Abstract
Introduction:
Topical drug delivery is highly attractive and yet faces tissue barrier challenges. Different physical and chemical methods have been explored to facilitate topical drug delivery.
Areas covered:
Ablative fractional laser (AFL) has been widely explored by the scientific community and dermatologists to facilitate topical drug delivery since its advent less than two decades ago. This review introduces the major efforts in exploration of AFL to facilitate transdermal, transungual, and transocular drug delivery in preclinical and clinical settings.
Expert opinion:
Most of the preclinical and clinical studies find AFL to be safe and highly effective to facilitate topical drug delivery with little restriction on physicochemical properties of drugs. Clinical studies support AFL to enhance drug efficacy, shorten treatment time, reduce pain, improve cosmetic outcomes, reduce systemic drug exposure, and improve safety. Considering most of the clinical trials so far involved a small sample size and were in early phase, future trials will benefit from enrolling a large group of patients for thorough evaluation of the safety and efficacy of AFL-assisted topical drug delivery. The manufacturing of small and less costly AFL devices will also facilitate the translation of AFL-assisted topical drug delivery.
Keywords: Ablative fractional laser, AFL, Transdermal, Topical, Ocular, Transungual, Microchannel, Micropore, Sustained drug delivery, Laser
1. Introduction
Topical drug delivery delivers drugs to readily accessible body locations, such as skin, nail, and eye, to treat local diseases with good patient compliance. Topical drug delivery bypasses harsh environment of the gastrointestinal tract and first-pass metabolism and usually has a good drug bioavailability. Topical drug delivery mainly relies on drug’s own properties to penetrate and reach disease sites [1]. Without help, only a small number of drugs with low molecular weight and good lipophilicity can be successfully delivered via topical routes [1].
Penetration enhancers have been explored to enhance topical drug delivery [2]. A variety of chemicals are able to enhance topical drug delivery by disruption of tissue barrier function [2]. Yet, chemical enhancers have a risk to cause tissue irritation [2]. Physical methods have also been explored to facilitate topical drug delivery, such as iontophoresis, ultrasound, and microneedles [3]. Iontophoresis utilizes electric currents to drive charged molecules into the tissue and can also breach tissue barrier function to facilitate topical drug delivery [4]. Yet, iontophoresis has a risk to cause electric burn or pain [4]. Ultrasound takes advantage of high-frequency sonophoresis to generate oscillating pressure waves or low-frequency sonophoresis to generate cavitation bubbles to disrupt lipid structures to facilitate topical drug delivery [5]. High-frequency sonophoresis mainly enhances transdermal delivery of small and lipophilic compounds and more aggressive conditions have a risk to cause tissue damage due to excessive heating [5]. Low-frequency sonophoresis can be used to enhance transdermal delivery of small chemicals and macromolecules [5]. Yet, the development of cost-effective devices suitable for delivery of diverse drugs in different patients presents a challenge [3]. Microneedles received tremendous attention to facilitate topical drug delivery in the last two decades due to its minimal invasiveness and low cost [3,6]. Drugs can be coated on microneedle surface or encapsulated during microneedle fabrication for release after insertion of microneedles into the skin [3,6]. Microneedles can also be used to generate skin microchannels (MCs) to facilitate topical drug delivery [3,6]. Yet, microneedles often induce rapid drug release and have a low drug delivery capacity.
Laser has a long history in disruption of tissue barriers to support topical drug delivery [7]. Laser parameters can be conveniently adjusted to achieve the desired ablative depths. Laser ablation can be achieved in a non-contact manner. In early times, lasers were used to ablate full-surface superficial skin to facilitate transdermal drug delivery [7]. Yet, full-surface laser ablation causes significant local reactions with slow skin recovery. Fractional photothermolysis was conceptualized in 2004 for improved skin resurfacing [8], which inspired the fabrication of non-ablative and ablative fractional lasers. Non-ablative fractional lasers deliver tiny laser beams and cause microscopic thermal damage, while ablative fractional lasers (AFLs) instantly ablate tiny skin tissues and generate skin MCs [9]. Microscopic skin damage was found to induce quick skin recovery [9], which makes fractional laser resurfacing highly attractive in aesthetic field. Almost at the same time, AFLs were explored to facilitate topical drug delivery in preclinical and clinical studies with unique advantages (Fig.1). Due to the complete removal of tissue barriers, AFLs greatly expand drugs that can be delivered via topical routes. AFL-generated MCs can be quickly and completely recovered in days, which ensures a good safety of AFL to facilitate topical drug delivery.
Figure 1.

Illustration of AFL-assisted topical drug delivery and advantages
The aim of this comprehensive review is to introduce preclinical development and clinical evaluation of AFL to facilitate transdermal, transungual, and transocular drug delivery, summarize the major findings, and discuss future directions.
2. AFL devices in topical drug delivery
There are 3 major types of AFL devices explored for topical drug delivery: carbon dioxide (CO2) laser, erbium-doped yttrium aluminum garnet (Er:YAG) laser, and handheld P.L.E.A.S.E. laser (Pantec Biosolutions). CO2 laser emits 10,600 nm wavelength light, while Er:YAG laser and P.L.E.A.S.E. laser emit 2,940 nm wavelength light. Water is the major tissue chromophore to absorb laser energies at 2,940 and 10,600 nm wavelengths. During tissue ablation, laser-generated heat dissipates to surrounding tissues and generates microthermal zones (MTZs), a phenomenon called tissue coagulation in skin resurfacing field. Tissue coagulation reduces bleeding risks during laser ablation. Er:YAG laser has an absorption coefficient of 2 × 107 cm−1 and requires less energy for tissue ablation than CO2 laser, which has an absorption coefficient of 2 × 106 cm−1 [10]. Thus, ablative fractional CO2 laser usually generates larger MTZs than the ablative fractional Er:YAG laser and the portable P.L.E.A.S.E. laser. The first two AFL types of different manufacturers and models were explored to facilitate topical drug delivery.
AFLs are usually designed for convenient adjustment of laser parameters, such as energy, percent coverage, pulse duration and frequency. Laser energy, pulse duration, and frequency affect skin MC diameter and depth and also MTZs, while percent coverage determines skin MC density. AFL-generated skin MCs are usually 50–300 µm in diameter and 50–1,850 µm in depth. Laser percent coverage is usually set at 5–20% to achieve quick skin recovery. The impact of laser parameters and skin MC depths on topical drug delivery was extensively studied in preclinical animal models.
3. Preclinical investigation of AFL to facilitate transdermal drug delivery
3.1. Skin barrier
Skin is the largest organ of human body with a surface area of 1.7 m2, which is the most accessible site for drug delivery [11]. Stratum Corneum (SC) is situated in the outmost layer of the skin and forms the major barrier for transdermal drug delivery. SC layer is comprised of highly packed and terminally differentiated corneocytes with only cellular membrane remaining. Interstitial space of corneocytes is filled with highly lipophilic materials, such as cholesterol, ceramides, and fatty acids. Only small (<500 Da) and lipophilic molecules (logP: 1–3), such as nicotine, fentanyl, estrogen, could readily permeate through the SC layer. Due to the major barrier function of the SC layer, there are only about 20 transdermal products approved for human use. The majority of large, lipophilic drugs and almost all hydrophilic drugs cannot be efficiently delivered through the transdermal route.
3.2. Franz Cell system
The ability of AFL to assist topical drug delivery in vitro has been mainly studied in Franz Cell systems. Franz Cell systems contain donor and receiver chambers and excised skin can be mounted in between to study drug deposition into or permeation across the skin. Receiver chamber often contains physiological solution, such as phosphate-buffered saline (PBS) or 0.9% saline solution. A small volume of samples can be harvested from receiver chambers to quantify drug concentrations and study cumulative drug delivery. Receiver chambers can also be surrounded by a water jacket to maintain skin temperature at around 32°C, resembling surface skin temperature of human and live animals. Donor chamber can also be removed if the drug is not in a liquid form. For example, drug creams can be topically applied after AFL treatment followed by mounting the skin on the receiver chamber to study intracutaneous and transcutaneous drug delivery. Franz Cell systems allow convenient study of the impact of laser parameters or skin MC depths on drug permeation into and across the skin. Various systems were used for in vivo delivery of topical drugs after AFL treatment depending on dosage forms and were introduced case by case.
3.3. AFL enhances transdermal delivery of different types of drugs
Drugs suitable for transdermal delivery have been mainly limited by their physicochemical properties. Relatively big lipophilic drugs and the majority of hydrophilic drugs face significant challenges to penetrate through the SC layer. AFL has been found to enhance transdermal delivery of drugs capable of penetrating the skin by their own or enable efficient transdermal delivery of chemical drugs, biologics, nano- and microparticles, and even live cells that cannot penetrate the skin by their own (Table 1 and Fig.2).
Table 1.
AFL enhances transdermal drug delivery in preclinical studies
| Laser | Drug and skin type | Delivery system and duration | Major findings | Ref |
|---|---|---|---|---|
| Drugs with the ability to permeate the skin | ||||
| Prototype CO2 laser | MAL cream/Pig skin | In vivo topical application/180 min |
|
[12] |
| Prototype CO2 laser | MAL cream/Pig skin | In vivo topical application/180 min |
|
[13] |
| Prototype CO2 laser | ALA and MAL cream/Pig skin | In vivo topical cream application/180 min |
|
[14] |
| P.L.E.A.S.E. laser | Lidocaine solution/Pig skin | In vitro Franz Cell system/24 h |
|
[15] |
| Fractional Er:YAG laser | Lidocaine cream/Pig skin | In vivo topical application/240 minutes |
|
[16] |
| P.L.E.A.S.E. laser | Diclofenac solution or gel formulation/Pig and human skin | In vitro Franz Cell system/24 h |
|
[17] |
| P.L.E.A.S.E. laser | IngMeb gel formulation/Pig skin | In vitro Franz Cell system/21 h |
|
[18] |
| Fractional Er:YAG laser | MTX solution (10 mg/mL)/Pig skin | In vitro Franz Cell system/24 h |
|
[19] |
| P.L.E.A.S.E. laser | MTX solution (10 mg/mL)/Pig skin | In vitro Franz Cell system/21 h |
|
[20] |
| P.L.E.A.S.E. laser PLGA microneedle |
MTX solution /Pig and human skin | In vitro Franz Cell system/24 h |
|
[21] |
| Chemical drugs unable to penetrate intact skin | ||||
| P.L.E.A.S.E. laser | Prednisone solution/Pig skin | In vitro Franz Cell system |
|
[23] |
| Fractional CO2 laser (UltraPulse) | SRB, MB, and TR-OVA solution/Mouse skin | In vivo gauze patch/18 h |
|
[24] |
| Fractional CO2 laser (UltraPulse) | SRB powder/Mouse, pig, and human skin | Topical application of powder array patches |
|
[25] |
| Prototype CO2 laser | PEG solution of varying MWs/Human skin | In vitro Franz Cell/16 h |
|
[26] |
| Fractional CO2 laser (UltraPulse) | Cisplatin solution/Pig skin | In vitro Franz Cell system/24 h |
|
[27] |
| Fractional CO2 laser (UltraPulse) | 5-FU cream/Pig skin | In vitro Franz Cell system/24 h |
|
[28] |
| Fractional CO2 laser (UltraPulse) | Cisplatin solution followed by 5-FU cream/Pig skin | In vivo delivery via customized wells (cisplatin/30 or 60 min and 5-FU/120 h) |
|
[29] |
| P.L.E.A.S.E. laser | Donepezil hydrochloride solution/Pig skin | In vitro Franz Cell system/24 h |
|
[30] |
| Biologics | ||||
| P.L.E.A.S.E. laser | Cyt c and FITC-BSA solution/Pig and human skin | In vitro Franz Cell system/24 h |
|
[31] |
| Fractional Er:YAG laser Fractional CO2 laser (eCO2) |
FITC, FD10, FD20, or siRNA and pDNA/Mouse skin | In vitro Franz Cell systems/24 h or in vivo glass cylinder attached to dorsal skin/2 h |
|
[32] |
| Ablative fractional Er:YAG laser and non-ablative Er:glass laser | Peptide and siRNA solution/ mouse and pig skin | In vitro Franz Cell systems/48 h |
|
[33] |
| P.L.E.A.S.E. laser | hGH solution/Pig and human skin | In vitro Franz Cell systems/48 h |
|
[34] |
| Fractional CO2 laser (UltraPulse) | Bleomycin solution/Pig skin | In vitro Franz Cell system/24 h |
|
[36] |
| Fractional CO2 laser (UltraPulse) | Bleomycin solution/Pig skin | In vivo delivery via customized wells/216 h |
|
[35] |
| Fractional CO2 laser (UltraPulse) | Bleomycin solution or 5-FU cream/Human and pig skin | In vitro Franz Cell system/19 h |
|
[37] |
| P.L.E.A.S.E. laser | ATG and Basiliximab solution/Mouse, pig, and human skin | In vitro Franz Cell systems or in vivo Franz Cell donor cell mounted on mouse skin/3 h |
|
[38] |
| Fractional CO2 laser (UltraPulse) | Nivolumab solution/Pig skin | In vitro Franz Cell systems/18 h |
|
[39] |
| Fractional CO2 laser (UltraPulse) | Nivolumab solution/Pig skin | In vivo delivery via Customized wells/4 h and 2 days |
|
[40] |
| Complex systems | ||||
| P.L.E.A.S.E. laser | FITC-BSA (in vitro), OVA and recombinant Phl p 5 (in vivo)/Mouse and pig skin | In vitro Franz Cell system (Pig skin) and in vivo mouse skin |
|
[57] |
| Fractional CO2 laser (UltraPulse) | OVA, BCG vaccine, LPS/CpG, VV-OVA powder/Mouse, pig, and human skin | In vitro and in vivo mouse and pig skin; In vitro human skin; Topical powder array patches |
|
[25] |
| Fractional CO2 laser (UltraPulse) | OVA and influenza vaccine powder/Mouse skin | In vivo immunization study |
|
[41] |
| P.L.E.A.S.E. laser | pDNA-loaded nanoparticle (dual rabies and contraceptive vaccine)/Mouse skin | In vivo immunization study |
|
[42] |
| P.L.E.A.S.E. laser | Measles vaccine-loaded microparticles/Mouse skin | In vivo immunization study |
|
[43] |
| P.L.E.A.S.E. laser | FITC and RD70, or PS-nano- and microparticle-incorporated dry PVA polymer films/Pig skin | In vitro Franz Cell systems |
|
[44] |
| Fractional CO2 laser (eCO2) Full ablative Er:YAG laser |
PRP; FITC-IFN-γ and FITC-PDGF-BB/Pig and mouse skin | In vitro Franz Cell systems (pig skin, /4 or 24 h); In vivo via Franz Donor Cells (mouse skin, 3 and 6 h) |
|
[45] |
| P.L.E.A.S.E. laser | Heparin-loaded poloxamer gel and solution/Pig skin | In vitro Franz Cell system/ |
|
[46] |
| Fractional Er:YAG laser | 20 × 106 BrdU–labeled ADSC/Pig skin | In vivo topical delivery of ADSC/4 or 48 h |
|
[47] |
| Fractional Er:YAG laser | Living human cells/Pig skin |
|
[48] | |
| Impact of dosage forms on MC filling | ||||
| Fractional CO2 laser (UltraPulse) | Shandon™ blue tissue-marking dye in 0.9% saline solution, gel, and cream/Pig skin | Ex vivo topical delivery and evenly distributed to cover the entire AFL field, and left to settle for 10 min. |
|
[49] |
| Techniques enhancing AFL-assisted drug permeation | ||||
| Fractional CO2 laser (UltraPulse) | Green biomarker liquid or PEG400/Pig skin | In vitro Franz Cell systems followed by PVP/4 h |
|
[50] |
| Fractional CO2 laser (UltraPulse) | Fluorescent drug ICG solution/Human skin | Topical ICG followed by massage, acoustic pressure wave, or PVP/24 h |
|
[51] |
| Sustained drug delivery | ||||
| P.L.E.A.S.E. laser | TA or Fluorescein/Nile Red microparticles/Pig skin | In vitro Franz Cell systems/2 days |
|
[52] |
| P.L.E.A.S.E. laser | Pentoxifylline and D-α-tocopherol succinate c-containing microparticle/Pig skin | In vitro Franz Cell system/48 h |
|
[53] |
| Fractional CO2 laser (UltraPulse) | Vismodegib oil-in-water microemulsion/Pig skin | In vitro Franz Cell system/24 h |
|
[54] |
| Fractional CO2 laser (UltraPulse) | Vismodegib oil-in-water microemulsion/Pig skin | In vivo delivery via customized wells for 4 h/evaluation lasted 9 days |
|
[58] |
| P.L.E.A.S.E. laser | Ketamine and brexanolone solution, brexanolone microemulsions/Human skin | In vitro Franz Cell systems/24 h (ketamine) |
|
[55] |
| Fractional CO2 laser (UltraPulse) | SRB, ZDV, Insulin, and anti-PD1 powder/Mouse skin | In vitro Franz Cell systems and in vivo powder array patch delivery/15 h |
|
[56] |
| Fractional CO2 laser (UltraPulse) | SRB, ZDV, BSA powder/Mouse skin | In vitro Franz Cell systems/7 days; In vivo topical reservoir patches/8 days |
|
[22] |
Figure 2.

Drug types suitable for AFL-assisted topical drug delivery
3.3.1. Chemical drugs capable of penetrating the skin by their own
Photodynamic therapy (PDT) is an attractive non-invasive treatment of actinic keratoses (AKs) and non-melanoma skin cancers (NMSCs). For this therapy, photosensitizer prodrug 5-aminolevulinate (ALA) or methyl aminolevulinate (MAL) is first applied on the lesion sites. Patients need to wait 3 h to allow ALA or MAL to penetrate the skin and converted into active photosensitizer protoporphyrin IX (PpIX) inside skin cells. The lesion sites are then cleaned and exposed to a source of red light to activate porphyrins and generate super oxygen species and other toxic compounds to destruct cancer cells. As compared to ALA, MAL is more lipophilic and can more readily penetrate the skin. Haedersdal et al. used a prototype CO2 AFL and generated deep MCs (1850 µm) in pig skin [12]. AFL treatment prompted deep tissue penetration of topically applied MAL, which further dissipated to entire dermal tissue in 3 h with MCs 3 mm apart. Haak et al. explored whether skin MC depths could affect topical MAL delivery with the same prototype CO2 AFL [13]. Skin MC depths (300, 1400, 2100 µm) showed no significant effects on porphyrin accumulation in the skin. In addition, 1 h MAL incubation with prior AFL treatment induced significantly higher porphyrin fluorescence in the surface of the skin and 2 h MAL incubation with prior AFL treatment induced significantly higher porphyrin fluorescence in the dermis when compared to 3 h MAL incubation without AFL treatment. This study supported the potential of AFL to shorten the waiting period in MAL-based PDT (MAL-PDT). Haedersdal et al. further compared the prototype CO2 AFL-assisted topical ALA and MAL delivery in pig models and found AFL treatment favorably enhanced the delivery of ALA over MAL to the deep layer of the skin in 3 h, while ALA and MAL similarly penetrated into the intact skin in 3 h [14].
Similar to ALA and MAL, lidocaine can penetrate the skin by its own. Yet, it takes 30 min to 1 h to reach deep layer of the skin to minimize the pain associated with procedures, such as blood collection and venipuncture. Bachhav et al. found AFL pretreatment could significantly increase lidocaine permeation across pig skin in Franz Cell systems [15]. Increase of pore density and lidocaine concentration significantly increased transdermal delivery, while increase of laser fluences showed a lesser effect [15]. AFL-enhanced lidocaine delivery was confirmed in vivo using a commercially available lidocaine cream. Oni et al. found AFL pretreatment could enhance transdermal delivery of lidocaine to reach systemic circulation [16]. Interestingly, skin MC depth at 250 µm elicited significantly higher peak serum lidocaine levels than other MC depths at 25, 50, and 500 µm. Although systemic delivery of lidocaine may not be desirable, this study indicated AFL was highly effective to enhance transdermal delivery of lidocaine and potentially shorten the waiting time. Diclofenac is a widely used non-steroid anti-inflammatory agent. Oral delivery of diclofenac faces rapid clearance and causes adverse reactions in gastric mucosa [17]. Bachhav et al. found AFL could be used to significantly enhance transdermal delivery of diclofenac in Franz Cell systems [17]. Furthermore, increase of pore density and laser fluence could significantly increase cumulative diclofenac permeation across the skin, while increase of laser fluence showed no significant effect on diclofenac deposition in the skin [17].
Topical Ingenol Mebutate (IngMeb) gel is approved to treat AKs due to its cytotoxic effects against fast-growing cancerous cells. Erlendsson et al. explored the ability of AFL to facilitate deep penetration of IngMeb to treat NMSCs [18]. IngMeb could readily deposit to the epidermal layer of intact skin, while dermal deposition was limited. AFL pretreatment was found to significantly enhance IngMeb deposition in the dermal tissue. Furthermore, skin MC depths did not significantly affect IngMeb uptake.
Methotrexate (MTX) is a chemotherapy and immunosuppressive drug and often prescribed as an oral dosage form with a high risk to cause adverse reactions, such as nausea/vomiting, thrombocytopenia, and leukopenia. Local delivery may reduce these adverse reactions. Taudorf et. al. explored two types of AFLs (Er:YAG, P.L.E.A.S.E.) to facilitate topical MTX delivery in Franz Cell systems [19,20]. Both AFLs were effective to enhance MTX penetration into the skin. Interestingly, coagulation zone (CZ) or MTZ provided no barrier for MTX diffusion into the surrounding tissues. Furthermore, deeper skin MCs favored MTX deposition into deep dermis. Nguyen et al. compared AFL and poly(lactic-co-glycolic acid) (PLGA) microneedles in facilitation of topical MTX delivery in pig and human skin in Franz Cell systems [21]. AFL was found to generate bigger skin MCs and was more efficient than PLGA microneedles to facilitate intracutaneous and transcutaneous delivery of MTX. This result was in line with our finding that CO2 AFL even at the relatively weak laser condition (2.5 mJ, 5% coverage) could more significantly increase transepidermal water loss (TEWL) than a stainless steel 2D microneedle (each microneedle: 700 µm in height, 200 µm in base width, and 50 µm in thickness) when similar numbers of skin MCs were created in a similar skin area (unpublished data). TEWL data hinted AFL created bigger skin MCs than microneedle insertion. This might be due to the diverse MC-creating mechanisms. AFL ablates tiny tissues to generate skin MCs, while microneedle insertion pushes tissues away to generate skin MCs [22]. After AFL treatment, skin MCs remain in its original size, while microneedle retraction leads to partial skin MC closure [22].
3.3.2. Chemical drugs unable to penetrate the skin
The majority of hydrophilic drugs are unable to penetrate the skin. Due to the aqueous feature of AFL-generated skin MCs, AFL has been tested to facilitate transdermal delivery of hydrophilic drugs including chemical and biologics drugs. Yu et al. found prednisone delivery could be significantly enhanced with prior AFL treatment [23]. Furthermore, micropore number could positively impact prednisone delivery [23]. Increase of laser fluence or micropore depths more significantly increased prednisone permeation across but not deposition into the skin especially when micropores already reached the deep epidermal layer [23]. Chen et al. evaluated the ability of CO2 AFL to enhance transdermal delivery of fluorescent model drugs sulforhodamine b (SRB), methylene blue (MB), and Texas-red ovalbumin (TR-OVA) in murine models [24]. The in vivo delivery was achieved by topical application of liquid drug-soaked gauze patches on AFL-treated skin. AFL was found to significantly enhance transdermal delivery of SRB, MB, and TR-OVA, while fluorescent drugs remained on skin surface without AFL treatment. Furthermore, AFL-treated skin achieved quick recovery in 1–2 days. This study demonstrated the high potency and safety of AFL to facilitate transdermal delivery of hydrophilic drugs. Chen et al. also explored direct powder drug delivery via AFL-generated skin MCs by coating powder drugs on the surface of adhesive patches in the same pattern as skin MCs [25]. Using SRB as a model, powder SRB was found to rapidly deposit into excised pig and human skins in vitro [25]. Haak et al. explored the prototype CO2 AFL treatment of human skin to facilitate intracutaneous and transcutaneous delivery of highly water-soluble polyethylene glycols (PEGs) with molecular weights (MWs) ranging from 400 to 3350 Da in Franz Cell systems [26]. AFL significantly enhanced intracutaneous delivery of PEGs with PEG delivery rate positively correlated with laser percent coverage. Furthermore, higher MW of PEGs showed more intracutaneous retention than transcutaneous delivery.
Cisplatin is the first FDA-approved platinum compound for cancer treatment and is effective against different types of cancer, such as lung, ovarian, head and neck, due to its ability to crosslink and cause DNA damage. Cisplatin also showed its cytotoxic effects against NMSCs. Local delivery is promising to enhance its therapeutic effects while limiting its adverse reactions. Wenande et al. found AFL could significantly enhance cisplatin delivery into and across the skin in Franz Cell systems and skin MC depth did not affect cisplatin deposition into the skin, while deeper skin MCs favored cisplatin permeation across the skin [27]. Wenande et al. also explored the ability of AFL to enhance topical delivery of 5-fluorouracil (5-FU) in Franz Cell systems. 5-FU is a hydrophilic chemotherapy agent used to treat skin cancer. AFL was found to enhance and accelerate 5-FU uptake and accumulation in deep skin layers [28]. Wenande et al. further explored the ability of AFL to enhance topical delivery of combined cisplatin and 5-FU therapy in pig skin in vivo [29]. For in vivo delivery, customized wells made from DuoDerm Hydroactive™ dressings (ConvaTec, Flintshire, UK) were secured on skin surface and further occluded with Tegaderm film after cisplatin solution loading. Cisplatin was allowed to deliver for 30 or 60 min and then removed followed by topical application of 5-FU cream. AFL enhanced peak cisplatin levels in the skin by 6 folds at 5 h when compared to non-laser-treated skin. AFL also allowed 5-FU to reach maximal skin levels within 1 h, while 5-day delivery on intact skin failed to reach such a level. Interestingly, AFL-assisted combinatorial cisplatin/5-FU therapy elicited the greatest cell apoptosis than either drug alone with no detectable systemic distribution of drugs.
Kale et al. explored AFL to assist transdermal delivery of Donepezil, a highly water-soluble medicine prescribed to treat all stages of Alzheimer’s disease [30]. AFL was further compared with microneedles to generate skin MCs to facilitate transdermal delivery of Donepezil across pig skin in Franz Cell systems [30]. Results showed that AFL could more efficiently enhance transdermal delivery of Donepezil than microneedles [30].
3.3.3. Biologics and complex systems
Besides chemical drugs, AFL has been also explored to facilitate transdermal delivery of biologics, including protein/peptide drugs, monoclonal antibodies (mAbs), and genetic materials. Bachhav et al. explored AFL to facilitate protein/peptide delivery across pig and human skin in Franz Cell systems and found AFL could significantly enhance the intracutaneous deposition of cytochrome c (Cyt c, 12.4 kDa), human growth hormone (hGH, 22 kDa), follicle-stimulating hormone (FSH, 30 kDa), and fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (FITC-BSA, 70 kDa) [31]. Further studies found increase of pore density and drug concentration significantly increased transcutaneous delivery of Cyt c and FITC-BSA, while increase of laser fluence only showed a positive effect on transcutaneous delivery of FITC-BSA [31].
Lee et al. used FITC-labeled Dextrin (FD) at 10 and 20 kDa as model systems and demonstrated significant enhancement of transcutaneous FD delivery by both Er:YAG and CO2 AFLs [32]. This study for the first time demonstrated high efficiency of AFL to enhance transdermal delivery of siRNA and plasmid DNA. Lee et al. also compared Er:YAG AFL and Er:glass non-AFL to enhance peptide and siRNA delivery across mouse Psoriasis and Atopic Dermatitis skin [33]. Results indicated AFL but not non-AFL enhanced macromolecule delivery on barrier-disrupted psoriasiform skin. Song et al. explored the potential of AFL to facilitate transdermal delivery of hGH [34]. Results showed that increase of laser energy from 34.1 to 45.4 J/cm2 or 68.1 J/cm2 but not from 45.4 to 68.1 J/cm2 significantly increased hGH delivery across AFL-treated pig and human skin. Increase of laser percent coverage also significantly increased hGH permeation.
Bleomycin is a non-ribosomal peptide and an anti-cancer chemotherapy drug to treat a variety of tumors, such as melanoma, sarcoma, and ovarian cancer. Bleomycin was also reported to be effective to treat non-malignant (e.g., keloids) and malignant skin conditions (e.g., basal cell carcinoma (BCC)) after intralesional delivery. Hendel et al. explored AFL to facilitate topical delivery of Bleomycin in pig models [35,36]. In vitro Franz Cell studies found AFL could enhance transdermal delivery of Bleomycin with deeper skin MCs leading to higher drug concentrations in the skin. In vivo studies found AFL-assisted delivery led to a horizontal belt-shaped BLM distribution, while needle injection resulted in a radiating BLM distribution from the injection site. In vivo studies further found electroporation (EP) did not significantly change BLM delivery at any time point in both deliveries. AFL-assisted delivery induced almost complete skin recovery in 9 days. Rosenberg et al. compared Bleomycin and 5-FU delivery across AFL-treated human and pig skin in Franz Cell systems and found Bleomycin uptake was similar between pig and human skins at all skin depths, while 5-FU showed similar uptake in superficial skin and mid-dermis but different uptake in deep dermis [37]. Overall, pig skin is a good model to study AFL-assisted topical drug delivery.
mAbs are an attractive type of biologics due to their high specificity to targets. Till now, there are over 100 mAb drugs approved by FDA. mAbs are usually delivered by systemic routes. Topical delivery is promising to increase local mAb concentration and reduce systemic side effects. Several groups explored the potential of AFL to facilitate topical delivery of mAbs in preclinical studies. Yu et al. explored the use of AFL to enhance transdermal delivery of two immunosuppressive mAbs (ATG (Thymoglobulin®) and Basiliximab (Simulect®)) in vitro and in vivo [38]. AFL was highly effective to enhance transdermal delivery of ATG and Basiliximab and increase of laser percent coverage and fluence enhanced mAb delivery. Nivolumab is a mAb drug targeting programmed death-1 (PD-1), which is an immune checkpoint. Nivolumab was first approved in 2014 for advanced melanoma and later expanded to treat other tumors, such as advanced lung cancer, metastatic renal cell carcinoma, and Hodgkin lymphoma. Christensen et al. explored AFL-assisted topical delivery of Nivolumab for treatment of advanced skin cancer to avoid systemic side effects in pig models. In vitro Franz Cell studies found AFL could significantly enhance Nivolumab delivery into the pig skin and Nivolumab further showed a horizontal and homogenous distribution in the upper and mid-dermis, while electronically controlled pneumatic injection (EPI) displayed a deep focal deposition extending into the deep dermis [39]. In vivo study confirmed the different biodistribution of AFL-assisted delivery and intradermal injection delivery [40]. AFL-assisted delivery induced peak Nivolumab levels in the upper dermis after 4 h [40]. These studies consistently indicated the safety and efficacy of AFL to facilitate topical mAb delivery despite of their high molecular weight and complex 3D structures.
More complex systems, such as vaccines, nanoparticles, plasma, and cells, have been also successfully delivered into or across the skin with the help of AFL. Weiss et al. found model antigen ovalbumin (OVA) and recombinant Phl p 5 (a grass pollen allergen) could be efficiently delivered via AFL-generated skin MCs after topical delivery in a liquid form and elicit potent antigen-specific immune responses. Chen et. al. found powder array patch-coated OVA could be efficiently delivered into mouse, pig, and human skin via AFL-generated skin MCs within 1 h [25]. Furthermore, AFL-assisted powder array patch delivery of lipopolysaccharide (LPS)/oligonucleotide CpG mixture (LPS/CpG), BCG vaccine, and OVA-encoding vaccinia virus (VV-OVA) induced significantly reduced local reactions as compared to intradermal injection [25]. Due to the induction of significant photothermal stress in MC-surrounding tissues by the Ultrapulse Lumenis CO2 AFL, Li et al. explored whether laser-based powder delivery (LPD) could elicit more potent immune responses than needle-based injection delivery. It was found LPD of OVA and influenza vaccine at relatively strong laser conditions (10 mJ and 10% coverage) elicited more potent antigen-specific immune responses than needle-based injection delivery [41]. AFL treatment stimulated NLRP3 inflammasome and MyD88 pathways and only MyD88 pathway was critical for the observed laser adjuvant effects [41]. Besides needle-free delivery and improved vaccine immunogenicity, direct powder vaccination eliminates reconstitution steps and is likely to have improved vaccine stability and reduced storage volume, ideal for immunization in resource-poor territories. Besides these studies, Bansal et al. prepared dual rabies and contraceptive vaccine antigen-encoding pDNA-loaded PLGA nanoparticles, while were further incorporated into poloxamer hydrogel for topical immunization on AFL-treated skin [42]. Interestingly, Alum and MF59 adjuvants could be also incorporated into the same poloxamer hydrogel to further enhance vaccine-induced immune responses [42]. Joshi et al. encapsulated measles vaccines in crosslinked BSA microparticles and successfully immunized mice with measles vaccine-encapsulated microparticles after topical application on AFL-treated skin [43]. The AFL-assisted topical immunization was found to elicit comparable immune responses to subcutaneous injection [43].
Engelke et al. prepared FITC, rhodamine B-labeled dextran (RD70), and polystyrene (PS) nano- and microparticle-incorporated dry polyvinyl alcohol (PVA) polymer films and explored the topical delivery via AFL-generated skin MCs [44]. Similar to LPD, water evaporated from AFL-generated skin MCs needed to transform topically applied dry polymer films into a wet form to initiate the transdermal drug delivery. It was found dry PVA films could be sufficiently wetted within 6 h, which led to a considerable intradermal delivery of RD70 and PS nano- but not microparticles [44].
Lee et al. explored superficial ablation by CO2 AFL and fully ablative Er:YAG laser to facilitate platelet-rich plasma (PRP) delivery to treat methicillin-resistant Staphylococcus Aureus (MRSA)-infected wounds and photoaging of the skin [45]. It was found CO2 and Er:YAG lasers were effective to enhance skin deposition of interferon γ (IFN-γ) and tumor necrosis α (TNF-α) from PRP in vitro and interestingly fully ablative Er:YAG laser showed a higher penetration enhancement than AFL. Both lasers also enhanced an extensive and deep distribution of IFN-γ and platelet-derived growth factor (PDGF)-BB in the pig skin in vitro. Both lasers were effective to enhance PRP delivery and induce wound closure in MRSA infection mouse models despite more reduction of bacterial load in fully ablative laser group. Topical PRP delivery with fully ablative laser rather than AFL or PRP alone was found to fully restore elastin and collagen deposition in the mouse photoaging model. This study remains one of the few studies that compared AFL and fully ablative laser side by side in assisting topical drug delivery to treat skin disorders. More therapeutic effects observed in fully ablative laser group might be related to the photomechanical waves that created transient delivery routes in the epidermis to facilitate macromolecule delivery. In support, tape stripping physically removed SC layer but failed to enhance macromolecule uptake in the skin. Vora et al. prepared heparin-loaded poloxamer gel and found AFL could facilitate topical heparin delivery across pig skin in Franz Cell systems [46]. Moreover, AFL-assisted poloxamer gel delivery might sustain heparin delivery over a prolonged period [46]. Oni et al. explored possible delivery of adipocyte-derived stem cells (ADSCs) via AFL-generated skin MCs to aid chronic wound healing [47]. Using in vivo pig models, it was found 12% applied ADSCs were alive in the skin at 4 h and 5.5% applied ADSCs were alive in the skin at 48 h. This study indicated AFL could be used to generate skin MCs to support topical cell therapy in wound healing or other skin conditions. Yu et al. found living human cells could be delivered via AFL-generated skin MCs in pig skin and increase of pore density could increase living cell delivery [48]. Furthermore, cells delivered via AFL-generated skin MCs were found to express GFP, confirming live cell status after AFL-assisted topical delivery [48].
3.4. Strategies to increase topical drug delivery
The above studies indicated increase of laser coverage and drug concentration had a major impact on intracutaneous and transcutaneous drug delivery, while increase of laser fluence and MC depth mainly enhances transcutaneous but not intracutaneous drug delivery [13,15,17,23,27,31,34,38], which is also summarized in Table 2. The less impact of MC depths on intracutaneous drug delivery reflects the major barrier function of the SC layer. When the SC layer is disrupted, drugs can readily transmigrate into the skin regardless of skin MC depths. Yet, deep skin MCs facilitate rapid drug deposition to deep tissue layers required to relieve the pain associated with blood collection and venipuncture and treat malignant skin neoplasm, such as MNSCs and BCCs. Generally, increase of laser coverage, laser fluence, and drug concentration is promising to increase transdermal drug delivery. Yet, increase of laser coverage and laser fluence also delays skin recovery (Table 2). The maximal drug concentration is limited by its solubility and also affected by dosage forms. Thus, strategies to increase transdermal drug delivery need to be carefully considered and balanced in real applications.
Table 2.
Increase of diverse parameters on intracutaneous and transcutaneous drug delivery and limitations
| Increase of | Intracutaneous delivery | Transcutaneous delivery | Limitations |
|---|---|---|---|
| Laser coverage | ↑ | ↑ | Delayed skin recovery |
| Laser fluence | Not much | ↑ | |
| Skin MC depths | Not much | ↑ | |
| Drug concentration | ↑ | ↑ | Controlled by drug solubility and dosage forms |
3.5. Drug dosage forms in AFL-assisted topical drug delivery
Different dosage forms were explored in AFL-assisted topical drug delivery in vivo (Fig.3). Traditional topical dosage forms, such as creams and gels, can be topically applied without modifications in AFL-assisted topical drug delivery. Drugs can also be blended into a cream or gel base for convenient delivery via AFL-generated skin MCs. For liquid dosage forms, gauze patches, customized wells, or Franz donor cells can be used to hold liquid drugs for topical delivery via AFL-generated skin MCs. Different patch systems were also fabricated to facilitate direct powder drug delivery via AFL-generated skin MCs. Different from other dosage forms, powder drugs need to be wetted and dissolved in situ by evaporated water from skin MCs before initiation of delivery. As of such, usually two phases of delivery were observed with the first phase contributed by drug powder in close proximity to skin MCs and the second phase contributed by the remaining drug powder. Drugs can also be incorporated into dry polymer films for topical delivery via AFL-generated skin MCs. Similar to direct powder delivery, dry polymer films need to be wetted by evaporated water from skin MCs to initiate the drug delivery. Dry polymer films share similar advantages to direct powder drug delivery in storage and stability.
Figure 3.
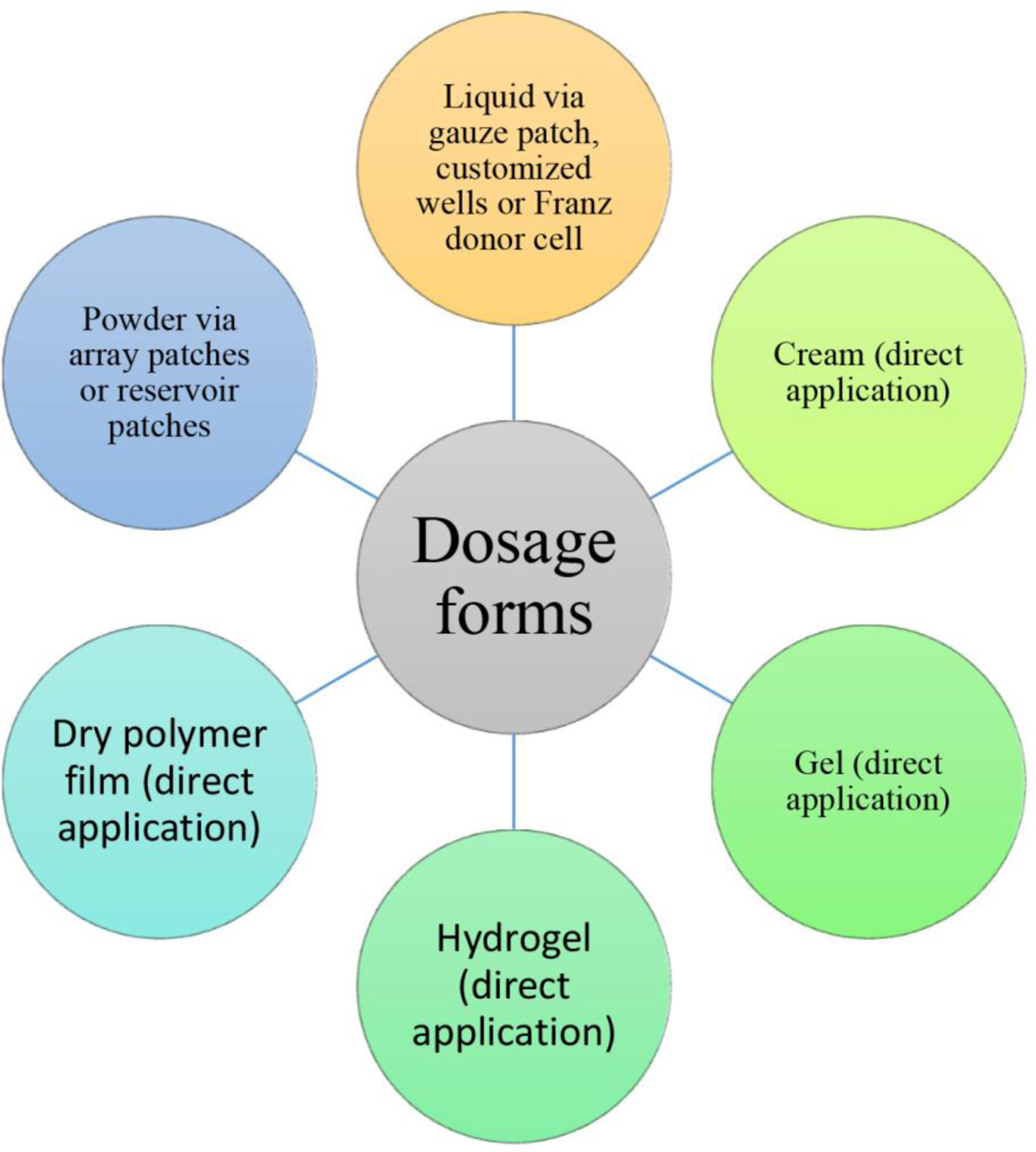
Dosage forms for AFL-assisted topical drug delivery
Rare studies directly compared the diverse dosage forms in AFL-assisted topical drug delivery. Olesen et al. compared topical dosage forms (liquid, gel, cream) on drug delivery via AFL-treated ex vivo pig skin and found drugs prepared in liquid and gel forms showed higher percentage of filling of AFL-generated skin MCs than drugs prepared in cream form regardless of skin MC depths [49].
3.6. Strategies to enhance liquid drug filling of skin MCs
Right after laser ablation, interstitial fluids were found to crowd the bottom of laser-generated skin MCs, leaving the upper part void. Due to the tiny skin MCs, topically applied liquid drugs face challenges to fill skin MCs. Erlendsson et al. explored altering pressure, vacuum, pressure (PVP) to facilitate liquid drug filling of AFL-generated skin MCs [50]. Theoretically, pressure is expected to push liquid drugs to fill skin MCs, while vacuum might extract entrapped tiny air bubbles out of skin MCs to facilitate liquid drug filling. AFL alone was found to generate an inhomogeneous uptake of PEG400 by skin MCs, which was greatly improved with the PVP application. Active filling with PVP also significantly enhanced intracutaneous delivery of PEG400.
Meesters et al. explored massage, acoustic pressure wave, and PVP to enhance topical delivery of liquid fluorescent drug indocyanine green (ICG) on AFL-treated ex vivo human skin [51]. Enhancement techniques were found to significantly enhance drug delivery at early time points and no difference could be observed regardless of the use of enhancement techniques or not at 24 h [51]. After 3 h, acoustic pressure wave showed the highest efficiency to improve drug accumulation in AFL-treated skin [51]. The above two studies indicated enhancement techniques could be used to increase liquid drug filling and transdermal drug delivery at early phases.
3.7. AFL facilitates sustained drug delivery
The majority of the above studies focused on relatively short duration of drug delivery. Several groups explored the potential of AFL to assist sustained transdermal drug delivery. Sustained drug delivery is promising to reduce dosing frequency and improve patient compliance. Sustained drug delivery may also improve therapeutic efficacy and reduce drug toxicity. One approach to induce sustained AFL-assisted drug delivery is to introduce slow-releasing drug formulations. Singhal et al. encapsulated triamcinolone acetate (TA) into polymeric microparticles (MPs) followed by topical delivery via AFL-generated skin MCs to form local depot for sustained release of TA [52]. TA was found to have more uniform distribution in the epidermis and upper dermis following AFL-assisted MP delivery as compared to TA suspension delivery. Furthermore, almost no transcutaneous delivery was found in the Franz Cell systems, indicating the dominant intracutaneous delivery in the 2-day study period. A similar approach was explored by Gou et al. to induce sustained release of Pentoxifylline and D-α-tocopherol from PLGA MPs after AFL-assisted transdermal delivery to reverse radiation injuries [53]. It was found this approach allowed successful deposition of drug-encapsulated PLGA MPs in AFL-treated skin at 48 h with potential release of drugs over an extended period of time [53].
Olesen et al. encapsulated Hedgehog inhibitor vismodegib, an approved chemotherapy drug to treat BCC, in oil-in-water (o/w) microemulsion and explored AFL-assisted topical delivery in pig skin in Franz Cell systems. The highest drug concentration was found in superficial AFL-treated skin at 24 h and significantly enhanced drug concentration was also found in deep skin layers [54]. Olesen et al. further explored AFL-assisted vismodegib microemulsion delivery in vivo and found 4 h-topical delivery of vismodegib microemulsion could sustain drug release for at least 9 days. AFL enhanced drug concentration in different skin layers at different time points and peak drug concentrations in dermis exceeded steady-state plasma concentration following oral delivery in humans. AFL-assisted vismodegib microemulsion delivery elicited mild local reactions with no systemic delivery observed. Bhattacchrjee et al. explored sustained delivery of brexanolone, a newly approved antidepressant to treat post-partum depression, across AFL-treated skin using novel microemulsion formulations [55]. It was found AFL was more effective than microneedles to support transdermal delivery of brexanolone [55]. Furthermore, percutaneous absorption of brexanolone across AFL-treated skin was significantly higher using o/w microemulation than w/o microemulsion [55]. Efficient delivery of brexanolone microemulsion via AFL-generated skin MCs might sustain drug release over an extended period of time.
Powder drugs were also coated in diverse patch systems to elicit sustained delivery via AFL-generated skin MCs by taking advantage of water evaporation from AFL-generated skin MCs to dissolve powder drugs in situ. Yan et al. formulated drugs into a powder form and further coated them in volumetric channels of thick patches to allow powder drugs to be slowly dissolved and delivered via AFL-generated skin MCs [56]. Using SRB, zidovudine (ZDV), insulin, and anti-PD-1 antibodies as examples, Yan et al. proved topical application of powder drug-coated array patches onto AFL-treated skin could elicit ~12 h sustained drug release in murine models. The sustained epidermal powder delivery (sEPD) was found to increase bioavailability of ZDV by ~100%. sEPD of insulin maintained blood glucose levels in the normal range much longer than needle injection delivery in a chemical-induced diabetes mouse model (Fig.4). sEPD of anti-PD-1 antibodies more significantly inhibited B16F10 melanoma growth than needle injection delivery. To increase drug coating capacity, reservoir patches were coated with a bulk amount of drug powder. Using SRB, ZDV, and BSA as examples, Kakar et al. found tapped coating could coat 10–20 mg drug per 0.5 cm2 reservoir patch to elicit 3-day sustained delivery, while compression coating could coat ~35–70 mg drug per 0.5 cm2 reservoir patch to elicit week-long sustained delivery [22]. The ability of AFL-generated skin MCs to support multi-day or week-long sustained drug delivery was due to the delayed skin MC resealing with topical patch occlusion, which prevented oxygen exposure and delayed re-epithelization. Yet, AFL-generated skin MCs could reseal in 1–2 days after completion of the week-long delivery to minimize infection risks [22]. By enlarging the reservoir patch size to about 10 cm2, similar to traditional patch size, AFL-assisted reservoir powder patch is promising to deliver high-dose drugs in grams for week-long sustained release.
Figure 4. Sustained insulin delivery.

A. Pictures of patches before (left) and 24 h after powder insulin delivery (middle) as well as picture of blank patches (right). Experiments were repeated 3 times and representative pictures were shown. Scale: 667μm. B–C. Powder insulin-coated patches were topically applied onto AFL-treated skin of STZ-induced diabetic mice or extracted for subcutaneous injection. At different time points, serum insulin levels (B) and BGLs (C) were measured. Non-treated mice served as control. n=7–9. Cited from [56].
4. Clinical investigation of AFL to assist transdermal drug delivery
Preclinical investigation of AFL in the last 12–15 years has proved its good safety, high efficiency, and broad applicability to enhance different types of drug delivery. AFL has been found to increase the delivery rate of skin permeable drugs and enable the delivery of skin impermeable chemical and biologics drugs. AFL-generated skin MCs can be conveniently adjusted to reach superficial, mid, or deep dermis and provide short-cuts for topical drugs to reach deep skin layers without being impeded by the epidermal layer, which also presents a barrier for transdermal drug delivery. In support, tape-stripping removal of SC layer failed to allow significant drug penetration to the dermal layer as observed in our studies and also others [24,25,57]. Different groups consistently found deep skin MCs favored drug permeation across the skin but not drug deposition into the skin especially when the skin MCs reached deep epidermis or dermal layers (Table 2). These results strongly encouraged clinical investigation of AFL to assist transdermal drug delivery (Table 3).
Table 3.
Clinical investigation of AFL-assisted transdermal drug delivery
| Laser | Drug and trial | Patient and skin condition | Methods | Major findings | Ref |
|---|---|---|---|---|---|
| MAL and ALA | |||||
| Fractional Er:YAG laser |
|
AKs (n=45, >18 years old, total 136 lesions) | Efficacy, recurrence, cosmetic outcomes, and safety were evaluated. |
|
[60] |
| P.L.E.A.S.E. laser |
|
Healthy male volunteers (n=11, >18 years old) | Impact of laser parameters, incubation time, and drug concentration on PpIX accumulation were studied. |
|
[61] |
| Fractional CO2 laser (eCO2) |
|
Healthy male subjects (n=10) | ALA-induced porphyrin fluorescence intensities were evaluated and compared. |
|
[63] |
| Fractional Er:YAG laser |
|
AKs (n=6) | Efficacy and treatment satisfaction |
|
[62] |
| Fractional Er:YAG laser |
|
AKs (n=41, >18 years old, total 160 lesions) | Efficacy, recurrence, cosmetic outcomes, and safety were evaluated. |
|
[64] |
| Fractional CO2 Laser (eCO2) |
|
AKs (n=46, >18 years old, total 69 lesions) | Safety and efficacy of the treatment were evaluated. |
|
[65] |
| Fractional CO2 laser (Pixel) |
|
AKs (n=15, 40–70 years old, total 638 lesions) | Efficacy, safety, cosmetic outcomes were evaluated. |
|
[66] |
| Fractional CO2 laser (Pixel) |
|
Field cancerized skin in association with AK (n=46) | Safety and efficacy were evaluated |
|
[67] |
| Other chemical drugs | |||||
| Fractional Er:YAG laser (Pixel) |
|
Healthy female subjects (n=22, age 40.05±5.38)/Normal skin | Pain levels were scored after induction with a 2,790-nm YSGG laser as a pain stimulus. |
|
[68] |
| Fractional CO2 laser (UltraPulse) |
|
Healthy subjects (n=10) | Pain levels were scored following a pain stimulus with a single AFL pass |
|
[69] |
| Fractional CO2 laser P.L.E.A.S.E. laser |
|
Healthy volunteer (n=15) | Subjects were asked to score pain after each pain stimulus. |
|
[70] |
| Fractional CO2 Laser (Pixel) |
|
Idiopathic palmar hyperhidrosis (n=10, 18–50 years old) | Treatment pain and sweat inhibition were evaluated. |
|
[71] |
| Fractional Er:YAG laser |
|
Keloids after BCG vaccination (n=10, 31–42 years old) | Treatment efficacy and safety were evaluated. |
|
[72] |
| Fractional Er:YAG laser |
|
Keloid scars (n=30, 19–50 years old) | Treatment efficacy and safety were evaluated |
|
[73] |
| Fractional CO2 Laser (UltraPulse) |
|
Cutaneous leishmaniasis (n=20, 18–60 years old, total 181 lesions) | Treatment pain and cosmetic outcomes were evaluated. |
|
[74] |
| Fractional CO2 Laser (UltraPulse) |
|
Cutaneous leishmaniasis (n=10 children, 5–14 years old) | Cosmetic outcomes and safety of treatment were evaluated. |
|
[75] |
| Fractional CO2 Laser (UltraPulse) |
|
Deep Infantile Hemangioma (n=9, 1–6 months old) | Treatment efficacy and safety were evaluated. |
|
[76] |
| Fractional CO2 laser (UltraPulse) |
|
Low-risk superficial or nodular BCCs (n=20, 56–89 years old) | Tumor response, local reactions, cosmetics, safety and drug distribution were analyzed. |
|
[77] |
| Fractional CO2 laser (UltraPulse) |
|
Low-risk superficial or nodular BCC (n=20, >18 years old) | Follow-up tumor clearance rate, tumor recurrence rate, cosmetic outcomes, and long-term safety were evaluated. |
|
[78] |
| Fractional CO2 laser (eCO2) |
|
Bowen’s disease (squamous cell carcinoma, n=17) | Treatment effects and safety were evaluated. |
|
[79] |
| Biologics | |||||
| P.L.E.A.S.E. laser |
|
Oocyte donor enrolled in a clinical study with transdermal delivery of highly purified urinary FSH | Follicular size was monitored and tranvaginal oocyte retrieval was conducted |
|
[80] |
| P.L.E.A.S.E. laser |
|
Chronic plaque-type psoriasis (n=8, a mean age of 43 ± 14 years) | Local and systemic side effects and treatment efficacy were evaluated. |
|
[81] |
| Cosmetics | |||||
| Fractional Er:YAG laser |
|
Melasma (n=32) | The efficacy to treatment and safety were evaluated. |
|
[82] |
| Fractional erbium laser |
|
Keloids resistant to first-line treatment (n=23, 12–51 years old, 70 lesions) | Treatment efficacy and side effects were evaluated. |
|
[83] |
| Fractional CO2 laser (UltraPulse) |
|
Hypertrophic scars (n=15, 17–52 years of age) | Treatment efficacy and safety were evaluated. |
|
[84] |
| Fractional Er:YAG laser |
|
Severe hypertrophic scars (n=24) | Treatment efficacy and safety were evaluated. |
|
[85] |
| Ablative fractional RF Fractional CO2 laser |
|
Areata alopecia (n=5, 18–47 years old) | Efficacy was evaluated. |
|
[86] |
| Fractional CO2 laser (DEKA Smartxide DOT/RF c60) |
|
Androgenetic Alopecia (n=45, 21–45 years old) | Efficacy was evaluated. |
|
[87] |
| Miscellaneous | |||||
| Palomar Lux2940 erbium laser |
|
Both ex vivo (n=4) and in vivo human volunteer skin (n=3) | Photograph and imaging study of particle delivery and local safety |
|
[88] |
| Fractional CO2 laser (UltraPulse) |
|
Healthy subjects (n=6)/Normal skin | In vivo imaging study of skin MC closure. |
|
[89] |
| Fractional CO2 laser (UltraPulse) |
|
Healthy volunteers (n=11) | Fluorescence photography and in vivo imaging and TEWL |
|
[90] |
| Fractional CO2 laser (eCO2) |
|
Skin aging (n=25, 27–62 years old) | Efficacy and safety were evaluated. |
|
[91] |
4.1. ALA and MAL
One application of AFL in topical drug delivery is to increase drug delivery rate and shorten the time to reach therapeutic levels in target tissues. As introduced earlier, MAL and ALA are FDA-approved photosensitizers used in PDT to treat AKs. However, standard therapies require 3 h incubation time to allow deep penetration and conversion of MAL and ALA to the active photosensitizer PpIX. Preclinical studies hinted pretreatment of lesion site with AFL might increase the rate of MAL or ALA delivery and shorten the incubation time.
Ko et al. found pretreatment of the lesion site with AFL followed by conventional MAL-PDT was more effective to treat all AK grades, reduce recurrence, and improve cosmetic outcomes [58]. Although more frequent side effects were observed in the AFL group, most of the adverse reactions were mild and well tolerated [58]. Haak et al. compared the impact of laser parameters on topical MAL cream delivery in healthy volunteers and found fluorescence intensities of PpIX could be markedly enhanced with AFL treatment at 1% coverage and further enhanced by increasing laser coverage to 5% [59]. Wang et al. found AFL-assisted ALA-PDT with 45 min of ALA occlusion achieved more than 95% clearance of AK lesions with a similar safety profile to traditional ALA-PDT [60]. In healthy subjects, Choi et al. found CO2 AFL pretreatment could significantly enhance ALA penetration into the skin and AFL-assisted ALA-PDT with 60 min incubation elicited similar PpIX fluorescence intensities to traditional ALA-PDT [61]. Interestingly, increase of laser fluence or combining with sonophoresis did not further increase ALA penetration. Choi et al. found AFL-assisted MAL-PDT with 2 h incubation in combination with iontophoresis elicited similar treatment efficacy against AK lesions to AFL-assisted MAL-PDT with 3 h incubation, while AFL-assisted MAL-PDT with 2 h incubation without iontophoresis showed a slightly weaker treatment efficacy than AFL-assisted MAL-PDT with 3 h incubation [62]. Interestingly, all treatments elicited similar cosmetic outcomes and safety. This study did not include conventional MAL-PDT group and thus the potential of AFL to shorten the incubation time of MAL could not be assessed. Yet, this study indicated combination of AFL and iontophoresis might further shorten the incubation time of MAL in PDT.
Song et al. found AFL-assisted MAL-PDT with 90 min incubation elicited similar treatment efficacy against AK lesions to conventional MAL-PDT with 3 h incubation [63]. Pires el al. explored AFL-assisted MAL-PDT in combination with acoustic pressure wave ultrasound to treat AK lesions and found the combined approach could shorten MAL incubation time to 1 h while generating similar treatment efficacy to conventional MAL-PDT [64]. Furthermore, the combined AFL/ultrasound approach was more effective to improve cosmetic outcomes as compared to conventional MAL-PDT although both treatments were well tolerated by patients [64]. Paasch et al. explored AFL-assisted MAL delivery in combination with indoor daylight exposure to treat skin field cancerization associated with AK and found this treatment showed a complete response rate of 71.7% and a partial response rate of 28.3% despite a high pain score [65].
The above studies support AFL pretreatment to enhance the treatment efficacy of conventional MAL/ALA-PDT against AK lesions. The majority of these studies also support AFL pretreatment to shorten the incubation time to achieve similar treatment efficacy. Several studies also support the combination of AFL with iontophoresis or acoustic pressure wave ultrasound to further shorten the incubation time. The above clinical studies also found AFL-assisted MAL/ALA-PDT was well tolerated by patients.
4.2. Anesthetics and other chemical drugs
AFL was also effective to increase the rate of topical anesthetics delivery. Five minutes after AFL-assisted topical lidocaine delivery, patients experienced significantly less pain after exposed to laser stimuli as compared to sham laser-assisted topical lidocaine delivery, which failed to reduce pain when compared to sham medication treatment [66]. Meesters et al. found 10 min of AFL-assisted articaine hydrochloride (40 mg/ml) and epinephrine (10 mg/ml) (AHES) delivery could significantly reduce pain induced by laser stimuli [67]. In another study, Meesters et al. compared two different AFLs (UltraPulse Lumenis CO2 and P.L.E.A.S.E. laser) to facilitate topical AHES delivery with 15 min occlusion in healthy volunteers [68]. Significantly reduced pain was observed in AFL groups and the two AFL groups showed no significant difference in reducing the pain scores [68]. Furthermore, increase of skin MC density from 5% to 15% led to significantly reduced pain scores in both AFLs [68]. These studies indicated AFL could be used to induce a quick response of topical anesthetics.
AFL can also be used for painless or less painful drug delivery especially when a number of injections are required to deliver drugs to cover a big skin area. Idiopathic palmar hyperhidrosis is a common disorder characterized as excessive hand sweating and has a substantial negative impact on quality of life. Intradermal injection of botulinum toxin (BTX) is an effective palliative therapy for palmar hyperhidrosis. However, over 20 intradermal injections are needed for BTX to cover the whole palm, which causes significant pain during treatment and limits its broad application. Issa et al. compared AFL-assisted BTX delivery and needle injection delivery of BTX [69]. In this study, BTX was dripped onto laser-treated right palm with an Impact Ultrasound handpiece after a pixel CO2 laser treatment, while the left palm was intradermally injected with the same total BTX amounts among 25 equidistant points across the palm. They found both treatments significantly reduced sweat production, while AFL-assisted BTX delivery showed significantly reduced pain during treatment. Intralesional injection of corticosteroids has been considered as the first-line therapy of keloids, which are formed by abnormal proliferation of scar tissues. Yet, intralesional injection of corticosteroids often causes severe and unbearable pain. Two split-lesion comparable studies compared AFL-assisted topical delivery and intralesional injection delivery of TA to treat keloid scars [70,71]. Both studies found AFL-assisted delivery and intralesional injection similarly decreased mean keloid scores, while AFL-assisted delivery induced less pain as compared to intralesional injection [70,71]. Another example to use AFL for less painful drug delivery is cutaneous leishmaniasis treatment. Cutaneous leishmaniasis is caused by parasite infection. The current gold standard for cutaneous leishmaniasis treatment is intralesional injection of sodium stibogluconate. This treatment is effective, however, often left with disfiguring strophic hypo- or hyper-pigmented post-inflammatory scars. Artzi et al. found AFL-assisted topical sodium stibogluconate delivery was equally effective to treat cutaneous leishmaniasis to intralesional injection [72]. Yet, AFL-assisted topical delivery resulted in remarkably improved cosmetic outcomes and was also less painful than intralesional injection. Hilerowicz et al. conducted a trial in pediatric patients diagnosed of cutaneous leishmaniasis and found AFL-assisted topical sodium stibogluconate delivery effectively treated 9 out of 10 pediatric patients with good cosmetic outcomes and safety [73].
AFL has been also explored to deliver drugs to deep layer of the skin to treat deep infantile hemangiomas (IHs). Topical timolol was found to be an effective treatment for superficial IHs. Yet, it showed a limited effect for deep IHs. Ma et al. explored CO2 AFL pretreatment to facilitate deep penetration of topical timolol to treat deep IHs [74]. They found 4 out of 9 patients showed excellent regression and another 4 out of 9 patients showed good response. Only one patient experienced moderate regression. Such a treatment was safe without significant local or systemic adverse reactions in infants.
Wenande et al. explored potential use of AFL to enhance topical drug delivery to treat BCC [75]. Prior CO2 AFL treatment followed by topical delivery of 0.1% cisplatin for 1 h and then topical 5-FU cream for 7 days resulted in a clinical clearance in 18/19 patients with 94% (17/18) achieving histological clearance at 3 months. Interestingly, AFL-assisted topical cisplatin/5-FU delivery did not induce detectable drug levels in the plasma. AFL-assisted topical cisplatin/5-FU delivery was well tolerated in the absence of pain or infection. In a follow-up study [76], complete BCC clearance was found to be 89% and 79% at 6 and 12 months, respectively, and 67% (2/3) recurrent tumors received only one treatment. The cosmetic outcomes were still rated as good or excellent in the follow-up study. Lee et al. found topical application of IngMeb gel alone showed limited efficacy for treatment of Bowen’s Disease (i.e., squamous cell carcinoma), while pretreatment with CO2 AFL followed by topical application of IngMeb gel elicited a complete response in 8 out of 9 patients and a partial response in the remaining patient [77].
4.3. Biologics
Besides chemical drugs, AFL has been also explored to facilitate biologics delivery in clinical trials. Zech el al. explored AFL-assisted topical delivery of FSH in a patch form for induction of superovulation in an attempt to address needle phobia in oocyte donors [78]. Transvaginal oocyte was successfully retrieved following AFL-assisted topical delivery of FSH that led to uneventful dichorionic-diamniotic twin pregnancy in a recipient [78]. Bauer et al. explored the potential use of AFL to facilitate topical delivery of biologics to treat psoriasis [79]. Psoriasis is an autoimmune disease of the skin affecting about 2% of the population. Besides topical corticosteroids, novel therapies have been developed or under development against pathogenic TNF/IL-23/Th17 pathway in psoriasis, such as anti-TNF, anti-p40, anti-p19, anti-IL17A, and anti-IL-17 receptor antibodies. Etanercept (ETA) is a genetically engineered fusion protein capable of binding and neutralizing TNFα and lymphotoxin α activities, which play a pathogenic role in psoriasis, and have been approved to treat moderate-to-severe plaque psoriasis in a number of countries, including the US. Topical delivery is highly attractive to increase its local concentration and reduce systemic side effects. It was found AFL-assisted topical ETA delivery improved the lesion-specific Target Plaque Severity Score (TPSS) score by 1.75, while ETA or AFL alone improved TPSS score only by 0.75 points, which was not statistically significant as compared to baseline. AFL-assisted topical ETA delivery also showed a favorable safety profile in this study.
4.4. Cosmetics
Other applications of AFL have been related to skin aesthetics. AFL was explored to facilitate topical cosmetics delivery to treat melasma, keloids, hypertrophic scars, and etc. In these applications, topical cosmetics alone work fine. Yet, topical cosmetics with prior AFL treatment usually result in more significant improvement in cosmetic outcomes. Hydroquinone (HQ) topical 4% cream is used to treat melasma characterized as brown or grey patches on the face. AFL pretreatment followed by topical HQ cream produced more significant improvement of treatment outcomes than topical HQ cream alone [80]. Cavalie et al. explored AFL-assisted topical betamethasone cream to treat keloids resistant to first-line treatment and found the median improvement reached 50% [81]. AFL pretreatment followed by topical triamcinolone suspension or 5-FU cream was found to be safe and effective to treat hypertrophic scars [82,83].
AFL followed by topical triamcinolone cream and then acoustic pressure ultrasound was found to be safe and effective to treat areata alopecia, which is an autoimmune disorder resulting in unpredictable patchy hair loss [84]. In this study, acoustic pressure ultrasound was used to enhance triamcinolone permeation into AFL-treated skin [84]. AFL followed by topical application of minoxidil also showed the most significant effect in stimulation of hair growth compared with AFL alone or topical minoxidil alone in treatment of androgenetic alopecia, a genetically predetermined disorder causing hair loss primarily in the top and front of the scalp [85].
4.5. Miscellaneous
A few other clinical trials explored AFL to assist topical delivery of nano- and microparticles, sodium fluorescein (NaF), and ADSC-conditioned medium (CM). Genina et al. found AFL-generated skin MCs in human skin enabled efficient delivery of topical TiO2 nanoparticles and Al2O3 microparticles into the skin [86]. Furthermore, nano- and microparticles could reach a depth of 230 µm and remained in the skin for more than one month [86]. Banzhaf et al. found AFL-generated skin MCs gradually close and higher laser energy resulted in delayed MC closure [87]. Banzhaf et al. further explored the impact of delayed topical NaF delivery on drug deposition in AFL-treated skin by measurement of fluorescence intensities (FI) of NaF [88]. It was found the highest FI occurred when NaF was applied within 30 minutes after laser exposure and significantly higher FI could be detected when NaF was applied up to 6 h after laser exposure [88]. This study confirmed that a significant number of AFL-generated skin MCs closed within 6 h. Lee et al. found AFL-assisted topical delivery of ADSC-CM in combination with niacinamide showed anti-aging effect on skin [89].
4.6. Summary of clinical studies
Clinical studies in the last 10 years find AFL to be highly effective to enhance transdermal delivery of various types of drugs, including small chemicals, biologics, and nanoparticles, with little restriction on their physicochemical properties, confirming findings from preclinical studies. AFL-assisted transdermal drug delivery is found to be well-tolerated in patients with transient and mild local reactions and lack of systemic adverse reactions. In these clinical trials, AFL showed a great promise to shorten the incubation time of MAL/ALA in PDT against AK lesions, induce rapid action of topical anesthetics, reduce pain of intralesional corticosteroid therapy against keloid scars, improve the cosmetic outcomes of cutaneous leishmaniasis therapy, induce the deep penetration of chemotherapy drugs against BCCs, and etc. Yet, most of the clinical trials are in early phase to test safety and efficacy of AFL-assisted transdermal drug delivery with a small sample size (<50 participants). Some clinical trials have no proper controls to evaluate the relative efficacy of AFL-assisted topical drug delivery. As of such, larger randomized, double-blinded, well-controlled clinical trials are needed to evaluate the full potential of AFL to assist topical drug delivery in treatment of diverse skin conditions.
5. AFL-assisted transungual drug delivery
Human nail consists of nail plate and four epithelial tissues including nail matrix, nail bed, hyponychium and perionychium. Human nail plate is about 0.25–0.60 mm in thickness and comprised of roughly 25 layers of closely packed dead keratinocytes within a matrix of keratin filaments [90]. The upper layer of the human nail plate is slightly elastic and poorly permeable and acts as a primary barrier for topical drug delivery. Nail illnesses are difficult to cure due to poor permeation of topical medicine into the nail bed, pain from intralesional injections, and patients' noncompliance with long-term therapy. AFL was used to treat nail plates to generate tiny channels to enhance topical drug delivery in vitro and in vivo (Table 4).
Table 4.
AFL-assisted transungual drug delivery
| Laser | Drug and trial | Study subjects | Methods | Major findings | Ref |
|---|---|---|---|---|---|
| P.L.E.A.S.E. laser | MTX solution 2 mg/mL | Finely sliced bovine hoof membrane has been validated to simulate human nail. | In vitro Franz Cell systems |
|
[92] |
| Fractional CO2 laser (UltraPulse) | 50 μl of 1% rhodamine B coated on a commercialized skin patch test device | Rabbit toenails of right forelimbs | In vivo delivery for 2 h followed by histological examination and confocal imaging |
|
[93] |
| Fractional CO2 laser (UltraPulse) | Normal saline solution | Fingernails of the same female volunteer | Imaging of AFL-generated nail micropores and saline diffusion process |
|
[94] |
| Fractional CO2 laser (UltraPulse) | 100 µM aqueous solution of the carboxy derivative of ATTO-647N in dimethyl sulfoxide | Nail samples from healthy participants (n = 18) and anonymous discard clippings from fungal nail disease (n = 12) | Nail samples placed on PBS-soaked gauze |
|
[95] |
| Fractional CO2 laser (eCO2) |
|
Periungual warts (n=17) | No control, all AFL/Drug |
|
[96] |
| Fractional CO2 laser (Ultrapulse) |
|
Bilateral fingernail psoriasis (n=22, age 24–63) | One hand treated and the other hand as non-treated control, monthly for 3 times |
|
[97] |
Nguyen et al. used bovine hoof membrane as an in vitro model of human nail and explored the potential use of AFL to generate vertical nail channels to facilitate topical drug delivery in Franz Cell systems [90]. The portable P.L.E.A.S.E. laser was used in this study and it was found increase of laser energy could increase pore depths. AFL treatment significantly increased topical MTX permeation through the human nail model and the permeation rate could be modified by laser parameters. Guan et al. evaluated AFL-assisted topical rhodamine B delivery in toenails of rabbit in vivo [91]. It was found AFL could successfully generate micropores in nail plates and the micropore depths and rhodamine B permeation could be significantly increased with increase of laser energy [91].
Several groups used clipped human nails and explored AFL-assisted topical drug delivery in vitro. Yang et al. found optical coherence tomography (OCT) could be an effective tool to study topical drug delivery through microscopic ablation channels after nail fractional laser treatment [92]. Ortner et al. found AFL treatment was highly effective to enhance deposition of fluorescent dye (ATTO-647N) in nail tissues [93]. Interestingly, AFL had a more profound effect to enhance ATTO-647N delivery into healthy nail as compared to fungal nail [93].
Several groups conducted human clinical trials to study AFL-assisted topical drug delivery to treat nail diseases. Suh et al. explored AFL-assisted topical bleomycin to treat periungual warts and found 68.4% lesions showed complete clearance with no recurrence and 7.8% had partial response with 75% improvement [94]. This study indicated AFL could be used to enhance efficacy of topical cytotoxic agents to treat periungual warts and limit the recurrence after complete clearance.
AFL-assisted topical drug delivery has been also explored to treat fingernail psoriasis. Shehadeh et al. used a combination of pulse-dye laser (PDL) therapy of the nail folds and CO2 AFL-assisted betamethasone propionate-calcipotriol gel drug delivery to the nail bed to treat all aspects of nail psoriasis. The Nail Psoriasis Severity Index (NAPSI) score improved dramatically after three months of treatment utilizing this combination method [95].
Overall, AFLs would be an excellent option for the treatment of nail illnesses when paired with topical medications.
6. AFL-assisted transocular drug delivery
Ocular drug delivery is challenging. Physiological barriers, such as cornea, sclera and blood-retina barriers, and short residence time of drugs in the eye due to tear drainage, lacrimation and conjunctival uptake seriously impeded the drug delivery into anterior and vitreous chambers of the eye [96,97]. Conventional intraocular injections could efficiently deliver both small molecules (corticosteroids) and biologics through the corneal or scleral tissues. However, their highly invasive nature has driven researchers to search for better alternative permeation enhancing strategies [98–100].
Laser, as a non-invasive tool to create micropores with controlled depth, density, and area for transdermal drug delivery, may also have potential for ocular drug delivery [15]. Besides, the wide application of laser in ocular disease treatment, such as laser photocoagulation surgeries, vision correction and cataract surgeries, provided evidence of its safety and potential feasibility for ocular drug delivery [101–103]. Although laser has not been well studied for ocular drug delivery yet, some studies have shown the promise of laser as an alternative minimally invasive tool for ocular drug delivery. Recently, a study using P.L.E.A.S.E. laser showed enhanced permeation of both rhodamine B and FD through porcine scleral and corneal tissues [104]. They also proved fast healing of laser-created micropores (<24 h) by measuring the transscleral water loss change after laser irradiation [104]. In another study, an ophthalmic Nd:YAG laser was used to disrupt the membrane of a device implanted in rabbits’ eyes to release the model drug 10% NaF dye into vitreous chamber, supporting a good potential of laser as a trigger for controlled drug delivery to treat ocular diseases [105].
7. Conclusion
Since its advent, AFL has been widely explored to facilitate topical drug delivery owing to its minimal invasiveness and high efficiency to breach tissue barrier function. AFL-assisted topical drug delivery involves two steps with the first step to generate MCs in tissue surface and the second step to apply drugs topically to initiate drug delivery. AFL-generated MCs allow efficient delivery of different types of drugs, such as chemical drugs, biologics, nano- and microparticles, and live cells, with little restriction on physicochemical properties of drugs. AFL-generated MCs also allow the efficient delivery of drugs in different dosage forms, such as cream, lotion, gel, liquid, and powder. AFL has been mostly explored to facilitate transdermal drug delivery. Studies consistently find laser percent coverage and drug concentration have a major impact on transcutaneous and intracutaneous drug delivery, while laser fluence and MC depths mainly impact transcutaneous but not intracutaneous drug delivery. AFL can be used to enhance therapeutic efficacy, shorten the waiting period, reduce pain, improve cosmetic outcomes, minimize systemic exposure, and reduce drug toxicity. Although microneedles can also be used to generate skin MCs to support transdermal drug delivery, several group found AFL was prone to generate relatively big skin MCs and more efficiently support transdermal drug delivery than microneedles. Furthermore, AFL may be a more suitable technology to support sustained drug delivery due to the slower rate of recovery of relatively big skin MCs. Besides transdermal drug delivery, AFL has been also explored to facilitate transungual drug delivery to treat nail diseases, which are otherwise hard to reach by topical delivery or face painful injection procedures. There are also a small number of studies that explored AFL to facilitate transocular drug delivery and results are highly encouraging in both safety and efficacy of delivery.
8. Expert opinion
Tissue barrier presents a big challenge for topical drug delivery. AFL emits high-energy micro-laser beams to ablate tiny tissues and create MCs in a non-contact, well controlled, and highly repeatable manner. A large number of preclinical and clinical studies support the good safety and efficacy of AFL to support topical drug delivery. Although the field has advanced well in the last two decades, several factors hinder further progress and clinical translation of AFL-assisted topical drug delivery. First, the majority of the AFL devices explored to facilitate topical drug delivery were originally fabricated for improved skin resurfacing. These AFLs are generally large and expensive. Although these cosmetic devices allow convenient adjustment of laser parameters, the field will benefit from the development of small and cost-effective AFL devices specifically designed for drug delivery applications. Challenges remain though considering this might need to attract investment from pharmaceutical companies to design and manufacture such a device and during this process a good input from potential users of such a device, such as clinicians and academic investigators, is required to guide the design and test. Second, appropriate dosage forms are required to use in conjunction with AFL to facilitate topical drug delivery. Topical dosage forms, such as creams, lotions, gels, can be directly applied on AFL-treated skin. However, the majority of the hydrophilic drugs are not prepared as topical dosage forms due to their inability to permeate across the intact tissue. Liquid drugs were frequently prepared in preclinical and even clinical studies to explore AFL-assisted topical drug delivery. However, liquid drugs are easy to leak and not convenient to deliver on AFL-treated skin or other tissues. Topical dosage forms also face challenges to control variations between applications and individuals. Topical dosage forms are highly likely to have an incomplete drug delivery, which may pose challenges to high-cost drug delivery, such as mAbs. Third, most of the clinical trials so far are in relatively early phase with the focus to establish safety and gain insights about efficacy in a small number of patients. Future trials would benefit by enrolling a bigger group of patients with well-designed controls included for comparison. One disease area that might benefit from AFL-assisted topical drug delivery in the near future is MAL/ALA-PDT against AKs considering a handful of clinical trials have been conducted so far. Larger clinical trials are needed to determine the time that can be shortened by the use of AFL, while eliciting similar therapeutic efficacy to traditional MAL/ALA-PDT. The other disease area that is likely to benefit from AFL-assisted topical drug delivery in the near future is the treatment of deep skin malignancies, such as BCC, NMSCs, and infantile hemangiomas. The safety and efficacy of AFL-assisted topical drug delivery need to be thoroughly evaluated and compared with current practices in larger clinical trials to justify its use.
Besides use in doctor’s office, AFL-assisted topical drug delivery can also be developed for at-home use. One potential at-home application is to use AFL for needle-free, painless insulin delivery in diabetes patients with needle phobia. However, at-home AFL-assisted topical drug delivery requires an appropriate topical dosage forms to minimize the variations between deliveries and applied by different subjects. For at-home use, the two-step AFL-assisted topical drug delivery is better integrated with one click of button to accomplish laser ablation and drug application processes. From this regard, the powder patches that enables high-efficient drug delivery via AFL-generated skin MCs present an ideal form for incorporation into AFL-assisted topical drug delivery for at-home use.
AFL holds a great promise to facilitate topical drug delivery due to its minimal invasiveness to breach tissue barrier and elicitation of quick and complete tissue recovery at the end of delivery. AFL greatly expands the number and types of drugs that can be delivered via topical routes. The development of small, less costly AFL devices suitable for delivery of diverse types of drugs remains the key to expand its use in preclinical and clinical settings. The various benefits of AFL-assisted topical drug delivery warrants further development for human use.
Article highlights.
This review introduces preclinical development and clinical evaluation of ablative fractional laser to facilitate transdermal/transungual/transocular drug delivery.
Ablative fractional laser allows efficient delivery of small chemicals, biologics, nano- and microparticles, and even live cells with little restriction on physicochemical properties of drugs.
Ablative fractional laser allows rapid drug penetration to deep tissue layers and is promising to enhance drug efficacy, shorten treatment time, reduce pain, and improve cosmetic outcomes.
Ablative fractional laser-assisted topical drug delivery enables quick and complete tissue recovery and is well-tolerated in humans.
The development of small, cost-effective ablative fractional laser devices is expected to facilitate its clinical translation in topical drug delivery.
Funding
This paper was funded by the National Institutes of Health (NIH). X Chen received grant support NIH (AI139473 and AI156510). J Voyer received support from the URI MARC U*STAR grant from the National Institute of General Medical Sciences of the National Institutes of Health under grant number T34GM131948.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Reference
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Roberts MS, Cheruvu HS, Mangion SE, et al. Topical drug delivery: History, percutaneous absorption, and product development. Advanced drug delivery reviews 2021. Oct;177:113929. [DOI] [PubMed] [Google Scholar]
- 2.Haque T, Talukder MMU. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv Pharm Bull 2018. Jun;8(2):169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prausnitz MR, Langer R. Transdermal drug delivery. Nature biotechnology 2008. Nov;26(11):1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawat S, Vengurlekar S, Rakesh B, et al. Transdermal delivery by iontophoresis. Indian journal of pharmaceutical sciences 2008. Jan;70(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polat BE, Hart D, Langer R, et al. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. Journal of controlled release : official journal of the Controlled Release Society 2011. Jun 30;152(3):330–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prausnitz MR. Microneedles for transdermal drug delivery. Advanced drug delivery reviews 2004. Mar 27;56(5):581–7. [DOI] [PubMed] [Google Scholar]
- 7.Sklar LR, Burnett CT, Waibel JS, et al. Laser assisted drug delivery: a review of an evolving technology. Lasers in surgery and medicine 2014. Apr;46(4):249–62. [DOI] [PubMed] [Google Scholar]
- 8. Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers in surgery and medicine 2004;34(5):426–38. **. Concept of fractional photothermolysis behind AFL technology.
- 9.Bodendorf MO, Grunewald S, Wetzig T, et al. Fractional laser skin therapy. J Dtsch Dermatol Ges 2009. Apr;7(4):301–8. [DOI] [PubMed] [Google Scholar]
- 10.Waibel JS, Rudnick A, Shagalov DR, et al. Update of Ablative Fractionated Lasers to Enhance Cutaneous Topical Drug Delivery. Adv Ther 2017. Aug;34(8):1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon GK. New insights into skin structure: scratching the surface. Advanced drug delivery reviews 2002. Nov 1;54 Suppl 1:S3–17. [DOI] [PubMed] [Google Scholar]
- 12. Haedersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers in surgery and medicine 2010. Feb;42(2):113–22. **. One of the first studies evaluating the feasibility of AFL to assist topical drug delivery
- 13.Haak CS, Farinelli WA, Tam J, et al. Fractional laser-assisted delivery of methyl aminolevulinate: Impact of laser channel depth and incubation time. Lasers in surgery and medicine 2012. Dec;44(10):787–95. [DOI] [PubMed] [Google Scholar]
- 14.Haedersdal M, Sakamoto FH, Farinelli WA, et al. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL). Lasers in surgery and medicine 2014. Aug;46(6):462–9. [DOI] [PubMed] [Google Scholar]
- 15. Bachhav YG, Summer S, Heinrich A, et al. Effect of controlled laser microporation on drug transport kinetics into and across the skin. Journal of controlled release : official journal of the Controlled Release Society 2010. Aug 17;146(1):31–6. *. One of the first studies evaluating the impact of laser parameters and MC depths on intracutaneous and transcutaneous drug delivery
- 16.Oni G, Brown SA, Kenkel JM. Can fractional lasers enhance transdermal absorption of topical lidocaine in an in vivo animal model? Lasers in surgery and medicine 2012. Feb;44(2):168–74. [DOI] [PubMed] [Google Scholar]
- 17.Bachhav YG, Heinrich A, Kalia YN. Using laser microporation to improve transdermal delivery of diclofenac: Increasing bioavailability and the range of therapeutic applications. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2011. Aug;78(3):408–14. [DOI] [PubMed] [Google Scholar]
- 18.Erlendsson AM, Taudorf EH, Eriksson AH, et al. Ablative fractional laser alters biodistribution of ingenol mebutate in the skin. Arch Dermatol Res 2015. Aug;307(6):515–22. [DOI] [PubMed] [Google Scholar]
- 19.Taudorf EH, Lerche CM, Vissing AC, et al. Topically applied methotrexate is rapidly delivered into skin by fractional laser ablation. Expert opinion on drug delivery 2015. Jul;12(7):1059–69. [DOI] [PubMed] [Google Scholar]
- 20.Taudorf EH, Lerche CM, Erlendsson AM, et al. Fractional laser-assisted drug delivery: Laser channel depth influences biodistribution and skin deposition of methotrexate. Lasers in surgery and medicine 2016. Jul;48(5):519–29. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen HX, Banga AK. Delivery of Methotrexate and Characterization of Skin Treated by Fabricated PLGA Microneedles and Fractional Ablative Laser. Pharmaceutical research 2018. Feb 21;35(3):68. **. Direct comparison of AFL and microneedle to generate skin MCs to support topical drug delivery
- 22. Kakar P, Li Z, Li Y, et al. Laser facilitates week-long sustained transdermal drug delivery at high doses. Journal of controlled release : official journal of the Controlled Release Society 2020. Jan 7;319:428–437. *. Report about AFL to support week-long sustained transdermal drug delivery.
- 23.Yu J, Bachhav YG, Summer S, et al. Using controlled laser-microporation to increase transdermal delivery of prednisone. Journal of controlled release : official journal of the Controlled Release Society 2010. Nov 20;148(1):e71–3. [DOI] [PubMed] [Google Scholar]
- 24. Chen X, Shah D, Kositratna G, et al. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. Journal of controlled release : official journal of the Controlled Release Society 2012. Apr 10;159(1):43–51. *. One of the first studies demonstrating the effectiveness of AFL to support transdermal macromolecule delivery and vaccination
- 25. Chen X, Kositratna G, Zhou C, et al. Micro-fractional epidermal powder delivery for improved skin vaccination. Journal of controlled release : official journal of the Controlled Release Society 2014. Oct 28;192:310–6. *. Report about AFL to support direct powder delivery across the skin
- 26.Haak CS, Bhayana B, Farinelli WA, et al. The impact of treatment density and molecular weight for fractional laser-assisted drug delivery. Journal of controlled release : official journal of the Controlled Release Society 2012. Nov 10;163(3):335–41. [DOI] [PubMed] [Google Scholar]
- 27.Wenande E, Olesen UH, Boesen MR, et al. Laser-assisted delivery enhances topical uptake of the anticancer agent cisplatin. Drug delivery 2018. Nov;25(1):1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenande E, Olesen UH, Nielsen MM, et al. Fractional laser-assisted topical delivery leads to enhanced, accelerated and deeper cutaneous 5-fluorouracil uptake. Expert opinion on drug delivery 2017. Mar;14(3):307–317. [DOI] [PubMed] [Google Scholar]
- 29.Wenande E, Tam J, Bhayana B, et al. Laser-assisted delivery of synergistic combination chemotherapy in in vivo skin. Journal of controlled release : official journal of the Controlled Release Society 2018. Apr 10;275:242–253. [DOI] [PubMed] [Google Scholar]
- 30.Kale M, Kipping T, Banga AK. Modulated delivery of donepezil using a combination of skin microporation and iontophoresis. International journal of pharmaceutics 2020. Nov 15;589:119853. [DOI] [PubMed] [Google Scholar]
- 31.Bachhav YG, Heinrich A, Kalia YN. Controlled intra- and transdermal protein delivery using a minimally invasive Erbium:YAG fractional laser ablation technology. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2013. Jun;84(2):355–64. [DOI] [PubMed] [Google Scholar]
- 32.Lee WR, Shen SC, Chen WY, et al. Noninvasive delivery of siRNA and plasmid DNA into skin by fractional ablation: erbium:YAG laser versus CO(2) laser. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2014. Apr;86(3):315–23. [DOI] [PubMed] [Google Scholar]
- 33.Lee WR, Shen SC, Sung CT, et al. Is the Fractional Laser Still Effective in Assisting Cutaneous Macromolecule Delivery in Barrier-Deficient Skin? Psoriasis and Atopic Dermatitis as the Disease Models. Pharmaceutical research 2018. Apr 26;35(7):128. [DOI] [PubMed] [Google Scholar]
- 34.Song Y, Hemmady K, Puri A, et al. Transdermal delivery of human growth hormone via laser-generated micropores. Drug Deliv Transl Res 2018. Apr;8(2):450–460. [DOI] [PubMed] [Google Scholar]
- 35.Hendel K, Hansen ACN, Bik L, et al. Bleomycin administered by laser-assisted drug delivery or intradermal needle-injection results in distinct biodistribution patterns in skin: in vivo investigations with mass spectrometry imaging. Drug delivery 2021. Dec;28(1):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendel KK, Bagger C, Olesen UH, et al. Fractional laser-assisted topical delivery of bleomycin quantified by LC-MS and visualized by MALDI mass spectrometry imaging. Drug delivery 2019. Dec;26(1):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg LK, Bagger C, Janfelt C, et al. A Comparison of Human and Porcine Skin in Laser-Assisted Drug Delivery of Chemotherapeutics. Lasers in surgery and medicine 2021. Jan;53(1):162–170. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Kalaria DR, Kalia YN. Erbium:YAG fractional laser ablation for the percutaneous delivery of intact functional therapeutic antibodies. Journal of controlled release : official journal of the Controlled Release Society 2011. Nov 30;156(1):53–9. [DOI] [PubMed] [Google Scholar]
- 39.Christensen RL, Omland SH, Persson DP, et al. Topical Delivery of Nivolumab, a Therapeutic Antibody, by Fractional Laser and Pneumatic Injection. Lasers in surgery and medicine 2021. Jan;53(1):154–161. [DOI] [PubMed] [Google Scholar]
- 40.Christensen RL, Hendel KK, Persson DP, et al. Topical delivery of PD-1 inhibitors with laser-assisted passive diffusion and active intradermal injection: Investigation of cutaneous pharmacokinetics and biodistribution patterns. Lasers in surgery and medicine 2022. Jan;54(1):170–181. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Cao Y, Li Y, et al. Vaccine delivery alerts innate immune systems for more immunogenic vaccination. JCI Insight 2021. Apr 8;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bansal A, Gamal W, Menon IJ, et al. Laser-assisted skin delivery of immunocontraceptive rabies nanoparticulate vaccine in poloxamer gel. Eur J Pharm Sci 2020. Dec 1;155:105560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi D, Gala RP, Uddin MN, et al. Novel ablative laser mediated transdermal immunization for microparticulate measles vaccine. International journal of pharmaceutics 2021. Sep 5;606:120882. [DOI] [PubMed] [Google Scholar]
- 44.Engelke L, Winter G, Engert J. Application of water-soluble polyvinyl alcohol-based film patches on laser microporated skin facilitates intradermal macromolecule and nanoparticle delivery. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2018. Jul;128:119–130. [DOI] [PubMed] [Google Scholar]
- 45.Lee WR, Hsiao CY, Huang TH, et al. Low-fluence laser-facilitated platelet-rich plasma permeation for treating MRSA-infected wound and photoaging of the skin. International journal of pharmaceutics 2021. Feb 15;595:120242. [DOI] [PubMed] [Google Scholar]
- 46.Vora D, Kim Y, Banga AK. Development and evaluation of a heparin gel for transdermal delivery via laser-generated micropores. Therapeutic delivery 2021. Feb;12(2):133–144. [DOI] [PubMed] [Google Scholar]
- 47.Oni G, Lequeux C, Cho MJ, et al. Transdermal delivery of adipocyte-derived stem cells using a fractional ablative laser. Aesthetic surgery journal 2013. Jan;33(1):109–16. [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Dubey S, Kalia YN. Needle-free cutaneous delivery of living human cells by Er:YAG fractional laser ablation. Expert opinion on drug delivery 2018. Jun;15(6):559–566. [DOI] [PubMed] [Google Scholar]
- 49.Olesen UH, Mogensen M, Haedersdal M. Vehicle type affects filling of fractional laser-ablated channels imaged by optical coherence tomography. Lasers in medical science 2017. Apr;32(3):679–684. [DOI] [PubMed] [Google Scholar]
- 50.Erlendsson AM, Doukas AG, Farinelli WA, et al. Fractional laser-assisted drug delivery: Active filling of laser channels with pressure and vacuum alteration. Lasers in surgery and medicine 2016. Feb;48(2):116–24. [DOI] [PubMed] [Google Scholar]
- 51.Meesters AA, Nieboer MJ, Almasian M, et al. Drug penetration enhancement techniques in ablative fractional laser assisted cutaneous delivery of indocyanine green. Lasers in surgery and medicine 2019. Oct;51(8):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singhal M, Del Rio-Sancho S, Sonaje K, et al. Fractional Laser Ablation for the Cutaneous Delivery of Triamcinolone Acetonide from Cryomilled Polymeric Microparticles: Creating Intraepidermal Drug Depots. Molecular pharmaceutics 2016. Feb 1;13(2):500–11. [DOI] [PubMed] [Google Scholar]
- 53.Gou S, Del Rio-Sancho S, Singhal M, et al. Er:YAG fractional laser ablation for cutaneous co-delivery of pentoxifylline and d-alpha-tocopherol succinate: A new approach for topical treatment of radiation-induced skin fibrosis. Eur J Pharm Sci 2019. Jul 1;135:22–31. [DOI] [PubMed] [Google Scholar]
- 54.Olesen UH, Clergeaud G, Lerche CM, et al. Topical delivery of vismodegib using ablative fractional laser and micro-emulsion formulation in vitro. Lasers in surgery and medicine 2019. Jan;51(1):79–87. [DOI] [PubMed] [Google Scholar]
- 55.Bhattaccharjee SA, Murnane KS, Banga AK. Transdermal delivery of breakthrough therapeutics for the management of treatment-resistant and post-partum depression. International journal of pharmaceutics 2020. Dec 15;591:120007. [DOI] [PubMed] [Google Scholar]
- 56.Cao Y, Kakar P, Hossen MN, et al. Sustained epidermal powder drug delivery via skin microchannels. Journal of controlled release : official journal of the Controlled Release Society 2017. Mar 10;249:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss R, Hessenberger M, Kitzmuller S, et al. Transcutaneous vaccination via laser microporation. Journal of controlled release : official journal of the Controlled Release Society 2012. Sep 10;162(2):391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olesen UH, Clergeaud G, Hendel KK, et al. Enhanced and Sustained Cutaneous Delivery of Vismodegib by Ablative Fractional Laser and Microemulsion Formulation. The Journal of investigative dermatology 2020. Oct;140(10):2051–2059. [DOI] [PubMed] [Google Scholar]
- 59.Andrews SN, Jeong E, Prausnitz MR. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharmaceutical research 2013. Apr;30(4):1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ko DY, Jeon SY, Kim KH, et al. Fractional erbium: YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol 2014. Nov;28(11):1529–39. *. One of the first studies evaluating AFL for topical drug delivery in human subjects
- 61.Haak CS, Christiansen K, Erlendsson AM, et al. Ablative fractional laser enhances MAL-induced PpIX accumulation: Impact of laser channel density, incubation time and drug concentration. J Photochem Photobiol B 2016. Jun;159:42–8. [DOI] [PubMed] [Google Scholar]
- 62.Wang JV, Griffin TD. Fractional Ablative Laser-Assisted Photodynamic Therapy as Field Treatment for Actinic Keratoses: Our Anecdotal Experience. Skinmed 2020;18(4):214–216. [PubMed] [Google Scholar]
- 63.Choi JH, Shin EJ, Jeong KH, et al. Comparative analysis of the effects of CO2 fractional laser and sonophoresis on human skin penetration with 5-aminolevulinic acid. Lasers in medical science 2017. Nov;32(8):1895–1900. [DOI] [PubMed] [Google Scholar]
- 64.Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther 2017. Jun;18:105–110. [DOI] [PubMed] [Google Scholar]
- 65.Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed 2015. Nov;31(6):296–301. [DOI] [PubMed] [Google Scholar]
- 66.Pires MTF, Pereira AD, Duraes SMB, et al. Laser-assisted MAL-PDT associated with acoustic pressure wave ultrasound with short incubation time for field cancerization treatment: A left-right comparison. Photodiagnosis Photodyn Ther 2019. Dec;28:216–220. [DOI] [PubMed] [Google Scholar]
- 67.Paasch U, Said T. Treating Field Cancerization by Ablative Fractional Laser and Indoor Daylight: Assessment of Efficacy and Tolerability. J Drugs Dermatol 2020. Apr 1;19(4):425–427. [DOI] [PubMed] [Google Scholar]
- 68.Tian T, Luo Y, Jiang T, et al. Clinical effect of ablative fractional laser-assisted topical anesthesia on human skin: A randomized pilot study. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology 2016. Nov;18(7):409–412. [DOI] [PubMed] [Google Scholar]
- 69.Meesters AA, Bakker MM, de Rie MA, et al. Fractional CO2 laser assisted delivery of topical anesthetics: A randomized controlled pilot study. Lasers in surgery and medicine 2016. Feb;48(2):208–11. [DOI] [PubMed] [Google Scholar]
- 70.Meesters AA, Nieboer MJ, Kezic S, et al. Parameters in fractional laser assisted delivery of topical anesthetics: Role of laser type and laser settings. Lasers in surgery and medicine 2018. Oct;50(8):813–818. [DOI] [PubMed] [Google Scholar]
- 71.Issa MC, Torreao PS, Boechat M, et al. Early investigations in drug delivery of onabotulinum toxin A using combined fractional ablative laser with impact ultrasound vs. injections of onabotulinum toxin A for palmar hyperhidrosis: a right-left comparison trial. Br J Dermatol 2018. Nov;179(5):1168–1169. [DOI] [PubMed] [Google Scholar]
- 72.Park JH, Chun JY, Lee JH. Laser-assisted topical corticosteroid delivery for the treatment of keloids. Lasers in medical science 2017. Apr;32(3):601–608. [DOI] [PubMed] [Google Scholar]
- 73.Abd El-Dayem DH, Nada HA, Hanafy NS, et al. Laser-assisted topical steroid application versus steroid injection for treating keloids: A split side study. Journal of cosmetic dermatology 2021. Jan;20(1):138–142. [DOI] [PubMed] [Google Scholar]
- 74.Artzi O, Sprecher E, Koren A, et al. Fractional Ablative CO2 Laser Followed by Topical Application of Sodium Stibogluconate for Treatment of Active Cutaneous Leishmaniasis: A Randomized Controlled Trial. Acta Derm Venereol 2019. Jan 1;99(1):53–57. [DOI] [PubMed] [Google Scholar]
- 75.Hilerowicz Y, Koren A, Mashiah J, et al. Fractional ablative carbon dioxide laser followed by topical sodium stibogluconate application: A treatment option for pediatric cutaneous leishmaniasis. Pediatr Dermatol 2018. May;35(3):366–369. [DOI] [PubMed] [Google Scholar]
- 76.Ma G, Wu P, Lin X, et al. Fractional carbon dioxide laser-assisted drug delivery of topical timolol solution for the treatment of deep infantile hemangioma: a pilot study. Pediatr Dermatol 2014. May-Jun;31(3):286–91. [DOI] [PubMed] [Google Scholar]
- 77.Wenande E, Hendel K, Mogensen M, et al. Efficacy and Safety of Laser-Assisted Combination Chemotherapy: An Explorative Imaging-Guided Treatment With 5-Fluorouracil and Cisplatin for Basal Cell Carcinoma. Lasers in surgery and medicine 2021. Jan;53(1):119–128. [DOI] [PubMed] [Google Scholar]
- 78.Fredman G, Wenande E, Hendel K, et al. Efficacy and safety of laser-assisted combination chemotherapy: A follow-up study of treatment with 5-fluorouracil and cisplatin for basal cell carcinoma. Lasers in surgery and medicine 2022. Jan;54(1):113–120. [DOI] [PubMed] [Google Scholar]
- 79.Lee DW, Ahn HH, Kye YC, et al. Clinical experience of ingenol mebutate gel for the treatment of Bowen's disease. J Dermatol 2018. Apr;45(4):425–430. [DOI] [PubMed] [Google Scholar]
- 80.Zech NH, Murtinger M, Uher P. Pregnancy after ovarian superovulation by transdermal delivery of follicle-stimulating hormone. Fertil Steril 2011. Jun 30;95(8):2784–5. [DOI] [PubMed] [Google Scholar]
- 81.Bauer M, Lackner E, Matzneller P, et al. Phase I Study to Assess Safety of Laser-Assisted Topical Administration of an Anti-TNF Biologic in Patients With Chronic Plaque-Type Psoriasis. Front Med (Lausanne) 2021;8:712511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Badawi AM, Osman MA. Fractional erbium-doped yttrium aluminum garnet laser-assisted drug delivery of hydroquinone in the treatment of melasma. Clin Cosmet Investig Dermatol 2018;11:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cavalie M, Sillard L, Montaudie H, et al. Treatment of keloids with laser-assisted topical steroid delivery: a retrospective study of 23 cases. Dermatol Ther 2015. Mar-Apr;28(2):74–8. [DOI] [PubMed] [Google Scholar]
- 84.Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers in surgery and medicine 2013. Mar;45(3):135–40. [DOI] [PubMed] [Google Scholar]
- 85.Tawfik AA, Fathy M, Badawi A, et al. Topical 5 fluorouracil cream vs combined 5 fluorouracil and fractional erbium YAG laser for treatment of severe hypertrophic scars. Clin Cosmet Investig Dermatol 2019;12:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Issa MC, Pires M, Silveira P, et al. Transepidermal drug delivery: a new treatment option for areata alopecia? Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology 2015. Feb;17(1):37–40. [DOI] [PubMed] [Google Scholar]
- 87.Salah M, Samy N, Fawzy MM, et al. The Effect of the Fractional Carbon Dioxide Laser on Improving Minoxidil Delivery for the Treatment of Androgenetic Alopecia. J Lasers Med Sci 2020. Winter;11(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Genina EA, Bashkatov AN, Dolotov LE, et al. Transcutaneous delivery of micro- and nanoparticles with laser microporation. Journal of biomedical optics 2013. Nov;18(11):111406. [DOI] [PubMed] [Google Scholar]
- 89.Banzhaf CA, Wind BS, Mogensen M, et al. Spatiotemporal closure of fractional laser-ablated channels imaged by optical coherence tomography and reflectance confocal microscopy. Lasers in surgery and medicine 2016. Feb;48(2):157–65. [DOI] [PubMed] [Google Scholar]
- 90.Banzhaf CA, Thaysen-Petersen D, Bay C, et al. Fractional laser-assisted drug uptake: Impact of time-related topical application to achieve enhanced delivery. Lasers in surgery and medicine 2017. Apr;49(4):348–354. [DOI] [PubMed] [Google Scholar]
- 91.Lee YI, Kim S, Kim J, et al. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. Journal of cosmetic dermatology 2021. Jun;20(6):1774–1781. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen HX, Banga AK. Effect of ablative laser on in vitro transungual delivery. International journal of pharmaceutics 2018. Jun 15;544(2):402–414. [DOI] [PubMed] [Google Scholar]
- 93.Guan XH, Xu TH, Chen X, et al. Fractionated carbon dioxide (CO2) laser treatment contributes to trans-nail penetration of rhodamine B and changes of cytokine microenvironment. Lasers in medical science 2021. Oct;36(8):1619–1623. [DOI] [PubMed] [Google Scholar]
- 94.Yang CH, Tsai MT, Shen SC, et al. Feasibility of ablative fractional laser-assisted drug delivery with optical coherence tomography. Biomed Opt Express 2014. Nov 1;5(11):3949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ortner VK, Nguyen N, Brewer JR, et al. Fractional CO2 laser ablation leads to enhanced permeation of a fluorescent dye in healthy and mycotic nails-An imaging investigation of laser-tissue effects and their impact on ungual drug delivery. Lasers in surgery and medicine 2022. Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suh JH, Lee SK, Kim MS, et al. Efficacy of bleomycin application on periungual warts after treatment with ablative carbon dioxide fractional laser: a pilot study. J Dermatolog Treat 2020. Jun;31(4):410–414. [DOI] [PubMed] [Google Scholar]
- 97.Shehadeh W, Matz H, Ellenbogen E, et al. Pulse-Dye Laser Followed by Betamethasone-Calcipotriol and Fractional Ablative CO2-Laser-Assisted Delivery for Nail Psoriasis. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2021. Apr 1;47(4):e111–e116. [DOI] [PubMed] [Google Scholar]
- 98.Gaudana R, Ananthula HK, Parenky A, et al. Ocular drug delivery. AAPS J 2010. Sep;12(3):348–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel A, Cholkar K, Agrahari V, et al. Ocular drug delivery systems: An overview. World J Pharmacol 2013;2(2):47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013. Jul;27(7):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patane MA, Cohen A, From S, et al. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol 2011;5:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thakur Singh RR, Tekko I, McAvoy K, et al. Minimally invasive microneedles for ocular drug delivery. Expert opinion on drug delivery 2017. Apr;14(4):525–537. [DOI] [PubMed] [Google Scholar]
- 103.Figueroa M, Schocket LS, DuPont J, et al. Effect of laser treatment for dry age related macular degeneration on foveolar choroidal haemodynamics. Br J Ophthalmol 2004. Jun;88(6):792–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tiwari NN, Sachdev GS, Ramamurthy S, et al. Comparative analysis of visual outcomes and ocular aberrations following wavefront optimized and topography-guided customized femtosecond laser in situ keratomileusis for myopia and myopic astigmatism: A contralateral eye study. Indian J Ophthalmol 2018. Nov;66(11):1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol 2014. Jan;25(1):49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thakur RRS, Adwan S, Tekko I, et al. Laser irradiation of ocular tissues to enhance drug delivery. International journal of pharmaceutics 2021. Mar 1;596:120282. [DOI] [PubMed] [Google Scholar]
- 107.Zaman RT, Gopal A, Starr K, et al. Micro-patterned drug delivery device for light-activated drug release. Lasers in surgery and medicine 2012. Jan;44(1):30–48. [DOI] [PubMed] [Google Scholar]


