Abstract
The troponin (Tn) complex, responsible for the Ca2+ activation of striated muscle, is composed of three interacting protein subunits: TnC, TnI, and TnT, encoded by TNNC, TNNI, and TNNT genes. TNNI and TNNT are sister gene families, and in mammals the three TNNI paralogs (TNNI1, TNNI2, TNNI3), which encode proteins with tissue-specific expression, are each in close genomic proximity with one of the three TNNT paralogs (TNNT2, TNNT3, TNNT1, respectively). It has been widely presumed that all vertebrates broadly possess genes of these same three classes, although earlier work has overlooked jawless fishes (cyclostomes) and cartilaginous fishes (chimeras, rays, and sharks), which are distantly related to other jawed vertebrates. With a new phylogenetic and synteny analysis of a diverse array of vertebrates including these taxonomic groups, we define five distinct TNNI classes (TNNI1-5), with TNNI4 and TNNI5 being only present in non-amniote vertebrates and typically found in tandem, and four classes of TNNT (TNNT1-4). These genes are located in four genomic loci that were generated by the 2R whole-genome duplications. TNNI3, encoding “cardiac TnI” in tetrapods, was independently lost in cartilaginous and ray-finned fishes. Instead, ray-finned fishes predominantly express TNNI1 in the heart. TNNI5 is highly expressed in shark hearts and contains a N-terminal extension similar to that of TNNI3 found in tetrapod hearts. Given that TNNI3 and TNNI5 are distantly related, this supports the hypothesis that the N-terminal extension may be an ancestral feature of vertebrate TNNI and not an innovation unique to TNNI3, as has been commonly believed.
Keywords: whole-genome duplication, Ohno's hypothesis, 2R, gnathostome, adrenergic regulation
Significance.
Troponin I (TnI) and troponin T (TnT) are striated muscle proteins, and mammals are known to have three copies of each gene, each of which exhibit tissue-specific expression. Cardiac TnI (TNNI3 gene) is exclusively expressed in the heart and contains a “unique” N-terminal extension. By studying the genomes as well as striated muscle gene and protein expression of a diverse cohort of vertebrates, we found a range of uncharacterized TnI and TnT genes. A newly described TnI (TNNI5) expressed in shark hearts bears a similar N-terminal extension to cardiac TnI of tetrapods (TNNI3). As these proteins are distantly related, this finding suggests the N-terminal extension is possibly an ancestral trait that has been differentially lost in some vertebrate TNNI lineages.
Introduction
Contraction of striated muscle is initiated when Ca2+ binds to the troponin (Tn) complex, which is located, along with tropomyosin, in association with the actin filament of the sarcomere (Solaro and Rarick 1998; van der Velden and Stienen 2019). Tn-Ca2+ binding induces a conformational change that moves tropomyosin and allows the formation of actin–myosin cross-bridges which generate contractile force. The Tn complex is composed of three proteins; the Ca2+-binding subunit (TnC), the inhibitory subunit (TnI), and the tropomyosin-binding subunit (TnT) (van der Velden and Stienen 2019; Solaro and Rarick 1998). TnC is a calmodulin-like protein that is part of the helix–loop–helix group of Ca2+ binding proteins (Strynadka and James 1989), whilst TnI and TnT, which are closely related to one another (Chong and Jin 2009; Rasmussen and Jin 2021; Wei and Jin 2016), indirectly affect Ca2+ affinity of Tn through protein–protein interactions within the complex (Lombardi et al. 2008; Evans and Levine 1980; Hwang et al. 2014). This mode of contraction activation is evolutionarily ancient and can be traced back to the earliest bilaterian animals ∼700 Ma (Barnes et al. 2016; Rasmussen et al. 2022; Cao et al. 2019; Yaguchi et al. 2017).
In vertebrates, each striated muscle type (i.e., cardiac muscle, slow, and fast twitch skeletal muscle) express a specific complement of TnC, TnI, and TnT genes (TNNC, TNNI, and TNNT, respectively). In mammals and most other vertebrates, two groups of genes encoding TnC are found, which are characterized by expression in fast skeletal muscle (fsTnC; TNNC2) or both cardiac and slow skeletal muscle (cTnC; TNNC1) (Gillis and Klaiman 2011). In some ray-finned fishes, including teleosts and gar, there are two TNNC1 genes due to a lineage-specific gene duplication (Genge et al. 2016). The evolutionary histories of TnI and TnT have received great attention (Wei and Jin 2016; Rasmussen et al. 2022; Sheng and Jin 2016; MacLean et al. 1997; Palpant et al. 2010; Gross and Lehman 2016; Chong and Jin 2009; Hastings 1997; Shaffer and Gillis 2010; Genge et al. 2016; Barnes et al. 2016), and it is generally believed that, like mammals, most vertebrates possess three genes encoding TnI; slow skeletal (ssTnI; TNNI1), fast skeletal (fsTnI; TNNI2), and cardiac (cTnI; TNNI3), and three genes for TnT; slow skeletal (ssTnT: TNNT1), fast skeletal (fsTnT; TNNT3), and cardiac (cTnT: TNNT2). Because, in the present study, we explore a range of previously uncharacterized TNNI and TNNT genes and proteins outside of the three defined in mammals, and because non-mammalian vertebrates are known to express a variety of TNNIs in a given muscle type (Alderman et al. 2012), we herein eschew the protein names that derive from muscle-specific expression and instead adopt protein names based on the corresponding numbered gene, that is, TnI1-3 corresponding to genes TNNI1-3 (instead of ssTnI, fsTnI, and cTnI, respectively).
Mammalian TNNI genes are located in close proximity to TNNT paralogs in human and mouse genomes: TNNI2 with TNNT3, TNNI3 with TNNT1, and TNNI1 with TNNT2 (Chong and Jin 2009) and there is also some limited evidence for this in fish (Genge et al. 2016). This is intriguing because whole-genome duplications (WGDs) have played a key role in expanding the gene repertoire of early vertebrates (Hoffmann et al. 2021, 2012; Zavala et al. 2017), and because the teleost whole-genome duplication has been linked to the functional diversification of the zebrafish TNNI paralogs (Genge et al. 2016). As such, the diversity in TNNI and TNNT families appears to have arisen through a tandem duplication followed by successive rounds of whole-genome duplication (Chong and Jin 2009; Rasmussen and Jin 2021).
Particular interest has been paid to the evolution of TNNI3, which in adult mammals is solely expressed in the heart and is distinguished from other vertebrate TNNI paralogs by a “unique” N-terminal extension peptide (Sheng and Jin 2016; Shaffer and Gillis 2010; Rasmussen et al. 2022). This N-terminal extension is an important regulatory structure (Sheng and Jin 2016) containing two protein kinase A (PKA) target serine residues that, when phosphorylated via ß-adrenergic stimulation, decrease myofilament Ca2+ sensitivity (Fentzke et al. 1999; Layland et al. 2005; Robertson et al. 1982; Solaro et al. 1976), thereby increasing the rate of relaxation during diastole (Kentish et al. 2001; Zhang et al. 1995). In teleost fishes, such as zebrafish (Fu et al. 2009) and rainbow trout (Alderman et al. 2012; Gillis and Klaiman 2011; Kirkpatrick et al. 2011), cardiac-expressed TnI lacks the N-terminal extension that characterizes mammalian TnI3, although a long N-terminal extension is present in amphibian (Drysdale et al. 1994; Warkman and Atkinson 2004) and lungfish (Rasmussen et al. 2022) TnI3. Currently, the most widely believed consensus, at least within the vertebrate TnI field, is that TNNI1 and TNNI3 are more closely related to each other than to TNNI2, and that all three evolved from a single gene in the ancestor of vertebrates (Shaffer and Gillis 2010; Sheng and Jin 2016). The N-terminal extension has been commonly interpreted as an evolutionary novelty that emerged in TnI3 from a TnI1-like ancestral form in the ancestor of lobe-finned fishes (Palpant et al. 2010; Shaffer and Gillis 2010; Sheng and Jin 2016; Rasmussen et al. 2022). However, given that some protostome invertebrate (Cao et al. 2019; Barnes et al. 2016) and tunicate (MacLean et al. 1997; Cleto et al. 2003) TNNI genes encode for alternatively spliced isoforms with and without a N-terminal extension, an alternative interpretation is that skeletal muscle paralogs secondarily lost an ancestral extension that has been differentially retained by TNNI3 (Hastings 1997; MacLean et al. 1997; Barnes et al. 2016).
Several fundamental questions remain open regarding the evolution of the troponin I and T genes in vertebrates. Both teleost fish and tetrapods have TNNI paralogs that encode for cardiac-expressed TnIs, however, it is not clear whether these subunits are encoded by orthologous genes (Genge et al. 2016; Rasmussen et al. 2022). Further, the duplicative history of these genes in the early stages of vertebrate evolution could not be properly resolved because of the limited availability of cartilaginous fish and jawless fish sequences. Cartilaginous fish sequences are particularly valuable as this lineage was the first to diverge from other gnathostomes (jawed vertebrates), meaning that orthologous genes identified in both cartilaginous fishes and other gnathostome clades can be traced back to the last common ancestor of all jawed vertebrates. The current consensus is that the three tetrapod TNNIs are monophyletic, and that tetrapod and ray-finned fish TNNI2s fall in a monophyletic group, implying these genes are orthologs. The evidence is inconclusive regarding TNNI1 and TNNI3 (Genge et al. 2016, Sheng and Jin 2016). Support to resolve relationships among the different vertebrate TNNI paralogs is also limited (Genge et al. 2016, Sheng and Jin 2016), which is critical to determine whether the N-terminal extension of cTnI is ancestral or derived, and to provide a robust evolutionary context to interpret the observed functional differences among the different TnI subunits, including the capacity to regulate contractile function via ß-adrenergic stimulation.
In the current study, we take advantage of improved assemblies of sea lamprey and cartilaginous fish genomes to answer long-standing questions on the duplicative history of the TNNI and TNNT gene families of vertebrates. We combine phylogenetic and synteny analyses from a representative set of vertebrates to reconstruct the early stages of evolution of these two closely related gene families in the group. Our reconstruction indicates that the last common ancestor of gnathostomes possessed five TNNI and four TNNT genes in its genome arranged in four different loci which derive from the 2R of WGD early in vertebrate evolution. Comparisons with lamprey and hagfish suggest that the tandem arrangement of TNNI and TNNT was present in the last common ancestor of vertebrates. We augment our analyses by assessing TNNI gene and protein expression, as well as PKA-mediated phosphorylation of cardiac-expressed TnI, in a diverse cohort of gnathostome vertebrates. In the context of our phylogenetic findings, our results suggest that the presence of an N-terminal extension in the TnI3 subunit of tetrapods represents the retention of an ancestral feature rather than an evolutionary innovation of tetrapods or sarcopterygian fish. Our findings also indicate that the genes encoding for the cardiac-expressed TnI subunits of teleost fish (TNNI1) and tetrapods (TNNI3) are not orthologs. Instead, these subunits are encoded by paralogous genes that were lost (TNNI3 in ray-finned fish lineage) or exhibit divergent expression patterns (TNNI1 being restricted to slow skeletal muscle and embryonic cardiac muscle in tetrapods).
Results
Data Description and Approach
We combined bioinformatic searches of the NCBI and Ensembl databases to collect the full TNNI and TNNT repertoires in a representative set of vertebrate genomes including two invertebrate chordates, as reference. In supplementary analyses (supplementary figs. S1 and S2, Supplementary Material online), we added further deuterostome invertebrate outgroups, including echinoderms and additional tunicates, which reaffirmed that the gnathostome TNNI and TNNT gene families are monophyletic and did not alter the relationships between paralogs. For clarity, herein we primarily concentrate on our focussed selection of chordates, composed of 19 different species including two cyclostomes (Sea lamprey, Petromyzon marinus and Inshore hagfish, Eptatretus burgeri); representatives of three different orders of cartilaginous fishes (class Chondrichthyes), elephant shark (Callorhinchus milii, order Chimaeriformes), thorny skate (Amblyraja radiata, order Rajiformes), and small-spotted catshark (Scyliorhinus canicula, order Carcharhiniformes); three non-teleost ray finned fishes, reedfish (Erpetoichthys calabaricus, order Polypteriformes), sterlet sturgeon (Acipenser ruthenus, order Acipenseriformes) and spotted gar (Lepisosteus oculatus, order Lepisosteiformes); two teleosts, Asian bonytongue (Scleropages formosus, order Osteoglossiformes), and zebrafish (Danio rerio, order Cypriniformes); African coelacanth (Latimeria chalumnae, order Coelacanthiformes); West African lungfish (Protopterus annectens, order Dipnoi); an amphibian, tropical clawed frog (Xenopus tropicalis, order Anura); a non-avian reptile, anole lizard (Anolis carolinensis, order Squamata); a bird, chicken (Gallus gallus, order Galliformes); a monotreme, Australian echidna (Tachyglossus aculeatus, order Monotremata); and a eutherian mammal, human (Homo sapiens, order Primates) (databases available in Supplementary material online). As outgroup references, we included the full repertoire of TNNI and TNNT genes from two invertebrate chordates: the sea squirt (Ciona intestinalis, a tunicate), and the Florida lancelet (Branchiostoma floridae, a cephalochordate). Because our aim was on the early stages of vertebrate evolution and on resolving relationships between teleost and tetrapod paralogs, our sampling included a focused number of amniotes and teleosts. Because we studied a broad range of taxonomic groups with different gene nomenclature practises (e.g., teleost “tnni”), to avoid confusion we standardized all gene names to the human convention for example “TNNI”.
Our bioinformatic searches combined information from the Ensembl comparative genomics assignments of orthology (Zerbino et al. 2018) with the results of BLAST searches (NCBI Resource Coordinators 2016) against the corresponding genomes. BLAST searches used the blastp and tblastn programs and were seeded with known TNNI and TNNT protein sequences. We validated our TNNI and TNNT candidates using reverse BLAST against the NCBI Reference Protein database of vertebrates, refseq_protein. Candidate records that did not include either a TNNI or TNNT as their top hit were discarded. We inferred that our sequences had captured the full range of TNNI and TNNT diversity present in each of the genomes surveyed because searches seeded with TNNI identified TNNT-like sequences, and searches seeded with TNNT sequences identified TNNI-like sequences.
Variation in TNNI and TNNT Gene Complements
After curating the results of our searches, our main datasets included a total of 81 TNNI and 72 TNNT sequences. As expected, vertebrates exhibit a wider range of variation in both gene families relative to the invertebrate chordates included as outgroups. In the case of TNNI, the number of genes in invertebrate chordates ranged from one in sea squirt (a tunicate) to two in the Florida lancelet (amphioxus), whereas in vertebrates the number ranged from two in reedfish, the least of any vertebrate we surveyed, to a maximum of 14 in zebrafish, which have undergone an additional WGD, and include two series of tandem duplications. The discovery of only two TNNI genes in reedfish (TNNI1 and TNNI2) is likely representative of the true state, and not an artefact of incomplete genome sequencing, because we could additionally verify that the Senegal bichir (Polypterus senegalus), which is found within the same early-diverging family as reedfish (Polypteridae), but had its genome sequenced separately, also appears to have only these two TNNI genes. In these species, we were also able to identify the genomic location of genes normally in synteny with other TNNIs (discussed for TNNI3 below). In general, our results agree with previous assessments of copy number variation for this gene family in invertebrate chordates and vertebrates (Shih et al. 2015; MacLean et al. 1997). In the case of the TNNT genes, the number of genes in invertebrate chordates matched the number of TNNI genes, one in sea squirt and two in the Florida lancelet, and in the case of vertebrates, the number ranged from three in gar, coelacanth, and tetrapods to the eight different copies identified in zebrafish.
Phylogenies Identify Additional Gnathostome TNNI and TNNT Paralogs
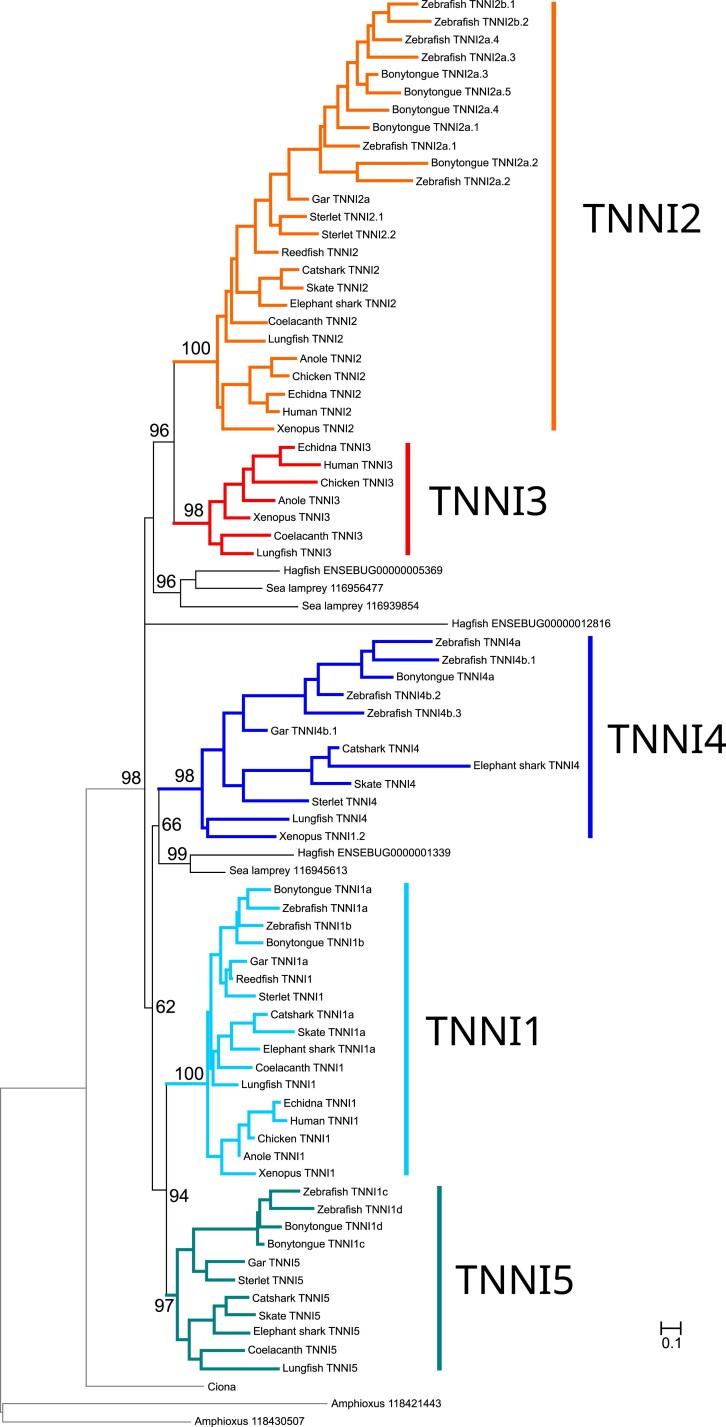
Our phylogenetic analyses place vertebrate TNNIs in a monophyletic clade and arrange gnathostome TNNIs into five strongly supported monophyletic groups (fig. 1; supplementary fig. S1, Supplementary Material online). Three of these groups can be defined by the presence of the mammalian TNNI1, TNNI2, and TNNI3 paralogs. The TNNI1 and TNNI2 groups include ray-finned, lobe-finned, and cartilaginous fish genes, whereas we only found TNNI3 copies in lobe-finned fishes. The fourth group, TNNI4, contains previously annotated (i.e., NCBI and ZFIN) tnni4 zebrafish genes, although these have not previously been formally described in the literature. For the remaining group, we coin the name TNNI5. TNNI4 and TNNI5, have restricted phyletic distributions, and we failed to find copies of these genes in any of the amniote genomes we surveyed. TNNI4 is present in the genomes of cartilaginous fishes, ray-finned fishes, lungfish, and amphibians, and includes the previously named tnni1.2 gene of the tropical clawed frog (NCBI Gene ID: 394556; Xenbase: XB-GENE-485710, supplementary Table S1, Supplementary Material online). In turn, TNNI5 is restricted to cartilaginous fishes, ray-finned fishes, and coelacanth, and includes the previously named zebrafish genes tnni1c (NCBI Gene ID: 751665, ZFIN:ZDB-GENE-060825-192, supplementary Table S1, Supplementary Material online) and tnni1d (NCBI Gene ID: 436902, ZFIN:ZDB-GENE-040718-374, supplementary Table S1, Supplementary Material online). Our analyses identify additional duplications, found in lineages that have undergone additional WGDs, such as teleosts or sterlet, some of which correspond to the already reported tandem expansions of TNNI2 in zebrafish.
Fig. 1.
Maximum-likelihood phylogenetic tree showing evolutionary relationships between vertebrate troponin I (TNNI) sequences. The five distinct TNNI groups that we infer were present in the common ancestor of gnathostome vertebrates are highlighted. The tree was rooted with the amphioxus and Ciona sequences. Ultrafast bootstrap support is shown above relevant nodes.
The five gnathostome TNNI genes are divided into two super-groups; TNNI3 is placed as sister to TNNI2 in the first group, and TNNI1, 4, and 5 are placed in the second one, where TNNI1 is placed as sister to TNNI5, and TNNI4 groups with the TNNI1 + TNNI5 clade (fig. 1). Lamprey and hagfish include 3 TNNI paralogs in their genomes that weakly clustered with the gnathostome TNNI2/3 or TNNI1/4/5 groups. The lamprey 116956477 and 116939854 genes are placed in a group with the hagfish ENSEBUG00000005369 gene in a clade that is placed within gnathostome TNNI2/3, and the lamprey 116945613 gene is sister to the hagfish ENSEBUG00000013390 in a clade within the TNNI1/4/5 clade of gnathostomes. The third hagfish TNNI, ENSEBUG00000012816, appears to be either incomplete in the current genome annotation or highly divergent and has unclear phylogenetic affinities.
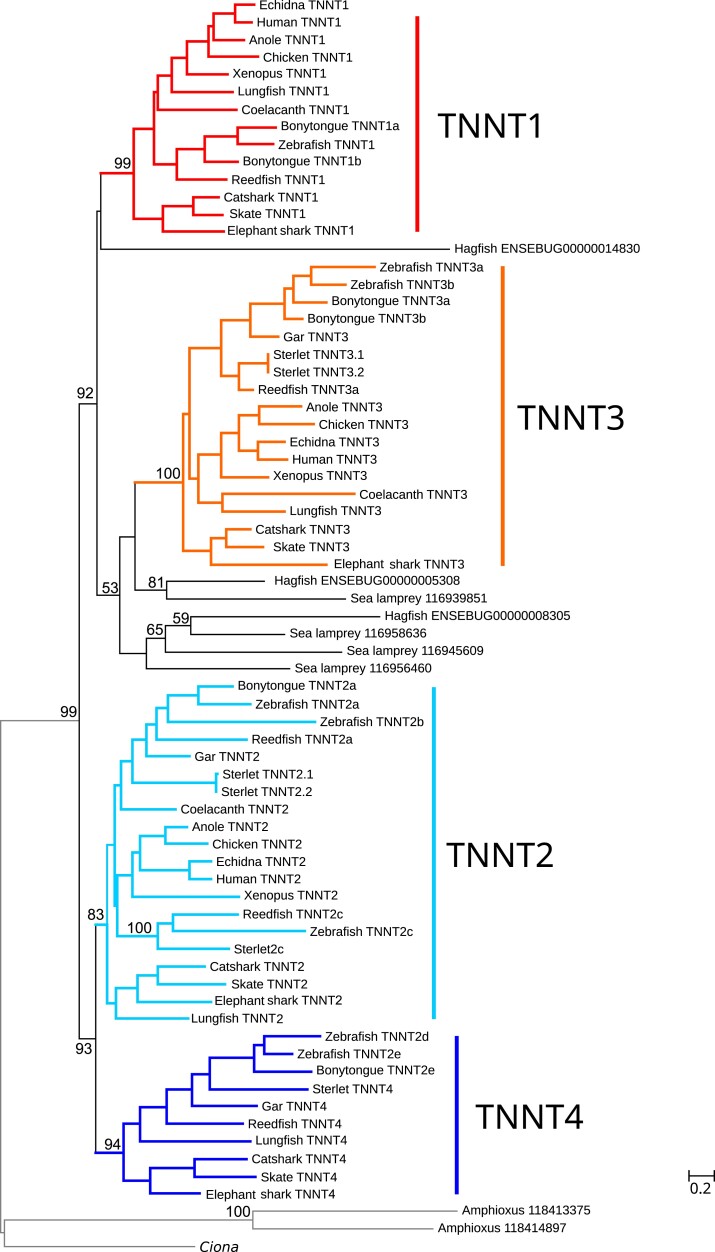
Like TNNIs, vertebrate TNNTs were monophyletic relative to invertebrate chordates, and the gnathostome sequences were arranged into four monophyletic groups with moderate to strong support (fig. 2; supplementary fig. S2, Supplementary Material online). The TNNT1, TNNT2, and TNNT3 groups can be defined by the presence of human paralogs and are found in the vast majority of species surveyed. The fourth group, which we label as TNNT4 is restricted to cartilaginous fishes, ray-finned fishes and lungfish. As with TNNIs, our analyses identify additional duplications which are mostly restricted to lineages that have undergone additional WGDs, such as teleosts or sterlet. The only exception to this is the presence of duplicate TNNT2s (i.e., duplicates named TNNT2c) in reedfish, sterlet, and zebrafish. Within the TNNT2 paralog, tetrapod and cartilaginous fish sequences fell in monophyletic clades, but ray-finned fish sequences were grouped in two separate groups, the first one including copies from all ray-finned fish species in our study, and the second one including copies from reedfish, sterlet, and zebrafish. This arrangement is suggestive of an old duplication in the last common ancestor of ray-finned fishes that has been differentially retained in some descendants. The TNNT genes of gnathostomes are arranged in two groups, with TNNT2 and TNNT4 in one, and TNNT1 and TNNT3 in the other.
Fig. 2.
Maximum-likelihood phylogenetic tree showing evolutionary relationships between vertebrate troponin T (TNNT) sequences. The four distinct TNNT groups that we infer were present in the common ancestor of gnathostome vertebrates are highlighted. The tree was rooted with the amphioxus and Ciona sequences. Ultrafast bootstrap support is shown above relevant nodes.
Cyclostomes also include multiple TNNT copies in their genomes: four in the case of lamprey and three in the case of hagfish. These genes are arranged into three separate groups, all of which fall within the TNNT1/3 clade of gnathostomes. The hagfish ENSEBUG00000014830 gene is placed as sister to TNNT1 with low support. Then a clade with the hagfish ENSEBUG00000005308 and lamprey 116939851 genes is placed as sister to the TNNT3 group of gnathostomes, and a second cyclostome TNNT clade which includes the hagfish ENSEBUG00000008305 gene plus the three remaining lamprey genes, 116945609, 116956460, and 116958636, is placed with gnathostome TNNT3 as well. Support for the nodes resolving affinities for these groups is low.
TNNIs and TNNTs are Found in Clusters of Conserved Synteny
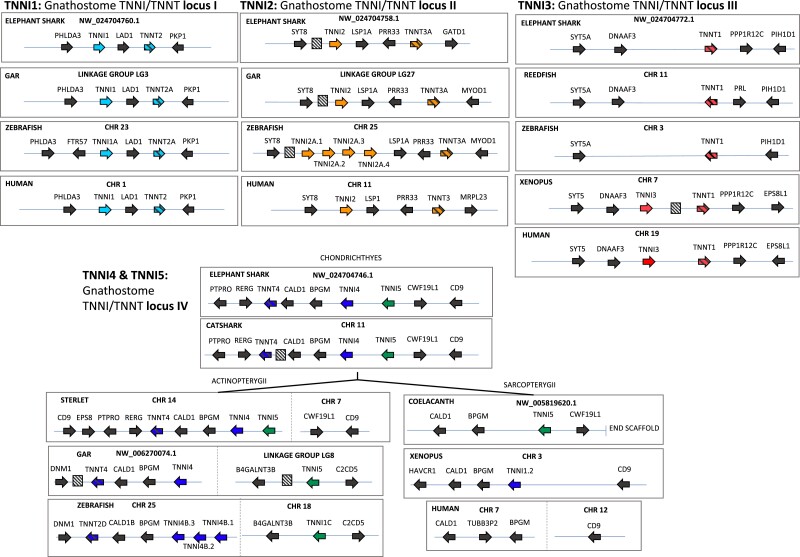
In the case of gnathostomes, the results of our synteny analyses (fig. 3) are consistent with our phylogenetic analyses and provide additional insights regarding the duplicative history of the gene families and the absence of some paralogs in some gnathostome genomes. Microsynteny is very conserved in the cases of the TNNI1-TNNT2 and TNNI2-TNNT3 clusters of gnathostomes (fig. 3). TNNI1 and TNNT2 are found in tandem (within the region we label gnathostome TNNI/T locus I), with LAD1 between them and PHLDA3 and PKP flanking the cluster. TNNI2 is found in tandem with TNNT3 (gnathostome TNNI/T locus II), with copies of LSP1 and PRR33 between them, and copies of SYT8 upstream of the cluster. TNNI3 is flanked by copies of DNAAF3 and TNNT1 in most tetrapods (gnathostome TNNI/T locus III). Interestingly, DNAAF3 and TNNT1 are adjacent to each other in the elephant shark and reedfish genomes, whereas we could not find copies of any of these three genes in the current release of the spotted gar genome (assembly name: LepOcu1; accession GCF_000242695.1). TNNI4 and TNNI5 are found in tandem in cartilaginous fishes and sterlet but are located on separate loci in gar and zebrafish. There are copies of CALD1 and BPGM between TNNT4 and the TNNI4-5 cluster (gnathostome TNNI/T locus IV). In gar and zebrafish, TNNI5 is on a different chromosome than the TNNI4-TNNT4 cluster, flanked by copies of B4GALNT3B and C2CD5. Orthologs of these two genes are found on separate chromosomes in humans. Further, the arrangement of TNNI and TNNT genes in the phylogenies is consistent with their position in the genome. The TNNI2 and TNNT3 genes, which are together found at locus II, on human chromosome 11, are grouped with TNNI3 and TNNT1, which are found on the same locus (locus III) on human chromosome 19. In turn, TNNI1 and TNNT3, which are found on the same locus (locus I), on human chromosome 1, are grouped with TNNI4 + TNNI5 and TNNT4, respectively, which are absent in the human genome, but are found in the same locus (locus IV) in cartilaginous fish, and sterlet.
Fig. 3.
Conserved synteny diagrams of genomic regions harboring TNNI/TNNT genes in gnathostome vertebrates. Hatched boxes indicate uncharacterized predicted protein coding genes. Non-coding and microRNAs are excluded.
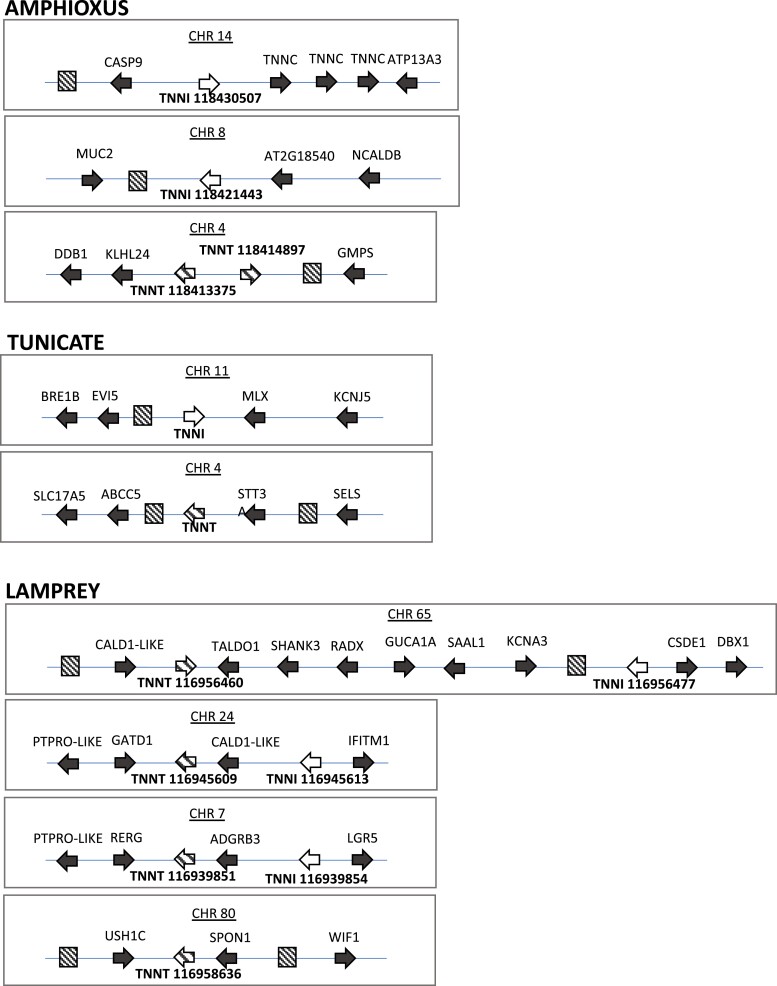
As in gnathostomes, we find pairs of TNNT and TNNI genes in close proximity in both cyclostome genomes. However, synteny comparisons are not as informative because of the reduced contiguity of the hagfish genome relative to the lamprey assembly and because there are discrepancies between the phylogenetic and synteny analyses in this group. The lamprey genome includes three TNNI-TNNT pairs, on chromosomes 7, 24, and 65, plus a single TNNT gene on chromosome 80, whereas the hagfish genome contains one pair on contig FYBX02009389. There are copies of GATD1 and CALD1 between the TNNI-TNNT pair on lamprey chromosome 24 (116945613 and 116945609) and the hagfish pair on contig FYBX02009389 (ENSEBUG00000013390 and ENSEBUG00000005308). However, the flanking genes are different, and the phylogenies are not congruent with the synteny. The corresponding TNNI genes are sister but not the TNNT genes. In the amphioxus and the tunicate, TNNI and TNNT were not found in close genomic proximity, although curiously in amphioxus we found TNNI in a cluster with three TNNC genes (fig. 4).
Fig. 4.
Conserved synteny diagrams of genomic regions harboring TNNI/TNNT genes in invertebrate chordates and a cyclostome.
There are similarities in genomic context between the lamprey and the gnathostome TNNI-TNNT pairs but results of synteny comparisons and phylogenetic analyses are not easy to reconcile. For example, the TNNT4 genes of cartilaginous fishes and sterlet are next to PTPRO and RERG copies, as is the 116939851 TNNT gene of the lamprey, but these genes are not placed together in the phylogeny, and the associated TNNI genes are not placed close together either. More generally, there are similarities in the genomic context shared by many of the TNNI-TNNT genomic loci. There are paralogs of CALD1 or LSP1, next to two of the TNNI-TNNT clusters of gnathostomes and two of the lamprey clusters (fig. 4), there are PTPRO paralogs close to the TNNI1-TNNT2 and the TNNI4-5-TNNT4 clusters of gnathostomes and the lamprey TNNI-TNNT clusters on chromosomes 7 and 24, and there are SYT paralogs close to the each of the three TNNI-TNNT pairs defined by the presence of mammalian TNNIs.
The Evolution of the N-terminal Extension in TnI
The TNNI5 sequence found in cartilaginous fishes, non-teleost ray-finned fishes, and sarcopterygian fishes included an N-terminal sequence bearing a similarity to TNNI3 previously described in tetrapods (Drysdale et al. 1994; Warkman and Atkinson 2004) and lungfish (Rasmussen et al. 2022). Although teleost fishes possessed genes of the TNNI5 family (previously named tnni1c and tnni1d), they did not contain the N-terminal extension, indicating it was lost in this protein lineage in teleosts. To provide a formal and unbiased comparison between TNNI paralogs, we used ancestral sequence reconstructions to predict ancestral protein sequences for TNNI1-5. The alignment highlights the strong resemblance between TNNI3 and TNNI5 N-terminal sequences, particularly with regard to glutamic acid and proline-rich stretches (fig. 5A). The TNNI3 and TNNI5 N-terminal extensions also showed similarities to TNNT, as represented by a reconstruction of the common ancestor of all gnathostome vertebrate TNNTs (TNNT1-4) (fig. 5B).
Fig. 5.
Alignments of N-terminal portions of TNNI and TNNT genes. (A) Similar glutamic acid and proline-rich N-terminal extensions in predicted ancestral TNNI3 and TNNI5. Bolded text (serines) indicates protein kinase A target site in TNNI3 that is absent in TNNI5. (B) Comparison of TNNI3 and TNNI5 with ancestral TNNT (common ancestral sequence of TNNT1-4) also shows similarities in N-terminal extensions.
Cardiac and Skeletal Muscle Gene and Protein Expression
Having established that cartilaginous and ray-finned fish TNNI5 shares a strikingly similar N-terminal extension with tetrapod and sarcopterygian fish TNNI3, we next investigated the expression of different TNNIs in cardiac and skeletal muscle of diverse cartilaginous fishes, non-teleost ray-finned fishes, early diverging teleosts, and a sarcopterygian fish. Gene expression (analysis of previously generated RNA-seq data; see Supplementary material online for full species list and data accession information) and protein expression (Western blotting and mass spectrometry) analysis were conducted with a particular focus on the expression and characterization of the TNNI5 paralog.
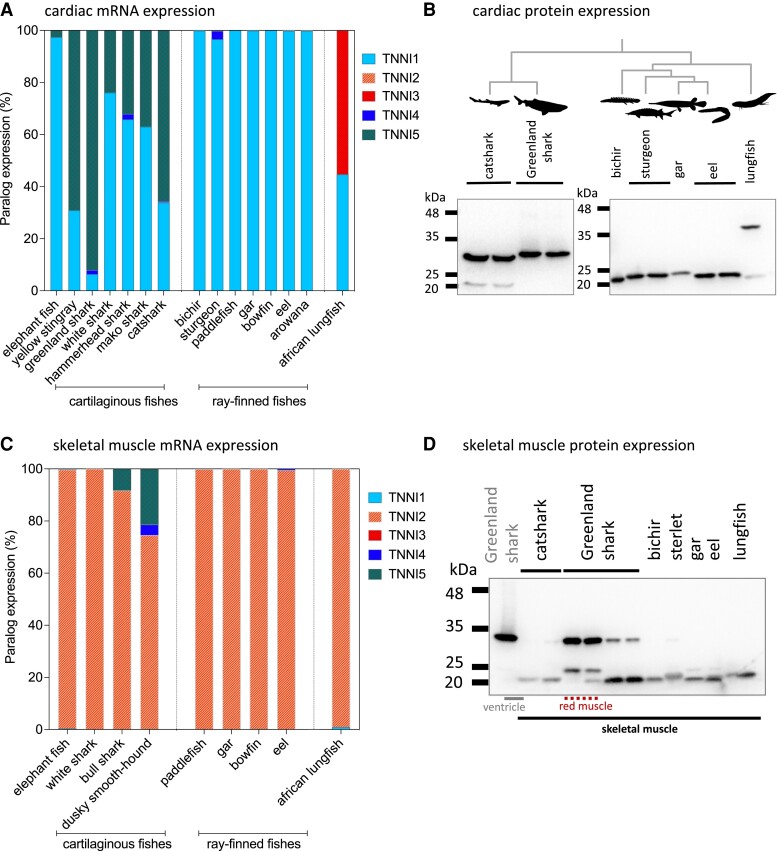
In the cardiac transcriptomes of cartilaginous fishes, we found the expression of a broad array of TNNIs. In small-spotted catshark (S. canicula), for instance, we identified transcripts for each of the four genes found in the genome, whereby TNNI1 and TNNI5 were dominantly expressed (∼33% TNNI1 and ∼66% TNNI5), TNNI4 exhibited only low expression (< 0.5% TNNI) and TNNI2 was found only at trace levels (0.1% TNNI) (fig. 6A). In most of the other sharks (i.e., Great white shark [Carcharodon carcharias], Great hammerhead shark [Sphyrna mokarran], shortfin mako shark [Surus oxyrinchus]) as well as yellow stingray (Urobatis jamaicensis), we likewise found mixed expression of TNNI1 and TNNI5 and that the other genes were also absent or expressed at negligible levels. In Greenland shark (Somniosus microcephalus) TNNI5 was strongly dominant (>90% TNNI). In the chimerid elephant shark (Ca. milii), TNNI1 was almost exclusively expressed (>97% TNNI expression), although TNNI5 transcripts were also detected (accounting for the remaining 3% TNNI). In almost all of the ray-finned fishes, including “basal” (early-diverging) actinopterygians (Senegal bichir [Po. senegalus], paddlefish [Polyodon spathula], spotted gar [Le. oculatus], bowfin [Amia calva]) as well as early-diverging teleosts (European eel [Anguilla anguilla] and silver arowana [Osteoglossum bicirrhosum]), TNNI1 was the virtually exclusively expressed paralog, although in Siberian sturgeon (Acipenser baerii) there was also low but evident (∼3% TNNI) expression of TNNI4 (fig. 6A). In African lungfish (Pr. annectens), we identified slightly predominant gene expression (55% TNNI) of TNNI3, with the remainder comprising of TNNI1.
Fig. 6.
TNNI gene and TnI protein expression in cardiac and skeletal muscle of gnathostome vertebrates. (A and C) gene expression as studied by transcriptomics. (B and D) immunoblots with general TnI antibody. Fish silhouettes are courtesy of phylopic.org.
We next studied TnI protein expression in two shark species (small-spotted catshark [S. canicula] and Greenland shark [So. microcephalus]), four early-diverging ray-finned fishes (Senegal bichir [Po. senegalus], sterlet [Ac. ruthenus], Florida gar [Lepisosteus platyrhincus], and European eel [An. anguilla]) and African lungfish [Pr. annectens] figure 6B), and in each case the predicted protein sizes correlated well with predictions from transcriptomics. In Greenland shark, only a relatively large TnI (∼32 kDa) was detected, aligning with the N-terminal extended TNNI5, whereas in catshark we were also able to observe the less abundant expression of a shorter TnI protein (∼20 kDa), corresponding with the complementary expression of TNNI1 indicated by the transcriptome (fig. 6B). Mass spectrometry for protein identification was used to confirm the protein sequence of the dominant band matched the predicted sequence for both catshark (28% coverage) and Greenland shark (65% coverage) protein predicted from TNNI5. In both cases, the peptide matches included a large proportion of the N-terminal extension (supplementary fig. S3, Supplementary Material online). In each of the ray-finned fishes, we observed only a band of lower molecular mass, corresponding with the N-terminal extension-absent TNNI1 sequences predicted from transcriptomics. In lungfish, we observed expression of both a high molecular weight (the dominant band) and lower molecular weight TnI. The dominant band was confirmed as that predicted from the TNNI3 sequence, with a long N-terminal extension, which was verified with mass spectrometry for protein identification (83% coverage).
Given that the genomes of some early-diverging actinopterygians contained an N-terminal extended TnI (TNNI5) that was not abundantly expressed in their hearts, we extended our survey to skeletal muscle (fig. 6C). However, none of the species’ transcriptomes that we were able to investigate (paddlefish, gar, bowfin) showed evidence of TNNI5 expression in skeletal muscle (fig. 6C). In all of these species, the TNNI2 paralog was strongly dominant, which indicates the preferential dissection of fast twitch muscle in skeletal muscle samples. Western blot analysis of skeletal muscle homogenates from bichir, sturgeon, gar, and eel also indicated that only one or more lower molecular weight TnIs were present (fig. 6D). Surprisingly, however, some shark skeletal muscle tissues (particularly the dusky smooth-hound, Mustelus canis) expressed the N-terminal extended TNNI5 mRNA (fig. 6C), and in Greenland shark skeletal muscle, higher molecular weight TnI was confirmed to be expressed as protein (fig. 6D), which appeared (qualitatively) more abundantly expressed in red than white skeletal muscle.
We additionally studied TNNT gene expression from the cardiac and skeletal muscle transcriptomes of the cartilaginous fishes, ray-finned fishes, and lungfish (supplementary fig. S4, Supplementary Material online). In most species, TNNT2 (the classical “cardiac troponin T” defined in mammals) was virtually exclusively expressed in the heart, with only bichir (∼25% TNNT) and arowana (∼5% TNNT) also expressing a minor proportion of TNNT1. In skeletal muscle samples from all species TNNT3 was dominantly expressed, which is consistent with the high expression of TNNI2, as both are characteristic of fast twitch muscle. TNNT4, which we have defined for the first time, was seldom detected, save for negligible expression (<1% TNNT) in some elasmobranch (such as yellow stingray and mako shark) hearts. It is possible that TNNT4 has a particular spatially or temporarily (i.e., developmentally) restricted expression pattern, and further dedicated studies will be required to establish whether it is expressed, and if so, where and when.
Phosphorylation of Cardiac-expressed TnI by PKA
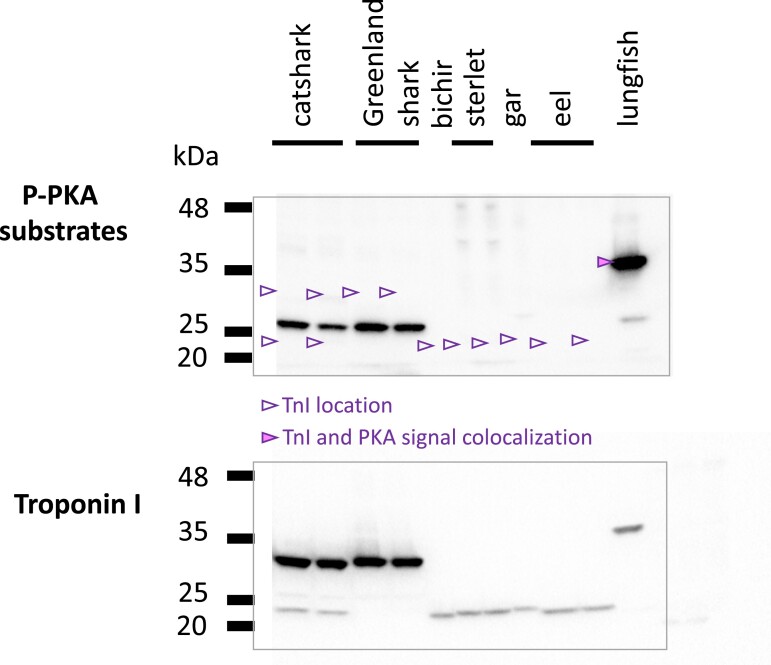
In the mammal TnI3 (TNNI3), PKA is known to primarily target two serine residues within a canonical PKA motif (Ser-23/24) (Martin-Garrido et al. 2018). Sequence alignment indicates the PKA motif is well conserved in all species with TNNI3, including coelacanth and lungfish (Rasmussen et al. 2022). However, despite containing an N-terminal extension and exhibiting cardiac expression, TNNI5 of sharks does not contain a predicted PKA phosphorylation site in the N-terminal extension (see fig. 5). To investigate if the TnI expressed in the hearts of sharks, diverse ray-finned fishes, or lungfish are targeted by PKA, we employed a phospho-PKA motif specific antibody in Western blots on cardiac homogenates before stripping the membrane and re-probing for TnI (fig. 7). Our data showed that in lungfish, a PKA phosphorylated band co-localized with the confirmed TnI location (in agreement with another recent study [Rasmussen et al. 2022]), whereas in sharks and ray-finned fish, there was no co-localization of PKA substrates and TnI band, consistent with predictions from the protein sequences and previous findings that the TnI of ray-finned fish exhibits little phosphorylation (Gillis and Klaiman 2011; Patrick et al. 2010). The PKA-phosphorylated band in both shark species at ∼26 kDa (fig. 7) remains unidentified, but non-TnI candidates were anticipated for the non-specific PKA substrate antibody. It is also possible that this band represented a TnI protein not identified by our TnI primary antibody, although we regard this as unlikely, given that the antibody we used detected a phylogenetically broad range of TnI proteins across different species (fig. 6).
Fig. 7.
Protein kinase A-mediated phosphorylation of cardiac expressed TnI in gnathostome vertebrates. The membrane was blotted with a phospho-PKA substrate antibody, then stripped and reprobed with general TnI antibody in order to identify if a canonical PKA site was phosphorylated in TnI. Arrows show localization of TnI on P-PKA blot. Filled arrows show co-localization of TnI and PKA band, non-filled arrows indicate no co-localization.
Discussion
In this study, we combined bioinformatic searches for phylogenetic and synteny analyses with gene and protein expression studies to reconstruct the evolution of the vertebrate TNNI and TNNT gene families. Our analyses suggest a novel hypothesis regarding the duplicative history of these gene families, identify additional paralogs that are absent from amniote genomes, and provide a strong hint that the presence of an N-terminal extension in mammalian cardiac TnI represents the retention of an ancestral state. A summary of our interpretation of TNNI and TNNT gene origins and losses is presented in figure 8. The lineage-specific gene losses resulted in different paralog repertoires being available for tissue-dependent expression in different vertebrate groups. This is well illustrated in the context of cardiac TNNI expression, where tetrapods and lobe-finned fishes characteristically express TNNI3, ray-finned fishes by and large express TNNI1, and cartilaginous fishes express a variable combination of TNNI1 and TNNI5 in the heart.
Fig. 8.
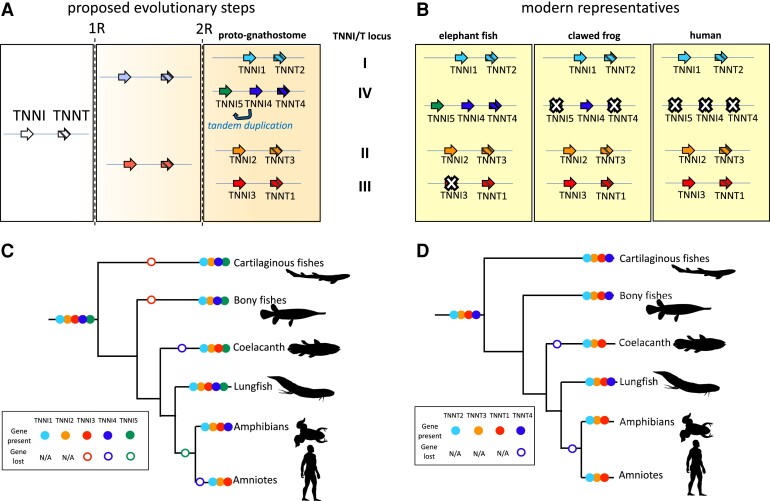
The origin and losses of TNNI and TNNT genes in vertebrates. (A) Predicted evolutionary steps in evolution showing how whole-genome duplications (1R and 2R) and tandem duplications generated gene diversity. White crosses (X) mark genes that have been lost in extant lineages. Whilst our maximum-likelihood tree suggests the tandem duplication giving rise to TNNI4 and TNNI5 occurred before the genome duplication that gave rise to TNNI1 and TNNI5, which would require subsequent gene loss of a paralog in both gnathostomes and cyclostomes independently, it is also possible that the tandem duplication occurred only in TNNI4 and TNNI5 after 2R (here shown by arrow marked “tandem duplication’). (B) Comparison of which duplicated genes were retained or lost in three specific gnathostomes (elephant shark, Ca. milii, tropical clawed frog, X. tropicalis and human, H. sapiens. (C and D) broader overview of patterns of TNNI and TNNT gene loss in major vertebrate lineages. Animal silhouettes are courtesy of phylopic.org.
TNNI and TNNT Paralogs Originated in 2R Whole-genome Duplication Events
Earlier convention has been to pigeon-hole the TNNI paralogs found in non-mammalian vertebrates into the three classes of TNNI defined in mammals. By contrast here, tree reconciliation allows us to trace the five different TNNIs, and the four different TNNTs of gnathostomes back to the last common ancestor of the group. Curiously, of extant lineages, only lungfish appear to retain the full ancestral complement of TNNI and TNNT genes (fig. 8). The variable lineage-specific muscle tissue expression patterns, and repeated evolutionary loss of some paralogs, indicates that there may be substantial functional redundancy between TnI proteins in particular. Genomic gene loss probably occurs in species that have already ceased to express a given TNNI in a specific muscle type, presumably after natural selection has favored the expression an alternative paralog.
Our trees suggest that at least three of the TNNI and four of TNNT cyclostome paralogs predate the split between hagfish and lamprey, but support for the corresponding nodes is low to move beyond this broad statement. Going deeper into the phylogeny, our maximum-likelihood tree placed gnathostome TNNI4 as sister to a cyclostome TNNI gene, implying that the tandem duplication giving rise to TNNI4 and TNNI5 would have predated the duplication that gave rise to TNNI5 and TNNI1 and the split between cyclostomes and gnathostomes. This would require multiple losses of TNNI and TNNT genes in both cyclostomes and gnathostomes to account for the extant repertoires. However, a tree where TNNI4 is constrained to be sister to TNNI5 (supplementary fig. S5, Supplementary Material online) is not significantly different from the unconstrained tree (supplementary Table S2, Supplementary Material online). This constrained tree requires fewer gene losses and maps the tandem duplication giving rise to TNNI4-TNNI5 to the last common ancestor of gnathostomes, which is consistent with the observed phyletic distribution of the genes. Thus, our analyses indicate the presence of four different TNNI-TNNT pairs in the last common ancestor of gnathostomes, and of at least three TNNI-TNNT clusters in the last common ancestor of cyclostomes.
Unfortunately, our phylogenies lack power to resolve relationships between gnathostome and cyclostome TNNIs and TNNTs and move ancestral reconstruction deeper. This is not surprising because cyclostome genomes are unusual with respect to nucleotide, codon, and amino acid composition (Qiu et al. 2011; Kuraku 2013) that complicate the resolution of orthology based on phylogenies. In some cases, synteny is informative to resolve ambiguous gene phylogenies (Kuraku and Meyer 2012; Hoffmann et al. 2010; Campanini et al. 2015), but in others such as the TNNI-TNNT pairs, synteny shows similarities with gnathostomes but ultimately is not informative, as in the globin X genes of vertebrates (Hoffmann et al. 2021). Nevertheless, the presence of multiple TNNI-TNNT pairs in both groups is consistent with the possibility that the last common ancestor of vertebrates possessed four TNNI-TNNT pairs in its genome.
Whole-genome duplications played a critical role in expanding the repertoire of vertebrate genes. The presence of four different TNNI-TNNT clusters in gnathostomes which are phylogenetically arranged by location and are flanked by additional gene families that appear to have co-duplicated with the TNNI-TNNT suggest that the TNNI-TNNT clusters of vertebrates also expanded via WGDs, a notion previously speculated on more limited evidence (Shaffer and Gillis 2010). The presence of independent duplications in cyclostomes and synteny similarities within the cyclostome clusters are also consistent with this interpretation. Further, the three human TNNI-TNNT pairs and the three lamprey TNNI-TNNT pairs all map to proto-vertebrate chromosome Pv11 from Nakatani et al. (2021), as does the tandem of TNNT genes in amphioxus (supplementary Table S3, Supplementary Material online). All of these observations suggest that the vertebrate TNNI-TNNT gene families expanded as a result of WGDs. There are competing hypotheses regarding the number and timing of the WGDs early in vertebrate evolution. There is consensus that gnathostomes underwent two rounds of WGD (Meyer and Schartl 1999; McLysaght et al. 2002; Dehal and Boore 2005), 1R and 2R, and that 1R predates the split of extant cyclostomes and gnathostomes. The placement of 2R on the vertebrate tree, however, is controversial (Kuraku et al. 2009). Whereas some authors place 2R in the common ancestor of cyclostomes and gnathostomes (Sacerdot et al. 2018), that is “2R-early”, more recent studies place 2R in the last common ancestor of gnathostomes (Simakov et al. 2020; Nakatani et al. 2021), that is “2R-late”, and suggest that cyclostomes underwent an independent polyploidization early in their evolution (Mehta et al. 2013; Nakatani et al. 2021). These competing explanations make alternative phylogenetic predictions (supplementary fig. S6, Supplementary Material online). Our results do not fit either the 2R-early or 2R-late hypotheses sensu stricto but they are easier to reconcile with the 2R-late hypothesis. Linking the duplicative history to the 1R and 2R WGDs is trivial in the case of gnathostomes, the TNNI2/3-TNNT1/3 and TNNI1/4/5-TNNT2/4 pro-orthologs would derive from 1R, which then expanded to the four different TNNI-TNNT pairs we see today, with the TNNI4/5 pro-ortholog in single copy state. Thus, we have defined four ancestral gnathostome TNNI/T genomic loci, which are given names deriving from the TNNI gene featured, at least in the ancestral (proto-gnathostome) state (fig. 8). Locus I includes the TNNI1-TNNT2 genes, locus II includes the TNNI2-TNNT3 genes, locus III includes the TNNI3-TNNT1 genes, and locus IV includes the TNNI4, TNNI5, and TNNT4 genes. The first three loci, I-III, have remained stable, whereas the fourth locus, IV, has become fragmented in human, gar, sterlet, and zebrafish (fig. 3), which probably explains the restricted distribution of the genes that can be traced back to it.
The case of cyclostomes is more complex. The TNNI-TNNT clusters of lamprey share synteny similarities that distinguish them from the TNNI-TNNT clusters of gnathostomes. This fits well with the 2R-late hypothesis, which posits that cyclostomes underwent an independent polyploidization event. Under this scenario, the TNNI116956477-TNNT116956460 and TNNI116939854-TNNT116939851 pairs of lamprey and the TNNI2-TNNT3 and TNNI3-TNNT1 pairs of gnathostomes would represent independent expansions of one of the post 1R TNNI-TNNT pairs, and whereas the TNNI1-TNNT2 and TNNI4/5-TNNT4 pairs of gnathostomes and the TNNI116945613-TNNT116945609 would derive from the other post-1R TNNI-TNNT pair. We favor this interpretation because it requires less changes relative to the observed trees and the synteny similarities within cyclostomes and within gnathostomes. Unfortunately, support for the relevant nodes is low, and topology tests are not informative (supplementary Table S2, Supplementary Material online).
Relationships Among the TNNI and TNNT Paralogs
In contrast to previous studies, which indicated that TNNI1 and TNNI3 were more closely related to one another than to TNNI2 (Hastings 1997; Shaffer and Gillis 2010; Sheng and Jin 2016), our expanded analyses place TNNI3 as sister to TNNI2, and place TNNI1 as sister to TNNI5, with TNNI4 grouping with the TNNI1 + TNNI5 clade. This has important implications regarding the origin of the TNNI3 N-terminal extension (see below). The parallel analyses of TNNI and TNNT, consistently found in close proximity in vertebrate genomes, provided a powerful tool to cross-examine predicted gene duplications. In support of the surprising TNNI2-TNNI3 sister relationship, we also found a sister relationship between their syntenically associated TNNT genes, TNNT3 and TNNT1, respectively. Likewise, the TNNT2-TNNT4 affinity supported the close relationship of TNNI1, TNNI4, and TNNI5.
TNNI1 is Expressed in the Fish Heart
Virtually all of the previous phylogenetic studies on vertebrate TNNI evolution (Sheng and Jin 2016; Shaffer and Gillis 2010; Gross and Lehman 2016; Rasmussen et al. 2022) have shown that the teleost cardiac-expressed TNNI gene clusters within TNNI1 (ssTnI) of tetrapods, yet it is still frequently labeled as a fish “cTnI” which implies orthology with TNNI3. Its phylogenetic “misplacement” has been repeatedly attributed to its lack of N-terminal extension (Shaffer and Gillis 2010; Rasmussen et al. 2022). By combining our phylogenetic analysis with comparisons of conserved synteny and a broad transcriptomic survey, we unequivocally conclude that ray-finned fishes, including teleosts, lack the TNNI3 gene and simply express a TNNI1 ortholog in the heart. This is consistent with the state in embryonic and neonatal mammals, which express TNNI1 in the heart before TNNI3 becomes exclusively expressed as juveniles and adults (Saggin et al. 1989; Reiser et al. 1994). In the adult mammalian heart, overexpression of ssTnI (TNNI1) at the expense of cTnI (TNNI3) confers increased tolerance to acidosis (Wolska et al. 2001), and may provide similar benefits in the fish heart, which in many species show exceptional performance during acidosis (Driedzic and Gesser 1994; Hanson et al. 2009; Joyce et al. 2015).
As both cartilaginous fishes and ray-finned fishes lack TNNI3, it is conceivable that the gene originated de novo in the lobe-finned fish lineage after their divergence from ray-finned fishes. However, TNNI2, the most closely related paralog to TNNI3, is universally found in vertebrates and our phylogenetic analysis shows it is robustly monophyletic. If TNNI3 were to have evolved from a duplication of TNNI2 only in the lobe-finned fish lineage, lobe-finned fish TNNI2 would be more closely related to TNNI3 than it is to cartilaginous fish and ray-finned fish TNNI2 (i.e., the TNNI2 gene family would be paraphyletic). As it is not, we strongly favor the hypothesis that TNNI3 and TNNI2 duplicated in the 2R WGD before the diversification of gnathostomes and TNNI3 was independently lost in cartilaginous fishes and ray-finned fishes. Because TNNI and TNNT genes are so often found in tandem, this is provided further support by the lone TNNT1 gene retained in cartilaginous and ray-finned fishes (at gnathostome TNNI/T locus III), where otherwise the extinct TNNI3 would be expected to be found.
Given that ray-finned fishes have lost the TNNI3 gene (which encodes for cTnI in tetrapods and lobe-finned fishes), we suggest that the use of the “cTnI” name for cardiac expressed TNNIs in these fish, as has been frequently applied (Sheng and Jin 2016; Shaffer and Gillis 2010; Alderman et al. 2012; Gillis and Klaiman 2011), should be discontinued. Indeed, more generally the currently used protein nomenclature is based on similarities to human genes, some of which are absent from vertebrate genomes, and incorporate information about the tissue where the protein is found and does not align well with our evolutionary hypothesis. Because non-mammalian species, such as teleost fish (Alderman et al. 2012; Shih et al. 2015), have multiple TNNI genes in different striated muscle types, and non-orthologous genes may be expressed in a given muscle type, it becomes ambiguous to use protein nomenclature based on muscle type specific expression of mammals. We therefore advocate that protein names be derived from the gene number (i.e., TnI1-5) in studies that include non-mammalian vertebrates.
Even in some cartilaginous fishes and lungfish, TNNI1 was relatively highly expressed in the heart, but unlike in ray-finned fishes it was found in combination with another paralog, that is TNNI5 or TNNI3, respectively. The ability to express two or more distinct TNNIs with different properties (i.e., TNNI multiplicity) may provide a substrate for acclimation to different environmental conditions (Alderman et al. 2012). Such an ability would be of obvious benefit for ectothermic species and has also been demonstrated to occur with respect to TnC paralog expression in the fish heart with thermal acclimation (Genge et al. 2013).
A Common Origin for the N-terminal Extension in Vertebrate TNNI
The N-terminal extension peptide in TnI3 is widely viewed as an evolutionary novelty that appeared in the sarcopterygian fish and tetrapod lineage (Sheng and Jin 2016; Palpant et al. 2010; Shaffer and Gillis 2010; Rasmussen et al. 2022). However, we identified that the TNNI5 in cartilaginous fishes, non-teleost ray-finned fishes, and coelacanth contained an N-terminal extension with obvious similarity to that found in TNNI3, particularly of lungfish and amphibians.
The sister relationships of TNNI3 with TNNI2, and TNNI5 with TNNI1 and TNNI4, were robustly supported (and cross-supported by the tree of syntenic TNNTs), indicating that TNNI3 and TNNI5 are only distantly related. Based on the molecular similarity of the N-terminal extensions in the proteins encoded by TNNI5 and TNNI3, it appears possible that it was found in the common ancestor of vertebrate TNNIs and was independently lost in TNNI1, TNNI2, and TNNI4 lineages. The common origin of the N-terminal extension is also supported by the N-terminal extension in the TNNI of Ci. intestinalis, which is structurally similar to TNNI3 of tetrapods such as the tropical clawed frog (MacLean et al. 1997). This earlier led Hastings to also conclude that the N-terminal extension could be ancestral (Hastings 1997), although this hypothesis has largely been overlooked in more recent work (Sheng and Jin 2016; Palpant et al. 2010; Shaffer and Gillis 2010; Rasmussen et al. 2022).
It remains plausible that gene conversion, that is the unidirectional transfer of genetic material from one homologous sequence to another (Chen et al. 2007), is responsible for the similar N-terminal extensions found in TNNI3 and TNNI5, or the extension could have evolved de novo in parallel. However, some protostome TnI genes also contain an N-terminal extension, and the possibility that it is homologous with vertebrate TNNI3 has also been previously acknowledged (Cao et al. 2019; Barnes et al. 2016). Indeed, TNNT, as the sister family to TNNI (Chong and Jin 2009) that diverged following a duplication before the separation of protostomes and deuterostomes (Cao et al. 2019), also contains a proline- and glutamic acid-rich N-terminal extension. An alignment of the ancestral vertebrate TNNT (prior to 2R) with ancestral TNNI3 and TNNI5 reveals stretches of similarity with TnT in the N-terminus (fig. 5B), indicating the TNNI N-terminal extension may date back to before the TNNI-TNNT separation. Taken together, this evidence for a conserved N-terminal extension in more distantly related proteins supports the hypothesis that the similarities between TNNI3 and TNNI5 are attributable to the retention of a homologous state, rather than gene conversion or parallel evolution. Nevertheless, we cannot irrefutably exclude the possibility that a similar N-terminal extension could have evolved multiple times in different TNNI lineages, and to further address this issue may be a goal for future studies. The functional significance of the proline and glutamic acid-rich stretches of the N-terminus, found in ancestral TNNI3, TNNI5, and TNNT, remains to be established, but likely affects Ca2+ affinity of the Tn complex.
That the N-terminal extension was likely lost multiple times in other vertebrate TNNI lineages is initially surprising but is also supported by evidence that the single TNNI gene of Ci. intestinalis is alternatively spliced, with the N-terminal extension expressed only in cardiac muscle but excluded in skeletal muscle (MacLean et al. 1997). This indicates that it may provide a benefit to lose the N-terminal extension in skeletal muscle, which was only afforded at the genomic level following the gene duplications that generated paralog diversity (Hastings 1997; MacLean et al. 1997).
In the mammalian heart, TnI3 is a major target for PKA following activation by ß-adrenergic stimulation (Bers et al. 2019) where it affects myofilament Ca2+ sensitivity (Fentzke et al. 1999; Robertson et al. 1982). Whilst TNNI3, in sarcopterygian fishes and tetrapods, and TNNI5, in sharks and rays, are both abundantly expressed in the heart, an important distinction is that TnI5 appears to lack functional PKA phosphorylation target sites in the N-terminal extension. This would presumably limit sensitivity of cardiac myofilaments to the effects of adrenergic stimulation (Pi et al. 2002) and reduce the functional scope of the heart. TNNI5 also appears to be expressed in shark skeletal muscle (red muscle in particular), whereas TNNI3 is strictly only found in the heart in mammals (Sheng and Jin 2016). Given its unique structure and expression pattern, it would be of interest for future work to establish the functional properties of cartilaginous fish TnI5, including the possible influence of its non-phosphorylatable N-terminal extension.
Conclusion
Our analyses suggest a novel hypothesis regarding the expansion of the TNNI and TNNT gene families of vertebrate, linking the presence of multiple TNNI-TNNT pairs in their genomes to the WGDs early in the history of the group. Under the 2R-late hypothesis, our analyses suggest that the presence of four TNNI-TNNT clusters in the genomes of gnathostomes are the product of the 1R and 2R WGDs. In cyclostomes, which share 1R with gnathostomes, the presence of multiple TNNI-TNNT pairs seems to be a combination of 1R and a polyploidization event specific to this lineage. Moving closer to present, we also identify additional paralogs present in the last common ancestor of gnathostomes that are absent from amniote genomes. The genes were retained by a subset of extant lineages, such as the TNNI3 from amniotes that is absent in cartilaginous fish or ray-finned fishes, or the TNNI4/5-TNNT4 locus, which has apparently been lost in amniotes. Our new evolutionary framework highlights the need for revised nomenclature to more faithfully portray the evolutionary affiliations of some previously mis-annotated genes (see supplementary Table S1, Supplementary Material online), and we also provide consistent names for previously unnamed TNNI genes (e.g., “slow skeletal-like” troponin I genes found across cartilaginous and ray-finned fish lineages that can now be identified as TNNI4 or TNNI5).
We found that two distantly related lineages, TNNI3 and TNNI5, encode TnI proteins with remarkably similar N-terminal extensions (fig. 5), which is most easily explained by it being present in their common ancestor and independently lost in TNNI1, TNNI2, and TNNI4 lineages. The discovery of a “second” vertebrate TNNI with an N-terminal extension provides the strongest evidence to date that the extension, which has been widely viewed as unique to TNNI3, could represent an ancestral state of gnathostome TnI prior to the 2R duplication events, and likely dates back even further to the origin of bilaterian TNNI. Shark hearts exhibited dominant protein expression of the N-terminal extended TNNI5. However, the heavily studied PKA-target phosphorylation sites present in mammal TnI3 were only found in the TNNI3 gene family and not TNNI5.
Materials and Methods
Bioinformatic Searches and Curation
Our strategy was to include all known TNNI and TNNT genes in a broad range of vertebrates with annotated whole genome assemblies. We pre-defined the following target species: three distantly related species of cartilaginous fish (elephant shark, Ca. milii, thorny skate, Am. radiata, and small-spotted catshark, S. canicula), three non-teleost ray finned fishes (reedfish, Er. calabaricus, sterlet sturgeon, Ac. ruthenus and spotted gar, Le. oculatus), two distantly related teleosts (Asian bonytongue, Scleropages formosus and zebrafish, D. rerio), African coelacanth (La. chalumnae), West African lungfish (Pr. annectens), an amphibian (tropical clawed frog, X. tropicalis), a non-avian reptile (anole lizard, Anolis carolinensis), a bird (chicken, G. gallus), a monotreme (Australian echidna, T. aculeatus), and a eutherian mammal (human, H. sapiens). We additionally included two cyclostomes, the sea lamprey (Pe. marinus) and inshore hagfish (Ep. burgeri). An amphioxus (Florida lancelet, B. floridae) and a tunicate (vase tunicate, Ci. intestinalis) were included as outgroups. In supplementary analyses (see supplementary figs. S1 and S2, Supplementary Material online) we added further invertebrate deuterostomes, which confirmed the monophyly of the vertebrate TNNI and TNNTs respectively, and reaffirmed the gene affinities amongst gnathostome TNNI and TNNT groups. In these additional analyses, we also included the TNNI and TNNT genes from a recent release (preprint) of the inshore hagfish genome (Yamaguchi et al., 2020), which were redundant with some of the genes present in the earlier assembly. We used both National Center for Biotechnology Information (NCBI) and Ensembl (Release 105) databases. The NCBI database gene pages were manually searched (i.e., “species name + “troponin I”) and also searched with protein–protein BLAST using known (i.e., mammalian or tropical clawed frog) TnI sequences, and TNNI genes were searched for a given species in Ensembl. Where genes of a given species appeared on both NCBI and Ensembl, the former was typically used. We included only one isoform for each protein; when gene pages included multiple alternative transcripts, we selected the isoform with the longest coding sequence, and throughout manually cross-compared between species to ensure that comparable isoforms were chosen. The African lungfish genome has only recently been sequenced (Wang et al. 2021) and predicted proteins from the gene annotations appeared inconsistent with other species. As we assembled transcriptomes for African lungfish cardiac and skeletal muscle (below), which generated more plausible and complete TNNI1, TNNI2 and TNNI3 sequences, these were used instead for the phylogenetic analyses. The transcriptome-predicted lungfish TNNI3 is consistent with that recently cloned by Rasmussen et al. (2022) in the same species. One annotated amphioxus TNNT gene (NCBI 118422967) was excluded as it shared little resemblance with TNNT in any other species.
Phylogenetic Analysis
TNNI and TNNT alignments were generated using MAFFT (v7.490) using the einsi and linsi strategies (Katoh and Standley 2013). The resulting alignments were compared using MUMSA (Lassmann and Sonnhammer 2006) and the alignments with the highest scores were selected for downstream processing. Phylogenetic relationships were estimated using IQ-TREE (multicore version 2.2.0-beta) (Minh et al. 2020). First, the best-fitting model of amino acid substitution was selected using the ModelFinder subroutine from IQ-Tree (Kalyaanamoorthy et al. 2017; Katoh and Standley 2013). Then, searches were run under the selected model using 10,000 pseudoreplicates of the ultrafast bootstrap procedure to assess support for the nodes (Hoang et al. 2018). Competing phylogenetic hypotheses were compared using the approximately unbiased test (Shimodaira 2002) as implemented in IQ-Tree. The alignments, tree files, and a log of the commands required to replicate our results are all available as a compressed file with the Supplementary material online.
Synteny
Synteny diagrams were generated for key species (amphioxus, tunicate, lamprey, elephant shark, catshark, spotted gar, zebrafish, coelacanth, tropical clawed frog, human) for genomic regions harboring select TNNI/TNNT genes using the “Genomic context” section on the relevant NCBI gene pages.
Reconstruction of Ancestral Protein Sequences
Common ancestral protein sequences for each TNNI paralog and the common vertebrate TNNT were predicted using FireProt ASR (ancestral sequence reconstruction) v1.1 web server using default parameter settings (Khan et al. 2021). An unrooted tree was generated with the same alignments as used for our phylogenetic trees, except that for TNNT the “bonytongue_tnnt2a” (XP_029102145.1) sequence was omitted as it contained an unknown amino acid (‘X”, i.e., low quality prediction) so was not recognized by the software. Human TNNI3 and TNNT1 were used as “query” sequences. Nodal sequences from the common ancestor of TNNI1, TNNI2, TNNI3, TNNI4, and TNNT5, and the common ancestor of vertebrate TNNT1-4 were exported and aligned using MAFFT (v. 7.503).
Gene Expression
Previously generated heart and skeletal muscle RNA-seq data for a broad cohort of cartilaginous fishes, ray-finned fishes, and lungfish were collated (see Supplementary material online for full list of species and SRA accession numbers). Sequences with a low-quality score (regions averaging a score <5 over a 4 bp sliding window, and leading/trailing sequences scoring <5) were removed using Trimmomatic (Bolger et al. 2014). The cleaned reads were processed in Trinity 2.2.0 using default parameters (Haas et al. 2013; Grabherr et al. 2011). Open reading frames (ORFs) were predicted from the transcripts using Transdecoder (https://github.com/TransDecoder/TransDecoder/wiki) with a minimum length threshold of 100 amino acids. CD-HIT (Li and Godzik 2006) was used to eliminate redundancy by clustering nucleotide sequences with ≥99% similarity. The reads were mapped using Bowtie-2 (Langmead and Salzberg 2012), and pseudo-aligned to the predicted ORFs using Kallisto (Bray et al. 2016), allowing relative abundance estimates of each transcript to be calculated. Annotation was performed using BLAST (blastp, default cut-offs) searches to query a broad range of TNNI genes (TNNI1,2,4,5 from catshark and TNNI3 from tropical clawed frog) and TNNT genes (TNNT1,2,3,4 from catshark). The top hits were manually curated to find TNNI and TNNT paralogs by blastp (default settings) against the NCBI database and candidate genes were cross checked against our conserved synteny diagrams to confirm their identity.
Protein Expression and Phosphorylation Immunoblots
Small-spotted catshark (S. canicula; 600–800 g; N = 2), Senegal bichir (Po. senegalus; ∼15 g, N = 2), sterlet (Ac. ruthenus; ∼40 g N = 2), Florida gar (Le. platyrhincus: ∼2 g, N = 2), European eel (An.anguilla; 600–800 g; N = 2), and West African lungfish (P. annectens; 410 g; N = 1) were obtained from local commercial dealers and euthanized with an overdose (1 g/L) of bicarbonate-buffered tricaine methanesulfonate (MS-222) followed by destruction of the brain, a procedure endorsed by the local animal experiments committee and in accordance with Schedule 1 of the Home Office Animals (Scientific Procedures) Act 1986. The heart (ventricle) and skeletal muscle (epaxial muscle) were dissected and rapidly frozen on dry ice and stored at −80 °C.
Samples from non-sexually mature Greenland sharks (So. microcephalus; N = 2, TL = 398 and 386 cm) were collected in 2021 in south-eastern Greenland from the Danish research vessel Dana. Sharks were caught via long lines at depths between 285 and 325 month. Immediately after capture and euthanization, samples of ventricle, and white skeletal muscle and red skeletal muscle, were flash frozen in liquid nitrogen and stored at −80 °C until use.
All experiments were conducted with tissue stored (at −80 °C) for less than 6 months, as initial pilot experiments indicated that catshark tissue that was several years old showed greatly reduced general phosphorylation levels. Tissue samples were homogenized in 10 µl m/g RIPA buffer (Millipore, 20–188) with 1% protease and phosphatase inhibitor (PPI) cocktail (PPC1010 Sigma-Aldrich). Protein concentration was measured with bicinchoninic acid (BCA) assay (71285-3 EMD Millipore) before samples were denatured with 2 × Laemmli buffer (S3401 Sigma-Aldrich) and boiled at 95 °C for 5 min. Tris-glycine gels (10 or 12.5% acrylamide) were run with an Invitrogen XCell SureLock Mini-Cell and transferred to a PVDF membrane with XCell II Blot Module (ThermoFisher). For the specific gel used for each blot, alongside raw uncropped blot images, see Supplementary material online. Gels were run at 200 V for 45 min. 15 µg protein was loaded in each lane and run alongside 5 µl BLUeye Prestained Protein Ladder (Sigma-Aldrich 94964). Blots were blocked for 1 h at room temperature using 5% skimmed milk in standard tris-buffered saline with 0.05% Tween 20 (TBS-T).
For the initial determination of TnI molecular weight, blots were incubated with a mouse monoclonal primary antibody against troponin I (C-4) (Santa-Cruz SC-133117). This antibody, which recognized the majority of the TnI protein except for the “cTnI-specific” N-terminal extension, is recommended by the manufacturer for detection of diverse (cardiac and skeletal) TnI proteins. 1:1000 dilutions of the 200 µg ml stock were diluted in 2% milk TBS-T to incubate the blot overnight on a shaker at 4 °C. The next day, a corresponding HRP-conjugated anti-mouse secondary antibody (Santa-Cruz sc-516102) at 1:5000 dilution of 400 µg ml stock in 2% milk TBS-T was used for a 1 h incubation at room temperature. Blots were imaged on a Bio-Rad ChemiDoc and chemiluminescent signals developed with Millipore/Immobilon Classico Western HRP substrate (Merck WBLUC0500) or Bio-Rad Clarity Western ECL Substrate (Bio-Rad 1705061).
To identify phosphorylated protein kinase A (phosho-PKA) substrates (RRXS*/T*) in heart samples, we used the New England Biolabs 9624S rabbit primary antibody (1:1000 dilution of unspecified stock concentration) at 4 °C overnight with gentle agitation followed by goat anti-rabbit secondary antibody (New England Biolabs 7074P2; 1:3000 dilution of unspecified stock concentration) for 1 h at room temperature. The blot was imaged as above, then stripped (Restore Western Blot Stripping Buffer, ThermoFisher 21059) and the TnI antibody protocol followed as described above.
Mass Spectrometry
To verify if the apparently N-terminal extended dominant TnI proteins present in catshark and Greenland shark as well as African lungfish corresponded with the predicted TNNI5 or TNNI3 sequences, protein identification with liquid chromatography-mass spectrometry (LC-MS) was performed with the University of Manchester Bio-MS Research Core Facility (RRID SCR_020987). 20 µg protein per lane was run on a 16% acrylamide Tris-glycine gel (Invitrogen XP00165BOX) which was then stained with SimplyBlue SafeStain (ThermoFisher LC6065). The band at the location corresponding with TnI identified by the immunoblot was excised and digested with elastase. The samples were analysed with LC-MS/MS using an UltiMate 3000 Rapid Separation LC (RSLC, Dionex Corporation, Sunnyvale, CA) coupled to an Orbitrap Exploris 480 (Thermo Fisher Scientific, Waltham, MA) mass spectrometer. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. The products were analyzed with Scaffold 5 (Proteome Software, Portland, OR, USA) and searched against an in-house database including the transcriptomics-predicted TNNI3 and TNNI5 sequences from each respective species.
Supplementary Material
Acknowledgements
This work was supported by Novo Nordisk Foundation (NNF19OC0055842) to WJ. Professors John Steffensen, Peter Bushnell, Diego Bernal, and Eurofleets+, and scientists and crew of the RV Dana are thanked for providing the Greenland shark heart and muscle samples.
Contributor Information
William Joyce, Department of Biology - Zoophysiology, Aarhus University, 8000 Aarhus C, Denmark; Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PL, United Kingdom.
Daniel M Ripley, Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PL, United Kingdom.
Todd Gillis, Department of Integrative Biology, University of Guelph, Guelph, Ontario N1G 2W1, Canada.
Amanda Coward Black, Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology, Mississippi State University, Starkville, Mississippi 39762, USA.
Holly A Shiels, Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PL, United Kingdom.
Federico G Hoffmann, Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology, Mississippi State University, Starkville, Mississippi 39762, USA; Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University, Starkville, Mississippi 39762, USA.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Data Availability
Specific accession information is provided, where possible, in the “Supplementary Materials, Supplementary Material online’ file “supplementary Sequence database” (.xlsx file), Supplementary Material online. Sequence alignments are also available in the Supplementary Materials (.txt files), Supplementary Material online.
Literature Cited
- NCBI Resource Coordinators . 2016. Database resources of the national center for biotechnology information. Nucleic Acids Res. 44:D7–D19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman SL, Klaiman JM, Deck CA, Gillis TE. 2012. Effect of cold acclimation on troponin I isoform expression in striated muscle of rainbow trout. American journal of physiology, Regulatory, integrative and comparative physiology 303:R168–R176. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Hwang H, Ono K, Lu H, Ono S. 2016. Molecular evolution of troponin I and a role of its N-terminal extension in nematode locomotion. Cytoskeleton (Hoboken) 73:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM, Xiang YK, Zaccolo M. 2019. Whole-cell cAMP and PKA activity are epiphenomena, nanodomain signaling matters. Physiology (Bethesda, Md.) 34:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34:525–527. [DOI] [PubMed] [Google Scholar]
- Campanini EB, et al. . 2015. Early evolution of vertebrate mybs: an integrative perspective combining synteny, phylogenetic, and gene expression analyses. Genome Biol Evol 7:3009–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T, Thongam U, Jin J-P. 2019. Invertebrate troponin: insights into the evolution and regulation of striated muscle contraction. Arch Biochem Biophys 666:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-M, Cooper DN, Chuzhanova N, Férec C, Patrinos GP. 2007. Gene conversion: mechanisms, evolution and human disease. Nat Rev Genet. 8:762–775. [DOI] [PubMed] [Google Scholar]
- Chong SM, Jin J-P. 2009. To investigate protein evolution by detecting suppressed epitope structures. J Mol Evol 68:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleto CL, et al. . 2003. Ascidian larva reveals ancient origin of vertebrate-skeletal-muscle troponin I characteristics in chordate locomotory muscle. Mol Biol Evol. 20:2113–2122. [DOI] [PubMed] [Google Scholar]
- Dehal P, Boore JL. 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3:e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedzic WR, Gesser H. 1994. Energy metabolism and contractility in ectothermic vertebrate hearts: hypoxia, acidosis, and low temperature. Physiol Rev. 74:221–258. [DOI] [PubMed] [Google Scholar]
- Drysdale TA, Tonissen KF, Patterson KD, Crawford MJ, Krieg PA. 1994. Cardiac troponin I is a heart-specific marker in the Xenopus embryo: expression during abnormal heart morphogenesis. Dev Biol. 165:432–441. [DOI] [PubMed] [Google Scholar]
- Evans JS, Levine BA. 1980. Protein-protein interaction sites in the calcium modulated skeletal muscle troponin complex. J Inorg Biochem 12:227–239. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, et al. . 1999. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol (Lond). 517(Pt 1):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C-Y, Lee H-C, Tsai H-J. 2009. The molecular structures and expression patterns of zebrafish troponin I genes. Gene Expr Patterns. 9:348–356. [DOI] [PubMed] [Google Scholar]
- Genge CE, et al. . 2016. Functional divergence in teleost cardiac troponin paralogs guides variation in the interaction of TnI switch region with TnC. Genome Biol Evol 8:994–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genge CE, Davidson WS, Tibbits GF. 2013. Adult teleost heart expresses two distinct troponin C paralogs: cardiac TnC and a novel and teleost-specific ssTnC in a chamber- and temperature-dependent manner. Physiol Genomics 45:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis TE, Klaiman JM. 2011. The influence of PKA treatment on the Ca2 + activation of force generation by trout cardiac muscle. J Exp Biol. 214:1989–1996. [DOI] [PubMed] [Google Scholar]
- Grabherr MG, et al. . 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol 29:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SM, Lehman SL. 2016. Functional phosphorylation sites in cardiac myofilament proteins are evolutionarily conserved in skeletal myofilament proteins. Physiol Genomics. 48:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, et al. . 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson LM, et al. . 2009. Intrinsic mechanical properties of the perfused armoured catfish heart with special reference to the effects of hypercapnic acidosis on maximum cardiac performance. J Exp Biol. 212:1270–1276. [DOI] [PubMed] [Google Scholar]
- Hastings KEM. 1997. Molecular evolution of the vertebrate troponin I gene family. Cell Struct. Funct 22:205–211. [DOI] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 35:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC, Storz JF. 2010. Gene cooption and convergent evolution of oxygen transport hemoglobins in jawed and jawless vertebrates. Proc Natl Acad Sci U S A 107:14274–14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC, Storz JF. 2012. Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol Biol Evol 29:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann FG, Storz JF, Kuraku S, Vandewege MW, Opazo JC. 2021. Whole-genome duplications and the diversification of the globin-X genes of vertebrates. Genome Biol Evol. 13:evab205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PM, Cai F, Pineda-Sanabria SE, Corson DC, Sykes BD. 2014. The cardiac-specific N-terminal region of troponin I positions the regulatory domain of troponin C. Proc Natl Acad Sci USA. 111:14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce W, Gesser H, Bayley M, Wang T. 2015. Anoxia and acidosis tolerance of the heart in an air-breathing fish (Pangasianodon hypophthalmus). Physiol Biochem Zool. 88:648–659. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. Modelfinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish JC, et al. . 2001. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 88(10):1059–1065. [DOI] [PubMed] [Google Scholar]
- Khan RT, Musil M, Stourac J, Damborsky J, Bednar D. 2021. Fully automated ancestral sequence reconstruction using FireProtASR. Curr Protoc 1:e30. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick KP, Robertson AS, Klaiman JM, Gillis TE. 2011. The influence of trout cardiac troponin I and PKA phosphorylation on the Ca2 + affinity of the cardiac troponin complex. Journal of Experimental Biology 214:1981–1988. [DOI] [PubMed] [Google Scholar]
- Kuraku S. 2013. Impact of asymmetric gene repertoire between cyclostomes and gnathostomes. Semin Cell Dev Biol 24:119–127. [DOI] [PubMed] [Google Scholar]
- Kuraku S, Meyer A. 2012. Detection and phylogenetic assessment of conserved synteny derived from whole genome duplications. Methods Mol Biol 855:385–395. [DOI] [PubMed] [Google Scholar]
- Kuraku S, Meyer A, Kuratani S. 2009. Timing of genome duplications relative to the origin of the vertebrates: did cyclostomes diverge before or after? Mol Biol Evol 26:47–59. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with bowtie 2. Nat Methods 9:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T, Sonnhammer ELL. 2006. Kalign, kalignvu and mumsa: web servers for multiple sequence alignment. Nucleic Acids Res 34:W596–W599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Solaro RJ, Shah AM. 2005. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66:12–21. [DOI] [PubMed] [Google Scholar]
- Li W, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22:1658–1659. [DOI] [PubMed] [Google Scholar]
- Lombardi R, et al. . 2008. Differential interactions of thin filament proteins in two cardiac troponin T mouse models of hypertrophic and dilated cardiomyopathies. Cardiovasc Res. 79:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DW, Meedel TH, Hastings KE. 1997. Tissue-specific alternative splicing of ascidian troponin I isoforms. Redesign of a protein isoform-generating mechanism during chordate evolution. J Biol Chem 272:32115–32120. [DOI] [PubMed] [Google Scholar]
- Martin-Garrido A, et al. . 2018. Monophosphorylation of cardiac troponin-I at Ser-23/24 is sufficient to regulate cardiac myofibrillar Ca2 + sensitivity and calpain-induced proteolysis. Journal of Biological Chemistry 293:8588–8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLysaght A, Hokamp K, Wolfe KH. 2002. Extensive genomic duplication during early chordate evolution. Nat Genet 31:200–204. [DOI] [PubMed] [Google Scholar]
- Mehta TK, et al. . 2013. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum). Proc Natl Acad Sci U S A 110:16044–16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Schartl M. 1999. Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol 11:699–704. [DOI] [PubMed] [Google Scholar]
- Minh BQ, et al. . 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37:1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, et al. . 2021. Reconstruction of proto-vertebrate, proto-cyclostome and proto-gnathostome genomes provides new insights into early vertebrate evolution. Nat Commun 12:4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpant NJ, et al. . 2010. Pathogenic peptide deviations support a model of adaptive evolution of chordate cardiac performance by troponin mutations. Physiol Genomics 42:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SM, et al. . 2010. Enhanced length-dependent Ca2 + activation in fish cardiomyocytes permits a large operating range of sarcomere lengths. J Mol Cell Cardiol. 48:917–924. [DOI] [PubMed] [Google Scholar]
- Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. 2002. Phosphorylation of troponin I controls cardiac twitch dynamics. Circ Res. 90:649–656. [DOI] [PubMed] [Google Scholar]
- Qiu H, Hildebrand F, Kuraku S, Meyer A. 2011. Unresolved orthology and peculiar coding sequence properties of lamprey genes: the KCNA gene family as test case. BMC Genomics 12:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M, Feng H-Z, Jin J-P. 2022. Evolution of the N-terminal regulation of cardiac troponin I for heart function of tetrapods: lungfish presents an example of the emergence of novel submolecular structure to lead the capacity of adaptation. J Mol Evol 90:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M, Jin J-P.. 2021. Troponin variants as markers of skeletal muscle health and diseases. Front Physiol. 12: 747214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Westfall MV, Schiaffino S, Solaro RJ. 1994. Tension production and thin-filament protein isoforms in developing rat myocardium. Am J Physiol 267:H1589–H1596. [DOI] [PubMed] [Google Scholar]
- Robertson SP, et al. . 1982. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. Journal of Biological Chemistry 257:260–263. [PubMed] [Google Scholar]
- Sacerdot C, Louis A, Bon C, Berthelot C, Roest Crollius H. 2018. Chromosome evolution at the origin of the ancestral vertebrate genome. Genome Biol 19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggin L, Gorza L, Ausoni S, Schiaffino S. 1989. Troponin I switching in the developing heart. J Biol Chem 264:16299–16302. [PubMed] [Google Scholar]
- Shaffer JF, Gillis TE. 2010. Evolution of the regulatory control of vertebrate striated muscle: the roles of troponin I and myosin binding protein-C. Physiol Genomics. 42:406–419. [DOI] [PubMed] [Google Scholar]
- Sheng J-J, Jin J-P. 2016. TNNI1, TNNI2 and TNNI3: evolution, regulation, and protein structure–function relationships. Gene 576:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y-H, et al. . 2015. Cardiac transcriptome and dilated cardiomyopathy genes in zebrafish. Circ Cardiovasc Genet 8:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H. 2002. An approximately unbiased test of phylogenetic tree selection. Syst Biol 51:492–508. [DOI] [PubMed] [Google Scholar]
- Simakov O, et al. . 2020. Deeply conserved synteny resolves early events in vertebrate evolution. Nat Ecol Evol 4:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ, Moir AJG, Perry SV. 1976. Phosphorylation of troponin I and the inotropic effect of Adrenaline in the perfused rabbit heart. Nature 262:615–617. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Rarick HM. 1998. Troponin and tropomyosin. Circ Res. 83:471–480. [DOI] [PubMed] [Google Scholar]
- Strynadka NCJ, James MNG. 1989. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 58:951–999. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Stienen GJM. 2019. Cardiac disorders and pathophysiology of sarcomeric proteins. Physiol Rev. 99:381–426. [DOI] [PubMed] [Google Scholar]
- Wang K, et al. . 2021. African Lungfish genome sheds light on the vertebrate water-to-land transition. Cell 184:1362–1376.e18. [DOI] [PubMed] [Google Scholar]
- Warkman AS, Atkinson BG. 2004. Amphibian cardiac troponin I gene's Organization, developmental expression, and regulatory properties are different from its mammalian homologue. Dev Dyn. 229:275–288. [DOI] [PubMed] [Google Scholar]
- Wei B, Jin J-P. 2016. TNNT1, TNNT2, and TNNT3: isoform genes, regulation, and structure-function relationships. Gene 582:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska BM, et al. . 2001. Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions. J Physiol (Lond). 536:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S, Yaguchi J, Tanaka H. 2017. Troponin-I is present as an essential component of muscles in echinoderm larvae. Sci Rep 7:43563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, et al. 2020. Inference of a genome-wide protein-coding gene set of the inshore hagfish Eptatretus burgeri. BioRxiv (Preprint). 2020.07.24.218818. doi: 10.1101/2020.07.24.218818. [DOI] [Google Scholar]
- Zavala K, Vandewege MW, Hoffmann FG, Opazo JC. 2017. Evolution of the β-adrenoreceptors in vertebrates. Gen Comp Endocrinol 240:129–137. [DOI] [PubMed] [Google Scholar]
- Zerbino DR, et al. . 2018. Ensembl 2018. Nucleic Acids Res. 46:D754–D761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhao J, Mandveno A, Potter JD. 1995. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 76:1028–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Specific accession information is provided, where possible, in the “Supplementary Materials, Supplementary Material online’ file “supplementary Sequence database” (.xlsx file), Supplementary Material online. Sequence alignments are also available in the Supplementary Materials (.txt files), Supplementary Material online.