Abstract
Background & Aims:
Populations consuming soy have reduced risk for breast cancer, but the mechanisms are unclear. We tested the hypothesis that soy isoflavones, which have ovarian hormone-like effects, can reduce fibroglandular breast tissue (FGBT, ‘breast density’), a strong risk marker for breast cancer.
Methods:
Premenopausal women (age 30–42 years) were randomized to consume isoflavones (136.6 mg as aglycone equivalents, n=99) or placebo (n=98) for 5 days per week up to 2 years, and changes in breast composition measured by magnetic resonance imaging at baseline and yearly intervals were compared after square root transformation using linear mixed effects regression models.
Results:
By intention-to-treat analyses (n=194), regression coefficients (β estimates) of the interaction of time and isoflavone treatment were −0.238 (P=0.06) and −0.258 (P<0.05) before and after BMI adjustment, respectively for FGBT, 0.620 (P<0.05) and 0.248 (P=0.160), respectively for fatty breast tissue (FBT), and −0.155 (P<0.05) and −0.107 (P<0.05), respectively for FGBT as percent of total breast (FGBT%). β Estimates for interaction of treatment with serum calcium were −2.705 for FBT, and 0.588 for FGBT% (P<0.05, before but not after BMI adjustment). BMI (not transformed) was related to the interaction of treatment with time (β=0.298) or with calcium (β=−1.248) (P<0.05). Urinary excretion of isoflavones in adherent subjects (n=135) significantly predicted these changes in breast composition. Based on the modeling results, after an average of 1.2, 2.2 and 3.3 years of supplementation, a mean decrease of FGBT by 5.3, 12.1, and 19.3 cc, respectively, and a mean decrease of FGBT% by 1.37, 2.43, and 3.50%, respectively, were estimated for isoflavone exposure compared to placebo treatment. Subjects with maximum isoflavone excretion were estimated to have 38 cc less FGBT (or ~3.13% less FGBT%) than subjects without isoflavone excretion. Decrease in FGBT and FGBT% was more precise with daidzein than genistein.
Conclusions:
Soy isoflavones can induce a time- and concentration-dependent decrease in FGBT, a biomarker for breast cancer risk, in premenopausal women, and moderate effects of calcium on BMI and breast fat, suggesting a beneficial effect of soy consumption. Trial registration: www.clinicaltrials.gov identifier: NCT00204490.
Keywords: soy, isoflavones, daidzein, genistein, breast density, breast cancer prevention
1. Introduction
Breast cancer is the second leading cause of death among women [1, 2]. Associations between amounts of mammographic parenchymal tissue (‘breast density’) and breast cancer risk have been recognized since the mid-1970’s [3, 4]. Risk of developing breast cancer in women with extremely dense breasts is estimated to be 4- to 6-fold in excess of that risk in women with minimal breast density [5–7]. Byrne et al. [8] showed that increased breast cancer risk after postmenopausal estrogen and progestin combination therapy is mediated by increased breast density. Tamoxifen is highly effective in preventing breast cancer and also reduces breast density [9].
Observational studies suggest that soy consumption reduces occurrence of breast cancer and the risk of death in women with breast cancer [10–13]. However, isoflavones had no effect on breast density in randomized clinical trials (RCTs) in postmenopausal women, [14–24]. We hypothesized that soy isoflavones would decrease fibroglandular breast tissue (FGBT) in premenopausal women, who have higher levels of endogenous ovarian hormones, and that this change would be best demonstrated by magnetic resonance imaging (MRI). We also studied the time-dependence of isoflavone exposure, effects of individual differences in isoflavone metabolism [25–27], and differential effects of daidzein and genistein. Premenopausal women were chosen because they have higher levels of endogenous ovarian hormones which may interact with soy isoflavones. Moreover, populations consuming legumes usually do so life-long and not just after menopause. Epidemiologic studies suggest that early or adolescent exposure to soy may be important in reducing breast cancer risk [28–30].
2. Study Design and Methods
2.2. Study Design
This single-site, parallel, two-arm, repeated measures, randomized, double-blind, placebo-controlled study examined the effects of an oral supplement containing soy isoflavones on the primary outcome of breast tissue composition measured at yearly intervals. Other outcomes including blood chemistries, blood pressure, and BMI were measured at quarterly intervals primarily as indicators of safety as previously described [31–34]. The protocol was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB), and written informed consent was obtained from all subjects. Trial outcomes remained unchanged, with no interim analysis. The trial was registered at www.clinicaltrials.gov under identifier NCT00204490.
2.2.1. Participants
As detailed previously [32], healthy women 30–42 years of age with monthly menstrual cycles were recruited from the Houston-Galveston area. Exclusion criteria were use of exogenous hormones, contraceptive medications, medications known to affect mammographic density, any medically prescribed diets within the past 6 months, current pregnancy or lactation, peri- or post-menopausal status, or a personal or family history of breast cancer, or any prior history of breast surgery including breast augmentation, reduction, or lifting.
2.2.2. Intervention agents
Isoflavone and placebo pills appeared identical and were designed and supplied at no cost to the study by Dr. Brent Flickinger of Archer Daniel Midland Co. (Decatur, IL) [31, 32]. Each isoflavone pill contained 246 mg of Nova soy from a single lot, providing 30 mg daidzein, 30 mg genistein, and 8.3 mg glycitein, totaling 68.3 mg of isoflavones as aglycone equivalents, of which 90 mol-% were glycosides (daidzin, genistin, and glycitin). In our previous studies these amounts of isoflavones decreased levels of ovarian hormones in premenopausal women [35]. Each placebo pill contained 246 mg of a carbohydrate filler. Both pills also contained 15 mg of riboflavin, 60 mg sorbitol, 3 mg magnesium stearate, and 676 mg dicalcium phosphate for a total of 1,000 mg per tablet. The stability of the isoflavones in the pills was monitored [31, 36].
2.2.3. Randomization.
The study statistician generated the randomization list using the PLAN procedure in SAS©. Using this list, the UTMB research pharmacy assigned subjects to isoflavone or placebo groups at a 1:1 ratio in blocks of six and to one of three sub-groups within each block for scheduling of first and subsequent treatment visits. Study pills were dispensed in blister packs, with each daily blister containing two assigned study pills and one prenatal vitamin pill (Rugby Prenavite Prenatal Formula, Swanson Health Products, Duluth, GA). Subjects ingested these three pills daily for five days per week for up to 2 years or 24 menstrual cycles. Subjects, research staff, and investigators were blinded to the treatment assignment.
2.2.4. Study visits
Study visits occurred only during the luteal phase of the subjects’ menstrual cycles. Subjects were required to contact the study team on the first day of menstrual spotting for every cycle during the study and for scheduling. Table 1 outlines the visit schedule. Due to cyclical nature of the ovarian hormones and to obtain more representative baseline values, four baseline visits were scheduled, two in each of two separate cycles to provide blood and urine samples. Treatment was started on the second day of the menstrual period that immediately followed the 4th baseline visit. All treatment visits occurred once every three menstrual cycles except for the first treatment visit, which for the three pre-randomized subgroups occurred after one, two, or three menstrual cycles on study pills, respectively. This staggering of the first visit eased the scheduling of subsequent study visits and importantly also provided study data for every menstrual cycle during the intervention phase.
Table 1:
Study visit schedule (X=1 visit) by menstrual cycle month (2 menstrual cycles at baseline and ~24 menstrual cycles during treatment) in a study of isoflavones-induced changes in breast density at yearly intervals and other outcomes at quarterly intervals in premenopausal women
| Group & Subgroup | Menstrual cycle month | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Intervention | |||||||||||||
| N | −1 & −2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12† | |
| Control/1 | 33 | X, X | X | X | X | X | ||||||||
| Control/2 | 33 | X, X | X | X | X | X | ||||||||
| Control/3 | 33 | X, X | X | X | X | X | ||||||||
| Soy/1 | 33 | X, X | X | X | X | X | ||||||||
| Soy/2 | 33 | X, X | X | X | X | X | ||||||||
| Soy/3 | 33 | X, X | X | X | X | X | ||||||||
Pattern continued through year 2 (N, up to 24 cycles)
2.2.5. Specimen collections
Subjects arrived at each study visit after an overnight fast and provided a 12-hr overnight urine collection for measurement of daidzein and genistein (as markers of isoflavone exposure) and riboflavin (an adherence marker for pill ingestion) [31]. Fasting blood chemistries, including serum calcium (Ca2+), were analyzed by the UTMB hospital certified clinical laboratory using a VITROS® 5.1 FS analyzer (Ortho-Clinical Diagnostics, Rochester, NY). Demographic and reproductive information was obtained at the first visit and anthropometric values at each visit.
2.2.5.1. Analyses of isoflavones
The amounts of daidzein and genistein in the study pills and excreted in urine were measured by a gas-chromatography flame-ionization detection method after enzymatic hydrolysis of the conjugated isoflavones, as described [31, 36, 37].
2.2.5.2. Adherence/compliance
Riboflavin was incorporated into both study pills and measured in urine by a HPLC-fluorescence detection method to document ingestion adherence [31, 36]. Urinary excretion of riboflavin at concentrations ≥ 1.42 μg/ml (Youden index) in a 12-hour urine collection was used to categorize each subject as adherent or non-adherent in taking study pills within 12 hours preceding each follow-up visit, as we reported previously [31, 36].
2.2.6. Breast imaging
Breast images were obtained using a Senographe 2000D Full Field Digital Mammographic unit and a Signa LX 1.5-Tesla MRI system (General Electric Healthcare Institute, Waukesha, WI) as described previously [38, 39] during the luteal phase once before, and yearly (or 12 menstrual cycles) after starting study pills. A negative pregnancy test on spot urine was ascertained immediately before each breast imaging. Patients were imaged using the same mammography unit and MRI scanner during the entire study using approved clinical protocols. An external standard was included with each mammogram to monitor machine drift. The reproducibility of these two breast imaging protocols have been described [38, 39]. Figure 1 highlights key steps of breast model generation (A-C) and breast tissue segmentation (D-F). Briefly, breast MRI images (A) were obtained using a clinically used pulse sequence, a 3-dimensional (3-D) T1-weighted gradient-echo pulse sequence with a body coil to maximize signal uniformity for each tissue type (A-B). A standard breast coil was not utilized because this coil’s geometrical design made some signal receiver elements closer to some breast tissue parts than others, which tends to produce non-uniform signal intensity throughout the breast and would hinder our ability to identify changes in breast tissue.
Figure 1:
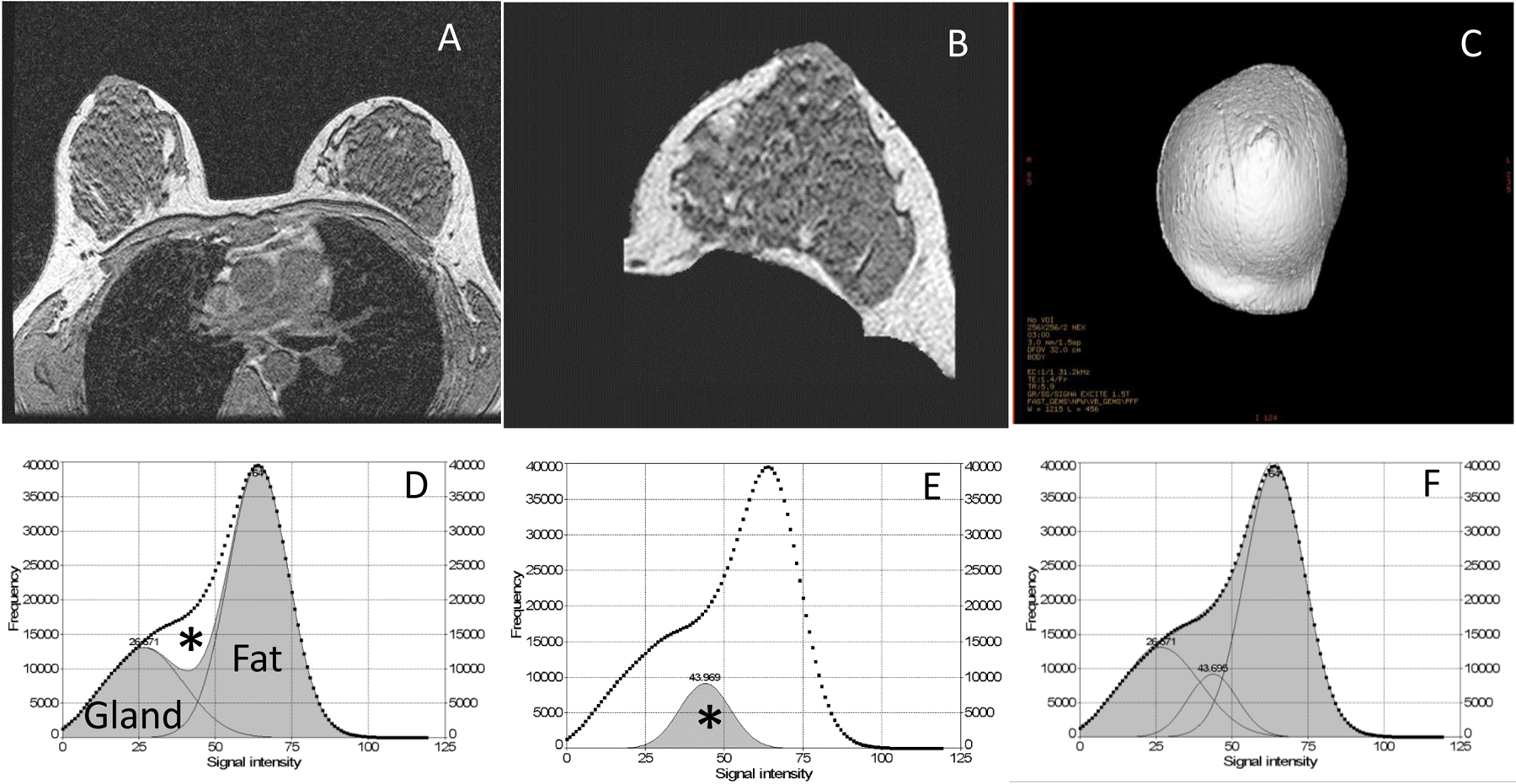
Steps in processing breast tissue magnetic resonance imaging (MRI) results and generating 3-dimensional (3-D) volume-rendered models (panels A-C) using the 3-D Advantage Windows Workstation (version 4.1, General Electric Healthcare Institute, Waukesha, WI). Voxel signal intensity histograms (panel D) of generated 3-D breast models (panel C) and a commercial PeakFit 4.0 software (SyStat Software Inc., San Jose, CA) were used to estimate percentage of glandular tissue (panels D-F). Image acquisition parameters [39] were most often a field of view of 320 mm × 320 mm, with adjustment for differences in breast size, a matrix size of 256 mm × 256 mm (reconstructed 512 mm × 512 mm), a constant slice thickness of 1.5 mm (interpolated allowing number to be varied with breast sizes), a zero interspace gap, a repetition time of 5.9 ms, echo time of 1.4 ms, and a total scan time of 3 min. Panel A shows a slice of a representative scan field of view consisting of both breasts and nearby tissue without other parts of the torso. Panel B shows a breast slice image after cropping off the chest wall and other non-breast tissue and subtracting air to finally represent only the breast region of interest. Panel C shows the final 3D view ready for tissue volume analysis after resampling and reconstructing the breast model from all MRI slices of breast regions of interest. Panel D shows a voxel signal intensity histogram from a representative breast model shown in panel C with two Gaussian curves fitted for fibroglandular and adipose breast tissue, respectively (shaded areas; the area under the dotted line is the sum of voxels of the total breast; minimizing residual curve fitting Rsqr of 0.92). Panel E shows a partial volume peak, a Gaussian curve fit corresponding to the unfitted area (*) in panel D and representing a typical mixture of both glandular and fatty breast tissue. Panel F shows the sum of all three Gaussian curve fits (glands, fat and mixture peaks), achieving a final curve fitting Rsqr of 0.999. This figure is modified from Lu et al. [39].
2.2.6.1. Breast tissue analysis
Cranial-caudal views of the same breast (most often left breast) before and after intervention were used for generating a 3-D breast model (Fig. 1C). Assuming a two-compartment model of fibroglandular and fatty breast tissue, the glandular peak area and half of the mixed tissue peak area (* in Fig. 1D–E) were combined as FGBT and the fat peak area plus half of the mixture tissue peak area as FBT. FGBT% is FGBT/(FGBT+FBT) × 100. The total breast volume for the resampled/reconstructed 3-D breast model (Fig. 1C) was estimated using the manufacturer’s software. Thus, FGBT volume (cc) = total breast volume (cc) × FGBT%/100, and the fatty breast tissue (FBT) (cc) = total breast volume (cc) × (1-FGBT%/100).
2.3. Statistical analyses
2.3.1. Sample size estimates
Breast composition was the primary outcome of interest. Since MRI can apply a standardized, and highly reproducible, set of imaging parameters to acquire breast images, MRI is more appropriate than X-ray mammography for measuring FGBT changes during intervention. The acquisition parameters for X-ray mammography machines are routinely adjusted to equalize the overall appearance of breast images across all breast densities, thereby regressing all breast density values toward the mean density value. To address these complexities, effects of isoflavones on mammographic density will be a subject of a separate communication. Because breast imaging is not a standard of care for 30–42 years old women, the sample size was estimated for standardized effect size. Assuming no change in breast composition with placebo over 2 years and an expected attrition rate of 15%, 100 subjects per arm would provide 80% power to detect a difference in change of ≥ 0.43 standard deviation (SD) with a 0.05 two-sided significance level using a two-sample t-test. Based on the density distribution of our subjects at baseline (see Table 2), we can detect a difference of ≥ 6% in FGBT%, ≥ 36 cc in FGBT, and ≥171 cc in FBT between the two comparison groups. Even greater power would be expected when analyzed by repeated measure linear mixed effect models (LME).
Table 2:
Baseline characteristics of all premenopausal women randomized to placebo or isoflavones
| Placebo | Isoflavones | |||||
|---|---|---|---|---|---|---|
| Characteristics* | n | Mean (SD) | n | Mean (SD) | ||
| Age at consent (year) | 98 | 37.35 | (3.45) | 99 | 37.58 | (2.95) |
| Race/ethnicity (%) | 98 | 99 | ||||
| Hispanic | 39 | 39.80 | 51 | 51.52 | ||
| African American | 12 | 12.24 | 14 | 14.14 | ||
| White | 47 | 47.96 | 34 | 34.34 | ||
| BMI (kg/m2) | 98 | 29.17 | (6.33) | 99 | 28.45 | (6.05) |
| Waist-to-hip ratio | 97 | 0.83 | (0.06) | 99 | 0.83 | (0.06) |
| Urinary level | ||||||
| Riboflavin (μg/mL) | 91 | 0.66 | (1.00) | 93 | 0.82 | (1.72) |
| Daidzein (mg/h) | 90 | 0.06 | (0.03) | 89 | 0.07 | (0.04) |
| Genistein (mg/h) | 90 | 0.08 | (0.05) | 89 | 0.08 | (0.04) |
| Serum level | ||||||
| Cholesterol (mg/dL) | 98 | 182.00 | (30.40) | 99 | 182.90 | (27.78) |
| HDL (mg/dL) | 98 | 53.05 | (12.35) | 99 | 55.87 | (10.47) |
| LDL (mg/dL) | 98 | 109.00 | (26.79) | 99 | 108.50 | (24.73) |
| Albumin (g/dL) | 98 | 4.28 | (0.34) | 99 | 4.28 | (0.26) |
| Calcium (mg/dL) | 98 | 9.01 | (0.35) | 99 | 9.05 | (0.28) |
| Magnetic resonance image† | ||||||
| Total breast (cc) | 97 | 829.20 | (448.50) | 95 | 807.60 | (424.30) |
| Fibroglandular breast tissue (FGBT) (cc) | 97 | 164.80 | (90.03) | 95 | 166.50 | (85.42) |
| Fatty breast tissue (FBT) (cc) | 97 | 664.40 | (427.40) | 95 | 641.00 | (399.30) |
| FGBT%, % of total breast | 97 | 23.86 | (13.99) | 95 | 24.76 | (14.54) |
| Mammographic density, % of total breast§ | 98 | 26.73 | (10.83) | 96 | 27.77 | (11.62) |
| Study visits | 98 | 8.60 | (3.00) | 99 | 8.70 | (3.10) |
Variables were average of 4 baseline screening visits except for race/ethnicity. Study visits were mean number of baseline and completed follow-up visit. All P values > 0.05 for two group comparisons.
One placebo and 4 isoflavone subjects had missing or unsatisfactory baseline MRI.
A signal intensity from a pixel signal intensity histogram of each digital mammogram was selected to best segment FGBT from FBT, and pixel counts in FGBT was divided by pixel counts in total breast. Baseline mammograms were missing for 3 isoflavone subjects.
2.3.2. Statistical analysis
MRI outcomes were FGBT (cc), FBT (cc), and FGBT% in the same breast at baseline and after 1 and 2 years of supplementation. For the intention-to-treat analyses, exposure was treatment assignment (categorical data). Treatment effects were also analyzed using the measured urinary excretion rates of daidzein (DE), genistein (GE) and the sum and difference in their excretion (DE+GE and DE–GE, respectively) as exposure, while excluding treatment assignment in the models. DE+GE and DE–GE tested for potential synergism or antagonism in effects of these two major isoflavones. The mean of 4 measurements of DE, GE, riboflavin excretion, BMI, and Ca2+ before each breast MRI was predictor variable for FGBT, FBT, and FGBT%. The time of exposure to treatment was the exact numbers of days (expressed as years) on supplements before each breast exam.
All LME regression models accounted for inter-subject heterogeneity and for within-subject dependence of repeated measures. These models included duration on treatment, Ca2+, and interaction between duration and isoflavones and between Ca2+ and isoflavones, with or without adjustment for race and ethnicity, age at entry to the study, and BMI at each study visit. Ca2+ and isoflavone excretion were mean centered. The corresponding assumption of LME models and potential outliers or influential points were assessed by residual analyses. Square root transformed FGBT, FGBT% and FBT accommodated non-symmetric distribution in residual analyses and were used for LME model analyses and for presenting results, unless stated otherwise. Effects of isoflavones on BMI (not transformed) were also analyzed. The model fit was assessed using the conditional Akaike information criterion (CAIC) [40]. All tests of statistical significance were two-sided with a P<0.05 indicating a significant difference. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
3. Results
Enrollment, treatment assignment and intervention:
This study was completed between January 2003 and August 2012 and prolonged unexpectedly due to drop-outs and disruption of study activities due to Hurricane Ike in 2008. This exhausted funds for immunoassays of ovarian hormones which the protocol stipulated batch analysis for all samples from a participant after completion of the study. Figure 2 is a CONSORT flow diagram of trial progress [41], and enrollment statistics as previously reported [32–34]. Subjects were randomized to isoflavones (N=99) or placebo (N=98) soon after initial screening mammography. One placebo and two isoflavone subjects dropped out after mammography and before baseline MRI leaving 97 placebo and 97 isoflavone subjects with suitable baseline and/or treatment breast MRIs for inclusion in the intention-to-treat analyses. Pregnancy testing performed immediately before breast MRI was positive in six subjects who were excluded from further participation and statistical analyses. At least one treatment MRI was completed in 67 placebo and 68 to isoflavone subjects. All baseline characteristics and number of study visits (a proxy for length of participation in the study) (Table 2) and retention statistics (Fig. 2) were balanced between study groups.
Figure 2:
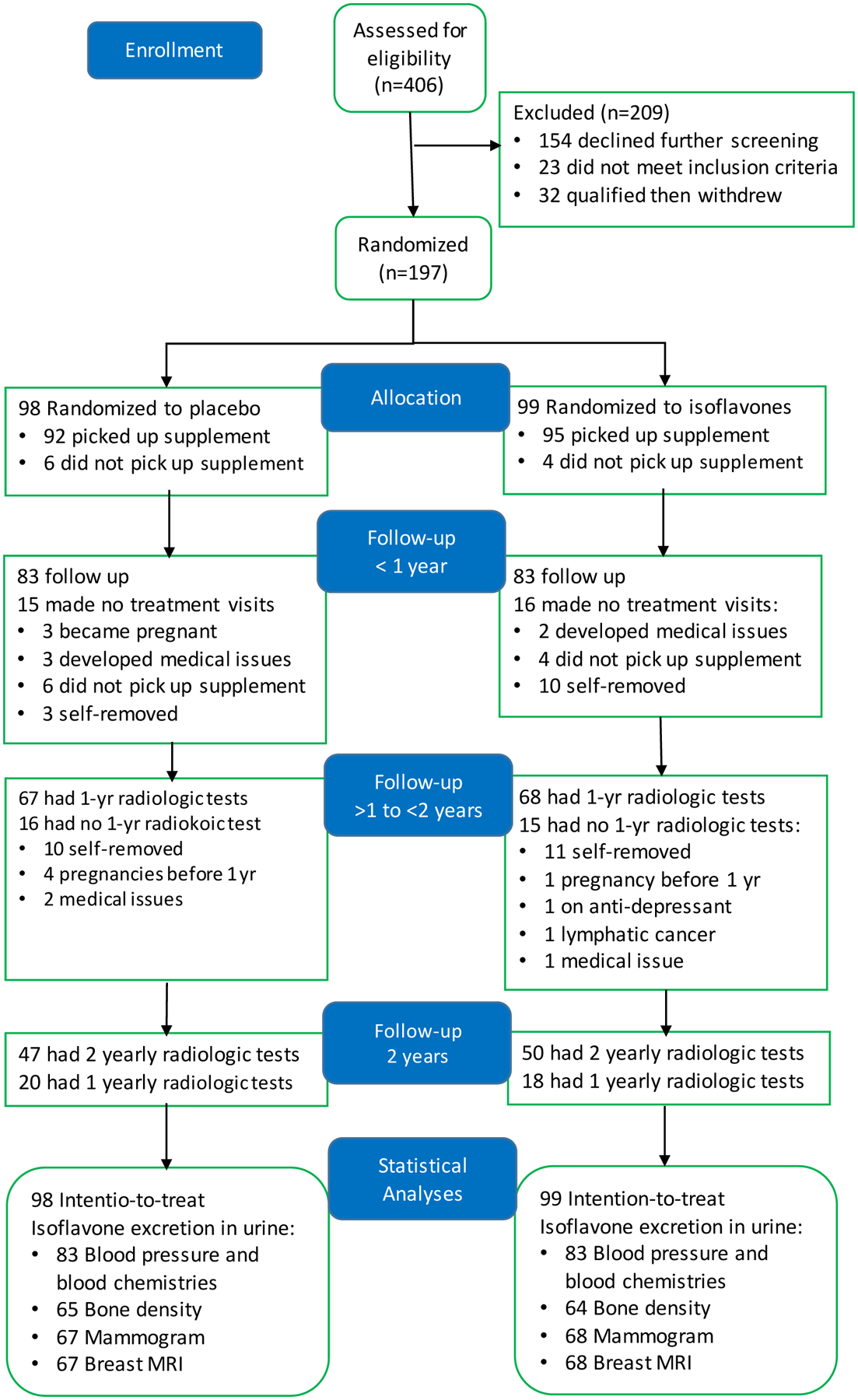
Flow diagram showing enrollment and allocation of subjects to treatment groups and observation during treatment for up to two years and prepared according to the guidelines specified in www.CONSORT-statement.org [41]. All 197 randomized subjects completed 4 baseline visits and provided blood and urine samples, and completed at least one of the three imaging tests (mammography, breast magnetic resonance imaging or bone density scan). Numbers of observations for various outcome analyses at the conclusion of the study are shown [32–34].
Effects of isoflavones on percent of changes in FGBT% from baseline.
This initial unadjusted analysis of the 135 adherent subjects, i.e., those with treatment MRI images and positive for pill ingestion assessed by urinary presence of riboflavin showed that FGBT% remained balanced at baseline between the 67 placebo and 68 isoflavone subjects (see box plots, Figure 3A). Figures 3B–C show percent of changes from baseline in FGBT% at the 1st and 2nd MRI during treatment. Differences of percent changes in FGBT% from baseline between the treatment groups at the first and second treatment MRIs were 7.96% (P=0.071) and 10.50% (P=0.080), respectively, with higher levels in the placebo group. Further statistical analyses considered effects of (i) the repeated measure study design and (ii) varying time of supplement exposure before MRI assessment due to varying menstrual cycle lengths.
Figure 3:
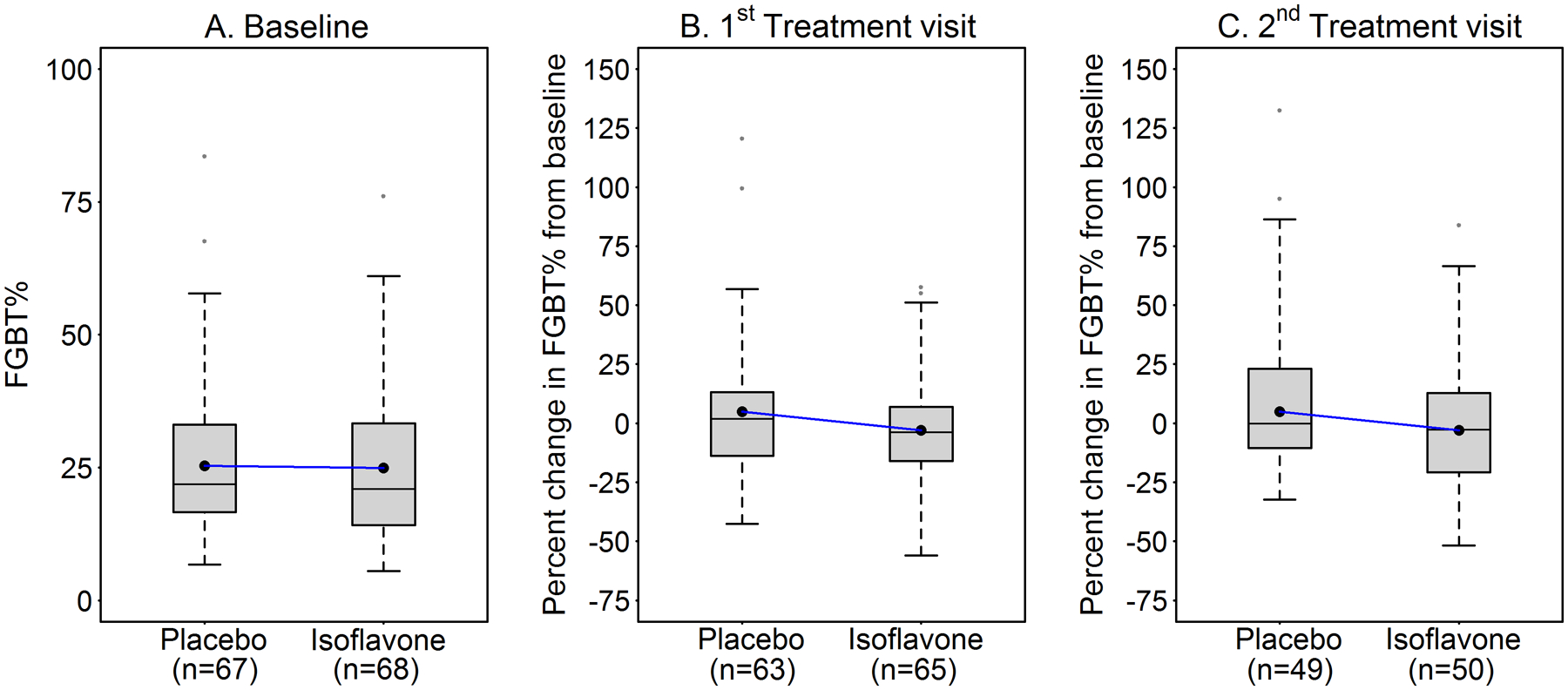
Effects of isoflavones on the unadjusted means (●---●) for fibroglandular breast tissue expressed as percent of total breast volume (FGBT%) assessed by MRI in premenopausal women at baseline and percent change from baseline at 1st and 2nd visits during treatment.
Time-dependent effects of soy isoflavones on breast composition by intention-to-treat analyses.
Results of multi-variable, hierarchical LME regression models on square-root transformed outcomes for 97 placebo subjects (referent) and 97 isoflavone subjects are shown in Table 3 and estimated means of FGBT%, FGBT and FBT are shown in Figures 4A–C, respectively, using regression model 4 adjusted for covariates at mean from Table 3. Due to timing of MRI to the luteal phase, the mean duration to the first and second treatment MRI were 1.2 and 2.2 years, respectively. Maximal duration of exposure for some subjects was 3.3 years due to interruption of Hurricane Ike. Thus, isoflavone treatment induced a time-dependent decrease in FGBT and FGBT% but without a significant effect on FBT after controlling for BMI measured at each study visit. Directions of change with time are opposite in the placebo and isoflavone groups. Note that β-estimate for time effect ranges from 0.141 to 0.161, an increase for FGBT (P from 0.054 to 0.101). Based on the modeling results, we estimated that after an average of 1.2, 2.2 and 3.3 years on supplement, the isoflavone group had a mean decrease of FGBT relative to the placebo group of 5.3 cc (95%CI: −17 to 28), 12.1 cc (95%CI: −12 to 36), and 19.3 cc (95%CI: −8 to 47), respectively, and a mean decrease of FGBT% by 1.37% (95%CI: −1.54 to 4.27); 2.43% (95%CI: −0.71 to 5.57), and 3.50% (95%CI: −0.11 to 7.12). These modelling differences remained after controlling for adherence and when the analyses were restricted to a subset of 143 subjects whose random assignment could be confirmed by urinary presence of daidzein, genistein and riboflavin [31, 32] (results not shown). Similar findings were also obtained for log-transformed outcomes (not shown).
Table 3.
Fibroglandular and fatty breast tissue in 97 premenopausal women treated with isoflavones and 97 with placebo compared by intention-to-treat analysis
| FGBT (cc)* | FBT (cc)* | FGBT%* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Effect‡,§ | β-Estimate (SE) | p value | β-Estimate (SE) | p value | β-Estimate (SE) | p value | |||
| 1 | Treatment | 0.085 | (0.470) | 0.856 | −0.318 | (1.135) | 0.780 | 0.070 | (0.196) | 0.721 |
| Duration | 0.161 | (0.083) | 0.054 | −0.062 | (0.174) | 0.720 | 0.034 | (0.035) | 0.331 | |
| Treatment × Duration | −0.184 | (0.118) | 0.122 | 0.379 | (0.247) | 0.125 | −0.101 | (0.049) | 0.040 | |
| 2 | Treatment | 0.075 | (0.470) | 0.873 | −0.267 | (1.133) | 0.814 | 0.060 | (0.195) | 0.758 |
| Duration | 0.141 | (0.086) | 0.101 | 0.022 | (0.173) | 0.900 | 0.016 | (0.035) | 0.653 | |
| Treatment × Duration | −0.188 | (0.118) | 0.113 | 0.385 | (0.240) | 0.109 | −0.104 | (0.048) | 0.032 | |
| Ca2+ | 0.265 | (0.252) | 0.295 | −1.017 | (0.469) | 0.031 | 0.225 | (0.102) | 0.028 | |
| 3 | Treatment | 0.117 | (0.472) | 0.805 | −0.466 | (1.130) | 0.681 | 0.103 | (0.195) | 0.597 |
| Duration | 0.159 | (0.087) | 0.069 | −0.063 | (0.173) | 0.716 | 0.034 | (0.035) | 0.325 | |
| Treatment × Duration | −0.238 | (0.126) | 0.061 | 0.620 | (0.250) | 0.014 | −0.155 | (0.051) | 0.003 | |
| Ca2+ | 0.030 | (0.328) | 0.928 | 0.068 | (0.600) | 0.910 | −0.017 | (0.131) | 0.894 | |
| Treatment × Ca2+ | 0.570 | (0.511) | 0.266 | −2.705 | (0.944) | 0.005 | 0.588 | (0.204) | 0.004 | |
| 4† | Treatment | 0.084 | (0.462) | 0.857 | 0.505 | (0.670) | 0.452 | −0.022 | (0.160) | 0.891 |
| Duration | 0.157 | (0.087) | 0.072 | −0.216 | (0.121) | 0.076 | 0.057 | (0.031) | 0.067 | |
| Treatment × Duration | −0.258 | (0.126) | 0.042 | 0.248 | (0.176) | 0.160 | −0.107 | (0.045) | 0.019 | |
| Ca2+ | −0.028 | (0.329) | 0.932 | −0.058 | (0.460) | 0.423 | −0.029 | (0.118) | 0.807 | |
| Treatment × Ca2+ | 0.717 | (0.514) | 0.164 | −1.223 | (0.718) | 0.090 | 0.388 | (0.184) | 0.035 | |
| BMI | 0.038 | (0.033) | 0.254 | 1.079 | (0.048) | < 0.001 | −0.141 | (0.012) | < 0.001 | |
Fibroglandular breast tissue expressed as volume (FGBT) or percent of total breast tissue (FGBT%) and fatty breast tissue as volume (FBT). Regression coefficient (β-Estimate), corresponding standard error (SE), and p value are for square-root transformed outcomes.
Model 4 also included age at entry to the study and race.
Duration expressed in years.
Serum Ca2+ is mean-centered.
Figure 4:
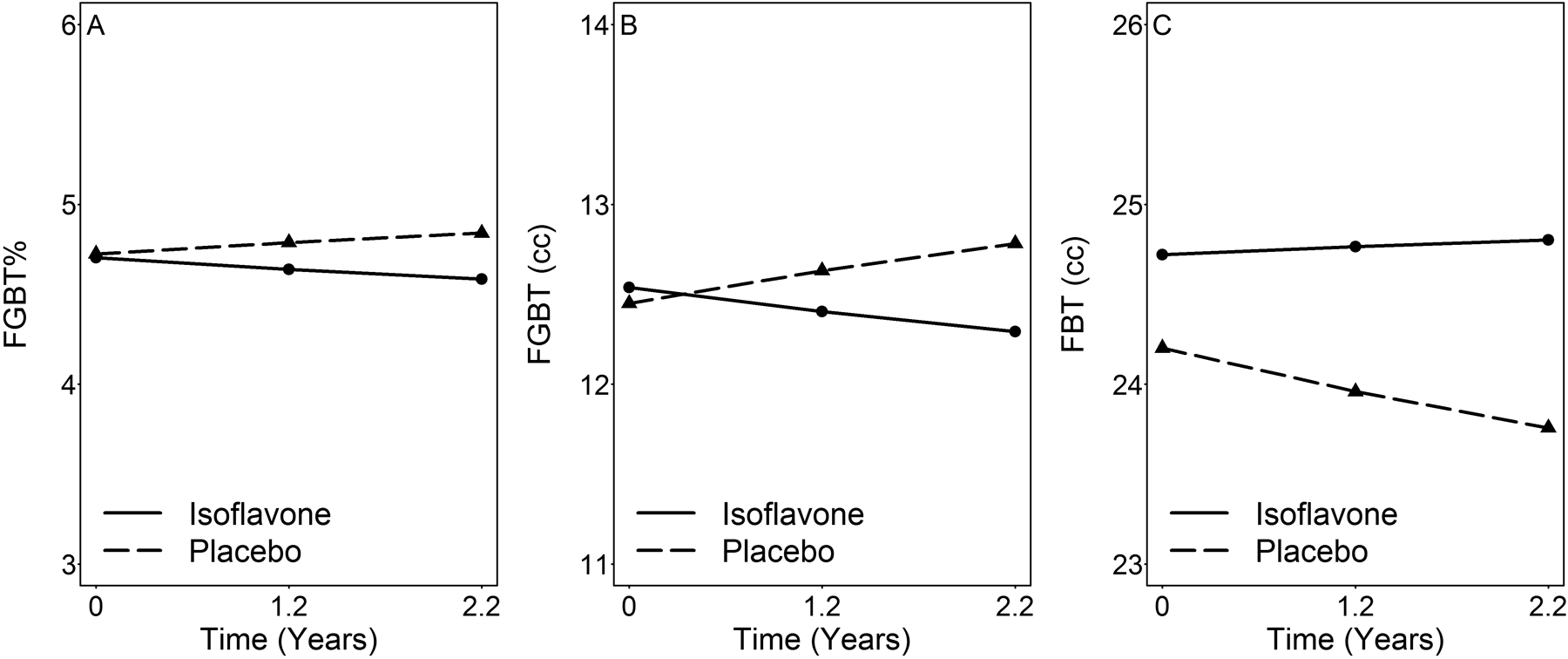
Time-dependent changes in the model-adjusted means of breast tissue components measured by MRI in premenopausal women treated with isoflavones (n=97) or placebo (n=97) after mean treatment periods of 1.2 and 2.2 years. These estimates using model 4 (Table 3) were for square root transformed outcomes. FGBT, fibroglandular breast tissue in cc, FGBT%, FGBT as percent of total breast, and FBT, fatty breast tissue in cc.
Soy isoflavones also affected breast composition when analyzed based on urinary excretion of isoflavone metabolites.
Although daidzein and genistein were ingested at a 1:1 ratio, the ratio of DE to GE varied from 0.9 to 8.9. In studying effects of this variability, we found that DE, GE, DE+GE, and DE–GE were all inversely associated with FGBT and FGBT% (among 67 placebo and 68 isoflavone adherent subjects, Table 4). For FBT, β-estimates were all negative for Ca2+ and interactions of Ca2+ with DE, GE, DE+GE or DE–GE were significant for before (model 3, all P<0.05) but not after controlling for age, race, and BMI (model 4), with BMI being the major confounder. Subjects with maximum levels of DE, GE, DE+GE and DE–GE were estimated to have 30 to 38 cc less FGBT (a decrease of 2.84 to 3.13% in absolute FGBT%, all back-transformed data) than did those with no isoflavone excretion, with this representing a decrease of about 18 to 23% from baseline values in glandular tissue after isoflavone exposure. The CAIC values for models predicting FGBT were 1458.6 for DE–GE as a predictor, 1459.3 for DE, 1460.0 for DE+GE, and 1463.2 for GE. The CAIC values for models predicting FGBT% were 720.1 for DE–GE or DE as a predictor, 720.2 for DE+GE, and 721.3 for GE [40, 42].
Table 4.
Effects of daidzein and genistein excretion rates as a measure of isoflavone exposure on square-root transformed FGBT, FBT and FGBT% in a double blind, placebo-controlled study of premenopausal women, 68 assigned to isoflavones and 67 to placebo.
| Outcome | Effect | Model 1† | Model 2† | Model 3† | Model 4† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoflavones | Model 1, Calcium (Ca2+) |
Model 2, Isoflavones × Ca2+ |
Model 3, Age at entry, Race, BMI |
||||||||||
| β-Estimate (SE) | p value | β-Estimate (SE) | p value | β-Estimate (SE) | p value | β-Estimate (SE) | p value | ||||||
| FGBT (cc) | Daidzein (DE) | −0.413 | (0.162) | 0.011 | −0.430 | (0.162) | 0.009 | −0.444 | (0.163) | 0.007 | −0.460 | (0.164) | 0.005 |
| Ca2+ | 0.287 | (0.255) | 0.262 | 0.326 | (0.258) | 0.207 | 0.372 | (0.260) | 0.155 | ||||
| DE×Ca2+ | 0.482 | (0.509) | 0.345 | 0.597 | (0.514) | 0.247 | |||||||
| Genistein (GE) | −1.064 | (0.567) | 0.062 | −1.135 | (0.569) | 0.047 | −1.151 | (0.569) | 0.044 | −1.180 | (0.572) | 0.040 | |
| Ca2+ | 0.283 | (0.257) | 0.271 | 0.308 | (0.260) | 0.237 | 0.348 | (0.262) | 0.185 | ||||
| GE×Ca2+ | 1.073 | (1.709) | 0.531 | 1.407 | (1.726) | 0.416 | |||||||
| DE + GE | −0.314 | (0.128) | 0.015 | −0.328 | (0.129) | 0.011 | −0.338 | (0.129) | 0.009 | −0.350 | (0.130) | 0.008 | |
| Ca2+ | 0.289 | (0.255) | 0.259 | 0.326 | (0.258) | 0.208 | 0.372 | (0.261) | 0.156 | ||||
| (DE + GE)×Ca2+ | 0.356 | (0.399) | 0.373 | 0.446 | (0.403) | 0.270 | |||||||
| DE − GE | −0.571 | (0.215) | 0.008 | −0.589 | (0.215) | 0.007 | −0.610 | (0.215) | 0.005 | −0.635 | (0.216) | 0.004 | |
| Ca2+ | 0.279 | (0.254) | 0.274 | 0.319 | (0.257) | 0.215 | 0.365 | (0.259 | 0.161 | ||||
| (DE − GE)×Ca2+ | 0.688 | (0.686) | 0.317 | 0.841 | (0.692) | 0.226 | |||||||
| FBT (cc) | Daidzein (DE) | 0.296 | (0.296) | 0.318 | 0.353 | (0.297) | 0.237 | 0.393 | (0.293) | 0.181 | 0.120 | (0.231) | 0.606 |
| Ca2+ | −1.096 | (0.476) | 0.022 | −1.268 | (0.475) | 0.008 | −0.571 | (0.368) | 0.122 | ||||
| DE×Ca2+ | −2.289 | (0.932) | 0.015 | −0.211 | (0.726) | 0.771 | |||||||
| Genistein (GE) | 1.446 | (1.018) | 0.157 | 1.670 | (1.027) | 0.105 | 1.705 | (1.016) | 0.095 | 0.981 | (0.801) | 0.222 | |
| Ca2+ | −1.117 | (0.474) | 0.019 | −1.254 | (0.475) | 0.009 | −0.585 | (0.366) | 0.112 | ||||
| GE×Ca2+ | −6.400 | (3.085) | 0.039 | −0.012 | (2.414) | 0.996 | |||||||
| DE + GE | 0.260 | (0.234) | 0.266 | 0.308 | (0.235) | 0.192 | 0.336 | (0.232) | 0.149 | 0.125 | (0.183) | 0.495 | |
| Ca2+ | −1.102 | (0.475) | 0.021 | −1.273 | (0.475) | 0.008 | −0.575 | (0.368) | 0.119 | ||||
| (DE + GE)×Ca2+ | −1.762 | (0.728) | 0.016 | −0.135 | (0.568) | 0.813 | |||||||
| DE − GE | 0.310 | (0.392) | 0.431 | 0.377 | (0.394) | 0.340 | 0.437 | (0.389) | 0.262 | 0.069 | (0.307) | 0.823 | |
| Ca2+ | −1.085 | (0.476) | 0.024 | −1.249 | (0.475) | 0.009 | −0.563 | (0.367) | 0.126 | ||||
| (DE − GE)×Ca2+ | −3.086 | (1.260) | 0.015 | −0.364 | (0.128) | 0.710 | |||||||
| FGBT% | Daidzein (DE) | −0.138 | (0.066) | 0.038 | −0.151 | (0.066) | 0.022 | −0.161 | (0.066) | 0.015 | −0.126 | (0.059) | 0.035 |
| Ca2+ | 0.239 | (0.104) | 0.022 | 0.267 | (0.104) | 0.011 | 0.145 | (0.094) | 0.127 | ||||
| DE×Ca2+ | 0.338 | (0.206) | 0.102 | 0.086 | (0.187) | 0.646 | |||||||
| Genistein (GE) | −0.396 | (0.229) | 0.086 | −0.453 | (0.230) | 0.050 | −0.466 | (0.229) | 0.043 | −0.380 | (0.207) | 0.068 | |
| Ca2+ | 0.240 | (0.104) | 0.022 | 0.260 | (0.105) | 0.014 | 0.140 | (0.095) | 0.140 | ||||
| GE×Ca2+ | 0.855 | (0.689) | 0.216 | 0.064 | (0.623) | 0.918 | |||||||
| DE + GE | −0.107 | (0.052) | 0.042 | −0.118 | (0.052) | 0.024 | −0.125 | (0.052) | 0.017 | −0.098 | (0.047) | 0.038 | |
| Ca2+ | 0.240 | (0.104) | 0.021 | 0.267 | (0.104) | 0.011 | 0.145 | (0.095) | 0.127 | ||||
| (DE + GE)×Ca2+ | 0.255 | (0.161) | 0.114 | 0.057 | (0.146) | 0.696 | |||||||
| DE − GE | −0.184 | (0.087) | 0.036 | −0.200 | (0.087) | 0.023 | −0.214 | (0.087) | 0.015 | −0.166 | (0.079) | 0.036 | |
| Ca2+ | 0.236 | (0.103) | 0.023 | 0.264 | (0.104) | 0.012 | 0.143 | (0.094) | 0.130 | ||||
| (DE − GE)×Ca2+ | 0.469 | (0.277) | 0.092 | 0.140 | (0.252) | 0.580 | |||||||
All models included treatment duration (years). β-estimates of treatment duration, age at entry, race, and BMI are not shown. DE and GE from 12 hr urine samples and Ca2+ from sera were mean-centered.
Effects of soy isoflavones on BMI.
Because isoflavone effects on FBT became insignificant after controlling for BMI (Tables 3–4), we examined effects of isoflavones and serum Ca on BMI. As shown in Table 5, intention-to-treat analyses showed a positive treatment × time interaction, and a negative treatment × Ca2+ interaction before (Model 3) and after (Model 4) controlling for age and race (all P<0.05). Interactions of DE, GE, DE+GE or DE–GE with Ca2+ were all negative (P=0.001).
Table 5.
Effects of isoflavone exposure on BMI (kg/m2) by intention-to-treat analysis or by isoflavone excretion rates and analyzed by linear mixed effects models.
| Analysis | Effect | Model 1† | Model 2† | Model 3† | Model 4† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoflavones | Model 1, Calcium (Ca2+) |
Model 2, Isoflavones × Ca2+ |
Model 3, Age at entry, Race |
||||||||||
| β-Estimate (SE) | p value | β-Estimate (SE) | p value | β-Estimate (SE) | p value | β-Estimate (SE) | p value | ||||||
| Intention-to-Treat (97 placebo, 97 isoflavone) | Treatment | −0.655 | (0.889) | 0.462 | −0.632 | (0.888) | 0.478 | −0.721 | (0.886) | 0.417 | −1.222 | (0.838) | 0.146 |
| Duration | 0.163 | (0.097) | 0.093 | 0.206 | (0.097) | 0.035 | 0.169 | (0.096) | 0.080 | 0.169 | (0.096) | 0.080 | |
| Treatment × Duration | 0.188 | (0.137) | 0.171 | 0.191 | (0.134) | 0.154 | 0.298 | (0.139) | 0.033 | 0.295 | (0.139) | 0.035 | |
| Ca2+ | −0.503 | (0.266) | 0.059 | −0.014 | (0.343) | 0.967 | −0.041 | (0.342) | 0.905 | ||||
| Treatment × Ca2+ | −1.248 | (0.539) | 0.022 | −1.193 | (0.539) | 0.028 | |||||||
| Urinary Excretion (67 placebo, 68 isoflavone) | Daidzein (DE) | 0.169 | (0.166) | 0.311 | 0.199 | (0.167) | 0.236 | 0.237 | (0.164) | 0.150 | 0.230 | (0.164) | 0.162 |
| Ca2+ | −0.545 | (0.268) | 0.043 | −0.696 | (0.266) | 0.009 | −0.691 | (0.265) | 0.010 | ||||
| DE×Ca2+ | −1.773 | (0.519) | 0.001 | −1.750 | (0.519) | 0.001 | |||||||
| Genistein (GE) | 0.268 | (0.577) | 0.643 | 0.396 | (0.583) | 0.498 | 0.451 | (0.570) | 0.430 | 0.435 | (0.569) | 0.445 | |
| Ca2+ | −0.539 | (0.269) | 0.047 | −0.669 | (0.266) | 0.013 | −0.664 | (0.266) | 0.013 | ||||
| GE×Ca2+ | −5.749 | (1.723) | 0.001 | −5.658 | (1.722) | 0.001 | |||||||
| DE + GE | 0.120 | (0.132) | 0.365 | 0.145 | (0.133) | 0.275 | 0.173 | (0.130) | 0.184 | 0.168 | (0.130) | 0.197 | |
| Ca2+ | −0.545 | (0.268) | 0.043 | −0.698 | (0.266) | 0.009 | −0.692 | (0.265) | 0.010 | ||||
| (DE + GE)×Ca2+ | −1.405 | (0.406) | 0.001 | −1.386 | (0.406) | 0.001 | |||||||
| DE − GE | 0.258 | (0.220) | 0.244 | 0.291 | (0.221) | 0.190 | 0.343 | (0.218) | 0.117 | 0.334 | (0.218) | 0.127 | |
| Ca2+ | −0.542 | (0.267) | 0.044 | −0.684 | (0.266) | 0.011 | −0.679 | (0.265) | 0.011 | ||||
| (DE − GE)×Ca2+ | −2.276 | (0.703) | 0.001 | −2.250 | (0.703) | 0.002 | |||||||
All models included duration (year) on supplement. Results of effects of duration, age at entry, and race are not shown. DE, GE, and Ca2+ were mean-centered.
4. Discussion
To our knowledge, this is the first RCT to demonstrate that soy isoflavones can decrease fibroglandular breast tissue in women. This decrease was time-dependent (Table 3 and Figure 4) and contrasted with a progressive increase in fibroglandular tissue in the placebo group, reflecting the continued effects of premenopausal levels of circulating ovarian steroids. Note that time effect on FGBT is also an increase albeit with marginal statistical significance (Table 3). The isoflavone effect was also dependent on differences in urinary isoflavone excretion (Table 4), which reflects a combination of well-documented individual differences in isoflavone metabolism [25–27], adherence to pill ingestion [31], and incidental exposure to isoflavones containing foods. We also found evidence for differential effects of daidzein and genistein.
Previous RCTs of mixtures of isoflavones derived from soy [14, 16–22, 24] or red clover [15, 23] or genistein alone [17] found no effects on breast density (amount of fibroglandular tissue in the breast). These trials were mostly in postmenopausal women, and only one assessed breast density by MRI [24] rather than by mammography, which is known to be less accurate [38, 39]. Wu et al [24] studied mostly post-menopausal women for just one year and found no effect of soy ingestion on glandular breast tissue measured by MRI. Breast density decreases about 0.36% per year after menopause [43]. Note that in our study the positive regression coefficients of time effect alone on FGBT indicated an increasing trend of FGBT before menopause (Table 3). We studied premenopausal women who experience a continuing increase in fibroglandular breast tissue in volume and as percentage of total breast (‘breast density’), as shown in the placebo group (Figure 4), which may make an isoflavone-induced decrease more apparent in the intervention group, and also found that the decrease became most apparent after >2 years of treatment. The findings of Marini et al [17] that genistein alone had no effect on mammographic density in postmenopausal women seems consistent with our modeling results showing that excretion of genistein was less predictive of fibroglandular tissue change than daidzein. Effects on blood chemistries, blood pressure and bone density were monitored primarily as indicators of safety of isoflavones. However, we previously found unexpectedly that isoflavones interacted with serum calcium levels to moderate the effects of isoflavones on bone mineral density [33] and systolic blood pressure [34], and now report that they also moderated the effects of calcium on FBT and BMI. To our knowledge, this is the first RCT on isoflavone effects to include serum calcium in statistical models. Our observations are consistent with the fact that calcium is essential for life and is either the first or second messenger for all physiological reactions, and therefore its serum levels are controlled within a very narrow range [44].
Isoflavone effects on both FBT and BMI are similarly complex. FBT and BMI increased over time (+β) with increasing duration of isoflavone exposure, which is likely compensatory to the observed decrease in FGBT. However, effects of isoflavones on FBT and BMI are inversely conditioned by serum calcium levels such that isoflavones decrease both FBT and BMI when calcium levels are higher than mean calcium levels for the group and increase FBT and BMI when serum calcium levels are lower than the group mean. We previously reported that isoflavones increase serum calcium [34] which made it more likely that isoflavone exposure decreases rather than increases FBT and BMI. The similarity between model predictors of FBT (Table 4) and BMI (Table 5) also explains why BMI attenuated the significant interaction terms of treatment with time and with calcium as a strong predictor of FBT. Further studies are needed to determine if isoflavones exert effects on fat tissue at sites other than the breast.
We found that daidzein and genistein both predicted decreases in glandular breast tissue, but with different degrees of precision (Table 4). All daidzein-containing variables, namely DE, DE+GE and DE–GE with smaller CAIC values produced better-fit models for changes in breast glandular tissue than genistein alone, namely GE. While DE+GE and DE-GE were both significant predictors of isoflavone effects, DE–GE with a smaller CAIC value produced a better fit model than DE+GE. These results suggest that the two isoflavones may synergize and antagonize the effects of each other on breast tissue. Daidzein appears to dominate FGBT change and was also the most abundant isoflavone metabolite in urine as we and others have reported [25–27]. However, in this study DE and GE moderated the effects of serum calcium on BMI and FBT (Tables 4–5). Although differential effects of these isoflavones have been noted in nonhuman models [21], this is to our knowledge the first indication of different biological effects of these two isoflavones in humans.
Strengths of this study include our focus on premenopausal women with higher levels of ovarian hormones and breast density than postmenopausal women. Moreover, evidence suggests a greater reduction in breast cancer risk with soy exposure earlier rather than later in life [28, 30, 45, 46]. Unlike mammography, MRI applies identical imaging parameters without adjustment for breast density and is considered more accurate than mammography for measuring breast composition. MRI also does not require breast compression and produces bell-shaped glandular and fatty tissue peaks in voxel signal intensity histograms (Fig. 1D–F) which are amenable to automatic peak analysis using commercial software [38, 39]. Therefore, use of MRI may have facilitated our detection of isoflavone effects [17–24, 47]. We will describe in a separate communication the importance of adjusting mammographic instrument settings for assessing effects of isoflavones on mammographic density. Other strengths noted previously [31, 32], include stringent inclusion criteria, repeated measurements specifically during the luteal phase of the menstrual cycle, and integrating urinary excretion of both daidzein and genistein on effect on breast density which complemented intention-to-treat analyses.
5. Conclusions
In this RCT, the major soy isoflavones, daidzein and genistein, attenuated the ongoing increase in breast fibroglandular tissue in premenopausal women, with daidzein appearing to predict this treatment effect better than genistein. We previously reported that these isoflavones increase serum calcium levels and now we show that when serum calcium levels are higher, isoflavones decrease breast fat tissue and BMI. Isoflavones in soy may reduce breast cancer risk by decreasing both fibroglandular and fatty breast tissues. Increasing soy exposure beginning earlier in life may be warranted for breast cancer prevention.
Acknowledgements
The authors wish to acknowledge the nursing staff of the Institute for Translational Sciences-Clinical Research Center (ITS-CRC). We are also very grateful for the women who volunteered as subjects in this study for up to 2 years.
Funding
This work was supported by grants from the National Institute of Health (NIH) R01 CA095545 and CA065628 and utilized the resources of the Institute for Translational Sciences at the UTMB, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, NIH and by NIEHS 2 P30 ES06676.
Abbreviations used:
- FGBT
fibroglandular breast tissue
- FGBT%
FGBT as percent of breast tissue (‘breast density’)
- FBT
fatty breast tissue
- DE
daidzein excretion
- GE
genistein excretion
- SD
standard deviation
- RCT
randomized clinical trial
Footnotes
Financial and non-financial competing interests: Fatima Nayeem, Nai-Wei Chen, Donald G. Brunder, Manubai Nagamani, Thomas K. Nishino, Karl E. Anderson, Tuenchit Khamapirad, and Lee Jane Lu declare that they have no conflict of interest.
Data Availability Statement:
The data underlying this article are subject to an embargo of 12 months from the publication date of the article. Once the embargo expires the data will be available upon reasonable request to Lee Jane Lu, Ph.D. at llu@utmb.edu and can be found at www.clinicaltrials.gov under the unique identifier of NCT00204490
References
- [1].Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2016. Cancer Statistics Facts: Female Breast Cancer (https://seercancergov/statfacts/html/breasthtml). Bethesda, MD,: National Cancer Institute; 2019. [Google Scholar]
- [2].ACS. Breast cancer facts & figures 2017–2018; American Cancer Society; (https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf). 2017. [Google Scholar]
- [3].Wolfe J. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–92. [DOI] [PubMed] [Google Scholar]
- [4].Wolfe J. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol. 1976;126:1130–7. [DOI] [PubMed] [Google Scholar]
- [5].Boyd N, Guo H, Martin L, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. [DOI] [PubMed] [Google Scholar]
- [6].McCormack V, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. [DOI] [PubMed] [Google Scholar]
- [7].Bertrand KA, Scott CG, Tamimi RM, Jensen MR, Pankratz VS, Norman AD, et al. Dense and nondense mammographic area and risk of breast cancer by age and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Byrne C, Ursin G, Martin CF, Peck JD, Cole EB, Zeng DL, et al. Mammographic Density Change With Estrogen and Progestin Therapy and Breast Cancer Risk. Jnci-Journal of the National Cancer Institute. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. [DOI] [PubMed] [Google Scholar]
- [10].Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang FF, Haslam DE, Terry MB, Knight JA, Andrulis IL, Daly MB, et al. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer. 2017;123:2070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qiu S, Jiang C. Soy and isoflavones consumption and breast cancer survival and recurrence: a systematic review and meta-analysis. European Journal of Nutrition. 2019;58:3079–90. [DOI] [PubMed] [Google Scholar]
- [14].Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:195–201. [PubMed] [Google Scholar]
- [15].Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, Healey CS, et al. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004;6:R170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities.[see comment]. Journal of Nutrition. 2004;134:3089–94. [DOI] [PubMed] [Google Scholar]
- [17].Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. United States2008. p. 4787–96. [DOI] [PubMed] [Google Scholar]
- [18].Verheus M, van Gils CH, Kreijkamp-Kaspers S, Kok L, Peeters PH, Grobbee DE, et al. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2632–8. [DOI] [PubMed] [Google Scholar]
- [19].Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, et al. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res (Phila). 2012;5:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK, Lewis RD, et al. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Delmanto A, Nahas-Neto J, Traiman P, Uemura G, Pessoa EC, Nahas EA. Effects of soy isoflavones on mammographic density and breast parenchyma in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Menopause. 2013;20:1049–54. [DOI] [PubMed] [Google Scholar]
- [23].Powles TJ, Howell A, Evans DG, McCloskey EV, Ashley S, Greenhalgh R, et al. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int. 2008;14:6–12. [DOI] [PubMed] [Google Scholar]
- [24].Wu AH, Spicer D, Garcia A, Tseng CC, Hovanessian-Larsen L, Sheth P, et al. Double-Blind Randomized 12-Month Soy Intervention Had No Effects on Breast MRI Fibroglandular Tissue Density or Mammographic Density. Cancer Prev Res (Phila). 2015;8:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu LJ, Lin SN, Grady JJ, Nagamani M, Anderson KE. Altered kinetics and extent of urinary daidzein and genistein excretion in women during chronic soya exposure. Nutr Cancer. 1996;26:289–302. [DOI] [PubMed] [Google Scholar]
- [26].Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. Journal of Nutrition. 1994;124:825–32. [DOI] [PubMed] [Google Scholar]
- [27].van der Velpen V, Hollman PC, van Nielen M, Schouten EG, Mensink M, van’t Veer P, et al. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur J Clin Nutr. 2014;68:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–6. [DOI] [PubMed] [Google Scholar]
- [29].Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:483–8. [PubMed] [Google Scholar]
- [30].Korde LA, Wu AH, Fears T, Nomura AM, West DW, Kolonel LN, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–9. [DOI] [PubMed] [Google Scholar]
- [31].Ramanujam VS, Nayeem F, Anderson KE, Kuo YF, Chen NW, Ju H, et al. Riboflavin as an independent and accurate biomarker for adherence in a randomized double-blind and placebo-controlled clinical trial. Biomarkers. 2017;22:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu L-JW, Chen N-W, Nayeem F, Ramanujam VMS, Kuo Y-F, Brunder DG, et al. Novel effects of phytoestrogenic soy isoflavones on serum calcium and chloride in premenopausal women: A 2-year double-blind, randomized, placebo-controlled study. Clinical Nutrition. 2018;37:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nayeem F, Chen N-W, Nagamani M, Anderson KE, Lu L-JW. Daidzein and genistein have differential effects in decreasing whole body bone mineral density but had no effect on hip and spine density in premenopausal women: A 2-year randomized, double-blind, placebo-controlled study. Nutrition Research. 2019;68:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lu LW, Chen NW, Nayeem F, Nagamani M, Anderson KE. Soy isoflavones interact with calcium and contribute to blood pressure homeostasis in women: a randomized, double-blind, placebo controlled trial. Eur J Nutr. 2020;59:2369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu L, Anderson K, Grady J, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res. 2000;60:4112–21. [PubMed] [Google Scholar]
- [36].Ramanujam VM, Anderson KE, Grady JJ, Nayeem F, Lu LJ. Riboflavin as an oral tracer for monitoring compliance in clinical research. Open Biomark J. 2011;2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu L, Broemeling L, Marshall M, Ramanujam V. A simplified method to quantify isoflavones in commercial soybean diets and human urine after legume consumption. Cancer Epidemiol Biomarkers Prev. 1995;4:497–503. [PubMed] [Google Scholar]
- [38].Lu L, Nishino T, Khamapirad T, Grady J, Leonard MJ, Brunder D. Computing mammographic density from a multiple regression model constructed with image-acquisition parameters from a full-field digital mammographic unit. Phys Med Biol. 2007;52:4905–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lu LJ, Nishino TK, Johnson RF, Nayeem F, Brunder DG, Ju H, et al. Comparison of breast tissue measurements using magnetic resonance imaging, digital mammography and a mathematical algorithm. Phys Med Biol. 2012;57:6903–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vaida F, Blanchard S. Conditional Akaike information for mixed-effects models. Biometrika. 2005;92:351–70. [Google Scholar]
- [41].Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. International Journal of Surgery. 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- [42].Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology. 2011;65:13–21. [Google Scholar]
- [43].Krishnan K, Baglietto L, Stone J, Simpson JA, Severi G, Evans CF, et al. Longitudinal Study of Mammographic Density Measures That Predict Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2017;26:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. [DOI] [PubMed] [Google Scholar]
- [45].Lee S, Shu X, Li H, Yang G, Cai H, Wen W, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89:1920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Messina M, Hilakivi-Clarke L. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer. 2009;61:792–8. [DOI] [PubMed] [Google Scholar]
- [47].Kataoka M, Atkinson C, Warren R, Sala E, Day NE, Highnam R, et al. Mammographic density using two computer-based methods in an isoflavone trial. Maturitas. 2008;59:350–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are subject to an embargo of 12 months from the publication date of the article. Once the embargo expires the data will be available upon reasonable request to Lee Jane Lu, Ph.D. at llu@utmb.edu and can be found at www.clinicaltrials.gov under the unique identifier of NCT00204490


