Abstract
Context
Blood pressure and plasma catecholamines normally decline during sleep and rapidly increase in early morning. This is blunted in adults with type 2 diabetes (T2D).
Objective
We hypothesize that increased sympatho-adrenal activity during sleep differentiates youth with T2D from nondiabetic obese youth and lean youth.
Methods
Fasting spot morning and 24-hour urines were collected in obese adolescents with and without T2D, and normal-weight controls. Fractionated free urine catecholamines (epinephrine, norepinephrine, and dopamine) were measured, and the ratio of fasting spot morning to 24-hour catecholamines was calculated.
Results
Urinary 24-hour catecholamine levels were comparable across the 3 groups. Fasting morning epinephrine and the ratio of fasting morning/24-hour epinephrine were higher in youth with T2D (P = 0.004 and P = 0.035, respectively). In males, the ratio of fasting morning/24-hour epinephrine was also higher in youth with T2D (P = 0.005). In females, fasting morning norepinephrine and the ratio of fasting morning/24-hour dopamine were lower in obese youth with and without T2D (P = 0.013 and P = 0.005, respectively) compared with lean youth. Systolic blood pressure was higher in diabetic participants than other groups; males trended higher than females.
Conclusion
Circadian rhythm in catecholamines is disrupted in youth-onset T2D, with a blunted overnight fall in urinary epinephrine in males. Conversely, fasting morning norepinephrine and dopamine levels were lower in obese females with or without T2D. Higher nocturnal catecholamines in males with T2D might associate with, or predispose to, hypertension and cardiovascular complications. Lower catecholamine excretion in females with obesity might serve an adaptive, protective role.
Keywords: catecholamine, type 2 diabetes, circadian rhythm
Type 2 diabetes (T2D) is a worldwide pandemic affecting about 460 million people, and the prevalence has been increasing [1]. With a similarly worsening childhood obesity epidemic, studies show that the diagnosis of T2D in children and adolescents is also increasing [2]. This has profound individual and community health consequences, since T2D is the major cause of retinopathy, leg amputation, nephropathy, and end-stage kidney disease and a principal cause of myocardial infarction and stroke [3, 4]. Additionally, many youths with T2D develop complications more rapidly and aggressively than adults, with comorbidities including hypertension, dyslipidemia, and fatty liver disease [5].
Even though the major determinants of T2D in children and adults are obesity and insulin resistance [6-8], the pathogenesis of T2D remains poorly understood. About one-half of children with obesity are insulin resistant, and far fewer will progress to T2D, about 2% to 8% [9, 10]. It is unclear why only some children with obesity develop metabolic dysfunction and T2D [11, 12]. Accordingly, derangements of insulin metabolism have been extensively studied; however, the role of “insulin counter-regulatory” hormones has not been explored widely in youth. We previously demonstrated that the 24-hour average plasma concentrations of cortisol are lower in nondiabetic youth with obesity than in lean controls [13, 14] and higher in youth with obesity and T2D than without T2D [15]. This suggests that adaptive suppression of cortisol in youth with obesity is lost during or following the development of T2D, contributing to worsening insulin resistance and glucose intolerance.
We have not previously investigated the role of catecholamines in T2D, which also function as “counter-regulatory” hormones. We hypothesized that obesity without T2D might be associated with adaptive suppression of catecholamines, similar to cortisol. Catecholamines not only antagonize the effects of insulin and increase blood glucose levels and insulin resistance, but also cause dysregulated insulin secretion and an increase in blood pressure and heart rate [16-18].
In healthy, normal-weight adults, there is a circadian rhythm in blood pressure and plasma catecholamines: blood pressure and the levels of catecholamines decline during sleep, followed by a rapid increase during the early morning hours [19, 20]. However, studies in adults with T2D show blunted reductions in blood pressure and plasma catecholamine levels during sleep, which are associated with increased risks for heart failure, stroke, myocardial infarction, and sudden death [21-23]. Even so, there are sex differences in circadian rhythm of blood pressure, since both adolescent and adult healthy males appear to show a blunted nocturnal blood pressure dip compared to females [24, 25]. This could be due to sex differences in regulation of catecholamines.
We hypothesized that there is increased sympatho-adrenal activity during sleep in youth with T2D compared to obese youth without T2D and lean controls. A relative increase in catecholamines over time could potentially increase future risk of hypertension and other cardiovascular complications. We also hypothesized that there would be sex differences in sympatho-adrenal activity during sleep. To test these hypotheses, we measured urine catecholamines (epinephrine, norepinephrine, and dopamine) in fasting spot morning urine and 24-hour urine samples in male and female obese youth with and without T2D and lean nondiabetic controls.
Methods
Participants
Participants were recruited from Duke Children's primary care clinics, diabetes clinics at Duke's Lenox Baker Children's Hospital, and the Duke Children's Healthy Lifestyles program. The Healthy Lifestyle program is a comprehensive clinic for pediatric obesity treatment that includes medical management as well as registered dieticians and pediatric physical therapists to create specialized plans for nutrition and fitness for the family. They use motivational interviewing to improve habits and encourage evidence-based lifestyle modifications to reduce excess weight [26, 27]. Three participants with T2D were recruited from the Pediatric Endocrine and Diabetes Clinic at the University of North Carolina (UNC) in Chapel Hill, NC, and 2 were also recruited from Pennington Biomedical Research Center in Baton Rouge, LA. The Clinical Trials Unit at Pennington Biomedical Research Center has extensive experience with studies enrolling adolescents with obesity and T2D and has the capability to conduct a variety of observational and interventional studies across the lifespan.
A total of 141 participants were enrolled; the cohort included 56 youth who were overweight/obese without T2D (“obese”), 42 youth who were obese with T2D (“T2D”), and 43 normal-weight controls (“lean”). The participants were ≥12 to 21 (inclusive) years of age. Normal weight was defined as a body mass index (BMI) between the 5th and 85th percentile, and overweight/obesity was defined as a BMI at or above the 85th percentile [28]. Diabetes was defined as glycated hemoglobin (HbA1c) ≥ 6.5%, fasting plasma glucose ≥126 mg/dL, 2-hours glucose ≥200 mg/dL during an oral glucose tolerance test (OGTT), and/or random plasma glucose ≥200 mg/dL with symptoms of hyperglycemia [28].
Participants were excluded if they had a genetic syndrome causing obesity, Cushing syndrome, untreated hypothyroidism, persistent hyperprolactinemia, current or recent (within the past month) use of systemic corticosteroids, antipsychotics, medications for weight loss, topiramate, medroxyprogesterone acetate, recent start of oral contraceptives (within the last 3 months), or chronic or recent (within the past week) use of over-the-counter drugs such as acetaminophen or aspirin. No lean or obese participants were diagnosed with hypertension or were on antihypertensive treatment. Only 1 participant with T2D had stage 2 hypertension and was on lisinopril 2.5 mg for antihypertensive treatment, although he was not compliant with the medication and was not using the medication during the week of the urine collection. Participants with T2D were on metformin and/or an insulin regimen. Three participants with T2D were taking liraglutide, 3 were taking dulaglutide, and 1 was taking sitagliptin for their diabetes management. The duration from onset of T2D to date of urine collection was 1.6 ± 1.5 years. No participants were ill or metabolically unstable at the time of urine collection. At least one parent/guardian provided informed consent for all participant children <18 years. If the participant was ≥18 years, informed consent was obtained from the participant. The protocol was approved by the Duke University Health System (DUHS) Institutional Review Board, Pediatrics Clinical Research Unit, as the Pro00057460 protocol.
Anthropometric and Demographic Measurements
Age, race, ethnicity, weight, height, BMI, BMI percentile (BMI%), blood pressure, and Tanner stage were extracted from medical charts or collected at the initial research visit. Weight was measured to the nearest 0.1 kg using the same calibrated scales and height was measured to the nearest 0.1 cm using wall-mounted stadiometers. Blood pressure was measured twice, and the average blood pressure was used for statistical analyses. Age, sex, and height-specific normal values for children are available at https://www.nhlbi.nih.gov/files/docs/bp_child_pocket.pdf. BMI, BMI percentiles, and BMI z-scores were calculated using an age- and sex-specific pediatric z-score calculator. Body fat percentage (BF%) was measured by a Tanita BC-418 segmental body composition analyzer, which is a simple, noninvasive, well-validated method in children. Physical exams were performed by clinic providers, including pediatricians and nurse practitioners who received training in pubertal staging and pediatric endocrinologists. For all cohorts, Tanner stage was divided into early to mid-puberty (Tanner 1-3) and late puberty (Tanner 4-5) stages. This was done because there appears to be differences in the circadian system between early/mid vs late puberty [29].
Because catecholamine metabolism is highly affected by stress and depression, we extracted data from the Patient Health Questionnaire (PHQ)-2/PHQ-9 Modified for Teens from medical charts and the initial research visits [30]. The PHQ-2/PHQ-9 Modified for Teens questionnaire is a reliable and valid method to detect depression and assess severity of depression; thus, all youth receive it as part of standard care for routine annual well child checks at Duke's primary clinics. Youth who are obese/overweight or have diabetes also receive the same surveys as standard of care at their first visits and annually at Duke's diabetes clinics and the Healthy Lifestyles program.
Laboratory Results and Urine Catecholamines
Laboratory studies including fasting glucose, insulin, HbA1c, liver function tests, kidney function tests, uric acid, and lipid panels are measured as standard of care at first visit for all youth who are obese/overweight or have diabetes. Data were extracted from their charts; however, not all participants included in this study had all these laboratory tests performed if this was not their first visit to the clinic.
Participants provided first morning, fasting, and spot urines, as well as preservative-free, 24-hour urines. Urine was collected in a container provided by the study team and was returned by the participant to the recruitment site. For the 24-hour urine sample, participants were instructed to discard the first urine specimen upon awakening; they then collected all urine during the next 24 hours. An aliquot of the first morning sample on the second day was considered the fasting spot urine sample; the remainder of this first morning urine was combined with the rest of the 24-hour sample. Urine samples were refrigerated throughout collection and processing.
Urine was analyzed by positive-ion, electrospray, tandem mass spectrometry for fractionated free urine catecholamines (epinephrine, norepinephrine, and dopamine) as per Kushnir et al [31]. To adjust for variation in dilution effects, sample volume, and rate of urine production, the free catecholamine levels were normalized to urinary creatinine; the rate of urinary creatinine excretion is fairly constant [32]. Urinary creatinine measurements were done on a Beckman DxC 600 clinical analyzer (Brea, CA). The ratio of fasting spot morning urine catecholamines to 24-hour urine catecholamines was calculated to assess circadian variation in urinary catecholamines. Of note, the methods used in the study to assess catecholamine circadian variation have not been previously validated. However, Därr et al [33] compared night to day ratios of urinary epinephrine and norepinephrine in adults to investigate nocturnal catecholamine excretion and its correlation with the circadian rhythm of blood pressure. Brossaud et al [34] also used the first morning void to measure nocturnal hormones, although in this case they were investigating cortisol metabolism.
Statistical Analysis
Chi-square testing was used to assess differences in ethnicity, race, sex, and Tanner stage across 3 groups. Kruskal–Wallis or ANOVA was used to assess differences across 3 groups for age, BMI%, BMI z-score, BF%, blood pressure, PHQ-2/9 scores, standard of care laboratory results if available, fractionated free urine catecholamines, and the ratio of fasting spot morning urine catecholamines to 24-hour urine catecholamines. For across 3-group contrasts, P ≤ 0.05 was considered statistically significant. For the same comparisons between 2 groups, t tests or Wilcoxon Rank Sum tests were performed and P values ≤ 0.01 were considered significant to be more stringent. Urine catecholamine group differences were also assessed using multivariate linear regression adjusting for Tanner stage, sex, BMI, age, and race. Data were adjusted for race as previous studies have shown racial differences in catecholamine excretion and circadian rhythm of blood pressure [35-37]. In regression analysis, P values ≤ 0.01 were considered nominally significant. To investigate sex differences, the data were separated by the participant's sex, and each group was separately analyzed as described above. Additionally, t tests were performed to compare the anthropometric and demographic measurements between males and females within the T2D group. To evaluate the association between urinary catecholamines with blood pressure, stress levels, and glycemic control, the Spearman Rho correlations were calculated. All analyses were performed in R version 4.1.2 (2021-11-01).
Results
Baseline Anthropometric and Demographic Characteristics
Baseline characteristics of study participants in the overall cohort are shown in Table 1, while for males only and females only are shown in Tables 2 and 3 respectively. In the overall cohort, lean, obese, and T2D groups were comparable for age, ethnicity, and pubertal status. Although age was comparable across groups, as clinically expected, youth with T2D trended older compared to youth with obesity without T2D, although it did not reach statistical significance (P = 0.031). Obese youth with and without T2D were predominantly female and African American. The T2D group had the highest BMI-z, BMI%, and BF% compared with the lean and obese groups. Patients with T2D also had the highest systolic blood pressure (P = 5.49e−10), while the diastolic blood pressure was comparable across groups. The PHQ-2 and PHQ-9 scores were also comparable across groups. However, as often seen in clinical practice, youth with T2D trended toward higher PHQ-2 and PHQ-9 scores, which did not reach statistical significance (P = 0.053 and P = 0.064, respectively).
Table 1.
Anthropometric and demographic comparisons across the 3 groups in the overall cohort
| Lean (n = 43) | Obese without T2D (n = 56) | Obese with T2D (n = 42) | P value | |
|---|---|---|---|---|
| Age (years) | 14.93 ± 1.87 | 14.77 ± 1.93 | 15.68 ± 2.09 | 0.067 |
| Sex (females/males) | 17 (39.5%)/26 (60.5%) | 33 (58.9%)/23 (41.1%) | 28 (66.7%)/14 (33.3%) | 0.033 |
| Race | AA = 21 (48.8%), Asian = 1 (2.3%), White = 15 (34.9%), More than 1 = 5 (11.6%), NA = 1 (2.3%) | AA = 37 (66.1%), Asian = 1 (1.8%), White = 8 (14.3%), More than 1 = 2 (3.6%), NA = 8 (14.3%) | AA = 31 (73.8%), NH = 1 (2.4%), White = 5 (11.9%), More than 1 = 2 (4.8%), NA = 3 (7.1%) | 0.034 |
| Ethnicity | Hispanic/Latino = 2 (4.7%), Not Hispanic/Latino = 40 (93.0%), NA = 1 (2.3%) | Hispanic/Latino = 11 (19.6%), Not Hispanic/Latino = 43 (76.8%), NA = 2 (3.6%) | Hispanic/Latino = 6 (14.3%), Not Hispanic/Latino = 36 (85.7%) | 0.177 |
| Tanner staging (early/mid, late) | Early/Mid = 5, Late = 35 (NA = 3) | Early/Mid = 12, Late = 44 (NA = 0) | Early/Mid = 3, Late = 35 (NA = 4) | 0.171 |
| BMI-z | 0.06 ± 0.63 | 2 ± 0.49 | 2.36 ± 0.41 | 4.22e−45 |
| BMI% | 52.41 ± 21.92 | 96.37 ± 3.68 | 98.51 ± 1.91 | 5.89e−40 |
| BF% | 20.11 ± 6.28 | 37.25 ± 9.50 | 42.9 ± 9.87 | 2.58e−22 |
| Systolic blood pressure (mmHg) | 111.65 ± 9.13 | 115.36 ± 11.54 | 127.83 ± 12.08 | 5.49e−10 |
| Diastolic blood pressure (mmHg) | 67.91 ± 7.54 | 69.11 ± 8.98 | 71.51 ± 10.53 | 0.182 |
| PHQ-2 score | 0.3 ± 0.62 | 0.54 ± 1.12 | 1.06 ± 1.56 | 0.053 |
| PHQ-9 score | 1.09 ± 3.04 | 3.55 ± 5.12 | 4.53 ± 6.19 | 0.064 |
Data are presented as mean ± 1 SD. Percentage distribution provided for sex, race, and ethnicity.
Abbreviations: AA, African American; BF, body fat; BMI, body mass index; NA, not answered/not available; NH, Native Hawaiian/Pacific Islander; PHQ, Patient Health Questionnaire; T2D, type 2 diabetes.
Table 2.
Anthropometric and demographic comparisons across 3 groups in males
| Lean (n = 26) | Obese without T2D (n = 23) | Obese with T2D (n = 14) | P value | |
|---|---|---|---|---|
| Age (years) | 15.44 ± 1.79 | 14.53 ± 1.55 | 15.68 ± 1.61 | 0.076 |
| Race | AA = 13 (50.0%), Asian = 1 (3.8%), White = 11 (42.3%), More than 1 = 1 (3.8%) | AA = 17 (73.9%), Asian = 1 (4.3%), White = 3 (13.0%), More than 1 = 1 (4.3%), NA = 1 (4.3%) | AA = 11 (78.6%), White = 2 (14.3%), NA = 1 (7.1%) | 0.314 |
| Ethnicity | Hispanic/Latino = 0, Not Hispanic/Latino = 26 (100.0%) | Hispanic/Latino = 2 (8.7%), Not Hispanic/Latino = 21 (91.3%) | Hispanic/Latino = 0, Not Hispanic/Latino = 14 (100.0%) | 0.166 |
| Tanner staging (early/mid, late) | Early/Mid = 2, Late = 21 (NA = 3) | Early/Mid = 7, Late = 16 (NA = 0) | Early/Mid = 3, Late = 10 (NA = 1) | 0.18 |
| BMI-z | 0.3 ± 0.67 | 1.98 ± 0.54 | 2.45 ± 0.51 | 1.33e−10 |
| BMI% | 51.51 ± 23.71 | 96.53 ± 3.46 | 98.32 ± 2.77 | 7.8e−16 |
| BF% | 16.04 ± 3.37 | 32.08 ± 8.22 | 36.11 ± 10.32 | 4.4e−12 |
| Systolic blood pressure (mmHg) | 115.08 ± 7.90 | 115.65 ± 11.62 | 132.14 ± 9.86 | 2.8e−6 |
| Diastolic blood pressure (mmHg) | 68.5 ± 8 | 67.91 ± 8.3 | 70.93 ± 8.07 | 0.53 |
| PHQ-2 score | 0.17 ± 0.58 | 0.11 ± 0.32 | 1.17 ± 1.17 | 0.002 |
| PHQ-9 score | 0.29 ± 0.73 | 1.28 ± 1.81 | 4.67 ± 7.37 | 0.022 |
Data are presented as mean ± 1 SD. Percentage distribution provided for race and ethnicity.
Abbreviations: AA, African American; BF, body fat; BMI, body mass index; NA, not answered; PHQ, Patient Health Questionnaire; T2D, type 2 diabetes.
Table 3.
Anthropometric and demographic comparisons across 3 groups in females
| Lean (n = 17) | Obese without T2D (n = 33) | Obese with T2D (n = 28) | P value | |
|---|---|---|---|---|
| Age (years) | 14.16 ± 1.76 | 14.93 ± 2.16 | 15.67 ± 2.32 | 0.074 |
| Race | AA = 8 (47.1%), White = 4 (23.5%), More than 1 = 4 (23.5%), NA = 1 (5.9%) | AA = 20 (60.6%), White = 5 (15.2%), More than 1 = 1 (3.0%), NA = 7 (21.2%) | AA = 20 (71.4%), NH = 1 (3.6%), White = 3 (10.7%), More than 1 = 2 (7.1%), NA = 2 (7.1%) | 0.130 |
| Ethnicity | Hispanic/Latino = 2 (11.8%), Not Hispanic/Latino = 14 (82.4%), NA = 1 (5.9%) | Hispanic/Latino = 9 (27.3%), Not Hispanic/Latino = 22 (66.7%), NA = 2 (6.1%) | Hispanic/Latino = 6 (21.4%), Not Hispanic/Latino = 22 (78.6%) | 0.494 |
| Tanner staging (Early/Mid, Late) | Early/Mid = 3, Late = 14 (NA = 0) | Early/Mid = 5, Late = 28 (NA = 0) | Early/Mid = 0, Late = 25 (NA = 3) | 0.103 |
| BMI-z | 0.11 ± 0.57 | 2.05 ± 0.46 | 2.3 ± 0.35 | 2.83e−10 |
| BMI% | 53.73 ± 19.62 | 96.26 ± 3.88 | 98.61 ± 1.27 | 2.83e−10 |
| BF% | 25.86 ± 4.71 | 40.86 ± 8.72 | 46.58 ± 7.52 | 5e−12 |
| Systolic blood pressure (mmHg) | 106.41 ± 8.55 | 115.15 ± 11.66 | 125.59 ± 12.67 | 2.7e−6 |
| Diastolic blood pressure (mmHg) | 67 ± 6.91 | 69.94 ± 9.46 | 71.81 ± 11.73 | 0.3 |
| PHQ-2 score | 0.5 ± 0.65 | 0.9 ± 1.41 | 1 ± 1.79 | 0.59 |
| PHQ-9 score | 2.33 ± 4.66 | 5.12 ± 6.05 | 4.45 ± 5.84 | 0.47 |
Data are presented as mean ± 1 SD. Percentage distribution provided for race and ethnicity.
Abbreviations: AA, African American; BF, body fat; BMI, body mass index; NA, not answered/not available; NH, Native Hawaiian/Pacific Islander; PHQ, Patient Health Questionnaire; T2D, type 2 diabetes.
As shown in Table 4, within the T2D group, males (n = 14) and females (n = 28) were comparable in age, race, ethnicity, BMI-z, and BMI%. Females were predominantly in later pubertal stages, but this did not reach statistical significance (P = 0.062), and they had higher BF% (P = 0.004). Systolic blood pressure trended higher in males (P = 0.077), while diastolic blood pressure was comparable. Additionally, PHQ-2 and PHQ-9 scores were comparable.
Table 4.
Male vs female anthropometric and demographic comparisons within T2D group
| T2D Males (n = 14) | T2D Females (n = 28) | P value | |
|---|---|---|---|
| Age (years) | 15.68 ± 1.61 | 15.67 ± 2.32 | 0.987 |
| Race | AA = 11 (78.6%), White = 2 (14.3%), NA = 1 (7.1%) | AA = 20 (71.4%), NH = 1 (3.6%), White = 3 (10.7%), More than 1 = 2 (7.1%), NA = 2 (7.1%) | 0.797 |
| Ethnicity | Hispanic/Latino = 0, Not Hispanic/Latino = 14 (100.0%) | Hispanic/Latino = 6 (21.4%), Not Hispanic/Latino = 22 (78.6%) | 0.161 |
| Tanner staging (Early/Mid, Late) | Early/Mid = 3, Late = 10 (NA = 1) | Early/Mid = 0, Late = 25 (NA = 3) | 0.062 |
| BMI-z | 2.45 ± 0.51 | 2.3 ± 0.35 | 0.58 |
| BMI% | 98.32 ± 2.77 | 98.61 ± 1.27 | 0.72 |
| BF% | 36.11 ± 10.32 | 46.58 ± 7.52 | 0.004 |
| Systolic blood pressure (mmHg) | 132.14 ± 9.86 | 125.59 ± 12.67 | 0.077 |
| Diastolic blood pressure (mmHg) | 70.93 ± 8.07 | 71.81 ± 11.73 | 0.78 |
| PHQ-2 score | 1.17 ± 1.17 | 1 ± 1.79 | 0.82 |
| PHQ-9 score | 4.67 ± 7.37 | 4.45 ± 5.84 | 0.95 |
Data are presented as mean ± 1 SD. Percentage distribution provided for race and ethnicity.
Abbreviations: AA, African American; BF, body fat; BMI, body mass index; NA, not answered/not available; NH, Native Hawaiian/Pacific Islander; PHQ, Patient Health Questionnaire; T2D, type 2 diabetes.
Laboratory Results and Urine Catecholamines
Lean controls did not provide blood samples for analysis. Lab studies were available for a subgroup of obese participants with and without T2D. Compared to obese youth without T2D, youth with T2D had higher levels of HbA1c (T2D 8.38 ± 2.70%, obese 5.54 ± 0.37%, P = 2.70e−10), alanine aminotransferase (T2D 43.8 ± 44 U/L, obese 20.03 ± 15.5 U/L, P = 0.003), and triglycerides (T2D 132.50 ± 63.49 mg/dL, obese 86.96 ± 23.26 mg/dL, P = 0.015), and a lower level of high-density lipoprotein (T2D 39.53 ± 8.63 mg/dL, obese 45.78 ± 9.54 mg/dL, P = 0.022). Levels of aspartate aminotransferase, uric acid, total cholesterol, and low-density lipoprotein were comparable in participants with obesity with and without T2D.
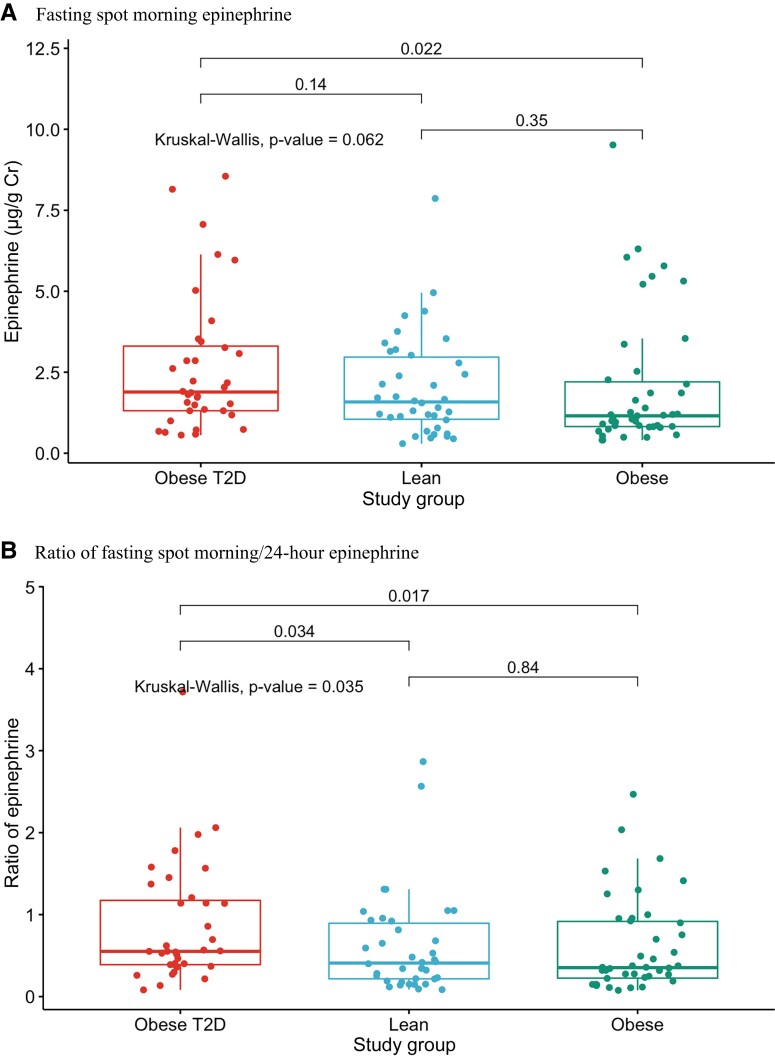
Fractionated free epinephrine was comparable across groups in fasting spot morning and 24-hour urine samples (Fig. 1A, Table 5). However, when models were adjusted for biological variables including Tanner stage, sex, BMI, age, and race, the fasting spot morning epinephrine level was higher in youth with T2D compared to other groups (P = 0.004). Additionally, as shown in Fig. 1B and Table 5, the ratio of fasting spot morning/24-hour epinephrine was higher in participants with T2D compared with other groups (P = 0.035). Obese youth without T2D and lean controls had comparable levels (P = 0.840). Norepinephrine and dopamine levels in fasting spot morning and 24-hour urine samples, and the ratios of fasting spot morning/24-hour urine norepinephrine were comparable across groups (Table 5).
Figure 1.
A, Fasting spot morning epinephrine levels across the 3 groups (lean, obese, T2D). B, Ratio of fasting spot morning/24-hour epinephrine across the 3 groups (lean, obese, T2D).
Table 5.
Fractionated free urine catecholamines across 3 groups in the overall cohort, normalized for creatinine (μg/g Cr)
| Lean (n = 43) | Obese without T2D (n = 56) | Obese with T2D (n = 42) | P value | |
|---|---|---|---|---|
| Fasting spot morning | ||||
| Epinephrine | 2.04 ± 1.57 | 2.04 ± 2.07 | 2.69 ± 2.13 | 0.062 |
| Norepinephrine | 18.47 ± 9.55 | 19.12 ± 12.87 | 16.48 ± 6.68 | 0.94 |
| Dopamine | 224.33 ± 82.23 | 243.41 ± 113.1 | 211.42 ± 70.7 | 0.51 |
| 24-hour | ||||
| Epinephrine | 4.39 ± 2.64 | 3.90 ± 1.95 | 3.74 ± 2.38 | 0.361 |
| Norepinephrine | 26.52 ± 13.06 | 25.18 ± 11.71 | 22.21 ± 8.75 | 0.424 |
| Dopamine | 211.41 ± 98.75 | 254.52 ± 103.34 | 256.45 ± 125.34 | 0.087 |
| Ratio fasting spot morning/24-hour | ||||
| Epinephrine | 0.61 ± 0.61 | 0.60 ± 0.57 | 0.86 ± 0.74 | 0.035 |
| Norepinephrine | 0.78 ± 0.41 | 0.85 ± 0.68 | 0.78 ± 0.31 | 0.61 |
| Dopamine | 1.96 ± 4.92 | 1.01 ± 0.51 | 0.88 ± 0.25 | 0.092 |
Data are presented as mean ± 1 SD. Abbreviation: T2D, type 2 diabetes.
There were no significant correlations between fasting spot morning or fasting spot morning/24-hour epinephrine and the systolic or diastolic blood pressures, PHQ-2/9 scores, or HbA1c levels. Interestingly, 24-hour urine epinephrine levels were negatively (though weakly) correlated with BMI (r = −0.19, P = 0.032), and trended to be negatively (and weakly) correlated with BMI-z (r = −0.17, P = 0.057) and BMI% (r = −0.17, P = 0.058).
Males
Baseline anthropometric and demographic characteristics
Baseline characteristics of males are shown in Table 2. Males were comparable for age, race, ethnicity, and pubertal status across the 3 groups. The T2D group had higher BMI-z, BMI%, and BF% compared with nondiabetic obese and lean groups. They also had higher mean systolic blood pressure (P = 2.8e−6), while diastolic blood pressure was comparable across groups. Additionally, the PHQ-2 and PHQ-9 scores were significantly higher in males with T2D (P = 0.002 and P = 0.022, respectively).
Urine catecholamines
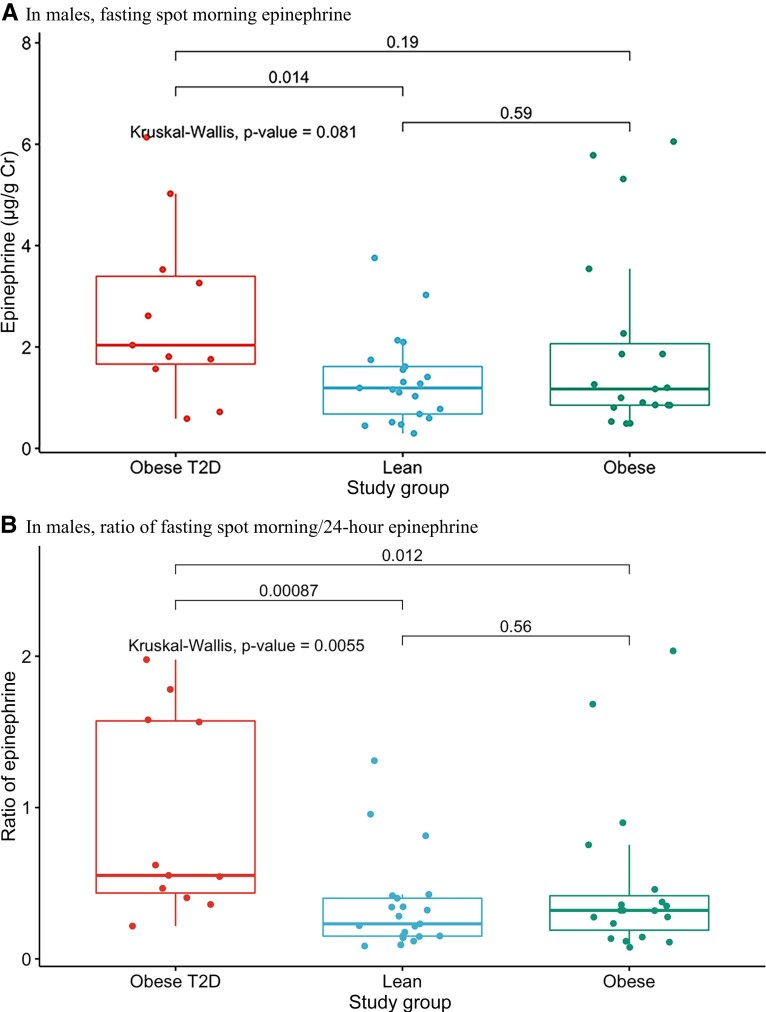
Epinephrine, norepinephrine, and dopamine levels in both fasting spot morning and 24-hour urine samples were comparable across the 3 groups (Table 6). However, as seen in Table 6 and Fig. 2A, fasting spot morning epinephrine was higher in males with T2D than in lean controls (P = 0.014), while 24-hour epinephrine and norepinephrine levels trended lower. Accordingly, the ratio of fasting spot morning/24-hour epinephrine was higher in males with T2D compared with other groups (Fig. 2B and Table 6, P = 0.005), and the ratio of fasting spot morning/24-hour norepinephrine trended higher. In contrast, the ratios for fasting spot morning/24-hour dopamine were comparable among the 3 groups. There were no differences between any 2 groups in fasting spot morning and 24-hour norepinephrine and dopamine levels.
Table 6.
Fractionated free urine catecholamines across 3 groups in males, normalized for creatinine (μg/g Cr)
| Lean (n = 26) | Obese without T2D (n = 23) | Obese with T2D (n = 14) | P value | |
|---|---|---|---|---|
| Fasting spot morning | ||||
| Epinephrine | 1.34 ± 0.87 | 1.95 ± 1.83 | 2.64 ± 1.73 | 0.081 |
| Norepinephrine | 12.52 ± 5.10 | 17.50 ± 8.76 | 14.64 ± 5.91 | 0.15 |
| Dopamine | 194.67 ± 83.73 | 228.74 ± 91.38 | 182.26 ± 57.75 | 0.19 |
| 24-hour | ||||
| Epinephrine | 4.61 ± 2.29 | 4.36 ± 1.67 | 3.38 ± 2.38 | 0.057 |
| Norepinephrine | 23.06 ± 11.13 | 26.47 ± 11.94 | 18.25 ± 6.24 | 0.078 |
| Dopamine | 206.15 ± 77.08 | 229.26 ± 69.98 | 183.02 ± 50.02 | 0.1 |
| Ratio fasting spot morning/24-hour | ||||
| Epinephrine | 0.35 ± 0.31 | 0.49 ± 0.53 | 0.92 ± 0.66 | 0.005 |
| Norepinephrine | 0.63 ± 0.30 | 0.75 ± 0.54 | 0.84 ± 0.22 | 0.076 |
| Dopamine | 0.94 ± 0.09 | 0.98 ± 0.43 | 1.01 ± 0.18 | 0.187 |
Data are presented as mean ± 1 SD. Abbreviation: T2D, type 2 diabetes.
Figure 2.
. A, Fasting spot morning epinephrine levels in males across the 3 groups (lean, obese, T2D). B, Ratio of fasting spot morning/24-hour epinephrine in males across the 3 groups (lean, obese, T2D).
Females
Baseline anthropometric and demographic characteristics
Baseline characteristics of females are shown in Table 3. Females were comparable for age, race, ethnicity, and pubertal status across the 3 groups. They also had higher BMI-z, BMI%, and BF% compared to obese and lean groups. Female youth with T2D also had higher systolic blood pressure than females without diabetes (P = 2.7e−6). Diastolic blood pressures were comparable across groups, as were the PHQ-2 and PHQ-9 scores.
Urine catecholamines
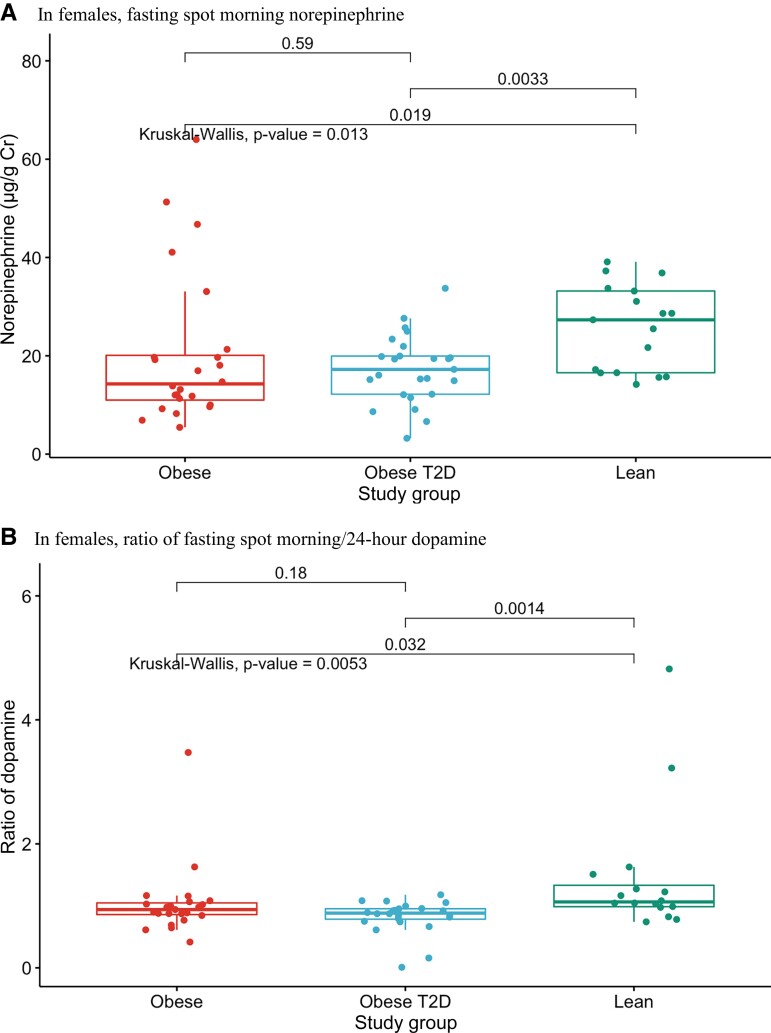
In fasting spot morning urines, epinephrine and dopamine were comparable across the 3 groups (Table 7). In contrast to males, fasting spot morning norepinephrine was lower in obese females with and without T2D than in lean controls (P = 0.013, Fig. 3A and Table 7). Likewise, the ratio of fasting spot morning/24-hour dopamine was lower in obese females with and without T2D than in lean controls (Fig. 3B and Table 7, P = 0.005). There were no differences among female groups in 24-hour norepinephrine, dopamine, or epinephrine levels, or in the ratios of fasting spot morning/24-hour epinephrine and norepinephrine.
Table 7.
Fractionated free urine catecholamines across 3 groups in females, normalized for creatinine (μg/g Cr)
| Lean (n = 17) | Obese without T2D (n = 33) | Obese with T2D (n = 28) | P value | |
|---|---|---|---|---|
| Fasting spot morning | ||||
| Epinephrine | 2.89 ± 1.84 | 2.10 ± 2.29 | 2.71 ± 2.32 | 0.069 |
| Norepinephrine | 25.81 ± 8.65 | 20.40 ± 15.44 | 17.30 ± 6.95 | 0.013 |
| Dopamine | 260.98 ± 65.51 | 255.03 ± 128.47 | 224.25 ± 73.07 | 0.22 |
| 24-hour | ||||
| Epinephrine | 4.07 ± 3.13 | 3.52 ± 2.10 | 3.92 ± 2.41 | 0.89 |
| Norepinephrine | 31.61 ± 14.31 | 24.12 ± 11.62 | 24.26 ± 9.24 | 0.13 |
| Dopamine | 219.14 ± 126.38 | 275.28 ± 121.63 | 294.52 ± 136.04 | 0.25 |
| Ratio fasting spot morning/24-hour | ||||
| Epinephrine | 0.94 ± 0.74 | 0.70 ± 0.60 | 0.84 ± 0.78 | 0.408 |
| Norepinephrine | 0.95 ± 0.47 | 0.94 ± 0.77 | 0.75 ± 0.35 | 0.259 |
| Dopamine | 3.21 ± 7.28 | 1.04 ± 0.58 | 0.83 ± 0.26 | 0.005 |
Data are presented as mean ± 1 SD. Abbreviation: T2D, type 2 diabetes.
Figure 3.
A, Fasting spot morning norepinephrine levels in females across the 3 groups (lean, obese, T2D). B, Ratio of fasting spot morning/24-hour dopamine in females across the 3 groups (lean, obese, T2D).
Discussion
In healthy individuals, there are circadian rhythms in blood pressure and plasma catecholamines; they decline during sleep and then rapidly increase during early morning hours [19, 20]. However, this nocturnal decline in both blood pressure and catecholamines appears to be blunted in adults with diabetes [21, 38]. These changes are likely associated, since studies have shown that increases in sympathetic activity are accompanied by, and likely contributory to, increases in blood pressure [21, 39, 40]. Importantly, Därr et al showed that higher night to day ratios of epinephrine and norepinephrine are associated with attenuated overnight declines in blood pressure in adults [33].
In this study, we investigated sympatho-adrenal activity during sleep in youth with T2D compared to obese youth without T2D and lean controls. We also assessed sex differences in sympatho-adrenal activity during sleep. Our findings include 3 important observations. First, there is a blunted nocturnal decline in epinephrine levels in youth with T2D compared to lean youth and nondiabetic youth with obesity, evident by higher fasting spot morning/24-hour urine epinephrine levels. Second, the increase in morning epinephrine in youth with T2D is detected in males but not females. Third, in contrast to males, fasting spot morning norepinephrine was lower in obese females with and without T2D than in lean controls as was the ratio of fasting spot morning/24-hour dopamine.
Blunted nocturnal decline in epinephrine in males with T2D is consistent with a study by Bernardi et al [41], who showed higher sympathetic activity overnight in adults with T2D compared to those without T2D. We also found that youth with T2D have higher systolic blood pressures compared to lean youth or nondiabetic youth with obesity. The cause of hypertension in T2D is not fully understood; however, the sustained nocturnal sympathetic activation likely contributes to increases in blood pressure and hypertension in young males with T2D, because increased catecholamine levels correlate with higher blood pressures in adults [21, 33, 39, 40]. Interestingly, we found that 24-hour epinephrine levels were negatively, though weakly, correlated with BMI-related metrics. Results of studies of the relationship among catecholamines and BMI in pediatrics are limited and conflicting [42-44]. However, recent studies in adults show that 24-hour norepinephrine positively correlates with BMI, while epinephrine is negatively correlated [45].
In contrast to males, the females in our study had comparable epinephrine levels across groups. In fact, relative to lean controls, obese females with and without diabetes had lower fasting spot morning norepinephrine and lower ratio of fasting spot morning/24-hour dopamine. Sex differences in catecholamine excretion are apparent in the adult literature. Gustafson and Kalkhoff [46] found that in response to isometric exercise, epinephrine levels in lean men exceeded those in lean women, and both were higher than epinephrine levels in women with obesity. They also found that the norepinephrine response was higher in lean men than both lean and obese women. Other studies have also shown that during stress or with hypertension, females excrete less catecholamine than men [47-49]. Consistent with those findings, our results suggest that young teenage girls have a suppressed catecholamine response in comparison to young teenage boys. This might explain in part the trend toward lower blood pressures in girls than boys with T2D observed in our study.
Indeed, women tend to have lower daytime blood pressures than men until they are postmenopausal [50], and their nocturnal systolic blood pressures are commonly lower than those of males [25, 51]. This association of sympathetic activation and blood pressure elevation specifically in males is also seen in mice. Male and female mice that have undergone early-life stress develop hypertension after placement on a high-fat diet. However, they appear to develop hypertension differently, with male mice displaying increased sympathetic activation, and female mice showing greater adiposity and metabolic compromise [52]. The higher PHQ-2 and PHQ-9 scores in our male participants with T2D might reflect increased stress and may be associated with sympathetic activation. It is possible that females with obesity demonstrate a greater reduction in sympatho-adrenal activity during sleep compared with lean females as an adaptive response to weight gain. It has been suggested that estrogen is protective; it induces activation of nitric oxide to promote vasodilation and exerts adaptive effects on the renin-angiotensin system to prevent hypertension [53, 54]. Studies have also shown that estrogen plays a role in inhibiting sympathetic activity. For example, Gomes et al [55] found that gonadectomy in female rats led to an increase in plasma catecholamine concentration, and Vongpatanasin et al [56] demonstrated that estrogen-replacement therapy decreased sympathetic nerve activity in postmenopausal women. The evolution of an adaptive response in obese female youth in the face of obesity and T2D complements our previous work, which found that obese African American youth without T2D have lower 24-hour cortisol levels than obese youth with T2D [15]. Higher cortisol levels likely reduce insulin sensitivity, thereby predisposing to, or exacerbating, glucose intolerance. One hypothesis, which requires more rigorous testing, is that obesity may predispose preferentially to insulin resistance and metabolic complications among females and to hypertension and cardiovascular disease in males. In that regard, the Type 2 Diabetes in Adolescents and Youth study showed that T2D is twice as common in adolescent females than males despite comparable BMI z-scores [57, 58]. Likewise, males were at 81% greater risk than females of developing hypertension in the same cohort (P = 0.0005). Studies in adults with hypertension or diabetes have shown that failure of blood pressure to decline overnight (“non-dippers”) is associated with higher cardiovascular morbidity and mortality [22, 33, 59]. The apparent increase in nocturnal sympatho-adrenal activity in youth with T2D may therefore portend risks of serious complications, especially in males.
This study has several strengths. Participants were matched for age, ethnicity, and pubertal status, and within the male and female groups, participants were additionally matched for race. Baseline characteristics of the participants were also consistent with clinical practice; the T2D group were predominantly female and African American, and had a higher BMI, body fat percentage, and PHQ-9 score. Another strength of this study was the successful collection of a relatively large number of fasting spot morning and 24-hour urine samples in children, especially in children with T2D, as well as the use of urinary creatinine to normalize samples.
Our study has several limitations. We did not perform 24-hour ambulatory blood pressure monitoring. This could have helped to determine if the blunted nocturnal reduction in catecholamines seen in T2D is specifically associated with changes in the circadian rhythm of blood pressure. This may explain why we did not see a significant correlation between catecholamines and blood pressures measured during clinic visits. Moreover, it is unknown if our participants had sleep apnea, as they did not routinely undergo sleep studies to qualify for inclusion; this could confound results for catecholamine excretion. However, none of the participants had a formal diagnosis of sleep apnea. Another limitation was the use of urinary catecholamine rather than plasma to measure sympatho-adrenal activity. However, many studies have shown success in measuring variations in sympathetic activity using measurements of urinary catecholamines [60, 61]. Of note, males with obesity and T2D had higher PHQ-2/9 scores (Table 2), suggesting that their increases in catecholamine excretion might be associated with depression. Some studies found that depression in adults is associated with higher blood pressure and catecholamine excretion [62-64]. For example, Shinagawa et al [62] found that a depressive mood was associated with higher 24-hour averages of systolic and diastolic blood pressure, and less dipping of the diastolic blood pressure overnight. On the other hand, Scalco et al [64] noted that the literature regarding depression and the circadian rhythm of blood pressure is varied. Some studies found that depression was associated with a higher night/day ratio of systolic blood pressure, while others show that depression was associated with a high day/night blood pressure ratio. Alternatively, the association of depression and hypertension might be mediated by higher BMI [65]. We did not include clinical diagnosis of depression as a confounding factor in the T2D group and we did not compare catecholamine excretion in those with high and low PHQ scores. Such multiple group comparisons would be statistically invalid due to our limited number of participants. Finally, our methods to assess catecholamine circadian variation have not been fully validated. Nevertheless, our findings suggest a novel approach for assessing circadian rhythm of catecholamines in adolescents and children.
In spite of these limitations, our findings demonstrate disruption of circadian catecholamine secretion in male youth with T2D and in female youth with obesity. The heightened overnight sympathetic activity might contribute to hypertension in diabetic males, while the reduction in norepinephrine and dopamine in obese females might serve an adaptive role to protect against blood pressure surges. These observations and others [26, 42, 46, 57, 58] underscore the roles of sex differences in the pathogenesis and complications of pediatric obesity and T2D.
Acknowledgments
We would like to thank our patients and their families, as well as Duke Children's primary care clinics, Duke's Lenox Baker Children's Hospital Diabetes Clinics, Duke Children's Healthy Lifestyles Program, The Metabolomics Core Laboratory at the Stedman Center/Duke Molecular Physiology Institute, Duke Center for Childhood Obesity Research, Pennington Biomedical Research Center, and University of North Carolina's Pediatric Endocrine and Diabetes Clinic. We would also like to thank Huaxia Cui for her technical assistance in running the Beckman DxC 600 clinical analyzer.
Abbreviations
- BF%
body fat percentage
- BMI
body mass index
- HbA1c
glycated hemoglobin
- OGTT
oral glucose tolerance test
- PHQ
Patient Health Questionnaire
- T2D
type 2 diabetes
Contributor Information
Stephanie Giessner, Email: stephanie.giessner@duke.edu, General Pediatrics, Duke University Medical Center, Durham, NC 27710, USA.
Megan E Ramaker, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA.
Kathryn Blew, Division of Pediatric Endocrinology and Diabetes, Duke University Medical Center, Durham, NC 27710, USA.
Matthew L Crawford, Department of Research and Development, LabCorp, Burlington, NC 27215, USA.
Russell P Grant, Department of Research and Development, LabCorp, Burlington, NC 27215, USA.
James R Bain, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA; Division of Endocrinology, Metabolism, and Nutrition, Duke University Medical Center, Durham, NC 27710, USA.
Michael Muehlbauer, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA.
Nina Jain, Division of Endocrinology, Department of Pediatrics, University of North Carolina, Chapel Hill, NC 27514, USA.
Daniel S Hsia, Clinical Trials Unit, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA.
Sarah Armstrong, Division of General Pediatrics and Adolescent Health, Duke University Medical Center, Durham, NC 27710, USA; Department of Family Medicine and Community Health, Duke University Medical Center, Durham, NC 27710, USA; Department of Population Health Sciences, Duke University Medical Center, Durham, NC 27701, USA; Duke Clinical Research Institute, Duke University Medical Center, Durham, NC 27701, USA.
Michael Freemark, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Division of Pediatric Endocrinology and Diabetes, Duke University Medical Center, Durham, NC 27710, USA.
Pinar Gumus Balikcioglu, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Division of Pediatric Endocrinology and Diabetes, Duke University Medical Center, Durham, NC 27710, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA.
Funding
P.G.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) under the award number DK117067, Children's Miracle Network Hospitals partnerships and programs benefiting Duke Children's, Derfner Foundation Research Grant, and Duke University Pediatric Departmental Support, Duke Strong Start Award Program. D.S.H. was supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center and in part by a NORC Center Grant # P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.R.B. receives salary support from National Institutes of Health 5P30DK124723, 5R01DK117491, 1U24DK129557, and 2P30AG027816, and USDA 2020-38640-31521.
Author Contributions
S.G. was responsible for interpretation of the data and writing the manuscript. M.E.R. was responsible for advanced statistical analysis. K.B. was responsible for interpretation of the data and critical review of the manuscript. M.L.C. and R.P.G. were responsible for mass spectrometry analysis of samples and critical review of the manuscript. J.R.B. and M.M. performed biochemical analysis and critical review of the manuscript. N.J., D.S.H., and S.A. contributed to data collection and critical review of the manuscript. M.F. was responsible for development of the research question, conception and design of the research project, interpretation of the data, and critical review of the manuscript. P.G.B. was responsible for development of the research question, conception and design of the research project, obtaining funding, acquisition of data, statistical analysis and interpretation of the data, and writing the manuscript.
Disclosures
M.L.C. and R.P.G. own stock and are paid salary by Laboratory corporation of America. J.R.B. has non-financial ties to Agilent Technologies, Inc. The other authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
ClinicalTrials.gov Identifier: NCT02326129.
References
- 1. Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin N Am. 2021;50(3):337–355. [DOI] [PubMed] [Google Scholar]
- 2. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14(8):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14(6):491–509. [DOI] [PubMed] [Google Scholar]
- 4. Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. [DOI] [PubMed] [Google Scholar]
- 5. Valaiyapathi B, Gower B, Ashraf AP. Pathophysiology of type 2 diabetes in children and adolescents. Curr Diabetes Rev. 2020;16(3):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374. [DOI] [PubMed] [Google Scholar]
- 7. Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369(9579):2059–2061. [DOI] [PubMed] [Google Scholar]
- 8. Artz E, Haqq A, Freemark M. Hormonal and metabolic consequences of childhood obesity. Endocrinol Metab Clin N Am. 2005;34(3):643–658. [DOI] [PubMed] [Google Scholar]
- 9. Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care. 2009;32(2):342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizokami-Stout K, Cree-Green M, Nadeau KJ. Insulin resistance in type 2 diabetic youth. Curr Opin Endocrinol Diabetes Obes. 2012;19(4):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28(4):902–909. [DOI] [PubMed] [Google Scholar]
- 12. Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802–810. [DOI] [PubMed] [Google Scholar]
- 13. Chalew SA, Lozano RA, Armour KM, Zadik Z, Kowarski AA. Reduction of plasma cortisol levels in childhood obesity. J Pediatr. 1991;119(5):778–780. [DOI] [PubMed] [Google Scholar]
- 14. Chalew SA, Lozano RA, Armour KM, Kowarski AA. Reduction of plasma insulin levels does not restore integrated concentration of growth hormone to normal in obese children. Int J Obes Relat Metab Disord. 1992;16(6):459–463. [PubMed] [Google Scholar]
- 15. Balikcioglu P G, Balikcioglu M, Soros A, Chalew S. The 24-hour average concentration of cortisol is elevated in obese African-American youth with type 2 diabetes. J Diabetes Complications. 2021;35(7):107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zawalich WS, Zawalich KC, Yamazaki H. Divergent effects of epinephrine and prostaglandin E2 on glucose-induced insulin secretion from perifused rat islets. Metab Clin Exp. 2007;56(1):12–18. [DOI] [PubMed] [Google Scholar]
- 17. Li R, Huang H, Limesand SW, Chen X. Pancreatic islets exhibit dysregulated adaptation of insulin secretion after chronic epinephrine exposure. Curr Issues Mol Biol. 2021;43(1):240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barth E, Albuszies G, Baumgart K, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35(Suppl):S508–S518. [DOI] [PubMed] [Google Scholar]
- 19. Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens Res. 2011;34(3):281–285. [DOI] [PubMed] [Google Scholar]
- 20. Rasch B, Dodt C, Mölle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32(8-10):884–891. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen FS, Hansen HP, Jacobsen P, et al. Increased sympathetic activity during sleep and nocturnal hypertension in type 2 diabetic patients with diabetic nephropathy. Diabet Med. 1999;16(7):555–562. [DOI] [PubMed] [Google Scholar]
- 22. Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17(5):360–364. [DOI] [PubMed] [Google Scholar]
- 23. Kwon Y, PL Stafford, DC Lim, et al. Blood pressure monitoring in sleep: time to wake up. Blood Press Monit. 2020;25(2):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenlund IM, Smoot CA, Carter JR. Sex differences in blood pressure responsiveness to spontaneous K-complexes during stage II sleep. J Appl Physiol (1985). 2021;130(2):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Driziene Z, Jakutiene E, Stakisaitis D, Pundziene B, Sveikata A. Characteristics of gender-related circadian arterial blood pressure in healthy adolescents. Medicina (Kaunas). 2008;44(10):768–774. [PubMed] [Google Scholar]
- 26. Newbern D, Gumus Balikcioglu P, Balikcioglu M, et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: metabolomic profiling and principal components analysis. J Clin Endocrinol Metab. 2014;99(12):4730–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jachthuber Trub C, Balikcioglu M, Freemark M, et al. Impact of lifestyle intervention on branched-chain amino acid catabolism and insulin sensitivity in adolescents with obesity. Endocrinol Diabetes Metab. 2021;4(3):e00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Diabetes Association Professional Practice Committee . 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Suppl_1):S17–S38. [DOI] [PubMed] [Google Scholar]
- 29. Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 2015;100(11):4067–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis M, Jones JD, So A, et al. Adolescent depression screening in primary care: who is screened and who is at risk? J Affect Disord. 2022;299:318–325. [DOI] [PubMed] [Google Scholar]
- 31. Kushnir MM, Urry FM, Frank EL, Roberts WL, Shushan B. Analysis of catecholamines in urine by positive-ion electrospray tandem mass spectrometry. Clin Chem. 2002;48(2):323–331. [PubMed] [Google Scholar]
- 32. Martin MD, Woods JS, Leroux BG, et al. Longitudinal urinary creatinine excretion values among preadolescents and adolescents. Transl Res. 2008;151(1):51–56. [DOI] [PubMed] [Google Scholar]
- 33. Därr R, Bursztyn M, Pamporaki C, et al. Dipping in ambulatory blood pressure monitoring correlates with overnight urinary excretion of catecholamines and sodium. J Clin Hypertens (Greenwich). 2016;18(9):921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brossaud J, Corcuff JB, Vautier V, et al. Altered cortisol metabolism increases nocturnal cortisol bioavailability in prepubertal children with type 1 diabetes mellitus. Front Endocrinol (Lausanne). 2021;12:742669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farrell MC, Giza RJ, Shibao CA. Race and sex differences in cardiovascular autonomic regulation. Clin Auton Res. 2020;30(5):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abate NI, Mansour YH, Tuncel M, et al. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38(3):379–383. [DOI] [PubMed] [Google Scholar]
- 37. Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kondo K, Matsubara T, Nakamura J, Hotta N. Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in type 2 diabetic patients. Diabet Med. 2002;19(5):359–365. [DOI] [PubMed] [Google Scholar]
- 39. Sowers JR, Whitfield LA, Catania RA, et al. Role of the sympathetic nervous system in blood pressure maintenance in obesity. J Clin Endocrinol Metab. 1982;54(6):1181–1186. [DOI] [PubMed] [Google Scholar]
- 40. Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5(1):86–99. [DOI] [PubMed] [Google Scholar]
- 41. Bernardi L, Ricordi L, Lazzari P, et al. Impaired circadian modulation of sympathovagal activity in diabetes. A possible explanation for altered temporal onset of cardiovascular disease. Circulation. 1992;86(5):1443–1452. [DOI] [PubMed] [Google Scholar]
- 42. Barkin S, Rao Y, Smith P, Po’e E. A novel approach to the study of pediatric obesity: a biomarker model. Pediatr Ann. 2012;41(6):250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Snow AB, Khalyfa A, Serpero LD, et al. Catecholamine alterations in pediatric obstructive sleep apnea: effect of obesity. Pediatr Pulmonol. 2009;44(6):559–567. [DOI] [PubMed] [Google Scholar]
- 44. Butte NF, Puyau MR, Adolph AL, Vohra FA, Zakeri I. Physical activity in nonoverweight and overweight Hispanic children and adolescents. Med Sci Sports Exerc. 2007;39(8):1257–1266. [DOI] [PubMed] [Google Scholar]
- 45. Hollstein T, Basolo A, Ando T, Votruba SB, Krakoff J, Piaggi P. Urinary norepinephrine is a metabolic determinant of 24-hour energy expenditure and sleeping metabolic rate in adult humans. J Clin Endocrinol Metab. 2020;105(4):1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gustafson AB, Kalkhoff RK. Influence of sex and obesity on plasma catecholamine response to isometric exercise. J Clin Endocrinol Metab. 1982;55(4):703–708. [DOI] [PubMed] [Google Scholar]
- 47. Frankenhaeuser M, Dunne E, Lundberg U. Sex differences in sympathetic-adrenal medullary reactions induced by different stressors. Psychopharmacology (Berl). 1976;47(1):1–5. [DOI] [PubMed] [Google Scholar]
- 48. Saxena AR, Chamarthi B, Williams GH, Hopkins PN, Seely EW. Predictors of plasma and urinary catecholamine levels in normotensive and hypertensive men and women. J Hum Hypertens. 2014;28(5):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang CC, Chung CM, Leu HB, et al. Sex difference in sympathetic nervous system activity and blood pressure in hypertensive patients. J Clin Hypertens (Greenwich). 2021;23(1):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2016;101(3):349–355. [DOI] [PubMed] [Google Scholar]
- 51. Perez-Lloret S, Toblli JE, Cardinali DP, Milei J. Gender differences in age-related increase of asleep blood pressure. Arch Gerontol Geriatr. 2010;50(3):319–322. [DOI] [PubMed] [Google Scholar]
- 52. Dalmasso C, Leachman JR, Ghuneim S, et al. Epididymal fat-derived sympathoexcitatory signals exacerbate neurogenic hypertension in obese male mice exposed to early life stress. Hypertension. 2021;78(5):1434–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Noah ML N, Adzika GK, Mprah R, Adekunle AO, Adu-Amankwaah J, Sun H. Sex-gender disparities in cardiovascular diseases: the effects of estrogen on eNOS, lipid profile, and NFATs during catecholamine stress. Front Cardiovasc Med. 2021;8:639946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Li C, Yang L, et al. Estrogen protects vasomotor functions in rats during catecholamine stress. Front Cardiovasc Med. 2021;8:679240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomes HL, Graceli JB, Gonçalves WL, et al. Influence of gender and estrous cycle on plasma and renal catecholamine levels in rats. Can J Physiol Pharmacol. 2012;90(1):75–82. [DOI] [PubMed] [Google Scholar]
- 56. Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103(24):2903–2908. [DOI] [PubMed] [Google Scholar]
- 57. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011; 96(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. TODAY Study Group . Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kario K, Hoshide S, Mizuno H, et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020; 142(19):1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Berge-Landry HM, Bovbjerg DH, James GD. Relationship between waking-sleep blood pressure and catecholamine changes in African-American and European-American women. Blood Press Monit. 2008;13(5):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O'Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12(5):483–488. [DOI] [PubMed] [Google Scholar]
- 62. Shinagawa M, Otsuka K, Murakami S, et al. Seven-day (24-h) ambulatory blood pressure monitoring, self-reported depression and quality of life scores. Blood Press Monit. 2002;7(1):69–76. [DOI] [PubMed] [Google Scholar]
- 63. Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30(5):842–851. [DOI] [PubMed] [Google Scholar]
- 64. Scalco AZ, Scalco MZ, Azul JB, Lotufo Neto F. Hypertension and depression. Clinics (Sao Paulo). 2005;60(3):241–250. [DOI] [PubMed] [Google Scholar]
- 65. Kabir AA, Whelton PK, Khan MM, Gustat J, Chen W. Association of symptoms of depression and obesity with hypertension: the Bogalusa Heart Study. Am J Hypertens. 2006;19(6):639–645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.