanh TH, Chung WY, Hung NT, Luo ST, Chang WC, Nhan L, Thinh LQ, Lee MS. Seroprevalence of ev-a71 neutralizing antibodies following the 2011 epidemic in hcmc, vietnam. PLoS Negl Trop Dis. 2020;14(3):e8124. doi: 10.1371/journal.pntd.0008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao F, Bian LL, Chen L, Zhou YP, Li GF, Mao QY, He Q, Wu X, Yao SS, Yang XM, et al. A cross-sectional seroepidemiology study of seven major enteroviruses causing hfmd in guangdong, china. J Infect. 2021;83(1):119–145. doi: 10.1016/j.jinf.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Javadi M, Nejati A, Yousefi M, Mahmoodi M, Shoja Z, Shahmahmoodi S. First seroepidemiological investigation of human enterovirus 71 in iran. Iran J Microbiol. 2021;13(4):502–508. doi: 10.18502/ijm.v13i4.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Boeckel TP, Takahashi S, Liao Q, Xing W, Lai S, Hsiao V, Liu F, Zheng Y, Chang Z, Yuan C, et al. Hand, foot, and mouth disease in china: critical community size and spatial vaccination strategies. Sci Rep. 2016 doi: 10.1038/srep25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Jiang L, Zhang C, He W, Tan Y, Ning C. The changes in the epidemiology of hand, foot, and mouth disease after the introduction of the ev-a71 vaccine. Vaccine. 2021;39(25):3319–3323. doi: 10.1016/j.vaccine.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Honkanen H, Oikarinen S, Pakkanen O, Ruokoranta T, Pulkki MM, Laitinen OH, Tauriainen S, Korpela S, Lappalainen M, Vuorinen T, et al. Human enterovirus 71 strains in the background population and in hospital patients in finland. J Clin Virol. 2013;56(4):348–353. doi: 10.1016/j.jcv.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 77.Witso E, Palacios G, Ronningen KS, Cinek O, Janowitz D, Rewers M, Grinde B, Lipkin WI. Asymptomatic circulation of hev71 in norway. Virus Res. 2007;123(1):19–29. doi: 10.1016/j.virusres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 78.Li P, Chen Y, Tang A, Gao F, Yan JB. Seroprevalence of coxsackievirus a16 antibody among people of various age groups: a systematic review and meta-analysis. Arch Public Health. 2021;79(1):166. doi: 10.1186/s13690-021-00688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.